Abstract
Black women (BLW) have a higher incidence of cardiovascular disease (CVD) morbidity and mortality compared to white women (WHW). Vascular dysfunction is a non-traditional risk factor for CVD and BLW demonstrate impaired vascular function when compared to WHW throughout the lifespan. Several previous studies assessed macrovascular and microvascular function in young BLW compared to WHW, but there has been no recent work exploring this disparity in young women using current, up-to-date methodologies. Therefore, the purpose of this study was to evaluate both macrovascular and microvascular function as assessed by hemodynamic responses to flow-mediated dilation (FMD), following current FMD guidelines, in young adult BLW and WHW. We hypothesized that BLW would demonstrate attenuated macrovascular and microvascular responses to FMD compared to WHW. Macrovascular function was assessed as the percent dilation of the brachial artery following FMD occlusion-cuff release (FMD%). Microvascular function was assessed by total reactive hyperemia area under the curve (RH AUC), calculated as the cumulative increase in brachial artery blood flow above baseline following FMD occlusion-cuff release. Participants were tested in the morning hours during the early follicular phase of their menstrual cycle. Twenty-eight young, apparently healthy women completed the study: 17 WHW (23±4 years) and 11 BLW (24±5 years). FMD% was lower in BLW (WHW: 8.0±1.6, BLW: 6.2±2.4%) (p=0.02), however significance was abolished when FMD% was normalized for shear (WHW: 0.1230±0.0388, BLW: 0.1132±0.0405) (p=0.53). RH AUC was lower in BLW (WHW: 438±133, BLW: 268±66 ml/min) (p<0.001). Young, otherwise healthy BLW demonstrated impaired microvascular function compared to WHW.
Keywords: Flow-mediated dilation, reactive hyperemia, vascular function, racial disparity
Introduction
Black women (BLW) experience a higher incidence of cardiovascular disease (CVD) morbidity and mortality compared to white women (WHW) (Virani et al., 2020; Williams, 2009). BLW also have a higher number of traditional CVD risk factors compared to WHW (Benjamin et al., 2019), likely contributing to the racial disparity in CVD morbidity and mortality. Interestingly, previous studies have also suggested that young and middle-aged black adults have a greater incidence of subclinical CVD compared to age-matched white adults even when controlling for traditional CVD risk factors (Breton et al., 2011; Morris et al., 2013), but these results were not differentiated by sex.
Vascular dysfunction is a non-traditional risk factor for CVD and is predictive of future CVD events, morbidity, and mortality (Anderson et al., 2011; A. C. Philpott et al., 2009; Shechter et al., 2009). Further, lower vascular function has been associated with higher odds of having CVD specifically in black older adults (Cooper et al., 2020). Vascular dysfunction can be more specifically classified as either macrovascular dysfunction, representing the large conduit arteries, or microvascular dysfunction, representing the smaller resistance arteries throughout the body. Generally, microvascular dysfunction precedes macrovascular dysfunction (Anderson et al., 2011), therefore, microvascular function assessments may be of greater utility in younger, otherwise healthy populations. Nevertheless, both macro- and microvascular dysfunction are predictive of future CVD and, when evaluated together, may provide the greatest insight into vascular health and future CVD risk compared to any one assessment alone (Gayda et al., 2015).
Flow-mediated dilation (FMD) is a commonly used, non-invasive assessment of macrovascular function and is strongly associated with future CVD risk (Inaba, Chen, & Bergmann, 2010). Studies comparing macrovascular function between young BLW and WHW using FMD have reported contrasting results. When FMD was traditionally quantified as percent dilation from baseline, Perregaux et al. reported lower FMD in young BLW compared to age-matched WHW, while Bransford and colleagues did not report a significant difference between races (Bransford, St Vrain, & Webb, 2001; Perregaux et al., 2000). Of note, both of these studies were conducted >20 years ago and their methodology does not meet current FMD methodological guidelines (Harris, Nishiyama, Wray, & Richardson, 2010; Thijssen et al., 2011). Therefore, it remains unclear if there is a difference in macrovascular function in young BLW as compared to WHW.
Previous studies have also suggested microvascular dysfunction in young black individuals compared to white individuals (Hurr, Patik, Kim, Christmas, & Brothers, 2018; Jones, Andrawis, & Abernethy, 1999; Ozkor et al., 2014; Patik et al., 2018; Pienaar et al., 2014; Sabbahi, Ellythy, Hwang, & Phillips, 2021). However, Patik et al. was the only study to stratify their results by sex and found that microvascular function was impaired in young BLW compared to WHW when assessed via skin blood flow in response to local heating (Patik et al., 2018). However, a noted drawback of this study was the limited generalizability of those findings to other vascular beds (Hill, Phillips, & Sandow, 2001). BLW also demonstrated microvascular dysfunction when assessed by peak reactive hyperemia (RH) following FMD cuff occlusion (Bransford et al., 2001), however, those findings are in disagreement with those of Perregaux et al., which did not find a difference in RH, quantified as a percent change in brachial artery blood flow from baseline, between races (Perregaux et al., 2000). As previously mentioned, the methodology used in those studies do not meet current FMD guidelines and to the best of our knowledge, there has been no recent work evaluating both macrovascular and microvascular function in the same cohort of young BLW and WHW.
Therefore, the purpose of the present study was to evaluate both macrovascular and microvascular function in young adult BLW and WHW. We hypothesized that both macrovascular and microvascular function, as assessed by hemodynamic responses to FMD while following current FMD guidelines, would be attenuated in young BLW compare to WHW.
Methods
Ethical Approval
This study was approved by the University of Delaware IRB (IRB study # 941369) and was conducted in accordance with the Declaration of Helsinki, except for registration in a database. Written informed consent was obtained from all participants prior to participation.
Participants and Experimental Procedures
Participants were recruited from the Newark, DE area and included healthy women aged 18-35 who self-identified as either white or black, were non-obese (body mass index <30 kg/m2), nontobacco users (<1 cigarette in the past month), and normotensive (resting blood pressure <130/80 mmHg). Following consenting procedures, participants completed a review of medical history, a physical activity questionnaire (GPAQ), and underwent body composition analysis (Tanita TBF-300A). All participants were naturally cycling (i.e., not using any form of hormonal birth control). The vascular function testing visit was scheduled for all participants in the morning hours (i.e., between 7:00am – 11:00am) and during the early follicular phase of their menstrual cycle defined as the first 5 days following the onset of menstruation, which was self-reported by participants. Participants were instructed to report to the laboratory following an overnight fast, and having abstained from caffeine, alcohol, exercise, vitamins, supplements, or over-the-counter medication for ≥24 hours prior to the visit. Upon arrival to the lab, participants underwent assessment of resting vitals and a subset of participants (15 WHW, 7 BLW) underwent intravenous blood sampling for the clinical analysis of fasting blood glucose, a lipid panel, and a complete blood count via spectrophotometry (Quest Diagnostics, Inc., Philadelphia, PA). Participants then rested quietly in the supine position for a minimum of 20-minutes prior to FMD testing.
Flow-Mediated Dilation
Brachial artery FMD was performed in accordance with current guidelines (Harris et al., 2010; Thijssen et al., 2011). A narrow rapidly-inflating cuff (E20 Rapid Cuff Inflation System, Hokanson, WA) was placed immediately proximal to the elbow joint and distal to the imaging site. Duplex ultrasound imaging (Logiq e, General Electric Medical Systems, Milwaukee, WI) was conducted using a linear array ultrasound probe (12 Hz) and a transducer with a Doppler frequency of 5 MHz, with the probe appropriately positioned to maintain an insonation angle of ≤60°. Sample volume was maximized according to vessel size and centered within the vessel based on real-time ultrasound visualization. Following 1-minute of baseline measures, the cuff was rapidly inflated to 250 mmHg for 5-minutes. Brachial artery diameter and blood flow velocity were measured continuously throughout baseline and 2-minutes immediately following cuff deflation. End diastolic electrocardiogram R-wave gated images were collected from the video output of the Logiq e for offline analysis of brachial artery diameter conducted using an automated edge-detection software (Brachial Analyzer for Research, Medical Imaging Applications, Coralville, IA).
Macrovascular function was assessed by the percent dilation of the brachial artery (FMD%), calculated as the maximal percent change in brachial artery diameter following occlusion-cuff release. In addition, the absolute change in brachial artery diameter from baseline to peak was calculated as peak diameter post cuff-occlusion – baseline diameter. Microvascular function was assessed by total RH area under the curve (AUC) (RH AUC) (Rosenberry & Nelson, 2020), quantified as the sum of brachial artery blood flow above baseline for each second during the 2-minutes following FMD occlusion-cuff release, calculated according to the trapezoidal rule using the equation Σ(yi(x(i+1) –xi) +(1/2) (y(i+1) –yi) (x(i+1) –xi)). Brachial artery blood flow was calculated as (Vmean∏) * (vessel diameter/2)2 * 60, where blood flow is in milliliters per minute. Peak RH was quantified as the peak brachial artery blood flow achieved during the 2 minutes following occlusion-cuff release. Shear rate AUC to peak arterial dilation was calculated as 8Vmean/arterial diameter. FMD% was normalized for shear rate AUC to peak dilation using the equation FMD%/shear rate AUC.
Statistical Analyses
Independent samples t-Tests were used to assess group differences and significance was set at α ≤0.05 (SPSS v. 26.0). Data are presented as mean ± standard deviation.
Results
Twenty-eight women (17 WHW, 11 BLW) completed the study protocol. Participant characteristics are displayed in Table 1. Groups were similar in age, body mass index, body fat percentage, blood pressure, self-reported moderate-vigorous physical activity, and most clinical blood values. There were significant differences between WHW and BLW for triglycerides, hemoglobin, and white blood cells, though values for both groups were within normal clinical ranges. On average, WHW were tested on day 3±1 of their menstrual cycle and BLW were tested on day 4±1 of their menstrual cycle.
Table 1.
Participant Characteristics
| Participant Characteristics | WHW | BLW | t-Test |
|---|---|---|---|
| N | 17 | 11 | - |
| Age (years) | 23 ± 4 | 24 ± 5 | p=0.90 |
| Body Mass Index (kg/m2) | 23.9 ± 3.1 | 23.2 ± 4.3 | p=0.62 |
| Body Fat (%) | 30.0 ± 5.6 | 28.5 ± 7.0 | p=0.53 |
| Systolic BP (mmHg) | 112 ± 6 | 113 ± 8 | p=0.87 |
| Diastolic BP (mmHg) | 69 ± 6 | 73 ± 4 | p=0.08 |
| MVPA (hours/week) | 9.8 ± 7.2 | 7.3 ± 5.0 | p=0.37 |
| Clinical Blood Values (n=22) | WHW | BLW | t-Test |
| #Total Cholesterol (mg/dl) | 140 ± 21 | 149 ± 16 | p=0.34 |
| #LDL Cholesterol | 67 ± 15 | 73 ± 11 | p=0.38 |
| #HDL Cholesterol | 59 ± 15 | 67 ± 11 | p=0.21 |
| #Triglycerides | 66 ± 28 | 37 ± 10 | p=0.02* |
| #Hematocrit | 37.3 ± 1.8 | 35.8 ± 2.0 | p=0.09 |
| #Hemoglobin | 12.3 ± 0.7 | 11.5 ± 0.6 | p=0.04* |
| #Red Blood Cells | 4.17 ± 0.21 | 4.26 ± 0.27 | p=0.43 |
| #White Blood Cells | 6.2 ± 1.1 | 4.7 ± 1.3 | p=0.01* |
| #Glucose (mg/dl) | 86 ± 5 | 86 ± 5 | p=0.93 |
Independent Samples t-Tests were used to assess group differences (n=28; 17 WHW, 11 BLW).
Clinical blood values were obtained in a subset of participants (n=22; 15 WHW, 7 BLW).
p < 0.05. WHW, white women; BLW, black women; BP, blood pressure; MVPA, moderate-vigorous physical activity. Data are presented as mean ± SD.
Baseline brachial artery diameters were similar between groups (WHW: 3.52±0.42, BLW: 3.28±0.37 mm) (p=0.14). Macrovascular function assessed by FMD% was lower in the BLW compared to the WHW (p=0.02) (Figure 1A). Though not significantly different between groups, shear rate AUC to peak dilation tended to be lower in the BLW (WHW: 69.0±17.8, BLW: 56.5±18.5 s¯1x103) (p=0.08). When FMD% was normalized for shear rate AUC there was no longer a difference between groups (Figure 1B). Additionally, the absolute change in brachial artery diameter during FMD was lower in the BLW compared to the WHW (WHW: 0.28±0.06, BLW: 0.20±0.08 mm) (p<0.01). Microvascular function assessed by RH AUC was lower in BLW compared to WHW (p<0.001) (Figure 2), while there were no differences in peak RH (WHW: 598±152, BLW: 506±148 ml/min) (p=0.13).
Figure 1. FMD% and FMD%/Shear.

The vasodilatory response to FMD (A) and the vasodilatory response to FMD normalized to shear rate area under the curve up to time of peak dilation (B) are shown for WHW (n=17) and BLW (n=11). Independent samples t-Tests were used to assess group differences. Data are displayed as mean ± SD. * p < 0.05. FMD, flow-mediated dilation; WHW, White women; BLW, Black women.
Figure 2. RH AUC.
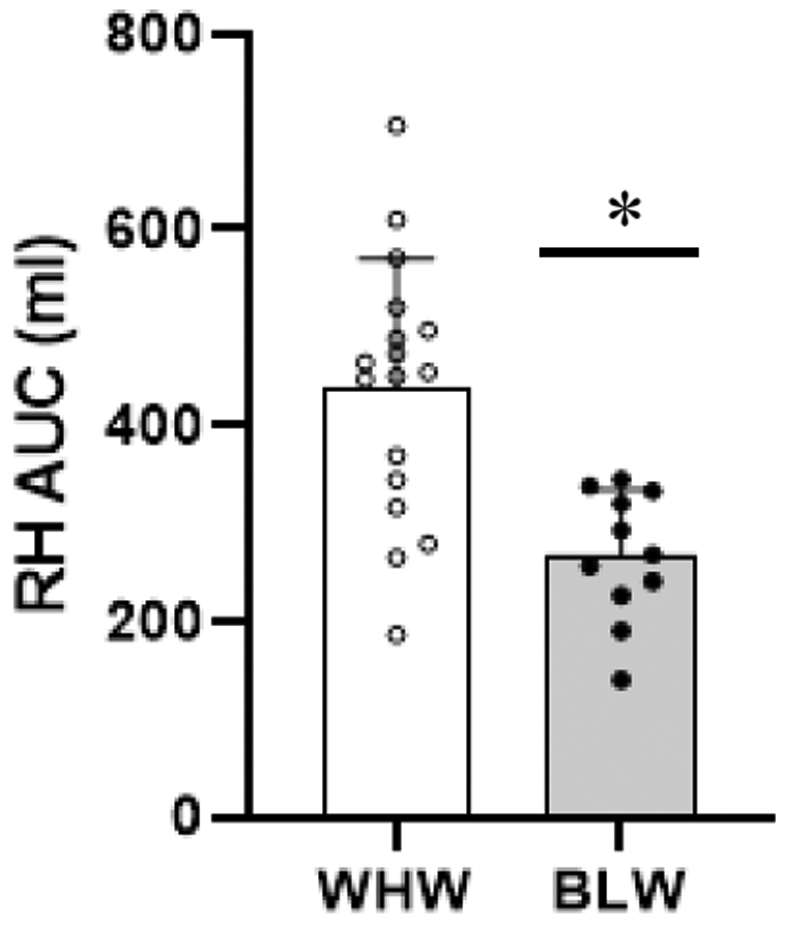
The total hyperemic response to FMD is shown for WHW (n=17) and BLW (n=11). An independent samples t-Test was used to assess group differences. Data are displayed as mean ± SD. * p < 0.001. RH AUC, reactive hyperemia area under the curve; WHW, White women; BLW, Black women.
Discussion
The main finding of the present study is that young BLW demonstrated impaired microvascular function assessed by RH AUC compared to young WHW. These data indicate that a racial disparity in microvascular function is identifiable between BLW and WHW as early as young adulthood, which is important as disturbances in microvascular function are predictive of CVD risk. Although macrovascular function assessed by FMD% was lower in the BLW compared to the WHW, when FMD% was normalized for shear AUC the differences were abolished, suggesting differences in the shear stimulus were driving these responses and macrovascular function appears to be intact. Therefore, these findings contribute to the limited body of literature suggesting that impairments in microvascular function, which likely precede subclinical CVD development, are present in young adult BLW compared to WHW, and may help explain the racial disparities in CVD that are apparent later in life.
Macrovascular Function is Not Different Between BLW and WHW
Over the years it has been clearly demonstrated that FMD% is an independent predictor of future cardiovascular events beyond traditional risk factors (Green, Jones, Thijssen, Cable, & Atkinson, 2011; Inaba et al., 2010). In the present study, macrovascular function, as assessed by FMD%, was lower in BLW compared to WHW. However, recognizing the FMD% response is highly dependent on the stimulus the brachial artery is exposed to and given that the BLW tended to have a lower shear rate AUC than the WHW, FMD% was normalized to shear rate AUC (Pyke & Tschakovsky, 2005; Thijssen et al., 2011). Normalization abolished group differences in FMD% suggesting that a lower stimulus in the BLW likely explains the lower FMD% in this group and thus there are no differences in macrovascular function when comparing BLW and WHW.
Our findings differ from those previously reported by Perregaux et al. (Perregaux et al., 2000) which did detect a racial disparity in macrovascular function assessed by FMD% between young BLW and WHW with no differences in shear stress found between groups. In contrast, previous work by Bransford et al. (Bransford et al., 2001) did not find differences in FMD% between young BLW and WHW. However, recognizing that both these studies were published >20 years ago, the discrepancy in findings may be partly explained by outdated methodology. Specifically, blood velocity and arterial diameter were not continuously measured throughout the protocols in either study, decreasing the likelihood that true peak vasodilation was captured (Harris et al., 2010; Thijssen et al., 2011). Additionally, in the study conducted by Bransford et al. the occlusion-cuff was placed proximal to the imaging site, the cuff was only inflated for 3-minutes, and the menstrual cycle was not controlled for, all of which may confound their findings (Harris et al., 2010; Thijssen et al., 2011). Therefore, by following current methodology guidelines, it is likely that the present study more accurately reflects the macrovascular profiles of young, otherwise healthy BLW and WHW.
Microvascular Function Is Lower in BLW Compared to WHW
The RH response following FMD occlusion-cuff release is dependent on the vasodilatory capacity of downstream micro-vessels and is therefore reflective of microvascular function (Anderson et al., 2011; Meininger, 1987; Rosenberry & Nelson, 2020). Using RH AUC as an assessment of microvascular function in the present study, young BLW demonstrated significantly attenuated RH AUC with a ~39% lower response compared to the young WHW. These findings are in agreement with previous data demonstrating attenuated microvascular function in young BLW compared to WHW when assessed via skin blood flow responses to 39° local heating (Patik et al., 2018) and peak RH quantified during the first 10-seconds following FMD occlusion-cuff release (Bransford et al., 2001). In contrast, Perregaux et al. did not demonstrate any differences in RH between young BLW and WHW when RH was quantified as a percent increase in baseline blood flow measured at 15 seconds post cuff deflation. However, as previously discussed, the methodology employed by both Perregaux et al. and Bransford et al. for assessing RH responses to FMD do not follow current FMD guidelines (Harris et al., 2010). Additionally, although there was no significant difference in peak RH between groups in the present study, the values were in the hypothesized direction such that ~15% lower peak RH values were observed in the BLW.
Currently, there are no universal guidelines for quantifying RH by Doppler ultrasound (Rosenberry & Nelson, 2020) and it is important to consider that studies that have assessed the utility of RH in predicting future CVD risk have mainly evaluated peak RH rather than RH AUC. The mechanisms of peak RH likely differ from the mechanisms controlling RH AUC such that RH AUC may be more reflective of endothelium-dependent vascular function (A. Philpott & Anderson, 2007). However, our findings align with previous studies that suggest reduced NO bioavailability in the microcirculation of young, otherwise healthy black individuals compared to white individuals (Ozkor et al., 2014; Patik et al., 2018). The attenuated RH AUC in the BLW in the present study may be more reflective of endothelium-dependent dysfunction as well as highlights the microvasculature as an important focus, particularly regarding CVD development in young BLW.
Experimental Considerations and Future Directions
It should be noted that although FMD analyses were performed by a single member of the research team, this person was not blinded to the analysis. We also did not assess vascular smooth muscle function; therefore, it remains unknown if the impaired microvascular function observed in the BLW was a result of endothelium-independent dysfunction. Additionally, our sample size was greatly limited by the fact that we only included women who were naturally cycling, therefore, future research should consider including women taking hormonal contraceptives to increase the generalizability of our findings. Further, we only assessed vascular function during the early follicular phase of the menstrual cycle to control for the potential influence of female sex hormones, which is also limiting as women spend a small percentage of their lives in this phase of the menstrual cycle. Therefore, future research should assess both macrovascular and microvascular function between BLW and WHW at multiple time points throughout the menstrual cycle as this will likely provide a more complete understanding of the vascular profiles of these young women. Finally, due to our small sample size and the described limitations, findings from the present study should be used to generate hypotheses for further research elucidating physiological mechanisms contributing to the racial disparity in vascular function observed in young, otherwise healthy BLW and WHW.
Conclusion
The findings from the present study suggest impaired microvascular function in young, otherwise healthy BLW compared to WHW. Given the higher incidence of CVD morbidity and mortality in BLW (Virani et al., 2020) and the utility of microvascular function assessments in predicting future CVD risk (Inaba et al., 2010; A. Philpott & Anderson, 2007), these data highlight the need to assess indices of microvascular function as early as young adulthood, even in otherwise healthy BLW. Identifying methods to limit the progression of microvascular dysfunction in young BLW may help mitigate the racial disparities in CVD that are apparent later in life.
New Findings.
What is the central question of this study?
Is there a racial disparity in macrovascular and/or microvascular function between young black and white women?
What is the main finding and its importance?
Black women (BLW) demonstrated impaired microvascular function but similar macrovascular function compared to white women (WHW). These findings suggest an identifiable racial disparity in microvascular function between BLW and WHW as early as young adulthood. Microvascular dysfunction is predictive of future CVD and generally precedes the development of macrovascular dysfunction. Therefore, these findings also suggest that evaluating microvascular function and CVD risk in young, otherwise healthy BLW are important, as there are known racial disparities in CVD morbidity and mortality in black adults.
Acknowledgements:
The authors would like to thank all study participants as well as Wendy Nichols, RN, and the staff at the Nurse Managed Primary Care Center for their assistance with blood collections and with the processing of clinical labs for this project.
Funding/Support:
This study was supported, in part, by NIH P20 GM113125
Footnotes
Conflict of Interest Disclosures: No conflicts of interest are reported by the authors.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, … Lonn EM (2011). Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation, 123(2), 163–169. doi: 10.1161/CIRCULATIONAHA.110.953653 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, … Stroke Statistics S (2019). Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation, 139(10), e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Bransford TL, St Vrain JA, & Webb M (2001). Abnormal endothelial function in young African-American females: discordance with blood flow. J Natl Med Assoc, 93(4), 113–119. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12653397 [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Wang X, Mack WJ, Berhane K, Lopez M, Islam TS, … Avol E (2011). Carotid artery intima-media thickness in college students: race/ethnicity matters. Atherosclerosis, 217(2), 441–446. doi: 10.1016/j.atherosclerosis.2011.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LL, Musani SK, Moore JA, Clarke VA, Yano Y, Cobbs K, … Fox ER (2020). Clinical Associations of Vascular Stiffness, Microvascular Dysfunction, and Prevalent Cardiovascular Disease in a Black Cohort: The Jackson Heart Study. J Am Heart Assoc, 9(18), e017018. doi: 10.1161/JAHA.120.017018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda M, Juneau M, Tardif JC, Harel F, Levesque S, & Nigam A (2015). Cardiometabolic and traditional cardiovascular risk factors and their potential impact on macrovascular and microvascular function: preliminary data. Clin Hemorheol Microcirc, 59(1), 53–65. doi: 10.3233/CH-141816 [DOI] [PubMed] [Google Scholar]
- Green DJ, Jones H, Thijssen D, Cable NT, & Atkinson G (2011). Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension, 57(3), 363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015 [DOI] [PubMed] [Google Scholar]
- Harris RA, Nishiyama SK, Wray DW, & Richardson RS (2010). Ultrasound assessment of flow-mediated dilation. Hypertension, 55(5), 1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Phillips JK, & Sandow SL (2001). Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev, 21(1), 1–60. doi: [DOI] [PubMed] [Google Scholar]
- Hurr C, Patik JC, Kim K, Christmas KM, & Brothers RM (2018). Tempol augments the blunted cutaneous microvascular thermal reactivity in healthy young African Americans. Exp Physiol, 103(3), 343–349. doi: 10.1113/EP086776 [DOI] [PubMed] [Google Scholar]
- Inaba Y, Chen JA, & Bergmann SR (2010). Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging, 26(6), 631–640. doi: 10.1007/s10554-010-9616-1 [DOI] [PubMed] [Google Scholar]
- Jones DS, Andrawis NS, & Abernethy DR (1999). Impaired endothelial-dependent forearm vascular relaxation in black Americans. Clin Pharmacol Ther, 65(4), 408–412. doi: 10.1016/S0009-9236(99)70135-9 [DOI] [PubMed] [Google Scholar]
- Meininger GA (1987). Responses of sequentially branching macro- and microvessels during reactive hyperemia in skeletal muscle. Microvasc Res, 34(1), 29–45. doi: 10.1016/0026-2862(87)90077-x [DOI] [PubMed] [Google Scholar]
- Morris AA, Patel RS, Binongo JN, Poole J, Al Mheid I, Ahmed Y, … Quyyumi A (2013). Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc, 2(2), e002154. doi: 10.1161/JAHA.112.002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkor MA, Rahman AM, Murrow JR, Kavtaradze N, Lin J, Manatunga A, … Quyyumi AA (2014). Differences in vascular nitric oxide and endothelium-derived hyperpolarizing factor bioavailability in blacks and whites. Arterioscler Thromb Vasc Biol, 34(6), 1320–1327. doi: 10.1161/ATVBAHA.113.303136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patik JC, Curtis BM, Nasirian A, Vranish JR, Fadel PJ, & Brothers RM (2018). Sex differences in the mechanisms mediating blunted cutaneous microvascular function in young black men and women. Am J Physiol Heart Circ Physiol, 315(4), H1063–H1071. doi: 10.1152/ajpheart.00142.2018 [DOI] [PubMed] [Google Scholar]
- Perregaux D, Chaudhuri A, Rao S, Airen A, Wilson M, Sung BH, & Dandona P (2000). Brachial vascular reactivity in blacks. Hypertension, 36(5), 866–871. doi: 10.1161/01.hyp.36.5.866 [DOI] [PubMed] [Google Scholar]
- Philpott A, & Anderson TJ (2007). Reactive hyperemia and cardiovascular risk. Arterioscler Thromb Vasc Biol, 27(10), 2065–2067. doi: 10.1161/ATVBAHA.107.149740 [DOI] [PubMed] [Google Scholar]
- Philpott AC, Lonn E, Title LM, Verma S, Buithieu J, Charbonneau F, & Anderson TJ (2009). Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. Am J Cardiol, 103(11), 1610–1615. doi: 10.1016/j.amjcard.2009.01.376 [DOI] [PubMed] [Google Scholar]
- Pienaar PR, Micklesfield LK, Gill JM, Shore AC, Gooding KM, Levitt NS, & Lambert EV (2014). Ethnic differences in microvascular function in apparently healthy South African men and women. Exp Physiol, 99(7), 985–994. doi: 10.1113/expphysiol.2014.078519 [DOI] [PubMed] [Google Scholar]
- Pyke KE, & Tschakovsky ME (2005). The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol, 568(Pt 2), 357–369. doi: 10.1113/jphysiol.2005.089755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry R, & Nelson MD (2020). Reactive hyperemia: a review of methods, mechanisms, and considerations. Am J Physiol Regul Integr Comp Physiol, 318(3), R605–R618. doi: 10.1152/ajpregu.00339.2019 [DOI] [PubMed] [Google Scholar]
- Sabbahi A, Ellythy A, Hwang CL, & Phillips SA (2021). Differential Responses of Resistance Arterioles to Elevated Intraluminal Pressure in Blacks and Whites. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.01023.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, … Feinberg MS (2009). Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol, 134(1), 52–58. doi: 10.1016/j.ijcard.2008.01.021 [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, … Green DJ (2011). Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol, 300(1), H2–12. doi: 10.1152/ajpheart.00471.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, … Stroke Statistics S (2020). Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation, 141(9), e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Williams RA (2009). Cardiovascular disease in African American women: a health care disparities issue. J Natl Med Assoc, 101(6), 536–540. doi: 10.1016/s0027-9684(15)30938-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


