Abstract
Autophagy refers to a ubiquitous set of catabolic pathways required to achieve proper cellular homeostasis. Aberrant autophagy has been implicated in a multitude of diseases including cancer. In this review, we highlight pioneering and groundbreaking research that centers on delineating the role of autophagy in cancer initiation, proliferation and metastasis. First, we discuss the autophagy-related (ATG) proteins and their respective roles in the de novo formation of autophagosomes and the subsequent delivery of cargo to the lysosome for recycling. Next, we touch upon the history of cancer research that centers upon ATG proteins and regulatory mechanisms that control an appropriate autophagic response and how these are altered in the diseased state. Then, we discuss the various discoveries that led to the idea of autophagy as a double-edged sword when it comes to cancer therapy. This review also briefly narrates how different types of autophagy—selective macroautophagy and chaperone-mediated autophagy, have been linked to different cancers. Overall, these studies build upon a steadfast trajectory that aims to solve the monumentally daunting challenge of finding a cure for many types of cancer by modulating autophagy either through inhibition or induction.
Keywords: Autophagy, cancer, chaperone-mediated autophagy, history, selective autophagy, treatment
1. Introduction
The term autophagy encompasses a set of evolutionarily conserved cellular processes that result in the delivery of intracellular material such as proteins and organelles to lysosomes for degradation and recycling (Fig. 1) [1]. Autophagy occurs at a basal level in all cells to prevent the accumulation of damaged proteins and organelles, thus playing a pivotal role in the quality control of cytoplasmic components and in the maintenance of cellular homeostasis. These processes also function as a survival mechanism employed by cells that can be rapidly upregulated under certain stress conditions, such as starvation, hypoxia, endoplasmic reticulum (ER) stress, infections and the absence of growth factors. Autophagy can also be appropriately modulated when cells are in preparation for structural remodeling during developmental transitions. Basal autophagy is relatively nonselective when induced by stressors such as nutrient deprivation or metabolic perturbation (Fig. 1A). For a long time, autophagy had been primarily considered as a nonspecific bulk degradation process; however, it has since been discovered that autophagy can also distinctively recognize and target specific cargos such as (but not limited to) certain proteins, aggregates, ribosomes, organelles such as mitochondria and the ER, and invading pathogens (Fig. 1A) [2]. In a similar fashion to “bulk” autophagy, selective autophagy occurs constitutively and can be induced in response to specific conditions. Apart from maintaining cellular homeostasis, autophagy also serves a wide variety of critical functions in cell differentiation and development, tissue homeostasis, anti-aging and immunity [3]. Given the importance of autophagy, it is not surprising that dysregulated autophagy is associated with many human diseases including cancer, neurodegenerative diseases, infectious disease and heart disease. In this review, we will chiefly focus on the implications of autophagy in cancer.
Fig. 1.
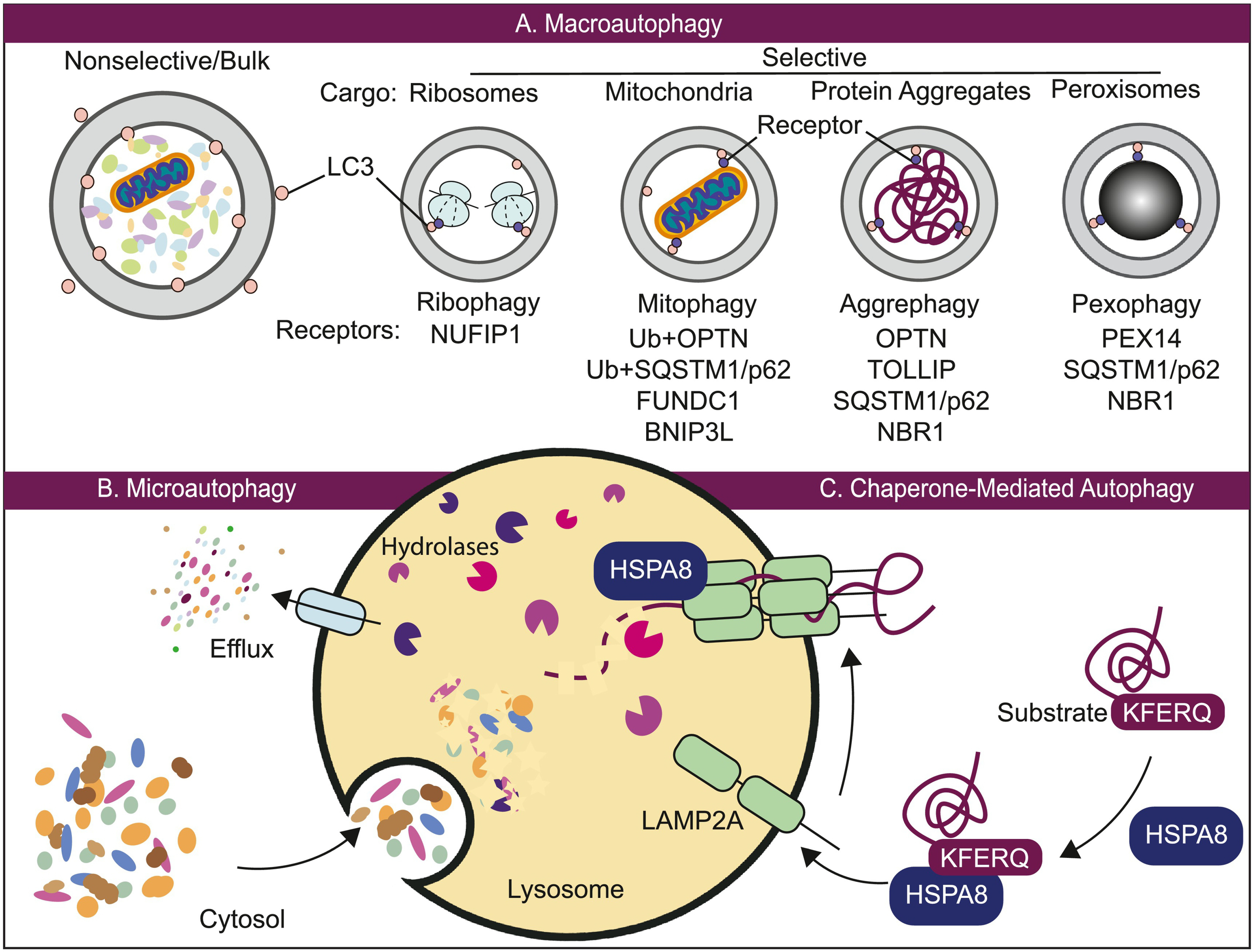
Three different types of autophagy. (A) Macroautophagy can be nonselective or selective. In the former, substrates may be engulfed randomly in bulk. In selective macroautophagy, specific cargo, such as ribosomes, mitochondria, protein aggregates and peroxisomes, are recognized by cargo receptors that can associate with LC3. (B) Microautophagy involves the direct uptake of cargo through the invagination of the lysosomal membrane. (C) Chaperone-mediated autophagy involves the direct translocation of an unfolded protein substrate across the lysosome via HSPA8 and the LAMP2A receptor. All three processes lead to the breakdown of cargo within the lysosome and the subsequent efflux of cellular building blocks back to the cytosol for use. See the text for details.
The most prevalent form of autophagy is macroautophagy (hereafter autophagy), which involves the de novo formation of double-membrane sequestering compartments termed phagophores, that mature into autophagosomes; the latter transport the enclosed cargo to a degradative organelle—the lysosome or vacuole. The process of autophagy is carried out and tightly regulated by a group of structurally and functionally conserved ATG proteins and can be broken down into several sequential steps: (1) initiation and nucleation of the phagophore, (2) expansion and closure of the phagophore to generate the autophagosome, (3) fusion with an endosome and/or a lysosome, and (4) cargo degradation and recycling (Fig. 2). Initiation begins with the activation of the ULK1 complex (consisting of ULK1/ULK2, ATG13, RB1CC1/FIP200 and ATG101) by integrating the nutrient and energy stress signals from the MTOR (mechanistic target of rapamycin kinase) complex 1 (MTORC1) and AMP-activated protein kinase (AMPK) [4]. Subsequently, the ULK1 complex phosphorylates and activates the class III phosphatidylinositol-3-kinase (PtdIns3K) complex, which is comprised of the catalytic subunit PIK3C3/VPS34, which phosphorylates the membrane lipid phosphatidylinositol (PtdIns) to produce phosphatidylinositol-3-phosphate (PtdIns3P), the putative tumor suppressor BECN1 (beclin 1), PIK3R4/VPS15, NRBF2, and the regulators ATG14 or UVRAG, and AMBRA1 [5,6]. Phagophores are nucleated on ER-emanating PtdIns3P-rich membrane domains known as omegasomes, which are characterized by the presence of the PtdIns3P-binding protein ZFYVE1/DFCP1 [7]. The recruitment of ATG9-containing vesicles to phagophore assembly sites where the ULK1 complex coalesces with the ER is also critical to autophagosome nucleation [8–10]. Following initiation, the phagophore proceeds to expand through the action of two ubiquitin-like (Ubl) protein conjugation systems: (1) the ATG12–ATG5 conjugation system comprised of ATG12 (a ubiquitin-like protein), ATG5 (an ATG12 substrate), ATG16L1, ATG7 (an E1 like enzyme), and ATG10 (an E2 like enzyme); (2) the Atg8-family proteins comprised of the MAP1LC3/LC3 and GABARAP subfamilies (ubiquitin-like proteins, homologous to yeast Atg8), phosphatidylethanolamine (PE, a substrate), ATG7 (an E1 enzyme), ATG3 (an E2 enzyme), ATG12–ATG5-ATG16L1 (an E3 ligase) and the protease ATG4 (which has four isoforms: ATG4A, ATG4B, ATG4C and ATG4D). PtdIns3P synthesized by PtdIns3K on the omegasome recruits the PtdIns3P-binding WIPI (WD repeat domain, phosphoinositide interacting) family proteins. Next, the WIPI proteins recruit the ATG12–ATG5-ATG16L1 complex through interaction with ATG16L1, which consequently allows targeting of the Atg8-family protein to the omegasome membrane by lipidation of the protein to form LC3-II, GABARAP-II, etc. [11]. The phagophore gradually expands into a cup-shaped structure that engulfs cytoplasmic components. The closure of the phagophore generates the autophagosome; maturation of the autophagosome involves clearance of ATG proteins from its outer membrane and recruitment of machinery that mediates fusion with the lysosome [12]. Ultimately, the outer membrane of the autophagosome fuses with the lysosome, which allows the degradation of the inner membrane and its engulfed contents.
Fig. 2.

Macroautophagy is a multi-step process. Following induction of the ULK1/2 complex, nucleation of the phagophore occurs. Expansion of the phagophore is facilitated by the class III PtdIns3K complex (PtdIns3K), the ATG9A system, the ATG12–ATG5-ATG16L1 complex and LC3/GABARAP conjugation system. Eventually, the expanding membrane closes around its cargo to form an autophagosome and LC3-II is cleaved from the outer membrane of this structure. Subsequently, the outer membrane of the autophagosome fuses with the lysosomal membrane to form an autolysosome. The contents of the autolysosome are then degraded and exported back into the cytoplasm for reuse by the cell. See the text for details.
Apart from macroautophagy, there are two other less well-characterized forms of lysosome-dependent autophagic degradation, namely, microautophagy and chaperone-mediated autophagy (CMA) (Fig. 1B and C), which mainly differ from macroautophagy in the nature of the cargo and how the cargos are delivered to lysosomes. In microautophagy, the lysosomal membrane is invaginated to form an autophagic tube or becomes protruded to wrap and engulf a portion of the cytosol along with other organelles—a key point being that cargo uptake occurs directly at the lysosome surface without a phagophore or autophagosome intermediate [13]. Currently, there are many types of microautophagic processes being identified, whose machineries and mechanisms are profoundly different from one another. The known functions of microautophagy include protein quality control, organelle remodeling and adaptation to stress, whereas its role in human disease remains unclear [14]. CMA is a form of selective autophagy that also directly takes up cytosolic cargos, but in this case the substrates are limited to individual proteins. In CMA, proteins containing the KFERQ-like pentapeptide targeting motif can be recognized by the chaperone HSPA8/HSC70 and then targeted to the lysosome through binding with LAMP2A (lysosomal associated membrane protein 2A) [15]. CMA plays various physiological roles and studies on the regulation of CMA have linked it to a variety of human diseases including neurodegenerative diseases, metabolic disorders and cancer [15,16].
2. An overview of autophagy and cancer
The process of autophagy was described morphologically at least as early as the 1950s, and in 1963 the term “autophagy” first appeared in print [17]. Thirty years later, studies in yeast identified core autophagy-related proteins (i.e., those required for autophagosome formation) which kickstarted the molecular era of autophagy research [18–20]. In the early 2000s, however, the focus of autophagy research began gradually shifting from identifying the core genes towards addressing questions that aim to understand how this essential cellular process is regulated, and its role in disease. Much attention has been placed on identifying both the major and nuanced regulatory mechanisms that control the extent of autophagy flux in response to particular environmental cues such as stress, infections, aging, etc., with an underpinning goal of utilizing and modulating autophagy for therapeutic purposes. Physiologically, dysregulated autophagy has been linked to a wide array of diseases including neurodegeneration, cardiovascular diseases, diabetes, myopathies, lung disease and cancer [21–25]. The significance of an exhaustive comprehension of autophagy becomes increasingly more pivotal in cancer where autophagy’s role becomes even more complex: autophagy controversially plays dual roles either as a suppressive or a promotive force, depending on the context and stages of tumor development (Fig. 3) [25,26]. In 1999, the Levine group showed that BECN1 is a tumor suppressor and first illustrated the implication of autophagy in cancer [5]. Subsequently, a tremendous amount of research has been conducted on the roles of autophagy in cancer in order to understand how it functions to either inhibit or promote cancer cell proliferation in different contexts. During the initial stages of tumor progression, autophagy plays a cytoprotective role by maintaining cellular and genomic integrity [27,28]. Conversely, multiple lines of evidence suggest that aberrant autophagy may enable the spread of cancer progenitor cells, aiding in the proliferation of oncogenic cells and continued tumor progression [29,30].
Fig. 3.

This timeline depicts a curated set of landmark discoveries in autophagy-related cancer research.
2.1. Interplay between autophagy and the V-ATPase
To further emphasize the complexity of this scenario, we briefly highlight recent studies regarding the emerging roles of the vacuolar-type H+-translocating ATPase (V-ATPase) and its interplay with autophagy in certain cancers. The V-ATPase, like other ATP-dependent H+-translocating transporters, is a multi-domain proton pump—and in this case, protons are pumped into the lumen of either lysosomes or endosomes to create and maintain an acidic environment within the corresponding cellular structures [31]. This acidification activates lumenal hydrolases, and is thus, critical in the degradation of autophagy-delivered cargo and endocytic contents into essential cellular building blocks inside the lysosome.
Mutations in multiple subunits of the V-ATPase have been identified to be closely associated with the development of various cancers. Recently, a study on follicular lymphoma found recurrent somatic mutations in the V-ATPase subunits ATP6AP1 and ATP6V1B2 by using discovery exome and extended targeted sequencing [32]. In addition, these mutations in ATP6AP1 and ATP6V1B2 frequently co-occur with mutations in RRAGC, which encodes a RAS-related GTP-binding protein that mediates the activation of MTORC1 by forming the complex of V-ATPase-RRAG-Ragulator [32], suggesting the involvement of MTORC1 signaling and autophagy regulation in follicular lymphoma. Other mutations in the genes that encode the V-ATPase protein subunits have been associated with other types of cancers. Somatic mutations in ATP6AP1 and ATP6AP2 (another accessory subunit of the V-ATPase) are found in 72% of granular cell tumors [33]. Apart from somatic mutations found in genes encoding the components of the V-ATPase, which may be genetically causal to cancers, changes in the expression levels of some V-ATPase subunits also correlate with cancer progression. For example, a decrease in the expression level of the V-ATPase stalk protein ATP6V1G1 is found in highly invasive breast cancer cells [34]. Similarly, upregulated expression levels of other V-ATPase subunits are observed in other types of cancers such as esophageal, ovarian and pancreatic cancers [35].
So far, investigations into the mechanism by which mutated V-ATPase affect tumorigenesis and cancer progression have not only been limited but are also contradictory. Because dysfunctional V-ATPase directly affects lysosomal acidification, which is essential for the final degradative step of autophagy, many studies have found that loss-of-function mutations of the V-ATPase lead to diminished autophagy activity [36]. For example, a very recent study shows that knockdown of ATP6AP2 increases both SQSTM1/p62 (sequestosome 1) and LC3-II levels in a lung adenocarcinoma cell line, indicating that defects in V-ATPase assembly lead to lower autophagy flux [37]. In direct contrast to these observations, however, it was recently discovered that hotspot mutations in ATP6V1B2 constitutively activate autophagy levels in lymphoma despite retaining MTORC1 kinase activity, enhancing the survival rate of these cancer cell lines by exploiting upregulated autophagy [38]. These results are intriguing in two ways: first, dual activation of autophagy and MTORC1 in follicular lymphoma-associated V-ATPase mutations contradicts the common understanding that MTORC1 negatively regulates autophagy; and, second, the various V-ATPase-related mutations found in different types of cancer highlight how deceptively simple the following question is when it comes to treating cancer: should we inhibit or stimulate autophagy? In this review, we provide a short summary of the history of autophagy research, with respect to cancer, focusing on the landmark discoveries that aim to address this controversy.
3. Autophagy suppresses tumorigenesis
Physiologically, autophagy is critical in both protein and organelle quality control and for the maintenance of cellular homeostasis through elimination of damaged cytoplasmic materials [39,40]. As mentioned, autophagy also plays a crucial role in cancer biology depending on the different context and stages of tumor development [25,26]. In this section, we highlight the discoveries that show autophagy’s active role in cancer prevention.
3.1. Defective autophagy promotes tumorigenesis
3.1.1. BECN1 and the role of its interacting proteins in cancer
The major pioneering study that first linked autophagy to cancer was published in 1999 by Beth Levine’s group where they demonstrated that BECN1 acts as a tumor-suppressing factor [5]. Monoallelic deletion of BECN1 has been found in up to 40% to 75% of various human cancers, including those of the prostate, breast and ovaries [41]. In a follow-up study in 2003, the Levine group demonstrated that in mice with monoallelic deletion of Becn1, higher rates of spontaneous tumor formation are observed [42]. Therefore, BECN1 is considered to be a haploinsufficient tumor suppressor. This observation was further supported by another study published later in the same year [43]. It is also of interest to note that BECN1 was first identified as an interacting partner of BCL2 and that elevated expression of BCL2 has been observed in many cancers, an observation that fits with the role of BCL2 as a BECN1 inhibitor [44,45].
In 2006, the interaction between BECN1 and UVRAG was first discovered, which further emphasized the importance of BECN1-dependent autophagy in tumor suppression. UVRAG induces autophagy and suppresses tumorigenesis via its direct interaction with BECN1 [46]. Similar to BECN1, UVRAG is monoallelically mutated in human breast and colon cancers, indicating that it may be a tumor suppressor [47].
A subsequent study from the Wang lab demonstrated that SH3GLB1/BIF1 (SH3 domain containing GRB2 like, endophilin B1) can also interact with BECN1 through UVRAG, leading to an increase in PtdIns3K lipid kinase activity and the induction of autophagosome formation during nutrient starvation [48]. Consistent with the observations made in the studies involving BECN1 and UVRAG, sh3glb1 knockout mice show a significantly higher frequency of spontaneous tumor occurrence compared to the wild type [48]. In addition, decreased expression of SH3GLB1 in gastric carcinomas [49] and homozygous deletion of SH3GLB1 in mantle-cell lymphomas has also been reported [50]. These studies suggest that SH3GLB1 functions as a potential activator of autophagy that allows for tumor suppression.
Along with these lines, another BECN1 interactor, AMBRA1, also displays a signature of cancer-associated mutations [50]. Furthermore, AMBRA1 loss-of-function mutations have been observed in various human tumors [51], which implies that AMBRA1 is a tumor suppressor. In the 2015 study mentioned above, mice xenografted with mouse embryonic fibroblasts lacking AMBRA1 develop tumors much more rapidly compared to those grafted with wild-type mouse embryonic fibroblasts. Furthermore, these tumors grow larger, demonstrating that depletion of AMBRA1 can lead to enhanced tumorigenic activity. Intriguingly, the expression of an AMBRA1 mutant partially restrains the tumorigenicity of AMBRA1-ablated cells. This special mutant of AMBRA1 does not affect canonical AMBRA1-mediated autophagy because this mutant can still bind to both BECN1 and ULK1; however, it was discovered that this AMBRA1 mutant partially affects its binding to the protein PPP2/PP2A (protein phosphatase 2; a cell cycle regulator). Therefore, it has been difficult to completely delineate the underlying mechanism by which AMBRA1 imparts tumor suppressive abilities. Is it due to its role in activating autophagy or its ability to regulate cell proliferation via its interaction with PPP2? Recently, subsequent analysis from the same group favored the latter option: the AMBRA1-CCND (cyclin D) pathway functions as a crucial cell cycle regulatory mechanism and is implicated in genomic stability in tumorigenesis [52]. Similarly, two other studies supported the idea that AMBRA1 can suppress tumorigenesis by regulating cellular levels of CCND, therefore restraining cancer cell proliferation [53,54]. It is important to note, however, that these studies do not exclude the possibility that AMBRA1-mediated autophagy can inhibit cancer progression. To this end, further studies to examine the tumor-suppressive function of AMBRA1 in human cancer are necessary.
3.1.2. Other core ATGs in cancer
Apart from BECN1 and AMBRA1, other core ATG proteins also have been reported to be oncogenically associated and may function as tumor suppressors. These include, but are not limited to ATG2B, ATG5, ATG7, ATG9B, ATG12 and ATG16L1. In a study published in 2009, frameshift mutations of ATG2B, ATG5, ATG9B and ATG12, along with mononucleotide repeats, were commonly found in gastric carcinomas and colorectal cancer (CRC) [55]. These particular types of cancer are considered to have high microsatellite instability (MSI-H), indicating that these mutations might contribute to the pathogenesis of human cancers by altering the autophagy process. Moreover, frameshift mutations in UVRAG discovered in colorectal and gastric cancer with MSI-H also have been observed [56,57]. Together, these studies reveal that autophagy may frequently be inactivated in MSI-H cancers. However, whether and how the frameshift mutations of various ATGs and UVRAG interact with each other in cancers with MSI-H are unclear and need to be further examined.
In another landmark study using mouse models, systemic mosaic deletion of Atg5 or liver-specific deletion of Atg7 result in the development of benign liver adenomas. In these autophagy-deficient hepatocytes, accumulation of SQSTM1/p62 is observed, along with oxidative stress and the induction of genomic damage response machinery [58]. Similarly, somatic mutation and loss of expression of ATG5 have been found in patients with gastrointestinal cancers and these ATG5 alterations may be involved in gastrointestinal cancer pathogenesis by perturbing apoptotic and autophagic cell death [59]. Another study in 2012 showed that the recombinant expression of ATG16L1 in epithelial cancer cells inhibits tumor growth, suggesting that ATG16L1 can function as tumor suppressor [59]. Overall, these studies demonstrate that core proteins in the autophagy machinery play an important role in the suppression of spontaneous tumorigenesis through a cell-intrinsic mechanism.
3.1.3. Inhibited autophagy results from activated MTOR in cancer
MTORC1 is a highly conserved Ser/Thr kinase functioning as a major molecular switchboard that interprets a wide variety of cellular stimuli, including the levels and bioavailability of amino acids, ATP and other nutrients, and, in response, appropriately regulate a multitude of fundamental cellular processes such as growth, cell cycle progression, protein synthesis and autophagy [60,61]. Furthermore, MTORC1 is vital in modulating anabolic and catabolic processes in response to various stresses. For example, this complex can directly regulate the autophagic machinery as well as lysosomal biogenesis: (1) by directly phosphorylating the ULK1/2 complex [62,63], (2) phosphorylation and subsequent inhibition of the PtdIns3K component ATG14, which plays a key role in autophagosome nucleation and maturation [64], and (3) by suppressing lysosome biogenesis through the inhibition of TFEB, a master regulator of autophagic and lysosomal gene expression. Thus, it is unsurprising that the deregulation of the MTORC1 pathway has also been implicated in many cancers [65–68]. Further investigation is necessary to determine whether MTORC1 could be further targeted for novel therapeutics targeting cancers where its activity is dysregulated.
3.2. Accumulation of the selective autophagy cargo receptor SQSTM1 contributes to tumorigenesis
SQSTM1/p62 is a ubiquitin-binding protein that acts as a selective autophagy receptor required to deliver specific types of cargo to the lysosome for degradation (Fig. 1A). SQSTM1 either recognizes and binds to cargo directly or through covalently linked ubiquitins. Targets include misfolded proteins and damaged organelles, which are and then delivered to nascent phagophores through SQSTM1 interaction with LC3 on the concave side of the phagophore membrane [69]. A study in 2009 showed that autophagy-defective tumor cells preferentially accumulate SQSTM1 caused by metabolic stress [70]. This study reported that defective autophagy contributes to the persistence of SQSTM1 by the accumulation of damage mitochondria, upregulated DNA damage response activation and increased oxidative stress. Hence the upregulation of SQSTM1 protein levels commonly observed in human tumors cells is at least partially due to defective autophagy [71,72]. Furthermore, the benign tumors formed in Atg5 and Atg7 knockdown mice exhibit an aberrant accumulation of SQSTM1. However, simultaneous deletion of Sqstm1 and Atg7 suppress tumor growth, associating cancer progression with SQSTM1 accumulation [58]. Consistent with these observations, another study also reported that SQSTM1 overexpression due to amplification of chromosome 5q, where SQSTM1 is located, can promote renal carcinogenesis both in vitro and in vivo [73]. Taken together, these studies demonstrate that irregular accumulation of SQSTM1 correlates with tumorigenesis and autophagy can act as a tumor suppressor by eliminating excess SQSTM1 [70]
4. Autophagy and tumor promotion
In the previous section, we summarized the landmark studies that investigated the roles of autophagy in tumor suppression. However, when it comes to cancer therapy, induction of autophagy is not necessarily a good choice as many studies have also pointed out that autophagy has the potential to contribute to chemotherapy resistance [74]. This is indicative of the fact that autophagy is primarily a mechanism for stress tolerance and, ultimately, cell survival, and can be commandeered by oncogenic cells to support disease progression and proliferation. In this section, we briefly review relevant discoveries on how autophagy can promote cancer cell growth, tumor metastasis, cancer stem cell maintenance and the tumor microenvironment.
4.1. Autophagy and tumor cell survival and growth
As tumors develop and expand, cancer cells are usually met with an environment that is lacking in both oxygen and nutrients. This is most especially true in the middle regions of a tumor, an area often observed to be severely hypovascularized. Therefore, in comparison to normal and healthy cells, cancer cells must adapt and mutate to deal with such conditions not just in order to survive but also to continue to grow. Indeed, in 2000 researchers discovered that multiple types of oncogenic cell lines—from liver, pancreatic, gastric and colon cancer cells—survive much longer than normal human fibroblasts [75] under conditions of nutrient starvation. Because autophagy is a critical cellular pathway that ensures survival under such stressful conditions, it is not surprising to see that the autophagic process can be requisitioned by these abnormal cells to support tumor maintenance and growth, in direct contrast to the initial discovery that autophagy acts in suppressing tumor initiation. A pioneering study suggesting that autophagy may promote cancer cell survival is from Eileen White’s group in 2006, where they found that autophagy is significantly induced in tumor cells, specifically in the regions with metabolic stress, where it can function to suppress tumor-induced inflammation and promote cell survival [76]. Consistent with these observations, in 2008, two other groups found that LC3, a key autophagy marker, is upregulated in gastrointestinal [77] and pancreatic [78] cancers, further demonstrating a putative role of autophagy in promoting progression of a tumor after its formation.
With the discovery of higher LC3 levels in pancreatic cancer cells [78], a more direct association between autophagy and pancreatic ductal adenocarcinoma (PDAC) growth was illustrated in a study conducted in 2011. Researchers showed that autophagy is essential for PDAC tumor growth; upon inhibition of autophagy through treatment with chloroquine or by knocking down ATG5, tumor growth is attenuated [79]. In the same year, another study in polyoma middle T antigen (PyMT)-driven mammary cancer demonstrated that deficient autophagy, by means of RB1CC1/FIP200 deletion, can also suppress tumor growth [80]. This discovery was further confirmed by a similar study published in 2014 [70]. In KRASG12D-driven lung tumors, deficiency in ATG7 leads to a reduction in both tumor burden and cell proliferation [81,82]. Similar results are also shown in an ATG5 deletion model [83,84]. In addition to KRASG12D -driven lung tumor, autophagy promotes growth of BRAFV600E-driven lung tumor cells [84]. Apart from the cancer types mentioned, tumor growth promotion through autophagy is also found in various cancers including lymphoma, prostate cancer, colorectal cancer, melanoma and glioblastoma [85–89].
With the advent of these publications, it was quite evident that autophagy contributes to tumor growth. Thus, many research groups became interested in mechanisms behind this phenomenon and whether autophagy could be a therapeutic target. Initial studies focused on RAS, a proto-oncogene. Mutations in RAS have been observed in 30% of cancers and even more than 90% of pancreatic cancers [90]. In 2011, a study from White’s group found that autophagy facilitates the growth and survival of multiple types of RAS-driven cancer cells including lung carcinoma cells, colorectal carcinoma cells and prostate carcinoma cells [91]. Additionally, they observed an accumulation of defective mitochondria and proposed a model suggesting that autophagy supports cancer cells by keeping the mitochondria functional, maintaining the energy levels required for survival and proliferation [91]. Another study from the White group further revealed an insight into the mechanism, showing that autophagy is used to maintain the quality of the mitochondrial genome and contribute to proper functioning of the pentose phosphate pathway and TCA cycle [92]. This is not only limited to RAS-driven lung cancers but was also found to be true in BRAFV600E-driven lung tumor cells wherein deletion of ATG7 also leads to the accumulation of defective mitochondria and overall metabolic impairment [84]. Together, these observations indicate that maintaining proper mitochondrial homeostasis may be one of the critical mechanisms by which autophagy supports the growth of cancer cells. Further studies into the relationship between autophagy and cancer cell growth show that the role of autophagy in tumors is not only dependent on the stage of the tumor [93], but is also determined by the genotype of the tumor cells, especially when it comes to the expression of TP53/p53. For instance, impairment of autophagy by loss of BECN1 only reduces PALB2-associated mammary tumorigenesis in a TP53 wild-type background but not in a null background [94]. Similarly, in non-small cell lung cancer (NSCLC), the deletion of TP53 in autophagy-deficient cells leads to comparable lung cancer development as seen in wild-type cells [83]. This finding indicates that TP53 inhibits cancer progression in autophagy-deficient tumors.
In PDAC, the role of TP53 is controversial. One study in 2013 shows that autophagy inhibition impedes the progression from pancreatic intraepithelial neoplasia lesion to PDAC in cell lines expressing TP53. Consequently, loss of autophagy does not block disease progression but instead accelerates tumor onset if TP53 is lacking [95]. However, another paper published a year later suggested that this process is independent of TP53 [96]. Therefore, more research is required to delineate how autophagy-dependent tumor progression is facilitated in different cellular genotypes, especially if the goal is to utilize autophagy inhibition as an avenue for cancer therapy. Clearly, this needs to be further resolved to determine if autophagy inhibition can be applied ubiquitously, or if it is more patient specific.
4.2. Autophagy and tumor metastasis
Metastasis is one of the hallmarks of cancers and causes most of the cancer-related death [97]. During metastasis, cancer cells leave the primary tumor to nucleate and form new tumors in other organs or tissues. Metastasis takes place in several steps: local invasion, intravasation, survival in the circulation, extravasation and colonization [98]. In each step of metastasis, cancer cells encounter altered microenvironments; therefore, they must utilize adaptive mechanisms to survive under these various types of stress. Thus, in this stage of cancer progression, autophagy may facilitate tumor metastasis. Indeed, multiple studies in the early 2010s found that high expression levels of LC3 and other autophagy-associated genes are linked with tumor metastasis [99–102], strongly suggesting that autophagy contributes to metastasis. In this section, we will summarize key studies defining the roles of autophagy in regulating tumor metastasis and the maintenance of cancer stem cells and dormant cancer cells.
4.2.1. Relationship between autophagy and tumor metastasis
For metastasis to occur, cancer cells must first gain: (1) motility to escape their primary tumor sites; and (2) the ability to adhere to and penetrate other organs. The direct link between metastasis of tumorigenic cells and autophagy was first established in 2014 when autophagy-dependent secretion factors such as IL6 (interleukin 6) and WNT5A were found to promote cancer cell invasion and proliferation [103]. In the same year, another study further demonstrated that TLR3- or TLR4-induced autophagy can promote the production of several cytokines, which are necessary to enhance lung cancer cell migration and invasion upon TLR activation [104]. With several studies suggesting that autophagy can promote cancer cell motility [99,103,104], two studies in 2016 established the molecular basis of this process: autophagy promotes focal adhesion turnover. These studies also pointed out that the selective autophagy cargo receptor NBR1 and the autophagy-dependent degradation of PXN (paxillin) are both critical for this process [105,106]. Additionally, MALAT1, a long non-coding RNA previously found to be associated with metastatic lung and pancreatic cancers [107,108], was found to accelerate tumor migration and invasion by stimulating autophagy [109]. Both WNT-CTNNB1/β-catenin and Hippo signaling pathways were also found to be crucial in this process in several recent studies [110,111].
Epithelial-mesenchymal transition (EMT) is a pro-metastatic process wherein epithelial cells gain mobility and invasiveness to become mesenchymal stem cells [98]. Several studies have pointed out that in hepatocellular carcinoma (HCC), autophagy promotes EMT through TGFB/TGF-β and SMAD signaling pathways [112,113]. Interestingly, autophagy is also reported to mediate TGFB-induced EMT in NSCLC cells: inhibition of autophagy attenuates TGFB-induced EMT [114]. Along these lines, suppression of ULK2 was found to promote both autophagy and EMT—directly implicating autophagy in the positive regulation of lung cancer cell motility [115]. Further studies have also shown that several known EMT regulators function through autophagy. For instance, the long non-coding RNA CPS1-IT1 suppresses EMT of colorectal cancer cells through inhibition of hypoxia-induced autophagy [116]. Another example is seen with PDCD1LG2/PD-L2, which stimulates osteosarcoma cell EMT by inducing autophagy [117].
Once cancer cells enter the circulatory system, they can disassociate from the extracellular matrix (ECM), which usually leads to anoikis, a form of programmed cell death [118]. In 2008, Jay Debnath’s group found that autophagy is induced when ECM detachment occurs, and that suppression of autophagy may lead to apoptosis [119]. The importance of autophagy in promoting cell survival despite anoikis induction was further evidenced in studies where it was shown that upon autophagy inhibition, both attenuation of anoikis and impairment of EMT are observed [120]. Follow-up investigations revealed the underlying mechanism by which autophagy promotes cell survival during ECM detachment: EIF2AK3/PERK (eukaryotic translation initiation factor 2 alpha kinase 3) and the IKBKB/IκB kinase complex (IKK) are activated to induce autophagy [121–123]. Additionally, MTDH/AEG-1, which is usually overexpressed in HCC is also proposed to be a contributor to anoikis resistance by stimulating autophagy [124].
4.2.2. Autophagy in cancer stem cells
EMT is considered to contribute to the formation of cancer stem cells (CSCs) [125]. CSCs are similar to tissue-specific stem cells—however, these are proposed to be critical for tumor metastasis because of their higher motility, invasiveness and their self-renewal ability [126]. CSCs were first identified in human acute myeloid leukemia in the 1990s [127,128] and the hypothesis that CSCs also exist in solid tumors was confirmed in a study in 2003 when researchers were able to isolate CSCs from breast cancer tumors [129]. Subsequently, CSCs have been found in multiple types of cancers, including brain [130], colon [131,132], pancreatic [133] and liver cancers [134]. CSCs are typically more resistant to radiation and chemotherapy [135], which suggests that CSCs potentially have an alternative means of sourcing energy to survive in the stressful microenvironments of tumors. Given the established role of autophagy in promoting EMT and in keeping the stemness of normal stem cells [136], it is reasonable to propose that autophagy is critical in promoting CSC persistence.
Chronic myeloid leukemia (CML) was initially treated using the chemotherapy drug imatinib mesylate (IM)—a tyrosine kinase inhibitor [127,128]. However, incidences of resistance to this treatment soon emerged. Researchers were eventually able to isolate CSCs from IM-resistant CML samples and were able to pursue clues as to how these CSCs persist through traditional therapy. The stem cells isolated from IM-resistant CML became widely used to investigate how autophagy can be modulated to produce more efficient therapeutic methods. A pilot study in 2009 found that autophagy is induced in IM-treated CML stem cells, serving as a survival mechanism therefore escaping cell death. Intriguingly, simultaneous treatment using a combination of IM and autophagy inhibitors achieves near complete clearance of CML stem cells [137]. A follow-up study in 2010 indicated that autophagy is critical for the BCR-ABL1-mediated leukemogenesis from precursor cells [138]. Later, it was found that ATG4B, as well as multiple other autophagy-related genes, including ATG4D, ATG5 and BECN1, have a higher expression level in CML stem cells [139]. Interestingly, the knockdown of ATG4B in CML stem cells impairs their ability to survive and makes these cancer cells susceptible to IM treatment. Researchers therefore suggest that ATG4B may work as a biomarker to predict therapeutic efficiency and can serve as a viable drug target [139]. Similarly, in acute myeloid leukemia, CD34+ cells, which are enriched in leukemic stem cells, utilize autophagy to maintain longevity [140].
In 2011, a study from Javier A. Menendez’ group was first to demonstrate that autophagy is mechanistically connected to the maintenance of breast cancer stem cells (BCSCs). They discovered that by knocking down certain ATGs, a decrease in the number of CD44+ CD24−/low cells (a marker of breast cancer) can be achieved [141]. Some follow-up studies further indicated the importance of autophagy-related genes in keeping the stem cell character of BCSCs, including BECN1 [142], ATG4A [143] and RB1CC1 [144]. ATM/ataxia-telangiectasia mutated kinase signaling, which maintains tumorigenicity in breast cancer [145], is also reported to keep BCSC sustainability through promoting ATG4C expression and, consequently, autophagy [146]. Similar to BCSCs, pancreatic CSCs show higher autophagy activity. Inhibition of autophagy leads to loss of the expression of CSC-related genes and tumorigenicity allowing for the re-sensitization of pancreatic CSCs to apoptosis [147]. In liver CSCs, autophagy is upregulated as well, and inhibition of autophagy leads to increased apoptosis under the hypoxic and nutrient-deprived microenvironment [148]. Autophagy is also critical for maintaining the stem-cell like features in osteosarcoma CSCs. Impaired autophagy results in the loss of its advantage of tolerance to low-nutrient conditions and the decreased expression of stemness markers [149]. A similar observation was also made in ovarian CSCs in two 2017 studies, where the researchers found that autophagy is upregulated compared with non-stem tumor cells and the inhibition of autophagy results in the loss of CSC properties and sensitivity to chemotherapy treatment [150,151]. In 2018, a study found that in glioblastoma stem-like cells (GSCs), autophagy is inversely correlated with susceptibility of GSCs to temozolomide treatment and impairment of autophagy increases sensitivity to such treatment [152]. In the same year, it was reported that in gastric CSCs, autophagy is upregulated and autophagy inhibition by chloroquine reduces cancer cell viability [153].
After determining the critical roles of autophagy in maintaining CSCs, researchers put substantial effort into revealing the underlying mechanisms. In BCSCs, two pathways mediating autophagy-dependent CSC maintenance were found. One study demonstrated that after autophagy inhibition, there is a decreased secretion of IL6 (a cytokine that induces BCSC formation) [154], suggesting that autophagy facilitates BCSC maintenance through IL6 secretion [155]. Another study reported that EGFR-STAT3 and TGFB-SMAD pathways both mediate BCSC maintenance support through autophagy, but the choice between the two pathways is dependent on the BCSC cell type (ALDH+ or ITGB1/CD29hi ITGB3/CD61+) [144]. Additionally, FOXA2, which helps maintain CSCs, is suggested to be involved in the autophagy-dependent sustainability of ovarian CSCs [156]. Experiments revealed that FOXA2 expression level in ovarian CSCs is maintained by autophagy and overexpression of FOXA2 can partially rescue the depressed self-renewal ability when autophagy is inhibited [151]. A recent study using lung CSCs as a model found that autophagy upregulates the stemness of CSC through the degradation of ubiquitinated TP53 [157]. The involvement of different pathways in this process indicates the complexity of how autophagy maintains CSCs. Further studies are needed to figure out whether different mechanisms are dependent on CSC cell type and whether autophagy can be an efficient clinical target to eliminate CSCs.
4.2.3. Autophagy and dormant cancer cells
One reason why tumor metastasis is hard to treat is that metastatic cells can become dormant and evade therapies. Dormant tumor cells are usually considered to be CSCs with arrested growth but retain the ability to proliferate that can lead to tumor outgrowth [158]. Considering autophagy is critical for the survival of CSCs, autophagy may be critical to the survival of dormant tumor cells. In 2008, Robert Bast Jr.’s group demonstrated the link between autophagy and ovarian tumor dormancy: the tumor suppressor ADPRH/ARH1 is required for autophagy and ADPRH-induced autophagy results in dormant tumor cells [159]. Evidence showing that autophagy plays a role in supporting dormant ovarian cancer cells is confirmed in many subsequent studies [160,161]. Furthermore, this also seems to be the case in multiple types of cancers, such as gastrointestinal stromal tumor cells [162], breast cancer [163,164], copper-deprivation induced dormant PDAC [165] and osteosarcoma [166]. The first studies using a dormant-to-proliferative model to determine the role of autophagy in tumor dormancy were conducted in 2018, when two groups found that inhibition of autophagy by chloroquine (CQ) leads to the cell death of dormant breast cancer cells but not the proliferative cells and that inhibition of autophagy leads to early escape from dormancy and recurrence of mammary carcinoma [167,168]. Another study the following year supports this conclusion and expands the scope of the finding by demonstrating the interaction between PFKFB3 and autophagy in governing metastatic outgrowth [169]. All these studies, figuring out the role of autophagy in sustaining dormant tumor cells, may provide a possible way to deal with some of the difficulties in cancer therapy. However, further research is still needed to demonstrate whether inhibiting autophagy could be used as a way to stimulate dormant cell death or to awaken dormant cancer cells so that they become more sensitive to the existing therapeutic methods.
4.3. Autophagy and the tumor microenvironment
Tumors are highly heterogeneous, which usually consist of cancer cells and stromal cells including fibroblasts, endothelial cells and immune cells. These cells, together with non-cellular structures, such as the extracellular matrix, create the tumor microenvironment [170]. In the past decades, increasing numbers of studies have found that the interaction between tumor cells and stroma are important for the initiation and progression of cancers [171]. In this subsection, we summarize how autophagy in cancer cells supports tumor growth though regulating its microenvironment and how non-cancer autophagy promotes tumor progression.
Cancer-associated fibroblasts (CAFs) are one of the key components of the tumor microenvironment. CAFs have quite diverse functions and their positive role on cancer cell growth has been well-established [172]. In 2010, researchers from the Lisanti and Sotgia groups published a series of papers, pointing out that loss of CAV1 (caveolin 1) on CAFs can induce autophagy. Furthermore, co-culturing of CAFs with breast cancer cells leads to oxidative stress in the former, which activates autophagy, or more specifically mitophagy. For one thing, these autophagic CAFs can protect cancer cells from apoptosis. In addition, these autophagic CAFs show a reduced mass of mitochondria and a metabolic shift, creating a negative energy balance in favor of tumor growth [173–176]. Based on these findings, these researchers put forward a model termed “the autophagic tumor stroma model of cancer”, proposing that CAFs provide recycled nutrients and chemical building blocks derived from autophagic degradation, a model confirmed by subsequent studies. In one such study, the researchers found that increased bulk autophagy and mitophagy in CAFs resulting from overexpression of some autophagy genes, including BNIP3, CTSB and ATG16L1, result in mitochondrial dysfunction and a shift from the TCA cycle to aerobic glycolysis, which produces ketone bodies and induces the growth and metastasis of the tumor [177]. In two other studies, induction of autophagy through activation of AMPK or overexpression of PPARG/PPARγ in CAFs showed similar results [178,179]. Autophagy in CAFs is critical for tumor growth through triggering metabolic reprogramming in several types of cancers [170–172]. In some cases, the non-contact coculture between CAFs and cancer cells also supports the tumor growth [171], indicating the significant role of secretory factors from CAFs in this context. It is found that the cytokines from CAFs such as IGF1, IGF2 and CXCL12 induce autophagy in cancer cells, which is critical for cancer cell recovery after radiation [173]. Additionally, IL6 and IL8 produced through secretory autophagy in CAFs, an autophagic pathway facilitating unconventional secretion of the cytosolic cargo [174], promote head and neck cancer progression [175].
Another important component of the tumor microenvironment is the endothelial cells (ECs), the cells lining tumor blood vessels. EC arrangement is the main driver of angiogenesis, defined as the formation of new blood vessels from the preexisting vasculature, which is critical for tumors to meet the high energy demands to allow proliferation [180]. Therefore, it is not surprising to see that tumor ECs are able to accelerate tumor metastasis [181]. Tumor-associated ECs have higher resistance to hypoxic stress, which may be explained by autophagy because tumor-associated ECs have a more pronounced autophagic phenotype than normal ECs when exposed to the hypoxic environment [182]. This observation suggests that autophagy could be critical for tumor ECs to maintain homeostasis. However, the role of autophagy in angiogenesis is not well studied and remains controversial. Two studies in 2012 indicated that autophagy is important for angiogenesis, maybe through its interplay with HMGB1 (high mobility group box 1) [176,177]. Even though the studies do not show the role of autophagy in tumor-associated ECs, the fact that HMGB1 is overexpressed in tumors but not normal ECs suggests the importance of autophagy in the former [183]. Another study in 2014 shows that atg5 knockout in ECs leads to smaller, more tortuous, and less mature tumor vessels with abnormal EC lining, further indicating a role of autophagy in EC homeostasis and potentially in normal angiogenesis [184]. However, in contrast, a study in 2011 shows that Becn1+/− mice have increased angiogenesis and tumor growth [185], which suggests that autophagy may confine angiogenesis in tumors. The studies related to autophagy in tumor-associated ECs are limited, probably due to the difficulty in separating pure tumor-associated ECs from neighboring cells. More studies are required before we can evaluate whether tumor-associated ECs are a good target for therapy. For one thing, the exact role of autophagy should be determined; for instance, whether autophagy in tumor-associated ECs inhibits or promotes angiogenesis is determined by cancer type or stage. For the other, because angiogenesis happens not only during the pathological state, how to specifically target tumor-associated ECs should also be taken into consideration.
Immune cells exist in the tumor microenvironment, and they are responsible for the anti-tumor immune response. CD8+ T cells are the major mediators of anti-tumor immunity; they recognize tumor-associated antigens presented by the major histocompatibility complex (MHC), become activated and kill tumor cells [186]. Cancer cells develop some strategies to evade from immunosurveillance [187]. Here, we summarize how autophagy helps tumor immune invasion in three ways.
First, autophagy in cancer cells can suppress antigen presentation and thus protect them from T-cell recognition. For instance, after uptake of apoptotic tumor cells, TIMD4/TIM-4 induces autophagy in macrophages through binding to AMPK, which leads to tumor-associated antigen degradation and reduced antigen presentation [188]. Two more recent studies indicate that MHC-I is degraded through autophagy in PDAC cells [189] and that ULK1 pathway inhibition in STK11/LKB1 mutant NSCLC restores antigen presentation [190].
Second, autophagy contributes to the resistance to immune-cell mediated lysis. For example, autophagy is responsible for the resistance to cytolytic T-cell lysis [191]. A more recent study supports this conclusion and further shows that it happens through inhibiting TNF/TNFα-induced apoptosis [192]. Also, autophagy in breast cancer cells can degrade GZMB (granzyme B), a cytotoxic protease released by natural killer cells, to increase cancer cell resistance to natural killer cell-mediated lysis [193].
Third, another function of autophagy is regulating immune cells, creating an immunosuppressive microenvironment that is favorable for tumor growth. Autophagosomes released from tumor cells inhibit T cells activity through inducing IL10-producing B cells [194] and enhancing apoptosis of neutrophils [195]. In addition, autophagy in T cells regulates metabolism and histone modification, and T cells with deficient autophagy have an increased tumor-killing ability [196]. Immunosuppressive cells, including regulatory T-cells, M2 macrophages and marrow-derived suppressor cells, are also important components of the cancer microenvironment, helping cancer cells escape from immunosurveillance [197]. Several studies show that autophagy in both tumor cells and macrophages induces macrophage M2 polarization [198–200]. In a recent study, researchers found that in PDAC, autophagy-dependent ferroptosis of cancer cells will induce the release of KRASG12D, which is taken up by macrophages and contributes to M2 polarization; the inhibition of this process suppresses PDAC growth in the mouse model [201]. Additionally, patients with melanoma have a higher autophagy activity in their marrow-derived suppressor cells, which is used for the degradation of MHC-II for decreasing activation of CD4+ T cells [202]. The function of regulatory T-cells is also found to be activated by hepatic autophagy in the tumor with high mutational burden, which contributes to a more cancer-favorable microenvironment [203].
The interaction between tumor cells and microenvironment is also important. A good example is seen with the observation that autophagy in HCC cells increases after coculture with macrophages and it contributes to drug resistance, suggesting that tumor-associated macrophages could be used as a therapeutic target [204]. All these studies, from different perspectives, indicate the role of autophagy in helping cancer cells escape from immunosurveillance, suggesting that autophagy may be a target for an improved cancer immunotherapy.
4.4. CMA and cancer promotion
As mentioned above, there are different types of autophagic processes including microautophagy and CMA (Fig. 1). Akin to autophagy, CMA has dual roles in cancer cells and its association with cancer cell proliferation was put forward by three independent studies in 2011 and 2012. The very first study found that PKM/PKM2 is degraded through CMA, which leads to the accumulation of glycolytic intermediates and promotes cell proliferation and tumor growth [205]. A later study in the same year shows an upregulation of CMA in many different cancers and confirms the positive role of CMA using human lung cancer xenografts in mice [206]. Consistently, another study published the following year found that overexpression of LAMP2A, a key protein in CMA, promotes lung cancer cell survival through CMA [207]. CMA is involved in the removal of many proteins, which may potentially explain the mechanism of how CMA promotes cancer cell proliferation; these substrates include proteins related to cell metabolism, such as PKM [205], proteins negatively regulating cell proliferation such as RND3 [208] and several known tumor-suppressing proteins, such as MST1 [209], unphosphorylated PEA15/PED [210] and CDKN1A/p21 [211]. Interestingly, a study in 2017 found that CMA promotes breast cancer cell growth through downregulation of ATG5-dependent autophagy [212]. With the basic research showing that CMA plays a role in promoting cancer cell proliferation, more studies are focusing on whether inhibiting CMA can work as a cancer therapy. A study in 2020 found that in colon carcinoma, inhibition of CMA by knocking down LAMP2A leads to increased cell apoptosis [213]. Two other very recent studies demonstrate that inhibition of CMA in both NSCLC cells and colorectal cancer cells not only suppresses cell proliferation, but also promotes chemotherapeutic drug sensitivity [214,215]. Overall, these studies indicate that proteins essential for CMA may be potential therapeutic targets to treat cancers.
5. Therapeutic targeting of autophagy in cancer
As noted previously, the role of autophagy in cancer is context dependent and could be either tumor suppressing or tumor promoting, based on factors including, but not limited to, the oncogenes involved and the stage of tumorigenesis [216]. However, as noted above, multiple lines of evidence now suggest that autophagy may aid the survival of cancer cells in established tumors [26,217], inspiring the development of autophagy inhibitors as cancer therapeutics. The multiple distinct steps of autophagy – initiation by the ULK1/2 complex, phagophore nucleation by the PtdIns3K complex, phagophore expansion, autophagosome-lysosome fusion and autolysosomal degradation of autophagic cargo – provide distinct targets for drug development (Fig. 2). Although inhibiting autophagy at any of these steps would potentially have a similar effect on reducing autophagy flux, given the autophagy-independent roles of autophagy-related proteins, other cellular implications of targeting each step would be variable and potentially detrimental [218]. In concert with this understanding, several autophagy inhibitors, targeting different steps in the pathway, are undergoing pre-clinical validation for their ability to safely diminish tumor growth and survival [219]. We focus on these inhibitors that are in development and then discuss the use of CQ and hydroxychloroquine (HCQ) for autophagy inhibition in clinical studies.
5.1. Therapies targeting early stages of autophagy
While several compounds for early-stage inhibition of autophagy are currently in development, we highlight three classes of inhibitors that have shown significant promise; those affecting ULK1, PIK3C3/VPS34 and ATG4B.
ULK1 inhibition is a potent mechanism to block autophagy and the small-molecule kinase inhibitor SBI-0206965 was identified as a selective ULK1 inhibitor that could effectively promote cell death when used in concert with MTOR inhibition [220]. Subsequently, the compound was identified to be a potent inhibitor of AMPK, another positive regulator of autophagy [221]. In several independent pre-clinical studies that have followed, SBI-0206965 has been demonstrated to promote tumor suppression. In NSCLC, ULK1 is identified to promote tumor survival by modulating both autophagy and apoptosis and the administration of SBI-0206965 sensitizes NSCLC to cisplatin [222]. In glioblastoma, increased autophagy through AMPK and ULK1 activation promotes resistance to temozolomide therapy; however, co-treatment with SBI-0206965 and high dose temozolomide promotes apoptosis [223]. A similar effect is seen in clear cell renal cell carcinoma cells, where ULK1 overexpression plays a protective role via autophagy upregulation [224].
Due to its critical role in phagophore nucleation, PIK3C3/VPS34 is an attractive target for autophagy inhibition. Inhibition of PIK3C3/VPS34 leads to the accumulation of autophagy substrates [225]. Two potent PIK3C3/VPS34 inhibitors, SB02024 and SAR405, have demonstrated pre-clinical promise in cancer therapy. SB02024, developed by the Karolinska Institute and Spirit Biosciences, reduces autophagy flux in vitro and the growth of breast cancer cell line-derived xenografts in vivo. Furthermore, co-administration with SB02024 increases the efficacy of the FDA-approved breast cancer drug sunitinib in monolayer cultures or multicellular spheroids of two breast cancer lines: MCF-7 and MDA-MB-231 [226]. SAR405, developed by Sanofi, is another highly potent inhibitor of PIK3C3/VPS34 that promotes reduction in autophagy flux [227] and has shown promise in the treatment of osteosarcoma when combined with celecoxib [228]. SAR405 was also successful in increasing the sensitivity of head and neck squamous cell carcinoma cells to cisplatin in a xenograft model [229]. Both SB02024 and SAR405 have the potential of bolstering the potency of immune checkpoint inhibitors by blocking autophagy flux. In murine models of melanoma and CRC blocking autophagy with either of these PIK3C3/VPS34 inhibitors leads to better infiltration of immune cells, including natural killer cells and CD8+ T-lymphocytes [230]. Another PIK3C3/VPS34 inhibitor, VPS34-IN-1, suppresses the expansion of CSCs in a mouse model of HCC but its effect in regard to the regulation of autophagy is still unclear [231,232].
The expansion of the phagophore requires the conjugation of Atg8-family proteins to the growing phagophore [233]. Prior to their conjugation, the proteins require proteolytic processing by the cysteine protease ATG4 [234]. Based on in vitro studies, the ATG4 isoform ATG4B is primarily involved in the processing of the LC3 subfamily of mammalian Atg8-family proteins and inhibition of ATG4B using the benzotropolone compound UAMC-2526 blocks autophagy within CRC tumor xenografts in mice. Co-administration of UAMC-2526 also increases the efficacy of oxaliplatin in inhibiting tumor growth [235]. Similar results had previously been obtained with a distinct ATG4B inhibitor, NSC185058, which was demonstrated to halt autophagy and the development of osteosarcoma tumors in vivo [236]. ATG4B expression is also significantly enhanced in CRC cells compared to normal cells and treatment with the ATG4B inhibitor S130 demonstrates reduced CRC tumor growth in vivo [237].
5.2. Therapies targeting late stages of autophagy
The current clinical focus on autophagy inhibition to improve cancer outcomes revolves around the inhibition of autophagosome-lysosome fusion and subsequent degradation of autophagic cargo by the administration of CQ or HCQ. Both CQ and HCQ promote lysosomal de-acidification and were initially developed for the treatment of other diseases [238,239] but showed promise as an adjuvant in a clinical trial for glioblastoma in 2003 [240,241]. CQ treatment has also demonstrated success in promoting the death of otherwise apoptosis-refractory tumor cells from MYC-induced models of lymphoma [242]. Several clinical trials followed using CQ/HCQ in combination with other therapies such a radiation in brain metastases [243,244], the HDAC-inhibitor vorinostat in solid tumors [245], the MTOR inhibitor temsirolimus in solid tumors and melanoma [246], temozolomide in solid tumors and melanoma [247], the proteasome inhibitor bortezomib [219], the RRM (ribonucleotide reductase) inhibitor gemcitabine in PDAC [248] and erlotinib in NSCLC [249].
The clinical trials with CQ/HCQ had some promising but largely inconclusive results, including the inconsistency in the maximum tolerated dose. Because inhibition with CQ/HCQ blocks autophagy both within and beyond the tumor, the maximum tolerated dose varies depending on the cytotoxicity of the drug used in combination as well as the tumor type [245,250]. Autophagy inhibition was also found to be inconsistent, especially because the CQ/HCQ uptake depends on the pH status of the tumor microenvironment – uptake is reduced under acidic conditions – which varies locally within the tumor and among different tumor types [251]. Additionally, some studies have revealed that CQ may play an autophagy-independent role in cancer mitigation that complicates the mechanism of action of the drug. CQ reduces cancer cell invasion and metastasis by promoting “tumor vessel normalization”, a process of restructuring the vasculature around a tumor to increase perfusion and reduce hypoxia. This action of CQ was determined to be independent of autophagy but dependent on NOTCH signaling [184,252]. Autophagy-independent sensitivity to chloroquine has also been noted in “autophagy-addicted” KRAS mutant tumors [253]. Similar observations have been made in breast cancer [254] and bladder cancer, with CQ shown to promote toxicity in bladder cancer cells by interfering with cholesterol metabolism [255]. Finally, pharmacodynamic studies revealed that the use of peripheral blood mononuclear cells as a surrogate for measuring autophagy inhibition within tumors – a conventional measure of CQ/HCQ potency – may be inaccurate [256].
5.3. Maximizing the chances of success with autophagy-targeted cancer therapies
Autophagy is a remarkably complex pathway that integrates itself with several other critical cellular processes, making nuanced therapeutic targeting a necessity. The host immune response as well as the regulation of apoptosis in tumor cells [257–259] is heavily influenced by autophagy. Therefore, autophagy inhibition may not be relevant for all tumors, meaning that proper patient selection is critical for successful treatment with autophagy inhibition.
First, autophagy inhibition may be particularly useful in treating “autophagy-addicted” tumors, while not having a significant effect on tumors that do not utilize autophagy for survival. Tumors derived from dysregulation of the RAS-RAF-MAP2K/MEK pathway exhibit autophagy-dependence [260–262] and may be good targets for autophagy-inhibition therapy. PDAC has a high incidence of KRAS mutations and is autophagy-dependent – pancreatic stellate cells in the tumor microenvironment use autophagy to supply PDAC cells with necessary nutrients [263]. Pre-clinical trials examining xenograft growth in vivo indicates promise for autophagy inhibition in treating PDAC [96], which was corroborated by clinical studies showing improved surgical outcomes for PDAC patients treated with HCQ [248]. The combination of targeting the RAS pathway was validated further in studies combining MAP2K/MEK inhibition with autophagy inhibition [262]. These findings led to the initiation of clinical trials targeting mutant RAS-driven melanoma with a combination of HCQ and trametinib (NCT03979651), RAS-driven pancreatic cancer with a combination of HCQ, gemcitabine and nab-Paclitaxel (NCT01506973) or BRAF-mutant melanoma with Vemurafenib and HCQ NCT01897116).
Second, the development of alternatives of CQ/HCQ promises to address the issue of variable uptake within and across tumors. The bisaminoquinoline autophagy inhibitor Lys01 is a significantly more potent autophagy inhibitor compared to HCQ. Lys05, the derivative salt of Lys01, accumulates within and de-acidifies the lysosome more potently than HCQ [264]. A derivative of Lys05 is the dimeric quinacrine DQ661 which binds and inhibits PPT1 (palmitoyl-protein thioesterase 1). PPT1 inhibition promotes the displacement of V-ATPase subunits leading to reduced lysosomal acidification and MTORC1 recruitment [265,266]. Together, these effects lead to a combination of reduced autophagy and protein synthesis making DQ661 an attractive therapeutic strategy against autophagy-dependent tumors. ROC-325, another dimeric compound structurally derived from HCQ has been demonstrated to promote autophagy inhibition and improved tumor responses in acute myeloid leukemia [267] and renal cell carcinoma [267,268].
Third, targeting cancers driven by specific mutations provides the availability of a biomarker (such a RAS or BRAF) to monitor therapy progress. This is crucial for complementing current biomarkers for autophagy inhibition, including measuring SQSTM1, LC3-II and CTSD (cathepsin D) levels [218,245], which may not always be consistent owing to the dynamic nature of autophagy and lysosomal function. Finally, because total autophagy inhibition can be detrimental to physiology and induce the development of contraindications, therapeutic considerations should include appropriate dosing and careful choice of drug for combination therapy [219]. Additional strategies could include rest periods between treatment regimens to allow restoration of critical physiological basal autophagy.
6. Conclusion and Future Perspectives
Although it has been more than 60 years since the first discovery of autophagosomes, scientists have barely begun to scratch the surface when it comes to completely unlocking the manipulation of autophagy as an effective therapeutic target, not only in cancer, but in several other diseases. The history of autophagy and cancer has been markedly short, relatively speaking, but has been intriguing and controversial. In the past couple of decades, we have seen the incredible advances in our knowledge of both topics. The ultimate goal is to comprehend and elucidate their contentious relationship, with the hopes of successfully targeting autophagy for treatment. However, as illustrated in this review, at least two critical issue remain: 1) the role of autophagy can shift from suppressing cancer initiation to advancing cancer progression in later stages of tumorigenesis, and 2) autophagy plays a fundamental role in normal cellular physiology. Furthermore, the same autophagy-related genes and proteins may act in different capacities depending on the conditions presented by specific cancer stages and types. Along with other factors summarized in this review, contradictory roles of autophagy make its targeted therapy problematic. Thus, more research needs to be carried out to clearly determine whether autophagy should be blocked or induced in cancer treatment. Currently, there are multiple autophagy inhibitors being investigated to treat different types of cancer. However, treatment by means of blocking autophagy needs to be carefully monitored—because autophagy performs critical roles in cell homeostasis, potential for toxicity is an issue. Overall, the current studies demonstrate that there are many significant questions that remain to be answered. There is a need for a more detailed mechanistic understanding of how autophagy is regulated in different stages of cancer, from initiation to proliferation, and in the context of what types of cells are affected. Further research on the matter is the key to a more effective approach when it comes to targeting autophagy to treat tumorigenesis.
Highlights.
We summarize findings on the role of autophagy in cancer from the past two decades.
Regarding cancer, either too little or too much autophagy can lead to disease.
How to modulate autophagy therapeutically remains unresolved and controversial.
Autophagy inhibitors for cancer treatment are currently undergoing clinical trials.
Funding sources
This work was supported by NIH grant GM131919 to DJK.
Abbreviations
- AMPK
AMP-activated protein kinase
- ATG
autophagy-related
- BCSCs
breast cancer stem cells
- CAFs
cancer-associated fibroblasts
- CMA
chaperone-mediated autophagy
- CML
chronic myeloid leukemia
- CQ
chloroquine
- CRC
colorectal cancer
- CSCs
cancer stem cells
- ECM
extracellular matrix
- HCC
hepatocellular carcinoma
- HCQ
hydroxychloroquine
- IM
imatinib mesylate
- MSI-H
high microsatellite instability
- MTORC1
mechanistic target of rapamycin kinase complex 1
- NSCLC
non-small cell lung cancer
- PDAC
pancreatic ductal adenocarcinoma
- PtdIns3K
class III phosphatidylinositol 3-kinase
- PtdIns3P
phosphatidylinositol-3-phosphate
- Ubl
ubiquitin-like
- V-ATPase
vacuolar-type H+-translocating ATPase
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Klionsky DJ, Emr SD, Autophagy as a regulated pathway of cellular degradation., Science (New York, N.Y.). 290 (2000) 1717–1721. 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gatica D, Lahiri V, Klionsky DJ, Cargo recognition and degradation by selective autophagy., Nature Cell Biology. 20 (2018) 233–242. 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yin Z, Pascual C, Klionsky DJ, Autophagy: machinery and regulation., Microbial Cell (Graz, Austria). 3 (2016) 588–596. 10.15698/mic2016.12.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xie Y, Kang R, Sun X, Zhong M, Huang J, Klionsky DJ, Tang D, Posttranslational modification of autophagy-related proteins in macroautophagy., Autophagy. 11 (2015) 28–45. 10.4161/15548627.2014.984267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B, Induction of autophagy and inhibition of tumorigenesis by beclin 1., Nature. 402 (1999) 672–676. 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- [6].Fimia GM, Stoykova A, Romagnoli A, Giunta L, Bartolomeo SD, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, Gruss P, Piacentini M, Chowdhury K, Cecconi F, Ambra1 regulates autophagy and development of the nervous system., Nature. 447 (2007) 1121–1125. 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- [7].Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT, Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum., The Journal of Cell Biology. 182 (2008) 685–701. 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC, Diverse autophagosome membrane sources coalesce in recycling endosomes., Cell. 154 (2013) 1285–1299. 10.1016/j.cell.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, Collinson LM, Tooze SA, Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy., Molecular Biology of the Cell. 23 (2012) 1860–1873. 10.1091/mbc.e11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Karanasios E, Walker SA, Okkenhaug H, Manifava M, Hummel E, Zimmermann H, Ahmed Q, Domart M-C, Collinson L, Ktistakis NT, Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles., Nature Communications. 7 (2016) 12420–17. 10.1038/ncomms12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dooley HC, Razi M, Polson HEJ, Girardin SE, Wilson MI, Tooze SA, WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12–5-16L1., Molecular Cell. 55 (2014) 238–252. 10.1016/j.molcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, Zhou Q, Wilz LM, Li J, Vivona S, Pfuetzner RA, Brunger AT, Zhong Q, ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes., Nature. 520 (2015) 563–566. 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oku M, Sakai Y, Three Distinct Types of Microautophagy Based on Membrane Dynamics and Molecular Machineries., BioEssays : News and Reviews in Molecular, Cellular and Developmental Biology. 40 (2018) e1800008. 10.1002/bies.201800008. [DOI] [PubMed] [Google Scholar]
- [14].Schuck S, Microautophagy - distinct molecular mechanisms handle cargoes of many sizes., Journal of Cell Science. 133 (2020). 10.1242/jcs.246322. [DOI] [PubMed] [Google Scholar]
- [15].Kaushik S, Cuervo AM, The coming of age of chaperone-mediated autophagy., Nature Reviews. Molecular Cell Biology. 19 (2018) 365–381. 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Alfaro IE, Albornoz A, Molina A, Moreno J, Cordero K, Criollo A, Budini M, Chaperone Mediated Autophagy in the Crosstalk of Neurodegenerative Diseases and Metabolic Disorders., Frontiers in Endocrinology. 9 (2018) 778. 10.3389/fendo.2018.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cameron MP, Duve CD, Reuck AVSD, Ciba Foundation symposium: lysosomes : [proceedings], J. & A. Churchill, London, 1963. [Google Scholar]
- [18].Tsukada M, Ohsumi Y, Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae., FEBS Letters. 333 (1993) 169–174. 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- [19].Harding TM, Morano KA, Scott SV, Klionsky DJ, Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway., The Journal of Cell Biology. 131 (1995) 591–602. 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Titorenko VI, Keizer I, Harder W, Veenhuis M, Isolation and characterization of mutants impaired in the selective degradation of peroxisomes in the yeast Hansenula polymorpha., Journal of Bacteriology. 177 (1995) 357–363. 10.1128/jb.177.2.357-363.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Leidal AM, Levine B, Debnath J, Autophagy and the cell biology of age-related disease., Nature Cell Biology. 20 (2018) 1338–1348. 10.1038/s41556-018-0235-8. [DOI] [PubMed] [Google Scholar]
- [22].Ariosa AR, Klionsky DJ, Autophagy core machinery: overcoming spatial barriers in neurons., Journal of Molecular Medicine (Berlin, Germany). 94 (2016) 1217–1227. 10.1007/s00109-016-1461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang Y, Klionsky DJ, Autophagy and disease: unanswered questions., Cell Death and Differentiation. 27 (2020) 858–871. 10.1038/s41418-019-0480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gatica D, Chiong M, Lavandero S, Klionsky DJ, Molecular mechanisms of autophagy in the cardiovascular system., Circulation Research. 116 (2015) 456–467. 10.1161/circresaha.114.303788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kimmelman AC, The dynamic nature of autophagy in cancer., Genes & Development. 25 (2011) 1999–2010. 10.1101/gad.17558811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].White E, The role for autophagy in cancer., The Journal of Clinical Investigation. 125 (2015) 42–46. 10.1172/jci73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nassour J, Radford R, Correia A, Fusté JM, Schoell B, Jauch A, Shaw RJ, Karlseder J, Autophagic cell death restricts chromosomal instability during replicative crisis., Nature. 565 (2019) 659–663. 10.1038/s41586-019-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E, Autophagy suppresses tumor progression by limiting chromosomal instability., Genes & Development. 21 (2007) 1367–1381. 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dou Z, Xu C, Donahue G, Shimi T, Pan J-A, Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, Catanzaro JM, Ricketts MD, Lamark T, Adam SA, Marmorstein R, Zong W-X, Johansen T, Goldman RD, Adams PD, Berger SL, Autophagy mediates degradation of nuclear lamina., Nature. 527 (2015) 105–109. 10.1038/nature15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].White E, Deconvoluting the context-dependent role for autophagy in cancer., Nature Reviews. Cancer. 12 (2012) 401–410. 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pamarthy S, Kulshrestha A, Katara GK, Beaman KD, The curious case of vacuolar ATPase: regulation of signaling pathways., Molecular Cancer. 17 (2018) 41–9. 10.1186/s12943-018-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Okosun J, Wolfson RL, Wang J, Araf S, Wilkins L, Castellano BM, Escudero-Ibarz L, Seraihi AFA, Richter J, Bernhart SH, Efeyan A, Iqbal S, Matthews J, Clear A, Guerra-Assunção JA, Bödör C, Quentmeier H, Mansbridge C, Johnson P, Davies A, Strefford JC, Packham G, Barrans S, Jack A, Du M-Q, Calaminici M, Lister TA, Auer R, Montoto S, Gribben JG, Siebert R, Chelala C, Zoncu R, Sabatini DM, Fitzgibbon J, Recurrent mTORC1-activating RRAGC mutations in follicular lymphoma., Nature Genetics. 48 (2016) 183–188. 10.1038/ng.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pareja F, Brandes AH, Basili T, Selenica P, Geyer FC, Fan D, Paula ADC, Kumar R, Brown DN, Gularte-Mérida R, Alemar B, Bi R, Lim RS, de Bruijn I, Fujisawa S, Gardner R, Feng E, Li A, da Silva EM, Lozada JR, Blecua P, Cohen-Gould L, Jungbluth AA, Rakha EA, Ellis IO, Edelweiss MIA, Palazzo J, Norton L, Hollmann T, Edelweiss M, Rubin BP, Weigelt B, Reis-Filho JS, Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors., Nature Communications. 9 (2018) 3533–13. 10.1038/s41467-018-05886-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Luca MD, Romano R, Bucci C, Role of the V1G1 subunit of V-ATPase in breast cancer cell migration, Sci Rep-Uk. 11 (2021) 4615. 10.1038/s41598-021-84222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Whitton B, Okamoto H, Packham G, Crabb SJ, Vacuolar ATPase as a potential therapeutic target and mediator of treatment resistance in cancer, Cancer Med-Us. 7 (2018) 3800–3811. 10.1002/cam4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ramachandran N, Munteanu I, Wang P, Ruggieri A, Rilstone JJ, Israelian N, Naranian T, Paroutis P, Guo R, Ren Z-P, Nishino I, Chabrol B, Pellissier J-F, Minetti C, Udd B, Fardeau M, Tailor CS, Mahuran DJ, Kissel JT, Kalimo H, Levy N, Manolson MF, Ackerley CA, Minassian BA, VMA21 deficiency prevents vacuolar ATPase assembly and causes autophagic vacuolar myopathy, Acta Neuropathol. 125 (2013) 439–457. 10.1007/s00401-012-1073-6. [DOI] [PubMed] [Google Scholar]
- [37].Ohba K, Endo M, Sato S, Kashio-Yokota Y, Hirose T, Takahashi K, (Pro)renin receptor/ATP6AP2 is required for autophagy and regulates proliferation in lung adenocarcinoma cells, Genes Cells. 25 (2020) 782–795. 10.1111/gtc.12812. [DOI] [PubMed] [Google Scholar]
- [38].Wang F, Gatica D, Ying ZX, Peterson LF, Kim PK, Bernard D, Saiya-Cork K, Wang S, Kaminski MS, Chang AE, Phillips T, Klionsky DJ, Malek SN, Follicular lymphoma-associated mutations in vacuolar ATPase ATP6V1B2 activate autophagic flux and MTOR, J Clin Invest. 129 (2019) 1626–1640. 10.1172/jci98288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Levine B, Kroemer G, Autophagy in the Pathogenesis of Disease, Cell. 132 (2008) 27–42. 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DCO, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC, Regulation of Mammalian Autophagy in Physiology and Pathophysiology, Physiol Rev. 90 (2010) 1383–1435. 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- [41].Aita VM, Liang XH, Murty VVVS, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B, Cloning and Genomic Organization of Beclin 1, a Candidate Tumor Suppressor Gene on Chromosome 17q21, Genomics. 59 (1999) 59–65. 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- [42].Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen E-L, Mizushima N, Ohsumi Y, Cattoretti G, Levine B, Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene, J Clin Invest. 112 (2003) 1809–1820. 10.1172/jci20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yue Z, Jin S, Yang C, Levine AJ, Heintz N, Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor, Proc National Acad Sci. 100 (2003) 15077–15082. 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sinha S, Levine B, The autophagy effector Beclin 1: a novel BH3-only protein, Oncogene. 27 (2008) S137–S148. 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Frenzel A, Grespi F, Chmelewskij W, Villunger A, Bcl2 family proteins in carcinogenesis and the treatment of cancer, Apoptosis. 14 (2009) 584–596. 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh B-H, Jung JU, Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG, Nat Cell Biol. 8 (2006) 688–698. 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- [47].Liang C, Feng P, Ku B, Oh B-H, Jung JU, UVRAG: A New Player in Autophagy and Tumor Cell Growth, Autophagy. 3 (2007) 69–71. 10.4161/auto.3437. [DOI] [PubMed] [Google Scholar]
- [48].Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mul JJ, Pledger WJ, Wang H-G, Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis, Nat Cell Biol. 9 (2007) 1142–1151. 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee JW, Jeong EG, Soung YH, Nam SW, Lee JY, Yoo NJ, Lee SH, Decreased expression of tumour suppressor Bax-interacting factor-1 (Bif-1), a Bax activator, in gastric carcinomas, Pathology. 38 (2006) 312–315. 10.1080/00313020600820880. [DOI] [PubMed] [Google Scholar]
- [50].Davoli T, Xu AW, Mengwasser KE, Sack LM, Yoon JC, Park PJ, Elledge SJ, Cumulative Haploinsufficiency and Triplosensitivity Drive Aneuploidy Patterns and Shape the Cancer Genome, Cell. 155 (2013) 948–962. 10.1016/j.cell.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Balakrishnan A, von Neuhoff N, Rudolph C, Kamphues K, Schraders M, Groenen P, van Krieken JH, Callet-Bauchu E, Schlegelberger B, Steinemann D, Quantitative microsatellite analysis to delineate the commonly deleted region 1p22.3 in mantle cell lymphomas, Genes Chromosomes Cancer. 45 (2006) 883–892. 10.1002/gcc.20352. [DOI] [PubMed] [Google Scholar]
- [52].Maiani E, Milletti G, Nazio F, Holdgaard SG, Bartkova J, Rizza S, Cianfanelli V, Lorente M, Simoneschi D, Marco MD, D’Acunzo P, Leo LD, Rasmussen R, Montagna C, Raciti M, Stefanis CD, Gabicagogeascoa E, Rona G, Salvador N, Pupo E, Merchut-Maya JM, Daniel CJ, Carinci M, Cesarini V, O’sullivan A, Jeong Y-T, Bordi M, Russo F, Campello S, Gallo A, Filomeni G, Lanzetti L, Sears RC, Hamerlik P, Bartolazzi A, Hynds RE, Pearce DR, Swanton C, Pagano M, Velasco G, Papaleo E, Zio DD, Maya-Mendoza A, Locatelli F, Bartek J, Cecconi F, AMBRA1 regulates cyclin D to guard S-phase entry and genomic integrity, Nature. 592 (2021) 799–803. 10.1038/s41586-021-03422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Simoneschi D, Rona G, Zhou N, Jeong Y-T, Jiang S, Milletti G, Arbini AA, O’Sullivan A, Wang AA, Nithikasem S, Keegan S, Siu Y, Cianfanelli V, Maiani E, Nazio F, Cecconi F, Boccalatte F, Fenyö D, Jones DR, Busino L, Pagano M, CRL4AMBRA1 is a master regulator of D-type cyclins, Nature. 592 (2021) 789–793. 10.1038/s41586-021-03445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chaikovsky AC, Li C, Jeng EE, Loebell S, Lee MC, Murray CW, Cheng R, Demeter J, Swaney DL, Chen S-H, Newton BW, Johnson JR, Drainas AP, Shue YT, Seoane JA, Srinivasan P, He A, Yoshida A, Hipkins SQ, McCrea E, Poltorack CD, Krogan NJ, Diehl JA, Kong C, Jackson PK, Curtis C, Petrov DA, Bassik MC, Winslow MM, Sage J, The AMBRA1 E3 ligase adaptor regulates the stability of cyclin D, Nature. 592 (2021) 794–798. 10.1038/s41586-021-03474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kang MR, Kim MS, Oh JE, Kim YR, Song SY, Kim SS, Ahn CH, Yoo NJ, Lee SH, Frameshift mutations of autophagy-related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and colorectal cancers with microsatellite instability, J Pathology. 217 (2009) 702–706. 10.1002/path.2509. [DOI] [PubMed] [Google Scholar]
- [56].Ionov Y, Nowak N, Perucho M, Markowitz S, Cowell JK, Manipulation of nonsense mediated decay identifies gene mutations in colon cancer Cells with microsatellite instability, Oncogene. 23 (2004) 639–645. 10.1038/sj.onc.1207178. [DOI] [PubMed] [Google Scholar]
- [57].Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ, Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability, Hum Pathol. 39 (2008) 1059–1063. 10.1016/j.humpath.2007.11.013. [DOI] [PubMed] [Google Scholar]
- [58].Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N, Autophagy-deficient mice develop multiple liver tumors, Gene Dev. 25 (2011) 795–800. 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Capparelli C, Guido C, Whitaker-Menezes D, Bonuccelli G, Balliet R, Pestell TG, Goldberg AF, Pestell RG, Howell A, Sneddon S, Birbe R, Tsirigos A, Martinez-Outschoorn U, Sotgia F, Lisanti MP, Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production, Cell Cycle. 11 (2012) 2285–2302. 10.4161/cc.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH, RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs, Cell. 78 (1994) 35–43. 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- [61].Brown EJ, Albers MW, Shin TB, ichikawa K, Keith CT, Lane WS, Schreiber SL, A mammalian protein targeted by G1-arresting rapamycin–receptor complex, Nature. 369 (1994) 756–758. 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- [62].Jung CH, Jun CB, Ro S-H, Kim Y-M, Otto NM, Cao J, Kundu M, Kim D-H, ULK-Atg13-FIP200 Complexes Mediate mTOR Signaling to the Autophagy Machinery, Mol Biol Cell. 20 (2009) 1992–2003. 10.1091/mbc.e08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim J, Guan K-L, Amino Acid Signaling in TOR Activation, Biochemistry-Us. 80 (2011) 1001–1032. 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- [64].Yuan H-X, Russell RC, Guan K-L, Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy, Autophagy. 9 (2013) 1983–1995. 10.4161/auto.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lim HJ, Crowe P, Yang J-L, Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer, J Cancer Res Clin. 141 (2015) 671–689. 10.1007/s00432-014-1803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mossmann D, Park S, Hall MN, mTOR signalling and cellular metabolism are mutual determinants in cancer, Nat Rev Cancer. 18 (2018) 744–757. 10.1038/s41568-018-0074-8. [DOI] [PubMed] [Google Scholar]
- [67].Faivre S, Kroemer G, Raymond E, Current development of mTOR inhibitors as anticancer agents, Nat Rev Drug Discov. 5 (2006) 671–688. 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- [68].Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo W-L, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung M-C, van de Vijver MJ, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT, An Integrative Genomic and Proteomic Analysis of PIK3CA, PTEN, and AKT Mutations in Breast Cancer, Cancer Res. 68 (2008) 6084–6091. 10.1158/0008-5472.can-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Pankiv S, Clausen TH, Lamark T, Brech A, Bruun J-A, Outzen H, Øvervatn A, Bjørkøy G, Johansen T, p62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy*, J Biol Chem. 282 (2007) 24131–24145. 10.1074/jbc.m702824200. [DOI] [PubMed] [Google Scholar]
- [70].Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen H-Y, Bray K, Reddy A, Bhanot G, Gelinas C, DiPaola RS, Karantza-Wadsworth V, White E, Autophagy Suppresses Tumorigenesis through Elimination of p62, Cell. 137 (2009) 1062–1075. 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Moscat J, Diaz-Meco MT, p62 at the Crossroads of Autophagy, Apoptosis, and Cancer, Cell. 137 (2009) 1001–1004. 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Moscat J, Diaz-Meco MT, p62: a versatile multitasker takes on cancer, Trends Biochem Sci. 37 (2012) 230–236. 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li L, Shen C, Nakamura E, Ando K, Signoretti S, Beroukhim R, Cowley GS, Lizotte P, Liberzon E, Bair S, Root DE, Tamayo P, Tsherniak A, Cheng S-C, Tabak B, Jacobsen A, Hakimi AA, Schultz N, Ciriello G, Sander C, Hsieh JJ, Kaelin WG, SQSTM1 Is a Pathogenic Target of 5q Copy Number Gains in Kidney Cancer, Cancer Cell. 24 (2013) 738–750. 10.1016/j.ccr.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, Wang X, He C, Pan H, Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment, Cell Death Dis. 4 (2013) e838–e838. 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H, Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy., Cancer Res. 60 (2000) 6201–7. [PubMed] [Google Scholar]
- [76].Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson DA, Jin S, White E, Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis, Cancer Cell. 10 (2006) 51–64. 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yoshioka A, Miyata H, Doki Y, Yamasaki M, Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y, Monden M, LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers., Int J Oncol. 33 (2008) 461–8. [PubMed] [Google Scholar]
- [78].Fujii S, Mitsunaga S, Yamazaki M, Hasebe T, Ishii G, Kojima M, Kinoshita T, Ueno T, Esumi H, Ochiai A, Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome, Cancer Sci. 99 (2008) 1813–1819. 10.1111/j.1349-7006.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’Antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC, Pancreatic cancers require autophagy for tumor growth, Gene Dev. 25 (2011) 717–729. 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wei H, Wei S, Gan B, Peng X, Zou W, Guan J-L, Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis, Gene Dev. 25 (2011) 1510–1527. 10.1101/gad.2051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, Snyder E, Santanam U, DiPaola RS, Jacks T, Rabinowitz JD, White E, Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis, Gene Dev. 27 (2013) 1447–1461. 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, White E, Autophagy Is Required for Glucose Homeostasis and Lung Tumor Maintenance, Cancer Discov. 4 (2014) 914–927. 10.1158/2159-8290.cd-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, Sigl V, Aumayr K, Schmauss G, Fellner N, Handschuh S, Glösmann M, Pasierbek P, Schlederer M, Resch GP, Ma Y, Yang H, Popper H, Kenner L, Kroemer G, Penninger JM, A dual role for autophagy in a murine model of lung cancer, Nat Commun. 5 (2014) 3056. 10.1038/ncomms4056. [DOI] [PubMed] [Google Scholar]
- [84].Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M, White E, Autophagy Sustains Mitochondrial Glutamine Metabolism and Growth of BrafV600E–Driven Lung Tumors, Cancer Discov. 3 (2013) 1272–1285. 10.1158/2159-8290.cd-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Santanam U, Banach-Petrosky W, Abate-Shen C, Shen MM, White E, DiPaola RS, Atg7 cooperates with Pten loss to drive prostate cancer tumor growth, Gene Dev. 30 (2016) 399–407. 10.1101/gad.274134.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Xie X, Koh JY, Price S, White E, Mehnert JM, Atg7 Overcomes Senescence and Promotes Growth of BrafV600E-Driven Melanoma, Cancer Discov. 5 (2015) 410–423. 10.1158/2159-8290.cd-14-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lévy J, Cacheux W, Bara MA, L’Hermitte A, Lepage P, Fraudeau M, Trentesaux C, Lemarchand J, Durand A, Crain A-M, Marchiol C, Renault G, Dumont F, Letourneur F, Delacre M, Schmitt A, Terris B, Perret C, Chamaillard M, Couty J-P, Romagnolo B, Intestinal inhibition of Atg7 prevents tumour initiation through a microbiome-influenced immune response and suppresses tumour growth, Nat Cell Biol. 17 (2015) 1062–1073. 10.1038/ncb3206. [DOI] [PubMed] [Google Scholar]
- [88].Gammoh N, Fraser J, Puente C, Syred HM, Kang H, Ozawa T, Lam D, Acosta JC, Finch AJ, Holland E, Jiang X, Suppression of autophagy impedes glioblastoma development and induces senescence, Autophagy. 12 (2016) 1–9. 10.1080/15548627.2016.1190053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, Koumenis C, ER stress–mediated autophagy promotes Myc-dependent transformation and tumor growth, J Clin Invest. 122 (2012) 4621–4634. 10.1172/jci62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zinatizadeh MR, Momeni SA, Zarandi PK, Chalbatani GM, Dana H, Mirzaei H, Akbari ME, Miri SR, The Role and Function of Ras-association domain family in Cancer: A Review, Genes Dis. 6 (2019) 378–384. 10.1016/j.gendis.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Guo JY, Chen H-Y, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JMS, Karantza V, Coller HA, DiPaola RS, Gelinas C, Rabinowitz JD, White E, Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis, Gene Dev. 25 (2011) 460–470. 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Guo JY, Teng X, Laddha SV, Ma S, Nostrand SCV, Yang Y, Khor S, Chan CS, Rabinowitz JD, White E, Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells, Gene Dev. 30 (2016) 1704–1717. 10.1101/gad.283416.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sun K, Guo X, Zhao Q, jing Y, Kou X, Xie X, Zhou Y, Cai N, Gao L, Zhao X, Zhang S, Song J, Li D, Deng W, Li R, Wu M, Wei L, Paradoxical role of autophagy in the dysplastic and tumorforming stages of hepatocarcinoma development in rats, Cell Death Dis. 4 (2013) e501–e501. 10.1038/cddis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Huo Y, Cai H, Teplova I, Bowman-Colin C, Chen G, Price S, Barnard N, Ganesan S, Karantza V, White E, Xia B, Autophagy Opposes p53-Mediated Tumor Barrier to Facilitate Tumorigenesis in a Model of PALB2-Associated Hereditary Breast Cancer, Cancer Discov. 3 (2013) 894–907. 10.1158/2159-8290.cd-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ, Ryan KM, p53 status determines the role of autophagy in pancreatic tumour development, Nature. 504 (2013) 296–300. 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- [96].Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, Hoff DDV, Maitra A, Kimmelman AC, Autophagy Is Critical for Pancreatic Tumor Growth and Progression in Tumors with p53 Alterations, Cancer Discov. 4 (2014) 905–913. 10.1158/2159-8290.cd-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y, Molecular principles of metastasis: a hallmark of cancer revisited, Signal Transduct Target Ther. 5 (2020) 28. 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Valastyan S, Weinberg RA, Tumor Metastasis: Molecular Insights and Evolving Paradigms, Cell. 147 (2011) 275–292. 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Galavotti S, Bartesaghi S, Faccenda D, Shaked-Rabi M, Sanzone S, McEvoy A, Dinsdale D, Condorelli F, Brandner S, Campanella M, Grose R, Jones C, Salomoni P, The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells, Oncogene. 32 (2013) 699–712. 10.1038/onc.2012.111. [DOI] [PubMed] [Google Scholar]
- [100].Han C, Sun B, Wang W, Cai W, Lou D, Sun Y, Zhao X, Overexpression of Microtubule-Associated Protein-1 Light Chain 3 Is Associated with Melanoma Metastasis and Vasculogenic Mimicry, Tohoku J Exp Medicine. 223 (2011) 243–251. 10.1620/tjem.223.243. [DOI] [PubMed] [Google Scholar]
- [101].Zhao H, Yang M, Zhao J, Wang J, Zhang Y, Zhang Q, High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer, Med Oncol. 30 (2013) 475. 10.1007/s12032-013-0475-1. [DOI] [PubMed] [Google Scholar]
- [102].Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM, Punctate LC3B Expression Is a Common Feature of Solid Tumors and Associated with Proliferation, Metastasis, and Poor Outcome, Clin Cancer Res. 18 (2012) 370–379. 10.1158/1078-0432.ccr-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lock R, Kenific CM, Leidal AM, Salas E, Debnath J, Autophagy-Dependent Production of Secreted Factors Facilitates Oncogenic RAS-Driven Invasion, Cancer Discov. 4 (2014) 466–479. 10.1158/2159-8290.cd-13-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhan Z, Xie X, Cao H, Zhou X, Zhang XD, Fan H, Liu Z, Autophagy facilitates TLR4- and TLR3-triggered migration and invasion of lung cancer cells through the promotion of TRAF6 ubiquitination, Autophagy. 10 (2013) 257–268. 10.4161/auto.27162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kenific CM, Stehbens SJ, Goldsmith J, Leidal AM, Faure N, Ye J, Wittmann T, Debnath J, NBR1 enables autophagy-dependent focal adhesion turnover, J Cell Biol. 212 (2016) 577–590. 10.1083/jcb.201503075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sharifi MN, Mowers EE, Drake LE, Collier C, Chen H, Zamora M, Mui S, Macleod KF, Autophagy Promotes Focal Adhesion Disassembly and Cell Motility of Metastatic Tumor Cells through the Direct Interaction of Paxillin with LC3, Cell Reports. 15 (2016) 1660–1672. 10.1016/j.celrep.2016.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gutschner T, Hämmerle M, Eibmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Groß M, Zörnig M, MacLeod AR, Spector DL, Diederichs S, The Noncoding RNA MALAT1 Is a Critical Regulator of the Metastasis Phenotype of Lung Cancer Cells, Cancer Res. 73 (2013) 1180–1189. 10.1158/0008-5472.can-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].JIAO F, HU H, YUAN C, WANG L, JIANG W, JIN Z, GUO Z, WANG L, Elevated expression level of long noncoding RNA MALAT-1 facilitates cell growth, migration and invasion in pancreatic cancer, Oncol Rep. 32 (2014) 2485–2492. 10.3892/or.2014.3518. [DOI] [PubMed] [Google Scholar]
- [109].Li L, Chen H, Gao Y, Wang Y-W, Zhang G-Q, Pan S-H, Ji L, Kong R, Wang G, Jia Y-H, Bai X-W, Sun B, Long Noncoding RNA MALAT1 Promotes Aggressive Pancreatic Cancer Proliferation and Metastasis via the Stimulation of Autophagy, Mol Cancer Ther. 15 (2016) 2232–2243. 10.1158/1535-7163.mct-16-0008. [DOI] [PubMed] [Google Scholar]
- [110].Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, Zong Z, Hua X, Su D, Li H, Liu J, Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells, J Exp Clin Canc Res. 37 (2018) 9. 10.1186/s13046-018-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen W, Bai Y, Patel C, Geng F, Autophagy promotes triple negative breast cancer metastasis via YAP nuclear localization, Biochem Bioph Res Co. 520 (2019) 263–268. 10.1016/j.bbrc.2019.09.133. [DOI] [PubMed] [Google Scholar]
- [112].Hu S, Wang L, Zhang X, Wu Y, Yang J, Li J, Autophagy induces transforming growth factor-β-dependent epithelial-mesenchymal transition in hepatocarcinoma cells through cAMP response element binding signalling, J Cell Mol Med. 22 (2018) 5518–5532. 10.1111/jcmm.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, Song Z, Zheng Q, Xiong J, Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transition, Carcinogenesis. 34 (2013) 1343–1351. 10.1093/carcin/bgt063. [DOI] [PubMed] [Google Scholar]
- [114].Alizadeh J, Glogowska A, Thliveris J, Kalantari F, Shojaei S, Hombach-Klonisch S, Klonisch T, Ghavami S, Autophagy modulates transforming growth factor beta 1 induced epithelial to mesenchymal transition in non-small cell lung cancer cells, Biochimica Et Biophysica Acta Bba - Mol Cell Res. 1865 (2018) 749–768. 10.1016/j.bbamcr.2018.02.007. [DOI] [PubMed] [Google Scholar]
- [115].Kim YH, Baek SH, Kim EK, Ha JM, Jin SY, Lee HS, Ha HK, Song SH, Kim SJ, Shin HK, Yong J, Kim D, Kim CD, Bae SS, Uncoordinated 51-like kinase 2 signaling pathway regulates epithelial-mesenchymal transition in A549 lung cancer cells, Febs Lett. 590 (2016) 1365–1374. 10.1002/1873-3468.12172. [DOI] [PubMed] [Google Scholar]
- [116].Zhang W, Yuan W, Song J, Wang S, Gu X, LncRNA CPS1-IT1 suppresses EMT and metastasis of colorectal cancer by inhibiting hypoxia-induced autophagy through inactivation of HIF-1α, Biochimie. 144 (2018) 21–27. 10.1016/j.biochi.2017.10.002. [DOI] [PubMed] [Google Scholar]
- [117].Ren T, Zheng B, Huang Y, Wang S, Bao X, Liu K, Guo W, Osteosarcoma cell intrinsic PD-L2 signals promote invasion and metastasis via the RhoA-ROCK-LIMK2 and autophagy pathways, Cell Death Dis. 10 (2019) 261. 10.1038/s41419-019-1497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gilmore AP, Anoikis, Cell Death Differ. 12 (2005) 1473–1477. 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- [119].Fung C, Lock R, Gao S, Salas E, Debnath J, Induction of Autophagy during Extracellular Matrix Detachment Promotes Cell Survival, Mol Biol Cell. 19 (2008) 797–806. 10.1091/mbc.e07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Peng Y-F, Shi Y-H, Ding Z-B, Ke A-W, Gu C-Y, Hui B, Zhou J, Qiu S-J, Dai Z, Fan J, Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells, Autophagy. 9 (2013) 2056–2068. 10.4161/auto.26398. [DOI] [PubMed] [Google Scholar]
- [121].Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA, PERK Integrates Autophagy and Oxidative Stress Responses To Promote Survival during Extracellular Matrix Detachment, Mol Cell Biol. 31 (2011) 3616–3629. 10.1128/mcb.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Avivar-Valderas A, Bobrovnikova-Marjon E, Diehl JA, Bardeesy N, Debnath J, Aguirre-Ghiso JA, Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK, Oncogene. 32 (2013) 4932–4940. 10.1038/onc.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chen N, Debnath J, IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway, Autophagy. 9 (2013) 1214–1227. 10.4161/auto.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhu H, Liu L, Deng H, Li Z, Sheng J, He X, Tian D, Li P, Astrocyte elevated gene 1 (AEG-1) promotes anoikis resistance and metastasis by inducing autophagy in hepatocellular carcinoma, J Cell Physiol. 235 (2020) 5084–5095. 10.1002/jcp.29377. [DOI] [PubMed] [Google Scholar]
- [125].Mani SA, Guo W, Liao M-J, Eaton E.Ng., Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA, The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells, Cell. 133 (2008) 704–715. 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Chaffer CL, Weinberg RA, A Perspective on Cancer Cell Metastasis, Science. 331 (2011) 1559–1564. 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- [127].Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE, A cell initiating human acute myeloid leukaemia after transplantation into SCID mice, Nature. 367 (1994) 645–648. 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- [128].Bonnet D, Dick JE, Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell, Nat Med. 3 (1997) 730–737. 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- [129].Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF, Prospective identification of tumorigenic breast cancer cells, Proc National Acad Sci. 100 (2003) 3983–3988. 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB, Identification of human brain tumour initiating cells, Nature. 432 (2004) 396–401. 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- [131].Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, Maria RD, Identification and expansion of human colon-cancer-initiating cells, Nature. 445 (2007) 111–115. 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- [132].O’Brien CA, Pollett A, Gallinger S, Dick JE, A human colon cancer cell capable of initiating tumour growth in immunodeficient mice, Nature. 445 (2007) 106–110. 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- [133].Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C, Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer, Cell Stem Cell. 1 (2007) 313–323. 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [134].Ma S, Chan K, Hu L, Lee TK, Wo JY, Ng IO, Zheng B, Guan X, Identification and Characterization of Tumorigenic Liver Cancer Stem/Progenitor Cells, Gastroenterology. 132 (2007) 2542–2556. 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- [135].Dean M, Fojo T, Bates S, Tumour stem cells and drug resistance, Nat Rev Cancer. 5 (2005) 275–284. 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- [136].Chang NC, Autophagy and Stem Cells: Self-Eating for Self-Renewal, Frontiers Cell Dev Biology. 8 (2020) 138. 10.3389/fcell.2020.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi T, Yacobi R, Etten RAV, Donato N, Hunter A, Dinsdale D, Tirrò E, Vigneri P, Nicotera P, Dyer MJ, Holyoake T, Salomoni P, Calabretta B, Targeting autophagy potentiates tyrosine kinase inhibitor–induced cell death in Philadelphia chromosome–positive cells, including primary CML stem cells, J Clin Invest. 119 (2009) 1109–1123. 10.1172/jci35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Altman BJ, Jacobs SR, Mason EF, Michalek RD, MacIntyre AN, Coloff JL, Ilkayeva O, Jia W, He Y-W, Rathmell JC, Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis, Oncogene. 30 (2011) 1855–1867. 10.1038/onc.2010.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Rothe K, Lin H, Lin KBL, Leung A, Wang HM, Malekesmaeili M, Brinkman RR, Forrest DL, Gorski SM, Jiang X, The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells, Blood. 123 (2014) 3622–3634. 10.1182/blood-2013-07-516807. [DOI] [PubMed] [Google Scholar]
- [140].Folkerts H, Hilgendorf S, Wierenga ATJ, Jaques J, Mulder AB, Coffer PJ, Schuringa JJ, Vellenga E, Inhibition of autophagy as a treatment strategy for p53 wild-type acute myeloid leukemia, Cell Death Dis. 8 (2017) e2927–e2927. 10.1038/cddis.2017.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Vellon L, Menendez JA, Autophagy positively regulates the CD44 + CD24 −/low breast cancer stem-like phenotype, Cell Cycle. 10 (2011) 3871–3885. 10.4161/cc.10.22.17976. [DOI] [PubMed] [Google Scholar]
- [142].Gong C, Bauvy C, Tonelli G, Yue W, Deloménie C, Nicolas V, Zhu Y, Domergue V, Marin-Esteban V, Tharinger H, Delbos L, Gary-Gouy H, Morel A-P, Ghavami S, Song E, Codogno P, Mehrpour M, Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells, Oncogene. 32 (2013) 2261–2272. 10.1038/onc.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wolf J, Dewi DL, Fredebohm J, Müller-Decker K, Flechtenmacher C, Hoheisel JD, Boettcher M, A mammosphere formation RNAi screen reveals that ATG4A promotes a breast cancer stem-like phenotype, Breast Cancer Res. 15 (2013) R109. 10.1186/bcr3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Yeo SK, Wen J, Chen S, Guan J-L, Autophagy Differentially Regulates Distinct Breast Cancer Stem-like Cells in Murine Models via EGFR/Stat3 and Tgfβ/Smad Signaling, Cancer Res. 76 (2016) 3397–3410. 10.1158/0008-5472.can-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Stagni V, Manni I, Oropallo V, Mottolese M, Benedetto AD, Piaggio G, Falcioni R, Giaccari D, Carlo SD, Sperati F, Cencioni MT, Barilà D, ATM kinase sustains HER2 tumorigenicity in breast cancer, Nat Commun. 6 (2015) 6886. 10.1038/ncomms7886. [DOI] [PubMed] [Google Scholar]
- [146].Antonelli M, Strappazzon F, Arisi I, Brandi R, D’Onofrio M, Sambucci M, Manic G, Vitale I, Barilà D, Stagni V, ATM kinase sustains breast cancer stem-like cells by promoting ATG4C expression and autophagy, Oncotarget. 5 (2014) 21692–21709. 10.18632/oncotarget.15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Rausch V, Liu L, Apel A, Rettig T, Gladkich J, Labsch S, Kallifatidis G, Kaczorowski A, Groth A, Gross W, Gebhard MM, Schemmer P, Werner J, Salnikov AV, Zentgraf H, Büchler MW, Herr I, Autophagy mediates survival of pancreatic tumour-initiating cells in a hypoxic microenvironment, J Pathology. 227 (2012) 325–335. 10.1002/path.3994. [DOI] [PubMed] [Google Scholar]
- [148].Song Y, Zhang S, Guo X, Sun K, Han Z, Li R, Zhao Q, Deng W, Xie X, zhang J, Wu M, Wei L, Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment, Cancer Lett. 339 (2013) 70–81. 10.1016/j.canlet.2013.07.021. [DOI] [PubMed] [Google Scholar]
- [149].Zhang D, Zhao Q, Sun H, Yin L, Wu J, Xu J, He T, Yang C, Liang C, Defective autophagy leads to the suppression of stem-like features of CD271+ osteosarcoma cells, J Biomed Sci. 23 (2016) 82. 10.1186/s12929-016-0297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pagotto A, Pilotto G, Mazzoldi EL, Nicoletto MO, Frezzini S, Pastò A, Amadori A, Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells, Cell Death Dis. 8 (2017) e2943–e2943. 10.1038/cddis.2017.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Peng Q, Qin J, Zhang Y, Cheng X, Wang X, Lu W, Xie X, Zhang S, Autophagy maintains the stemness of ovarian cancer stem cells by FOXA2., J Exp Clin Canc Res. 36 (2017) 171. 10.1186/s13046-017-0644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Buccarelli M, Marconi M, Pacioni S, Pascalis ID, D’Alessandris QG, Martini M, Ascione B, Malorni W, Larocca LM, Pallini R, Ricci-Vitiani L, Matarrese P, Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis, Cell Death Dis. 9 (2018) 841. 10.1038/s41419-018-0864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Li L-Q, Pan D, Zhang S-W, D. -Y-Xie, Zheng X-L, Chen H, Autophagy regulates chemoresistance of gastric cancer stem cells via the Notch signaling pathway., Eur Rev Med Pharmaco. 22 (2018) 3402–3407. 10.26355/eurrev_201806_15162. [DOI] [PubMed] [Google Scholar]
- [154].Iliopoulos D, Hirsch HA, Wang G, Struhl K, Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion, Proc National Acad Sci. 108 (2011) 1397–1402. 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Maycotte P, Jones KL, Goodall ML, Thorburn J, Thorburn A, Autophagy Supports Breast Cancer Stem Cell Maintenance by Regulating IL6 Secretion, Mol Cancer Res. 13 (2015) 651–658. 10.1158/1541-7786.mcr-14-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Perez-Balaguer A, Ortiz-Martínez F, García-Martínez A, Pomares-Navarro C, Lerma E, Peiró G, FOXA2 mRNA expression is associated with relapse in patients with Triple-Negative/Basal-like breast carcinoma, Breast Cancer Res Tr. 153 (2015) 465–474. 10.1007/s10549-015-3553-6. [DOI] [PubMed] [Google Scholar]
- [157].Wang J, Liu D, Sun Z, Ye T, Li J, Zeng B, Zhao Q, Xing HR, Autophagy augments the self-renewal of lung cancer stem cells by the degradation of ubiquitinated p53, Cell Death Dis. 12 (2021) 98. 10.1038/s41419-021-03392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Sosa MS, Bragado P, Aguirre-Ghiso JA, Mechanisms of disseminated cancer cell dormancy: an awakening field, Nat Rev Cancer. 14 (2014) 611–622. 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, Kondo S, Kondo Y, Yu Y, Mills GB, Liao WS-L, Bast RC, The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells, J Clin Invest. 118 (2008) 3917–3929. 10.1172/jci35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Correa RJM, Valdes YR, Peart TM, Fazio EN, Bertrand M, McGee J, Préfontaine M, Sugimoto A, DiMattia GE, Shepherd TG, Combination of AKT inhibition with autophagy blockade effectively reduces ascites-derived ovarian cancer cell viability, Carcinogenesis. 35 (2014) 1951–1961. 10.1093/carcin/bgu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lu Z, Baquero MT, Yang H, Yang M, Reger AS, Kim C, Levine DA, Clarke CH, Liao WS-L, R.C.B. Jr, DIRAS3 regulates the autophagosome initiation complex in dormant ovarian cancer cells, Autophagy. 10 (2014) 1071–1092. 10.4161/auto.28577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Gupta A, Roy S, Lazar AJF, Wang W-L, McAuliffe JC, Reynoso D, McMahon J, Taguchi T, Floris G, Debiec-Rychter M, Schőffski P, Trent JA, Debnath J, Rubin BP, Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST), Proc National Acad Sci. 107 (2010) 14333–14338. 10.1073/pnas.1000248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].de Prati AC, Butturini E, Rigo A, Oppici E, Rossin M, Boriero D, Mariotto S, Metastatic Breast Cancer Cells Enter Into Dormant State and Express Cancer Stem Cells Phenotype Under Chronic Hypoxia, J Cell Biochem. 118 (2017) 3237–3248. 10.1002/jcb.25972. [DOI] [PubMed] [Google Scholar]
- [164].Chaterjee M, van Golen KL, Breast Cancer Stem Cells Survive Periods of Farnesyl-Transferase Inhibitor-Induced Dormancy by Undergoing Autophagy, Bone Marrow Res. 2011 (2011) 362938. 10.1155/2011/362938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Yu Z, Zhou R, Zhao Y, Pan Y, Liang H, Zhang J, Tai S, Jin L, Teng C, Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death, Cell Proliferat. 52 (2019) e12568. 10.1111/cpr.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Shimizu T, Sugihara E, Yamaguchi-Iwai S, Tamaki S, Koyama Y, Kamel W, Ueki A, Ishikawa T, Chiyoda T, Osuka S, Onishi N, Ikeda H, Kamei J, Matsuo K, Fukuchi Y, Nagai T, Toguchida J, Toyama Y, Muto A, Saya H, IGF2 Preserves Osteosarcoma Cell Survival by Creating an Autophagic State of Dormancy That Protects Cells against Chemotherapeutic Stress, Cancer Res. 74 (2014) 6531–6541. 10.1158/0008-5472.can-14-0914. [DOI] [PubMed] [Google Scholar]
- [167].Vera-Ramirez L, Vodnala SK, Nini R, Hunter KW, Green JE, Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence, Nat Commun. 9 (2018) 1944. 10.1038/s41467-018-04070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Aqbi HF, Tyutyunyk-Massey L, Keim RC, Butler SE, Thekkudan T, Joshi S, Smith TM, Bandyopadhyay D, Idowu MO, Bear HD, Payne KK, Gewirtz DA, Manjili MH, Autophagy-deficient breast cancer shows early tumor recurrence and escape from dormancy, Oncotarget. 9 (2018) 22113–22122. 10.18632/oncotarget.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Flynn ALB, Calhoun BC, Sharma A, Chang JC, Almasan A, Schiemann WP, Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression, Nat Commun. 10 (2019) 3668. 10.1038/s41467-019-11640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P, Tumor microenvironment complexity and therapeutic implications at a glance, Cell Commun Signal. 18 (2020) 59. 10.1186/s12964-020-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Pietras K, Östman A, Hallmarks of cancer: Interactions with the tumor stroma, Exp Cell Res. 316 (2010) 1324–1331. 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- [172].Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z, A framework for advancing our understanding of cancer-associated fibroblasts, Nat Rev Cancer. 20 (2020) 174–186. 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Caro J, Lisanti MP, Sotgia F, Autophagy in cancer associated fibroblasts promotes tumor cell survival, Cell Cycle. 9 (2010) 3515–3533. 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, Tanowitz HB, Casimiro MC, Wang C, Pestell RG, Grieshaber P, Caro J, Sotgia F, Lisanti MP, HIF1-alpha functions as a tumor promoter in cancer-associated fibroblasts, and as a tumor suppressor in breast cancer cells, Cell Cycle. 9 (2014) 3534–3551. 10.4161/cc.9.17.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Martinez-Outschoorn UE, Balliet RM, Rivadeneira D, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer K, Lin Z, Witkiewicz A, Flomenberg N, Howell A, Pestell R, Knudsen E, Sotgia F, Lisanti MP, Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution, Cell Cycle. 9 (2014) 3276–3296. 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP, The autophagic tumor stroma model of cancer, Cell Cycle. 9 (2010) 3485–3505. 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Salem AF, Whitaker-Menezes D, Lin Z, Martinez-Outschoorn UE, Tanowitz HB, Al-Zoubi MS, Howell A, Pestell RG, Sotgia F, Lisanti MP, Two-compartment tumor metabolism, Cell Cycle. 11 (2012) 2545–2559. 10.4161/cc.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Avena P, Anselmo W, Whitaker-Menezes D, Wang C, Pestell RG, Lamb RS, Hulit J, Casaburi I, Andò S, Martinez-Outschoorn UE, Lisanti MP, Sotgia F, Compartment-specific activation of PPARγ governs breast cancer tumor growth, via metabolic reprogramming and symbiosis, Cell Cycle. 12 (2013) 1360–1370. 10.4161/cc.24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Chaudhri VK, Salzler GG, Dick SA, Buckman MS, Sordella R, Karoly ED, Mohney R, Stiles BM, Elemento O, Altorki NK, McGraw TE, Metabolic Alterations in Lung Cancer–Associated Fibroblasts Correlated with Increased Glycolytic Metabolism of the Tumor, Mol Cancer Res. 11 (2013) 579–592. 10.1158/1541-7786.mcr-12-0437-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Sewduth R, Santoro MM, “Decoding” Angiogenesis: New Facets Controlling Endothelial Cell Behavior, Front Physiol. 7 (2016) 306. 10.3389/fphys.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Maishi N, Hida K, Tumor endothelial cells accelerate tumor metastasis, Cancer Sci. 108 (2017) 1921–1926. 10.1111/cas.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Filippi I, Saltarella I, Aldinucci C, Carraro F, Ria R, Vacca A, Naldini A, Different Adaptive Responses to Hypoxia in Normal and Multiple Myeloma Endothelial Cells, Cell Physiol Biochem. 46 (2018) 203–212. 10.1159/000488423. [DOI] [PubMed] [Google Scholar]
- [183].van Beijnum JR, Dings RP, van der Linden E, Zwaans BMM, Ramaekers FCS, Mayo KH, Griffioen AW, Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature, Blood. 108 (2006) 2339–2348. 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- [184].Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion A-C, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquière B, Annaert W, Agostinis P, Carmeliet P, Tumor Vessel Normalization by Chloroquine Independent of Autophagy, Cancer Cell. 26 (2014) 190–206. 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- [185].Lee S-J, Kim H-P, Jin Y, Choi AMK, Ryter SW, Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis, Autophagy. 7 (2011) 829–839. 10.4161/auto.7.8.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Jhunjhunwala S, Hammer C, Delamarre L, Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion, Nat Rev Cancer. 21 (2021) 298–312. 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- [187].Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS, Immune evasion in cancer: Mechanistic basis and therapeutic strategies, Semin Cancer Biol. 35 (2015) S185–S198. 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- [188].Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S, Akiba H, Foretz M, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Takeya M, Viollet B, Yagita H, Jinushi M, TIM-4 Glycoprotein-Mediated Degradation of Dying Tumor Cells by Autophagy Leads to Reduced Antigen Presentation and Increased Immune Tolerance, Immunity. 39 (2013) 1070–1081. 10.1016/j.immuni.2013.09.014. [DOI] [PubMed] [Google Scholar]
- [189].Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, Banh RS, Paulo JA, Wen KW, Debnath J, Kim GE, Mancias JD, Fearon DT, Perera RM, Kimmelman AC, Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I, Nature. 581 (2020) 100–105. 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Deng J, Thennavan A, Dolgalev I, Chen T, Li J, Marzio A, Poirier JT, Peng DH, Bulatovic M, Mukhopadhyay S, Silver H, Papadopoulos E, Pyon V, Thakurdin C, Han H, Li F, Li S, Ding H, Hu H, Pan Y, Weerasekara V, Jiang B, Wang ES, Ahearn I, Philips M, Papagiannakopoulos T, Tsirigos A, Rothenberg E, Gainor J, Freeman GJ, Rudin CM, Gray NS, Hammerman PS, Pagano M, Heymach JV, Perou CM, Bardeesy N, Wong K-K, ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1-mutant lung cancer, Nat Cancer. 2 (2021) 503–514. 10.1038/s43018-021-00208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Noman MZ, Janji B, Kaminska B, Moer KV, Pierson S, Przanowski P, Buart S, Berchem G, Romero P, Mami-Chouaib F, Chouaib S, Blocking Hypoxia-Induced Autophagy in Tumors Restores Cytotoxic T-Cell Activity and Promotes Regression, Cancer Res. 71 (2011) 5976–5986. 10.1158/0008-5472.can-11-1094. [DOI] [PubMed] [Google Scholar]
- [192].Young TM, Reyes C, Pasnikowski E, Castanaro C, Wong C, Decker CE, Chiu J, Song H, Wei Y, Bai Y, Zambrowicz B, Thurston G, Daly C, Autophagy protects tumors from T cell–mediated cytotoxicity via inhibition of TNFα-induced apoptosis, Sci Immunol. 5 (2020) eabb9561. 10.1126/sciimmunol.abb9561. [DOI] [PubMed] [Google Scholar]
- [193].Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, Medves S, Zimmer J, Oudin A, Niclou SP, Bleackley RC, Goping IS, Chouaib S, Janji B, Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia, Proc National Acad Sci. 110 (2013) 17450–17455. 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Zhou M, Wen Z, Cheng F, Ma J, Li W, Ren H, Sheng Y, Dong H, Lu L, Hu H-M, Wang L-X, Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-κB signal pathway, Oncoimmunology. 5 (2016) e1180485. 10.1080/2162402x.2016.1180485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Gao R, Ma J, Wen Z, Yang P, Zhao J, Xue M, Chen Y, Aldarouish M, Hu H-M, Zhu X-J, Pan N, Wang L-X, Tumor cell-released autophagosomes (TRAP) enhance apoptosis and immunosuppressive functions of neutrophils, Oncoimmunology. 7 (2018) 00–00. 10.1080/2162402x.2018.1438108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].DeVorkin L, Pavey N, Carleton G, Comber A, Ho C, Lim J, McNamara E, Huang H, Kim P, Zacharias LG, Mizushima N, Saitoh T, Akira S, Beckham W, Lorzadeh A, Moksa M, Cao Q, Murthy A, Hirst M, DeBerardinis RJ, Lum JJ, Autophagy Regulation of Metabolism Is Required for CD8+ T Cell Anti-tumor Immunity, Cell Reports. 27 (2019) 502–513.e5. 10.1016/j.celrep.2019.03.037. [DOI] [PubMed] [Google Scholar]
- [197].Liu Y, Cao X, Immunosuppressive cells in tumor immune escape and metastasis, J Mol Med. 94 (2016) 509–522. 10.1007/s00109-015-1376-x. [DOI] [PubMed] [Google Scholar]
- [198].Wen Z-F, Liu H, Gao R, Zhou M, Ma J, Zhang Y, Zhao J, Chen Y, Zhang T, Huang F, Pan N, Zhang J, Fox BA, Hu H-M, Wang L-X, Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1, J Immunother Cancer. 6 (2018) 151. 10.1186/s40425-018-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Chang C-P, Su Y-C, Hu C-W, Lei H-Y, TLR2-dependent selective autophagy regulates NF-κB lysosomal degradation in hepatoma-derived M2 macrophage differentiation, Cell Death Differ. 20 (2013) 515–523. 10.1038/cdd.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Yang M, Liu J, Shao J, Qin Y, Ji Q, Zhang X, Du J, Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization, Mol Cancer. 13 (2014) 43. 10.1186/1476-4598-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D, Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein, Autophagy. 16 (2020) 1–15. 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Alissafi T, Hatzioannou A, Mintzas K, Barouni RM, Banos A, Sormendi S, Polyzos A, Xilouri M, Wielockx B, Gogas H, Verginis P, Autophagy orchestrates the regulatory program of tumor-associated myeloid-derived suppressor cells, J Clin Invest. 128 (2018) 3840–3852. 10.1172/jci120888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Poillet-Perez L, Sharp DW, Yang Y, Laddha SV, Ibrahim M, Bommareddy PK, Hu ZS, Vieth J, Haas M, Bosenberg MW, Rabinowitz JD, Cao J, Guan J-L, Ganesan S, Chan CS, Mehnert JM, Lattime EC, White E, Autophagy promotes growth of tumors with high mutational burden by inhibiting a T-cell immune response, Nat Cancer. 1 (2020) 923–934. 10.1038/s43018-020-00110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Fu X-T, Song K, Zhou J, Shi Y-H, Liu W-R, Shi G-M, Gao Q, Wang X-Y, Ding Z-B, Fan J, Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma, Cancer Cell Int. 19 (2019) 71. 10.1186/s12935-019-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Lv L, Li D, Zhao D, Lin R, Chu Y, Zhang H, Zha Z, Liu Y, Li Z, Xu Y, Wang G, Huang Y, Xiong Y, Guan K-L, Lei Q-Y, Acetylation Targets the M2 Isoform of Pyruvate Kinase for Degradation through Chaperone-Mediated Autophagy and Promotes Tumor Growth, Mol Cell. 42 (2011) 719–730. 10.1016/j.molcel.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Kon M, Kiffin R, Koga H, Chapochnick J, Macian F, Varticovski L, Cuervo AM, Chaperone-Mediated Autophagy Is Required for Tumor Growth, Sci Transl Med. 3 (2011) 109ra117–109ra117. 10.1126/scitranslmed.3003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Saha T, LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy, Autophagy. 8 (2012) 1643–1656. 10.4161/auto.21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Zhou J, Yang J, Fan X, Hu S, Zhou F, Dong J, Zhang S, Shang Y, Jiang X, Guo H, Chen N, Xiao X, Sheng J, Wu K, Nie Y, Fan D, Chaperone-mediated autophagy regulates proliferation by targeting RND3 in gastric cancer, Autophagy. 12 (2016) 515–528. 10.1080/15548627.2015.1136770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Li L, Fang R, Liu B, Shi H, Wang Y, Zhang W, Zhang X, Ye L, Deacetylation of tumor-suppressor MST1 in Hippo pathway induces its degradation through HBXIP-elevated HDAC6 in promotion of breast cancer growth, Oncogene. 35 (2016) 4048–4057. 10.1038/onc.2015.476. [DOI] [PubMed] [Google Scholar]
- [210].Quintavalle C, Costanzo SD, Zanca C, Tasset I, Fraldi A, Incoronato M, Mirabelli P, Monti M, Ballabio A, Pucci P, Cuervo AM, Condorelli G, Phosphorylation-Regulated Degradation of the Tumor-Suppressor Form of PED by Chaperone-Mediated Autophagy in Lung Cancer Cells, J Cell Physiol. 229 (2014) 1359–1368. 10.1002/jcp.24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zhang S, Hu B, You Y, Yang Z, Liu L, Tang H, Bao W, Guan Y, Shen X, Sorting nexin 10 acts as a tumor suppressor in tumorigenesis and progression of colorectal cancer through regulating chaperone mediated autophagy degradation of p21Cip1/WAF1, Cancer Lett. 419 (2018) 116–127. 10.1016/j.canlet.2018.01.045. [DOI] [PubMed] [Google Scholar]
- [212].Han Q, Deng Y, Chen S, Chen R, Yang M, Zhang Z, Sun X, Wang W, He Y, Wang F, Pan X, Li P, Lai W, Luo H, Huang P, Guan X, Deng Y, Yan J, Xu X, Wen Y, Chen A, Hu C, Li X, Li S, Downregulation of ATG5-dependent macroautophagy by chaperone-mediated autophagy promotes breast cancer cell metastasis, Sci Rep-Uk. 7 (2017) 4759. 10.1038/s41598-017-04994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Peng J, Han S, Chen Z, Yang J, Pei Y, Bao C, Qiao L, Chen W, Liu B, Chaperone-mediated autophagy regulates apoptosis and the proliferation of colon carcinoma cells, Biochem Bioph Res Co. 522 (2020) 348–354. 10.1016/j.bbrc.2019.11.081. [DOI] [PubMed] [Google Scholar]
- [214].Ichikawa A, Fujita Y, Hosaka Y, Kadota T, Ito A, Yagishita S, Watanabe N, Fujimoto S, Kawamoto H, Saito N, Yoshida M, Hashimoto M, Minagawa S, Hara H, Motoi N, Yamamoto Y, Ochiya T, Araya J, Kuwano K, Chaperone-mediated autophagy receptor modulates tumor growth and chemoresistance in non–small cell lung cancer, Cancer Sci. 111 (2020) 4154–4165. 10.1111/cas.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Xuan Y, Zhao S, Xiao X, Xiang L, Zheng H-C, Inhibition of chaperone-mediated autophagy reduces tumor growth and metastasis and promotes drug sensitivity in colorectal cancer, Mol Med Rep. 23 (2021) 360. 10.3892/mmr.2021.11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Mizushima N, Levine B, Autophagy in Human Diseases, New Engl J Med. 383 (2020) 1564–1576. 10.1056/nejmra2022774. [DOI] [PubMed] [Google Scholar]
- [217].Poillet-Perez L, White E, Role of tumor and host autophagy in cancer metabolism, Gene Dev. 33 (2019) 610–619. 10.1101/gad.325514.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Levy JMM, Towers CG, Thorburn A, Targeting autophagy in cancer, Nat Rev Cancer. 17 (2017) 528–542. 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Levy JMM, Thorburn A, Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients, Cell Death Differ. 27 (2020) 843–857. 10.1038/s41418-019-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Egan DF, Chun MGH, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang C-C, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford NDP, Shaw RJ, Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates, Mol Cell. 59 (2015) 285–297. 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Dite TA, Langendorf CG, Hoque A, Galic S, Rebello RJ, Ovens AJ, Lindqvist LM, Ngoei KRW, Ling NXY, Furic L, Kemp BE, Scott JW, Oakhill JS, AMP-activated protein kinase selectively inhibited by the type II inhibitor SBI-0206965, J Biol Chem. 293 (2018) 8874–8885. 10.1074/jbc.ra118.003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Tang F, Hu P, Yang Z, Xue C, Gong J, Sun S, Shi L, Zhang S, Li Z, Yang C, Zhang J, Xie C, SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways, Oncol Rep. 37 (2017) 3449–3458. 10.3892/or.2017.5635. [DOI] [PubMed] [Google Scholar]
- [223].Zheng Y, Liu L, Wang Y, Xiao S, Mai R, Zhu Z, Cao Y, Glioblastoma stem cell (GSC)-derived PD-L1-containing exosomes activates AMPK/ULK1 pathway mediated autophagy to increase temozolomide-resistance in glioblastoma, Cell Biosci. 11 (2021) 63. 10.1186/s13578-021-00575-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [224].Lu J, Zhu L, Zheng L, Cui Q, Zhu H, Zhao H, Shen Z, Dong H, Chen S, Wu W, Tan J, Overexpression of ULK1 Represents a Potential Diagnostic Marker for Clear Cell Renal Carcinoma and the Antitumor Effects of SBI-0206965, Ebiomedicine. 34 (2018) 85–93. 10.1016/j.ebiom.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, Cantwell J, Luu C, Cornella-Taracido I, Harrington E, Fekkes P, Lei H, Fang Q, Digan ME, Burdick D, Powers AF, Helliwell SB, D’Aquin S, Bastien J, Wang H, Wiederschain D, Kuerth J, Bergman P, Schwalb D, Thomas J, Ugwonali S, Harbinski F, Tallarico J, Wilson CJ, Myer VE, Porter JA, Bussiere DE, Finan PM, Labow MA, Mao X, Hamann LG, Manning BD, Valdez RA, Nicholson T, Schirle M, Knapp MS, Keaney EP, Murphy LO, Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo, Nat Cell Biol. 16 (2014) 1069–1079. 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- [226].Dyczynski M, Yu Y, Otrocka M, Parpal S, Braga T, Henley AB, Zazzi H, Lerner M, Wennerberg K, Viklund J, Martinsson J, Grandér D, Milito AD, Tamm KP, Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 (Vps34) improves the sensitivity of breast cancer cells to Sunitinib, Cancer Lett. 435 (2018) 32–43. 10.1016/j.canlet.2018.07.028. [DOI] [PubMed] [Google Scholar]
- [227].Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, Bachelot M-F, Lamberton A, Mathieu M, Bertrand T, Marquette J-P, El-Ahmad Y, Filoche-Romme B, Schio L, Garcia-Echeverria C, Goulaouic H, Pasquier B, A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy, Nat Chem Biol. 10 (2014) 1013–1019. 10.1038/nchembio.1681. [DOI] [PubMed] [Google Scholar]
- [228].Zhou P, Li Y, Li B, Zhang M, Xu C, Liu F, Bian L, Liu Y, Yao Y, Li D, Autophagy inhibition enhances celecoxib-induced apoptosis in osteosarcoma, Cell Cycle. 17 (2018) 997–1006. 10.1080/15384101.2018.1467677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].New J, Arnold L, Ananth M, Alvi S, Thornton M, Werner LR, Tawfik O, Dai H, Shnayder Y, Kakarala K, Tsue TT, Girod DA, Ding W-X, Anant S, Thomas SM, Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target, Cancer Res. 77 (2017) canres.1077.2017. 10.1158/0008-5472.can-17-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Noman MZ, Parpal S, Moer KV, Xiao M, Yu Y, Arakelian T, Viklund J, Milito AD, Hasmim M, Andersson M, Amaravadi RK, Martinsson J, Berchem G, Janji B, Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti–PD-1/PD-L1 immunotherapy, Sci Adv. 6 (2020) eaax7881. 10.1126/sciadv.aax7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E, Shpiro N, Ward R, Cross D, Ganley IG, Alessi DR, Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase, Biochem J. 463 (2014) 413–427. 10.1042/bj20140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Liu F, Wu X, Qian Y, Jiang X, Wang Y, Gao J, PIK3C3 regulates the expansion of liver CSCs and PIK3C3 inhibition counteracts liver cancer stem cell activity induced by PI3K inhibitor, Cell Death Dis. 11 (2020) 427. 10.1038/s41419-020-2631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Martens S, Fracchiolla D, Activation and targeting of ATG8 protein lipidation, Cell Discov. 6 (2020) 23. 10.1038/s41421-020-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Agrotis A, Pengo N, Burden JJ, Ketteler R, Redundancy of human ATG4 protease isoforms in autophagy and LC3/GABARAP processing revealed in cells., Autophagy. 15 (2019) 976–997. 10.1080/15548627.2019.1569925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Kurdi A, Cleenewerck M, Vangestel C, Lyssens S, Declercq W, Timmermans J-P, Stroobants S, Augustyns K, Meyer GRYD, Veken PVD, Martinet W, ATG4B inhibitors with a benzotropolone core structure block autophagy and augment efficiency of chemotherapy in mice, Biochem Pharmacol. 138 (2017) 150–162. 10.1016/j.bcp.2017.06.119. [DOI] [PubMed] [Google Scholar]
- [236].Akin D, Wang SK, Habibzadegah-Tari P, Law B, Ostrov D, Li M, Yin X-M, Kim J-S, Horenstein N, W.A.D. Jr, A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors, Autophagy. 10 (2014) 2021–2035. 10.4161/auto.32229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Fu Y, Hong L, Xu J, Zhong G, Gu Q, Gu Q, Guan Y, Zheng X, Dai Q, Luo X, Liu C, Huang Z, Yin X-M, Liu P, Li M, Discovery of a small molecule targeting autophagy via ATG4B inhibition and cell death of colorectal cancer cells in vitro and in vivo, Autophagy. 15 (2018) 295–311. 10.1080/15548627.2018.1517073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Jensen M, Mehlhorn H, Seventy-five years of Resochin® in the fight against malaria, Parasitol Res. 105 (2009) 609. 10.1007/s00436-009-1524-8. [DOI] [PubMed] [Google Scholar]
- [239].Bell CL, Hydroxychloroquine sulfate in rheumatoid arthritis: Long-term response rate and predictive parameters, Am J Medicine. 75 (1983) 46–51. 10.1016/0002-9343(83)91270-6. [DOI] [PubMed] [Google Scholar]
- [240].Briceño E, Calderon A, Sotelo J, Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme, Surg Neurol. 67 (2007) 388–391. 10.1016/j.surneu.2006.08.080. [DOI] [PubMed] [Google Scholar]
- [241].Briceño E, Reyes S, Sotelo J, Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine., Neurosurg Focus. 14 (2003) e3. [DOI] [PubMed] [Google Scholar]
- [242].Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB, Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma, J Clin Invest. 117 (2007) 326–336. 10.1172/jci28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Eldredge HB, DeNittis A, DuHadaway JB, Chernick M, Metz R, Prendergast GC, Concurrent whole brain radiotherapy and short-course chloroquine in patients with brain metastases: a pilot trial, J Radiat Oncol. 2 (2013) 315–321. 10.1007/s13566-013-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Rojas-Puentes LL, Gonzalez-Pinedo M, Crismatt A, Ortega-Gomez A, Gamboa-Vignolle C, Nuñez-Gomez R, Dorantes-Gallareta Y, Arce-Salinas C, Arrieta O, Phase II randomized, double-blind, placebo-controlled study of whole-brain irradiation with concomitant chloroquine for brain metastases, Radiat Oncol. 8 (2013) 209. 10.1186/1748-717x-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi RK, Davis LE, Mita AC, Curiel TJ, Espitia CM, Nawrocki ST, Giles FJ, Carew JS, Combined autophagy and HDAC inhibition, Autophagy. 10 (2014) 1403–1414. 10.4161/auto.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, Tan K-S, Heitjan DF, DeMichele AM, Vaughn DJ, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O’Dwyer PJ, Amaravadi RK, Combined MTOR and autophagy inhibition, Autophagy. 10 (2014) 1391–1402. 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Rangwala R, Leone R, Chang YC, Fecher LA, Schuchter LM, Kramer A, Tan K-S, Heitjan DF, Rodgers G, Gallagher M, Piao S, Troxel AB, Evans TL, DeMichele AM, Nathanson KL, O’Dwyer PJ, Kaiser J, Pontiggia L, Davis LE, Amaravadi RK, Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma, Autophagy. 10 (2014) 1369–1379. 10.4161/auto.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Boone BA, Bahary N, Zureikat AH, Moser AJ, Normolle DP, Wu W-C, Singhi AD, Bao P, Bartlett DL, Liotta LA, Espina V, Loughran P, Lotze MT, Zeh HJ, Safety and Biologic Response of Pre-operative Autophagy Inhibition in Combination with Gemcitabine in Patients with Pancreatic Adenocarcinoma, Ann Surg Oncol. 22 (2015) 4402–4410. 10.1245/s10434-015-4566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Goldberg SB, Supko JG, Neal JW, Muzikansky A, Digumarthy S, Fidias P, Temel JS, Heist RS, Shaw AT, McCarthy PO, Lynch TJ, Sharma S, Settleman JE, Sequist LV, A Phase I Study of Erlotinib and Hydroxychloroquine in Advanced Non–Small-Cell Lung Cancer, J Thorac Oncol. 7 (2012) 1602–1608. 10.1097/jto.0b013e318262de4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, Mikkelson T, Wang D, Chang YC, Hu J, McAfee Q, Fisher J, Troxel AB, Piao S, Heitjan DF, Tan K-S, Pontiggia L, O’Dwyer PJ, Davis LE, Amaravadi RK, A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme, Autophagy. 10 (2014) 1359–1368. 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Pellegrini P, Strambi A, Zipoli C, Hägg-Olofsson M, Buoncervello M, Linder S, Milito AD, Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine, Autophagy. 10 (2014) 562–571. 10.4161/auto.27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Maes H, Kuchnio A, Carmeliet P, Agostinis P, How to teach an old dog new tricks: Autophagy-independent action of chloroquine on the tumor vasculature, Autophagy. 10 (2014) 2082–2084. 10.4161/auto.36259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Eng CH, Wang Z, Tkach D, Toral-Barza L, Ugwonali S, Liu S, Fitzgerald SL, George E, Frias E, Cochran N, Jesus RD, McAllister G, Hoffman GR, Bray K, Lemon L, Lucas J, Fantin VR, Abraham RT, Murphy LO, Nyfeler B, Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy, Proc National Acad Sci. 113 (2016) 182–187. 10.1073/pnas.1515617113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A, Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy, Autophagy. 8 (2012) 200–212. 10.4161/auto.8.2.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].King MA, Ganley IG, Flemington V, Inhibition of cholesterol metabolism underlies synergy between mTOR pathway inhibition and chloroquine in bladder cancer cells, Oncogene. 35 (2016) 4518–4528. 10.1038/onc.2015.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Barnard RA, Wittenburg LA, Amaravadi RK, Gustafson DL, Thorburn A, Thamm DH, Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma, Autophagy. 10 (2014) 1415–1425. 10.4161/auto.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G, Self-consumption: the interplay of autophagy and apoptosis, Nat Rev Mol Cell Bio. 15 (2014) 81–94. 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Fitzwalter BE, Thorburn A, Recent insights into cell death and autophagy, Febs J. 282 (2015) 4279–4288. 10.1111/febs.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Fitzwalter BE, Towers CG, Sullivan KD, Andrysik Z, Hoh M, Ludwig M, O’Prey J, Ryan KM, Espinosa JM, Morgan MJ, Thorburn A, Autophagy Inhibition Mediates Apoptosis Sensitization in Cancer Therapy by Relieving FOXO3a Turnover, Dev Cell. 44 (2018) 555–565.e3. 10.1016/j.devcel.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, Debnath J, Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation, Mol Biol Cell. 22 (2011) 165–178. 10.1091/mbc.e10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, Gunda V, Pierobon M, Waters AM, George SD, Tomar G, Papke B, Hobbs GA, Yan L, Hayes TK, Diehl JN, Goode GD, Chaika NV, Wang Y, Zhang G-F, Witkiewicz AK, Knudsen ES, Petricoin EF, Singh PK, Macdonald JM, Tran NL, Lyssiotis CA, Ying H, Kimmelman AC, Cox AD, Der CJ, Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer, Nat Med. 25 (2019) 628–640. 10.1038/s41591-019-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, Schuman SS, Shea JE, Seipp MT, Yap JT, Burrell LD, Lum DH, Whisenant JR, Gilcrease GW, Cavalieri CC, Rehbein KM, Cutler SL, Affolter KE, Welm AL, Welm BE, Scaife CL, Snyder EL, McMahon M, Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers, Nat Med. 25 (2019) 620–627. 10.1038/s41591-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC, Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion, Nature. 536 (2016) 479–483. 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].McAfee Q, Zhang Z, Samanta A, Levi SM, Ma X-H, Piao S, Lynch JP, Uehara T, Sepulveda AR, Davis LE, Winkler JD, Amaravadi RK, Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency, Proc National Acad Sci. 109 (2012) 8253–8258. 10.1073/pnas.1118193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Nicastri MC, Rebecca VW, Amaravadi RK, Winkler JD, Dimeric quinacrines as chemical tools to identify PPT1, a new regulator of autophagy in cancer cells, Mol Cell Oncol. 5 (2017) e1395504. 10.1080/23723556.2017.1395504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Rebecca VW, Nicastri MC, Fennelly C, Chude CI, Barber-Rotenberg JS, Ronghe A, McAfee Q, McLaughlin NP, Zhang G, Goldman AR, Ojha R, Piao S, Noguera-Ortega E, Martorella A, Alicea GM, Lee JJ, Schuchter LM, Xu X, Herlyn M, Marmorstein R, Gimotty PA, Speicher DW, Winkler JD, Amaravadi RK, PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer., Cancer Discov. 9 (2018) CD-18–0706. 10.1158/2159-8290.cd-18-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Nawrocki ST, Han Y, Visconte V, Przychodzen B, Espitia CM, Phillips J, Anwer F, Advani A, Carraway HE, Kelly KR, Sekeres MA, Maciejewski JP, Carew JS, The novel autophagy inhibitor ROC-325 augments the antileukemic activity of azacitidine, Leukemia. 33 (2019) 2971–2974. 10.1038/s41375-019-0529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Jones TM, Espitia C, Wang W, Nawrocki ST, Carew JS, Moving beyond hydroxychloroquine: the novel lysosomal autophagy inhibitor ROC-325 shows significant potential in preclinical studies, Cancer Commun. 39 (2019) 72. 10.1186/s40880-019-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


