Abstract
Objective:
This study aims to evaluate the role of scalp electroencephalography (EEG; ictal and interictal patterns) in predicting resective epilepsy surgery outcomes. We use the data to further develop a nomogram to predict seizure freedom.
Methods
We retrospectively reviewed the scalp EEG findings and clinical data of patients who underwent surgical resection at three epilepsy centers. Using both EEG and clinical variables categorized into 13 isolated candidate predictors and 6 interaction terms, we built a multivariable Cox proportional hazards model to predict seizure freedom 2 years after surgery. Harrell's step-down procedure was used to sequentially eliminate the least-informative variables from the model until the change in the concordance index (c-index) with variable removal was less than 0.01. We created a separate model using only clinical variables. Discrimination of the two models was compared to evaluate the role of scalp EEG in seizure-freedom prediction.
Results:
Four hundred seventy patient records were analyzed. Following internal validation, the full Clinical + EEG model achieved an optimism-corrected c-index of 0.65, whereas the c-index of the model without EEG data was 0.59. The presence of focal to bilateral tonic-clonic seizures (FBTCS), high preoperative seizure frequency, absence of hippocampal sclerosis, and presence of nonlocalizable seizures predicted worse outcome. The presence of FBTCS had the largest impact for predicting outcome. The analysis of the models’ interactions showed that in patients with unilateral interictal epileptiform discharges (IEDs), temporal lobe surgery cases had a better outcome. In cases with bilateral IEDs, abnormal magnetic resonance imaging (MRI) predicted worse outcomes, and in cases without IEDs, patients with extratemporal epilepsy and abnormal MRI had better outcomes.
Significance:
This study highlights the value of scalp EEG, particularly the significance of IEDs, in predicting surgical outcome. The nomogram delivers an individualized prediction of postoperative outcome, and provides a unique assessment of the relationship between the outcome and preoperative findings.
Keywords: epilepsy surgery, focal epilepsy, scalp EEG, surgery outcome
1 |. INTRODUCTION
For patients with pharmacoresistant focal epilepsy, resective brain surgery offers the best chance for seizure freedom. Even though the surgical treatment of epilepsy has improved with the advent of new noninvasive evaluation techniques like magnetic resonance imaging (MRI), positron emission tomography (PET), ictal single-photon emission tomography (SPECT), and magnetoencephalography (MEG), as well as invasive techniques like electrocorticography (ECoG) and stereo-electroencephalography (SEEG), the current chances of achieving seizure freedom following resective surgery are still highly variable. Between 40% and 80% of patients achieve complete postoperative seizure freedom.1 This variability highlights the need to pursue a deeper understanding of the predictive factors involved in surgical outcome.
Previous studies have examined the associations between preoperative findings and postoperative seizure outcome and have identified possible outcome determinants.2 Overall, patients attain a higher rate of seizure freedom when there is a high degree of agreement between multiple diagnostic modalities and when this agreement suggests a well-localized epileptogenic zone. However, the prediction of surgical outcome performed by clinicians lacks precision because it is based on the overall impression of how concordant the preoperative findings are. This type of prediction can sometimes be highly subjective and experience dependent, as often reflected by the lack of agreement between clinicians.3 The development of statistical models that provide objective and quantifiable predictions of seizure freedom on a case-by-case basis is crucial to improving presurgical counseling and to expand our understanding of the surgical outcome.
Since its inception, electroencephalography (or EEG) has been paramount in the evaluation of epilepsy. Scalp EEG is a valuable “front line” tool to localize and delineate the relative focality of the epileptic zone.4–6 Except for using scalp EEG to determine potential candidacy for surgery, most of the current literature focusing on resective intervention comprises studies using invasive methods.7,8 Furthermore, available studies that address the relationship between scalp EEG and the surgical outcome are often outdated, rely on relatively small samples, focus on a single lobe of resection, or only focus on a particular feature of the EEG (eg, spikes, ictal spread, onset pattern).6,9–12
In this study, we evaluate the predictive value of scalp EEG in epilepsy surgery outcome and specifically measure its prognostic contribution beyond a patient’s obvious clinical characteristics. We accomplish this by developing nomograms, models for individualized risk prediction, using scalp EEG data to predict seizure freedom at 2 postoperative years in a large, multicenter, deeply phenotyped, epilepsy surgery cohort.
2 |. METHODS
2.1 |. Patient selection
In this retrospective cohort study, we identified 470 patients who underwent epilepsy surgery at Cleveland Clinic (n = 334), Mayo Clinic (n = 53), and University of Campinas (n = 73) from 2010 to 2017. These study patients had at least 6 months of postoperative follow-up, and no prior brain surgery, no multilobar resections, and no postoperative events of unclear nature. All patients were at least 10-years-old. Younger patients were excluded to avoid the potential confounding effects of age-related differences observed in EEG patterns and in neuroplasticity.
Clinical data were collected from patients’ medical charts. All patients underwent a preoperative evaluation that included, at minimum, MRI and EEG. When appropriate, SPECT, PET, MEG, invasive evaluation, and neuropsychological testing were also performed.
2.2 |. EEG variables
We reviewed scalp video-EEG reports. In all study sites, patients were admitted for a few days until seizures were recorded. EEG studies were performed using an extended International 10–20 system (31 electrodes that include the inferior temporal chain).13,14
Interictal findings were coded based on laterality as bilateral interictal epileptiform discharges (IEDs), unilateral IEDs (more than 80% of the discharges on one side15,16), or no IEDs. Based on the EEG report description, we collected data on the typology of the IEDs (sharp waves, spikes/polyspikes, both, or no IEDs), on the percent of temporal lobe IEDs, and on the frequency of discharges (rare or frequent). The frequency of spikes was based on the impression reported by the EEG reader and not by a precise number. The percent of temporal lobe IEDs was based on the location and the estimated frequency of the different subtypes of IEDs reported per patient. The location was defined as the region with maximum electronegativity. When the maximum electronegativity was located in-between lobes (eg, F7/F8, P7/P8) we used the spread of the activity to define the location of the IEDs. For example, if a patient had two types of IEDs—that is,(1) sharp wave maximum F8 spreading to F4 (10%); (2) sharp wave maximum F8 spreading to T4 (90%)—we would classify this patient as having 90% of temporal IEDs. Ictal findings were classified according to the number of ictal patterns (one or fewer vs two or more) and according to the presence or absence of nonlocalizable ictal patterns, which included generalized and nonlocalized ictal onset.
Interictal and ictal EEG concordance was coded as concordant with resection, discordant with resection (including EEG findings that extended beyond the resection), normal EEG (interictal only), or nonlocalizable (ictal only).
2.3 |. Seizure outcomes
The primary outcome in this study was complete seizure freedom at last follow-up, allowing for isolated auras. Time to seizure recurrence was measured as time (in months) to the first postoperative seizure. Patients who presented seizures exclusively in the acute phase (first month after surgery) were classified as being seizure-free. If seizures persisted beyond the first postoperative month, the date of recurrence during the acute phase was used as the date of the first postoperative seizure.
2.4 |. Statistical methods
2.4.1 |. Candidate predictors
Demographic, clinical, and EEG candidate predictors were selected a priori, based on published literature and clinical experience, and included the following: sex,17 age at surgery,17–19 age at epilepsy onset,17,19–21 disease duration,1 monthly seizure frequency,1,19,22,23 lobe of surgery (temporal vs extratemporal),24–26 MRI findings (normal vs abnormal),24,27,28 presence or absence of focal to bilateral tonic-clonic seizures (FBTCS),17,22,24,29,30 pathologic cause (mesial temporal sclerosis [MTS], malformation of cortical development [MCD], unknown, tumor, and other),23,24,28 interictal EEG side (unilateral vs bilateral),16,31 presence or absence of nonlocalizable ictal patterns,7,32 number of ictal patterns, EEG concordance to resected area,33 invasive evaluation, interictal typology (sharp waves, spike waves, both, and none), IEDs (frequent vs rare), and location of IEDs (percent of temporal lobe IEDs). To account for the expected variable correlations of EEG with outcome across different epilepsy etiologies and localization, we also included several pre-specified interaction terms as candidates for the model: MRI findings and lobe of surgery each by interictal laterality, presence of nonlocalizable seizures, and the number of ictal patterns.
2.4.2 |. Model-building
Descriptive statistics were calculated for the sample and are presented using mean with standard deviation, median with interquartile range, or count with proportion, as appropriate. A Cox proportional hazards regression model was fit to include all candidate clinical and EEG variables, including pre-specified interaction terms. Harrell's step-down procedure was used to sequentially eliminate the least informative variables from the model until the change in the concordance index (c-index) with variable removal was less than 0.01.34 The c-index ranges from 0.50 and 1.0 and measures the model's ability to discriminate between high- and low-risk patients. For a Cox model, the c-index is an extension of the area under the receiver-operating characteristic (ROC) curve (AUC), but calculation of the c-index considers the censoring inherent in time-to-event data, unlike the simple AUC more commonly used for logistic regression. A value of 0.50 indicates that the model is no better than chance, and a value of 1.0 indicates the model correctly classifies 100% of patients. Restricted cubic splines were fit to each continuous variable to relax the assumption of linearity between the predictor and the outcome, but nonlinear terms were retained only in the final model if significant. Visual examination of Schoenfield residuals confirmed the proportional hazards assumption.
2.4.3 |. Model validation
A model will always perform better in the data set on which it was built than when it is applied to a new set of data. Therefore, accurate assessment of discrimination and calibration should be corrected for the optimism that arises when evaluating a model using model-building data. Thus we subjected our final model to internal validation to correct the c-index for optimism using bootstrap resampling. Bootstrap resampling has been found repeatedly to be a more stable, more efficient, and less biased method for model validation than the more traditional split-sample approaches.35–37 In detail, 500 bootstrapped data sets—the same size as the original—were created through resampling the original data with replacement. The 500 bootstrapped data sets are meant to simulate new samples from the population, as each will vary in how similar it is to the original, training data set, and the c-index will be lower in each bootstrapped data set when compared to the training data. The Cox model is then applied to each bootstrapped data set, and the c-index is calculated for each. The differences in c-index between each bootstrapped sample and the original data were averaged to provide an estimate of optimism, which is subtracted from the raw c-index to yield the optimism-corrected c-index.35
Although external validation on a separate sample is ideal to validate prediction models, our external sample size precluded this work. Thus we elected to combine data from all sites and perform an internal validation using bootstrap resampling. Bootstrap-based internal validation methods have been shown to be reasonable approximations of how a model would likely perform in an external sample.36
An optimism-corrected calibration curve was created to compare predicted probabilities generated by the model to actual seizure freedom. A 45-degree calibration curve indicates the model is perfectly calibrated. A nomogram was created to provide a visual representation of each model.
2.4.4 |. Comparison with the clinical model
To investigate the relative effect of EEG predictors on seizure freedom at 2 years, EEG predictors were removed from the model and model validation procedures were repeated for the reduced model containing clinical predictors only (“Clinical Model”). After confirming adequate calibration of the Clinical Model, the c-index was compared to the Clinical + EEG model to determine whether EEG variables increased discriminative ability compared to clinical predictors alone.
2.4.5 |. Online risk calculator
We generated an online risk calculator presenting the estimated individualized chance for any given patient for seizure-freedom 2 years after surgery, using the final Cox model, R software, and previously published methods.38
2.4.6 |. Invasive evaluation
Considering the difficulty in interpreting the value of scalp EEG without considering the influence of invasive data, we tested the variable “Invasive evaluation” as a potential stratification variable. The full data set was divided based on invasive status and the model was applied separately to each group.
2.4.7 |. Statistical tools
Analyses were conducted on complete cases using SAS Studio v.3.5 (SAS Institute) and R Studio v.3.3.0 (“rms” package).
2.5 |. Classification of evidence
This study provides Class IV evidence that scalp EEG findings could enhance the individualized surgical outcome prediction.
2.6 |. Standard protocol approvals, registrations, and patient consent
The Cleveland Clinic and Mayo Clinic Institutional Review Boards, and the University of Campinas Ethics Committee approved this study.
2.7 |. Data availability statement
Data not provided in the article will be available to any qualified investigator upon request.
3 |. RESULTS
3.1 |. Demographics and clinical data
The sample included 470 patients from three centers and was 51% female with an average age at surgery of 37.5 years. Forty-eight percent of patients experienced seizure recurrence following surgery, with a median time to the first seizure of 4.1 months (interquartile range [IQR] 1.0–12.2). Among patients who did not experience seizure recurrence, the median follow-up was 42.1 months (IQR 21.0–69.4). Additional demographic and clinical descriptions of the sample can be found in Table 1. The median follow-up time in the total sample was 44 months (IQR 22.0–67.0).
TABLE 1.
Characteristics of the sample
| n (%) unless otherwise specified | |
|---|---|
| Site | |
| Cleveland Clinic | 344 (73.2) |
| Mayo | 53 (11.3) |
| UNICAMP | 73 (15.5) |
| Age at surgery (years), mean (SD) | 37.5 (14.0) |
| Female | 238 (50.6) |
| Age at epilepsy onset (years), median (IQR) | 15 (6–28) |
| Time from epilepsy onset to surgery (years), median (IQR) | 16 (7–28) |
| Preoperative monthly seizure frequency | 5 (2–14) |
| Side of surgery | |
| Left | 253 (53.8) |
| Right | 217 (46.2) |
| Temporal surgery | 376 (80.0) |
| Pathological cause | |
| MTS* | 157 (33.4) |
| Unknown | 121 (25.7) |
| MCD | 88 (18.7) |
| Tumor | 36 (7.7) |
| Other | 59 (12.6) |
| Missing | 9 (1.9) |
| Normal MRI | 91 (19.4) |
| Nonlocalizable seizures | 104 (22.1) |
| Interictal EEG side | |
| >80% Unilateral | 316 (67.2) |
| Bilateral | 110 (23.4) |
| No epileptiform discharges | 44 (9.4) |
| Ictal EEG concordance with resection | |
| Concordant | 262 (55.7) |
| Discordant | 182 (38.7) |
| Nonlocalized | 18 (3.8) |
| Missing | 8 (1.7) |
| Interictal EEG concordance with resection | |
| Concordant | 221 (47.0) |
| Discordant | 205 (43.6) |
| Normal EEG | 44 (9.4) |
| Two or more ictal patterns | 133 (28.3) |
| Interictal epileptiform typology | |
| Sharp waves | 255 (54.3) |
| Spike waves | 31 (6.6) |
| Both spike and sharp waves | 129 (27.5) |
| None | 44 (9.4) |
| Missing | 11 (2.3) |
| IED | |
| Frequent | 245 (52.1) |
| Rare | 137 (29.2) |
| Missing | 88 (18.7) |
| Percent of temporal lobe interictal epileptiform discharges | |
| 0% | 100 (21.3) |
| 100% | 281 (59.8) |
| Between 0% and 100% | 74 (15.7) |
| Missing | 15 (3.2) |
| Invasive evaluation | |
| Yes | 150 (31.9) |
| No | 320 (68.1) |
Includes two patients coded mesial temporal sclerosis + other.
Abbreviations: IQR, interquartile range; SD, standard deviation.
3.2 |. Predictors of seizure freedom
3.2.1 |. Multivariable analysis and nomogram development
The final model included the following variables: presence or absence of FBTCS, preoperative monthly seizure frequency, normal or abnormal MRI, pathological cause (recoded during model-building to MTS vs non-MTS), temporal vs extratemporal surgery, presence or absence of nonlocalizable seizures, interictal EEG laterality, and two interactions (between surgery site and interictal EEG laterality and between normal/abnormal MRI and interictal EEG laterality). Full model coefficients can be found in Table 2.
TABLE 2.
Coefficients for each variable
| Variable | Coefficient |
|---|---|
| Presence of focal to bilateral tonic-clonic seizures | 0.7551 |
| Preoperative monthly seizure frequency | 0.001 |
| Pathological cause = MTS | −0.3278 |
| Presence of nonlocalizable seizures | 0.4283 |
| Interictal EEG side | |
| >80% unilateral | 0 (reference) |
| Bilateral | −0.1314 |
| No epileptiform discharges | −1.2614 |
| Normal MRI | |
| If >80% unilateral interictal EEG | −0.0218 |
| If bilateral EEG | −.3957 |
| If no epileptiform discharges | 0.4615 |
| Temporal resection | |
| If >80% unilateral interictal EEG | −0.4442 |
| If bilateral EEG | 0.2128 |
| If no epileptiform discharges | 0.6642 |
Note: Coefficients for Normal MRI and Temporal Resection represent interactions with Interictal EEG side.
Abbreviations: MTS, mesial temporal sclerosis; Ref, reference.
Following internal validation, the full Clinical + EEG model achieved an optimism-corrected c-index of 0.65, indicating that when presented with two patients at a given follow-up time, one who had experienced seizure recurrence and one who had not, the model would correctly assign the patient with recurrence a lower probability of seizure freedom 65% of the time. The optimism-corrected calibration curve for the model is presented in Figure 1. The model displays good calibration, as evidenced by the calibration curve (blue) not substantially deviating from the ideal 45-degree line (gray). The pattern of slight deviation observed is likely due to regression to the mean: patients assigned a probability of seizure freedom below 0.4 in reality have a slightly higher probability of seizure freedom, whereas patients assigned a probability of above 0.7 in reality have a slightly lower probability of seizure freedom.
FIGURE 1.
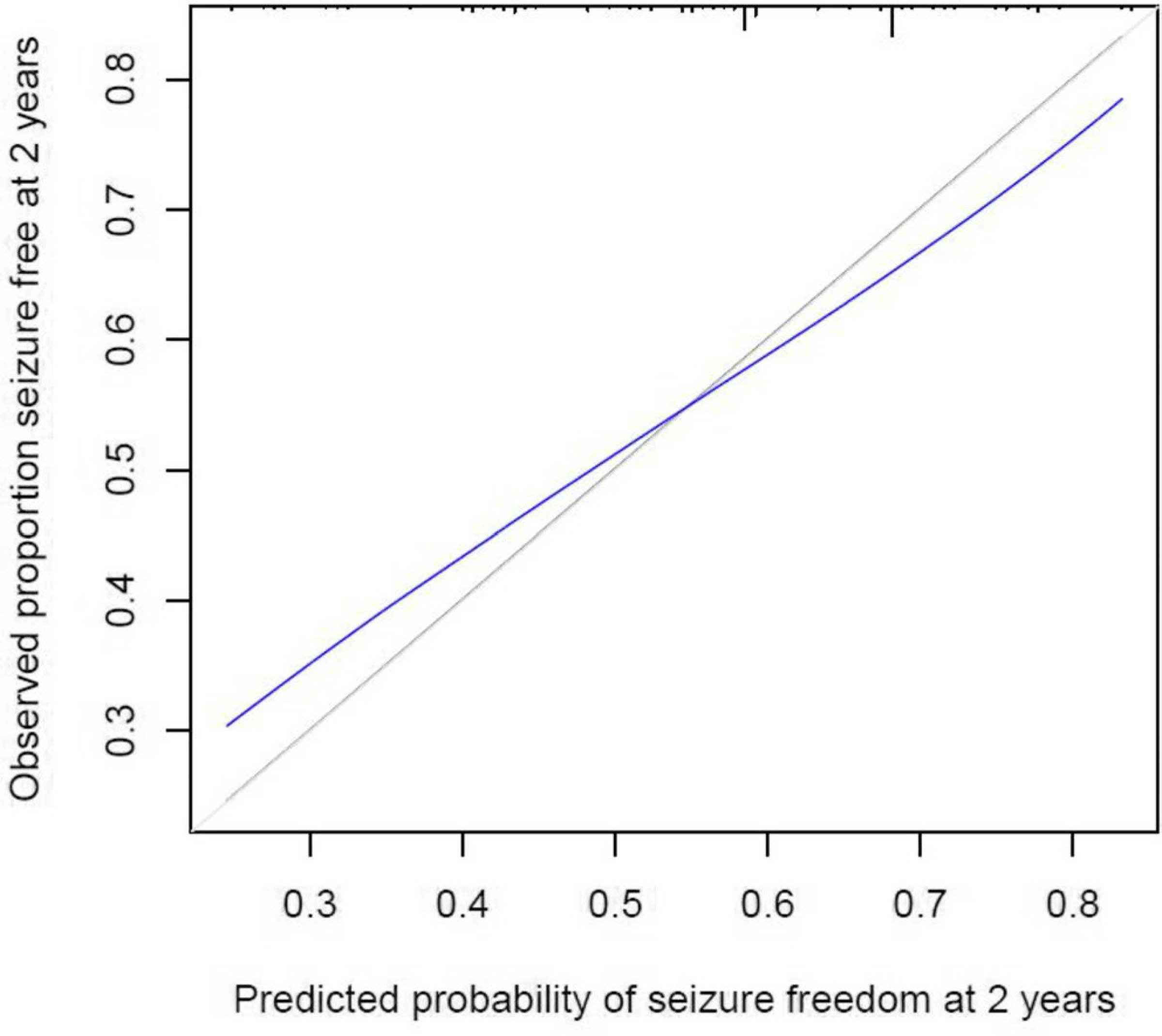
Calibration curve for the model predicting probability of seizure freedom at 2 years. The x-axis displays the predicted probabilities generated by the statistic model and the y-axis shows the proportion of the patients who were seizure-free at each predicted probability. The 45-degree line (gray) indicates perfect calibration, whereas the blue line represents model-predicted probabilities compared with observed seizure freedom. The curve presented indicates that the model is well calibrated. The very minor deviation from the ideal line likely represents the common phenomenon of regression to the mean: patients with predicted probabilities of less than 0.5 likely have a slightly higher actual probability, while patients with predicted probabilities of more than 0.5 likely have a slightly lower actual probability
Reduced clinical model
After removal of all EEG variables from the model and following internal validation, the Clinical Model achieved a c-index of 0.59, a drop in the c-index of 0.06 from the Clinical + EEG model.
3.2.2 |. Nomogram
We created a nomogram to predict complete seizure freedom at 2 years (Figure 2). The manual application of the nomogram to predict complete seizure freedom requires drawing a vertical line from the appropriate value of each outcome predictor up to the “Points” line on top (eg, Normal MRI + extratemporal + no IEDs = 40 points). This is repeated for each individual patient characteristic. All the points are then added to yield a total. The user will then find the total points in the ‘Total Points Line” (second to last line on figure), and draw a vertical line down to the estimated 2-year probability of seizure freedom.
FIGURE 2.
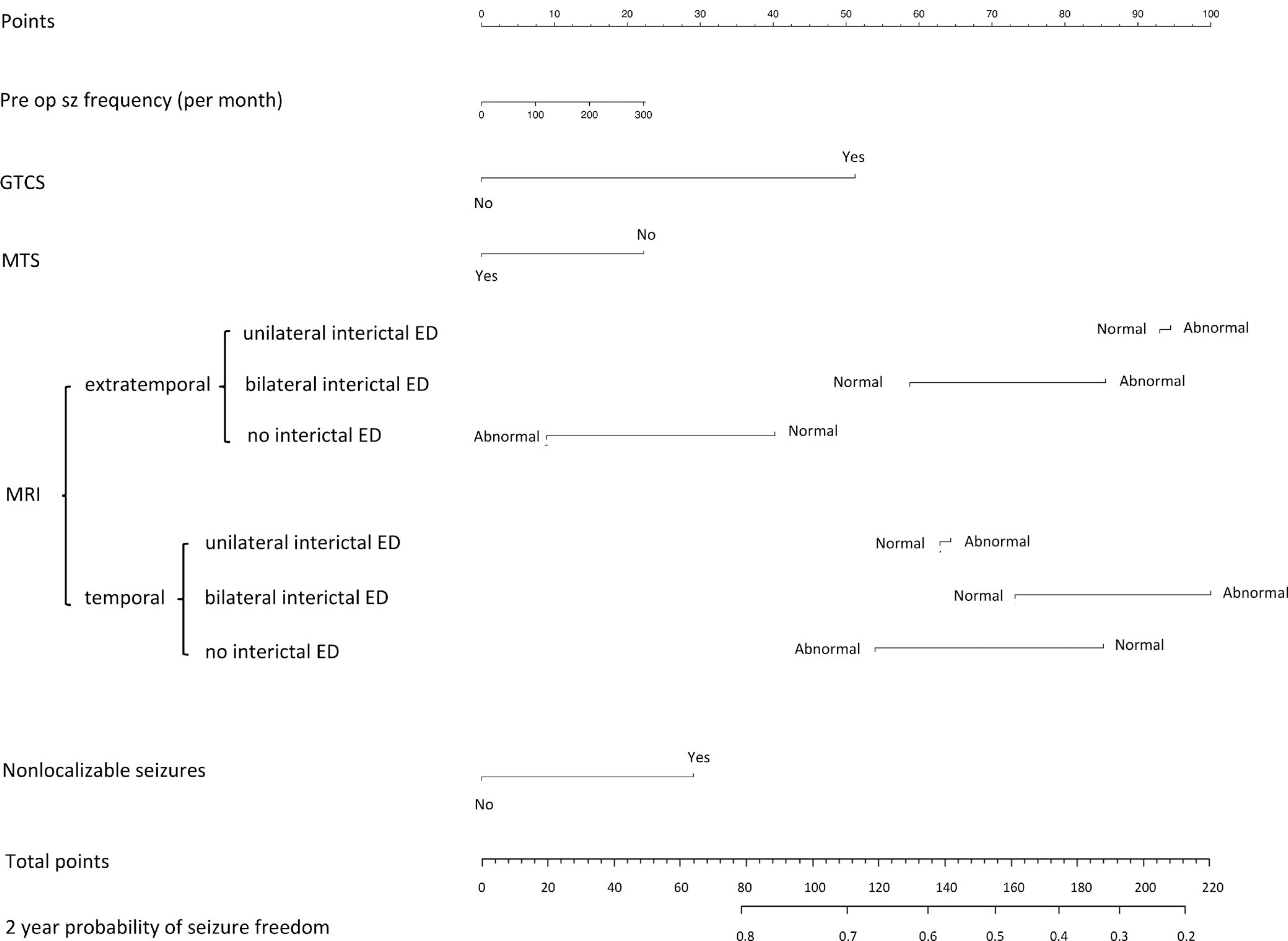
Nomogram to predict the probability of complete seizure freedom after 2 years. The manual application of the nomogram to predict complete seizure freedom requires drawing a vertical line from the appropriate value of each outcome predictor up to the “Points” line on top. This is repeated for each individual patient characteristic. All the points are then added to yield a total. The end-user would then find the total points in the ‘Total Points Line” (second to the last line on figure), and draw a vertical line down to the estimated 2-year probability of seizure freedom
Online risk calculator
The calculator runs all calculations in the background and generates an individualized seizure-freedom probability (The model is available at: https://riskcalc.org/ClinicalAndEEGPredictionofSeizureFreedomAt2Years/). Figure 3 shows an example demonstrating the use and the output from the unified online risk calculator.
FIGURE 3.

Example of online risk calculator to predict seizure outcome. The online risk calculator requires users to enter a numerical value for “Preoperative Monthly Seizure Frequency” and select options from drop-down menus for the remaining variables. After clicking “Run Calculator,” a percent chance value of predicted probability of seizure freedom at 2 years will be displayed under the “Probability” heading. For example, a patient presenting without generalized tonic-clonic, 30 seizures a month preoperatively, mesial temporal sclerosis, abnormal magnetic resonance imaging (MRI), sometimes non-localizable seizures, unilateral interictal epileptiform discharges, and a temporal resection will have a 75.1% chance of achieving seizure freedom following surgery
3.2.3 |. Invasive evaluation
There was not a difference in predictive performance of the model based on invasive status.
4 |. DISCUSSION
Our results provide an updated assessment of extended 10–20 scalp EEG (31 electrodes with a subtemporal chain)13 in surgical epilepsy and its ability to predict postoperative seizure freedom. Scalp EEG has its limitations, with a relatively low spatial resolution and inability to assess subcortical structures, thereby limiting its role for independently defining the exact location of the epileptogenic zone. However, it has excellent temporal resolution and is a pillar of epilepsy surgery evaluations. In essence, scalp EEG is a ubiquitous test that is integral to getting the patient to the point of surgery: optimizing its utility for individualized outcome prediction after surgery becomes a low-hanging fruit.
Statistical models are not yet part of the current clinical epilepsy practice; however, these algorithms have a promising role in the era of precision care and personalized medicine.39–41 The addition of a calculated risk score to the traditionally subjective and variable prediction of seizure outcome would enhance and modernize the preoperative epilepsy surgery counseling. Our study advances that goal by providing individualized clinical and scalp EEG analysis based on a large multicenter cohort.
Our statistical methods represent current best practice for prediction modeling and have been used successfully in other fields. Nomograms provide a visual assessment of the model's results, and this is the first nomogram including scalp EEG to predict outcomes of epilepsy surgery.
4.1 |. Interpretation of the predictive variables
The final Clinical + EEG model achieved an optimism-corrected c-index of 0.65 with good calibration (Figure 1), and performed better than the Clinical model alone (c-index 0.59). Albeit modest, this improvement in predictive performance is still clinically helpful since this EEG information is obtained on every surgical patient and does not therefore require any additional resources beyond routine clinical practice.
The clinical variables included in the final model are consistent with previously described predictors of surgical outcomes. Our findings are in accordance with the literature showing that the presence of FBTCS,24 high preoperative seizure frequency,1 and nonlocalizable seizures7 predicts worse outcome. Our finding that better outcomes are seen in patients with MTS as compared to those with other epilepsy causes is also in agreement with previous studies.24 It is important to highlight that even though some variables statistically added some predictive value to the model, the individual contribution of each variable was not necessarily substantial.
A clinical history of FBTCS has a major impact on the model. Unsurprisingly, FBTCS were associated with worse surgical outcomes, which is a well-established finding.29 Because FBTCS can indicate a more widespread epileptogenic zone,42,43 and at times can extend into eloquent cortex, full removal of the epileptogenic zone is exceedingly difficult, resulting in lower rates of seizure freedom.
Nonlocalizable seizure pattern on EEG was the only ictal pattern included in the final model, which predicted worse surgical outcome. This finding agrees with the literature on patients who underwent both scalp and invasive EEG studies.7 A few explanatory hypotheses were raised: The presence of nonlocalizable seizures might indicate a more widespread region of epileptogenicity and might suggest a faster pattern of propagation. Prior studies suggest an association between rapid spread and worse surgical outcome.7,44
4.2 |. Interpretation of model's interactions
The nomogram also provides an interesting analysis of the interactions between the location of the IEDs, MRI findings, and type of surgery.
4.2.1 |. Unilateral interictal epileptiform discharges
In patients with unilateral interictal EEG, MRI findings did not influence risk prediction. For these same patients, resection of the temporal lobe imparts a higher probability of seizure freedom when compared to an extratemporal resection. These findings are in accordance with published studies showing that patients who underwent temporal lobe resections had either better24 or similar25 outcomes compared to extratemporal cases.
4.2.2 |. Bilateral interictal epileptiform discharges
In this patient subgroup, a normal MRI was correlated with a higher probability of seizure freedom than an abnormal MRI. This unexpected and seemingly contradictory finding could be explained by the fact that patients with normal MRI and bilateral IED might require a more extensive, and therefore more precise, preoperative evaluation to define the epileptogenic zone. The increased attention given to these patients may result in a more accurate demarcation of the epileptogenic zone. In our study cohort, 23 of 28 patients (82.1%) with bilateral IEDs and normal MRI had an invasive EEG evaluation as opposed to 23 of 85 (27.1%) of those with bilateral IEDs and an abnormal MRI (p < .0001). The presence of a lesion may provide clinicians with a false sense of security, thereby biasing them toward assuming that the epileptogenic zone comprises solely the lesion when, in reality, the true epileptogenic zone extends beyond it. This conclusion would lead clinical teams to suggest more restrictive resections that do not adequately remove the epileptogenic zone, resulting in worse surgical outcomes. The sample size limitation could also be interfering in these unexpected results. To evaluate the model's interactions, we end up creating subgroups with smaller sample sizes. For example, even though we had a total of 113 patients with bilateral IEDs, in the end, the subgroup “normal MRI, with extratemporal resection and bilateral IED” included only 9 patients. Taking into consideration the limitations described here, this is still an interesting finding that should be further investigated in future studies.
4.2.3 |. No interictal epileptiform discharges
For patients with no IEDs on EEG, a normal MRI imparts a lower probability of seizure freedom than an abnormal MRI, as would be expected.45,46 However, in this group of patients, a temporal lobe surgery imparts a lower probability of seizure freedom than an extratemporal surgery, the opposite of what is reported in the literature on surgical outcome in general.24,25 We could hypothesize that in extratemporal epilepsies, the absence of IEDs could point to a more localized epileptogenic zone or more restricted recruitment of the epilepsy network, thereby explaining our finding. However, more studies are needed to confirm this hypothesis.
Even though, in theory, we would expect patients with unilateral IEDs to have better outcomes compared to patients with no IEDs, there are scant data in the literature to support this hypothesis. A study comparing “oligospikers” vs patients with frequent IEDs found similar surgical outcomes.47 Another study evaluating patients with nonlesional intractable TLE with rare or absent IEDs even suggested that the rarity of spikes could reflect a less severe disease with excellent surgical outcomes.48 In other words, the real meaning of the absence of IEDs is still unclear49 and needs to be further investigated, as emphasized by our results.
4.2.4 |. Interpreting interactions from the direction of the surgery site
As discussed previously, extratemporal resections with no IEDs correlated with higher postoperative seizure freedom compared to extratemporal cases with unilateral or bilateral IED. Temporal resections correlate with higher postoperative seizure freedom in the setting of unilateral IEDs but lower seizure freedom for bilateral EEG and no IEDs. This finding corresponds to the existing literature, which reports better outcomes in temporal lobe epilepsy patients with strictly unilateral IED.16
4.3 |. Nomogram
In summary, the relationship between scalp EEG findings and the surgical outcome might be more complicated than initially thought. The current concept of an abnormal MRI and unilateral IEDs always being associated with better surgical outcomes could be an example of how the oversimplification of a complex relationship could contribute to the lack of precision seen in the current surgical outcome prediction. To compare our findings to the existing literature, we discussed some of the nomogram results individually. However, it is important to emphasize that the final prediction is based on the combination and interaction of all variables and we cannot use these individual variables as independent predictors.
The inclusion of the variable interictal side in the final model was unexpected and highlights the potential predictive value of IEDs, a variable frequently considered less important in the surgical decision-making process. One possible explanation would be that interictal discharges outside of the suspected epileptic zone could work as biomarkers of a larger epileptogenic region. Interictal discharges may also point toward areas that will possibly develop as new seizure foci in patients who experience seizure recurrence after surgery. These findings also correlate with the literature on interictal discharges observed in MEG.50 Although IEDs have often been considered a secondary factor in surgical planning, our findings argue for an increased importance to be placed on interictal activity in determining the surgical outcome.
In the current study an extended 10–20 (31-electrode scalp montage) was used, which includes an inferior temporal row of scalp electrodes. Although the extended 10–20 montage is superior to the standard 10–20 (19-electrode scalp montage) for identifying temporal lobe IEDs,14 our study does suggest the potential value of high spatial resolution scalp EEG for extratemporal lobe epilepsy.51 The addition of scalp EEG data improved the model’s performance by 0.06. This increase was not as high as initially expected, highlighting the multifactorial and complex nature of surgical outcome prediction as it cannot be reduced to any single patient characteristic.
4.4 |. Study limitations
Even though pathology and MRI results are frequently similar, it is not uncommon to have a normal MRI with “abnormal” pathology results and vice versa. To build the predictive model we included both pathology (MCD, MTS, unknown, tumor, other) and MRI findings (normal and abnormal). One limitation of this model is that pathology results are usually not available prior to surgery; however, we kept this variable given that pathology results can usually be predicted based on MRI findings and later confirmed if needed.
For the correct interpretation of our results, the distinction between explanatory and predictive statistical models is necessary. Explanatory modeling aims to understand relationships, whereas predictive models aim to accurately predict new observations.52 Because of complex relationships across variables, in a predictive model, the data are evaluated as a whole and not individually. When we create subgroups that contain smaller sample sizes, there is a risk of over-interpreting the model's interactions. In addition, because models are calculated based on real data, the prediction of unusual combinations of predictive variables might lack precision. This is a limitation that needs to be considered when individually interpreting the data and the multiple interactions.
Another limitation is that we did not quantify the frequency of IEDs. We acknowledge that the frequency and percentage of IEDs are largely subjective measurements and working with more objective measures will be a future line of research.
Despite its limitations, this work attempts to generate an individualized, evidence-based surgical outcome prediction deviating from traditional individual physicians’ experience-dependent prediction. Further accumulation of the data is needed to enhance our ability to predict surgical outcome.
4.5 |. Discussion: Summary
Although individual electroencephalographic features are important in determining the postoperative outcome after epilepsy surgery, we describe here the role of scalp EEG using a large multicenter cohort. Our findings support several widely held beliefs of the predictive value of certain preoperative variables, but we also point toward variables whose influence may not be entirely understood. The nomogram created emphasizes the importance of IEDs in the prediction of surgery outcome and confirms the predictive value of other clinical variables, with clinical history of FBTCS having the largest impact in the model. We highlight the complex interaction between variables when predicting surgical outcome, justifying the need for advanced statistical techniques to enhance our ability to predict surgical outcome further.
Key Points.
We developed a nomogram and an online risk calculator using scalp electroencephalography (EEG) data to facilitate clinical counseling on seizure outcome following resective surgery.
The full Clinical + EEG model achieved a concordance index of 0.65, whereas the concordance index of the model without EEG data was 0.59.
This nomogram highlights the importance of interictal epileptiform discharges in the prediction of resective epilepsy surgery outcome.
The analysis of the model's interactions provides a unique assessment of the relationship between the preoperative laterality of interictal epileptiform discharges and surgical outcome.
ACKNOWLEDGMENTS
Study Funding: Lara Jehi, National Institutes of Health (NIH) grant R01 NS097719.
Funding information
NIH, Grant/Award Number: R01 NS097719
CONFLICT OF INTEREST
Dr. Worrell has licensed intellectual property to NeuroOne Inc., and licensed intellectual property to Cadence Neuroscience Inc. Dr. Brinkmann reports that he has licensed intellectual property to Cadence Neuroscience Inc. Dr. Cendes reports personal fees from UCB Pharma, grants from Sao Paulo Research Foundation (FAPESP), and grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), outside the submitted work; and member of the editorial boards of the following journals: (1) Neurology (2) Epilepsy Research (3) Epilepsia (Associate Editor), (4) Frontiers in Neurology (Specialty Chief Editor in Epilepsy). All other authors have nothing to disclose. “We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.”
REFERENCES
- 1.Jehi L, Yardi R, Chagin K, Tassi L, Russo GL, Worrell G, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14(3):283–90. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Xu J, Mao K, Wang M, Ren P, Lei D, et al. Risk factors analyses for seizure recurrence in different periods after refractory epilepsy surgery: a prospective single-center study. World Neurosurg. 2018;112:e454–64. 10.1016/j.wneu.2018.01.060 [DOI] [PubMed] [Google Scholar]
- 3.Gracia CG, Chagin K, Kattan MW, Ji X, Kattan MG, Crotty L, et al. Predicting seizure freedom after epilepsy surgery, a challenge in clinical practice. Epilepsy Behav. 2019;95:124–30. 10.1016/j.yebeh.2019.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Koubeissi MZ. Electroencephalography in Epilepsy Evaluation. Contin Lifelong Learn Neurol. 2019;25(2):431–53. [DOI] [PubMed] [Google Scholar]
- 5.Mesraoua B, Deleu D, Al Hail H, Melikyan G, Boon P, Haider HA, et al. Electroencephalography in epilepsy: look for what could be beyond the visual inspection. Neurol Sci. 2019;40(11):2287–91. [DOI] [PubMed] [Google Scholar]
- 6.Sadler M, Desbiens R. Scalp EEG in temporal lobe epilepsy surgery. Can J Neurol Sci. 2000;27(SUPPL. 1):22–8. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Gotman J, Khoo HM, Olivier A, Hall J, Dubeau F, et al. Neurophysiological seizure-onset predictors of epilepsy surgery outcome: a multivariable analysis. J Neurosurg. 2019;133(6):1863–72. [DOI] [PubMed] [Google Scholar]
- 8.Bulacio JC, Jehi L, Wong C, Gonzalez-Martinez J, Kotagal P, Nair D, et al. Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. Epilepsia. 2012;53(10):1722–30. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Wu S, Daif A, Sun T, Chauhan V, Issa NP, et al. Clinical implications of scalp ictal EEG pattern in patients with temporal lobe epilepsy. Clin Neurophysiol. 2019;130(9):1604–10. [DOI] [PubMed] [Google Scholar]
- 10.Raghavendra S, Nooraine J, Mirsattari SM. Role of electroencephalography in presurgical evaluation of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmeiser B, Zentner J, Steinhoff BJ, Brandt A, Schulze-Bonhage A, Kogias E, et al. The role of presurgical EEG parameters and of reoperation for seizure outcome in temporal lobe epilepsy. Seizure. 2017;51:174–9. [DOI] [PubMed] [Google Scholar]
- 12.Zakaria T, Noe K, So E, Cascino GD, Wetjen N, Van Gompel JJ, et al. Scalp and intracranial EEG in medically intractable extratemporal epilepsy with normal MRI. ISRN Neurol. 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- 14.Bach Justesen A, Eskelund Johansen AB, Martinussen NI, Wasserman D, Terney D, Meritam P, et al. Added clinical value of the inferior temporal EEG electrode chain. Clin Neurophysiol. 2018;129(1):291–5. 10.1016/j.clinph.2017.09.113 [DOI] [PubMed] [Google Scholar]
- 15.Holmes MD, Dodrill CB, Wilensky AJ, Ojemann LM, Ojemann GA, et al. Unilateral focal preponderance of interictal epileptiform discharges as a predictor of seizure origin. Arch Neurol. 1996;53:228–32. [DOI] [PubMed] [Google Scholar]
- 16.Chung MY, Walczak TS, Lewis DV, Dawson DV, Radtke R. Temporal lobectomy and independent bitemporal interictal activity: what degree of lateralization is sufficient? Epilepsia. 1991;32(2):195–201. [DOI] [PubMed] [Google Scholar]
- 17.Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2008;49(8):1308–16. [DOI] [PubMed] [Google Scholar]
- 18.Simasathien T, Vadera S, Najm I, Gupta A, Bingaman W, Jehi L. Improved outcomes with earlier surgery for intractable frontal lobe epilepsy. Ann Neurol. 2013;73(5):646–54. [DOI] [PubMed] [Google Scholar]
- 19.Edelvik A, Rydenhag B, Olsson I, Flink R, Kumlien E, Kallen K, et al. Long-term outcomes of epilepsy surgery in Sweden: a national prospective and longitudinal study. Neurology. 2013;81(14):1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jutila L, Immonen A, Mervaala E, Partanen J, Partanen K, Puranen M, Kälviäinen R, et al. Long term outcome of temporal lobe epilepsy surgery: analyses of 140 consecutive patients. J Neurol Neurosurg Psychiatry. 2002;73(5):486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paglioli E, Palmini A, Paglioli E, da Costa JC, Portuguez M, Martinez JV, et al. Survival analysis of the surgical outcome of temporal lobe epilepsy due to hippocampal sclerosis. Epilepsia. 2004;45(11):1383–91. [DOI] [PubMed] [Google Scholar]
- 22.Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess-Walsh P, Morris HH, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66(12):1938–40. [DOI] [PubMed] [Google Scholar]
- 23.Boesebeck F, Janszky J, Kellinghaus C, May T, Ebner A. Presurgical seizure frequency and tumoral etiology predict the outcome after extratemporal epilepsy surgery. J Neurol. 2007;254(8):996–9. [DOI] [PubMed] [Google Scholar]
- 24.Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Shinnar S, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65(6):912–8. [DOI] [PubMed] [Google Scholar]
- 25.De Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WFJ, Sander JW, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378(9800):1388–95. 10.1016/S0140 [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Valentín A, Humayon D, Longbottom AL, Jimenez-Jimenez D, Mullatti N, et al. Preoperative estimation of seizure control after resective surgery for the treatment of epilepsy. Seizure. 2013;22(10):818–26. [DOI] [PubMed] [Google Scholar]
- 27.Englot DJ, Wang DD, Rolston JD, Shih TT, Chang EF. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. Clinical article. J Neurosurg. 2012;116(5):1042–8. [DOI] [PubMed] [Google Scholar]
- 28.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. 2014;37(3):389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell GS, De Tisi J, Gonzalez-Fraile JC, Peacock JL, McEvoy AW, Harkness WFJ, et al. Factors affecting seizure outcome after epilepsy surgery: an observational series. J Neurol Neurosurg Psychiatry. 2017;88(11):933–40. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh AM, Kalnins RM, Mitchell LA, et al. Temporal lobectomy: Long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(9):2018–30. [DOI] [PubMed] [Google Scholar]
- 31.Schulz R, Lüders HO, Hoppe M, Tuxhorn I, May T, Ebner A. Interictal EEG and ictal scalp EEG propagation are highly predictive of surgical outcome in mesial temporal lobe epilepsy. Epilepsia. 2000;41(5):564–70. [DOI] [PubMed] [Google Scholar]
- 32.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Luders H, et al. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130(2):574–84. [DOI] [PubMed] [Google Scholar]
- 33.Radhakrishnan K, So EL, Silbert PL, Jack CR, Cascino GD, Sharbrough FW, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51(2):465–71. [DOI] [PubMed] [Google Scholar]
- 34.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. [DOI] [PubMed] [Google Scholar]
- 35.Steyerberg EW, Harrell FE, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81. [DOI] [PubMed] [Google Scholar]
- 36.Steyerberg EW, Harrell FE. Prediction models need appropriate internal, internal–external, and external validation. J Clin Epidemiol. 2016;69:245–7. 10.1016/j.jclinepi.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molinaro AM, Simon R, Pfeiffer RM. Prediction error estimation: a comparison of resampling methods. Bioinformatics. 2005;21(15):3301–7. [DOI] [PubMed] [Google Scholar]
- 38.Ji X, Kattan MW. Tutorial: development of an online risk calculator platform. Ann Transl Med. 2018;6(3):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortese G. How to use statistical models and methods for clinical prediction. Ann Transl Med. 2020;8(4):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jehi L. Algorithms in clinical epilepsy practice: can they really help us predict epilepsy outcomes? Epilepsia. 2021;62(S2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Josephson CB, Wiebe S. Precision medicine: academic dreaming or clinical reality? Epilepsia. 2021;62(S2):1–12 [DOI] [PubMed] [Google Scholar]
- 42.Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonicclonic seizures. Brain. 2009;132(4):999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caciagli L, Allen LA, He X, Trimmel K, Vos SB, Centeno M, et al. Thalamus and focal to bilateral seizures: a multiscale cognitive imaging study. Neurology. 2020;95(17):e2427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holtkamp M, Sharan A, Sperling MR. Intracranial EEG in predicting surgical outcome in frontal lobe epilepsy. Epilepsia. 2012;53(10):1739–45. [DOI] [PubMed] [Google Scholar]
- 45.Chapman K, Wyllie E, Najm I, Ruggieri P, Bingaman W, Lüders J, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76(5):710–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fong JS, Jehi L, Najm I, Prayson RA, Busch R, Bingaman W. Seizure outcome and its predictors after temporal lobe epilepsy surgery in patients with normal MRI. Epilepsia. 2011;52(8):1393–401. [DOI] [PubMed] [Google Scholar]
- 47.Di Gennaro G, Quarato PP, Sebastiano F, Esposito V, Onorati P, Mascia A, et al. Postoperative EEG and seizure outcome in temporal lobe epilepsy surgery. Clin Neurophysiol. 2004;115(5):1212–9. [DOI] [PubMed] [Google Scholar]
- 48.Basiri R, Shariatzadeh A, Wiebe S, Aghakhani Y. Focal epilepsy without interictal spikes on scalp EEG: a common finding of uncertain significance. Epilepsy Res. 2019;150:1–6. [DOI] [PubMed] [Google Scholar]
- 49.Rosati A, Aghakhani Y, Bernasconi A, Olivier A, Andermann F, Gotman J, et al. Intractable temporal lobe epilepsy with rare spikes is less severe than with frequent spikes. Neurology. 2003;60(8):1290–5. [DOI] [PubMed] [Google Scholar]
- 50.Murakami H, Wang ZI, Marashly A, Krishnan B, Prayson RA, Kakisaka Y, et al. Correlating magnetoencephalography to stereo-electroencephalography in patients undergoing epilepsy surgery. Brain. 2016;139(11):2935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feyissa AM, Britton JW, Van Gompel J, Lagerlund TL, So E, Wong-Kisiel LC, et al. High density scalp EEG in frontal lobe epilepsy. Epilepsy Res. 2017;129:157–61. 10.1016/j.eplepsyres.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 52.Doherty C, Nowacki AS, McAndrews MP, McDonald CR, Reyes A, Kim MS, et al. Response: predicting mood decline following temporal lobe epilepsy surgery in adults. Epilepsia. 2021;62(2):450–9. 10.1111/epi.16800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article will be available to any qualified investigator upon request.


