Abstract
As the cerebellar molecular stress response is understudied, we assessed protein expression levels of hypothalamic-pituitary-adrenal (HPA) axis regulators and neurostructural markers in the cerebellum of a male PTSD mouse model and of unstressed vs. stressed male FK506 binding protein 51 (Fkbp5) knockout (KO) vs. wildtype mice. We explored the translatability of our findings in the Fkbp5 KO model to the situation in humans by correlating mRNA levels of candidates with those of FKBP5 in two whole transcriptome datasets of post-mortem human cerebellum and in blood of unstressed and stressed humans. Fkbp5 deletion rescued the stress-induced loss in hippocampal, prefrontal cortical, and, possibly, also cerebellar FKBP52 expression and modulated post-stress cerebellar expression levels of the glucocorticoid receptor (GR) and possibly (trend) also of glial fibrillary acidic protein (GFAP). Accordingly, expression levels of genes encoding for these three genes correlated with those of FKBP5 in human post-mortem cerebellum, while other neurostructural markers were not related to Fkbp5 either in mouse or human cerebellum. Also, gene expression levels of the two immunophilins correlated inversely in the blood of unstressed and stressed humans. We found transient changes in FKBP52 and persistent changes in GR and GFAP in the cerebellum of PTSD-like mice. Altogether, upon elucidating the cerebellar stress response we found first evidence for a novel facet of HPA axis regulation, i.e., the ability of FKBP51 to modulate the expression of its antagonist FKBP52 in the mouse and, speculatively, also in the human brain and blood and, moreover, detected long-term single stress-induced changes in expression of cerebellar HPA axis regulators and neurostructural markers of which some might contribute to the role of the cerebellum in fear extinction.
Keywords: FKBP5 knockout, PTSD mouse model, HPA axis, FKBP52, Cerebellum
1. Introduction
The hippocampus, prefrontal cortex (PFC) and the amygdala have been intensely studied in stress-related psychiatric disorders such as posttraumatic stress disorder (PTSD) and depression while reports on the cerebellum in these psychopathological syndromes and related animal models are comparably scarce. In addition to its well-known sensorimotor functions, the cerebellum increasingly emerges to be involved in functional domains highly relevant for stress-related psychopathology, namely in fear extinction (Carletto and Borsato, 2017), fear and anxiety (e.g. (Moreno-Rius, 2018)), cognition (e.g. (Shipman and Green, 2020; Stoodley, 2012)) and even in emotion (Pierce and Péron, 2020). These findings and recent MRI studies that illuminated the role of the cerebellum in stress-related disorders such as PTSD (Metz et al., 2019; Rabellino et al., 2018; Terpou et al., 2019; Verger et al., 2020), monopolar and bipolar depression (Lupo et al., 2019; Minichino et al., 2014), motivated us to explore the molecular stress response in the cerebellum which has been little studied so far. One of the few studies on this topic recently revealed that cerebellar and multi-system metabolic reprogramming was associated with trauma exposure and PTSD-like behavior in mice (Preston et al., 2021).
One of the major stress response systems is the hypothalamic-pituitary-adrenal (HPA) axis. It is widely accepted to play a role in stress-related psychiatric disorders (e.g., (Ferrer et al., 2020; Fries et al., 2015; Schumacher et al., 2019; Soria et al., 2018)) with its regulator FK 506 binding protein 51 (FKBP51) being one of the most studied molecules in psychiatric research. A brand-new study highlights the importance of brain region and cell-type specific expression regulation of HPA axis modulators for HPA axis physiology by demonstrating that expression of the Fkbp5 gene in a specific neuronal population of the paraventricular nucleus shaped HPA axis function (Häusl et al., 2021). Against this background, studying cerebellar expression levels of major HPA axis regulators seemed an auspicious endeavor for exploring the cerebellar molecular stress response. FKBP51 and the closely related FKBP52 compete for binding to the major heat shock protein (HSP) 90 which is required for binding of the glucocorticoid (GC) cortisol to the glucocorticoid receptor (GR) and to the mineralocorticoid receptor (MR) in vivo (Kirschke et al., 2014). Binding of the co-chaperone FKBP51 to HSP90 antagonizes FKBP52 effects and results inter alia in inhibition of steroid receptors such as GR through which cortisol regulates HPA axis activity via negative feedback (Galigniana et al., 2012).
Searching for promising molecules regulated by the HPA axis led us to neurostructural marker proteins as, for instance, the presynaptic vesicle (PSV) proteins synapsin and synaptophysin, the astroglial marker glial fibrillary acidic protein (GFAP) and the dendritic marker microtubule-associated protein 2 (MAP-2) which all have been reported to be regulated by GC (Antonow-Schlorke et al., 2003; Gomes et al., 1999; Pascual et al., 2017; Piazza et al., 2014; Revest et al., 2010). There is a considerable number of studies on the brain expression of neurostructural markers and HPA axis regulators in foot-shock-stressed animals and in animal models of PTSD. However, the far majority of them did not analyze the cerebellum. In particular, we found no studies on the long-term course of the protein expression of FKBP51, GR, MR, FKBP52, HSP90, MAP-2, GFAP, the PSV synaptophysin and the postsynaptic marker HOMER1 or the pan-neuronal marker NF–H in the cerebellum in response to foot-shock stress. In contrast, there are many studies on hippocampal, prefrontal cortical (pc) and amygdalar expression of GR and FKBP51 in various animal models of stress, íncluding such of PTSD. In general, expression studies in animal models show that brain expression of stress-regulated genes and proteins is highly dynamic and depends on various endo – and exogenous factors such as age, sex and species as well as on type, duration, and timing of the stressor. Several neurostructural markers have been found regulated in various tissues of PTSD animal models, for instance amygdalar synaptophysin (Campos et al., 2013) and hippocampal GFAP (Han et al., 2015). In mice modeling major depression, hippocampal NF–H expression was found reduced (Sanna et al., 2017). In our fear conditioning mouse model of PTSD (Golub et al., 2011; Herrmann et al., 2012; Siegmund and Wotjak, 2007), we previously found a loss in hippocampal synapsin, synaptophysin and NF–H on day 2 (d2) and d60 and of HOMER1 b/c on d60 after exposure to one single foot-shock while levels of hippocampal MAP-2 and GFAP as well as pc and cerebellar synapsin remained unaltered (Herrmann et al., 2012). Synapsin is the only neurostructural marker protein that we have yet analyzed in the cerebellum of our PTSD mouse model.
Thus, here, we quantified protein expression levels of several HPA axis regulators and neurostructural markers in our PTSD mouse model as well as in mice with a whole body-knockout (KO) of the above-introduced Fkbp5. Together with their wildtype (WT) littermates, they were exposed to two combined restraint and forced swim stressors of different intensities (RSI, RSII). We previously found that Fkbp5 KO mice exhibited a higher stress resilience (Touma et al., 2011) which was accompanied by an RSII-induced loss in hippocampal and pc GR and FKBP51 and pc synapsin. In contrast, expression levels of hippocampal or cerebellar synapsin as well as hippocampal and pc MAP-2, synaptophysin and GFAP remained uninfluenced by deletion of Fkbp5 (Schmidt et al., 2015; Touma et al., 2011). Except from synapsin protein (Schmidt et al., 2015) and from GR protein in mice subjected to dexamethasone-corticotropin-releasing hormone tests (Touma et al., 2011), molecular alterations in the cerebellum of Fkbp5 KO animals have not been analyzed so far. Furthermore, to the best of our knowledge, neither MR nor FKBP52 protein expression levels have ever been assessed at all in Fkbp5 KO or Fkbp5 transgenic animals.
2. Materials and methods
2.1. Animals
We have assessed two batches of mice, i.e., the PTSD mouse model and the Fkbp5KO-WT batches. All experimental procedures were approved by the Committee on Animal Health and Care of Upper Bavaria, Germany, and were accomplished in strict compliance with the European Union Directive for the care and use of laboratory animals (86/609/EEC). We made all efforts to minimize animal suffering. All experiments have been performed between 9:30 a.m. and 6:00 p.m. and all animals received food and water ad libitum.
2.2. Fkbp5 KO mouse model
As we have previously described (Touma et al., 2011), male Fkbp5 KO mice (whole body knockout of Fkbp5) and WT littermates were generated in a C57BL/6 background using blastocysts from 129SvJ mice and were single-housed under standard laboratory conditions (lights off: 08:00 p.m.; light-dark cycle 12:12 h) two weeks prior to experiments. At the time-point of experiments they were aged 10–16 weeks. We exposed both Fkbp5 KO mice and WT littermates to two different combined restraint and forced swim stressors of different intensities (RSI, RSII): RSI mice were exposed to RS for 15 min, then, after a 15 min break, to forced swim stress (FSS) followed by a 24 h recovery phase and a subsequent second FSS (FSS 2). RSII mice were exposed to RS for 60 min and were then allowed to rest in their cages for 24 h before subjection to FSS. RSI and RSII mice were sacrificed and brains were dissected eight days after the last FSS. We used n = 6 Fkbp5 KO and n = 6 WT mice per group (control, RSI, RSII), i.e., 36 mice in total. Note that behavioral and endocrine data as well as immunoblot data on hippocampal and pc proteins (except from FKBP52) in the Fkbp5 KO-WT batch studied here have been published previously (Schmidt et al., 2015; Touma et al., 2011) - previous analyses revealed that Fkbp5 KO mice showed an improved coping behavior vs. their WT littermates in both RSI and RSII paradigms (Touma et al., 2011).
2.3. PTSD mouse model
23 days old male C57BL/6 NCrl mice purchased from Charles River GmbH (Charles River Germany GmbH, Sulzfeld, Germany) were housed in groups of four animals for six weeks under an inverse 12:12 h light-dark cycle (lights off: 09:00 a.m.). Either two, 28 or 60 days after a 1.5 mA electric foot-shock or mock treatment (handling), mice were sacrificed, and cerebella as well as bilateral hippocampus were immediately dissected and snap frozen at – 80 °C. We employed n = 14 shocked and mock-treated mice per group (three groups: d2, d28 and d60), i.e., 84 mice in total. Six out of the 27 groups (9 proteins x 3 days of analysis) were smaller due to an accidental loss of cerebellar lysates, i.e., synaptophysin d2, d28 and d60 n = 7, FKBP51 d28 and d60 n = 6 and HSP90 d60 n = 12. We had previously demonstrated in several independent mouse batches that this foot-shock treatment leads to a pronounced PTSD-like syndrome in mice that lasts for longer than 60 days and is characterized by an elevated startle response as well as by elevated conditioned and generalized fear responses (e.g., (Golub et al., 2011; Herrmann et al., 2012; Siegmund and Wotjak, 2007)). Here, we refrained from another repeat of behavioral analysis as we aimed to assess the long-term consequences of the foot-shock stressor without the stress-enhancing effect (Kao et al., 2015) of the behavioral testing procedure.
2.4. Western blot
In lysed total cerebella, bilaterally pooled hippocampus and PFC of mice, immunoblot analyses were performed as we have described previously (Herrmann et al., 2012; Schmidt et al., 2015). For details, see Suppl. Methods.
2.5. In silico analysis of mRNA expression levels of candidate genes in post-mortem human brain
Using the GEO2R tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/), we extracted expression data of genes encoding for the ten proteins we analyzed in two mouse models of stress (Fig. 1-3) from two publicly available gene expression microarray datasets, i.e., from dataset 1 containing post-mortem cerebellum specimens of 50 individuals without psychiatric diagnoses (GSE35974 (Chen et al., 2013)) as well as from dataset 2 containing 1231 post-mortem specimens of ten brain regions (cerebellar cortex, frontal cortex, hippocampus, medulla, occipital cortex, putamen, substantia nigra, temporal cortex, thalamus, white matter) originating from 134 Caucasian neuropathologically confirmed control individuals that died of various reasons (GSE46706 (platform GPL517) (Trabzuni and Thomson, 2014)). Extracted data were used for correlation analyses (Table 1) – see below (statistics and results chapters) for further details. We found no information on the medication status of individuals. Both datasets comprised female and male donors. Gene expression data of both datasets were gained with the Affymetrix Human Gene 1.0 ST Array (Affymetrix; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Further details on specimen processing, RNA extraction and microarray experiments are reported in (Chen et al., 2013) and (Trabzuni and Thomson, 2014), respectively.
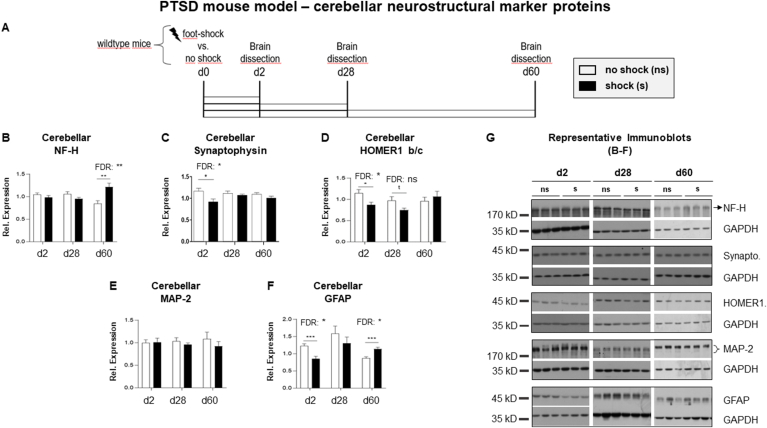
Fig. 1.
Expression of neurostructural marker proteins in the cerebellum of the PTSD mouse model
(A) Mouse model of posttraumatic stress disorder (PTSD): brains were dissected two, 28 or 60 days (d2, d28, d60) after mock – or foot-shock treatment (n = 14 per group except from synaptophysin n = 7 per group). We previously described foot-shock exposure to robustly induce a PTSD-like syndrome. Graphs show results of two-tailed unpaired t-tests of relative protein expression levels of (B) neurofilament H (NF–H), (C) synaptophysin, (D) HOMER1 b/c, (E) microtubule-associated protein 2 (MAP-2) and (F) glial fibrillary acidic protein (GFAP) after normalization to glycerinaldehyd-3-phosphat-dehydrogenase (GAPDH) in lysates of total cerebellum in foot-shocked (shock (s)) vs. mock-treated (no shock (ns)) mice including unadjusted and Benjamini-Hochberg (FDR)-adjusted significant p-values. Plotted immunoblot data represent means of two technical replicates ± standard error of the mean (SEM). Representative immunoblots showing expression levels of candidate proteins (B–F) and GAPDH are presented in (G). Significances are indicated with t, p ≤ 0.01; *, p ≤ 0.05; **, p ≤ 0.01, ***, p ≤ 0.001. Statistical details of significant results are reported in the results section.
Table 1.
Spearman rank correlation of FKBP5 with candidate genes including FKBP4 in human post-mortem brain specimens and in blood samples from PTSD patients vs. controls before and after psychosocial stress.
| FKBP5 |
FKBP4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Post-mortem human brain |
Blood samples from total (fused) PTSD patients + controls cohort |
Dataset 1 (GSE35974) |
|||||||
| Dataset 1 (GSE35974) |
Dataset 2 (GSE46706) |
||||||||
| cerebellum | hippoc. | ten brain regions (incl. hippoc.) | baseline | Immediately after stress exposure | baseline | Immediately after stress exposure | cerebellum | ||
| NEFH | 0.403 ** | 0.0459 | – | – | – | – | – | – | −0.179 |
| HOMER1 | 0.120 | −0.0679 | – | – | – | – | – | – | 0.027 |
| SYP | 0.336 | −0.344 *** | – | – | – | – | – | – | 0.272 |
| MAP2 | −0.043 | −0.104 | – | – | – | – | – | – | 0.196 |
| GFAP | 0.570 *** | 0.301 *** | – | – | – | – | – | – | −0.164 |
| NR3C1 | −0.530 *** | −0.368 *** | – | – | −0,505*** | −0,500*** | 0,634*** | 0,604*** | 0.078 |
| NR3C2 | −0.065 | 0.0461 | – | – | – | – | – | – | −0.065 |
| FKBP4 | −0.279 * | −0.204 * | 0.0941 | −0.172*** | −0.477** | −0.354* | – | – | – |
| HSP90AA1 | 0.063 | 0.101 | – | – | – | – | – | – | 0.333** |
First, using the GEO2R tool, we extracted expression data of selected genes from two publicly available gene expression microarray datasets, i.e., from dataset 1 (post-mortem cerebellum specimens of 50 human subjects without psychiatric diagnoses (GSE35974 (Chen et al., 2013)) as well as from a dataset comprising 1231 post-mortem specimens of ten brain regions originating from 134 control individuals (GSE46706 (Trabzuni and Thomson, 2014) – dataset 2). Analyses of cerebellum specimens of dataset 1 and 2: Extracted gene expression levels of FKBP5 (FK 506 binding protein 51) were correlated with those of the following genes (names of encoded proteins in brackets): NEFH (neurofilament H), HOMER1 (HOMER1), SYP (synaptophysin), MAP2 (microtubule-associated protein 2), GFAP (glial fibrillary acidic protein), NR3C1 (glucocorticoid receptor), NR3C2 (mineralocorticoid receptor), FKBP4 (FK 506 binding protein 52), HSP90AA1 (heat shock protein 90 alpha transcript variant 2). Analyses of hippocampal specimens and of specimens from ten brain regions in dataset 2: Extracted gene expression levels of FKBP5 were correlated with those of FKBP4. Second, we correlated relative FKBP5, FKBP4 and, for control, NR3C1 levels in peripheral whole blood of patients suffering from severe posttraumatic stress disorder (PTSD) vs. non-traumatized healthy controls before vs. after a Trier Social Stress Test (TSST) – the patient and control groups were fused before correlational analyses were performed. Significances are indicated with *, p ≤ 0.05; **, p ≤ 0.01, ***, p ≤ 0.001.
2.6. Correlational analyses of FKBP5, FKBP4 and NR3C1 in the blood of PTSD patients vs. controls before vs. after psychosocial stress
In addition, we correlated relative whole blood gene expression levels of FKBP5 with those of FKBP4 and NR3C1 (gene encoding the GR), respectively, in 23 female patients suffering from severe PTSD vs. 19 age and sex-matched non-traumatized healthy controls before vs. after the Trier Social Stress Test (TSST) as we described previously (Hofmann et al., 2021; Zaba et al., 2015). Gene expression levels were measured with reverse transcription quantitative polymerase chain reaction (RT-qPCR). Due to lack of RNA samples of sufficient quality of control subjects, we could analyze only these three genes at only two of the four assessment time-points of the TSST, i.e., at baseline (−30min, pre-stress) and immediately after exposure to the 10-min psychosocial stressor (+10min, after stress). Note that raw expression data of the patient group have previously been used for other calculations (Zaba et al., 2015). Also, except from one control subject integrated in addition, the PTSD/control cohort studied here is identical to that published in (Zaba et al., 2015). Thus, exclusion and inclusion criteria as well as clinical and demographical characteristics of study participants can be found in that previous manuscript. Briefly, all study participants were physically healthy and controls were free from any lifetime psychiatric DSM-IV diagnosis while patients suffered from a full DSM-IV PTSD syndrome of severe intensity. All participants have been studied, and in case of patients also treated, in the former Trauma Outpatient Clinic (then-head: U. Schmidt) of the Max Planck Institute of Psychiatry in Munich.
2.7. In silico identification of glucocorticoid-responsive elements (GREs)
is described in Suppl. Methods.
2.8. Statistics
Statistical analyses were performed with Sigma Plot 14 (Systat Software Inc., San Jose, CA) except from corrections for multiple testing which were calculated with the online tool of (Hemmerich, 2016). Graphical illustrations have been accomplished with GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA). The Shapiro-Wilk and the Brown-Forsythe tests have been used to test for normality and equal variance, respectively, and revealed that assumptions for unpaired t-tests and ANOVA were met in the far majority of groups. Fkbp5 KO-WT batch: Between-group comparisons of protein expression levels were calculated by one-way or two-way ANOVA followed by Bonferroni-adjusted post-hoc tests. PTSD mouse model batch: expression levels between shock and no shock groups were calculated with the two-tailed unpaired Student's t-test and outliers were excluded with the Grubbs test (p = 0.05). All outliers excluded are documented in the figure legends.
Immunoblot analyses were not performed in an array-like manner since, except from GR and FKBP51 as well as from HSP90 and FKBP52, candidates have been analyzed on separate blots. Nevertheless, since we analyzed a considerable number of candidates and groups per mouse batch and, moreover, since multiple testing of exploratory analyses and the definition of the latter are of constant debate (e.g., Groenwold et al., 2021), we corrected significant p-values of t-tests and of the ANOVA main effects and interactions for multiple comparisons (Benjamini-Hochberg False Discovery Rate (FDR)) (Fig. 1, Fig. 2, Fig. 3). As except from FKBP5 and NR3C1 in human post-mortem samples, human blood and post-mortem data were not normally distributed, we chose the Spearman's rank-order method for correlational analyses (Table 1).
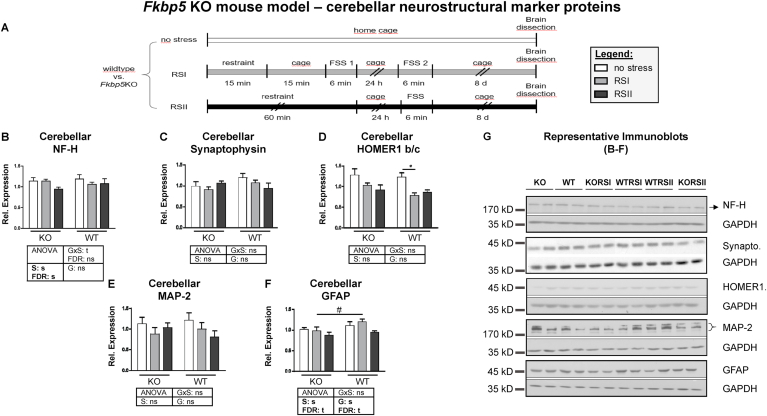
Fig. 2.
Expression of neurostructural marker proteins in the cerebellum of the Fkbp5 KO mouse model
(A) Fkbp5 KO-WT mouse batch: FK 506 binding protein (Fkbp5) knockout (KO) and wildtype (WT) mice have been either subjected to different combination stressors (RSI and RSII) or remained unstressed (no stress); n = 6 per group. For details, see methods chapter. We previously reported Fkbp5 KO mice of the same Fkbp5 KO-WT mouse batch to exhibit a diminished endocrine and behavioral stress response (Touma et al., 2011). Graphs show results of the two-way ANOVA (followed by Bonferroni-adjusted post-hoc tests) of relative protein expression levels of (B) neurofilament H (NF–H), (C) synaptophysin, (D) HOMER1 b/c, (E) microtubule-associated protein 2 (MAP-2) and (F) glial fibrillary acidic protein (GFAP) after normalization to glycerinaldehyd-3-phosphat-dehydrogenase (GAPDH) in lysates of total cerebellum. Plotted immunoblot data represent means of three technical replicates ± standard error of the mean (SEM). Representative immunoblots showing expression levels of candidate proteins (B–F) and GAPDH are presented in (G). Note that the sequence of experimental groups in blot inserts differs from that shown in the corresponding graphs since blots were not cut apart. Main effects (G, Genotype; S, Stressor) and their interaction (G x S) were FDR-corrected for multiple testing and their unadjusted and FDR-adjusted p-values are depicted below the graphs (s, significant; ns, not significant; t, trend). Significances of post-hoc tests are indicated with *, p ≤ 0.05 (between stress group significance), #; p ≤ 0.05 (between-genotype significance). Statistical details of significant results are reported in the results section.
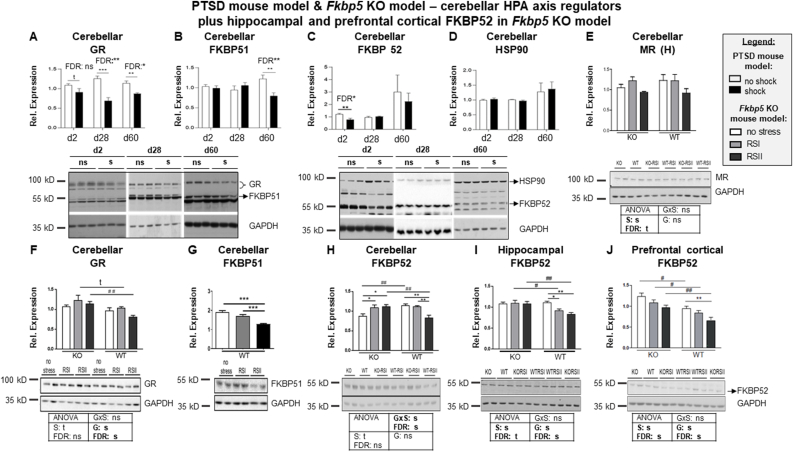
Fig. 3.
Expression of HPA axis regulating proteins in the PTSD and the Fkbp5 KO mouse models plus hippocampal and prefrontal cortical FKBP52 in the Fkbp5 KO mouse model.
Mouse models are described in detail in the methods section and graphically outlined in Fig. 1A and 2A. PTSD mouse model: n = 14 per group except from FKBP51 d28 and d60 (n = 6) and HSP90 d60 (n = 12); FK 506 binding protein (Fkbp5) KO mouse model: n = 6 per group. (A–D): PTSD mouse model: Graphs show results of two-tailed unpaired t-tests of relative protein expression levels of (A) glucocorticoid receptor (GR), (B) FKBP 51, (C) FKBP52 and (D) heat shock protein 90 (HSP90) after normalization to glycerinaldehyd-3-phosphat-dehydrogenase (GAPDH) in lysates of total cerebellum including unadjusted and Benjamini-Hochberg False Discovery Rate (FDR)-adjusted significant p-values. (E–J): Fkbp5 KO mouse model: Graphs show results of the two-way ANOVA (followed by Bonferroni-adjusted post-hoc tests) of relative protein expression levels of (E) mineralocorticoid receptor (MR), (F) GR, (G) FKBP51 (not FDR-corrected) and (H) FKBP52 as well as of (I) hippocampal and (J) prefrontal cortical FKBP52 after normalization to GAPDH in lysates of (E–H) total cerebellum, (I) bilaterally pooled hippocampus and (J) total prefrontal cortex. Main effects (G, Genotype; S, Stressor) and their interaction (G x S) were corrected for multiple testing employing FDR and their unadjusted and FDR-adjusted p-values are depicted below the graphs (s, significant; ns, not significant; t, trend). Plotted immunoblot data represent means of three (Fkbp5 KO model) or two (PTSD mouse model) technical replicates ± standard error of the mean (SEM). Inserts depict corresponding representative immunoblots showing expression levels of analyzed proteins. Note that the sequences of experimental groups in blot inserts belonging to H-J differ from those shown in the corresponding graphs since blots were not cut apart. Significances of t-tests and post-hoc tests are indicated with t, p ≤ 0.1; *, p ≤ 0.05; **, p ≤ 0.01, ***, p ≤ 0.001 for between-stress group significances and #, p ≤ 0.05; ##, p ≤ 0.01 for between-genotype significances. Outliers: (D) HSP90 d28: 1 no shock; HSP90 d60: 1 no shock and 1 shock. Statistical details of significant results are reported in the results section.
3. Results
3.1. Expression of neurostructural marker proteins in the cerebellum of PTSD-like mice
First, we analyzed a set of neurostructural marker proteins in our mouse model of PTSD. The protocol of the experiment is outlined in Fig. 1A. Note that we have already shown several times that foot-shocked mice develop a PTSD-like syndrome on d28 at the latest (e.g., (Golub et al., 2011; Herrmann et al., 2012; Siegmund and Wotjak, 2007)) and that this syndrome lasts at least 60 days after foot-shock (Herrmann et al., 2012). We refrained from assessing the behavior of the mouse batch analyzed here since we aimed to study the long-term effects of foot-shock stress and thus had to avoid application of any additional stressors such as behavioral testing. As expected, all trends for statistical significance did not survive FDR correction. In Figss. 1–3, both unadjusted and FDR-corrected significant p-values are presented.
We compared relative protein concentrations of NF–H, synaptophysin, HOMER1 b/c, MAP-2 and GFAP in the cerebellum of foot-shocked vs. control mice two, 28 and 60 days (d2, d28, d60) after foot-shock and mock-treatment, respectively. Two-tailed unpaired Student's t-tests revealed that cerebellar NF–H protein levels remained unchanged in foot-shocked mice until they exceeded those of non-shocked control mice on d60 (Fig. 1B, FDR p = 0.0098). In contrast, cerebellar synaptophysin and HOMER1 b/c protein expression changed relatively rapidly (Fig. 1C, FDR p = 0.0483; Fig. 1D, FDR p = 0.044) after foot-shock and had returned to its initial level on d28. MAP-2 was the only neurostructural marker protein we analyzed that did not change at all in response to foot-shock treatment (Fig. 1E). Interestingly, GFAP expression levels followed an undulating course: they decreased on d2, normalized on d28 and finally increased on d60 after foot-shock treatment (Fig. 1F d2 and d60: FDR p = ≤ 0.031). Representative blots for analyses shown in Fig. 1B-F are presented in Fig. 1G.
3.2. Expression of neurostructural marker proteins in the cerebellum of Fkbp5 KO mice
Next, we explored whether these cerebellar neurostructural markers are regulated by the major HPA axis regulator and PTSD candidate molecule FKBP51 (Wilker et al., 2014; Xie et al., 2010). We compared their expression levels in the cerebellum of Fkbp5 KO and corresponding WT mice that were subjected to two combination stressors of different intensities (RSI, RSII). Fig. 2A gives an overview over the course of experiments. We published previously that the batch of Fkbp5 KO and WT mice analyzed here exhibited both an attenuated behavioral stress response and a diminished HPA axis reactivity (Touma et al., 2011).
The insignificant main effects of Genotype of the two-way ANOVA indicate that the Fkbp5 genotype did not regulate the expression levels of NF–H, synaptophysin, HOMER1 b/c and MAP-2 in the mouse cerebellum (Fig. 2B-E). In contrast, we found provisional evidence that cerebellar GFAP levels depended on Fkbp5 as indicated by the trend for statistical significance of the main effect of Genotype (Fig. 2F, F = 4.660, FDR p = 0.94) and the significant between-genotype Bonferroni-adjusted post-test in the RSI group: eight days after RSI exposure - GFAP protein levels were lower in the cerebellum of Fkbp5 KO than in corresponding WT mice. Also, the main effect of Stressor shows a trend for statistical significance (Fig. 2F, F = 3.388, FDR p = 0.047). Note that both the latter main effects had been fully significant prior to FDR correction. Also, cerebellar NF–H levels were regulated by stress (significant main effect of Stressor (Fig. 2B, F = 7.639, FDR p = 0.015). In contrast, synaptophysin, HOMER1 b/c and MAP-2 were not regulated by the RSI and RSII stressors as revealed by the insignificant main effects of Stressor and the lack of significant between-stress group post-hoc tests (Fig. 2C-E). Representative blots for analyses are shown in Fig. 2G.
3.3. Expression of HPA axis regulating proteins in the cerebellum of PTSD-like mice
Then, we switched to analyzing HPA axis regulating molecules. First, we quantified expression levels of GR, FKBP51, FKBP52 and HSP90 in the cerebellum of foot-shocked, i.e., PTSD-like, vs. non-shocked, i.e., control mice (Fig. 3A-D). Interestingly, cerebellar concentrations of GR remained persistently reduced after one single electric inescapable foot-shock (Fig. 3A, d2 p = 0.078 (trend that did not survive FDR correction: p = 0.1808); d28 FDR p = 0.006; d60 FDR p = 0.0216) while those of its inhibitor FKBP51 remained unaltered two and 28 days after stress exposure but decreased significantly on d60 (Fig. 3B, p = 0.004, FDR p = 0.021). We cannot exclude that the decrease in cerebellar GR levels commenced even earlier than d2. However, the fact that there was only a trend for a statistically significant loss in GR on d2 that did not survive FDR correction tends to speak against it. Speculatively, this disinhibition of the GR inhibitor FKBP51 might represent a compensation process for the persistent GR loss. In contrast, relative protein expression levels of the FKBP51 antagonist FKBP52 decreased relatively rapidly after foot-shock, i.e., on d2, in the mouse cerebellum (Fig. 3C, FDR p = 0.012). This alteration was transient and fully regressed on d28 and d60. The immunophilins FKBP51 and FKBP52 compete for binding to HSP90 which we found unaltered at all three assessment points in the cerebellum of foot-shocked mice (Fig. 3D). Unfortunately, we have not analyzed MR in the PTSD mouse model.
3.4. Expression of HPA axis regulating proteins in the brain of Fkbp5 KO mice
Next, we assessed the influence of Fkbp5 on cerebellar expression levels of the HPA axis regulators GR, MR and FKBP52 in the Fkbp5 KO-WT mouse batch. Of course, FKBP51 levels could be analyzed only in WT littermates (Fig. 3E-J). As expected from our previous analyses of GR expression in the hippocampus (Touma et al., 2011) and the PFC (Schmidt et al., 2015), also cerebellar GR protein expression was regulated by the Fkbp5 genotype as indicated by the significant main effect of Genotype (Fig. 3F, F = 11.464, FDR p = 0.01), by the significant between-genotype post-test indicating that deletion of Fkbp5 prevented the RSII-induced decrease in GR levels (Fig. 3F, p = 0.004) and, in addition, by the between RSI-group post-hoc test that showed at least a trend for statistical significance and therewith provides provisional evidence that Fkbp5 might prevent also the RSI-induced decrease in GR levels (Fig. 3F, p = 0.083). In contrast, cerebellar MR expression remained uninfluenced by the Fkbp5 genotype (Fig. 3E) as indicated by the lack of a significant FDR-corrected main effect of Genotype (Fig. 3E). Like in the hippocampus (Touma et al., 2011) and the PFC (Schmidt et al., 2015), RSII exposure reduced FKBP51 protein concentrations also in the cerebellum (Fig. 3H). FDR-corrected main effects of the ANOVA of cerebellar expression levels of the FKBP51 antagonist FKBP52 are not significant (Fig. 3H). However, they cannot be properly interpreted as their interaction (S x G) is also significant (Fig. 3H, F = 12.457, FDR p = 0.009) suggesting that Fkbp5 modulation of cerebellar FKBP52 expression in stressed mice might depend on the intensity of the stressor. Post-hoc tests suggested that the RSII-induced decrease in cerebellar FKBP52 expression (Fig. 3H, WT: RSII vs. no stress p = 0.002) was possibly prevented by deletion of Fkbp5 (Fig. 3H, p = 0.002) and that, moreover, the Fkbp5 genotype might have also influenced baseline cerebellar FKBP52 protein concentrations as they were lower in Fkbp5 deficient mice (Fig. 3H, p = 0.002). They rose significantly in response to RSI and RSII exposure (Fig. 3H, KO: no stress vs. RSI p = 0.012; no stress vs. RSII: p = 0.033).
As, to the best of our knowledge, the study at hand is the first studying FKBP52 expression in KO animals, we wanted to test whether this interrelation extends beyond the cerebellum. Thus, we analyzed FKBP52 also in the hippocampus and PFC of the Fkbp5 KO-WT batch. Both brain regions are well-known for being associated with stress-related disorders such as PTSD (e.g., (Henigsberg et al., 2019). Indeed, significant FDR-corrected main effects and significant between-genotype post hoc-tests indicated that deletion of Fkbp5 prevented the stress-induced loss in FKBP52 both in the PFC and the hippocampus (Fig. 3I, F = 10.171, FDR p = 0.001; post-hoc tests: RSI WT vs. KO p = 0.019; RSII WT vs. KO p = 0.002; Fig. 3J, F = 20.529, FDR p = 0.001; post-hoc tests: no stress WT vs. KO p = 0.019; RSI WT vs. KO p = 0.05; RSII WT vs. KO p = 0.002). Furthermore, FKBP52 was regulated by stress in the PFC and potentially also in the hippocampus since the two-way ANOVA of pc FKBP52 revealed a significant main effect of Stressor (Fig. 3I, F = 7.012, FDR p = 0.15) and that of hippocampal FKBP52 showed a trend for statistical significance (Fig. 3I, F = 3.504, FDR p = 0.96). Accordingly, between-group post-hoc tests showed that RSII-treated WT mice exhibited lower FKBP52 protein levels in both these brain regions (Fig. 3I, p = 0.002; Fig. 3J, p = 0.0014), and, furthermore, also in the cerebellum (Fig. 3H, p = 0.001).
3.5. Expression of genes encoding for GFAP, GR and FKBP52 were related to FKBP5 expression in the human brain
In mice, cerebellar expression of GFAP (Fig. 2F), GR (Fig. 3F) and FKBP52 (Fig. 3H) as well as of hippocampal and pc FKBP52 (Fig. 3I and J) depended on the Fkbp5 genotype. To explore whether these results can be translated to the situation in humans, we correlated gene expression levels of FKBP5 (transcript variant 2) with those of the candidates analyzed in mice, i.e., with NEFH (encoding for neurofilament H), HOMER1, SYP (synaptophysin), MAP2, GFAP, NR3C1 (glucocorticoid receptor), NR3C2 (mineralocorticoid receptor), FKBP4 (FKBP52) and HSP90AA1 in two publicly available whole transcriptome microarray datasets, i.e., from dataset 1 containing post-mortem cerebellum specimens of 50 individuals without psychiatric diagnoses (GSE35974 (Chen et al., 2013)) as well as from dataset 2 containing 1231 post-mortem specimens of ten brain regions including the cerebellum that originated from 134 Caucasian neuropathologically-confirmed control individuals (GSE46706 (Trabzuni and Thomson, 2014)).
In both samples of human cerebellum, HSP90AA1 expression did not correlate with FKBP5 (Table 1). This meets our expectations; however, we cannot make any statement on the translatability of these findings as we did not analyze HSP90 in the Fkbp5KO-WT batch. Results for NEFH and SYP were inconsistent among the two human samples (Table 1). In both mice and humans, we found no evidence for a relation of cerebellar gene or protein levels of HOMER1, MAP2 and NR3C2 with FKBP5 protein or gene expression levels, respectively (Fig. 2D-E, Fig. 3E, Table 1). Most interestingly, expression levels of GFAP, NR3C1 and FKBP4 were related to FKBP5 in the cerebellum of human control subjects (Table 1, two different samples) and mice (Figs. 2F, 3F and 3H). In contrast, except from HSP90AA1 and FKBP5, none of the genes analyzed correlated with cerebellar FKBP4 levels (Suppl.Table 1). As we found Fkbp5 to regulate FKBP52 not only in the mouse cerebellum but also in the mouse hippocampus (Fig. 3I), we correlated FKBP5 and FKBP4 levels also in human hippocampal control specimens of dataset 2. However, this analysis did not reveal a significant result. Finally, we correlated FKBP4 and FKBP5 mRNA expression levels in the total of 10 different brain regions (dataset 2) and found a significant inverse correlation (Table 1).
3.6. FKBP5 correlated inversely with FKBP4 and NR3C1 expression in peripheral blood of unstressed and stressed humans
Next, we analyzed the relation of relative FKBP5, FKBP4 and NR3C1 mRNA expression concentrations in peripheral blood of a cohort consisting of 23 female patients with a severe DSM-IV PTSD syndrome and 19 healthy controls that all had been subjected to a psychosocial stress test. Demographic, clinical and endocrine characteristics of this cohort have been reported in one of our previous publications (Zaba et al., 2015). We performed Spearman rank correlations in the total cohort (Table 1) and found significant inverse correlations of FKBP5 and FKBP4 both in unstressed and acutely stressed study participants. Moreover, in human blood, as expected, gene expression of NR3C1 correlated negatively with those of its inhibitor FKBP5 and positively with those of the FKBP5 antagonist FKBP4 (Table 1).
In summary, brain protein expression analyses of the Fkbp5 KO-WT batch and correlational analyses of selected mRNAs in human tissues revealed that FKBP4/FKBP52 expression was inversely related to that of FKBP5/FKBP51, respectively, in the cerebellum of both humans and stressed mice, and, furthermore, in different tissues of unstressed and stressed female humans (blood) and male mice (hippocampus, PFC) as well as in a total of 10 human brain regions.
3.7. The promoters of the human and the mouse genes contain putative GRE elements
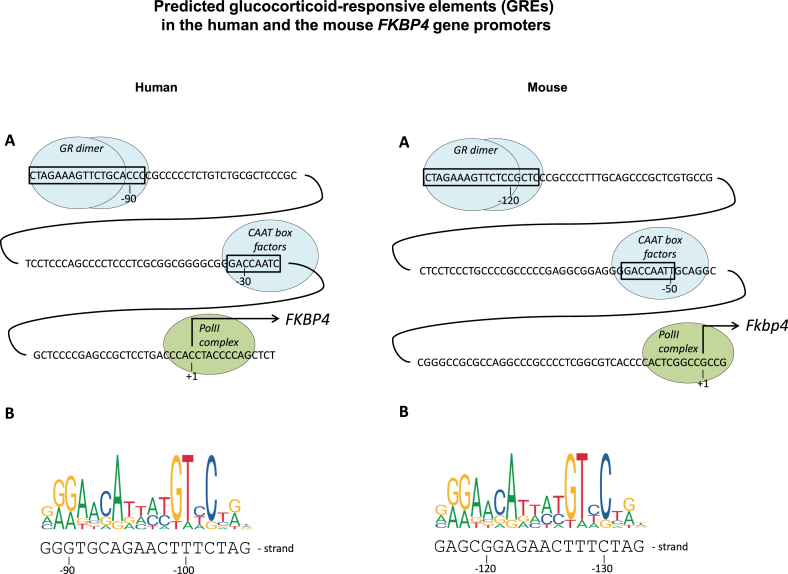
Finally, our results led us to consider the possibility that GR might regulate FKBP4 promoter activity. Since FKBP51 is established as potent inhibitor of GR's transcriptional activity (Galigniana et al., 2012; Wochnik et al., 2005), we searched for the presence of GREs in the FKBP4 promoter. To the best of our knowledge, previous publications specifically devoted to FKBP4/52 promoter analysis did not comment on the presence of GREs in FKBP4 (Massol et al., 2003; Scammell et al., 2003). We used the LASAGNA (Length-Aware Site Alignment Guided by Nucleotide Association) algorithm (Lee and Huang, 2013) to search for putative GREs in the human FKBP4 promoter and found the GRE with the highest score to be located about 90 nt upstream of the transcription start site (Fig. 4, Suppl.Fig.1A). This GRE is part of a GR ChIP-seq peak as well as of the Pol2 ChIP-seq peak listed in the UCSC genome browser (Suppl.Fig.1B), lies in a conserved region and also comes up as the one with the highest score in the mouse Fkbp4 promoter (Fig. 4, right panel). Collectively, this is highly suggestive of a (potentially functional) GRE in the mouse and human FKBP4 promoter, repectively.
Fig. 4.
Glucocorticoid-responsive elements (GREs) in the FKBP4 promoters of humans and mice
In silico search revealed a potential GRE in both the human and the mouse FKBP4 gene promoter. A, the program LASAGNA (Lee and Huang, 2013) was used to identify predicted GREs in the promoter of the FKBP4 gene. Depicted are the GREs with the highest score identified by this program, along with the previously identified CAAT boxes (Scammell et al., 2003) and transcription start sites. B, comparison of the identified GREs with the consensus sequence for NR3C1 binding sites (human, rat, mouse, JASPAR id MAO113.1, (http://jaspar.genereg.net/matrix/MAO113.1/). For further details, see methods chapter and Suppl.Fig. 1.
4. Discussion
We consider our finding that Fkbp5 regulated the expression of its antagonist FKBP52 in the brain of mice (Fig. 3H-J) and possibly also in the brain and blood of humans (Table 1) to be the most important result of our study since FKBP52 is a central HPA axis regulator (Fries et al., 2015) and has, to the best of our knowledge, so far never been analyzed in Fkbp5 KO or Fkbp5 transgenic animals.
Others showed previously that siRNA-mediated knockdown of Fkbp5 in cultured mouse neuroblastoma cells did not influence FKBP52 expression (Quintá et al., 2010). However, this does not contradict our results since, I, that experiment has been performed under unstressed conditions only (we will address this urgent issue in a future study) and, II, regulation in living animals is influenced by a plethora of systemic factors that is absent in cultured immortalized cells. As we found FKBP52 to be regulated by stress in mice (Fig. 3C,H-I) and in one of our PTSD patient cohorts (Zaba et al., 2015), we hypothesized that the GR might regulate FKBP4 promoter activity and performed the, to the best of our knowledge, first in silico search for GREs in the FKBP4 promoter. And indeed, we found substantial evidence for GREs in the FKBP4 promoters of both humans and mice (Fig. 4). Thus, speculatively, the GR might play a pivotal role in FKBP51 modulation of FKBP52 expression. We will test the functionality of the here newly detected GREs in a follow-up study. Alternatively, or additionally, other transcription factors might be involved in FKBP51 modulation of FKBP52 expression, and, thus, a further characterization of the human FKBP4 promoter is of urgent need.
GFAP (provisional evidence) and the GR were regulated by Fkbp5 in the mouse cerebellum (Figs. 2F and 3F) and, accordingly, expression levels of their genes, as well as those of FKBP4, correlated with Fkbp5 mRNA in the cerebellum as well as in a total of 10 brain regions in human control subjects (Table 1). Since we previously reported Fkbp5 KO mice to exhibit improved stress coping (Touma et al., 2011), our findings allow the conclusion that cerebellar GR as well as pc, hippocampal and speculatively also cerebellar FKBP52 (lack of significant main effects) and GFAP (statistical trend) might be involved in Fkbp5 modulation of stress responsiveness. In contrast, expression of cerebellar HOMER1 b/c and MAP-2 proteins/genes were not related to Fkbp5 in mice (Fig. 1D-E, Fig. 2D-E) or humans (Table 1), respectively. As we have already shown that Fkbp5 regulates the GR in the mouse hippocampus (Touma et al., 2011) and PFC (Schmidt et al., 2015), and as FKBP51 is a well-known GR regulator (Fries et al., 2015), our finding that Fkbp5 regulated the GR in the cerebellum did not surprise us. Neither did the RSII-induced decrease in cerebellar FKBP51 expression (Fig. 3G) since we previously detected it also in the PFC and the hippocampus (Schmidt et al., 2015; Touma et al., 2011). In contrast, we had not expected that GFAP protein expression might possibly be influenced by the Fkbp5 genotype in the mouse cerebellum (Fig. 2F) since we previously found that this applied neither to the hippocampus nor to the PFC (Schmidt et al., 2015). As far as we are aware, there was hitherto only one study that analyzed the role of Fkbp5 in GFAP expression. It employed transgenic Fkbp5 overexpressing mice and revealed inconclusive results on this topic, despite its excellent design (Criado-Marrero et al., 2020).
Moreover, our study adds to the scant literature on molecular changes of the cerebellum under stress (Moreno-Rius, 2018). To the best of our knowledge, the study at hand is the first analyzing the long-term course of cerebellar NF–H, HOMER1 b/c, synaptophysin, GFAP, FKBP51, FKBP52 and HSP90 expression levels in response to single acute stress. In contrast, a relatively long-lasting (14 days) stress-induced decrease in GR expression in the cerebellum and, furthermore, also in the hippocampus has been already reported by others (Kitraki et al., 1999). Nevertheless, we did not expect GR loss to last until d60 after foot-shock (Fig. 3A). Both the GR and FKBP51 are well-known targets of stress-related behavioral syndromes in rodents and psychiatric disorders in humans. Importantly, two pharmacological mouse studies suggested the GR-FKBP51 complex to be essential for fear extinction (Sawamura et al., 2016; H. H. Li et al., 2020), a functional domain highly relevant for PTSD and anxiety disorders. Our study adds to these findings as it shows that fear conditioning leads to substantial expression changes in GR protein in the mouse cerebellum.
In the study at hand, we employed two types of stressors, i.e., an immediate acute stressor (foot-shock treatment) and the prolonged RSI and RSII stressors. Post-stress incubation times varied between these two mouse models, i.e., eight days and two, 28 and 60 days in RSI/RSII-treated and foot-shocked mice, respectively. This overall design of our animal experiments can, evidently, be seen as inconsistent and thus as a limitation. However, we consider it rather multifaceted and thus particularly suitable for promoting the identification of cerebellar stress markers with robust cross-model validity. In detail, in the cerebellum, NF–H, GFAP, GR, FKBP51 and FKBP52 protein expression levels were regulated by both types of stressors while synaptophysin and HOMER1 b/c were altered in response to immediate acute stress only. Interestingly, in both mouse models of stress, MAP-2 levels remained unaltered in any brain region (Figss. 1–3). Table 2 summarizes the directions of changes in expression levels of candidate proteins in the hippocampus and cerebellum in response to foot-shock in our PTSD mouse model as well as in the hippocampus, PFC and cerebellum in response to RSI and RSII in WT mice of the Fkbp5 KO-WT batch; data on cerebellar expression levels as well as on hippocampal and pc FKBP52 expression were taken from Figs. 1–3 and the other data from three of our previous publications (Herrmann et al., 2012; Schmidt et al., 2015; Touma et al., 2011). Table 2 illustrates that, in response to stress, the expression levels of the HPA axis regulators GR, FKBP51 and FKBP52 changed in a similar pattern across brain regions, i.e., they decreased in response to different stressors in the cerebellum of both mouse models as well as in the hippocampus and PFC in WT mice of the Fkbp5 KO-WT batch at different assessment points while, in contrast, the neurostructural markers NF–H, synaptophysin and GFAP increased in some regions and decreased in others. It is highly likely that epigenetic mechanisms and noncoding RNAs play a role at both regulation levels, i.e., at the levels of the HPA axis regulators and that of the HPA axis targets. Epigenetic mechanisms also play a role in the age-related upregulation of Fkbp5 expression. However, as it occurs in months rather than weeks (Sabbagh et al., 2014), we do not expect it to bias comparisons between our two differently aged (nine vs. 10–16 weeks) mouse batches.
Table 2.
Summary of the directions of changes in expression levels of HPA axis regulating and neurostructural marker proteins in response to single acute (foot-shock) or prolonged (RSI, RSII) stress in the WT mouse brain.
| Proteins | PTSD mouse model (t-tests) |
WT mice of Fkbp5 KO mouse model (Bonferroni post-tests) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hippocampus |
Cerebellum |
Hippocampus |
PFC |
Cerebellum |
||||||||
| d2 | d28 | d60 | d2 | d28 | d60 | RSI | RSII | RSI | RSII | RSI | RSII | |
| GR | – | – | – | ↓ (t) FDR: ns |
↓ | ↓ | ↔ | ↓ | ↔ | ↓ | ↔ | ↔ |
| FKBP51 | – | – | – | ↔ | ↔ | ↓ | ↔ | ↓ | ↔ | ↓ | ↔ | ↓ |
| FKBP52 | – | – | – | ↓ | ↔ | ↔ | ↔ | ↓ | ↔ | ↓ | ↔ | ↓ FDR S: ns |
| MR | – | – | – | – | – | – | – | – | – | – | ↔ | ↔ |
| HSP90 | – | – | – | ↔ | ↔ | ↔ | – | – | – | – | – | – |
| NF–H | ↓ | ↔ | ↓ (t) | ↔ | ↔ | ↑ | – | – | – | – | ↓ | ↔ |
| Synaptophysin | ↓ (t) | ↔ | ↓ | ↓ | ↔ | ↔ | ↔ | ↑ | ↔ | ↑ | ↔ | ↔ |
| HOMER1 b/c | ↔ | ↔ | ↓ | ↓ | ↓ (t) FDR: ns |
↔ | – | – | – | – | ↔ | ↔ |
| MAP-2 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| GFAP | ↔ | ↔ | ↔ | ↓ | ↔ | ↑ | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ |
Data on cerebellar expression levels as well as on hippocampal and prefrontal cortical FKBP52 expression were taken from Figss. 1–3 (t-tests and Bonferroni post-tests from ANOVAs) and the other data from three of our previous publications (Herrmann et al., 2012; Schmidt et al., 2015; Touma et al., 2011). Symbols: ↑, increase in relation to unstressed controls; ↓, decrease in relation to unstressed controls; ↔ no statistically significant change in relation to unstressed controls; (t), statistical trend for a significant change; FDR, Benjamini-Hochberg false discovery rate (FDR) correction; S FDR, FDR-correction of main effect of Stressor (ANOVA). Note that FDR and FDR S are only listed in case FDR-correction has diminished the significance level of the respective result and that both FDR and FDR S have only been calculated for results gained in cerebellar samples. Abbreviations: PTSD, posttraumatic stress disorder; Fkbp5, gene encoding for FK 506 binding protein 51; KO, knockout; WT, wildtype; d2, d28 and d60: 2, 28 or 60 days after exposure to a single foot-shock or mock-treatment; abbreviations of proteins are explained in the main text. RSI and RSII are explained in detail in the methods chapter and are illustrated in Fig. 2A.
The very long-lasting cerebellar GR loss (Fig. 3A) might result from stress-induced epigenetic (re)-programming of its gene, Nr3c1, which has repeatedly been suggested to constitute an important adaptation mechanism to (traumatic) stress in humans (Watkeys et al., 2018). Besides GR expression, also expression of GFAP persistently changed between d2 and d60 after foot-shock, however in contrast to that of the GR, with an undulating course. Our previous finding that the mouse PTSD-like syndrome lasts at least until d60 (Herrmann et al., 2012) suggests that proteins that are persistently changed in their expression until d60, such as cerebellar GFAP and GR (Figs. 1F and 3A) as well as hippocampal synapsin (Herrmann et al., 2012), might possibly be involved in the maintenance of the behavioral PTSD-like syndrome while those exhibiting transient changes in expression levels, such as cerebellar FKBP52, synaptophysin and HOMER 1b/c (Figs. 3C and 1C,D), might rather be involved in its onset. The promoter of GFAP contains GREs and thus its expression can be directly modulated by GC (Dore et al., 2009). Of note, blood plasma GFAP has been found elevated both on d60 in the cerebellum of PTSD-like mice (Fig. 1F) and in veterans suffering from PTSD (Brahmajothi and Abou-Donia, 2020).
Yet, we have not studied the long-term course of HPA axis reactivity in response to foot-shock in our PTSD mouse model but aim to address this in a future study. However, we found that inescapable foot-shock caused, as expected, an immediate increase in corticosterone in the PFC and the hippocampus (Kao et al., 2015). In case the long-term reduction in cerebellar GR protein expression (Fig. 3A) also occurred in the key brain region orchestrating the HPA axis response, i.e. the paraventricular nucleus (PVN) (Herman et al., 2016), it would persistently change the set-point of HPA axis reactivity and shifted it towards HPA axis overactivity. Thus, experiencing a single strong stressor might change the HPA axis set-point in the mouse brain through attenuation of GR expression. This supposition is supported by our observations in the Fkbp5 KO-WT batch since, in response to stress, Fkbp5 KO mice exhibited a comparably lower HPA axis reactivity (Touma et al., 2011) that was paralleled by higher GR levels in their hippocampus, PFC (Herrmann et al., 2012; Touma et al., 2011), and cerebellum (Fig. 3F). In case our findings on stress-induced changes in expression levels of FKBP51 and FKBP52 in the cerebellum could be translated to the PVN, the foot-shock-induced dip in FKBP52 expression on d2 would transiently reinforce foot-shock-induced HPA axis overactivity while the late reduction in FKBP51 on d60, i.e., the inhibition of the GR inhibitor, might possibly represent a late compensation process targeting GR loss and might shift the HPA axis set-point back towards baseline. We aim to explore this hypothesis together with the epigenetic make-up of Fkbp5, Nr3c1 and Gfap in a future study. However, besides these possible systemic influences, stress-induced changes in expression levels of GR, FKBP52 and FKBP51 in the cerebellum (Fig. 3A-C, Fig. 3.F–H) might likely also have local, brain region-specific consequences some of which might be reflected in the here-observed changes in expression levels of neurostructural marker proteins. Brain region-specificity appeared to play a role also in stress modulation of FKBP52 expression since, in stressed mice, the PFC was the only region tested in which deletion of Fkbp5 led to an increase in FKBP52 levels (Fig. 3H-J).
Further elucidation of the role of the neurostructural proteins studied here will require additional experimental analysis techniques such as electrophysiology and immunohistochemistry. The lack of the latter is, in addition to the small sample sizes and the fact that we have studied only male mice, a major limitation of the study presented here as immunoblot analyses in lysates of the total brain regions allow conclusions on sum but not on subregional effects of expression. For instance, we can conclude that the sum expression of MAP-2 in the total cerebellum (Figs. 1E and 2E) and the bilateral hippocampus (Schmidt et al., 2015; Touma et al., 2011) were not influenced by stress in both mouse models but, however, we cannot fully rule out tiny changes in MAP-2 expression levels in distinct hippocampal or cerebellar subregions. With regard to the obligation to minimize the number of animals used in research and to the fact that the sample size of six mice per group was sufficient to detect between-genotype differences in GR protein expression in another brain region, i.e., the hippocampus, of the same Fkbp5 KO-WT mouse batch (Touma et al., 2011), we adopted the same sample size for the present study, although it lies below that estimated by the a priori power analysis and must thus been regarded a major limitation, in particular since it might leave smaller effects undetected.
In conclusion, upon elucidating the cerebellar molecular stress response we discovered a potential novel facet of HPA axis regulation, i.e., the ability of FKBP51 to modulate the expression of its antagonist FKBP52 in the mouse and, speculatively, also in the human brain and, moreover, found long-term single stress-induced changes in expression of cerebellar HPA axis regulating and neurostructural markers of which some might contribute to the role of cerebellum in fear extinction (Carletto and Borsato, 2017). For instance, the late increase in cerebellar GFAP levels in our PTSD mouse model might possibly reflect astrocyte activation which was recently shown to disrupt memory consolidation and to reduce contextual but not cued fear (Y. Y. Li et al., 2020).
Funding
All animal experiments including immunoblots and all clinical experiments (PTSD patient cohort) including mRNA analyses have been performed at the Max Planck Institute of Psychiatry in Munich. Consumables for expression analyses performed at the Max Planck Institute of Psychiatry in Munich have been funded by the Private Horst Kübler Stiftung, München. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Leonie Herrmann: Investigation, Formal analysis, Visualization, Data curation. Tim Ebert: Writing – review & editing, Conceptualization. Helena Rosen: Writing – original draft, Formal analysis. Bozidar Novak: Investigation, Validation. Alexandra Philipsen: Resources, Supervision. Chadi Touma: Methodology, Investigation, Supervision, Validation. Monika Schreckenbach: Investigation. Nils C. Gassen: Supervision, Resources. Theo Rein: Formal analysis. Ulrike Schmidt: Writing – original draft, Writing – review & editing, Conceptualization, Supervision, Software, Formal analysis, Project administration.
Declaration of competing interest
US and AP are consultants at Boehringer Ingelheim, Ingelheim, Germany. AP has served on advisory boards, given lectures, performed phase 3 studies, or received travel grants within the last 5 years from Eli Lilly and Co, Lundbeck, MEDICE Arzneimittel, Pütter GmbH and Co KG, Novartis, Servier and Shire/Takeda. TR is coinventor of the patent “FKBP51: a novel target for antidepressant therapy” (WO2005054500). TE, LH, HR, BN, CT, MS and NCG declare no conflict of interest.
Acknowledgement
We thank Dipl.biol.Dipl.inf. Cornel Babel for invaluable help with data logistics and Carsten T. Wotjak for substantial support with animal experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100401.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Antonow-Schlorke I., Schwab M., Li C., Nathanielsz P.W. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J. Physiol. 2003;547:117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmajothi M.V., Abou-Donia M.B. PTSD susceptibility and challenges: pathophysiological consequences of behavioral symptoms. Mil. Med. 2020;185:279–285. doi: 10.1093/milmed/usz321. [DOI] [PubMed] [Google Scholar]
- Campos A.C., Ferreira F.R., da Silva W.A., Guimarães F.S. Predator threat stress promotes long lasting anxiety-like behaviors and modulates synaptophysin and CB1 receptors expression in brain areas associated with PTSD symptoms. Neurosci. Lett. 2013;533:34–38. doi: 10.1016/j.neulet.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Carletto S., Borsato T. Neurobiological correlates of post-traumatic stress disorder: a focus on cerebellum role. European Journal of Trauma & Dissociation. 2017;1:153–157. doi: 10.1016/j.ejtd.2017.03.012. [DOI] [Google Scholar]
- Chen C., Cheng L., Grennan K., Pibiri F., Zhang C., Badner J.A., Members of the Bipolar Disorder Genome Study (BiGS) Consortium. Gershon E.S., Liu C. Two gene co-expression modules differentiate psychotics and controls. Mol Psychiatry. 2013;18:1308–1314. doi: 10.1038/mp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado-Marrero M., Smith T.M., Gould L.A., Kim S., Penny H.J., Sun Z., Gulick D., Dickey C.A., Blair L.J. FKBP5 and early life stress affect the hippocampus by an age-dependent mechanism. Brain, Behavior, & Immunity - Health. 2020;9:100143. doi: 10.1016/j.bbih.2020.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore J.J., DeWitt J.C., Setty N., Donald M.D., Joo E., Chesarone M.A., Birren S.J. Multiple signaling pathways converge to regulate bone-morphogenetic-protein-dependent glial gene expression. Dev. Neurosci. 2009;31:473–486. doi: 10.1159/000210187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer A., Labad J., Salvat-Pujol N., Monreal J.A., Urretavizcaya M., Crespo J.M., Menchón J.M., Palao D., Soria V. Hypothalamic-pituitary-adrenal axis-related genes and cognition in major mood disorders and schizophrenia: a systematic review. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2020;101:109929. doi: 10.1016/j.pnpbp.2020.109929. [DOI] [PubMed] [Google Scholar]
- Fries G.R., Gassen N.C., Schmidt U., Rein T. The FKBP51-glucocorticoid receptor balance in stress-related mental disorders. Curr. Mol. Pharmacol. 2015;9:126–140. doi: 10.2174/1874467208666150519114435. [DOI] [PubMed] [Google Scholar]
- Galigniana N.M., Ballmer L.T., Toneatto J., Erlejman A.G., Lagadari M., Galigniana M.D. Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51: FKBP51-regulated stress response. J. Neurochem. 2012;122:4–18. doi: 10.1111/j.1471-4159.2012.07775.x. [DOI] [PubMed] [Google Scholar]
- Golub Y., Kaltwasser S.F., Mauch C.P., Herrmann L., Schmidt U., Holsboer F., Czisch M., Wotjak C.T. Reduced hippocampus volume in the mouse model of posttraumatic stress disorder. J. Psychiatr. Res. 2011;45:650–659. doi: 10.1016/j.jpsychires.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Gomes F.C.A., Paulin D., Moura Neto V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz. J. Med. Biol. Res. 1999;32:619–631. doi: 10.1590/S0100-879X1999000500016. [DOI] [PubMed] [Google Scholar]
- Groenwold R.H., Goeman J.J., Le Cessie S., Dekkers O.M. Multiple testing: when is many too much? Eur. J. Endocrinol. 2021;184:E11–E14. doi: 10.1530/EJE-20-1375. [DOI] [PubMed] [Google Scholar]
- Han F., Xiao B., Wen L. Loss of glial cells of the Hippocampus in a rat model of post-traumatic stress disorder. Neurochem. Res. 2015;40:942–951. doi: 10.1007/s11064-015-1549-6. [DOI] [PubMed] [Google Scholar]
- Häusl A.S., Brix L.M., Hartmann J., Pöhlmann M.L., Lopez J.-P., Menegaz D., Brivio E., Engelhardt C., Roeh S., Bajaj T., Rudolph L., Stoffel R., Hafner K., Goss H.M., Reul J.M.H.M., Deussing J.M., Eder M., Ressler K.J., Gassen N.C., Chen A., Schmidt M.V. The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice. Mol Psychiatry. 2021 Mar 1 doi: 10.1038/s41380-021-01044-x. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich W. 2016. StatistikGuru: Rechner zur Adjustierung des α-Niveaus.https://statistikguru.de/rechner/adjustierung-des-alphaniveaus.html Verfügbar unter: [Google Scholar]
- Henigsberg N., Kalember P., Petrović Z.K., Šečić A. Neuroimaging research in posttraumatic stress disorder – focus on amygdala, hippocampus and prefrontal cortex. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2019;90:37–42. doi: 10.1016/j.pnpbp.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann L., Ionescu I.A., Henes K., Golub Y., Wang N.X.R., Buell D.R., Holsboer F., Wotjak C.T., Schmidt U. Long-lasting hippocampal synaptic protein loss in a mouse model of posttraumatic stress disorder. PloS One. 2012;7 doi: 10.1371/journal.pone.0042603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J., Huber C., Novak B., Schreckenbach M., Schubert C.F., Touma C., Rutten B.P., Schmidt U. Oxytocin receptor is a potential biomarker of the hyporesponsive HPA axis subtype of PTSD and might be modulated by HPA axis reactivity traits in humans and mice. Psychoneuroendocrinology. 2021;129:105242. doi: 10.1016/j.psyneuen.2021.105242. [DOI] [PubMed] [Google Scholar]
- Kao C.-Y., Stalla G., Stalla J., Wotjak C.T., Anderzhanova E. Norepinephrine and corticosterone in the medial prefrontal cortex and hippocampus predict PTSD-like symptoms in mice. Eur. J. Neurosci. 2015;41:1139–1148. doi: 10.1111/ejn.12860. [DOI] [PubMed] [Google Scholar]
- Kirschke E., Goswami D., Southworth D., Griffin P.R., Agard D.A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157:1685–1697. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitraki E., Karandrea D., Kittas C. Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinology. 1999;69:331–338. doi: 10.1159/000054435. [DOI] [PubMed] [Google Scholar]
- Lee C., Huang C.-H. LASAGNA-Search: an integrated web tool for transcription factor binding site search and visualization. Biotechniques. 2013;54:141–153. doi: 10.2144/000113999. [DOI] [PubMed] [Google Scholar]
- Li H., Su P., Lai T.K., Jiang A., Liu J., Zhai D., Campbell C.T., Lee F.H., Yong W., Pasricha S., Li S., Wong A.H., Ressler K.J., Liu F. The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J. Clin. Invest. 2020;130:877–889. doi: 10.1172/JCI130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li L., Wu J., Zhu Z., Feng X., Qin L., Zhu Y., Sun L., Liu Y., Qiu Z., Duan S., Yu Y.-Q. Activation of astrocytes in hippocampus decreases fear memory through adenosine A1 receptors. eLife. 2020;9 doi: 10.7554/eLife.57155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo M., Siciliano L., Leggio M. From cerebellar alterations to mood disorders: a systematic review. Neurosci. Biobehav. Rev. 2019;103:21–28. doi: 10.1016/j.neubiorev.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Massol N., Lebeau M.-C., Schumacher M., Baulieu E.-E. Promoter activity and gene structure of rabbit FKBP52. DNA Cell Biol. 2003;22:505–511. doi: 10.1089/10445490360708919. [DOI] [PubMed] [Google Scholar]
- Metz S., Fleischer J., Gärnter M., Golde S., Duesenberg M., Roepke S., Wolf O.T., Otte C., Wingenfeld K. Effects of hydrocortisone on autobiographical memory retrieval in patients with posttraumatic stress disorder and borderline personality disorder: the role of childhood trauma. Neuropsychopharmacology. 2019;44:2038–2044. doi: 10.1038/s41386-019-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichino A., Bersani F.S., Trabucchi G., Albano G., Primavera M., Delle Chiaie R., Biondi M. The role of cerebellum in unipolar and bipolar depression: a review of the main neurobiological findings. Riv. Psichiatr. 2014;49:124–131. doi: 10.1708/1551.16907. [DOI] [PubMed] [Google Scholar]
- Moreno-Rius J. The cerebellum in fear and anxiety-related disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018;85:23–32. doi: 10.1016/j.pnpbp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Pascual R., Cuevas I., Santander O., Valencia M. Influence of antenatal synthetic glucocorticoid administration on pyramidal cell morphology and microtubule-associated protein type 2 (MAP2) in rat cerebrocortical neurons. Clin. Pediatr. Endocrinol. 2017;26:9–15. doi: 10.1297/cpe.26.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza F.V., Segabinazi E., Centenaro L.A., Do Nascimento P.S., Achaval M., Marcuzzo S. Enriched environment induces beneficial effects on memory deficits and microglial activation in the hippocampus of type 1 diabetic rats. Metab. Brain Dis. 2014;29:93–104. doi: 10.1007/s11011-013-9467-2. [DOI] [PubMed] [Google Scholar]
- Pierce J.E., Péron J. Social Cognitive and Affective Neuroscience; 2020. The Basal Ganglia and the Cerebellum in Human Emotion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G., Emmerzaal T., Radenkovic S., Lanza I.R., Oglesbee D., Morava E., Kozicz T. Cerebellar and multi-system metabolic reprogramming associated with trauma exposure and post-traumatic stress disorder (PTSD)-like behavior in mice. Neurobiology of Stress. 2021;14:100300. doi: 10.1016/j.ynstr.2021.100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintá H.R., Maschi D., Gomez-Sanchez C., Piwien-Pilipuk G., Galigniana M.D. Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth: FKBP balance modulates neurite outgrowth. J. Neurochem. 2010;115:716–734. doi: 10.1111/j.1471-4159.2010.06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Théberge J., McKinnon M.C., Lanius R.A. The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 2018;39:3354–3374. doi: 10.1002/hbm.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest J.-M., Kaouane N., Mondin M., Le Roux A., Rougé-Pont F., Vallée M., Barik J., Tronche F., Desmedt A., Piazza P.V. The enhancement of stress-related memory by glucocorticoids depends on synapsin-Ia/Ib. Mol. Psychiatr. 2010;15:1140–1151. doi: 10.1038/mp.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh J.J., O'Leary J.C., Blair L.J., Klengel T., Nordhues B.A., Fontaine S.N., Binder E.B., Dickey C.A. Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency. PloS One. 2014;9 doi: 10.1371/journal.pone.0107241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna M.D., Ghelardini C., Galeotti N. Effect of amitriptyline treatment on neurofilament-H protein in an experimental model of depression. Brain Res. Bull. 2017;128:1–6. doi: 10.1016/j.brainresbull.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Klengel T., Armario A., Jovanovic T., Norrholm S.D., Ressler K.J., Andero R. Dexamethasone treatment leads to enhanced fear extinction and dynamic Fkbp5 regulation in amygdala. Neuropsychopharmacol. 2016;41:832–846. doi: 10.1038/npp.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell J.G., Hubler T.R., Denny W.B., Valentine D.L. Organization of the human FK506-binding immunophilin FKBP52 protein gene (FKBP4) Genomics. 2003;81:640–643. doi: 10.1016/S0888-7543(03)00090-9. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Buell D.R., Ionescu I.A., Gassen N.C., Holsboer F., Cox M.B., Novak B., Huber C., Hartmann J., Schmidt M.V., Touma C., Rein T., Herrmann L. A role for synapsin in FKBP51 modulation of stress responsiveness: convergent evidence from animal and human studies. Psychoneuroendocrinology. 2015;52:43–58. doi: 10.1016/j.psyneuen.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Schumacher S., Niemeyer H., Engel S., Cwik J.C., Laufer S., Klusmann H., Knaevelsrud C. HPA axis regulation in posttraumatic stress disorder: a meta-analysis focusing on potential moderators. Neurosci. Biobehav. Rev. 2019;100:35–57. doi: 10.1016/j.neubiorev.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Shipman M.L., Green J.T. Cerebellum and cognition: does the rodent cerebellum participate in cognitive functions? Neurobiol. Learn. Mem. 2020;170:106996. doi: 10.1016/j.nlm.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Siegmund A., Wotjak C.T. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J. Psychiatr. Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Soria V., González-Rodríguez A., Huerta-Ramos E., Usall J., Cobo J., Bioque M., Barbero J.D., García-Rizo C., Tost M., Monreal J.A., Labad J. Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: a systematic review and narrative synthesis. Psychoneuroendocrinology. 2018;93:8–19. doi: 10.1016/j.psyneuen.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Terpou B.A., Densmore M., Thome J., Frewen P., McKinnon M.C., Lanius R.A. The innate alarm system and subliminal threat presentation in posttraumatic stress disorder: neuroimaging of the midbrain and cerebellum. Chronic Stress. 2019;3 doi: 10.1177/2470547018821496. 247054701882149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C., Gassen N.C., Herrmann L., Cheung-Flynn J., Büll D.R., Ionescu I.A., Heinzmann J.-M., Knapman A., Siebertz A., Depping A.-M., Hartmann J., Hausch F., Schmidt M.V., Holsboer F., Ising M., Cox M.B., Schmidt U., Rein T. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Trabzuni D., Thomson P.C. Analysis of gene expression data using a linear mixed model/finite mixture model approach: application to regional differences in the human brain. Bioinformatics. 2014;30:1555–1561. doi: 10.1093/bioinformatics/btu088. [DOI] [PubMed] [Google Scholar]
- Verger A., Rousseau P.F., Malbos E., Chawki M.B., Nicolas F., Lançon C., Khalfa S., Guedj E. Involvement of the cerebellum in EMDR efficiency: a metabolic connectivity PET study in PTSD. Eur. J. Psychotraumatol. 2020;11:1767986. doi: 10.1080/20008198.2020.1767986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkeys O.J., Kremerskothen K., Quidé Y., Fullerton J.M., Green M.J. Glucocorticoid receptor gene (NR3C1) DNA methylation in association with trauma, psychopathology, transcript expression, or genotypic variation: a systematic review. Neurosci. Biobehav. Rev. 2018;95:85–122. doi: 10.1016/j.neubiorev.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Wilker S., Pfeiffer A., Kolassa S., Elbert T., Lingenfelder B., Ovuga E., Papassotiropoulos A., De Quervain D., Kolassa I.-T. The role of FKBP5 genotype in moderating long-term effectiveness of exposure-based psychotherapy for posttraumatic stress disorder. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.49. e403–e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wochnik G.M., Rüegg J., Abel G.A., Schmidt U., Holsboer F., Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Xie P., Kranzler H.R., Poling J., Stein M.B., Anton R.F., Farrer L.A., Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacol. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba M., Kirmeier T., Ionescu I.A., Wollweber B., Buell D.R., Gall-Kleebach D.J., Schubert C.F., Novak B., Huber C., Köhler K., Holsboer F., Pütz B., Müller-Myhsok B., Höhne N., Uhr M., Ising M., Herrmann L., Schmidt U. Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology. 2015;55:102–115. doi: 10.1016/j.psyneuen.2015.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.