Abstract
The prevalence and incidence of early-onset dementia among adults with Autism Spectrum Disorder (ASD) is currently unknown. In this case-control study, the prevalence and incidence of early-onset dementia in individuals with ASD was examined during 2008 – 2012 using Medicaid Analytic eXtract files. Participants were 30–64 year-old adults who were Medicaid beneficiaries and had either a diagnosis of ASD only (n=12,648), a diagnosis of ASD with co-occurring Intellectual Disability (n=26,168), a diagnosis of ID without ASD (n=406,570), or no ASD nor ID diagnoses (n=798,828). The 5-Year prevalence of dementia was 4.04% among adults with ASD only, and 5.22% for those with ASD and co-occurring ID. This prevalence was higher compared to the prevalence of dementia in individuals with no ASD and no ID (0.97%), but lower compared to individuals with ID only (7.10%). Risk factors associated with the increased prevalence in the general population were similarly associated with the increased risk of dementia in individuals with ASD. Even after adjusting for these risk factors, compared to the general population, dementia was found to occur more frequently in individuals with ASD only (adjusted hazard ratio, 1.96; 95% CI, 1.69–2.28), as well as individuals with ASD and co-occurring ID (adjusted hazard ratio, 2.89; 95% CI, 2.62–3.17).
In conclusion, adults with ASD under the age of 65 were approximately 2.6 times more likely to be diagnosed with dementia compared to the general population in our study.
Lay Abstract
It is unclear whether adults diagnosed with Autism Spectrum Disorder are at higher risk of being diagnosed with early-onset dementia compared to those who are not on the autism spectrum. In this study we examined for the first time the nationwide prevalence and incidence of Alzheimer’s Disease and other types of dementia in ASD in a sample of adults with ASD aged 30–64 years who were enrolled in Medicaid, the largest insurer of behavioral health services in the US. Medicaid claims data, which include information on the diagnoses that beneficiaries receive, suggested that the adults with Autism Spectrum Disorder were approximately 2.6 times more likely to be diagnosed with early-onset Alzheimer’s disease and related dementias compared to the general population.
Research on Autism Spectrum Disorder (ASD) in the past decade has witnessed a growing focus on early diagnosis and intervention. Conversely, less research attention has been devoted to the characteristics and treatment needs of adults with ASD, including co-occurring conditions and manifestations associated with aging. For example, it is currently unclear whether individuals with ASD, compared to the general population, are at a higher risk of being diagnosed with early-onset Alzheimer’s disease and other forms of dementias (hereinafter early-onset dementia; i.e., dementia manifesting before age 65). This is a critical question, given the personal and societal impact of early-onset dementia, and the importance of adequate service provision for affected individuals (Mendez, 2019; Rossor et al., 2010).
ASD has a growing prevalence, currently estimated at 1 in 54 among school-age children in the U.S. (Maenner et al., 2020), and its lifetime cost of care is estimated at $3.6 million per person in the U.S. (Cakir et al., 2020). Similarly, the number of individuals diagnosed with Alzheimer’s disease and other forms of dementias is increasing, currently affecting 10% of the North American population age 65 and older, and increasing direct costs of care, with per person costs estimated to reach $ 350,000 in 2018, and global costs expected to reach $1.1 trillion by 2050 (Alzheimer’s Association, 2019). Additionally, 3–5% of individuals with Alzheimer’s disease and other forms of dementias develop symptoms before age 65, resulting in additional personal and societal costs (Mendez, 2019; Rossor et al., 2010). Recent data indicate a prevalence of early-onset dementia of 74.3 per 100,000 in the general population under 65 (Chiari et al., 2021). Against this background, the intersectionality between these conditions represents a pressing public health issue. Indeed, the lack of knowledge on the co-occurrence of these conditions is likely to result in inadequate service provision and poor understanding of the social and economic implications for affected individuals, their care partners, and the society, leaving policymakers without an evidence base for formulating policies and programs. Additionally, understanding the co-occurrence of ASD with early-onset dementia might provide insight on the nature, mechanisms and treatment for each condition. For example, studies documenting the high prevalence of dementia in Down syndrome have paved the way for research uncovering a partial overlap in neuropathology between the two conditions, suggesting shared biological mechanisms and directions for intervention (Hartley et al., 2015).
Although the importance of building an evidence base in this area has been repeatedly highlighted by scholars in the field (Happé & Charlton, 2012; Howlin & Taylor, 2015), only a few studies have examined the co-occurrence of ASD and dementia, providing inconsistent findings. In a study surveying caregivers of 142 older persons with cognitive impairment, it was found that those reported to have ASD (n= 23) had significantly younger age at the onset and more pronounced severity of cognitive impairment compared to non-ASD samples, suggesting that ASD might be a risk factor for earlier onset of dementias (Rhodus et al., 2020). An important limitation of the study, however, was the use of a survey instrument to identify ASD that was not validated for older adults. Another investigation involving 1,754 individuals reported to have ASD as a cause of death in the National Vital Statistics System (Barnard-Brak, Richman, & Yang, 2019) identified 105 individuals (6%) with a dementia-related disorder listed as an additional cause of death, which was lower than for individuals without ASD (11%).
Through a database examination of Harvard Clinical and Translational Science Center records involving a sample of 265 individuals with ASD older than 55, Oberman and Pascual-Leone (2014) documented that fewer than 10 individuals (less than 3%) were reported to have an additional diagnosis of dementia, in contrast to 17% of individuals with a schizophrenia diagnosis (prevalence in the general population was not reported in the study). In the same study, an experimental protocol involving a sample of 35 individuals with ASD between the ages of 18 and 64 evidenced atypically heightened cortical plasticity compared to a matched control group, as indexed by greater duration of modulation to a repetitive transcranial magnetic stimulation paradigm. As Alzheimer’s disease and other forms of dementias are associated with cortical hypoplasticity (Pascual-Leone et al., 2011), the authors theorized that cortical hyperplasticity confers a protection against developing dementia in adults with ASD. Additional explanations for the putative safeguard effect of ASD against dementia have focused on the larger brain volume and increases in white matter connections often documented in ASD, which might provide additional “cognitive reserve” protecting against the impact of aging and/or dementia (Happe’ & Charlton, 2012). Another potential scenario is that individuals with ASD engage less in some behaviors that increase risk for dementia compared to the general population, such as substance abuse (Croen et al., 2015) leading to the putative safeguard effect.
Nevertheless, additional research has provided only partial support for the safeguard effect of ASD against age-related cognitive deterioration, or evidence for the contrary. For example, Lever and Geurts (2016) reported reduced age-related visual memory declines in aging individuals with ASD compared to a comparison group without ASD; however, typical age-related declines in ASD were reported across other cognitive domains. Further, a study by Croen et al. (2015) based on a sample of 1507 adults with ASD and 15,070 individuals without ASD from the Kaiser Permanente Medical Care Program in Northern California reported a higher prevalence of dementia in those with ASD (2.3% versus 0.5% in the control group). Another large study (Hand et al., 2020) comparing physical and mental health conditions in Medicare beneficiaries with ASD (n=4,685) and without ASD (n=46,850), reported increased prevalence of cognitive disorders in the ASD population (25.2% versus 4.9% in the general population). However, as the diagnosis of dementia was included in a broader category encompassing several conditions, limited inference can be drawn from the study regarding the specific risk for dementia. Another study using National Core Indicators surveys (Kats et al., 2013) reported the prevalence of dementia in adults with ASD with co-morbid intellectual disability to be approximately 2% – higher than for individuals without ASD in the same age group (1%), although the difference was not significant. The notion of increased risk for dementia in ASD has been examined by scholars arguing that cognitive impairments that frequently occur in ASD (e.g., executive functioning difficulties) might interact with age-related cognitive changes (e.g., declines in working memory and processing speed) resulting in a steeper cognitive decline in this population (Geurts & Vissers, 2012).
Additional research has documented a potential overlap in the etiologic factors across ASD and dementia, with shared genetic mutations being recently identified (Ivashko-Pachima et al., 2017). Further, treatment options for adults across both conditions may share promising yields; for example, use of dementia medications can mitigate impulsivity and improve social, communicative, and cognitive functioning for adults with ASD (Rossignol & Frye, 2014). While these research efforts raise intriguing questions on the intersectionality of dementia and ASD, conclusive inferences on dementia-related risk or protection are precluded by the paucity of data and challenges with the ascertainment of dementia in individuals with ASD, including communication challenges that complicate the identification of dementia symptoms in ASD (particularly in those with co-occurring intellectual disability), the presence of overlapping manifestations between the two conditions (e.g., difficulties in executive functioning), and the lack of practice guidelines to guide diagnosis of dementia in this population. Additionally, the lack of conclusive evidence on the risk of dementia in ASD reflects methodological shortcomings of existing research, including limitations with size and representativeness of samples, as well as limited consideration of the medical and psychiatric conditions that might confound the ascertainment of the true co-occurrence between ASD and dementia. Furthermore, previous literature has not provided information specific to the rates of dementia in individuals with ASD only versus those with co-occurring ASD and intellectual disability. Intellectual disability is a prominent risk factor in dementia, including early-onset dementia (Coppus, 2013; Huang et al., 2018), and a highly prevalent comorbidity in ASD, affecting up to 40% of school age children (Christensen et al., 2014) - although statistics vary substantially across studies (Lord et al., 2018). Examination of the prevalence of early-onset dementia in individuals with ASD with and without intellectual disability might provide critical insight on the nature of the association between ASD and early-onset dementia, including whether risk for early-onset dementia in ASD is linked to ASD itself or the co-occurring presence of intellectual disability. Additionally, no research effort to our knowledge has focused on the co-occurrence of early-onset as opposed to late-onset forms of dementia in ASD.
The current study examined for the first time the nationwide prevalence and incidence of Alzheimer’s Disease and other types of dementia in ASD using a Medicaid-enrolled sample of adults with ASD aged 30–64 years. Medicaid is the largest insurer of behavioral health services in the US, and among the only insurers available to individuals with ASD across the lifespan. We used this data source to characterize the prevalence and incidence of early-onset dementia in individuals with ASD as compared to individuals with ID who do not have ASD, as well as to a national random sample of Medicaid enrollees without ASD or ID.
Methods
Standard protocol approvals, registrations, and patient consents
The use of this deidentified database for research was approved by the institutional review board of Drexel University (protocol # 1603004379). The institutional review board granted a waiver of informed consent.
Data and study populations
We used Medicaid Analytic eXtract (MAX) data from 2008 – 2012, which include individual demographic information and eligibility classification (e.g., poverty, disability) in a personal summary file, as well as service files for inpatient, other outpatient/ambulatory therapy, long-term care, and prescription drug claims that contain information on diagnoses, treatments, and reimbursement. A unique Medicaid identifier allows linking of multiple claims for an individual over time. All Medicaid information for the study years was included in the dataset used in the study. The process used to identify the study population and analytic datasets from the MAX dataset is illustrated in figure 1.
Figure 1 -.
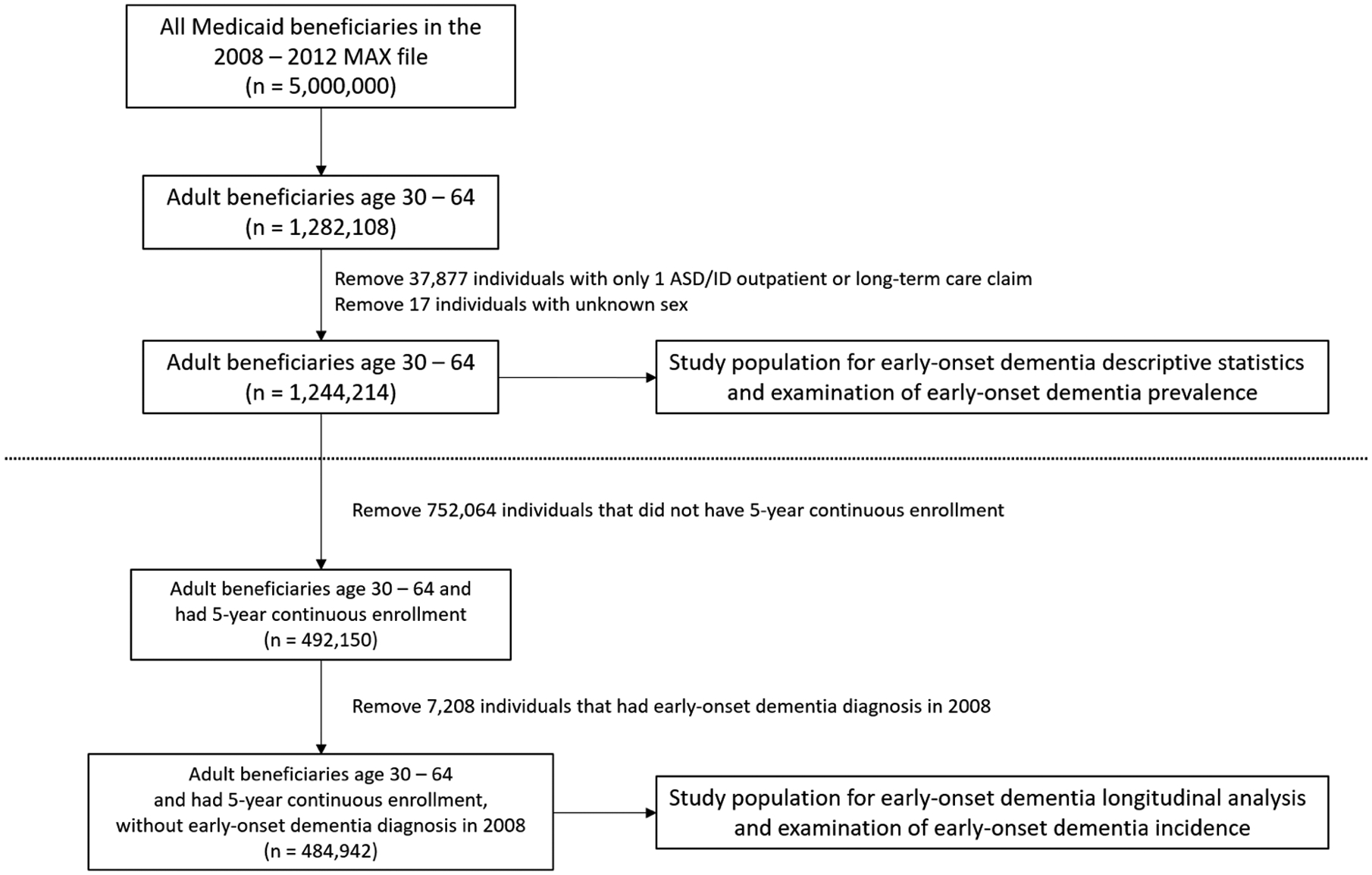
Process used to identify the study population and analytic datasets
Our initial sample included all individuals with an ASD (299.xx) or intellectual disability (317.xx-319.xx) ICD-9 diagnosis codes (total = 1.6M Medicaid enrollees) and a 3.4M person random sample of non-ASD, non-ID diagnosed Medicaid enrollees, ages 0–64. Following exclusion of those outside our target age range (which was ages 30–64, based on the age of early-onset dementia onset) and/or missing information on sex, 1,244,214 individuals were included for analyses of prevalence of dementia in the population. For analyses of incidence of early-onset dementia in the ASD population, following further exclusions of those without continuous enrollment over our 5-year follow-up period and those with dementia diagnoses at baseline, 484,942 individuals were included for analysis.
Data for individuals age 30 to 64 were extracted for the following four diagnostic categories of interest, further described in Table 1.
Table 1.
Characteristics of the four groups of interest stratified according to demographics and features known to be associated with risk of early-onset dementia, including sample compositions used for the prevalence analysis and for the incidence analyses sample
| Prevalence analyses sample | Incidence analyses sample | |||||||
|---|---|---|---|---|---|---|---|---|
| ASD only | ASD + ID | ID only | GP | ASD only | ASD + ID | ID only | GP | |
| N | 12,648 | 26,168 | 406,570 | 798,828 | 7,573 | 22,229 | 310,059 | 145,081 |
| Age at First Enrollment 1 (%) | ||||||||
| 30–50 | 80.42 | 81.33 | 69.04 | 74.74 | 82.29 | 82.30 | 71.08 | 65.20 |
| 51–64 | 19.58 | 18.67 | 30.96 | 25.26 | 17.71 | 17.70 | 28.92 | 34.80 |
| Sex Male (%) | 67.39 | 69.60 | 53.41 | 37.94 | 68.08 | 70.08 | 52.88 | 36.32 |
| Race/Ethnicity (%) | ||||||||
| White | 73.25 | 72.07 | 69.11 | 45.05 | 72.84 | 71.88 | 69.27 | 48.74 |
| Black | 13.12 | 17.30 | 18.97 | 20.47 | 14.26 | 17.41 | 18.84 | 25.09 |
| Asian/Pacific Islander | 1.79 | 1.68 | 1.64 | 5.02 | 1.90 | 1.72 | 1.68 | 5.37 |
| Hispanic/Latino | 5.09 | 3.99 | 5.58 | 22.65 | 4.87 | 4.07 | 5.68 | 15.25 |
| Other | 6.74 | 4.96 | 4.70 | 6.81 | 6.13 | 4.92 | 4.53 | 5.55 |
| Urbanicity | ||||||||
| Urban | 81.72 | 82.10 | 77.65 | 83.49 | 81.71 | 82.01 | 77.14 | 80.92 |
| Suburban | 10.56 | 10.69 | 13.29 | 9.32 | 10.70 | 10.77 | 13.67 | 10.57 |
| Rural | 7.50 | 6.86 | 8.68 | 6.88 | 7.36 | 6.86 | 8.81 | 8.36 |
| Missing | 0.22 | 0.35 | 0.38 | 0.32 | 0.24 | 0.35 | 0.38 | 0.15 |
| Medicaid Eligibility Category 2 (%) | ||||||||
| Poverty | 4.25 | 0.28 | 0.40 | 30.10 | 1.65 | 0.09 | 0.15 | 13.40 |
| Disability | 88.46 | 98.15 | 98.47 | 39.32 | 96.17 | 98.73 | 99.20 | 72.69 |
| Other | 7.29 | 1.57 | 1.13 | 30.59 | 2.18 | 1.18 | 0.66 | 13.90 |
| Depression (%) | 42.93 | 24.81 | 27.88 | 19.60 | 37.46 | 23.65 | 26.75 | 34.52 |
| Any Other Mental Disorder(s) (%) | 73.77 | 75.50 | 58.90 | 33.86 | 72.80 | 75.32 | 58.61 | 54.84 |
| Cardiovascular Disease Risk Factor(s) (%) | 42.98 | 39.23 | 43.27 | 33.32 | 42.63 | 39.01 | 43.02 | 56.39 |
Age at first observed Medicaid enrollment, starting in 2008.
The most common Medicaid eligibility category for the enrolled period.
ASD only group.
Inclusion in the ASD only diagnostic group required 2 outpatient or 1 inpatient claim with a diagnosis associated with ASD (ICD-9 codes 299.xx) and with no co-occurring intellectual disability diagnosis (ICD-9 codes 317.xx – 319.xx). This strategy has been used to select individuals with ASD in previous research using claims data (Burke et al., 2014).
ASD+ID group.
The ASD+ID group was based on the same ASD requirement as the ASD only group, but also had a co-occurring ID diagnosis (2 outpatient claims or 1 inpatient claim associated with an ID diagnosis of 317.xx – 319.xx) during the study period.
ID only group.
The ID only group had an ID diagnosis (2 outpatient claims or 1 inpatient claim associated with an ID diagnosis of 317.xx – 319.xx) and no ASD diagnosis during the study period. Individuals with Down syndrome were excluded due to the high prevalence of dementia in the Down syndrome population.
General population group.
The general population group (GP group) included a random sample of individuals who were Medicaid enrolled with no ASD or ID diagnosis during the study period. Individuals with any claims (including a single claim) of ASD or ID were not included in the sample.
Statistical analyses
Descriptive statistics were calculated for each of the diagnostic categories of interest. Common risk factors for dementia were compared across these groups in univariate analysis. Prevalence of dementia in the four groups was examined as a period prevalence estimate over the 5 years of enrollment and by year, with denominators calculated according to the total enrolled. Incidence rates were calculated for each group as early-onset dementia cases arising over the 2009–2012 period (2008 was used as the washout period). Cox regression models were used to calculate crude and adjusted hazard ratios and 95% confidence intervals for dementia in each of the ASD only, ASD + ID, and ID only groups, relative to the general population group. Adjusted models included the following covariates: sex, age (in years, at first month of observed enrollment), race/ethnicity (as indicator variables for Asian/Pacific Islander, Black, Hispanic, and Other, with White as the referent group), urbanicity (as an indicator for rural/suburban, with urban as the referent group), poverty status (as indicated by Medicaid eligibility category), a diagnosis of depression, presence of another mental health diagnosis, cardiovascular disease risk factors (see supplementary materials for the specific diagnostic codes) and state. Selection of the covariate variables was motivated by previous literature indicating associations between increased risk of dementia and sex (Mielke, 2018), age (Alzheimer’s Association, 2019), race/ethnicity (Mehta & Yeo, 2017), urbanicity (Weden et al., 2017), poverty status (Samuel et al., 2020), cardiovascular disease (Stefanidis et al., 2018), depression (Almeida et al., 2017), and other mental health diagnoses (Cai & Huang, 2018). Additionally, we included states to account for potential state-level variations in Medicaid eligibility assessment requirements. Urbanicity was coded using zip codes and Rural-Urban Commuting Area Codes, which denote metropolitan, micropolitan, small town, and rural commuting areas. If an individual resided in a metropolitan area, they were coded as “urban”; if they resided in a micropolitan area, they were coded as “suburban”; and if they resided in a small town or rural area, they were coded as “rural”. As 0.34% of the data on urbanicity were missing, the missing indicator method was used to handle these missing data. There were no additional missing covariate data in the dataset. Specific ICD-9 codes for each variable of interest are reported on Appendix 1.
Data Availability
Because of the data use agreements associated with the use of the Medicaid data, we are not permitted to release the raw data used in our analyses.
Results
Characteristics of each group and sample compositions for the prevalence and incidence analyses are reported in table 1, where it can be seen that, consistent with the previous literature, males were over-represented in the ASD samples, and many individuals with ASD, particularly those without co-occurring ID, had a diagnosis of depression or other psychiatric conditions. Contrary to the general population group, the preponderant Medicaid enrollment category in the ASD groups, as well as the ID only group, was Disability. Additionally, unlike in the general population group, most individuals in the ASD and ID groups were White.
Prevalence
Of 38,816 Medicaid beneficiaries with ASD, 1877 had dementia (4.84%). When stratified according to the presence/absence of co-occurring ID, dementia was present in 511 out of the 12,648 individuals who had ASD only (4.04%), and in 1,366 out of 26,168 individuals who had ASD+ID (5.22%). This prevalence was higher than the general population of Medicaid beneficiaries who had no ID nor ASD (n=798,828, prevalence of dementia=0.97 %). Finally, dementia was present in 28,871 out of 406,570 individuals who had ID only (7.10%), indicating an increased prevalence of dementia in individuals with ID (not attributable to Down syndrome) compared to the general population as well as individuals with a diagnosis of ASD (with or without co-occurring ID) in the Medicaid population <65. These data are illustrated in Figure 2. The prevalence of dementia in each group by year followed a similar pattern; individuals with ID had the highest prevalence rate, followed respectively by individuals with ASD and ID, individuals with ASD and no ID, and the general population.
Figure 2.
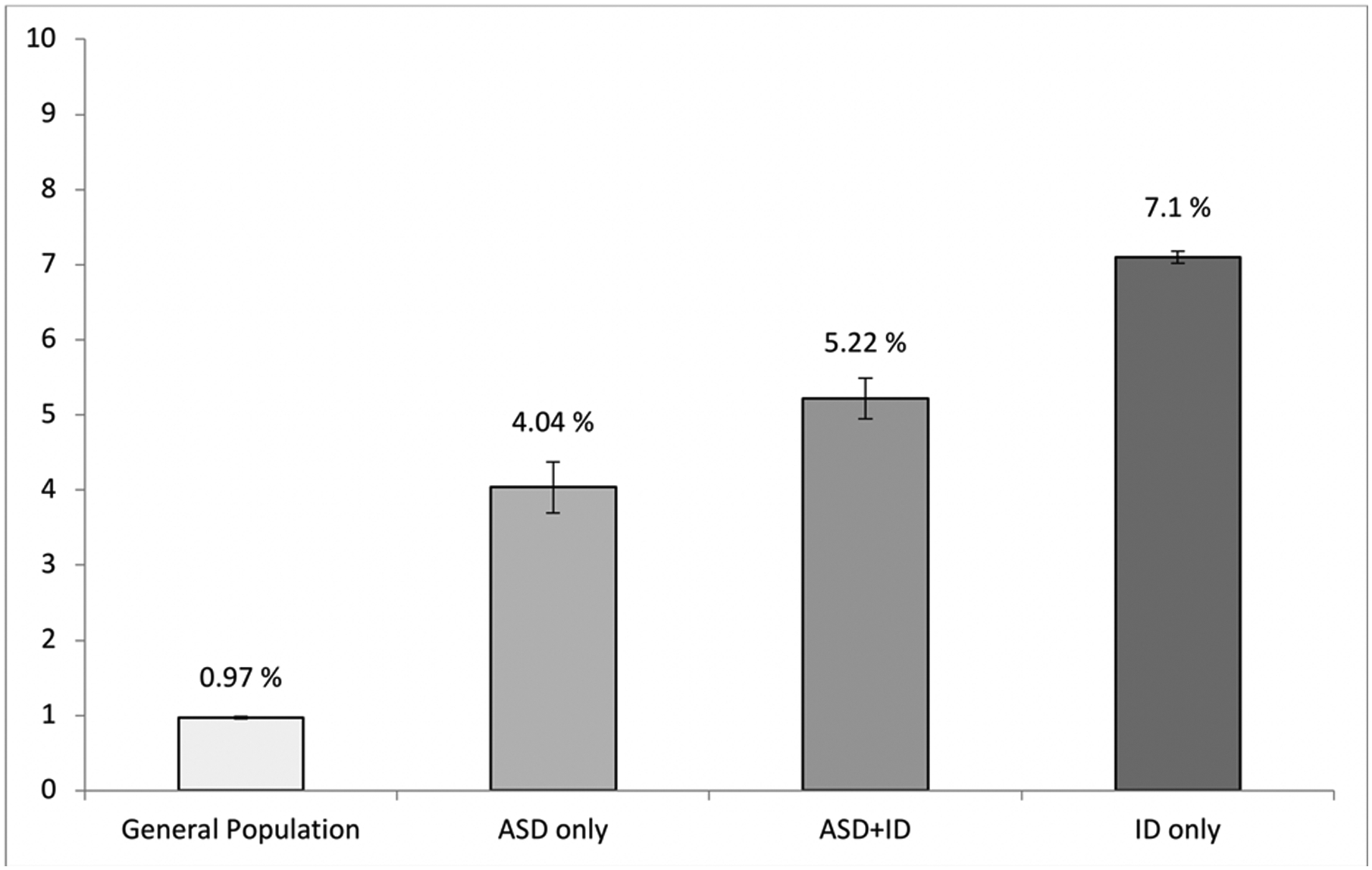
Prevalence of early-onset dementia in the four groups. Error bars represent 95% confidence interval
Additionally, we examined the prevalence of dementia in the four groups of interest according to age group (dichotomized as 30–50 and 51–64 age groups), sex, race, urbanicity status, poverty status, presence/absence of depression, other psychiatric conditions, and presence/absence of cardiovascular disease risk factors (which included diagnoses of obesity, hypertension and diabetes; see appendix 1 in the supplementary materials for diagnostic codes). As reported in table 2, across all groups the prevalence of dementia was higher in individuals who were older than 50, and had depression or any other psychiatric condition, or cardiovascular risk factors (p < 0.01 across all risk factors).
Table 2.
Prevalence of early-onset dementia in the 4 groups according to sex, age group, race, urbanicity, poverty, presence/absence of depression, other psychiatric conditions, and cardiovascular disease risk factors
| ASD only | ASD + ID | ID only | GP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 12,648) | (n = 26,168) | (n = 406,570) | (n = 798,828) | ||||||
| N | % | N | % | N | % | N | % | ||
| Dementia | 511 | 4.04 | 1,366 | 5.22 | 28,871 | 7.10 | 7,767 | 0.97 | |
| Age at First Enrollment1 | 30–50 | 288 | 2.83 | 899 | 4.22 | 13,061 | 4.65 | 2,517 | 0.42 |
| 51–64 | 223 | 9.01 | 467 | 9.56 | 15,810 | 12.56 | 5,250 | 2.60 | |
| Sex | Male | 321 | 3.77 | 908 | 4.99 | 14,975 | 6.90 | 3,855 | 1.27 |
| Female | 190 | 4.61 | 458 | 5.76 | 13,896 | 7.34 | 3,912 | 0.79 | |
| Race | White | 362 | 3.91 | 1,014 | 5.38 | 20,722 | 7.37 | 4,149 | 1.15 |
| Black | 75 | 4.52 | 207 | 4.57 | 5,422 | 7.03 | 2,070 | 1.27 | |
| Asian/Pacific Islander | 7 | 3.08 | 29 | 6.61 | 342 | 5.13 | 214 | 0.53 | |
| Hispanic/Latino | 28 | 4.35 | 52 | 4.99 | 1,196 | 5.27 | 804 | 0.44 | |
| Other | 39 | 4.58 | 64 | 4.93 | 1,189 | 6.22 | 530 | 0.97 | |
| Urbanicity | Urban | 423 | 4.09 | 1,109 | 5.16 | 22,252 | 7.05 | 6,380 | 0.96 |
| Suburban | 37 | 2.77 | 161 | 5.76 | 3,786 | 7.01 | 781 | 1.05 | |
| Rural | 51 | 5.38 | 93 | 5.18 | 2,756 | 7.81 | 585 | 1.06 | |
| Missing | 0 | 0.00 | 3 | 3.30 | 77 | 5.01 | 21 | 0.83 | |
| Medicaid Eligibility Category2 | Poverty | 10 | 1.86 | 1 | 1.39 | 39 | 2.42 | 268 | 0.11 |
| Disability | 489 | 4.37 | 1,350 | 5.26 | 28,611 | 7.15 | 7,039 | 2.24 | |
| Other | 12 | 1.30 | 15 | 3.64 | 221 | 4.79 | 460 | 0.19 | |
| Depression | Yes | 313 | 5.76 | 527 | 8.12 | 13,046 | 11.51 | 4,320 | 2.76 |
| No | 198 | 2.74 | 839 | 4.26 | 15,825 | 5.40 | 3,447 | 0.54 | |
| Any Other Mental Disorder(s) | Yes | 441 | 4.73 | 1,205 | 6.10 | 22,566 | 9.42 | 6,107 | 2.26 |
| No | 70 | 2.11 | 161 | 2.51 | 6,305 | 3.77 | 1,660 | 0.31 | |
| Cardiovascular Disease Risk Factor(s) | Yes | 339 | 6.24 | 753 | 7.34 | 16,237 | 9.23 | 5,793 | 2.18 |
| No | 172 | 2.38 | 613 | 3.85 | 12,634 | 5.48 | 1,974 | 0.37 | |
Age at first observed Medicaid enrollment, starting in 2008.
The most common Medicaid eligibility category for the enrolled period.
Mean age of dementia diagnosis was 49.35 (8.67) in individuals with ASD only, 47.51 (8.68) for those with ASD and co-occurring ID, 51.66 (7.51) for those with ID only, and 53.77 (7.33) in the general population. Results of two-sample t-tests indicate that individuals with an ASD diagnosis were diagnosed with dementia at an earlier age compared to the general population. This was the case for both the ASD only group, t(8276)= −13.04, p<.0001, and the ASD+ID group, t(9131)= −28.25, p<.0001).
Incidence
Cox regression analyses were conducted to determine the unadjusted and adjusted hazard ratios of dementia in the two ASD groups (ASD only and ASD+ID), as well as the ID only group in comparison to the general population. The adjusted model included sex, age, race, urbanicity status, poverty status, a diagnosis of depression or any other psychiatric, and cardiovascular disease risk factors as covariates (as these factors were shown to be associated with the risk of dementia in previous literature), as well as state. Results, reported in Table 3, indicate that the risk of dementia was highest in those with ASD and co-occurring ID reflecting a 2.9-fold increased risk relative to the general population, though individuals with ID only had a similar and only modestly lower HR. Individuals with ASD only had 1.9-fold increased risk compared to the general population. Therefore, when all individuals with ASD (including those with and without ASD) were combined in the same category, they were approximately 2.6 times more likely to be diagnosed with early-onset dementia compared to the general population.
Table 3.
Cox Regression Hazard Ratio comparing the incidence of early-onset dementia in the two ASD groups (ASD only and ASD+ID), and the ID only group relative to the general population.
| Crude Model | Adjusted Model1 | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |||
| Any ASD | 2.20 | 2.04 | 2.37 | 2.62 | 2.40 | 2.85 |
| ASD Only | 1.81 | 1.57 | 2.08 | 1.96 | 1.69 | 2.28 |
| ASD + ID | 2.34 | 2.16 | 2.53 | 2.89 | 2.62 | 3.17 |
| ID only | 2.94 | 2.82 | 3.08 | 3.01 | 2.87 | 3.15 |
Adjusted for: age, sex, race/ethnicity, urbanicity status, Medicaid eligibility category (poverty, disability, other), a diagnosis of depression, a diagnosis of any other psychiatric condition, cardiovascular disease risk factors and state.
As some of these factors included in the adjusted model, such as depression and other psychiatric conditions, could potentially mediate the pathway from ASD to dementia, we also examined simple mediation models comparing nested models with and without adjustment for these factors. The results of these additional analyses, reported in the supplementary materials (appendix 5), indicate no substantial changes to the estimates with or without adjustment for these factors. Full regression models including estimates for association between covariates and dementia outcome across the diagnostic groups are reported in the supplementary materials (Appendix 2–3–4)
Discussion
In this study we examined the prevalence and incidence of early-onset dementia in Medicaid-enrolled individuals aged 30– 64 diagnosed with ASD, including individuals diagnosed with ASD only and individuals with ASD and co-occurring ID. The prevalence of early-onset dementia in ASD was higher than that observed in the Medicaid beneficiary comparison group who did not have ASD or ID. This higher prevalence was observed for both individuals with ASD only and those with ASD and co-occurring ID. However, the prevalence of early-onset dementia in ASD was lower compared the prevalence observed in individuals without ASD who had an ID diagnosis.
Risk factors associated with the increased prevalence of dementia in the general population, including older age, depression, the presence of additional psychiatric conditions, and cardiovascular disease risk factors, were similarly associated with an increased risk of dementia in individuals with ASD (both with and without co-occurring ID), as well as in those with ID only (importantly, this latter group did not include individuals with Down syndrome, which was excluded due to known high prevalence of dementia in this population). After adjusting for these factors, individuals with ASD had a 2.6-fold increased risk compared to the general population. Although the risk was higher compared to general population both for individuals with ASD with and without co-occurring ID, the hazard of early-onset dementia was increased for individuals with ASD and co-occurring ID compared to those with ASD only.
These results do not support the theoretical framework positing that ASD serves as a safeguard against the development of cognitive deterioration and dementia (Lever & Geurts, 2016; Oberman & Pascual-Leone, 2014), as participants with ASD had an increased risk to receive a dementia diagnosis relative to the general population (although not relative to the ID group). Differences between our findings and the literature supporting the safeguard hypothesis might be due to methodological factors, including the larger and more representative sample size in the current study, or different compositions of the ASD group (e.g., inclusion of both individuals with ASD with and without ID).
Although the current study is not equipped to address the question of why early-onset dementia appeared to be more prevalent in ASD compared to the general population, several hypotheses can be advanced. It is possible that co-morbid features that are frequently observed in ASD, such as depression and intellectual disability, which are associated with increased risk of dementia in the general population (Chi et al., 2014; Strydom et al., 2007) confer additional risk in the ASD population. Consistent with this hypothesis, we found that the prevalence of early-onset dementia was higher in those with ASD and co-occurring ID compared to individuals with ASD only, and higher in those with an additional depression diagnosis. However, the prevalence of early-onset dementia appeared to be higher than expected even in the population with ASD only, and even when depression was taken into account in the adjusted model, suggesting that factors related to ASD in and of itself might confer additional risk of being diagnosed with early-onset dementia. Additionally, it is possible that dementia in this population results from lifestyle related or service need factors, including barriers to accessing intellectual, educational and social opportunities (Kivipelto, Mangialasche & Ngandu, 2018). It is also possible that increased service utilization in this population (Shea et al., 2018) leads to earlier recognition of dementia, by virtue of increasing opportunities for medical attention. However, there continue to exist important gaps in access and quality to healthcare for individuals with ASD (Malik-Soni et al., 2021), which may have biased estimates in the opposite direction, leading to an underestimation of the true occurrence of dementia in ASD.
Finally, it is possible that the increased risk of dementia in ASD reflects biological mechanisms. Previous research has identified several genes involved in brain growth that appear to be implicated in both ASD and dementia (Opris & Casanova, 2014). Additionally, alterations in temporal lobe cortical thickness and volume similar to those observed in frontotemporal dementia have been shown in individuals with ASD (Raznahan et al., 2010; Zielinski et al., 2014), as have higher plasma levels of beta-amyloid precursor protein (which is associated with Alzheimer disease; Sokol et al., 2019), and cognitive improvements in response to dementia medications (Rossignol & Frye, 2014). These results suggest a degree of shared pathophysiology in neurodevelopmental and neurodegenerative conditions.
Several limitations in the current study must be acknowledged. First, our data are limited to individuals enrolled in Medicaid. Use of Medicaid diagnoses precludes ability to address whether results are related to frontotemporal dementia, early-onset Alzheimer disease, or other types of dementia. As ASD, ID and dementia are umbrella terms encompassing diverse conditions, the examination of etiologically-defined subgroups would provide critical insight on which neurodevelopmental and neurodegenerative processes underlie the association documented in our data. Future research should include the examination of subgroups within these conditions, as well as the incidence and prevalence of dementia in individuals older than 65. Patterns of medication and service utilization should also be explored in future research in the area. Additionally, given the scope of the dataset, we did not independently ascertain the symptoms of dementia of participants with ASD in the study, and therefore we had to rely on claims data to address our research question. However, previous studies have found that the correspondence between ASD case identification via claims data and clinical diagnoses approaches 90% (Burke et al., 2014; Coo et al., 2017). Accordingly, Medicaid claims have been used in previous literature to examine individuals with dementia (Callahan et al., 2012; Geldmacher et al., 2013, 2014) and across different groups, including individuals with intellectual disability (Huang et al., 2018), with data showing that Medicare claims have a sensitivity and specificity of 0.85 and 0.89 for dementia compared with clinical assessment (Taylor et al., 2009). Previous studies using claims data have detected higher rates of dementia among individuals with intellectual disability, which echoes the findings in this study, and under-identification of younger and more clinically complex individuals (Zhu et al., 2019).
Individuals with ASD might be at higher risk of being misdiagnosed with dementia, due to communication challenges that complicate differential diagnoses and ascertainment of comorbidities, as well as the presence of overlapping manifestations between the two conditions (e.g., difficulties in executive functioning). However, barriers to healthcare access and diagnostic overshadowing could also result in under-diagnosing dementia in this population. Therefore, estimates of co-morbidity in the current dataset might under-estimate or over-estimate the true prevalence.
It is also possible that individuals may utilize private insurance or private pay to receive services for ASD and/or dementia external to Medicaid. However, Medicaid provides coverage in all US states for ASD and broad coverage to older adults in the US, especially those in need of long-term or nursing facility care such as dementia patients. Further, we only focused on the 2008–2012 period as those were the most recently available national Medicaid claims data. It is possible that individuals may have had prior diagnoses that are not represented in the codes for the years examined. Another limitation is that Medicaid is primarily a billing system and coding errors may occur. However, technical reports from the Centers for Medicare and Medicaid Services have validated MAX data and there are processes to purge problematic data from MAX data before release. Finally, the study sample is representative of the population of individuals with ASD who are Medicaid beneficiaries rather than the general ASD population, therefore caution is needed in terms of generalization to the broader ASD population. The same is true with regards to the general population sample used in the study, which reflects that general population of Medicaid beneficiaries, rather than the broader population. Accordingly, the prevalence of early-onset dementia in the general population sample observed in the current study appears to be higher compared to the figures reported in non-at-risk general populations (e.g., Chari et al., 2021), likely reflecting risk factors that are specific to being a Medicaid beneficiary (e.g., poverty, medical conditions). Despite these limitations, as Medicaid is the largest insurer of behavioral health services in the US, and among the only insurers available to individuals with ASD across the lifespan, examination of Medicaid data provides an unprecedented opportunity for large-scale examination of factors associated with the health of those with ASD, including co-morbidities.
Conclusions
Early-onset dementia was more prevalent in adults with ASD compared to the general population in this study. Incidence analyses suggested that, among Medicaid beneficiaries under the age of 65, there was a 2.9-fold increased risk of early-onset dementia in individuals with ASD and co-occurring ID and a 1.9-fold increased risk in individuals with ASD only relative to the general population. This finding points to the importance of understanding and addressing intervention needs in the ASD population with comorbid dementia, including planning long term care resources and formulating appropriate programs.
Supplementary Material
Acknowledgements
The authors wish to thank Katherine Verstreate for her assistance in data analysis
Funding/Support:
This work was supported by a National Institute of Mental Health award R01 MH117653 (Dr Shea).
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Lindsay Shea provides data, policy and program support through a contracting position with the Pennsylvania Department of Human Services, Office of Developmental Programs, Bureau of Supports for Autism and Special Populations. Diana L. Robins is co-owner of M-CHAT, LLC, which receives royalties from companies that incorporate the M-CHAT(-R) into commercial products, and she serves on the advisory board of Quadrant Bioscience, Inc. No disclosures were reported for other authors.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Almeida OP, Hankey GJ, Yeap BB, Golledge J, & Flicker L (2017). Depression as a modifiable factor to decrease the risk of dementia. Translational psychiatry, 7(5), e1117–e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & dementia, 15(3), 321–387. [Google Scholar]
- Barnard-Brak L, Richman D, & Yang Z (2019). Age at death and comorbidity of dementia-related disorders among individuals with autism spectrum disorder. Advances in Autism. 10.1108/AIA-11-2018-0045 [DOI] [Google Scholar]
- Burke JP, Jain A, Yang W, Kelly JP, Kaiser M, Becker L, … & Newschaffer CJ (2014). Does a claims diagnosis of autism mean a true case?. Autism, 18(3), 321–330. [DOI] [PubMed] [Google Scholar]
- Cai L, & Huang J (2018). Schizophrenia and risk of dementia: a meta-analysis study. Neuropsychiatric disease and treatment, 14, 2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CM, Arling G, Tu W, Rosenman MB, Counsell SR, Stump TE, & Hendrie HC (2012). Transitions in care for older adults with and without dementia. Journal of the American Geriatrics Society, 60(5), 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S, Yu JT, Tan MS, & Tan L (2014). Depression in Alzheimer’s disease: epidemiology, mechanisms, and management. Journal of Alzheimer’s disease, 42(3), 739–755. [DOI] [PubMed] [Google Scholar]
- Chiari A, Vinceti G, Adani G, Tondelli M, Galli C, Fiondella L, … & Vinceti M (2021). Epidemiology of early onset dementia and its clinical presentations in the province of Modena, Italy. Alzheimer’s & Dementia, 17(1), 81–88. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Braun KVN, Baio J, Bilder D, Charles J, Constantino JN, … & Yeargin-Allsopp M (2018). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveillance Summaries, 65(13), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir J, Frye RE, & Walker SJ (2020). The lifetime social cost of autism: 1990–2029. Research in Autism Spectrum Disorders, 72, 101502. [Google Scholar]
- Coo H, Ouellette-Kuntz H, Brownell M, Shooshtari S, & Hanlon-Dearman A (2017). Validating an administrative data-based case definition for identifying children and youth with autism spectrum disorder for surveillance purposes. Canadian Journal of Public Health, 108(5), e530–e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppus AMW (2013). People with intellectual disability: what do we know about adulthood and life expectancy?. Developmental disabilities research reviews, 18(1), 6–16. [DOI] [PubMed] [Google Scholar]
- Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, & Kripke C (2015). The health status of adults on the autism spectrum. Autism, 19(7), 814–823. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS, Kirson NY, Birnbaum HG, Eapen S, Kantor E, Cummings AK, & Joish VN (2014). Implications of early treatment among Medicaid patients with Alzheimer’s disease. Alzheimer’s & Dementia, 10(2), 214–224. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS, Kirson NY, Birnbaum HG, Eapen S, Kantor E, Cummings AK, & Joish VN (2013). Pre-diagnosis excess acute care costs in Alzheimer’s patients among a US Medicaid population. Applied health economics and health policy, 11(4), 407–413. [DOI] [PubMed] [Google Scholar]
- Geurts HM, & Vissers ME (2012). Elderly with autism: Executive functions and memory. Journal of autism and developmental disorders, 42(5), 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand BN, Angell AM, Harris L, & Carpenter LA (2020). Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism, 24(3), 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, & Charlton RA (2012). Aging in autism spectrum disorders: A mini-review. Gerontology, 58(1), 70–78. [DOI] [PubMed] [Google Scholar]
- Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K, … & Wisniewski T (2015). Down syndrome and Alzheimer’s disease: Common pathways, common goals. Alzheimer’s & Dementia, 11(6), 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, & Taylor JL (2015). Addressing the need for high quality research on autism in adulthood. Autism: the International Journal of Research and Practice, 19(7), 771–773. [DOI] [PubMed] [Google Scholar]
- Huang AR, Strombotne KL, Horner EM, & Lapham SJ (2018). Adolescent cognitive aptitudes and later-in-life Alzheimer disease and related disorders. JAMA network open, 1(5), e181726–e181726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats D, Payne L, Parlier M, & Piven J (2013). Prevalence of selected clinical problems in older adults with autism and intellectual disability. Journal of Neurodevelopmental Disorders, 5(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Mangialasche F, & Ngandu T (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nature Reviews Neurology, 14(11), 653–666. [DOI] [PubMed] [Google Scholar]
- Ivashko-Pachima Y, Hadar A, Grigg I, Korenková V, Kapitansky O, Karmon G, … & Gozes I (2019). Discovery of autism/intellectual disability somatic mutations in Alzheimer’s brains: mutated ADNP cytoskeletal impairments and repair as a case study. Molecular psychiatry, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever AG, & Geurts HM (2016). Age‐related differences in cognition across the adult lifespan in autism spectrum disorder. Autism Research, 9(6), 666–676. [DOI] [PubMed] [Google Scholar]
- Lord C, Elsabbagh M, Baird G, & Veenstra-Vanderweele J (2018). Autism spectrum disorder. The Lancet, 392(10146), 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, & Baio J (2020). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveillance Summaries, 69(4), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik-Soni N, Shaker A, Luck H, Mullin AE, Wiley RE, Lewis MS, … & Frazier TW (2021). Tackling healthcare access barriers for individuals with autism from diagnosis to adulthood. Pediatric Research, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF (2019). Early-onset Alzheimer disease and its variants. Continuum (Minneapolis, Minn.), 25(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM (2018). Sex and gender differences in Alzheimer’s disease dementia. The Psychiatric times, 35(11), 14. [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, & Yeo GW (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & Dementia, 13(1), 72–83. [DOI] [PubMed] [Google Scholar]
- Oberman LM, & Pascual-Leone A (2014). Hyperplasticity in autism spectrum disorder confers protection from Alzheimer’s disease. Medical hypotheses, 83(3), 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, & Casanova MF (2014). Prefrontal cortical minicolumn: from executive control to disrupted cognitive processing. Brain, 137(7), 1863–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, … & Rotenberg A (2011). Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain topography, 24(3–4), 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, … & Murphy DG (2010). Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cerebral cortex, 20(6), 1332–1340. [DOI] [PubMed] [Google Scholar]
- Rhodus EK, Barber J, Abner EL, Duff D, Bardach SH, Caban-Holt A, … & Jicha GA (2020). Behaviors characteristic of autism spectrum disorder in a geriatric cohort with mild cognitive impairment or early dementia. Alzheimer Disease & Associated Disorders, 34(1), 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, & Frye RE (2014). The use of medications approved for Alzheimer’s disease in autism spectrum disorder: a systematic review. Frontiers in pediatrics, 2, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor MN, Fox NC, Mummery CJ, Schott JM, & Warren JD (2010). The diagnosis of young-onset dementia. The Lancet Neurology, 9(8), 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel LJ, Szanton SL, Wolff JL, Ornstein KA, Parker LJ, & Gitlin LN (2020). Socioeconomic disparities in six-year incident dementia in a nationally representative cohort of US older adults: an examination of financial resources. BMC geriatrics, 20, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea LL, Xie M, Turcotte P, Marcus S, Field R, Newschaffer C, & Mandell D (2018). Brief report: Service use and associated expenditures among adolescents with autism spectrum disorder transitioning to adulthood. Journal of Autism and Developmental Disorders, 48(9), 3223–3227. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Maloney B, Westmark CJ, & Lahiri DK (2019). Novel contribution of secreted amyloid-β precursor protein to white matter brain enlargement in autism spectrum disorder. Frontiers in psychiatry, 10, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis KB, Askew CD, Greaves K, & Summers MJ (2018). The effect of non-stroke cardiovascular disease states on risk for cognitive decline and dementia: a systematic and meta-analytic review. Neuropsychology review, 28(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Strydom A, Livingston G, King M, & Hassiotis A (2007). Prevalence of dementia in intellectual disability using different diagnostic criteria. The British Journal of Psychiatry, 191(2), 150–157. [DOI] [PubMed] [Google Scholar]
- Taylor DH Jr, Østbye T, Langa KM, Weir D, & Plassman BL (2009). The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. Journal of Alzheimer’s Disease, 17(4), 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weden MM, Shih RA, Kabeto MU, & Langa KM (2018). Secular trends in dementia and cognitive impairment of US rural and urban older adults. American journal of preventive medicine, 54(2), 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CW, Ornstein KA, Cosentino S, Gu Y, Andrews H, & Stern Y (2019). Misidentification of dementia in Medicare claims and related costs. Journal of the American Geriatrics Society, 67(2), 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, … & Lainhart JE (2014). Longitudinal changes in cortical thickness in autism and typical development. Brain, 137(6), 1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of the data use agreements associated with the use of the Medicaid data, we are not permitted to release the raw data used in our analyses.


