Abstract
Dectin-1 is a C-type lectin receptor (CLR) expressed on the surface of various mammalian myeloid cells. Dectin-1 recognizes β-glucans and elicits antifungal proinflammatory immune responses. Recent studies have begun to examine the biology of Dectin-1 in previously less explored settings, such as homeostasis, sterile inflammation, and in the central nervous system. Indeed, in certain contexts, Dectin-1 is now known to promote tolerance, and anti-inflammatory and neuroprotective responses. In this review, we provide an overview of the current understanding of the roles of Dectin-1 in immunology beyond the context of fungal infections, mainly focusing on in vivo neuroimmunology studies, which could reveal new therapeutic approaches to modify innate immune responses in neurologic disorders.
Keywords: Dectin-1, Clec7a, CARD9, fungal infections, experimental autoimmune encephalomyelitis (EAE), Alzheimer’s disease, Disease-Associated Microglia (DAM), zymosan, central nervous system (CNS)
Dectin-1: beyond a β-glucan receptor for fungal detection
Dectin-1 is a mammalian C-type lectin receptor (CLR), expressed on the cell surface of myeloid cells. Human and mouse intestinal microfold cells (M cells) [1,2] and airway epithelial cells in the human and mouse lung [3,4] also express Dectin-1/DECTIN-1. In this review, we use “Dectin-1” to indicate mammalian Dectin-1 generally. However, if it is limited to human Dectin-1, it is denoted “DECTIN1.” Dectin-1 was initially identified and studied mainly as a mammalian receptor that detects fungal infections and elicits inflammatory responses. However, recent studies indicate that Dectin-1 can also detect endogenous ligands (Figure 1, Table 1, Box 1), and the outcomes of Dectin-1 stimulation could be anti-inflammatory and tolerogenic. Furthermore, Dectin-1 signaling (Box 2) has been implicated in multiple neuroimmune contexts (Figure 2, Table 2), and dynamic upregulation of Dectin-1 in microglia under pathogenic conditions, or in neurodevelopment in the absence of infections has become apparent. These new findings are changing our traditional understanding of Dectin-1 as a mere receptor for fungal detection or for eliciting inflammation. Importantly, human DECTIN1 polymorphisms have been linked to various diseases (Box 3). In this review, we focus on non-traditional aspects of Dectin-1 biology, particularly its role as a receptor for endogenous ligands, as well as its increasingly appreciated anti-inflammatory and neuroimmune functions.
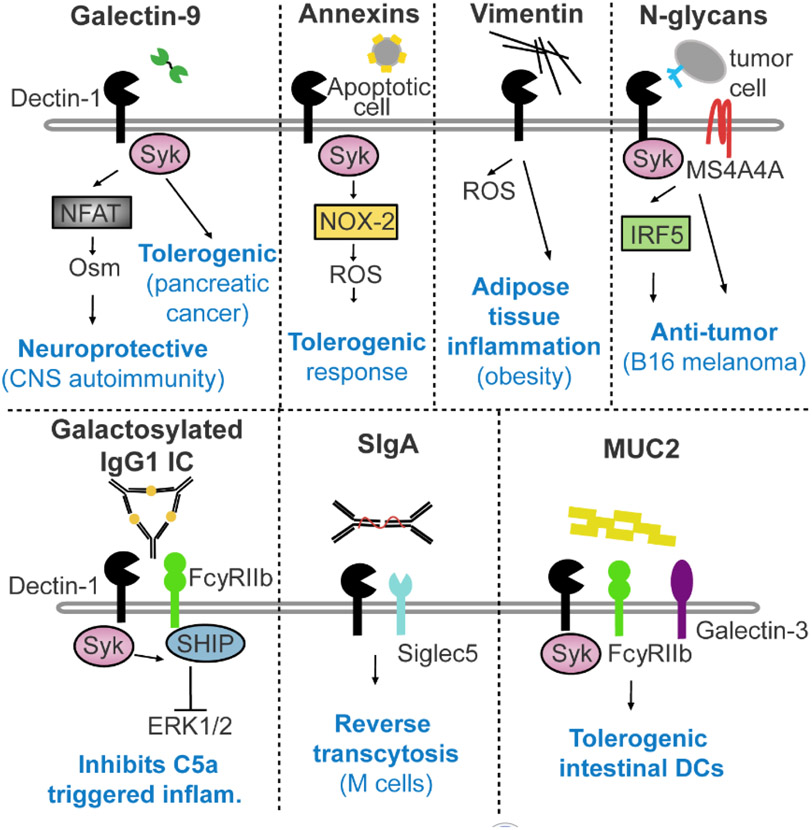
Figure 1. Endogenous Dectin-1 ligands and outcomes.
Endogenous Dectin-1 ligands, which have been previously reported from studies in mice and humans, are indicated. Galectin-9 has been implicated in driving Syk/NFAT-dependent expression of Oncostatin M (Osm) in a mouse model of CNS autoimmunity [15] and promoting a tolerogenic immune response in a mouse model of pancreatic cancer [30]. Annexins on apoptotic cells can trigger a tolerogenic response in myeloid cells via NOX-2 signaling [75], while vimentin can induce reactive oxygen species (ROS) production by human monocytes via Dectin-1 signaling [73,74]. In some cases, Dectin-1 is known to cooperate with other cell surface molecules upon detection of endogenous ligands, specifically working with MS4A4A to detect N-glycans on tumor cells [76], partnering with FcγRIIB to recognize galactosylated IgG1 immune complexes (ICs) [25], binding secretory IgA (SIgA) with Siglec5 [1], and acting with FcγRIIB and Galectin-3 to recognize MUC2 [26]. Signaling mechanisms downstream of Dectin-1 and outcomes of Dectin-1 ligation have been shown to vary depending on the specific ligands. Inflam: inflammation.
Table 1.
Endogenous Dectin-1 ligands
| Initial identification | Downstream effects |
Glycosylation dependent? |
Binding blocked by β- glucan? |
Species | Citations | |
|---|---|---|---|---|---|---|
| Unknown protein expressed on the surface of T cells | Extracellular domain of Dectin-1 bound to surface of T cell lines | T cell proliferation | No | No | Mouse, human | [72,94] |
| Vimentin | Affinity purification MS using anti-zymosan Ab and human atherosclerotic plaque tissue | ROS production | Unk. | Unk. | Human | [73,74] |
| IgG antibody | ||||||
| Galactosylated IgG1 ICs | Dectin-1 dependent function of IgG immune complexes in vivo | With FcγRIIb, inhibited C5a chemotaxis | Yes | Unk. | Mouse | [25] |
| Yes | No | Mouse | [77] | |||
| Core fucose of IgG | Surface plasmon resonance | (IgG) | ||||
| IgA antibody | Dectin-1 dependent reverse transcytosis of SIgA (in vitro and in vivo) | SIgA transcytosis, with Siglec5 | Yes | Yes | Mouse, human | [1] |
| MUC2 | Dectin-1 dependent binding of MUC2 by DCs | With galectin-3 and FcγRIIb, activates β-catenin and tolerogenic immune response | Yes | Unk. | Mouse, human | [26] |
| Galectin-9 | Affinity-purification MS using anti-Dectin1 Ab and mouse pancreatic cancer tissue | p-Syk; tolerogenic immune response; pro-tumorigenic (PDA) | No | Yes | Mouse, human | [15,30] |
| Annexins | Surface plasmon resonance | p-Syk (Tyr348/352); NOX-2; tolerogenic immune response | Unk. | No | Mouse, human | [75] |
| N-glycosylated proteins on cancer cells | Dectin-1 dependent, N-glycan dependent anti-tumor activity (B16 melanoma) | IRF5 activation; promotes anti-tumor activity (B16 melanoma) | Yes | Unk. | Mouse, human | [29,76] |
Unk.: Unknown
Box 1. Endogenous Dectin-1 ligands.
Although Dectin-1 is known to recognize pathogen-associated molecular patterns (PAMPs), accumulating evidence supports the biological significance of endogenous Dectin-1 ligands (Fig. 1, Table 1). Endogenous Dectin-1 signaling may be triggered by cell death, as multiple Dectin-1 ligands are associated with apoptosis or apoptotic cells (annexins [69], vimentin [70], and galectin-9 [71]) as damage-associated molecular patterns (DAMPs). An early study in mice described Dectin-1 on DCs detecting an unknown ligand on the surface of T cells [72]. The ligand on T cells was sensitive to proteases [72], suggesting it was a protein. Dectin-1 also binds N-glycans of some mouse and human tumor cell lines, while the specific N-glycosylated proteins responsible for triggering anti-tumor Dectin-1 signaling also remain unknown [29]. In addition, Dectin-1 can bind proteins in a glycan-independent fashion, e.g., detecting Gal-9 [30]. We also found Gal-9-mediated Dectin-1 signaling induced oncostatin-M (OSM) production, particularly in mouse neutrophils [15]. Vimentin, an intermediate filament protein, was found to bind to human DECTIN1 protein and induced reactive oxygen species (ROS) production in human monocytes [73]. A study using a high-fat diet mouse model suggested that Dectin-1 signaling triggered by Vimentin in adipose tissue promoted obesity and metabolic syndrome [74]. Moreover, recent work demonstrated that human and mouse Dectin-1 functions as a tolerogenic receptor for annexins on the surface of apoptotic cells through selective Syk phosphorylation [75], suggesting a potential bias towards downstream signaling. Recognition of at least some Dectin-1 endogenous ligands requires collaboration with other cell surface molecules (Fig. 1). For example, Dectin-1 associates with Membrane Spanning 4-Domains A4A (MS4A4A), which promotes Dectin-1-mediated response to B16 melanoma, as well as β-glucans [76]. Another study showed that glycosylated IgG1 immune complexes (IC) ligate Dectin-1 through the core fucose -- a major modification of N-glycans [77]. The interaction between Dectin-1 and Siglec-5 in M cells is also involved in reverse transcytosis of ICs, secretory IgA (SIgA), and antigens [1], FcyRIIB and Dectin-1, together with Galectin-3 (Gal-3), also recognize glycosylated residues of MUC2 [26], Gal-3-associated Dectin-1 promotes responses to β-glucans in macrophages [78], Thus, an increasing number of studies have identified the involvement of Dectin-1 in non-infectious settings, and Dectin-1 does not always appear to enhance inflammation. Interrogating Dectin-1 intracellular signaling may help elucidate how distinct endogenous ligands can elicit differing effector functions (e.g. immunogenic vs. tolerogenic) in various physiologic contexts.
Box 2. Dectin-1 signaling pathways.
Dectin-1 possesses an ITAM-like motif (also called a hemi-ITAM) in its cytoplasmic moiety and activates spleen tyrosine kinase (Syk) [79], A main Dectin-1 pathway includes CARD9, which interacts with Maltl and Bell, eventually activating NFkB and extracellular signal-regulated kinase (ERK) to induce the expression of proinflammatory molecules such as TNFa and IL-Ιβ [80-82], Dectin-1 signaling also includes other pathways, such as a Syk-independent Raf-1 pathway [81], Raf-1 promotes β-glucan-induced “trained immunity” in monocytes [83], Dectin-1 signaling also leads to nuclear localization of nuclear factor of activated T-cells (NFAT) via PLCy2 and intracellular Ca2+ flux [84]
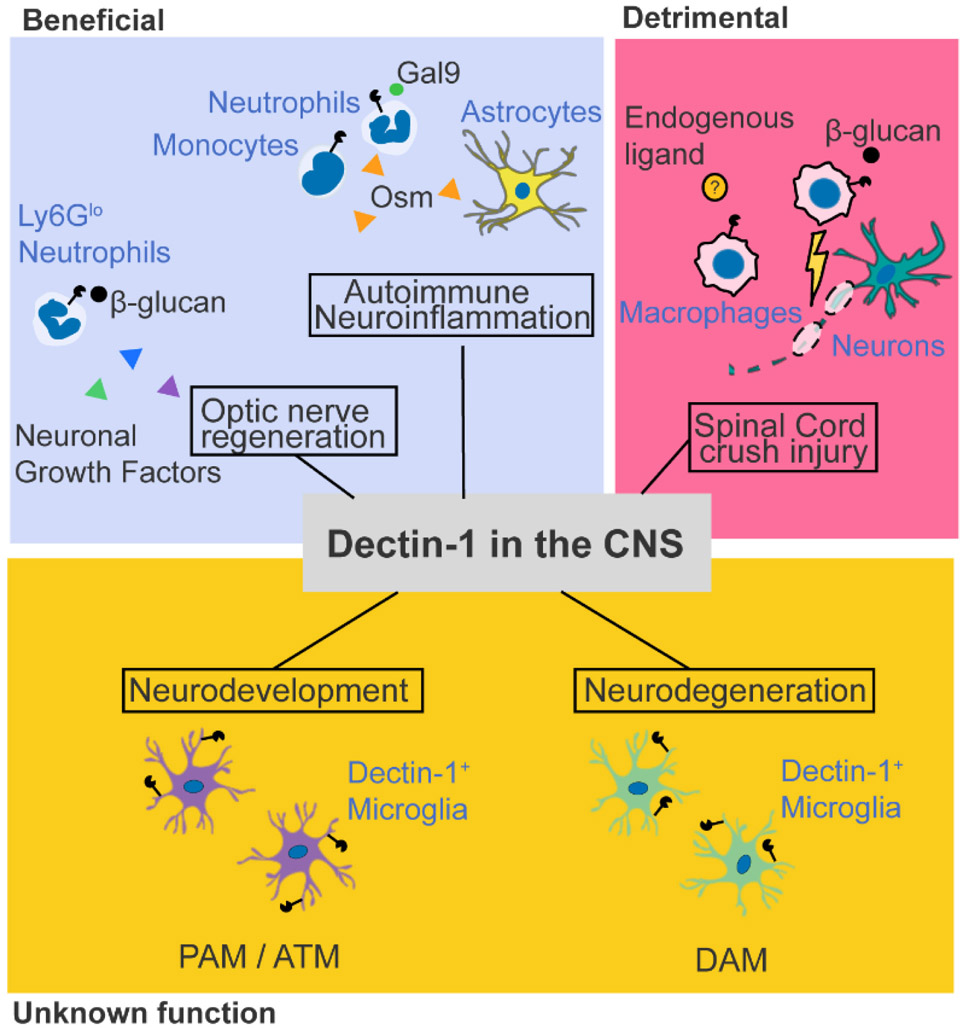
Key figure, Figure 2. Reported Dectin-1 effects in the CNS during sterile pathogenic and non-pathogenic conditions in mice.
Dectin-1, expressed by neutrophils and monocytes, is beneficial in autoimmune neuroinflammation in EAE [15]. Dectin-1, expressed in a subset of Ly6Glo neutrophils, is also beneficial in optic nerve regeneration [45,46] (left upper panel). In contrast, Dectin-1 on macrophages works as a detrimental molecule in spinal cord injury [36] (right upper panel). Enhanced Dectin-1 expression is also observed on subsets of microglia during neurodevelopment (proliferative region-associated microglia, PAM; axon-tract associated microglia, ATM) and neurodegenerative diseases (disease-associated microglia, DAM), although the function of Dectin-1 in these contexts remains unknown [34,65,66] (bottom panel).
Table 2.
Studies of Dectin-1 function in animal models of CNS pathology
| CNS disease model |
Reported Effect |
Approach | Species | Citation |
|---|---|---|---|---|
| Stroke | Detrimental | Laminarin (i.p.); Piceatannol (Syk inhibitor) | Mouse B6 | [95] [96] |
| Spinal cord injury | Detrimental | Zymosan, d-zymosan; Clec7a−/− mice | Mouse B6 | [36] |
| Optic nerve injury | Beneficial | Zymosan, curdlan (i.o.); Clec7a−/−; Card9−/− mice | Mouse B6 | [45,46] |
| EAE | Beneficial [TLR2 focus] | Zymosan (i.p.) | Mouse SJL, B6 | [47] |
| Detrimental [TLR2 focus] | Zymosan (i.c.v.) | Mouse SJL | [48] | |
| Beneficial [Dectin-1 focus] | Clec7a−/−; Card9−/− mice; d-zymosan (i.v.) | Mouse B6 | [15] | |
| CNS infection: Candida albicans | Beneficial | Clec7a−/−; Card9−/− mice | Mouse B6 | [62,63] |
| CNS infection: Neospora caninum | Detrimental | Clec7a−/− mice | Mouse B6 | [63] |
Box 3. DECTIN1 in human genetics.
Human DECTIN1 (CLEC7A) deficiency has been linked to increased susceptibility to fungal infections in multiple studies. In particular, the DECTIN1 Y238X (rsl6910526) variant, which leads to reduced cell surface expression of DECTIN1 [85], has been linked to recurrent vulvovaginal candidiasis [86] and increased risk of opportunistic fungal infections in immunocompromised patients. Specifically, The Y238X (rsl6910526) variant in hematopoietic stem cell transplant recipients has been associated with increased oral and gastrointestinal colonization with Candida sp. [85] and with increased susceptibility to aspergillosis [87], In addition, the Y238X allele has been associated with an increased risk of fungal pathogens and graft dysfunction in patients undergoing lung transplants [88], Beyond rsl6910526, other CLEC7A SNPs have also been linked to increased susceptibility to pulmonary invasive fungal infection in patients with acute myeloid leukemia (rs3901533 and rs7309123) [89] and invasive pulmonary aspergillosis (rs7309123_G/G) [90].
Beyond the context of fungal infections, DECTIN1 polymorphisms have been linked to the severity of asthma [3] and Ulcerative Crohn’s disease [91], although DECTIN1 variants have not been significantly associated with susceptibility to either disease. An association between Y238X and Rheumatoid Arthritis (RA) was investigated, but no significant association between Y238X and RA susceptibility or clinical severity was observed [92].
DECTIN1 polymorphisms have not been studied in the context of neurologic or psychiatric disorders to our knowledge, except one study, which found that the CLEC7A rs2078178 G allele was significantly more frequent in individuals with Asperger syndrome compared to other individuals with autism spectrum disorder (ASD) and correlated with higher IQ scores among ASD patients [93]. The G allele was also more prevalent in control subjects compared to ASD subjects but did not reach statistical significance [93]. The function of Dectin-1 in mouse models of ASD has not been investigated to our knowledge. Future studies are required to clarify whether genetic variation in CLEC7A may impact the susceptibility or severity of CNS disorders, particularly those with emerging evidence from mouse models for Dectin-1 involvement.
Immune functions of Dectin-1
Dectin-1 in infections
Dectin-1 has a well-described role in promoting an antifungal immune response and protects mammalian hosts by detecting fungal β-glucans to trigger reactive oxygen species (ROS) production, phagocytosis, as well as proinflammatory cytokine expression (including TNFα, IL-6, and IL-12). Dectin-1 operates via various signaling pathways (Box 2), including CARD9. Indeed, Dectin-1/CARD9-mediated cytokine production by dendritic cells (DCs) is crucial for promoting Th17 responses via the secretion of IL-1β, IL-6, and IL-23 [5]. Of note, susceptibility to Coccidioides fungal species was linked to a Dectin-1 isoform expressed in C57BL/6 (B6) mice [6]. The isoform is an alternative splice variant devoid of the Dectin-1 stalk region. When the stalkless Dectin-1 was expressed and compared to full-length Dectin-1 in RAW 264.7 cells, the production of TNFα and MIP2/CXCL2 was reduced relative to controls [6]. Thus, this study suggested that the stalk region of Dectin-1 was essential for fungal detection and ensuing cell responses. (For detailed information on Dectin-1 in its signal transduction and fungal infections, we recommend recent excellent review articles [7-10].) Dectin-1 also contributes to regulating immune responses to pathogens other than fungi. These roles include promoting antigen presentation by murine DCs in Salmonella enterica serovar Typhimurium infection in mice [11], enhancing cytokine release by human cell lines in response to Haemophilus influenza infection [12], triggering a microbicidal response to Leishmania infantum in mouse macrophages [13], and promoting TLR2-driven response to Mycobacterium tuberculosis (Mtb) in mouse macrophages [14]. Of note, by using a Dectin-1 signaling reporter cell line, our group demonstrated that Dectin-1 does not recognize heat-killed Mtb H37 Ra in complete Freund’s adjuvant (CFA) [15]. In addition, Dectin-1 is not required for host resistance to Mtb in mice, as well as in vivo survival, suggesting a minor contribution to susceptibility to Mtb infection in mice [16]. Thus, it is questionable if Dectin-1 can detect Mtb. Nevertheless, Dectin-1 has been well characterized in host protection against at least several fungal genera and other microbes.
Dectin-1 in non-infectious diseases
In autoimmunity and allergy, Dectin-1 has both protective and pathogenic functions, depending on context. For example, a single intraperitoneal (i.p). injection of a Dectin-1 agonist, curdlan or laminarin, can exacerbate autoimmune arthritis using the SKG mouse model through pathologic stimulation of DCs [17]. Moreover, in the mouse model of experimental autoimmune uveitis (EAU), Dectin-1 has been reported to be detrimental in one [18] of three studies [19,20]. Dectin-1 can also be detrimental to the host in mouse models of allergy triggered by fungus Aspergillus fumigatus by enhancing proinflammatory cytokine production [21] and ovalbumin (OVA)-induced airway inflammation [22]. In contrast, Dectin-1 limits house-dust mite tropomyosin-mediated allergic asthma in mouse and non-human primates [3,23]. In addition, intraperitoneal (i.p.) injections of β-glucan reduce the development of Type-1 diabetes in the NOD mouse model by inducing regulatory T cells (Tregs) [24]. In experimental epidermolysis bullosa acquisita (EBA) in mice, Dectin-1 inhibits complement C5a-mediated inflammation and resulting skin blisters by recognizing IgG1 immune complexes (ICs) via FcγRIIB [25]. In the mouse gastrointestinal tract, Dectin-1-expressing DCs forms a receptor complex with FcγRIIB and Galectin-3 (Gal3) and recognize MUC2 to induce tolerogenic DCs [26] (Fig. 1). Dectin-1 also limits autoimmune neuroinflammation in experimental autoimmune encephalomyelitis (EAE) (mouse model of multiple sclerosis (MS)) by upregulating oncostatin M (OSM) production via central nervous system (CNS)-infiltrated myeloid cells which detect endogenous Galectin-9 (Gal-9)[15]. In summary, Dectin-1 is increasingly being recognized as a molecula that can regulate autoimmunity and allergy through multiple tolerogenic or anti-inflammatory mechanisms, some of which are triggered by endogenous or non-traditional ligands.
In mouse tumor models, Dectin-1-mediated immune responses appear to alter in vivo outcomes. For example, mouse tumor-associated macrophages stimulated with β-glucan ex vivo gain potent immunostimulatory activity and have limited the growth of Lewis lung carcinoma (LLC) cells when subcutaneously injected together with LLCs in mice [27]. In addition, the adjuvant curdlan triggered Syk-dependent expression of IL-12p70 by DCs and promoted OVA-specific CD8+ T cell cytotoxicity in a mouse tumor model using intravenously injected OVA-expressing B16 melanoma cells [28]. N-glycans on tumor cells can also act as endogenous Dectin-1 ligands to activate IRF-5 in DCs and macrophages and promote anti-tumor immunity via natural killer (NK) cells, as shown in a mouse model of B16F1 cell metastasis [29]. However, Dectin-1 can also elicit detrimental functions by attenuating the anti-tumor immune response: Specifically, Gal-9-triggered Dectin-1 signaling in macrophages enhances tolerogenic T cells using a mouse pancreatic ductal adenocarcinoma (PDA) model [30]. Thus, Dectin-1 can promote or inhibit anti-tumor immunity. These studies suggest that Dectin-1 signaling can be either protective or pathogenic in non-infectious diseases and has context-dependent functions. A better understanding of cell-type and ligand-specific Dectin-1 functions may facilitate a more unified understanding of Dectin-1.
Dectin-1 in the central nervous system
Dectin-1 Impacts microglia
In addition to peripheral innate immune cells, Dectin-1 can also be expressed by CNS-resident macrophages under certain conditions. Indeed, expression of Dectin-1/DECTIN1 (encoded by Clec7a in mice and CLEC7A in humans) is a key feature of the disease-associated microglia (DAM) phenotype, as revealed by single-cell RNA sequencing (scRNAseq) in mice and humans [31-33]. The DAM phenotype was identified in multiple types of neuropathologies, such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), aging, and multiple sclerosis (MS) in humans and their mouse models. (For detailed information on DAM, we recommend the comprehensive review in [32]). As injection of apoptotic neurons in naïve adult mouse brains also induces Dectin-1 expression on microglia, Dectin-1 is thought to be related to sensing cellular damage [31]. Although microglia in adult naïve mice do not express Dectin-1 [15], a subset of microglia in the developing postnatal brain also upregulate Dectin-1 expression [34]. Dectin-1 is also expressed by primary mouse microglia in cultures and in the BV-2 microglial cell line [35]. These cell culture models have been used to study the mechanisms of Dectin-1 signaling in microglia, but whether these findings can translate to Dectin-1 functions in microglia in vivo during development or disease remain to be seen.
Notably, outcomes of Dectin-1 stimulation in microglia can be dissimilar to those in macrophages. For example, Dectin-1 in ex vivo mouse microglia does not elicit robust proinflammatory cytokine expression as macrophages do, despite Dectin-1-mediated phagocytosis and reactive oxygen species (ROS) production [35]. This low cytokine production in primary microglia compared to macrophages appears specific to Dectin-1 signaling, as a robust cytokine response can be observed when these cells are stimulated with zymosan (toll-like receptor TLR2/Dectin-1 ligand) [35]. Dectin-1 signaling in microglia can also inhibit cytokine production during TLR stimulation because primary mouse microglia have shown reduced TNFα and IL-6 production compared to TLR ligands alone, when co-stimulated with β-glucan and TLR ligands (e.g. Pam3CSK4, LPS) [35]. Another study using a rat dorsal column crush (DCC) spinal cord injury model showed that TLR2 and Dectin-1 on macrophages could lead to either neuroprotection or neurotoxicity, respectively, as assessed from the differential outcomes following selective in vivo stimulation with either TLR2 or Dectin-1 [36]. In contrast to these polarized responses, another study using human monocytes and macrophages demonstrated the ability of DECTIN1 and TLR2 (or TLR4) to synergistically promote TNFα [37]. Also, a collaborative induction of inflammatory responses by Dectin-1 and TLR2 was also reported in mouse macrophages [38,39]. We have shown that large particulates such as fungi and zymosan (also a TLR2 ligand), enable the simultaneous activation and crosslinking of Dectin-1 and TLR2 via support from intracellular osteopontin (iOPN); this crosslinking “tethers” the two receptors at their cytoplasmic moieties and lead to elevated antifungal responses in mouse macrophages [40]. Thus, the spatial proximity between Dectin-1 and TLR2 may determine the two pattern recognition receptors (PRR) to be synergistic [40]. This aspect has been studied in macrophages, but it is also possible that microglia do not function as macrophages do in this context. Regarding phagocytosis of zymosan by primary microglia, Dectin-1 acts with complement receptor 3 (CR3) to promote phagocytosis of nonopsonized zymosan. This is unlike mouse macrophages (peritoneal), in which CR3 does not appear to play a significant role in zymosan phagocytosis [41]. Collectively, these studies have suggested that Dectin-1 signaling in microglia is distinct from myeloid cells of hematopoietic origin. Whether these cell type-specific Dectin-1 signaling mechanisms are consistent between ex vivo and in vivo contexts also merits further investigation. The following sections review emerging findings regarding the role of Dectin-1 in multiple myeloid cell types using in vivo models of neuropathology (Fig. 2, Table 2).
Dectin-1 in neuropathology
Dectin-1 in CNS injury
Multiple studies have identified a role for zymosan (Dectin-1/TLR2 agonist) in modulating CNS injury and repair. Microinjection of zymosan into the CNS can lead to axonal injury and demyelination in rodents [42], but zymosan is also known to promote inflammation-triggered regenerative effects. Specifically, local zymosan administration promotes regeneration of axons after optic nerve crush (ONC) injury [43] and regeneration of sensory neurons after dorsal root crush injury in mice [44]. In mouse ONC injury, zymosan promotes axon regeneration via both Dectin-1 and TLR2 signaling [45]. However, TLR2 and MyD88 have not been deemed necessary for axon regeneration. Instead, the axon generation is achieved by the Dectin-1/CARD9 axis that activates cAMP response element binding protein (CREB) [45]. When an intraocular (i.o.) injection of zymosan in ONC injury was combined with CXCR2 antibody i.p. treatment, the neuroprotective and axonogenic effects of zymosan became clearer [46]. The protective effects of zymosan are mediated by the CD14+Ly6Glo neutrophil subset with an ‘alternatively activated’ gene expression profile and the production of multiple growth factor (e.g., NGF, IGF-1) [46]. Thus, zymosan can promote a pro-regenerative and tolerogenic neutrophil phenotype in the setting of ONC injury.
In summary, zymosan and curdlan can elicit neurotoxic and regenerative responses in various myeloid cells via Dectin-1 signaling. Further studies on context and cell-type dependent mechanisms are needed to clarify what determines whether Dectin-1 is neurotoxic or neuroregenerative. However, current studies suggest that zymosan-elicited Dectin-1 signaling may be neurotoxic if primarily mediated by macrophages [36] or as found in healthy tissue [42]. However, Dectin-1 signaling may modulate the ongoing neuroinflammatory response to become more protective and pro-regenerative in the context of pre-existing neuronal injury (as in ONC) and CD14+Ly6Glo neutrophil-mediated cell repair [45,46]. Another question is whether any endogenous molecules can ligate Dectin-1 to contribute to axon dieback and regeneration during CNS injury in the absence of exogenously administered Dectin-1 ligands. If so, this would substantially increase the significance of Dectin-1 function in physiologic responses to CNS injury. In addition, given that endogenous Dectin-1 ligands have been shown to elicit more targeted downstream signaling pathways than zymosan and curdlan, understanding endogenous Dectin-1 signaling in CNS injury may further explain how Dectin-1 can be both neurotoxic and neuroprotective/regenerative.
Dectin-1 in autoimmune neuroinflammation
Initial studies on Dectin-1 in autoimmune neuroinflammation using the EAE mouse model of MS, primarily tested the effect of zymosan on disease development and the peripheral immune response. In particular, intraperitoneal (i.p.) administration of zymosan limited EAE severity in both B6 and SJL mice (immunized with MOG35-55 and PLP139-151, respectively) [47]. The beneficial effect of zymosan administration was proposed to be attributable to the expression of an immunosuppressive cytokine, IL-10; indeed, increased IL-10 production was observed in ex vivo antigen recall assays using splenocytes from zymosan-treated EAE mice [47]. However, whether i.p. zymosan elicited IL-10 expression in EAE depends on TLR2 or Dectin-1 signaling was not tested [47]. Of note, in contrast to i.p. zymosan administration, intracerebroventricular (i.c.v.) zymosan administration during peak EAE triggered an acute severe toxic response and high mortality of mice; however, in these experiments, the relative contributions of TLR2 and Dectin-1 signaling mechanisms in conjunction with zymosan to abate pathology were not examined, and the distinct effects of these mechanistic pathways remain unclear [48]. Thus, this may represent a fruitful area of future investigation.
Recently, by comparing Dectin-1-deficient (Clec7a−/−) B6 mice to wild-type (WT) mice, our group showed that Dectin-1 specifically limits EAE severity [15]. Compared to WT mice, Clec7a−/− mice showed mild autoimmune experimental uveitis (EAU) [18]. The data in the EAU study suggest that Dectin-1 is involved in the enhanced production of IL-23 [18]-- a well-characterized process to sustain pathogenic Th17 responses [49]. The increased IL-23 production in EAU was found in draining lymph nodes of the autoantigen immunization site [18]; moreover, the Dectin-1-mediated proinflammatory response in peripheral lymphoid organs was aligned with the traditionally conceived role for Dectin-1 [18]. Nevertheless, the reasons for the observed different outcomes between EAE and EAU in the absence of Dectin-1 are unexpected and remain unclear, particularly because EAE and EAU models share certain similarities, including protocols of autoantigen peptide subcutaneous injections for disease induction (albeit, between EAE and EAU, not the same peptides are used), as well as the resulting CD4+ T cell activation [15,18]. However, in EAE, the impact of Dectin-1 deficiency has been observed in the CNS, rather than in peripheral lymphoid organs [15]; indeed, the protective effect of Dectin-1 in EAE has been reported to be elicited by CNS-infiltrated myeloid cells producing neuroprotective OSM -- detected by astrocytes [15].
Dectin-1 limits EAE through a mechanism which does not depend on CARD9 and is CNS-specific: Dectin-1- mediated Osm mRNA expression was shown to depend on NFAT but not CARD9 [15]. Specifically, Dectin-1 agonist-induced Osm upregulation was blocked by the NFAT inhibitor, VIVIT, but was preserved in Card9- deficient (Card9−/−) cells. In addition, an endogenous Dectin-1 agonist, Galectin-9 (Gal-9), acted in the CNS to mediate Dectin-1 function, as demonstrated by intrathecal anti-Gal9 blocking antibody administration which exacerbated disease severity in WT but not in Card9−/− mice [15]. Therefore, since astrocytes generate Gal-9 [50,51], the study suggested that crosstalk might occur between astrocytes and CNS-infiltrated myeloid cells in EAE, which limits disease severity [15]. Indeed, understanding the interactions between immune cells and astrocytes is an emerging area of study in multiple types of neuropathology [52], and this particular Dectin-1- mediated interaction could have potential relevance beyond CNS autoimmunity, meriting further investigation. Also, while microglia increased Clec7a gene expression and Dectin-1 protein levels during EAE relative to microglia from naïve mice [15,31], the involvement of microglia in Dectin-1-mediated protection was not apparent. This suggested that hematopoietic-derived myeloid cells are the primary mediators of Dectin-1 function in EAE [15]. Accordingly, active human MS lesions highly express CLEC7A relative to inactive lesions and control tissue samples [15]. Thus, although we still do not know if human DECTIN1 elicits protective responses in the CNS of MS patients, the expression of CLEC7A in MS lesions indicates that DECTIN1 may be poised to elicit its protective CNS function in MS.
Clec7a/Dectin-1 expression in neurodegenerative diseases
Although the function of Dectin-1 in neurodegenerative disorders has not been largely explored, studies have identified Clec7a as one of the major DAM genes, which were initially characterized by scRNAseq in the 5XFAD and APP/PS1 mouse models of AD [31,33]. The DAM phenotype also parallels the microglial neurodegenerative phenotype identified by bulk RNAseq in the SOD1693A mouse model of ALS, as well as in EAE [31]. A study using TREM2-deficient (Trem2−/−) 5XFAD mice suggested a two-stage model of DAM development: The first stage of microglia activation involves downregulation of homeostatic genes along with upregulation of Apoe and the TREM2-signaling adaptor Tyrobp. While the first stage is TREM2-independent, the second stage involving upregulation of Clec7a, Itgax, Trem2, and Axl is TREM2-dependent [33]. This indicates that Dectin-1/Clec7a expression by microglia is a significant feature of the TREM2-dependent stage of DAM phenotype establishment, and understanding the mechanisms driving its upregulation via TREM2 awaits further investigation. In the APP/PS1 mouse model, RNAseq and fluorescence in situ hybridization (FISH) demonstrated that Clec7a was upregulated by APOE4 in the plaque microenvironment, suggesting that CLEC7A expression, modulated by APOE4, might represent a human AD risk protein [53]. In addition, by exhibiting elevated H3K4me3 (trimethylation) and H3K27Ac (acetylation) histone marks, the Clec7a locus was deemed to show increased chromatin accessibility in the APP/PS1 mouse model of AD, relative to WT control mice [54]. Moreover, consistent with a role for TREM2-APOE signaling in promoting Clec7a expression, DAP12 (an adaptor for TREM2, encoded by Tyrobp) promoted Clec7a expression in the APP/PS1 mouse model of AD. Specifically, DAP12-deficient (Tyrobp−/−) APP/PS1 mice showed reduced expression of Clec7a in the prefrontal cortex by RNAseq compared to APP/PS1 control mice [55]. Lastly, deficiency of REV-ERBα (Nr1d1−/−)-- a cellular circadian clock system factor, decreased Clec7a and Trem2 expression and amyloid plaque formation in the 5XFAD mouse model of AD, compared with REV-ERBα-sufficient 5XFAD controls [56]. Collectively, these findings indicate that Dectin-1 (Clec7a) is upregulated by microglia in multiple mouse models of neurodegeneration -- particularly in AD models — and have involved TREM2/DAP12-APOE and REV-ERBα signaling. However, no studies to date have investigated the function of DECTIN1 in the pathogenesis of AD and neurodegeneration more broadly.
Dectin-1 in CNS infections by fungi and protozoans
From another angle, Dectin-1 has been reported to promote ex vivo phagocytosis of Candida albicans fungus by retinal microglia from B6 mice [57] as well as phagocytosis of Lomentospora prolificans by the BV-2 microglial cell line [58]. In in vivo settings, CARD9 studies establish a rationale for understanding the impact of Dectin-1 on CNS infection. As mentioned, CARD9 is a key signaling molecule downstream of Dectin-1 in myeloid cells. For instance, genetic mutations in the CARD9 locus are a major risk factor for acquiring fungal infections in the CNS in humans [59], and have been particularly observed for C. albicans [60] and Aspergillus fumigatus [61]. In B6 mice, microglia-specific Card9 deletion (Card9fl/flCx3cr1CreER, tamoxifen-pulsed 4-6wk prior to infection) impaired neutrophil recruitment to the CNS following systemic (i.v.) C. albicans infection relative to littermate control mice (Card9fl/fl) [62]. Among multiple CARD9-coupled CLRs tested (Dectin-1, Dectin-2, Dectin-3/MCL, Mincle), deletion of any individual receptor was not sufficient to modify CNS neutrophil recruitment. However, combined deletion of Dectin-1 and FcγR (required for Dectin-2, Dectin-3/MCL, and Mincle signaling) did indeed impair CNS neutrophil recruitment in C. albicans infection [62]. This indicates that there may be functional redundancy between CARD9-coupled CLRs in triggering antifungal responses in the CNS. Of note, Dectin-1 (Clec7a−/−) and Dectin-2 (Clec4n−/−) deletion each independently impacted brain fungal burden in C. albicans infected mice without modifying neutrophil recruitment [62]. Further studies are warranted to dissect the CLR-specific pathways upstream of CARD9 in CNS fungal infections and specifically, to identify which CLRs may regulate microglia-specific CARD9 signaling. Indeed, cell type-specific knock-out mice of CLRs will be valuable resources for such studies.
Beyond responding to fungal pathogens, Dectin-1 is also involved in CNS infections by intracellular protozoan parasites, Neospora caninum and Toxoplasma gondii [63,64]. Specifically, Clec7a−/− mice are less susceptible to N. caninum CNS infection than WT mice, suggesting that Dectin-1 might play a pathogenic rather than protective role in this scenario, but this remains to be thoroughly investigated [63]. Regarding T. gondii, the protozoan parasite upregulates Dectin-1 expression in the brain and spleen of outbred mice and has also been found to induce Clec7a expression in BV-2 microglial cells in vitro [64]. Nevertheless, Clec7a−/− mice have not shown significant differences compared to WT mice in terms of animal survival or brain parasite burdens. Thus, although Dectin-1 may be upregulated, it may not be necessary for immunity to CNS infection by T. gondii [63]. In summary, Dectin-1 involvement in CNS infections with fungi and intracellular protozoan parasites has been described, but further studies are required to understand the effects of Dectin-1 and whether it responds directly to pathogens, host DAMPs, or both.
Dectin-1 in neurodevelopment
The function of Dectin-1 in the CNS in non-pathogenic conditions remains largely unknown. However, multiple studies in mice have demonstrated that Dectin-1 is transiently upregulated in a unique subset of white-matter microglia during early postnatal murine brain development [34,65,66]. CD11c+ microglia emerge during postnatal neurodevelopment and highly upregulate Clec7a expression together with other DAM genes, including Spp1 (osteopontin, OPN) and Igf1 (IGF1) [66]. Another study also demonstrated upregulation of Clec7a, along with Itgax (CD11c), Spp1, and Igf1, in microglia specifically located in the corpus callosum of P7 mice [65]. A scRNAseq study further documented Clec7a mRNA and Dectin-1 protein expression in microglia localized in the corpus callosum, cerebellar white matter, and neurogenic niches near the lateral ventricles of P7 mice [34]. Moreover, co-expression of Clec7a and DAM-associated genes (Spp1, Igf1) in microglia with transient early postnatal Itgax expression was noted in these mice [34,67]. Of note, Dectin-1-expressing microglia from WT early postnatal mice displayed more amoeboid-shaped cell bodies than microglia, which did not express Dectin-1. Also, based on immunostaining, Dectin-1-expressing microglia appeared to have phagocytosed dying oligodendrocytes [34,65]. Due to their localization within white matter and neurogenic niches, the Dectin-1 expressing microglia subset was named as “proliferative region-associated microglia” (PAM) [34]. Both the PAM phenotype and a concurrently identified “axon-tract associated microglia” (ATM) phenotype described in a separate study [67] were characterized by expression of multiple adult DAM phenotype marker genes beyond Clec7a. This suggests that there may be phenotypic or even functional parallels between a subset of developmental microglia and DAM. However, unlike the adult DAM phenotype [31], Dectin-1 expression by microglia was not dependent on TREM2 or APOE in early postnatal mice [34], indicating that distinct signaling mechanisms may be regulating the PAM/ATM and DAM phenotypes. An increase in Dectin-1+ microglia was also observed in white-matter regions of the developing cerebellum (P10-14) of mice with a deficiency in Npc1 (Npcnmf164)-- a genetic risk factor for Nieman Pick Disease [68]. This suggested that changes in Dectin-1+ microglial populations might play a role in certain neurodevelopmental disorders. Further work with microglia-specific Dectin-1 deletion will be necessary to understand the putative functional roles of Dectin-1 in PAM (or ATM) during neurodevelopment and potentially reveal possible new functions of innate immunity in the brain, in health and disease.
Concluding Remarks
Although Dectin-1 has not been traditionally studied in the context of sterile inflammation and CNS physiology, multiple studies now indicate that Dectin-1 might regulate neuroinflammation across multiple types of neuropathology, acting through both hematopoietic-derived myeloid cells and microglia. Future studies on Dectin-1 in homeostasis and disease would benefit from considering the role of recently described endogenous Dectin-1 ligands and their potential for eliciting ligand-specific downstream signaling pathways (See Outstanding Questions). However, untangling the distinct roles of emerging endogenous Dectin-1 ligands remains an obstacle to understanding the function of Dectin-1 in non-infectious settings. In addition, using genetic approaches to comprehend the cell type-specific contributions of Dectin-1 will be essential, as microglial Dectin-1 may have a distinct role from Dectin-1 in CNS-infiltrating myeloid cells. In summary, emerging evidence demonstrates that Dectin-1 may have diverse previously unappreciated functions in non-infectious settings, and its role in the CNS is a promising area for future research.
Outstanding Questions.
Do endogenous Dectin-1 ligands trigger specific intracellular signaling responses that are distinct from those triggered by microbial ligands?
Do microglia harbor specific Dectin-1 signal transduction mechanisms compared to myeloid cells of hematopoietic origin?
How do intracellular signal transduction pathways involving Dectin-1 and other pattern- recognition receptors interact at the molecular level?
What are the key factors determining whether Dectin-1-mediated outcomes are beneficial or detrimental? In what contexts?
What are the roles of Dectin-1 in the CNS during neurodegenerative diseases, neurodevelopment, and aging?
What are the roles of Dectin-1 in DAM, ATM, and PAM?
Do rodent Dectin-1 and human DECTIN1 share similar roles?
Can Dectin-1 be tested as a putative therapeutic target to treat specific diseases?
Highlights.
Dectin-1 is a mammalian C-type lectin receptor with essential functions in innate immunity — particularly against fungal infections.
Multiple Dectin-1 endogenous ligands and functions have been uncovered beyond itd role in fungal infections
Depending on context, the outcomes of Dectin-1 signaling can be either beneficial or detrimental during sterile inflammation, as evidenced from mouse models.
New roles of Dectin-1 in the central nervous system are emerging
Acknowledgments
This study was supported by the National Institute of Health (F30-AI140497 to M.E.D.; R01-AI088100, R01-NS120417 to M.L.S.) and National Multiple Sclerosis Society (RG-1801-30040; PP-1509-06274 to M.L.S.).
Glossary
- amyloid plaque:
plaque accumulation consisting primarily of a 40–42 amino acid peptide of amyloid-β (Aβ) and a hallmark of AD pathology. Plaques become insoluble and deposit within the brain extracellular space; typically associated with swollen, dystrophic neurites, astrogliosis, and activated microglia which together comprise a neuritic plaque.
- axon dieback:
axonal retraction; axons in spinal tracts retract away from the initial site of injury.
- axon-tract associated microglia (ATM):
microglia subset associating closely with axonal tracts and highly expressing Spp1, Gpnmb, Igf1, CD68, and Lgals3. ATM were identified by scRNAseq in neonatal (P4-5) mouse corpus callosum and cerebellum.
- β-glucan:
Naturally occurring β-D-glucose polysaccharides. These glucose polymers are constituents of the cell walls of cereals, bacteria, and fungi. Fungal β-glucans contain 1-6 side branches, while cereal β-glucans contain both β-1,3 and β-1,4 backbone bonds.
- CARD9:
member of the CARD (caspase-associated recruitment domain) protein family. CARD9 is an adaptor protein in signaling pathways triggered by multiple pattern recognition receptors; activates cytokine expression, regulates inflammation and apoptosis
- Complement receptor 3 (CR3):
Mac-1, integrin αMβ2, or CD11b/CD18; expressed on phagocytic cells, minor subsets of B and T cells, and NK cells; functions as an adhesion molecule and a membrane receptor-mediating recognition of diverse ligands, such as ICAM-1 and iC3b on complement-opsonized objects.
- complete Freund’s adjuvant (CFA):
emulsion with antigen peptides for immunization to elicit cell-mediated immunity in experimental settings.
- DAMPs:
endogenous molecules released when cells are under stress and/or undergoing cell death. Many pattern recognition receptors (PRRs) can detect both PAMPs and DAMPs. An example is TLR4.
- Disease-associated microglia:
subset of microglia showing a unique transcriptional and functional signature associated with the expression of genes mainly linked to AD and other neurodegenerative conditions.
- Experimental autoimmune encephalomyelitis:
Animal model of MS. EAE is an autoimmune inflammatory CNS disease in which both innate and adaptive immune systems are involved.
- Immune Complex (IC):
Formed by the binding of an antibody to a soluble antigen (antigen-antibody complex).
- Nieman Pick Disease:
group of metabolic disorders known as lipid storage diseases; result from the deficiency of a lysosomal enzyme, acid sphingomyelinase. Lipids accumulate in the spleen, liver, lungs, bone marrow, and brain.
- NOD mouse model:
Nonobese diabetic (NOD) mice spontaneously develop destructive autoimmune pancreatic insulitis as early as four weeks of age and are used as a Type-1 diabetes (T1D) model.
- PAMPs;
derived from microorganisms; mainly drive innate immune responses to infections. An example is lipopolysaccharide (LPS), a ligand for TLR4. If the microbe of interest is not pathogenic, the term microbe-associated molecular patterns (MAMPs) is used.
- Plaque microenvironment:
In plaque-forming diseases, such as AD and atherosclerosis, plaque microenvironments significantly impact cellular pathology.
- Proliferative region-associated microglia (PAM):
early-postnatal microglial subset identified in P7 mouse brain white matter. PAM highly express genes such as Spp1, Gpnmb, Igf1, Itgax, and Lgals, sharing a characteristic gene signature with DAM, although the appearance of PAM do not depend on a TREM2-APOE axis in DAM. PAM have amoeboid morphology, are metabolically active, and phagocytose newly-formed oligodendrocytes.
- Regulatory T cells (Tregs):
subpopulation of T cells that suppress immune responses to maintain homeostasis and self-tolerance; generally Foxp3+CD4+ T cells.
- SKG mouse model:
spontaneous mutation in ZAP-70 in a BALB/c colony; develop chronic autoimmune arthritis.
- Th17 responses:
CD4+ T helper cells characterized by the production of IL-17; are involved in the pathogenicity of various conditions, particularly in autoimmune diseases, and host protection against fungi. Th17 responses constitute IL-17-producing cells but also refer to any Th17-associated immune response.
- Toll-like receptors:
cell surface or endosomal pattern-recognition receptors; play various crucial roles in innate immunity. TLRs are well-known to be expressed by myeloid cells, but lymphoid cells can express some TLRs.
- Trained Immunity:
Innate immune ‘memory’ induced by epigenetic reprogramming; can be elicited by exogenous or endogenous stimulation of innate immune cells and lead to an altered response towards a second challenge after returning to a non-activated state from the first stimulation. β-glucan, LPS, and the bacillus Calmette-Guerin (BCG) vaccine have been often used to induce trained immunity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rochereau N et al. (2013) Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 11, e1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rochereau N et al. (2021) NOD2 deficiency increases retrograde transport of secretory IgA complexes in Crohn's disease. Nat Commun. 12, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gour N et al. (2018) Dysregulated invertebrate tropomyosin-dectin-1 interaction confers susceptibility to allergic diseases. Sci Immunol. 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yong HM et al. (2021) Epigenetic regulation of epithelial dectin-1 through an IL-33-STAT3 axis in allergic disease. Allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeibundGut-Landmann S et al. (2007) Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 8, 630–638 [DOI] [PubMed] [Google Scholar]
- 6.del Pilar Jimenez AM et al. (2008) Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes Immun. 9, 338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolakopoulou C et al. (2020) C-Type Lectin Receptors in Antifungal Immunity. Adv Exp Med Biol. 1204, 1–30 [DOI] [PubMed] [Google Scholar]
- 8.Tang J et al. (2018) Regulation of C-Type Lectin Receptor-Mediated Antifungal Immunity. Front Immunol. 9, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond RA and Lionakis MS (2016) Mechanistic Insights into the Role of C-Type Lectin Receptor/CARD9 Signaling in Human Antifungal Immunity. Front Cell Infect Microbiol. 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tone K et al. (2019) C-type lectin receptors of the Dectin-1 cluster: Physiological roles and involvement in disease. Eur J Immunol. 49, 2127–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson N et al. (2014) Recognition of Salmonella by Dectin-1 induces presentation of peptide antigen to type B T cells. Eur J Immunol. 44, 962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyl KA et al. (2014) Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. mBio. 5, e01492–01414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefevre L et al. (2013) The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity. 38, 1038–1049 [DOI] [PubMed] [Google Scholar]
- 14.Yadav M and Schorey JS (2006) The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 108, 3168–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deerhake ME et al. (2021) Dectin-1 limits autoimmune neuroinflammation and promotes myeloid cell-astrocyte crosstalk via Card9-independent expression of Oncostatin M. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marakalala MJ et al. (2011) The Syk/CARD9-coupled receptor Dectin-1 is not required for host resistance to Mycobacterium tuberculosis in mice. Microbes Infect. 13, 198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshitomi H et al. (2005) A role for fungal {beta}-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med. 201, 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoppelkamp S et al. (2015) Murine pattern recognition receptor dectin-1 is essential in the development of experimental autoimmune uveoretinitis. Mol Immunol. 67, 398–406 [DOI] [PubMed] [Google Scholar]
- 19.Brown BR et al. (2017) Fungal-derived cues promote ocular autoimmunity through a Dectin-2/Card9-mediated mechanism. Clin Exp Immunol. 190, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EJ et al. (2016) Mincle Activation and the Syk/Card9 Signaling Axis Are Central to the Development of Autoimmune Disease of the Eye. J Immunol. 196, 3148–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lilly LM et al. (2012) The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J Immunol. 189, 3653–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han W et al. (2021) Ovalbumin-Induced Airway Inflammation Is Ameliorated in Dectin-1-Deficient Mice, in Which Pulmonary Regulatory T Cells Are Expanded through Modification of Intestinal Commensal Bacteria. J Immunol. 206, 1991–2000 [DOI] [PubMed] [Google Scholar]
- 23.Gu C et al. (2021) Dectin-1 Controls TSLP-Induced Th2 Response by Regulating STAT3, STAT6, and p50-RelB Activities in Dendritic Cells. Front Immunol. 12, 678036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karumuthil-Melethil S et al. (2014) Fungal beta-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J Immunol. 193, 3308–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsten CM et al. (2012) Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 18, 1401–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan M et al. (2013) Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 342, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M et al. (2015) Dectin-1 Activation by a Natural Product beta-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype. J Immunol. 195, 5055–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leibundgut-Landmann S et al. (2008) Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 112, 4971–4980 [DOI] [PubMed] [Google Scholar]
- 29.Chiba S et al. (2014) Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. Elife. 3, e04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daley D et al. (2017) Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med. 23, 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krasemann S et al. (2017) The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity. 47, 566–581 e569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deczkowska A et al. (2018) Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell. 173, 1073–1081 [DOI] [PubMed] [Google Scholar]
- 33.Keren-Shaul H et al. (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 169, 1276–1290 e1217 [DOI] [PubMed] [Google Scholar]
- 34.Li Q et al. (2019) Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron. 101, 207–223 e210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah VB et al. (2008) Beta-glucan activates microglia without inducing cytokine production in Dectin-1-dependent manner. J Immunol. 180, 2777–2785 [DOI] [PubMed] [Google Scholar]
- 36.Gensel JC et al. (2015) Toll-Like Receptors and Dectin-1, a C-Type Lectin Receptor, Trigger Divergent Functions in CNS Macrophages. J Neurosci. 35, 9966–9976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferwerda G et al. (2008) Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 10, 2058–2066 [DOI] [PubMed] [Google Scholar]
- 38.Gantner BN et al. (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 197, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown GD et al. (2003) Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 197, 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue M et al. (2011) Cutting edge: critical role of intracellular osteopontin in antifungal innate immune responses. J Immunol. 186, 19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown GD et al. (2002) Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 196, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popovich PG et al. (2002) The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 61, 623–633 [DOI] [PubMed] [Google Scholar]
- 43.Yin Y et al. (2003) Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 23, 2284–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinmetz MP et al. (2005) Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 25, 8066–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baldwin KT et al. (2015) Neuroinflammation triggered by beta-glucan/dectin-1 signaling enables CNS axon regeneration. Proc Natl Acad Sci U S A. 112, 2581–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sas AR et al. (2020) A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H et al. (2013) Low dose zymosan ameliorates both chronic and relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 254, 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luz A et al. (2015) The role of CNS TLR2 activation in mediating innate versus adaptive neuroinflammation. Exp Neurol. 273, 234–242 [DOI] [PubMed] [Google Scholar]
- 49.Bettelli E et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441, 235–238 [DOI] [PubMed] [Google Scholar]
- 50.Itoh N et al. (2018) Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc Natl Acad Sci U S A. 115, E302–E309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steelman AJ and Li J (2014) Astrocyte galectin-9 potentiates microglial TNF secretion. J Neuroinflammation. 11, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanmarco LM et al. (2021) Functional immune cell-astrocyte interactions. J Exp Med. 218, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitz NF et al. (2020) Trem2 deficiency differentially affects phenotype and transcriptome of human APOE3 and APOE4 mice. Mol Neurodegener. 15, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y et al. (2020) Characterization of the chromatin accessibility in an Alzheimer's disease (AD) mouse model. Alzheimers Res Ther. 12, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haure-Mirande JV et al. (2019) Integrative approach to sporadic Alzheimer's disease: deficiency of TYROBP in cerebral Abeta amyloidosis mouse normalizes clinical phenotype and complement subnetwork molecular pathology without reducing Abeta burden. Mol Psychiatry. 24, 431–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J et al. (2020) Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer's disease. Aging Cell. 19, e13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maneu V et al. (2011) Dectin-1 mediates in vitro phagocytosis of Candida albicans yeast cells by retinal microglia. FEMS Immunol Med Microbiol. 63, 148–150 [DOI] [PubMed] [Google Scholar]
- 58.Pellon A et al. (2018) Microglial immune response is impaired against the neurotropic fungus Lomentospora prolificans. Cell Microbiol. 20, e12847. [DOI] [PubMed] [Google Scholar]
- 59.Snarr BD et al. (2020) It's all in your head: antifungal immunity in the brain. Curr Opin Microbiol. 58, 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drummond RA et al. (2015) CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog. 11, e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rieber N et al. (2016) Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI Insight. 1, e89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drummond RA et al. (2019) CARD9(+) microglia promote antifungal immunity via IL-1beta- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 20, 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.da Silva MV et al. (2017) Dectin-1 Compromises Innate Responses and Host Resistance against Neospora caninum Infection. Front Immunol. 8, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan J et al. (2014) Dectin-1-CD37 association regulates IL-6 expression during Toxoplasma gondii infection. Parasitol Res. 113, 2851–2860 [DOI] [PubMed] [Google Scholar]
- 65.Hagemeyer N et al. (2017) Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 134, 441–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wlodarczyk A et al. (2017) A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammond TR et al. (2019) Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity. 50, 253–271 e256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyle BR et al. (2020) NPC1 deficiency impairs cerebellar postnatal development of microglia and climbing fiber refinement in a mouse model of Niemann-Pick disease type C. Development. 147, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weyd H et al. (2013) Annexin A1 on the surface of early apoptotic cells suppresses CD8+ T cell immunity. PLoS One. 8, e62449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moisan E and Girard D (2006) Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J Leukoc Biol. 79, 489–498 [DOI] [PubMed] [Google Scholar]
- 71.Wiersma VR et al. (2013) Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 33 Suppl 1, E102–126 [DOI] [PubMed] [Google Scholar]
- 72.Ariizumi K et al. (2000) Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 275, 20157–20167 [DOI] [PubMed] [Google Scholar]
- 73.Thiagarajan PS et al. (2013) Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc Res. 99, 494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castoldi A et al. (2017) Dectin-1 Activation Exacerbates Obesity and Insulin Resistance in the Absence of MyD88. Cell Rep. 19, 2272–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bode K et al. (2019) Dectin-1 Binding to Annexins on Apoptotic Cells Induces Peripheral Immune Tolerance via NADPH Oxidase-2. Cell Rep. 29, 4435–4446 e4439 [DOI] [PubMed] [Google Scholar]
- 76.Mattiola I et al. (2019) The macrophage tetraspan MS4A4A enhances dectin-1- dependent NK cell-mediated resistance to metastasis. Nat Immunol. 20, 1012–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manabe Y et al. (2019) The Core Fucose on an IgG Antibody is an Endogenous Ligand of Dectin-1. Angew Chem Int Ed Engl. 58, 18697–18702 [DOI] [PubMed] [Google Scholar]
- 78.Esteban A et al. (2011) Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci U S A. 108, 14270–14275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dambuza IM and Brown GD (2015) C-type lectins in immunity: recent developments. Curr Opin Immunol. 32, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jia XM et al. (2014) CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med. 211, 2307–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gringhuis SI et al. (2009) Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 10, 203–213 [DOI] [PubMed] [Google Scholar]
- 82.Gross O et al. (2006) Card9 controls a non-TLR signalling pathway for innate antifungal immunity. Nature. 442, 651–656 [DOI] [PubMed] [Google Scholar]
- 83.Quintin J et al. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 12, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goodridge HS et al. (2007) Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 178, 3107–3115 [DOI] [PubMed] [Google Scholar]
- 85.Plantinga TS et al. (2009) Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 49, 724–732 [DOI] [PubMed] [Google Scholar]
- 86.Ferwerda B et al. (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 361, 1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cunha C et al. (2010) Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 116, 5394–5402 [DOI] [PubMed] [Google Scholar]
- 88.Calabrese DR et al. (2019) Dectin-1 genetic deficiency predicts chronic lung allograft dysfunction and death. JCI Insight. 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen MJ et al. (2019) Dectin-1 rs3901533 and rs7309123 Polymorphisms Increase Susceptibility to Pulmonary Invasive Fungal Disease in Patients with Acute Myeloid Leukemia from a Chinese Han Population. Curr Med Sci. 39, 906–912 [DOI] [PubMed] [Google Scholar]
- 90.Sainz J et al. (2012) Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS One. 7, e32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iliev ID et al. (2012) Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 336, 1314–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plantinga TS et al. (2010) Functional consequences of DECTIN-1 early stop codon polymorphism Y238X in rheumatoid arthritis. Arthritis Res Ther. 12, R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennabi M et al. (2015) Dectin-1 Polymorphism: A Genetic Disease Specifier in Autism Spectrum Disorders? PLoS One. 10, e0137339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willment JA et al. (2001) Characterization of the human beta -glucan receptor and its alternatively spliced isoforms. J Biol Chem. 276, 43818–43823 [DOI] [PubMed] [Google Scholar]
- 95.Ye XC et al. (2020) Dectin-1/Syk signaling triggers neuroinflammation after ischemic stroke in mice. J Neuroinflammation. 17, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu X et al. (2021) Inhibition of Dectin-1 Ameliorates Neuroinflammation by Regulating Microglia/Macrophage Phenotype After Intracerebral Hemorrhage in Mice. Transl Stroke Res. [DOI] [PubMed] [Google Scholar]