Abstract
Oxytocin (OT) and vasopressin (AVP) are endogenous ligands for OT and AVP receptors in the brain and in the peripheral system. Several studies demonstrate that OT and AVP have opposite roles in modulating stress, anxiety and social behaviours. Interestingly, both peptides and their receptors exhibit high sequence homology which could account for the biased signalling interaction of the peptides with OT and AVP receptors. However, how and under which conditions this crosstalk occurs in vivo remains unclear. In this review we shed light on the complexity of the roles of OT and AVP, by focusing on their signalling and behavioural differences and exploring the crosstalk between the receptor systems. Moreover, we discuss the potential of OT and AVP receptors as therapeutic targets to treat human disorders, such as autism, schizophrenia and drug abuse.
Keywords: anxiety, crosstalk, GPCR, oxytocin, social behaviour, substance use disorder, vasopressin
1 |. INTRODUCTION
OT and vasopressin (AVP) are endogenous peptides that bind to the OT receptors and the V1A, V1B and V2 receptors respectively (Figure 1b). These peptides can function as hormones when peripherally released or as neuropeptides when released in the CNS (Stoop, 2012). As hormones, OT and AVP are synthesized in the hypothalamus, transported via neuronal axons to the posterior pituitary and released into the bloodstream in response to sexual stimulation and suckling (OT), and dehydration (AVP), thereby regulating parturition/lactation and BP/diuresis, respectively (Gimpl & Fahrenholz, 2001; Koshimizu et al., 2012). As neuropeptides, OT and AVP are involved in the regulation of behaviours, such as anxiety, social bonding, stress, aggression and reward (Albers, 2012; Gimpl & Fahrenholz, 2001). Initial studies suggested that OT plays a role in reproduction and social bonding, and AVP modulates aggressive behaviours (Song & Albers, 2018). This simplistic assumption has been challenged through the years, most recently by studies showing that under specific conditions, OT can enhance social fear (Guzmán et al., 2013), whereas AVP can promote bonding to a sexual partner (Winslow et al., 1993). Therefore, the exact roles of OT and AVP in regulating social behaviours need to be further characterized.
FIGURE 1.
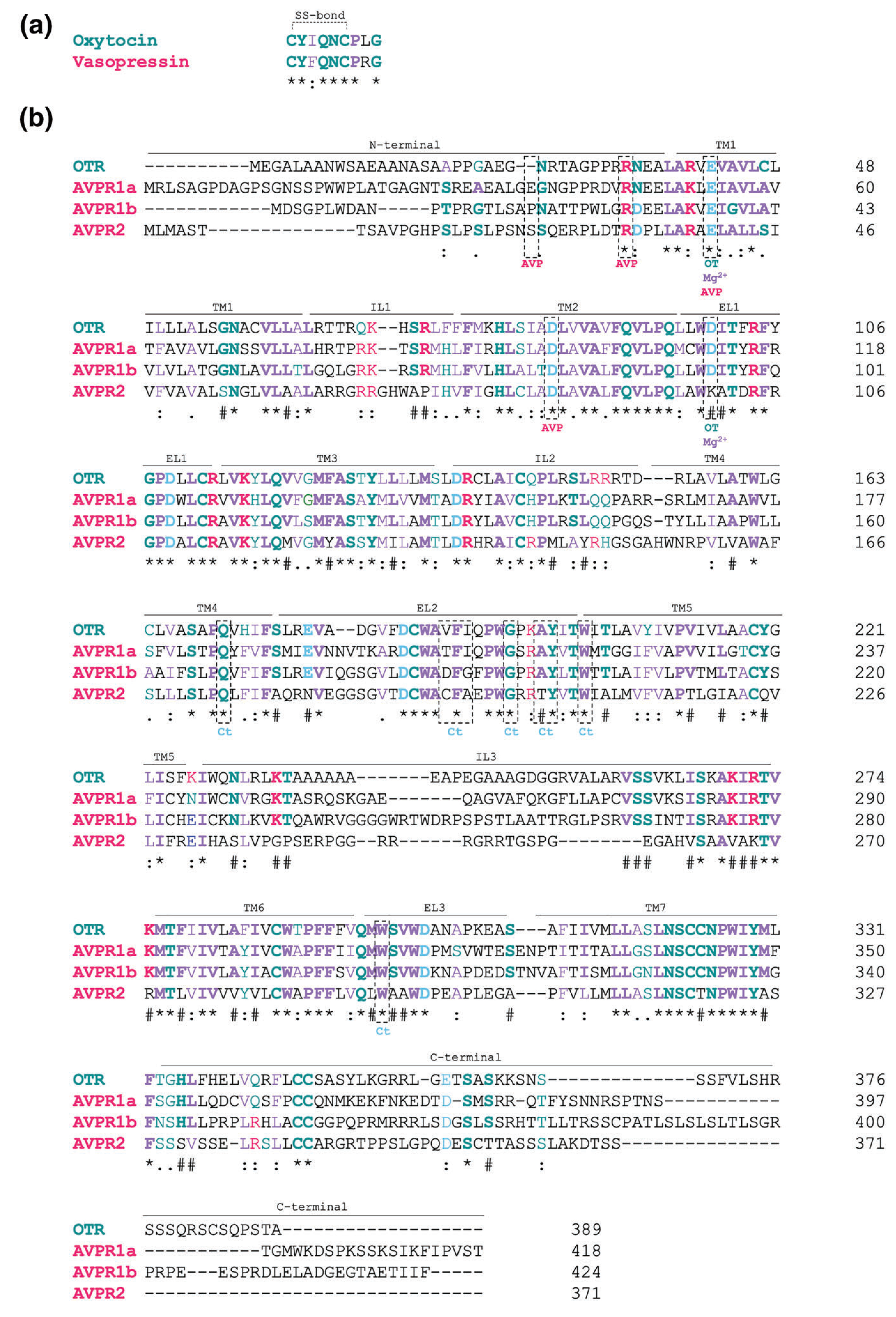
Amino acid sequences of human oxytocin and vasopressin neuropeptides (a) and their receptors (b). (a) Primary sequence of oxytocin and vasopressin in single letter amino acid code. (b) Primary sequence of human OT receptors (OTR), and V1A (AVPR1a), V1B (AVPR1b) and V2 (AVPR2) receptors. The amino acid residues that have been described to interact with the endogenous ligands vasopressin (AVP) and oxytocin (OT), as well as the co-factors Mg2+ and cholesterol (Ct) are highlighted by the dashed rectangles. Amino acids in bold represent residues that are conserved in the OT, V1A, V1B and V2 receptors. * marks residues that are conserved among all the receptors. # marks residues that are conserved among OT, V1A and V1B receptors.: marks residues that have same functional groups. Blue represents amino acids with negative charged side chain; magenta represents residues with positive charge; green represents residues with polar uncharged side chain; purple represents residues with hydrophobic side chain. OTR, oxytocin receptor; V1A, V1B and V2, subtypes of vasopressin receptors; TM, transmembrane; EL, extracellular loop; IL, intracellular loop. Sequence obtained from uniport.org
In this review, we focus on the roles of OT and AVP in the CNS. More specifically, we explore signalling by these neuropeptides at OT and AVP receptors, including potential crosstalk between these receptor systems and behavioural outcomes. Lastly, we provide an overall analysis of the pharmacological potential of these neuropeptides in the treatment of disorders such as autism, schizophrenia and substance use disorder (SUD).
2 |. OT AND AVP PEPTIDES: SYNTHESIS AND RELEASE
OT and AVP are nine-amino acid cyclic amidated peptides. They differ in only two amino acid residues, one at the third position and the other at the eighth position (Figure 1a). Both OT and AVP contain cysteine residues at positions 1 and 6 that form a disulphide bridge, leading to a cyclic secondary structure (Stoop, 2012). In mammals, at the third position, OT contains isoleucine whereas AVP contains phenylalanine, whereas at the eighth position OT contains leucine whereas AVP contains arginine.
OT and AVP are synthesized in the cell body of the hypothalamic-hypophyseal tract, specifically in magnocellular and parvocellular neurons of the paraventricular nucleus (PVN) and supraoptic nucleus (SON) (Stoop, 2012). OT and AVP synthesis in the accessory magnocellular neurosecretory nuclei has also been reported (Stoop, 2012). In addition, AVP synthesis has been described in the central amygdala (CeA), suprachiasmatic nucleus and bed nucleus of stria terminalis (BSNT) (Veenema & Neumann, 2008). Usually, magnocellular neurons express mRNA for both OT and AVP, although specific magnocellular neuronal populations express mRNA for either OT or AVP (Brown, 2016). Because only AVP secretion in magnocellular cells increases in rats subjected to water restriction stress (da Silva et al., 2015), this would suggest that OT and AVP synthesis can be independent from each other in these neurons (for a review, see Brown, 2016).
OT and AVP are released from PVN axon terminals to different brain regions via direct projections (Caldwell et al., 2008; Chini et al., 2017). Axonal projections to the forebrain have been described in rats, with branches projecting to the amygdala, BNST, hippocampus, lateral septum, nucleus accumbens (NAc) and spinal cord (Stoop, 2012). Besides PVN axonal terminals, OT and AVP can be released from different neuronal sites, such as dendrites, in a process known as volumetric transmission (Chini et al., 2017). This form of transmission allows the peptides to have local effects and reach other brain regions via the cerebral spinal fluid (CSF).
3 |. OT AND AVP RECEPTORS
OT and AVP bind and activate members of the class A family of GPCRs (Stoop, 2012). To date, one receptor for OT and three AVP receptors, V1A, V1B and V2, have been described (Stoop, 2012). The OT receptor and the V1A and V1B receptors are widely expressed in the brain, whereas V2 receptors are mostly found in the peripheral system. Considering our interest in exploring the role of OT and AVP in the CNS, this review will not discuss the V2 receptors any further.
3.1 |. Brain distribution
In the brain, the OT, V1A and V1B receptors are present in regions implicated in anxiety, social behaviour, memory, reward, motivation and stress (Albers, 2012; Gimpl & Fahrenholz, 2001).
In rats and mice, OT receptors are highly expressed in the olfactory bulb (OB), hypothalamus, PVN, CeA, BNST, piriform cortex and lateral septum. Medium expression of OT receptors is found in the basolateral amygdala, hippocampus and ventral tegmental area (VTA). Low expression is observed in NAc and striatum (Jurek & Neumann, 2018). In humans, recent findings described high levels of OT receptor mRNA in the caudate putamen, pallidum, thalamus, olfactory regions, hippocampus and amygdala (Quintana et al., 2019). OT receptor binding in the substantia nigra, piriform and cingulate cortex has also been reported (Gimpl & Fahrenholz, 2001; Jurek & Neumann, 2018).
In rats, the V1A receptor is highly expressed in the olfactory bulb, hypothalamus, hippocampus, lateral septum, suprachiasmatic nucleus, NAc, PVN, lateral habenula, amygdala, VTA, substancia nigra, inferior olive and dorsal raphe. It is moderately expressed in the spinal cord (Caldwell et al., 2008). In contrast, the V1B receptors are highly expressed in anterior pituitary corticotrophs in rats, mice and humans. Lower expression in the piriform cortex, septum, NAc, hippocampus, PVN, SCN and cerebellum has also been reported in rats (Caldwell et al., 2008).
In humans, AVP binding sites were described to be high in the lateral septum, thalamus and BNST and low in dentate gyrus and amygdala (Loup et al., 1991). However, these studies were performed using low affinity ligands, and therefore, other brain regions could contain AVP receptors, despite not being detected in these studies.
The distribution of receptors for OT and AVP across the CNS can impart some information about their potential physiological role. For instance, the distribution of V1B receptors indicates a role in regulation of the activation of the hypothalamic–pituitary–adrenal (HPA) axis (Koshimizu et al., 2012) (described below under Section 5.1.1), whereas expression of V1A and OT receptors in the VTA and NAc indicate modulation of rewarding behaviours.
3.2 |. Receptor structural characteristics
OT and V1 receptors across different species exhibit a 40%–85% sequence similarity (Chini & Manning, 2007). In humans, these receptors have 102 invariant amino acids among 371–424 amino acids, with the highest sequence similarity seen in the transmembrane regions and the extracellular loops (Figure 1b). OT and V1 receptors contain a 35–52 aa long extracellular N-terminal tail, followed by seven transmembrane domains (TM1-TM7), and a 43–83aa long intracellular C-terminal region (Barberis et al., 1998) (Figure 1b).
OT and AVP bind their receptors by interaction with the N-terminal domain, the extracellular loops and the transmembrane domains TM1 and TM2 (Hawtin et al., 2005; Wootten et al., 2011). More specifically, Glu42 in OTR and Glu37, Arg46, Glu54 and Asp95 in V1A receptors are found to play a critical role in OT and AVP binding, respectively (Hawtin et al., 2005; Waltenspühl et al., 2020). Sequence alignment of OT and V1 receptors across species shows that these amino acid residues are highly conserved (Waltenspühl et al., 2020). In addition, studies show that Tyr115 in V1A receptors, located in extracellular loop 2 (ECL2), is required for high affinity binding to AVP (Chini et al., 1995). This residue does not appear to be conserved in OT receptors (Figure 1b). In the OT receptor, Glu42 is critical for high-affinity binding of agonists (full and partial) but not required for antagonist binding (peptide and non-peptide) (Wootten et al., 2011). In addition, studies show that mutation of Tyr200 and Trp203 in the ECL2 of OT receptors reduces antagonist and agonist binding (Waltenspühl et al., 2020). Interestingly, these residues are conserved between OT and V1 receptors (Figure 1b).
Like other GPCRs, the activity of OT receptors is modulated by co-factors. Among them, ions and cholesterol play an important role by controlling thermostability and acting as allosteric modulators of these receptors. Thus, Na+ acts as a negative allosteric modulator by stabilizing the inactive receptor conformation (Katritch et al., 2014; Schiffmann & Gimpl, 2018), whereas Mg2+ acts as a positive allosteric modulator by favouring high-affinity binding of OT to its receptor (Waltenspühl et al., 2020). Finally, cholesterol acts as a co-factor by binding to hydrophobic residues in TM4 and TM5 of OT receptors; this shifts the receptor towards a high affinity state for agonist/antagonist binding (Waltenspühl et al., 2020). Understanding the differences between agonist and antagonist binding sites and their modulation by co-factors is fundamental to a rational drug design for the treatment of diseases involving the OT and V1 receptor systems.
4 |. MECHANISMS OF SIGNALLING BY OT AND V1 RECEPTORS AND MOLECULAR CROSSTALK
Studies show that OT and V1A receptors activate Gq/11 and Gi/o-mediated signalling pathways (Chini et al., 2017; Thibonnier et al., 1998), whereas V1B receptors activate Gs-, Gq- and Gi/o-mediated signalling (Thibonnier et al., 1998). For the OT receptor, studies by Busnelli and collaborators (2012) showed that activation of different G protein-mediated signalling pathways depends on the concentration of OT, lower concentrations facilitate Gq, whereas higher concentrations facilitate Gi/o signalling. For V1B receptors, Thibonnier and collaborators (1998) showed that receptor density influences the type of G protein it couples to, with low receptor density promoting coupling to Gq alone (or in combination with Gi), and high receptor density promoting additional coupling to Gs protein. Finally, the subs type of G protein coupling to the receptor appears to depend on the ligand used in the study. Atosiban, classically used as an OT receptor antagonist, was a competitive antagonist of OT receptor-Gq-mediated signalling and a selective agonist of the OT receptor-Gi pathway (Chini & Manning, 2007). Thus, the observed antagonistic effects could be mediated via blockade of Gq-mediated signalling or activation Gi signalling pathways.
OT and V1 receptors are internalized via a clathrin-dependent mechanism involving GPCR kinase (GRK)-mediated receptor phosphorylation followed by β-arrestin recruitment (Busnelli et al., 2012). However, the OT and V1 receptors exhibit differences in dynamics of receptor endocytosis, recycling, and degradation. OT receptors activated by OT, stably bind to β-arrestin, and are either slowly recycled back to cell surface (up to 4 h) or degraded in lysosomes (Chini et al., 2017). V1A receptors activated by AVP rapidly dissociates from bound β-arrestin, allowing the receptor to rapidly return to the cell membrane (Bowen-Pidgeon et al., 2001).
Differences in G-protein activation and internalization and recycling dynamics could be partly responsible for the differences in behavioural outcome discussed in the next section.
4.1 |. Molecular crosstalk and implications for future studies
The high sequence similarity between OT and AVP and their respective receptors raises the possibility of a crosstalk between both systems, in which AVP could bind to and activate OT receptors, and OT could bind to and activate V1 receptors, which led to the proposal that these peptides function as one ‘neurophyseal system’ (Young & Flanagan-Cato, 2012). Support for this comes from in vitro studies by Busnelli and collaborators (Busnelli et al., 2013) (in COS7 cells expressing mouse OT and V1 receptors) showing that AVP binds to OT and V1 receptors with similar affinity (Ki in the low nanomolar range) whereas OT binds to OT receptors with a higher affinity (Ki ~0.9 nM) compared with V1A (Ki ~20 nM) and V1B receptors (Ki ~35 nM) (Table 1). The difference in the binding affinity of OT for OT and V1 receptors could be due the presence of Leu8 in OT instead of the Arg8 in AVP (Figure 1b), given that Arg8 is a determinant for agonist binding selectivity (Chini et al., 1995). Taken together, the sequence similarity between OT and AVP, the amino acid sequence conservation between their receptors, and the binding of OT to V1 receptors and AVP to OT receptors allows for signalling crosstalk, thereby enhancing the repertoire of signalling by the OT/AVP systems.
TABLE 1.
Affinity of oxytocin, AVP and synthetic analogues for OT, V1A, V1B and mutant V1A receptors in rats, mice and humans
| Affinity of AVP and OT to V1A, V1B, OT and mutant V1A receptors (Ki, nM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat | Mouse | Human | Mutant V1A receptorse | ||||||||
|
|
|||||||||||
| Ligands | rV1A | rV1B | rOT | mV1A | mV1B | mOT | hV1A | hV1B | hOT | rV1A Y115F | hV1A Y115D |
| AVP | 1.54 ± 97a | 0.29 ± 0.05a | 1.17 ± 0.11a | 1.11 ± 27c | 0.43 ± 12c | 0.87 ± 8c | 1.73 ± 0.08d | 1.10 ± 0.05d | 1.65 ± 0.49e | 1.04 ± 0.25 | 2.2 ± 0.99 |
| OT | 71b | 294b | 1.0b | 20.38 ± 26c | 36.32 ± 7c | 0.83 ± 8c | 64 ± 12d | 1782 ± 79d | 0.79 ± 0.22e | 45 ± 13 | 839 ± 28 |
| [Thr4,Gly7]OTb | >10,000 | 8000 | 0.8 | n.d | n.d | n.d | 305 | >10,000 | 6.6 | n.d | n.d |
| d[Cha4]AVPb | 2297 | 1.4 | 1430 | n.d | n.d | n.d | 151 | 1.2 | 240 | n.d | n.d |
| d[Leu4,Lys8]VPb | 3786 | 0.16 | 64 | 1933 | 1.4 | n.d | 69.3 | 0.51 | 29 | n.d | n.d |
| FE202158b | n.d | n.d | n.d | n.d | n.d | n.d | 4.4 | >5200 | >840 | n.d | n.d |
| F180b | 480 | 750 | n.d | n.d | n.d | n.d | 11.7 | 2100 | 520 | n.d | n.d |
| dLVTf | n.d | n.d | n.d | n.d | n.d | n.d | 35.5 | 71.7 | 3.88 | n.d | n.d |
Abbreviation: n.d, not determined.
Pena et al. (2007; at-T20).
Manning et al. (2012; mixed cell lines).
Busnelli et al. (2013; COS7).
Thibonnier et al. (1997; CHO).
Chini et al. (1995; COS7).
Although binding studies suggest crosstalk between the OT and V1 receptor systems, carefully conducted signalling assays are needed in order to delineate the mechanisms involved in OT-AVP crosstalk. Such studies would compare the signalling pathways activated by OT and AVP in cells expressing OT and V1 receptors, in order to establish whether each peptide exhibits biased signalling at individual receptors. Moreover, examination of signalling in cells co-expressing both receptors in the absence or presence or receptor-selective antagonists would help establish pathways involved in signalling crosstalk. The latter studies could be facilitated by using highly receptor selective ligands (Manning et al., 2012) (Table 1). Given the potential therapeutic use of OT and V1 receptor ligands, signalling by agonists that are specific for the human receptor should be examined. Some of these ligands and their affinities for hOT receptors and hV1 receptors are described in Table 1.
Behavioural studies showing crosstalk between the OT and AVP systems have been described in a review by Song and Albers (2018). We discuss some of these studies using specific OT and V1A receptor antagonists in Section 5.1. Another approach to examine crosstalk between OT and AVP is to use genetically modified animals (see Song & Albers, 2018). An example is OT receptor knockout mice which have deficits in social exploration and recognition and exhibit increased aggressiveness. These behaviours are restored to wild-type levels upon intracerebroventricular (i.c.v.) administration of AVP and this can be blocked by a selective V1A receptor antagonist. Although genetically modified animals can provide relevant information on the ability of OT and AVP to activate each other's receptors, care has to be used in data interpretation because genetic manipulation could result in crosstalk under situations that do not mimic what happens normally in individuals, particularly due to the fact that crosstalk appears to be dependent on fiber density, receptor availability and peptide concentration (Song & Albers, 2018 and Smith et al., 2019).
As described in Section 5.2, OT and AVP could potentially be used as therapeutic agents for the treatment of these conditions. However, not much is known how crosstalk between these peptide-receptor systems could affect their involvement in these disorders or their therapeutic potential. Studies using selective ligands, agonists, and antagonists to OT and V1 receptors could help elucidate this further.
5 |. BEHAVIOURS INVOLVING OT AND AVP IN RODENTS
5.1 |. OT and AVP in social and anxiety related behaviours
OT and AVP have been implicated in the modulation of numerous behaviours related to sociability, trust, mating, aggressiveness and territory marking (Albers, 2012; Gimpl & Fahrenholz, 2001). Here, we present studies that suggest that (i) OT acts as an anxiolytic and bond-promoting neuropeptide and AVP acts as a stress inducer and modulator of aggressive behaviour (Table 2); (ii) as well as recent findings that challenge the preconceived idea that OT is purely a pro social and anti-stress peptide and that both neuropeptides play largely opposite roles in social and anxiety-related behaviours (Albers, 2012; Bartz et al., 2011).
TABLE 2.
Summary of the behavioural outcomes from classical oxytocin and vasopressin studies and in SUD
| OT- and AVP- related behaviors in rodents | |||||||
|---|---|---|---|---|---|---|---|
| Behaviour | Treatment | Route of administration | Test | Result | Behaviour | Specie/sex | Reference |
| Stress and anxiety | OT | i.c.v. | EPM | (−) Anxiety | (+) Time in the open arms | Sprague–Dawley rats/female | Windle et al., 1997 |
| OT | i.c.v. (high doses) | Chronic psychosocial stress | (+) Anxiety | (−) Time in the open arms and (−) time in the light side | C57BL/6 mice/male | Peters et al., 2014 | |
| OT | i.p. (chronic) | Isolation stress | (−) Corticosterone plasma - RIA | Praire voles/female | Stevenson et al., 2019 | ||
| OT | i.c.v. (acute) | EPM, light/dark box | (−) Anxiety | (+) Time in the open arms and (+) time in the light side | Wistar rats/female and male | Winter et al., 2020 | |
| OT | i.c.v. (chronic) (high dose) | Light/dark box | (+) Anxiety | (−) Time in the light side | Wistar rats/female and male | Winter et at, 2020 | |
| OT (mPFC) | Infusion | EPM | (−) Anxiety | (+) Time in the open arms | Sprague-Dawley rats/male | Sabihi et al., 2017 | |
| OT (CeA) | Local blue light | Fear conditioning | (−) Anxiety | (−) Freezing | Rats | Knobloch et al., 2012 | |
| OT (CeA) | Infusion | EZMT | (−) Anxiety | (+) Time in the open quadrant | C57/BL6 mice/male | Han et al., 2018 | |
| OT receptor antagonist (LS) | Retrodialysis (acute) | Elevated platform | (+) ACTH release - RIA | Rats/male | Neumann et al., 2000 (reviewed by) | ||
| OT receptor antagonist (LS) | Retrodialysis (acute) | FST | No effect on ACTH release versus CT | Rats/male | Neumann et al., 2000 (reviewed by) | ||
| AVP | i.c.v. (acute) | Steriotypic behaviour | (+) Scratching and grooming | C57/BL6 mice/male | Bielsky et al., 2004 | ||
| V1 receptor antagonist | Inverse microdialysis | EPM | (−) Anxiety | (+) Time in the open arms | Wistar rats/male | Liebsch et al., 1996 | |
| V1A receptor antagonist (Hyp) | Infusion (acute) | Intruder attack | (−) Aggression | (−) Biting attacks on the intruder | Hamster/male | Ferris & Potegal, 1988 | |
| V1A receptor antagonist (Amy) | Microinfusion (acute) | EPM | (−) Anxiety | (+) Time in the open arms | Wistar rats/male | Hernández et al., 2016 | |
| V1A receptor antagonist (Amy) | Inverse microdialysis | Forced swim | (−) Anxiety | (−) Floating time | Wistar rats/male | Ebner et al., 2002 | |
| V1A/B receptor antagonist | i.v (jugular venous catheter) | Dexamethasone/corticotropin-releasing hormone test | (−) HPA activation | (−) ACTH relase mediated by CRF (commercial kit) plasma | Wistar rats/female and male | Keck et al., 2002 | |
| V1B receptor antagonist | Oral | EPM, light/dark box | (−) Anxiety | (+) Time in the open arms and (+) time in the light side | Wistar rats male/BALB/c male | Griebel et al., 2002 | |
| V1B receptor antagonist | s.c cannula | Restraint stress and LPS | (−) ACTH plasma (IRMA) | Sprague-Dawley rats/male | Spiga et al., 2009 | ||
| AVP KO | Social defeat and restraint stress | (−) ACTH and corticosterone plasma (RIA) | Brattleboro rats/male | Zelena et al., 2009 | |||
| AVP KO | Ether or social avoidance | No effect on ACTH and corticosterone plasma versus CT (RIA) | Brattleboro rats/male | Zelena et al., 2009 | |||
| AVP KO | Foot shock | (−) ACTH and corticosterone plasma (RIA) | Brattleboro rats/male | Zelena et al., 2009 | |||
| V1A receptor KO | EPM, light/dark box, OF | (−) Anxiety | (+) Time in the open arms; (+) time in the light side; (+) time in the centre | C57/BL6 mice/male | Bielsky et al., 2004 | ||
| V1B receptor KO | LPS stress (acute) | (−) ACTH and corticosterone plasma (RIA) | C57BL/6J and 129·1/SvJ mix mice/male and female | Lolait et al., 2007 | |||
| Social interaction | OT (OB) | Infusion (acute) | Social discrimination paradigm | (+) Social memory | (+) Time spent with novel versus familiar counterpart | Wistar rats/male | Dluzen et al., 2000 |
| OT and AVP (OT receptor) | Social CPP | (+) Social reward | (+) Time spent on the side paired with animal versus empty | Syrian hamster/male | Song & Albers, 2018 (reviewed by) | ||
| OT receptor antagonist (OB) | Infusion (acute) | Social discrimination paradigm | (−) Social memory | Same time spent with familiar versus novel animal | Wistar rats/male | Dluzen et al., 2000 | |
| OT receptor antagonist | Intranasal | Social interaction test (after social defeat) | (+) Social approach | (+) Time in the interaction zone and exploration of animal | California mice/female | Duque-Wilckens et al., 2018 | |
| OT receptor knockdown (LS) | Acute social defeat + contextual fear conditioning | (−) Fear | (−) Freezing | C57BL/6 N mice/male | Guzmán et al., 2013 | ||
| OT receptor KO | Socila defeat | (−) Social stress | (−) Defeat posture | C57BL/6J mice/male | Nasanbuyan et al., 2018 | ||
| V1A receptor KO | Social recognition | (−) Social memory | (+) Time spent with familiar female | C57/BL6 mice/male | Bielsky et al., 2004 | ||
| Pair bonding | OT receptor antagonist | i.c.v. | Three chamber preference test | (−) Pair bonding | (−) Time spent with partner versus stranger | Praire voles/female | Insel & Hulihan, 1995 |
| V1 receptor antagonist | i.c.v. (acute) | Three chamber preference test; resident-intruder paradigm | (−) Pair bonding and aggression | (−) Attacks on the intruder (−) time spent with partner versus stranger | Praire voles/male | Winslow et al., 1993 | |
| V1A receptor down-regulation (VP) | RNA interference | Three chamber preference test | (−) Pair bonding | (−) Time spent with partner versus stranger | Praire voles/male | Barrett et al., 2013 | |
| V1A receptor overexpression | Viral vector | Three chamber preference test | (+) Pair bonding | (+) Time spent with partner | Meadow voles/male | Lim et al., 2004 | |
| Drug abuse | |||||||
| Cocaine | OT | s.c.(acute) | OF | (+) Behavioural sensitization | (+) Locomotor activity | CFPL mice of an inbred strain/male | Sarnyai et al., 1992 |
| OT | s.c. | OT + cocaine administration | (−) DA efflux (spectrofluorimetric assay) | CFLP albino mice/male | Kovács et al., 1990 | ||
| AVP | s.c.(acute) | OF | (−) Behavioural sensitization | Male CFPL mice of an inbred strain | Sarnyai et al., 1992 | ||
| AVP | s.c.(acute) | Cocaine-induced sniffing behaviour | (+) Stereotypic behaviour | Wistar rats/male | Sarnyai et al., 1991 | ||
| AVP | s.c.(acute) | Intravenous self-administration | (−) Self-administration | Wistar rats/male | Van Ree et al., 1988 | ||
| Opioids | CBT (OT analogue) | i.p. | Morphine- CPP | (−) Reinstatement induced by stress | C57/BL6 mice/male | Zanos et al., 2014 | |
| AVP | s.c.(acute) | Heroine - intravenous self-administration | (−) Self-administration | Wistar rats/male | Van Ree et al., 1988 | ||
| V1B receptor antagonist/V1B receptor KO | Morphine - hotplate anf tail flick tests | (+) Tolerance | Hybrid 129/Sv and C57BL/6 background/male | Koshimizu et al., 2018 | |||
| Alcohol | OT | i.p. | DID paradigm | (−) Self-administration | C57BL/6J mice/male | King et al., 2017 | |
| OT | i.c.v. (acute) | Alcohol injection - microdialysis; two-bottle choice | (−) Dopamine release - Nac; alcohol consumption | Wistar rats/male | Peters et al., 2017 | ||
| OT | i.c.v. (acute) | Chronic intermittent alcohol vapour exposure | (−) Self-administration and cue-induced reisntatement (dependent animals) | Wistar rats/male | Hansson et al., 2018 | ||
| OT | i.p., i.c.v., intranasal | Operant self-administration | (−) Self-administration (dependent animals) | Wistar rats/male | Tunstall et al., 2019 | ||
| CBT (OT analogue) | i.p. (chronic) | CPP | (−) Acquisition; (+) extinction | C57BL/6 mice/male | Bahi, 2015 | ||
| CBT (OT analogue) | i.p. (chronic) | CPP | (+) Preference | Swiss mice/male | Rae et al., 2018 | ||
| OT receptor KO | Two-bottle choice | (+) Consumption (in females); (=) consumption (male) - pre and post stress | C57BL/6J-backcrossed/male and female | Rodriguez et al., 2020 | |||
| AVP | i.c.v. (acute) | Two-bottle choice | (+) Self-administration | Brattleboro diabetes insipidus rats/male | Rigter & Crabbe, 1985 | ||
| AVP | Two-bottle choice | (+) Consumption/preference (correlation with AVP levels) | C57BL/6 mice/male | Nelson et al., 2018 | |||
| V1B receptor antagonist | i.p. (acute) | Operant self-administration and ethanol vapour chamber | (−) Self-administration (dependent animals) | Wistar rats/male | Edwards et al., 2012 | ||
| V1B receptor antagonist- | i.p. (acute) | Two-bottle choice | (−) Alcohol intake | Sardinian alcohol-preferring rats/male | Zhou et al., 2011 | ||
| AVP (via V1B receptors) (CeA) | Microinjections | Social interaction test | (+) Anxiety in animals subjected to intermitent alcohol consumption | (−) Interaction with conspecific | Sprague-Dawley rats/male | Harper et al., 2019 | |
| V1A receptor heterozygous | Two-bottle choice | (+) Alcohol consumption | C57BL/6JX 129/SvJ | Caldwell et al., 2006 | |||
| V1A receptor KO | Two-bottle choice | (+) Alcohol consumption and preference | C57BL/6Cr Sic mice | Sanbe et al., 2008 | |||
| Methamphetamine | OT | i.p. | Nose poke | (−) Self-administration and reinstatement | Sprague-Dawley rats/male and female | Everett et al., 2021 | |
Note: Symbols indicate (+) increase, (−) decrease or (=) no change in the tested behaviour.
Abbreviations: AVP, vasopressin; V1A and V1B receptors, AVP receptor subtypes; CBT, carbetocin; CPP, conditioned place preference; CT, control; DID, drinking in the dark; EPM, elevated plus maze; EZMT, elevated zero maze test; i.c.v., intracerebroventricular; i.p., intraperitoneal; KO, knockout; OF, open field; OT, oxytocin.
5.1.1 |. Stress and anxiety
Stress response is mainly mediated by the HPA axis, via corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH) and glucocorticoid release (corticosterone, in rodents, or cortisol, in humans) (Koshimizu et al., 2012). Brain regions such as the amygdala and medial prefrontal cortex (mPFC) have also been implicated in stress regulation (Sabihi et al., 2017).
OT is known as an anti-stress neuropeptide, particularly due to its effects on HPA axis activation. For example, adrenalectomization of rats results in blunted OT release (measured by microdialysis) from the PVN in response to forced swim test; normal release was restored following an intravenous infusion of corticosterone (Torner et al, 2016). On the other hand, central administration of OT to female rats reduced plasma corticosterone release after noise stress and increased the time spent in the open arms of the elevated plus maze (EPM) (Windle et al., 1997). In addition, OT can regulate CRF release by modulating GABA and suppressing the response of CRF neurons (for a detailed review, see Winter & Jurek, 2019). Interestingly, isolated animals that received OT showed lower corticosterone levels compared with group-housed controls (Stevenson et al., 2019), suggesting a modulatory effect of OT on HPA axis activity.
OT can also reduce amygdalar activity, particularly via GABA modulation. OT axonal projections from the hypothalamus to the amygdala have been described, and its release in this brain region decreased freezing response in rats exposed to fear-conditioning (Knobloch et al., 2012). Also, social isolation of male mice elicits anxiety-related behaviours by decreasing GABAergic transmission induced by OT in the CeA, an effect reversed by OT administration in this brain region, but not by i.c.v peptide administration (Han et al., 2018). In addition, OT can modulate the activity of the prelimbic region of mPFC in male rats by increasing activation of GABAergic neurons and ultimately decreasing amygdalar function (Sabihi et al., 2017). These results suggest a molecular mechanism whereby OT regulates amygdalar responses by modulating the GABAergic system in different brain regions, culminating in anti-stress responses.
A potential explanation for the anxiety modulatory effects of OT could be related to induced transcriptional changes. For instance, acute OT negatively regulates transcription of the crf gene (Jurek et al., 2015). On the other hand, chronic OT treatment increases phosphorylation of myocyte enhancing factor 2A (MEF2A), a transcription factor that induces alternative splicing of the crf2a gene, leading to the formation of a soluble form sCRF2α (Winter et al., 2020). Indeed, studies have shown a positive correlation between sCRF2α and anxiety-like behaviour (Jurek & Meyer, 2020). These differences need to be further investigated when considering OT as a potential therapy for stress related disorders.
The nature of the stressor, physical or psychological, produces different OT-mediated responses. For instance, administration of an OT receptor antagonist in the lateral septum of rats followed by exposure to the elevated plate test (a type of psychological stress) increased plasma ACTH levels, suggesting that OT in this brain region is involved with blunted HPA axis response. On the other hand, exposure of rats to forced swim test (a combination of psychological and physical stress) increased ACTH levels despite the presence of OT receptor antagonists (see Neumann et al., 2000). Taken together, these results point to the complexity of anxiety regulation by OT, including involvement of different brain circuits and pathways, dose and duration of treatment. This can affect the potential of OT as a pharmacotherapeutic agent for anxiety-related disorders.
AVP is known to potentiate the HPA axis response to stress (Stevenson & Caldwell, 2012). Under stressful conditions, AVP is co-released with CRF in the hypothalamus potentiating its actions (Koshimizu et al., 2012). Studies with HAB (high anxiety-related behaviour) mice showed that an V1A/B receptor antagonist reduced HPA hyperactivation, suggesting the involvement of V1 receptors in anxiety responses (Keck et al., 2002). This is supported by studies showing that V1 receptor antagonists decreased anxiety-like behaviour in the EPM test (Liebsch et al., 1996). Conversely, V1A receptor-knockout animals display significantly less anxiety-like behaviour in the EPM, light/dark box and open field tests (Bielsky et al., 2004). In addition, acute i.c.v administration of AVP causes increased scratching and grooming (behaviours related with stress and anxiety) in wild type but not in V1A receptor-knockout mice (Bielsky et al., 2004).
The amygdala appears to play a key role in the effects of AVP on stress, via V1A receptors. Thus, increased activity of AVP magnocellular neurons leads to higher anxiety-like behaviour in the EPM test that is blocked by administration of a V1A receptor antagonist into the CeA (Hernández et al., 2016). Moreover, a 10 min exposure of rats to the forced swim test increased AVP release in the amygdala (measured by microdialysis followed by RIA), that was blocked by administration of a V1A receptor antagonist (Ebner et al., 2002).
Regarding V1B receptors, knockout mice showed decreased ACTH and corticosterone levels following acute stress, compared with wild type (Lolait et al., 2007). Furthermore, animals treated with V1B receptor antagonist had anxiolytic effects in the EPM and light/dark box tests (Griebel et al., 2002). This suggests that V1B receptors are necessary to produce proper HPA axis response to acute stress through mechanisms involving stimulatory action of AVP on corticotropes.
The magnitude of the effect of AVP on stress response is stressor dependent. A study showed that acute treatment with V1B receptor antagonists decreased ACTH response to restraint and LPS stress but had no effect in response to noise stress (Spiga et al., 2009). Another study showed that AVP deficient rats exposed to social defeat or restraint stress had lower ACTH and corticosterone levels compared with wild types; no differences were seen following exposure to ether inhalation or social avoidance (Zelena et al., 2009). Also, foot shock stress caused increases in ACTH and corticosterone levels despite the presence of AVP. These results suggest that the role of AVP in stress response is dependent on the type of stressor and that HPA axis activation can happen independently from AVP.
Taken together, these studies show that AVP and OT can modulate anxiety and stress, although further studies on the dynamics of this modulation are required, particularly regarding stressor type, brain circuits and peptide levels.
5.1.2 |. Social interaction
Social interaction is evolutionary conserved in species such as mice, primates and humans and is important to promote mating and pair bonding as well as social support and protection (Gimpl & Fahrenholz, 2001). OT and AVP play a fundamental role in this process.
Several studies describe OT as having prosocial properties (reviewed by Gimpl & Fahrenholz, 2001). In rodents, the olfactory system is crucial for social recognition. Administration of OT into the olfactory bulb facilitates social recognition and memory (Dluzen et al., 2000) whereas administration of an OT receptor antagonist leads to an inability to distinguish between familiar and novel counterparts in a social recognition test (Dluzen et al., 2000).
Moreover, Johnson and Young (2015) proposed the following neurobiological mechanism for social approach and bonding based on studies in prairie voles, but not limited to this species: when the first encounter between two or more subjects occurs, OT is responsible for diminishing social anxiety, acting via OT receptors in the amygdala to facilitate social approach and bonding (Figure 2). This is followed by OT-mediated activation of OT receptors in brain regions responsible for recognizing counterparts such as the visual cortex in humans and the olfactory system in rodents, which promotes social memory (Johnson & Young, 2015). If the social contact is rewarding, there is an increase in dopamine release in the NAc and striatum that reinforces the social interaction (Johnson & Young, 2015). As the social interaction becomes stronger or pair bonding occurs, there is a shift from dopamine D2 receptor activation in the ventral striatum to dopamine D1 receptor up-regulation and activation in the dorsal striatum, which could encode sensory information from unfamiliar counterparts as aversive, contributing to increased aggressive behaviours. Supporting this is the observation that oxytocinergic and dopaminergic fibres are often located in the same brain regions, such as amygdala, PVN, NAc and VTA (Baskerville & Douglas, 2010). Moreover, studies show that in the PVN, OT neurons contain dopamine receptors and vice-versa (Baskerville & Douglas, 2010), which would enable OT and dopamine control over behaviours such as social bonding.
FIGURE 2.
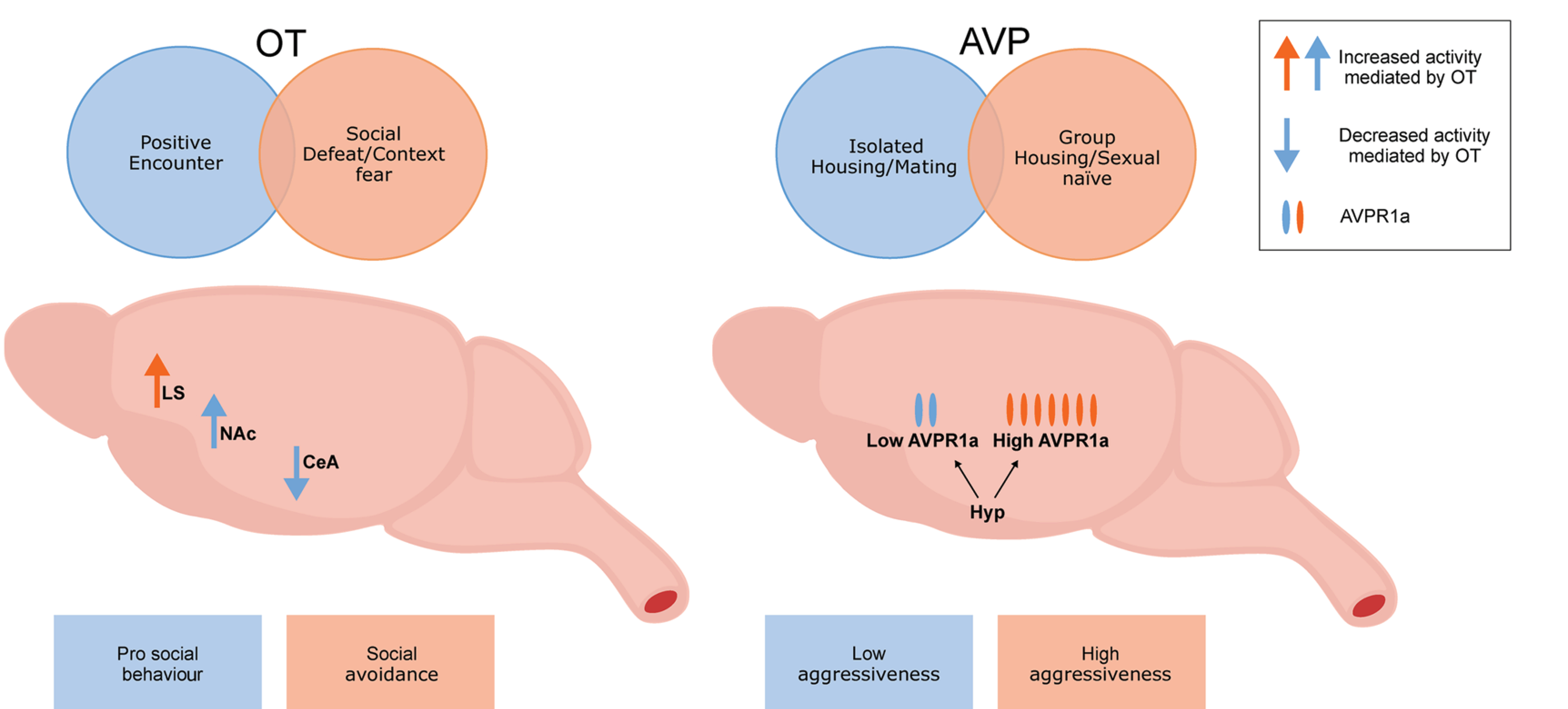
Schematic representation of context-dependent modulations of oxytocin and vasopressin. Oxytocin can act on different brain regions depending on the social context, leading to different behavioural outcomes. Expression of V1A receptors (AVPR1a) is modulated by housing or sexual experience, which increases or decreases aggression. CeA, central amygdala; Hyp, hypothalamus; LS, lateral septum; OT, oxytocin; AVP, vasopressin. Figure was generated using information obtained from Albers, 2012; Johnson & Young, 2015; Guzmán et al., 2013
The generalization of OT as a pro-social neuropeptide may be oversimplified due to the complexity of social behaviours, species, sex and the nature of the social encounter being studied. Regarding the nature of the social encounter, mice subjected to acute social defeat followed by contextual fear conditioning exhibited an increase in freezing (a fear-related behaviour) as well as in OT receptor binding in the lateral septum analysed via immunohistochemistry; these effects were blocked by an OT receptor antagonist (Guzmán et al., 2013) (Figure 2). Interestingly, behavioural responses do not seem to be sex specific. For example, socially defeated female rats treated with an OT receptor antagonist exhibited increased social approach behaviour (Duque-Wilckens et al., 2018), and OT receptor knockout male mice subjected to social defeat exhibited less defeat posture compared with wild types (Nasanbuyan et al., 2018). These results attest to the importance of previous social experiences in the outcome of social behaviours, as well as to the role of OT. As proposed by Bartz and collaborators, ‘it is not a question of “does OT improve social cognition?” but rather “under what circumstances does OT improve social cognition?”’(Bartz et al., 2011). Therefore, understanding the context in which animals are tested could help explain and even predict the social behaviour outcome and contribute to the elaboration of studies design.
Studies with AVP have also described its role in social behaviours, such as social memory, mating and bonding (Albers, 2012). Yet AVP became better known for having effects opposite to OT on social behaviour and greater focus has been given to its participation in aggression (Song & Albers, 2018). Administration of an V1A receptor antagonist into the hypothalamus was found to inhibit aggression in male hamsters (Ferris & Potegal, 1988). Interestingly, studies showed that AVP (and OT)-induced social preference and olfactory investigation are blocked by an antagonist to OT receptors but not to V1A receptors (Song & Albers, 2018), and in Syrian hamsters, AVP and OT modulate flank marking (a form of odour marking behaviour) via OT receptors and not via V1A receptor activation (Song et al., 2016). This suggests that crosstalk between both systems can affect social behaviour. Therefore, this should be taken into account when designing experiments and interpreting data, particularly on translational approach to human social disorders.
AVP's effects seem to be context dependent, particularly regarding male aggressive behaviour. For instance, sex naïve males have fewer V1A receptors in the hypothalamus compared with mating-males (Winslow et al., 1993). Also, socially isolated males have higher binding of V1A receptors and are more aggressive than group-housed males (Albers et al., 2006) (Figure 2). Despite being more often linked with aggressive behaviour, AVP seems to be involved in social recognition, because V1A receptor knockout animals present decreased olfactory investigation behaviour and are unable to distinguish a previously known female (Bielsky et al., 2004). Most recently, Song and Albers (2018) described that AVP, like OT, increases the rewarding effects of social interaction, via VTA activation.
Taken together, these studies show that context, peptide and receptor availability can modulate the responses caused by OT and AVP. Therefore, appropriate design and interpretation of social-related studies is crucial for understanding the behavioural outcomes of these neuropeptides. Furthermore, the crosstalk between AVP, OT and their receptors reveals the complexity of these peptide-receptor systems as well as the need for further studies to elucidate how and when this crosstalk occurs.
5.1.3 |. Pair bonding behaviour
OT and AVP play an important role in pair bonding (Veenema & Neumann, 2008). A classic example is the difference in pair bonding behaviour between prairie voles (a well-established monogamic species) and meadow or montane voles (non-monogamic species). Interestingly, sex differences in activation of OT and V1 receptors seem to interfere with pair bonding behaviour. In female prairie voles, i.c.v. administration of an OT receptor antagonist, but not an V1 receptor antagonist, blocked pair bonding, but did not affect mating (Insel & Hulihan, 1995). On the other hand, in males, administration of a V1 receptor antagonist, but not an OT receptor antagonist, decreased pair bonding and aggression, without affecting mating behaviour (Winslow et al., 1993).
Besides evaluating OT and AVP effects, studies have examined OT and V1 receptor levels and brain distribution in prairie voles and meadow voles and shown that the ventral pallidum of male prairie voles exhibits higher expression of V1A receptors compared with meadow voles (for details, see Young et al., 2011). Moreover, male prairie voles with down-regulation of V1A receptors in the ventral pallidum showed impaired female preference (Barrett et al., 2013) whereas overexpression of V1A receptors in the same brain region of meadow voles induces pair bonding behaviour (Lim et al., 2004). In the case of OT receptor expression levels, female prairie voles exhibit higher levels in the NAc, a brain region strongly related to pleasure and reward, and in the mPFC (for more details, see Young et al., 2011). Regarding the NAc, it is possible that OT interacts with dopaminergic neurons to modulate social reward, as dopamine is released from the NAc during social encounters (Liu & Wang, 2003). How this interaction happens to establish monogamy is not entirely understood. Liu and Wang (2003) proposed that OT and dopamine may act on different aspects necessary for partner preference, where OT could be responsible for creating preference and OT would mediate the expression of this behaviour. Similarly, Lim and collaborators (2004) proposed that concomitant activation of V1A receptors and D2 receptors in the ventral forebrain of prairie voles creates an association between partner recognition and reward from the social encounter. This would provide a conditioned response for partner preference.
Interestingly, a study demonstrated that social recognition in prairie voles can be context dependent, as single males can discriminate between male but not female conspecifics and display stronger male discrimination in a familiar territory (own cage) compared with a novel (male conspecific cage) or a neutral one (new cage) (Zheng et al., 2013). Taken together, these studies highlight the importance of OT and AVP and particularly their receptor brain distribution in determining monogamy and social affiliation across different species. Finally, OT is an important peptide in regulating anxiety and therefore facilitating mating in promiscuous species.
5.1.4 |. Drugs of abuse
An interaction between OT and dopamine in the reward system for drugs of abuse has been well established. OT containing neurons send direct projections to dopaminergic brain regions, including NAc and VTA (Baskerville & Douglas, 2010), and OT administration alters dopamine release in limbic structures (Estes et al., 2019). In addition, OT administration blocks cocaine-induced (Kovács et al., 1990) and ethanol-induced (Peters et al., 2017) dopamine efflux within the NAc, in non-dependent animals. However, an elegant study by Tunstall et al. (2019) also revealed a role of the CeA GABAergic system in the effects of OT on motivation for alcohol in dependent rats. These studies suggest that OT acts on different brain systems and circuits to promote its effects on drug motivation in non-dependent and dependent animals. Several animal models have been use to study various aspects of SUD, some of them distinguish alcohol dependent from non-dependent rats (see Spanagel, 2017). Although OT reduces alcohol drinking in mice using different models, such as binge-like drinking, two bottle-choice and operant self-administration procedures (King et al., 2017), cue-induced reinstatement excessive-drinking was blocked only in dependent- but not in non-dependent rats (Hansson et al., 2018). Similarly, carbetocin (an OT analogue and OT receptor agonist) prevented stress-induced reinstatement to morphine (Zanos et al., 2014). Using different routes of administration (intraperitoneal, intranasal and intracerebroventricular), OT blocked the high motivation for alcohol drinking in dependent rats, revealing a central action in reducing ethanol drinking (Tunstall et al., 2019). Unfortunately, most studies do not look at gender as a factor for the effects of OT on reward behaviours, which could reveal important differences. Indeed, a recent study found that female OT receptor knockout mice displayed increased alcohol consumption prior to and after exposure to forced swim test, whereas males showed no change in this behaviour (Rodriguez et al., 2020). Moreover, an up-regulation of OT receptors in frontal and striatal brain regions was found in male alcohol-dependent rats and in post-mortem brain tissue of male alcoholic patient (Hansson et al., 2018) but not in female rats or in alcohol-dependent women (Hansson & Spanagel, 2021). It should be noted that sex, route, duration of treatment, animal strain and most importantly timing of OT administration were found to affect the outcome of OT treatment (see King et al., 2020). For example, conditioned place preference studies have revealed reduced (Bahi, 2015) or enhanced (Rae et al., 2018) rewarding effects of ethanol, depending on the OT treatment regime.
Studies on the effects of AVP on drugs of abuse revealed interesting results, such as reduction in heroin and cocaine self-administration (Van Ree et al., 1988), decreased sensitization to cocaine in a dose-dependent manner (Sarnyai et al., 1992) and delayed morphine tolerance (Koshimizu et al., 2018), despite its tendency to increase cocaine stereotypic behaviour (Sarnyai et al., 1991). Studies have also examined the effects of AVP on alcohol consumption. These studies observed a correlation between high AVP mRNA levels in different brain regions (measured by qPCR) and high alcohol preference/consumption in male mice, although the cause and effect need to be elucidated (Nelson et al., 2018). Interestingly, rats homozygous for diabetes insipidus (di/di), which are vasopressin-deficient, showed reduced ethanol preference but enhanced intake (Rigter & Crabbe, 1985).
Regarding AVP receptors, V1B receptors appear to be associated with excessive alcohol drinking, because antagonism of these receptors reduced self-administration in dependent rats (Edwards et al., 2012) and excessive alcohol drinking in mice (Zhou et al., 2011). These effects could be related with modulation of anxiety-like behaviours by AVP, because activation of V1B receptors increased anxiety-like behaviour in animals exposed to chronic intermittent alcohol consumption (Harper et al., 2019). On the other hand, V1A receptors appear to be related to reduced alcohol use, because studies show that V1A receptor knockout mice (Sanbe et al., 2008) and heterozygous female and male mice (Caldwell et al., 2006) exhibit increased alcohol consumption and preference.
Together, these preclinical studies show that the OT and AVP receptor systems are promising targets in the treatment of drug abuse and addiction.
5.2 |. A potential role for OT and AVP in the treatment of neuropsychological disorders
As pleiotropic neuromodulators with sites of action in numerous brain regions, OT and AVP could potentially be involved in human disorders such as autism, schizophrenia and substance use disorders (King et al., 2020; Meyer-Lindenberg et al., 2011; Sarnyai et al., 1992) and thus be used as pharmacotherapeutics or plasma level biomarkers for these disorders.
To date, it is still not clear if OT and AVP can cross the blood brain barrier (BBB) following peripheral administration. However, studies show that following intranasal administration of AVP or OT increased levels can be detected in the brain of humans (Born et al., 2002; Striepens et al., 2013). Due to positive feedback regulation of OT and AVP, it is possible that even a small increase in the amount of these peptides can induce their release (Mitre et al., 2016) and induce behavioural changes. Therefore, further studies on the minimum dose of peptide needed to cross the BBB to modulate central behavioural responses are needed. In the case of OT, it has been proposed that peripheral OT could induce CNS changes via vagal afferent nerve stimulation; the latter contain OT receptors and project to the nucleus tractus solitarius, a brain region that expresses the same receptors (Iwasaki et al., 2015). In support of this, Everett and collaborators (2021) showed that peripheral OT can block methamphetamine self-administration via vagal nerve stimulation.
With regard to the use of OT and AVP as reliable plasma biomarkers for psychiatric diseases, it is not clear if peripheral peptide levels correspond to central (i.e. CNS) levels. For example, there are reports of increased central AVP release, without change in plasma levels (Wotjak et al., 1998). An important question to be addressed is if differences in plasma levels are the consequence or cause of the studied disorder. This issue gains complexity with the difficulty in measuring peptide level using commercial kits, particularly in regard to sample preparation, and differences observed between extracted and unextracted plasma preparation (Neumann & Landgraf, 2012). These are important aspects to be considered when interpreting results and should be further explored in order to increase our understanding of OT and AVP in disorders and improved therapeutic strategies. Below, we describe studies showing the role of these neuropeptides in neuropsychological disorders, as well as their pharmacotherapeutic potential following intranasal administration (Figure 3).
FIGURE 3.
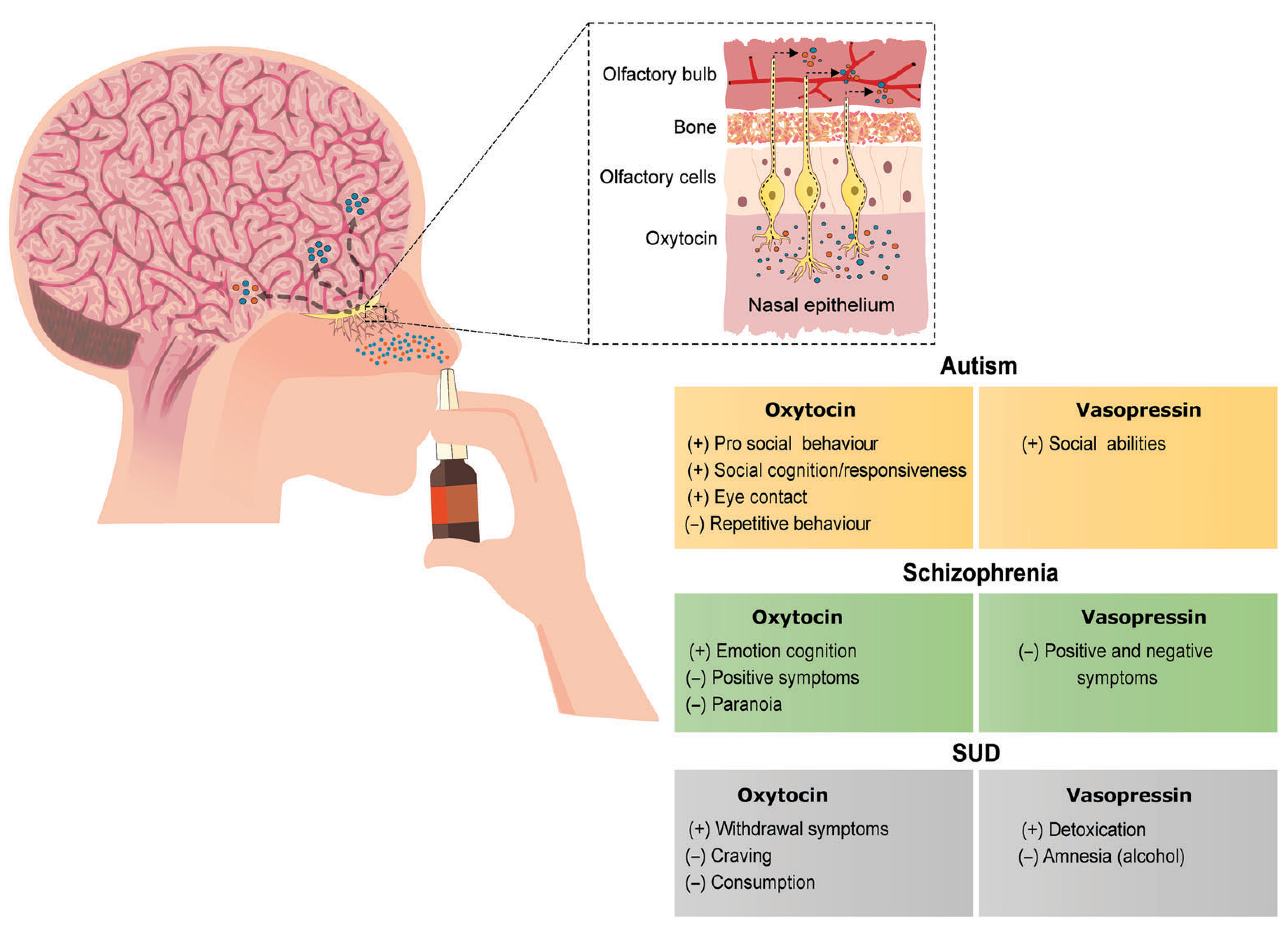
Behaviours modulated by oxytocin and vasopressin and their potential pharmacotherapeutic applications in autism, schizophrenia and SUD. Intranasal manipulation of oxytocinergic and vasopressinergic systems lead to improvement in symptoms observed in these disorders. Symbols indicate (+) increase or (−) decrease in the respective behaviour. SUD, substance use disorder
5.2.1 |. Involvement of OT and AVP in social behaviour
Preservation of social relationships is fundamental for species conservation and society's functionality. Therefore, understanding how OT and AVP can influence social bonding is valuable for maintenance of healthy social systems. In humans, social behaviour including approximation and memory have been reported to be mediated by OT (Groppe et al., 2013). Although very few studies examined the effects of AVP on human social behaviour, they suggest a correlation with aggressive behaviour (Coccaro et al., 1998), and increases in salivary cortisol levels following social stress (Ebstein et al., 2009). Interestingly, human variants of otr and avpr genes have been associated with sociability (Meyer-Lindenberg et al., 2011).
Studies show that intranasal administration of OT (OT-IN) increases trust in interpersonal interactions (Kosfeld et al., 2005), a behaviour characterized by reduced activation of the amygdala, midbrain regions and dorsal striatum (Baumgartner et al., 2008), leading to a decrease in fear and stress responses. OT-IN also enhances eye gazing, a behaviour linked to social interaction, in adults (Guastella et al., 2008). In addition, committed heterosexual males that received OT-IN preferred to be socially distant from attractive females, compared with single men and the placebo group (Scheele et al., 2012). Although OT did not increase partner preference in humans, higher social interest, irrespective of gender, was reported (Liu et al., 2013), suggesting that OT does not modulate partner preference in the same way as described in studies with prairie voles.
Similarly to animal studies on social behaviour, effects of OT are context-dependent in humans. For example, OT increases jealousy (Shamay-Tsoory et al., 2009), favours in-group well-being and out-group aggressive behaviour (De Dreu et al., 2011), which could be linked to violence and xenophobia. These studies question the common idea that OT is a pro social peptide and introduces a new perception of this peptide's role in human social behaviour (for a review, see Bartz et al., 2011). This should be kept in mind when designing studies with this peptide in humans, particularly taking into consideration the context in which it is administered.
In the case of AVP, studies showed that a selective V1A receptor antagonist blocked intranasal AVP (AVP-IN)-mediated effects on amygdalar activity associated with pictures of angry faces (Lee et al., 2013), suggesting that AVP activation of this brain region is at least partially responsible for perception of negative social emotions. AVP-IN administration was also found to increase tendency to engage in mutual cooperative behaviour (Brunnlieb et al., 2016).
5.2.2 |. Involvement of OT and AVP in autism
Autism is a developmental disorder that mainly involves social impairment and repetitive motor behaviours and is associated with genetic, phenotypic and environmental factors (Lord et al., 2018). Studies reporting lower OT plasma levels in children with autism (Modahl et al., 1998), showing higher OT receptor expression in the nucleus basalis of Meynert (a region associated with visual attention) and lower expression in the ventral pallidum (a region involved with reward) in post-mortem brain of autistic humans (Freeman et al., 2018) and associating genetic variations of the genes for OT receptors and V1A receptors with the disorder suggest an involvement of OT and AVP. In the case of genetic variations, the intronic SNP rs2254298 in the third intron of the otr gene was linked to autism in Caucasian patients (Jacob et al., 2007), and microsatellite polymorphisms RS1 and RS3 for V1A receptors were found to increase amygdalar activity (Meyer-Lindenberg et al., 2009).
Studies have looked at the potential therapeutical effect of OT on autism, and the results appear to be dependent on duration and dose of OT administration. Single OT-IN administration improved retention of social cognition in adults (Hollander et al., 2007) and increased emotion recognition in children (Guastella et al., 2010) diagnosed with autism. In children and adolescents, acute administration of OT-IN enhanced activity of brain regions related to social stimuli, such as mPFC and striatum (Gordon et al., 2013), suggesting potential targets for OT action in autism.
Studies using repeated OT-IN administration showed diverse results. Thus, treatment of adult males with higher dose (32 IU day 1) but not of lower dose (16 IU day 1) of OT for 18 weeks improved social symptoms (Kosaka et al., 2016). However, administration of OT-IN (48 IU day 1) for 6 weeks did not affect social approach but decreased repetitive behaviour (Yamasue et al., 2020); this was also observed with male adult patients receiving OT-IN (24 IU day 1) for 4 weeks (Bernaerts et al., 2019). Children that received OT-IN (24 IU day 1) for 5 weeks exhibited improved social responsiveness (Yatawara et al., 2016). Finally, a meta-analysis of 16 clinical trial studies revealed that OT had little or no effects on autism symptoms (Wang et al., 2019), which calls for additional studies and possibly changes to protocol design.
In the case of AVP, very few clinical trials results have been published. One study showed that an orally administered V1A receptor antagonist, balovaptan, improved adaptive behaviour scales scores, particularly behaviours related to socialization and communication in adult males (Bolognani et al., 2019). Another study showed that AVP-IN (24–32 IU day-1) administration for 4 weeks to autistic children led to an improvement in social abilities (Parker et al., 2019).
Although OT and AVP have potential as therapeutics for the treatment of autism further studies are needed to determine the dose to be used, the duration of the behavioural effects, and to characterize the brain circuits involved. Also, safety protocols for use in different age groups and sex need to be determined. This is important for OT, as it is highly involved with lactation and parturition, which could be an impediment for its use in females. Also, the peripheral side effects of OT and AVP need to be considered, especially because gynecomastia, urinary incontinence, thirst and constipation have been reported after OT treatment (Yamasue et al., 2020; Yatawara et al., 2016).
5.2.3 |. Involvement of OT and AVP in schizophrenia
Schizophrenia has a complex symptomology in which, like autism, social disfunction is one of the criteria used for diagnosis (Tandon et al., 2013). Studies with nonprimate animals suggest that OT, AVP and their receptors could play a role in modulating abnormal behaviours associated with schizophrenia (see Meyer-Lindenberg et al., 2011). This would suggest the use of ligands targeting OT receptors and/or V1 receptors as potential therapeutic agents in the treatment of this disorder.
In humans, a decrease in OT and AVP plasma levels was observed in male schizophrenic patients (Jobst et al., 2014). In addition, SNPs such as rs53576 (change from G to A) located on intron 3 of otr gene have been associated with lower amygdalar activity in schizophrenia patients (Haram et al., 2016). Multiple SNPs on the avpr1a gene appear to be involved in the regulation of gene expression and were associated with behaviours implicated with schizophrenia (Meyer-Lindenberg et al., 2011). Although the amygdala (Haram et al., 2016) has been implicated in the effects of OT on schizophrenia, the precise brain circuit involved remains unclear.
Clinical trials have examined the effect of OT on schizophrenia. Studies showed that acute OT-IN administration (24 IU) improved emotion and cognition in schizophrenic patients (Averbeck et al., 2012), and chronic OT-IN administration (24 IU day 1) for 14 days, improved positive symptoms and paranoia (Pedersen et al., 2011). Interestingly, OT-IN (40 IU/2 day) for 3 weeks was found to potentiate antipsychotic effects (Feifel et al., 2010) whereas OT-IN (40 IU day 1) treatment for 8 months had no effect on adult patients regarding positive and negative symptoms (Dagani et al., 2016). These results warrant novel studies to help elucidate the effects of OT on schizophrenia. For instance, gender-specific studies should be pursued, with a focus on female patients, because differences in OT and OT receptor expression, along with effects of sex steroid hormones, have been reported (Mendrek & Stip, 2011).
In the case of AVP, treatment of humans diagnosed with schizophrenia with a synthetic AVP analogue caused modest improvement in an array of reported symptoms (evaluated thorough negative symptom rating scale), although many subjects experienced electrolyte and water imbalance, due to the peripheral effects of AVP (Iager et al., 1986). Clinical studies revealed that AVP-IN administration (400 ng AVP per nostril, four times every day, for 12 weeks), improved positive and negative symptoms of Han Chinese first-episode in schizophrenic adult patients (Geng et al., 2017). AVP's effects on schizophrenia appear to be sex-dependent, because a study showed that males treated with AVP displayed decreased angry face recognition whereas females displayed decreased sad face recognition (Bloch et al., 2019).
Together these studies show the potential of OT and AVP as therapeutic agents for the treatment of schizophrenia. However, further studies are needed to determine the dose to be used, the duration of the behavioural effects, to characterize the brain circuits involved, and to identify analogues with reduced side-effects.
5.2.4 |. Involvement of OT and AVP in substance use disorder (SUD)
SUD is a complex psychiatric condition that is characterized by compulsive use and loss of control over drug intake (Koob, 2009). Because both social- and stress-related behaviours are linked to SUD, this places the OT and AVP systems as potential modulators of this disorder. Studies using various models of SUD (see Godino & Renard, 2018 and King et al., 2020) show that OT and AVP engage in neural and behavioural changes to a number of drugs of abuse including cocaine, morphine, methamphetamine, nicotine and ethanol and therefore could have a significant effects on the prevention and treatment of SUD in humans.
Studies in humans have suggested positive effects of the manipulation of the OT and AVP receptor systems in ameliorating SUD. For example, OT-IN administration blocked alcohol withdrawal (24 IU/2X day for 3 days) and consumption (40 IU/2X day for 12 days) (Pedersen, 2017), dampened craving in high attachment anxiety patients (40 IU/acute) (Mitchell et al., 2016), and reduced cue-reactivity in male heavy alcohol drinkers (24 IU/acute) (Hansson et al., 2018), suggesting a potential role in decreasing drug-craving. The latter seems to be related with activation of the NAc (Bach et al., 2019). However, another study reported no differences in alcohol withdrawal after OT-IN administration (24 IU/2X day for 3 days) (Melby et al., 2020). The differences in results could be due to number of participants, inclusion criteria (e.g. severity of withdrawal symptoms) and type of protocol used to access patients' symptom score. Regarding different classes of drugs of abuse, administration of OT reduced cigarette consumption (Van Hedger et al., 2018) and decreased cocaine and heroin craving in patients receiving methadone treatment (Stauffer et al., 2016). In the case of AVP, studies showed that it facilitated heroin detoxication when administered to patients receiving methadone (Fraenkel et al., 1983), and its analogue reduced alcohol-induced amnesia in alcohol drinkers (Millar et al., 1987). In addition, a novel selective antagonist of V1B receptors decreased cigarette consumption in smokers but had modest effects in its ability to reduce alcohol drinking (Ryan et al., 2017).
Together, these studies show that the OT and AVP systems might contribute to the treatment of SUD. However, further studies in humans are warranted because little is known about the dose, treatment regimen, the long-lasting effects, and possible side-effects that this pharmacotherapy can induce in people with SUD.
5.3 |. Future perspectives and conclusions
Here, we described the major roles of OT and AVP and their respective receptors, as well as the molecular and pharmacological aspects that require further investigation and consideration. The OT and AVP systems are complex as they are relevant for the regulation of several biological and behavioural responses. This complexity, together with strong evidence suggesting a crosstalk in signalling and behavioural responses, raises the need to use a systematic approach for studies involving these neuropeptides and their receptors.
Given the ability of OT, V1A and V1B receptors to couple to more than one type of G protein that depends on receptor and neuropeptide expression, it could be beneficial to map the brain location of receptors that bind to either Gq, Gi or Gs and under which conditions this happens. At the physiological level, measurement of OT and AVP levels, particularly in the plasma, could be improved either by developing more sensitive methods or by standardizing extract preparation. Also, evaluating if and how much OT and AVP cross the blood brain barrier following peripheral or intra-nasal administration could be helpful in using these neuropeptides as drugs to treat disorders.
There is a great need to explore the use of specific agonists and antagonists for each receptor system. This would help elucidate which behaviours or physiological responses are exclusively mediated by OT and AVP, as well as the biological relevance of the crosstalk between these peptides. It is to be noted that although most binding and signalling crosstalk between OT and AVP has been evaluated in vitro, using transfected cells or tissue, the in vivo results could be different given the possibility of modulation by other neuropeptide-receptor systems. Moreover, factors such as timing and dose of drug administration, and the brain region involved need to be explored in order to better understand the therapeutic potential of OT and AVP.
In conclusion, OT and AVP are peptides with high physiological relevance and increasing our knowledge about how their in vivo effects are modulated including perfecting methods for their detection in bodily fluids could be promising in developing treatments for disorders such as autism, schizophrenia and SUD.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
ACKNOWLEDGMENTS
MR was recipient of scholarships from Coordination for the Improvement of Higher Education Personnel (CAPES) (PROAP-Social Demand) and National Council for Scientific and Technological Development (CNPq) #141698/2016-0. Funding was provided by grant #2018/05038-0, from S~ao Paulo Research Foundation (Fundaç~ao de Amparo à Pesquisa do Estado de S~ao Paulo-FAPESP) and grant #470070/2012-9, CNPq to R.C. R.C. is a research fellow of CNPq. L.A.D. was supported by National Institute of Health grants DA008863 and NS026880.The sponsors had no involvement in the writing of the review and the decision to submit it for publication.
Funding information
National Council for Scientific and Technological Development, Grant/Award Numbers: #141698/2016-0, #470070/2012-9; Coordination for the Improvement of Higher Education Personnel, Grant/Award Number: PROAP-Social Demand; Sãao Paulo Research Foundation, Grant/Award Number: #2018/05038-0; National Institute of Health, Grant/Award Numbers: DA008863, NS026880
Abbreviations:
- ACTH
adrenocorticotropic hormone
- AVP
vasopressin
- AVP-IN
intranasal vasopressin
- BBB
blood brain barrier
- BSNT
bed nucleus of stria terminalis
- CBT
carbetocin
- CeA
central amygdala
- CPP
conditioned place preference
- CRF
corticotropin-releasing factor
- ECL
extracellular loop
- EPM
elevated plus maze
- EZMT
elevated zero maze test
- GRK
GPCR kinase
- HAB
high anxiety related behaviour
- HPA
hypothalamic–pituitary–adrenal
- Ki
inhibitory constant
- KO
knockout
- LS
lateral septum
- MEF2A
myocyte enhancing factor 2A
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- OF
open field
- OT
oxytocin
- OT-IN
intranasal oxytocin
- PVN
paraventricular nucleus
- sCRF2α
soluble corticotropin-releasing factor 2α
- SNP
single nucleotide polymorphism
- SON
supraoptic nucleus
- SUD
substance use disorder
- TM
transmembrane
- VTA
ventral tegmental area
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article because no new data were created or analysed in this study.
REFERENCES
- Albers HE (2012). The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Hormones and Behavior, 61, 283–292. 10.1016/j.yhbeh.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Albers HE, Dean A, Karom MC, Smith D, & Huhman KL (2006). Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Research, 1073–1074, 425–430. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, & CGTP Collaborators. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, & Shergill SS (2012). Emotion recognition and oxytocin in patients with schizophrenia. Psychological Medicine, 42, 259–266. 10.1017/S0033291711001413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Reinhard I, Bühler S, Vollstädt-Klein S, Kiefer F, & Koopmann A (2019). Oxytocin modulates alcohol-cue induced functional connectivity in the nucleus accumbens of social drinkers. Psychoneuroendocrinology, 109, 104385. [DOI] [PubMed] [Google Scholar]
- Bahi A (2015). The oxytocin receptor impairs ethanol reward in mice. Physiology & Behavior, 139, 321–327. 10.1016/j.physbeh.2014.11.046 [DOI] [PubMed] [Google Scholar]
- Barberis C, Mouillac B, & Durroux T (1998). Structural bases of vasopressin/oxytocin receptor function. The Journal of Endocrinology, 156, 223–229. 10.1677/joe.0.1560223 [DOI] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, & Young LJ (2013). Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Hormones and Behavior, 63, 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, & Ochsner KN (2011). Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences, 15, 301–309. 10.1016/j.tics.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Baskerville TA, & Douglas AJ (2010). Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neuroscience & Therapeutics, 16, e92–e123. 10.1111/j.1755-5949.2010.00154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, & Fehr E (2008). Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron, 58, 639–650. [DOI] [PubMed] [Google Scholar]
- Bernaerts S, Boets B, Bosmans G, Steyaert J, & Alaerts K (2019). Behavioral effects of multiple-dose oxytocin treatment in autism: A randomized, placebo-controlled trial with long-term follow-up. Molecular Autism, 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, & Young LJ (2004). Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology, 29, 483–493. 10.1038/sj.npp.1300360 [DOI] [PubMed] [Google Scholar]
- Bloch B, Levin R, Vadas L, Shalev I, Israel S, Uzefovsky F, Bachner-Melman R, Reshef A, Ebstein RP, & Kremer I (2019). Sex-specific effect of intranasal vasopressin, but not oxytocin, on emotional recognition and perception in schizophrenia patients. Israel Journal of Psychiatry, 56, 21–25. [Google Scholar]
- Bolognani F, Valle R, Del M, Squassante L, Wandel C, Derks M, Murtagh L, Sevigny J, Khwaja O, Umbricht D, & Fontoura P (2019). A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Science Translational Medicine, 11, 1–15. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, & Fehm HL (2002). Sniffing neuropeptides: A transnasal approach to the human brain. Nature Neuroscience, 5, 514–516. 10.1038/nn0602-849 [DOI] [PubMed] [Google Scholar]
- Bowen-Pidgeon D, Innamorati G, Sadeghi HM, & Birnbaumer M (2001). Arrestin effects on internalization of vasopressin receptors. Molecular Pharmacology, 59, 1395–1401. 10.1124/mol.59.6.1395 [DOI] [PubMed] [Google Scholar]
- Brown CH (2016). Magnocellular neurons and posterior pituitary function. Comprehensive Physiology, 6, 1701–1741. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Nave G, Camerer CF, Schosser S, Vogt B, Münte TF, & Heldmann M (2016). Vasopressin increases human risky cooperative behavior. Proceedings of the National Academy of Sciences of the United States of America, 113, 2051–2056. 10.1073/pnas.1518825113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Bulgheroni E, Manning M, Kleinau G, & Chini B (2013). Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. The Journal of Pharmacology and Experimental Therapeutics, 346, 318–327. 10.1124/jpet.113.202994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Saulière A, Manning M, Bouvier M, Galés C, & Chini B (2012). Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. The Journal of Biological Chemistry, 287, 3617–3629. 10.1074/jbc.M111.277178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee H-J, Macbeth AH, & Young WS (2008). Vasopressin: Behavioral roles of an “original” neuropeptide. Progress in Neurobiology, 84, 1–24. 10.1016/j.pneurobio.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Stewart J, Wiedholz LM, Millstein RA, Iacangelo A, Holmes A, Young WS 3rd, & Wersinger SR (2006). The acute intoxicating effects of ethanol are not dependent on the vasopressin 1a or 1b receptors. Neuropeptides, 40, 325–337. 10.1016/j.npep.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Chini B, & Manning M (2007). Agonist selectivity in the oxytocin/vasopressin receptor family: New insights and challenges. Biochemical Society Transactions, 35, 737–741. 10.1042/BST0350737 [DOI] [PubMed] [Google Scholar]
- Chini B, Mouillac B, Ala Y, Balestre MN, Trumpp-Kallmeyer S, Hoflack J, Elands J, Hibert M, Manning M, & Jard S (1995). Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. The EMBO Journal, 14, 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Verhage M, & Grinevich V (2017). The action radius of oxytocin release in the mammalian CNS: From single vesicles to behavior. Trends in Pharmacological Sciences, 38, 982–991. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, & Ferris CF (1998). Cerebrospinal fluid vasopressin levels. Archives of General Psychiatry, 55, 708–714. [DOI] [PubMed] [Google Scholar]
- da Silva MP, Merino RM, Mecawi AS, Moraes DJ, & Varanda WA (2015). In vitro differentiation between oxytocin- and vasopressin-secreting magnocellular neurons requires more than one experimental criterion. Molecular and Cellular Endocrinology, 400, 102–111. 10.1016/j.mce.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Dagani J, Sisti D, Abelli M, Di Paolo L, Pini S, Raimondi S, Rocchi MB, Saviotti FM, Scocco P, Totaro S, & Balestrieri M (2016). Do we need oxytocin to treat schizophrenia? A randomized clinical trial. Schizophrenia Research, 172, 158–164. 10.1016/j.schres.2016.02.011 [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, & Handgraaf MJJ (2011). Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences of the United States of America, 108, 1262–1266. 10.1073/pnas.1015316108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Ebner K, & Landgraf R (2000). Oxytocin induces preservation of social recognition in male rats by activating a-adrenoceptors of the olfactory bulb. The European Journal of Neuroscience, 12, 760–766. 10.1046/j.1460-9568.2000.00952.x [DOI] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, & Trainor BC (2018). Oxytocin receptors in the anteromedial bed nucleus of the Stria Terminalis promote stress-induced social avoidance in female California mice. Biological Psychiatry, 83, 203–213. 10.1016/j.biopsych.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, & Engelmann M (2002). Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. The European Journal of Neuroscience, 15, 384–388. 10.1046/j.0953-816x.2001.01869.x [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, Riebold M, Salomon S, & Yirmiya N (2009). Arginine vasopressin and oxytocin modulate human social behavior. Annals of the New York Academy of Sciences, 1167, 87–102. 10.1111/j.1749-6632.2009.04541.x [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, & Koob GF (2012). Evidence that vasopressin V 1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addiction Biology, 17, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK, Freels TG, Prater WT, & Lester DB (2019). Systemic oxytocin administration alters mesolimbic dopamine release in mice. Neuroscience, 408, 226–238. [DOI] [PubMed] [Google Scholar]
- Everett NA, Turner AJ, Costa PA, Baracz SJ, & Cornish JL (2021). The vagus nerve mediates the suppressing effects of peripherally administered oxytocin on methamphetamine self-administration and seeking in rats. Neuropsychopharmacology, 46, 297–304. 10.1038/s41386-020-0719-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, MacDonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, & Lefebvre M (2010). Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry, 68, 678–680. 10.1016/j.biopsych.2010.04.039 [DOI] [PubMed] [Google Scholar]
- Ferris CF, & Potegal M (1988). Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiology & Behavior, 44, 235–239. 10.1016/0031-9384(88)90144-8 [DOI] [PubMed] [Google Scholar]
- Fraenkel H, Van Beek-Verbeek G, Fabriek A, & Van Ree JM (1983). Desglycinamide 9-arginine 8-vasopressin and ambulant methadone-detoxification of heroin addicts. Alcohol and Alcoholism, 18, 331–335. [Google Scholar]
- Freeman SM, Palumbo MC, Lawrence RH, Smith AL, Goodman MM, & Bales KL (2018). Effect of age and autism spectrum disorder on oxytocin receptor density in the human basal forebrain and midbrain. Translational Psychiatry, 8(257), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng CH, Wang C, Yang J, Wang H, Ma RQ, Liu X, & Wang CH (2017). Arginine vasopressin improves the memory deficits in Han Chinese patients with first-episode schizophrenia. Peptides, 97, 8–15. 10.1016/j.peptides.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Gimpl G, & Fahrenholz F (2001). The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews, 81, 629–683. 10.1152/physrev.2001.81.2.629 [DOI] [PubMed] [Google Scholar]
- Godino A, & Renard GM (2018). Effects of alcohol and psychostimulants on the vasopressin system: Behavioural implications. Journal of Neuroendocrinology, 30, e12611. [DOI] [PubMed] [Google Scholar]
- Gordon I, Wyk BCV, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, Zagoory-Sharon O, Leckman JF, Feldman R, & Pelphrey KA (2013). Oxytocin enhances brain function in children with autism. Proceedings of the National Academy of Sciences of the United States of America, 110, 20953–20958. 10.1073/pnas.1312857110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, & Soubrié P (2002). Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proceedings of the National Academy of Sciences of the United States of America, 99, 6370–6375. 10.1073/pnas.092012099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, & Spreckelmeyer KN (2013). Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biological Psychiatry, 74, 172–179. 10.1016/j.biopsych.2012.12.023 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, & Hickie IB (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry, 67, 692–694. 10.1016/j.biopsych.2009.09.020 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry, 63, 3–5. 10.1016/j.biopsych.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Guzmán YF,Tronson NC,Jovasevic V,Sato K, Guedea AL, Mizukami H, Nishimori K, & Radulovic J (2013). Fear-enhancing effects of septal oxytocin receptors. Nature Neuroscience, 16, 1185–1187. 10.1038/nn.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han RT, Kim YB, Park EH, Kim JY, Ryu C, Kim HY, Lee J, Pahk K, Shanyu C, Kim H, & Back SK (2018). Long-term isolation elicits depression and anxiety-related behaviors by reducing oxytocin-induced GABAergic transmission in central amygdala. Frontiers in Molecular Neuroscience, 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Koopmann A, Uhrig S, Bühler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstädt-Klein S, & Spanagel R (2018). Oxytocin reduces alcohol cue-reactivity in alcohol-dependent rats and humans. Neuropsychopharmacology, 43, 1235–1246. 10.1038/npp.2017.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, & Spanagel R (2021). No changes in the oxytocin system in alcohol-dependent female rodents and humans: Towards a sex-specific psychopharmacology in alcoholism. Addiction Biology, 26, 1–2. [DOI] [PubMed] [Google Scholar]
- Haram M, Bettella F, Brandt CL, Quintana DS, Nerhus M, Bjella T, Djurovic S, Westlye LT, Andreassen OA, Melle I, & Tesli M (2016). Contribution of oxytocin receptor polymorphisms to amygdala activation in schizophrenia spectrum disorders. BJPsych Open, 2, 353–358. 10.1192/bjpo.bp.116.003376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Butler RK, Cook CA, Criswell HE, Stuber GD, & Breese GR (2019). Amygdala arginine vasopressin modulates chronic ethanol withdrawal anxiety-like behavior in the social interaction task. Alcoholism, Clinical and Experimental Research, 43, 2134–2143. 10.1111/acer.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtin SR, Wesley VJ, Simms J, Argent CCH, Latif K, & Wheatley M (2005). The N-terminal juxtamembrane segment of the V1a vasopressin receptor provides two independent epitopes required for high-affinity agonist binding and signaling. Molecular Endocrinology, 19, 2871–2881. 10.1210/me.2005-0148 [DOI] [PubMed] [Google Scholar]
- Hernandez VS,Hernandez OR, PerezdelaMora M,Gomora MJ, Fuxe K, Eiden LE, & Zhang L (2016). Hypothalamic vasopressinergic projections innervate central amygdala GABAergic neurons: Implications for anxiety and stress coping. Frontiers in Neural Circuits, 10, 92. 10.3389/fncir.2016.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, & Wasserman S (2007). Oxytocin increases retention of social cognition in autism. Biological Psychiatry, 61, 498–503. [DOI] [PubMed] [Google Scholar]
- Iager AC, Kirch DG, Bigelow LB, & Karson CN (1986). Treatment of schizophrenia with a vasopressin analogue. The American Journal of Psychiatry, 143, 375–377. [DOI] [PubMed] [Google Scholar]
- Insel TR, & Hulihan TJ (1995). A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behavioral Neuroscience, 109, 782–789. 10.1037/0735-7044.109.4.782 [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Maejima Y, Suyama S, Yoshida M, Arai T, Katsurada K, Kumari P, Nakabayashi H, Kakei M, & Yada T (2015). Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: A route for ameliorating hyperphagia and obesity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 308, R360–R369. [DOI] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, & Cook EH (2007). Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letters, 417, 6–9. 10.1016/j.neulet.2007.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst A, Dehning S, Ruf S, Notz T, Buchheim A, Henning-Fast K, Meibner D, Meyer S, Bondy B, Müller N, & Zill P (2014). Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatrica, 26, 347–355. 10.1017/neu.2014.20 [DOI] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2015). Neurobiological mechanisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences, 3, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, & Meyer M (2020). Anxiolytic and anxiogenic? How the transcription factor MEF2 might explain the manifold behavioral effects of oxytocin. Frontiers in Endocrinology (Lausanne), 11, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, & Neumann ID (2018). The oxytocin receptor: From intracellular signaling to behavior. Physiological Reviews, 98, 1805–1908. [DOI] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G, Neumann ID, & van den Burg EH (2015). Oxytocin regulates stress-induced Crf gene transcription through creb-regulated transcription coactivator 3. The Journal of Neuroscience, 35, 12248–12260. 10.1523/JNEUROSCI.1345-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, & Stevens RC (2014). Allosteric sodium in class a GPCR signaling. Trends in Biochemical Sciences, 39, 233–244. 10.1016/j.tibs.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Wigger A, Welt T, Müller MB, Gesing A, Reul JMHM, Holsboer F, Landgraf R, & Neumann ID (2002). Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: Implications for pathogenesis of affective disorders. Neuropsychopharmacology, 26(1), 94–105. [DOI] [PubMed] [Google Scholar]
- King CE, Gano A, & Becker HC (2020). The role of oxytocin in alcohol and drug abuse. Brain Research, 1736, 146761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, & Becker HC (2017). Oxytocin reduces ethanol self-administration in mice. Alcoholism, Clinical and Experimental Research, 41, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, & Grinevich V (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron, 73, 553–566. [DOI] [PubMed] [Google Scholar]
- Koob GF (2009). Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology, 56, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H, Okamoto Y, Munesue T, Yamasue H, Inohara K, Fujioka T, Anme T, Orisaka M, Ishitobi M, Jung M, Fujisawa TX, Tanaka S, Arai S, Asano M, Saito DN, Sadato N, Tomoda A, Omori M, Sato M, … Wada Y (2016). Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: A 24-week randomized clinical trial. Translational Psychiatry, 6, e872. 10.1038/tp.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, & Fehr E (2005). Oxytocin increases trust in humans. Nature, 435, 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Honda K, Nagaoka-Uozumi S, Ichimura A, Kimura I, Nakaya M, Sakai N, Shibata K, Ushijima K, Fujimura A, Hirasawa A, Kurose H, Tsujimoto G, Tanoue A, & Takano Y (2018). Complex formation between the vasopressin 1b receptor, ß-arrestin-2, and the μ-opioid receptor underlies morphine tolerance. Nature Neuroscience, 21, 820–833. 10.1038/s41593-018-0144-y [DOI] [PubMed] [Google Scholar]
- Koshimizu T-A, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, & Tanoue A (2012). Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiological Reviews, 92, 1813–1864. 10.1152/physrev.00035.2011 [DOI] [PubMed] [Google Scholar]
- Kovács GL,Sarnyai Z,Babarczi E,Szab G,&Telegdy G(1990).The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology, 29(4), 365–368. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Coccaro EF, Cremers H, McCarron R, Lu S-F, Brownstein MJ, & Simon NG (2013). A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: An fMRI study. Frontiers in Systems Neuroscience, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Wotjak CT, Landgraf R, & Engelmann M (1996). Septal vasopressin modulates anxiety-related behaviour in rats. Neuroscience Letters, 217(2–3), 101–104. [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE,Ren X,Terwilliger EF,& Young, L. J. (2004). Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature, 429, 754–757. [DOI] [PubMed] [Google Scholar]
- Liu JCJ, Guastella AJ, & Dadds MR (2013). Exploring the role of intra-nasal oxytocin on the partner preference effect in humans. Psychoneuroendocrinology, 38, 587–591. [DOI] [PubMed] [Google Scholar]
- Liu Y, & Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience, 121, 537–544. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Roper JA, Harrison G, Jessop DS, Young WS, & O'Carroll AM (2007). Attenuated stress response to acute lipopolysaccharide challenge and ethanol administration in vasopressin V1b receptor knockout mice. Journal of Neuroendocrinology, 19, 543–551. 10.1111/j.1365-2826.2007.01560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Elsabbagh M, Baird G, & Veenstra-Vanderweele J (2018). Autism spectrum disorder. Lancet, 392, 508–520. 10.1016/S0140-6736(18)31129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, & Dreifuss JJ (1991). Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research, 555, 220–232. 10.1016/0006-8993(91)90345-v [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, & Guillon G (2012). Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology, 24, 609–628. 10.1111/j.1365-2826.2012.02303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby K, Gråwe RW, Aamo TO, Skovlund E, & Spigset O (2020). Efficacy of self-administered intranasal oxytocin on alcohol use and craving after detoxification in patients with alcohol dependence. A double-blind placebo-controlled trial. Alcohol and Alcoholism, 133, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, & Stip E (2011). Sexual dimorphism in schizophrenia: Is there a need for gender-based protocols? Expert Review of Neurotherapeutics, 11, 951–959. 10.1586/ern.11.78 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews. Neuroscience, 12, 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, & Weinberger DR (2009). Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Molecular Psychiatry, 14, 968–975. 10.1038/mp.2008.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar K, Jeffcoate WJ, & Walders CP (1987). Vasopressin and memory: Improvement in normal short-term recall and reduction of alcohol-induced amnesia. Psychological Medicine, 17, 335–341. 10.1017/S0033291700024879 [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Arcuni PA, Weinstein D, & Woolley JD (2016). Intranasal oxytocin selectively modulates social perception, craving, and approach behavior in subjects with alcohol use disorder. Journal of Addiction Medicine, 10, 182–189. 10.1097/ADM.0000000000000213 [DOI] [PubMed] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, & Froemke RC (2016). A distributed network for social cognition enriched for oxytocin receptors. The Journal of Neuroscience, 36, 2517–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modahl C, Green LA, Fein D, Morris M, Waterhouse L, Feinstein C, & Levin H (1998). Plasma oxytocin levels in autistic children. Biological Psychiatry, 43, 270–277. 10.1016/S0006-3223(97)00439-3 [DOI] [PubMed] [Google Scholar]
- Nasanbuyan N, Yoshida M, Takayanagi Y, Inutsuka A, Nishimori K, Yamanaka A, & Onaka T (2018). Oxytocin–oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology, 159, 763–775. 10.1210/en.2017-00606 [DOI] [PubMed] [Google Scholar]
- Nelson BS, Sequeira MK, & Schank JR (2018). Bidirectional relationship between alcohol intake and sensitivity to social defeat: Association with Tacr1 and Avp expression. Addiction Biology, 23, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Krömer SA, Toschi N, & Ebner K (2000). Brain oxytocin inhibits the (re)activity of the hypothalamo–pituitary–adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regulatory Peptides, 96, 31–38. [DOI] [PubMed] [Google Scholar]
- Neumann ID, & Landgraf R (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35, 649–659. 10.1016/j.tins.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, Summers JE, Hinman KE, Motonaga KS, Phillips JM, & Carson DS (2019). A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Science Translational Medicine, 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA (2017). Oxytocin, tolerance, and the dark side of addiction. International Review of Neurobiology, 136, 239–274. (Elsevier Inc.) [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, & Penn DL (2011). Intranasal oxytocin reduces psychotic symptoms and improves theory of mind and social perception in schizophrenia. Schizophrenia Research, 132, 50–53. [DOI] [PubMed] [Google Scholar]
- Pena A, Murat B, Trueba M, Ventura MA, Wo NC, Szeto HH, Cheng LL, Stoev S, Guillon G, & Manning M (2007). Design and synthesis of the first selective agonists for the rat vasopressin V1b receptor: Based on modifications of deamino-[cys 1]arginine vasopressin at positions 4 and 8. Journal of Medicinal Chemistry, 50, 835–847. 10.1021/jm060928n [DOI] [PubMed] [Google Scholar]
- Peters S, Slaterry DA, Uschold-Schmidt N, Reber SO, & Neumann ID (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology, 42, 225–236. 10.1016/j.psyneuen.2014.01.021 [DOI] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, & Neumann ID (2017). Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addiction Biology, 22, 702–711. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Rokicki J, van der Meer D, Alnæs D, Kaufmann T, Cordova-Palomera A,Dieset I, Andreassen OA,&Westlye LT (2019). Oxytocin pathway gene networks in the human brain. Nature Communications, 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae M, Zanos P, Georgiou P, Chivers P, Bailey A, & Camarini R (2018). Environmental enrichment enhances conditioned place preference to ethanol via an oxytocinergic-dependent mechanism in male mice. Neuropharmacology, 138, 267–274. 10.1016/j.neuropharm.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Rigter H, & Crabbe JC (1985). Vasopressin and ethanol preference. I. Effects of vasopressin and the fragment DGAVP on altered ethanol preference in Brattleboro diabetes insipidus rats. Peptides, 6(4), 669–676. [DOI] [PubMed] [Google Scholar]
- Rodriguez KM, Smith BL, & Caldwell HK (2020). Voluntary alcohol consumption is increased in female, but not male, oxytocin receptor knockout mice. Brain and Behavior: A Cognitive Neuroscience Perspective, 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, Strain EC, Dunn KE, Kampman K, Mahoney E, & Ciraulo DA (2017). A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology, 42, 1012–1023. 10.1038/npp.2016.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Dong SM, Maurer SD, Post C, & Leuner B (2017). Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action. Neuropharmacology, 125, 1–12. 10.1016/j.neuropharm.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbe A, Takagi N, Fujiwara Y, Yamauchi J, Endo T, Mizutani R, Takeo S, Tsujimoto G, & Tanoue A (2008). Alcohol preference in mice lacking the Avpr1a vasopressin receptor. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 294, 1482–1490. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M,Szabo G,Kovacs GL,Barth T,& Telegdy G (1991). Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: Putative role of basal forebrain target sites. Neuropeptides, 19, 51–56. 10.1016/0143-4179(91)90073-R [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Szab G, Kovacs GL,&Telegdy G(1992).Oppositeactions of oxytocin and vasopressin in the development of cocaine-induced behavioral sensitization in mice. Pharmacology Biochemistry and Behavior, 43, 491–494. 10.1016/0091-3057(92)90182-F [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Gunturkun O, Deutschlander S, Maier W, Kendrick KM, & Hurlemann R (2012). Oxytocin modulates social distance between males and females. The Journal of Neuroscience, 32, 16074–16079. 10.1523/JNEUROSCI.2755-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann A, & Gimpl G (2018). Sodium functions as a negative allosteric modulator of the oxytocin receptor. Biochimica et Biophysica Acta - Biomembranes, 1860, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, & Levkovitz Y (2009). Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biological Psychiatry, 66, 864–870. [DOI] [PubMed] [Google Scholar]
- Smith CJW, DiBenedictis BT, & Veenema AH (2019). Comparing vasopressin and oxytocin fiber and receptor density patterns in the social behavior neural network: Implications for cross-system signaling. Frontiers in Neuroendocrinology, 53, 1–43. 10.1016/j.yfrne.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, & Albers HE (2018). Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Frontiers in Neuroendocrinology, 51, 14–24. 10.1016/j.yfrne.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Borland JM, Larkin TE, O'Malley M, & Albers HE (2016). Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology, 74, 164–172. 10.1016/j.psyneuen.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R (2017). Animal models of addiction. Dialogues in Clinical Neuroscience, 19, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Harrison LR, Wood S, Knight DM, MacSweeney CP, Thomson F, Craighead M, & Lightman SL (2009). Blockade of the V1b receptor reduces ACTH, but not corticosterone secretion induced by stress without affecting basal hypothalamic-pituitary-adrenal axis activity. The Journal of Endocrinology, 200, 273–283. 10.1677/JOE-08-0421 [DOI] [PubMed] [Google Scholar]
- Stauffer CS, Musinipally V, Suen A, Lynch KL, Shapiro B, & Woolley JD (2016). A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addiction Research and Theory, 24, 490–498. 10.3109/16066359.2016.1173682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, & Caldwell HK (2012). The vasopressin 1b receptor and the neural regulation of social behavior. Hormones and Behavior, 61, 277–282. 10.1016/j.yhbeh.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, McMahon EK, Boner W, & Haussmann MF (2019). Oxytocin administration prevents cellular aging caused by social isolation. Psychoneuroendocrinology, 103, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R (2012). Neuromodulation by oxytocin and vasopressin. Neuron, 76, 142–159. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, & Hurlemann R (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports, 3, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, Malaspina D, Owen MJ, Schultz S, Tsuang M, & Van Os J (2013). Definition and description of schizophrenia in the DSM-5. Schizophrenia Research, 150, 3–10. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Berti-Mattera LN, Dulin N, Conarty DM, & Mattera R (1998). Signal transduction pathways of the human V1-vascular, V2-renal, V3-pituitary vasopressin and oxytocin receptors. Progress in Brain Research, 119, 147–161. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, & Mattera R (1997). The human V3 pituitary vasopressin receptor: Ligand binding profile and density-dependent signaling pathways. Endocrinology, 138(10), 4109–4122. 10.1210/endo.138.10.5432 [DOI] [PubMed] [Google Scholar]
- Torner L, Plotsky PM, Neumann ID, & de Jong TR (2016). Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology, 77, 165–174. [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Kirson D, Zallar LJ, McConnell SA, Vendruscolo JCM, Ho CP, Oleata CS, Khom S, Manning M, Lee MR, & Leggio L (2019). Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biology, 17, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hedger K, Kushner MJ, Lee R, & De Wit H (2018). Oxytocin reduces cigarette consumption in daily smokers. Nicotine & Tobacco Research, 21, 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ree JM, Burbach-Bloemarts EMM, & Wallace M (1988). Vasopressin neuropeptides and acquisition of heroin and cocaine self-administration in rats. Life Sciences, 42, 1091–1099. [DOI] [PubMed] [Google Scholar]
- Veenema AH, & Neumann ID (2008). Central vasopressin and oxytocin release: regulation of complex social behaviours. In Progress in brain research (pp. 261–276). Elsevier. [DOI] [PubMed] [Google Scholar]
- Waltenspühl Y, Schöppe J, Ehrenmann J, Kummer L, & Plückthun A (2020). Crystal structure of the human oxytocin receptor. Science Advances, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang M-J, Rong Y, He H-Z, & Yang C-J (2019). Oxytocin therapy for core symptoms in autism spectrum disorder: An updated meta-analysis of randomized controlled trials. Research in Autism Spectrum Disorder, 64, 63–75. 10.1016/j.rasd.2019.03.007 [DOI] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, & Ingram CD (1997). Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology, 138, 2829–2834. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, & Insel TR (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature, 365, 545–548. [DOI] [PubMed] [Google Scholar]
- Winter J, & Jurek B (2019). The interplay between oxytocin and the CRF system: Regulation of the stress response. Cell and Tissue Research, 375, 85–91. 10.1007/s00441-018-2866-2 [DOI] [PubMed] [Google Scholar]
- Winter J, Meyer M, Berger I, Peters S, Royer M, Bianchi M, Stang S, Langgartner D, Reber SO, Kuffner K, & Schmidtner AK (2020). Chronic oxytocin-driven alternative splicing of CRFR2a induces anxiety. BioRxiv, 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten DL, Simms J, Massoura AJ, Trim JE, & Wheatley M (2011). Agonist-specific requirement for a glutamate in transmembrane helix 1 of the oxytocin receptor. Molecular and Cellular Endocrinology, 333, 20–27. 10.1016/j.mce.2010.11.029 [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, & Engelmann M (1998). Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience, 85, 1209–1222. 10.1016/S0306-4522(97)00683-0 [DOI] [PubMed] [Google Scholar]
- Yamasue H, Okada T, Munesue T, Kuroda M, Fujioka T, Uno Y, Matsumoto K, Kuwabara H, Mori D, Okamoto Y, & Yoshimura Y (2020). Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: A randomized clinical trial. Molecular Psychiatry, 25, 1849–1858. [DOI] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, & Guastella AJ (2016; ). The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Molecular Psychiatry, 21, 1225–1231. 10.1038/mp.2015.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, & Wang Z (2011). The neurobiology of pair bonding: Insights from a socially monogamous rodent. Frontiers in Neuroendocrinology, 32, 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, & Flanagan-Cato LM (2012). Editorial comment: Oxytocin, vasopressin and social behavior. Hormones and Behavior, 61, 227–229. 10.1016/j.yhbeh.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, & Bailey A (2014). The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology, 39, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena D, Domokos A, Jain SK, Jankord R, & Filaretova L (2009). The stimuli-specific role of vasopressin in the hypothalamus-pituitary-adrenal axis response to stress. The Journal of Endocrinology, 202, 263–278. 10.1677/JOE-09-0096 [DOI] [PubMed] [Google Scholar]
- Zheng D-J, Foley L, Rehman A, & Ophir AG (2013). Social recognition is context dependent in single male prairie voles. Animal Behaviour, 86, 1085–1095. 10.1016/j.anbehav.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MAM, Ho A, Gessa GL, & Kreek MJ (2011). Involvement of arginine vasopressin and V1b receptor in alcohol drinking in sardinian alcohol-preferring rats. Alcoholism, Clinical and Experimental Research, 35, 1876–1883. 10.1111/j.1530-0277.2011.01532.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.


