Abstract
Introduction
Antimicrobial resistance (AMR) is an emerging global threat. It increases mortality and morbidity and strains healthcare systems. Health care professionals can counter the rising AMR by promoting antibiotic stewardship and facilitating new drug development. Even with the economic and scientific challenges, it is reassuring that new agents continue to be developed.
Methods
This review addresses new antibiotics in the pipeline. We conducted a review of the literature including Medline, Clinicaltrials.org, and relevant pharmaceutical companies for approved and in pipeline antibiotics in phase 3 or new drug application (NDA).
Results
We found a number of new antibiotics and reviewed their current development status, mode of action, spectra of activity, and indications for which they have been approved. The included studies from phase 3 clinical trials were mainly utilized for the treatment of acute bacterial skin and skin structure infections, community-acquired bacterial pneumonia, and pneumonia acquired in the healthcare settings. The number of these agents is limited against high priority organisms. The identified antibiotics were based mainly on previously known molecules or pre-existing antimicrobial agents.
Conclusion
There are a limited number of antibiotics against high priority organisms such as multi-drug-resistant Pseudomonas aeruginosa, and carbapenem-resistant Enterobacteriaceae. New antimicrobial agents directed against the top priority organisms as classified by the World Health Organization are urgently needed.
Keywords: Antibiotics, Pipeline, Novel antibiotics, New antibiotics
Introduction
Antibiotics have provided protection against life-threatening bacterial infections for more than a century. However, indiscriminate use of antibiotics and organism evolution have led to the emergence of multi-drug-resistant organisms (MDRO), and at times resistant to most or even all currently available antibiotic classes, extensively drug-resistant or pan-resistant organisms (XDRO, PDRO). Antibiotic resistance is a serious emerging global health threat [1] and certain geographic areas might be affected more than others due to the pattern of antibiotic usage [2]. Thus, there is a great demand to search for novel antibiotics that are effective and safe. Antibiotic development has had several scientific and economic challenges over the years. A major hindrance for industrial support for new antimicrobial development is the low return of investment [3]. That said, antibiotics are indispensable for global health. This paper reviews the anti-bacterial agents launched worldwide since 2017 and details their development status, mode of action, spectra of activity and the indications for which these antibiotics have been approved.
Methodology
Search strategy
Two investigators initially reviewed the listed databases and then additional two investigators did a follow-up search to identify new antibiotics in development by searching the FDA, WHO, European medicine agency, and Central Drugs Standard Control Organization (India) platforms. Using the 25 new antibiotics identified between January 1st, 2017 and January 31, 2021, we formulated keywords and a search strategy for further databases. Two investigators independently searched electronic databases MEDLINE, NIH U. S. National Library of Medicine (clinicaltrial.gov), and Science Direct for articles as per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for the period January 2017 to November 30th, 2020. When necessary, websites of pharmaceutical companies responsible for the development of the drug were accessed for further relevant information. Only English language articles were selected.
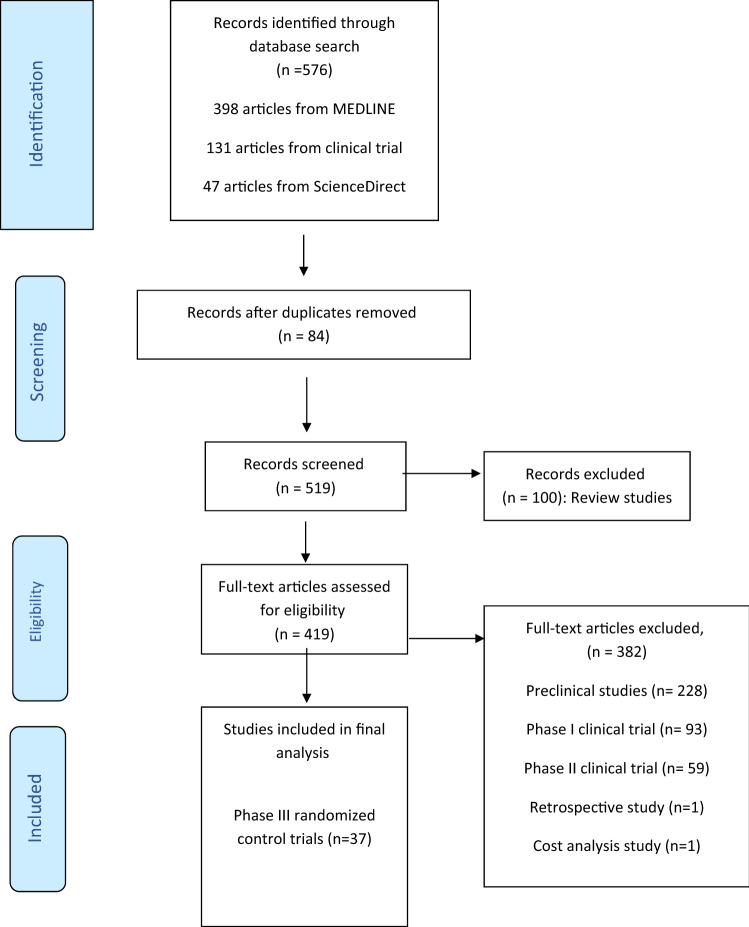
We used the following search terms (Fig. 1): Delafloxacin, Ridinilazole, Afabicin, Gepotidacin, Meropenem–Vaborbactam, Imipenem–Relebactam, Cefepime “AAI101”, Sulbactam–Diazabicyclooctane, Plazomicin, Cefiderocol, Cefilavacin, Nafithromycin, Eravacycline, Lefamulin, Sulopenem Zoliflodacin, Omadacycline, Iclaprim, Solithromycin, Levonadifloxacin, Contezolid, Pretomanid, Taniborbactam, DprE1 inhibitor, Lascufloxacin and the umbrella term “novel antibiotics”.
Fig. 1.
A flow diagram of the search strategy according to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines
Selection criteria
Four investigators independently extracted the data from the full text of the selected literature. We selected antibiotics currently in phase III new drug application (NDA), or were FDA approved. We also included drug trials and NDA in China, India, and Japan. We excluded phase I and phase II clinical trials, observational studies, case reports, cost analysis studies, and animal models. In the data extraction form, we included method-of-action, spectrum of activity, data from clinical trials, and the included antibiotics’ adverse event profiles. Two reviewers assessed the quality of the included literature independently. The information extracted was compared to information provided on the drug manufacturer’s website to confirm its development phase. We relied on data published in databases to confirm the spectrum of activity and adverse events.
Results
Of the 576 articles identified from database searching, 37 phase III clinical trials were included. Table 1 shows a summary of the reviewed antibiotics in the pipeline and their spectrum of activities.
Table 1.
Summary of antibiotics in the pipeline and their spectrum of activities
| Name | FDA approved | EMA Approved | Antibiotic class | Indication/usage | CRAB | CRPA | CRE | KPC | MRSA | VRE | ESBL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | ||||||||||||
| 1. | Delafloxacin | ✓ | ✓ | Fluoroquinolone | ABSSSI | X | X | X | X | ✓ | X | X |
| 2. | Meropenem/vaborbactam | ✓ | ✓ | Carbapenem/β-lactamases inhibitor | cUTI; cIAI | X | X | X | ✓ | X | X | X |
| 2018 | ||||||||||||
| 1. | Plazomicin | ✓ | ✓ | Aminoglycoside | cUTI | X | X | ✓ | X | ✓ | X | ✓ |
| 2. | Eravacycline | ✓ | ✓ | Tetracycline | cIAI | ✓ | X | X | X | ✓ | ✓ | ✓ |
| 3. | Omadacycline | ✓ | X | Tetracycline | CAP; ABSSSI | X | X | X | ✓ | ✓ | ✓ | |
| 2019 | ||||||||||||
| 1. | Lefamulina | X | X | Pleuromutilin | CABP | X | X | X | X | ✓ | ✓ | X |
| 2. | Cefiderocol | ✓ | ✓ | Cephalosporin | cUTI, HAP/VAP, bloodstream infection, and sepsis | ✓ | ✓ | ✓ | ✓ | X | X | ✓ |
| 3. | Pretomanid | ✓ | ✓ | Nitroimidazooxazine | XDR-TB; MDR-TB | |||||||
| 2020 | ||||||||||||
| 1. | Levonadifloxacin(EMROK)/alalevonadifloxacin; (approved by DCGI for use in India) | X | X | Fluoroquinolone | ABSSSI | ✓ | ||||||
| 2. | Iclaprima | X | Withdrawn | DHFR inhibitors | ABSSSI | X | X | X | X | ✓ | X | X |
| 3. | Imipenem/cilastatin + relebactam | ✓ | Carbapenem/β-lactamases inhibitor | cUTI, AP, cIAI, HAP, VAP | X | ✓ Except MBL Producer | ✓ | ✓ | X | |||
| Phase III trial | ||||||||||||
| 1. | Solithromycin | (Phase III) | 4th Generation macrolide | CAP | X | X | X | X | X | X | X | |
| 2. | Sulopenem | (Phase III) | Thiopenem b-lactam | UTI; cIAI | X | X | X | X | X | X | ✓ | |
| 3. | Cefilavacin | (Phase III) | Glycopeptide-β-lactam (Cephalosporin) hybrid | ABSSSI | X | X | X | X | ✓ | ✓ | X | |
| 4. | Cefepime + AAI101 | (Phase III) | 4th generation cephalosporin/β-lactamases inhibitor | cUTI | X | X | ✓ | X | X | X | ✓ | |
| 5. | Ridinilazole | (Phase III) | Inhibition of cell division, inhibition of toxin production | CDI | X | X | X | X | X | X | X | |
| 6. | Gepotidacin | (Phase III) | Triazaacenaphthylene | ABSSSI; Uncomplicated urogenital gonorrhea | X | X | X | X | ✓ | X | X | |
| 7. | Sulbactam/diazabicyclooctane | (Phase III) | β-Lactam/β-lactamases inhibitor | cUTI | ✓ | X | ✓ | X | X | X | ✓ | |
| 8. | Zoliflodacin | (Phase III) | Spiropyrimidinetrione | Uncomplicated gonorrhea | X | X | X | X | X | X | X | |
| 9. | Taniborbactam | (Phase III) | β-Lactam/β-lactamases inhibitor | cUTI | X | ✓ | ||||||
| 11. | Contezolid | (Phase III in china whereas phase II in USA) | Oxazolidinone | ABSSSI | ✓ | |||||||
CRAB, carbapenem-resistant Acinetobacter baumannii; CRPA, carbapenem-resistant Pseudomonas aeruginosa; CRE, carbapenem-resistant Enterobacteriaceae; KPC, Klebsiella pneumoniae carbapenemase; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococcus; ESBL, extended-spectrum beta-lactamases; ABSSSI, acute bacterial skin and skin structure infections; cUTI, complicated urinary-tract infections; cIAI, complicated intra-abdominal infections; CAP, community-acquired pneumonia; CABP, community-acquired bacterial pneumonia; HAP/VAP, hospital-acquired pneumonia (nosocomial pneumonia) and ventilator-associated pneumonia; XDR-TB, extensively drug-resistant tuberculosis; MDR-TB, multi-drug-resistant tuberculosis; CDI, Clostridium difficile infection
aNew drug application (NDA)
Delafloxacin
Delafloxacin is a novel anionic fluoroquinolone antibiotic approved by the FDA on June 19th, 2017 [4]. It has the European Medicines Agency’s (EMA) approval for the treatment of acute bacterial skin and skin structure infections (ABSSSI) [5]. Delafloxacin inhibits DNA replication, transcription, repair, and recombination by inhibiting the two primary enzymes: DNA gyrase (topoisomerase II) and topoisomerase IV enzymes [6]. Delafloxacin, as other fluoroquinolones, has a high pulmonary concentration of 13:1 compared to plasma and is primarily (65%) excreted as unchanged in the urine. Delafloxacin does not seem to prolong the QTc interval on ECG or cause phototoxicity [6]. Similar to other fluoroquinolones, delafloxacin has concentration-dependent antimicrobial activity. It has a low minimum inhibitory concentration (MIC) against Streptococcus pneumoniae and Staphylococcus aureus (including MRSA). Some levofloxacin-resistant S. pneumoniae strains are susceptible to delafloxacin. Delafloxacin is also active against Pseudomonas aeruginosa, anaerobes, and atypical organisms [6].
Delafloxacin was shown to be non-inferior to vancomycin plus aztreonam in the treatment of ABSSSI [7, 8]. In the PROCEED study, 660 adult patients received either delafloxacin 300 mg IV every 12 h or vancomycin IV 15 mg/kg every 12 h plus aztreonam IV 2 g every 12 h [7]. In an intent-to-treat (ITT) analysis, the response was similar in both arms (78.2% in delafloxacin versus 80.9% in vancomycin + aztreonam arm). Adverse event incidence was higher in the vancomycin plus aztreonam arm (4.5% versus 0.9%) [7]. In the second trial, delafloxacin was compared with vancomycin plus aztreonam in 850 adults [8]. In ITT analysis, response was similar in both arms (83.7% in delafloxacin arm versus 80.6% in vancomycin + aztreonam arm) [8]. Vancomycin plus aztreonam had a higher adverse events than delafloxacin (1.2% versus 2.4%) [8]. The efficacy and safety of delafloxacin in community-acquired bacterial pneumonia (CABP) was compared with moxifloxacin in a phase III, multi-center, randomized trial (DEFINE-CABP) [9]. The study showed similar early clinical response of about 89% in each arm [9].
Meropenem/vaborbactam
Meropenem/vaborbactam is a fixed combination of meropenem and vaborbactam and was approved by FDA on August 29th, 2017 for treatment of complicated urinary tract infection (cUTI) including pyelonephritis [4] and by EMA on September 20th, 2018 for treatment of cUTI including pyelonephritis, complicated intra-abdominal infection (cIAI), and hospital-acquired bacterial pneumonia (HABP), ventilator-associated bacterial pneumonia (VABP) [10]. Vaborbactam does not have any anti-bacterial activity and its main function is to protect meropenem from degradation by B-lactamases especially Klebsiella pneumoniae carbapenemase (KPC). However, verobactam has no activity against OXA-48 and Metallo-β-lactamases (MBL) carbapenemases or carbapenem-resistant P. aeruginosa [10].
The TANGO I is a phase III randomized clinical trial and it compared meropenem–vaborbactam and piperacillin–tazobactam for 10 days in 550 adult patients with cUTI [11]. The overall success rate was higher in the meropenem/vaborbactam arm (98.4% versus 94%) in a non-inferiority trial. Microbial eradication in the modified ITT analysis occurred in 66.7% of the meropenem–vaborbactam arm compared with 57.7% in the piperacillin–tazobactam group [11]. Subsequently, in the TANGO II phase 3 randomized clinical trial, the study evaluated the efficacy and safety of meropenem–vaborbactam in 77 adults with carbapenem-resistant Enterobacteriaceae (CRE) confirmed or suspected infections versus best available therapy (BAT) [12]. In microbiologic CRE-modified ITT, meropenem–vaborbactam achieved a higher clinical cure with no difference in microbiologic cure at the end of therapy [12]. There was no significant difference in the mortality at day 28 (15.6% versus 33.3% P = 0.20). Meropenem–vaborbactam had fewer adverse events (84% versus 92%) and fewer renal adverse event (4% versus 24%) [12]. The efficacy and safety evaluations of meropenem–vaborbactam in adults with HABP or VABP are expected to be completed in December 2020 in a phase IIIb TANGO III randomized clinical trial [13].
Imipenem–cilastatin + relebactam
Imipenem–cilastatin + relebactam is a fixed combination of imipenem, cilastatin, an imipenem renal metabolism inhibitor, and relebactam, a b-lactamase inhibitor. Relebactam is active against Class A (including ESBL and KPC) and Class C (AmpC) b-lactamases, has activity against ESBL-producing Enterobacteriaceae, KPC, CRE, and possibly carbapenem-resistant P. aeruginosa. It has no activity against MBL and OXA-48 producers, Acinetobacter baumannii, or Stenotrophomonas maltophilia [14].
RESTORE-IMI 1 was a phase III randomized trial in 47 adults with HAP/VAP, cIAI, and cUTI caused by Gram-negative imipenem-resistant organisms [15–17]. The treated patients received either imipenem + cilastatin + relebactam or colistin base activity (CBA) plus imipenem + cilastatin. In supplemental microbiological modified ITT, the overall response was 75% in imipenem/relebactam group compared with 76.9% in colistin/imipenem group (difference − 4.5; 95% CI − 24.2 to 20.7). All-cause 28-day mortality in imipenem/relebactam compared with colistin/imipenem was 10.7% versus 23.1%, respectively [15].
RESTORE-IMI 2 trial was completed on April 3rd, 2019 and was a phase III clinical trial comparing imipenem + cilastatin + relebactam with piperacillin + tazobactam in 536 adults with HAP/VAP and all patients also received linezolid. Imipenem/cilastatin/relebactam was non-inferior (P < 0.001) to piperacillin/tazobactam when comparing 28-day all-cause mortality (15.9% vs. 21.3%) and showed a favorable clinical response (61.0% and 55.8%) at early follow-up [18].
Plazomicin
Plazomicin is a novel aminoglycoside antibiotic approved by the FDA on June 25th, 2018 and by the EMA on March 19th, 2018 to treat cUTI. Plazomicin binds to the 30S ribosomal subunit and inhibits protein synthesis in a concentration-dependent manner. It has activity against ESBL-producing Enterobacteriaceae, CRE, MRSA, and organisms producing aminoglycoside-modified enzymes. Like other aminoglycosides, plazomicin is associated with nephrotoxicity, ototoxicity and fetal harm in pregnant women [19].
A phase III randomized trial compared plazomicin with meropenem in 609 adults with cUTI or acute pyelonephritis followed by optional oral therapy. The microbiologic eradication rate at test-of-cure (TOC) visit was higher in plazomicin group vs. meropenem group (81.7% vs 70.1%; 95% CI 2.7–20.3) [20]. In Phase III open-label, non-inferiority (CARE) trial, 39 adults with bloodstream infection, HABP, or VABP due to CRE were treated with plazomicin and all-cause mortality at day 28 or significant complications was 23.5% in the plazomicin arm compared with 50% of the patients in colistin arm [21].
Eravacycline
Eravacycline is a novel fluorocycline of the tetracyclines group and as such inhibits bacterial protein synthesis by binding to 30S ribosomal subunit. Eravacycline overcomes tetracycline-efflux and ribosomal protection mechanism. Eravacycline has activity against MRSA, VRE, ESBL Enterobacteriaceae, A. baumannii, and CRE, but has no activity against P. aeruginosa and Burkholderia cenocepacia [22, 23]. In a phase III, randomized, double-blind (IGNITE 1) clinical trial, an MITT showed a clinical cure of 87% in the eravacycline arm compared to 88.8% in the ertapenem arm [23]. Microbiological cure was 91.4% in the eravacycline group versus 95% in ertapenem group [23]. In the IGNITE4, a second phase III clinical trial, he clinical cure was 90.8% compared to 91.2% in the eravacycline and meropenem groups, respectively [24].
Omadacycline
Omadacycline is a tetracycline antibiotic and was approved in 2018 for treatment of CABP and ABSSSI. It overcomes the resistance by tetracycline-efflux and ribosomal protection mechanisms and has activity against Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydia pneumoniae [25–27]. Thus, it can be used as a single agent to treat CABP as an alternative to the empirical combination of beta-lactam and macrolide.
The efficacy and safety of omadacycline were tested on a phase III randomized control trial (OPTIC) comparing omadacycline in 388 patients with moxifloxacin in 386 patients with CABP followed by oral omadacycline or Moxifloxacin [28]. The early clinical response was 81.1% in omadacycline group compared with 82.7% in the comparator group [28]. In the post-treatment evaluation, clinical response rate was 87.6% in omadacycline compared with 85.1% in moxifloxacin arm [28]. The rate of adverse events leading to treatment discontinuation was 5.5% in omadacycline compared with 7% in moxifloxacin [28].
Omadacycline had been tested in 316 patients and linezolid in 311 patients with ABSSSI in phase 3 randomized controlled (OASIS-1) trial of either omadacycline or linezolid followed by oral omadacycline or linezolid [29]. In the MITT, the early response rate was 84.8% vs. 85.5%, respectively, compared with linezolid. [29]. The clinical response of omadacycline was 83% for MRSA compared with 86% in the linezolid arm. Treatment-related adverse events were 18% in omadacycline arm versus 18.3% with linezolid [29].
Lefamulin
Lefamulin is a novel pleuromutilin antibiotic and was approved in August 2019 by U.S. FDA for use in CABP. Lefamulin inhibits protein synthesis by inhibition of 50S bacterial ribosome. It has activity against S. pneumoniae, MRSA, VRE, MDR Neisseria gonorrhoeae, Chlamydophila pneumonia, L. pneumophila, M. pneumoniae, and Haemophilus influenzae. Lefamulin has a time-dependent killing with higher concentrations in epithelial lining fluid than in plasma [30]. Lefamulin was non-inferior to moxifloxacin in 551 adults with CABP in a phase III (LEAP-1) clinical trial [31]. Early clinical response was 87.3% versus 90.2%[31]. The rate of drug discontinuation was 2.9% in the lefamulin arm and 4.4% in the moxifloxacin arm [31]. In the second phase III clinical trial (LEAP-2), oral lefamulin was compared to moxifloxacin in 738 patients with CABP [32]. Lefamulin was non-inferior to moxifloxacin for CABP (90.8% versus 90.8%), clinical response (87.5% versus 89.1%), and clinically evaluable population (89.7% versus 93.6%) [32].
Cefiderocol
Cefiderocol is the first in a class of siderophore cephalosporins with activity against carbapenemase-producing Gram-negative bacteria (CRE, CRPA, and CRAB), MDR S. maltophilia, and Burkholderia cepacia, and ESBL- and MBL-producing organisms. Potential indications include cUTI, HAP/VAP, bloodstream infection, and sepsis caused by MDR Gram-negative isolate [33, 34]. A phase III trial compared cefiderocol in APEKS-NP trial with meropenem in 300 adults with healthcare-associated pneumonia (HCAP), HABP, or VABP with all-cause mortality at day 14 of 12·4% vs. 11·6%, respectively [35]. The second Phase III clinical trial (CREDIBLE-CR) will compare cefiderocol with best available therapy in 150 adults with HAP, VAP, HCAP, cUTI, or BSI/Sepsis caused by carbapenem-resistant Gram-negative pathogens [36].
Levonadifloxacin/alalevonadifloxacin
Levonadifloxacin and its prodrug, alalevonadifloxacin, are broad-spectrum benzoquinolizine sub-class of quinolones and are active against multi-drug-resistant Gram-positive pathogens including MRSA, hVISA, VRSA, and quinolone-resistant strains [36, 37]. A phase III clinical trial compared oral/IV levonadifloxacin to oral/IV Linezolid in a multi-center, randomized, open-label trial [38]. Clinical cure rates for levonadifloxacin were higher compared to linezolid in the IV sub-group (91.0% versus 87.8%) and in the oral sub-group (95.2% versus 93.6%) [38].
Pretomanid
Pretomanid is a nitroimidazooxazine antibiotic [37] and is being proposed for use in a combination regimen to treat adults with pulmonary XDR-TB, or treatment-intolerant or nonresponsive MDR-TB [39]. It exhibits both mycobactericidal activity against replicating and static M. tuberculosis [39]. In a phase III trial, 11 patients (10%) from 109 had an unfavorable outcome (7 deaths, 2 relapses, 1 lost to follow-up, and 1 withdrawal of treatment) [40].
Iclaprim
Iclaprim is a dihydrofolate reductase inhibitor (DHFR) which inhibits bacterial nucleic acid and protein synthesis and has superior activity to trimethoprim and overcomes trimethoprim resistance. It is bactericidal against Gram-positive MDR bacteria. Two phase III trials (ASSSIST 1 and 2) compared IV iclaprim and IV linezolid in the treatment of complicated ABSSSI. These studies failed to show non-inferiority of iclaprim and caused QT-prolongation [41, 42]. A two-phase III clinical trial (REVIVE-1 and 2) assessed iclaprim non-inferiority against vancomycin in ABSSSI [43, 44]. In REVIVE-1, early clinical response was 83.5% and 79.7% to iclaprim and vancomycin, respectively. In REVIVE-2, the clinical response was 82.7% in the iclaprim arm and 76.3% in the vancomycin arm [43, 44].
Sulopenem
Sulopenem is a novel thiopenem B-lactam antibiotic, developed as a prodrug of sulopenem-etzadroxil for therapy of UTI and cIAI. It has good activity against ESBL-producing Enterobacteriaceae [45, 46]. The first Phase III clinical trial (SURE 1) will evaluate the efficacy and safety of PO sulopenem-etzadroxil/probenecid versus ciprofloxacin PO in women with uncomplicated UTI [47]. The second ongoing phase III (SURE 2) clinical trial is comparing sulopenem IV followed by sulopenem-etzadroxil/probenecid PO versus ertapenem IV followed by ciprofloxacin PO or amoxicillin-clavulanate PO in adults with cUTI [48]. The SURE 3 trial is the third, ongoing, phase III clinical trial comparing sulopenem IV followed by sulopenem-etzadroxil/probenecid PO to ertapenem IV followed by ciprofloxacin PO or amoxicillin–clavulanate PO in adults with cIAI [49].
Contezolid
Contezolid (MRX-I) is an oxazolidinone and is being considered for the treatment of complicated skin and soft tissue infections (cSSTI) caused by resistant Gram-positive bacteria [50]. It has activity against MRSA, vancomycin-resistant E. faecium, and resistant S. pneumoniae. A Phase II trial of contezolid acefosamil in patients with ABSSSI has been completed (NCT02269319) in the United States by MicuRx, and compared MRX-I (Contezolid) with vancomycin [50]. In a phase III clinical trial, contezolid was non-inferior (93.0%) compared to linezolid (93.4%) for the clinical cure rate of patients with cSSSIs [51].
Solithromycin
Solithromycin is a novel, fourth-generation macrolide, known as fluoroketolide, which inhibits protein synthesis by binding to bacterial ribosome. It has activity against macrolide-resistant S. pneumonia, H. Influenzae, and atypical pathogens with potential indication for use in CABP [52, 53]. Oral solithromycin was non-inferior to oral moxifloxacin in 860 adults with CABP in a phase III trial (SOLITAIRE-ORAL) [54]. Early clinical response was 78.2% versus 77.9% Elevation of ALT was observed in 5.4% of the solithromycin group compared with 3.3% in moxifloxacin group and elevated AST in 2.5% of solithromycin group compared with 1.9% in the comparator group [54]. Solithromycin (IV to PO) was non-inferior to moxifloxacin (IV to PO)in 863 adults with CABP in a phase III clinical trial (SOLITAIRE-IV) [55]. The early clinical response in ITT was 79.3% in solithromycin arm compared with 79.7% in moxifloxacin arm with a higher rate of adverse drug reactions in the solithromycin arm [55]. The efficacy of PO solithromycin compared with standard treatment (IV ceftriaxone with oral azithromycin) in 264 adults with uncomplicated gonorrhea has been tested in a phase III clinical trial (SOLITAIRE-U) [56]. The cure rate was 80.5% in solithromycin arm compared with 84.5% in ceftriaxone/azithromycin arm [56].
Cefepime + AAI101 (Enmetazobactam)
Cefepime + AAI101 is a B-lactam and B-lactamase inhibitor [57]. AAI101 is a novel inhibitor of ESBL, and classes A and D carbapenemases [57, 58]. The addition of AAI101 to cefepime resulted in a significant in vivo reduction in the MIC50 against Enterobacteriaceae isolates [57]. Cefepime/AAI101 is being studied in a phase III randomized clinical trial in cUTI adults in comparison with piperacillin/tazobactam. The study was started on September 24, 2018 and completed on January 30th, 2020 (Clinical registration NCT03687255) [59].
Ridinilazole
Ridinilazole is a novel, non-absorbable, oral antimicrobial and is restricted to the gastrointestinal tract. An in vitro study showed that ridinilazole is a potent inhibitor of C. difficile by inhibiting cell division and reducing toxin production [60, 61]. Ridinilazole is being studied in a phase III randomized, controlled trial in comparison with fidaxomicin in one study (Clinical registration NCT02784002) [62] and with vancomycin (Clinical registration NCT03595566) in another study [63] for the treatment of C. difficile infection.
Gepotidacin
Gepotidacin (GSK2140944) is a novel triazaacenaphthylene antimicrobial agent and is an inhibitor of bacterial type II topoisomerase [64]. It shows excellent activity against MDR N. gonorrhoeae and Gram-positive bacteria including MRSA [65]. It had undergone phase II trial for the treatment of uncomplicated urogenital gonorrhea (Clinical registration NCT02294682) [66] and in treatment of ABSSSI caused by Gram-positive bacteria (Clinical registration NCT02045797) [67]. It is currently being used in a phase III, randomized study comparing efficacy and safety of Gepotidacin to Nitrofurantoin in uncomplicated urinary tract infection (Clinical registration—NCT04020341) [68].
Sulbactam/Diazabicyclooctane
Sulbactam/Diazabicyclooctane is a combination of B-lactam/B-lactamase inhibitors with a wide range of B-lactamases inhibition, including class A, C, D, CRE, and CRAB [69]. A Phase III randomized study will evaluate efficacy and safety of intravenous Sulbactam-ETX2514 in treating patients with A. baumannii-calcoaceticus Complex infection (Clinical registration NCT03894046) [70].
Zoliflodacin
Zoliflodacin is a novel Spiropyrimidinetrione is a type II topoisomerase inhibitor with a good activity against MDR N. gonorrhea [71]. Another non-inferiority phase III clinical trial is evaluating the safety and efficacy of zoliflodacin vs. a combination of ceftriaxone and azithromycin for the treatment of uncomplicated gonorrhea (Clinical registration NCT03959527) [72].
Taniborbactam
Taniborbactam is an injectable, beta-lactamase inhibitor. Taniborbactam (VNRX-5133) can inhibit metallo-beta-lactamase and serine-beta-lactamases and has a broad-spectrum inhibitory activity against Ambler Class A (ESBLs), B (NDM and VIM), C (AmpC from P. aeruginosa) and, to a lesser extent, D (OXA) β-lactamase [72]. It is currently undergoing phase III clinical trial (ClinicalTrials.gov—NCT03840148) comparing cefepime–taniborbactam to meropenem in adults with cUTI [73].
Discussion
The current review details the clinical outcome of the use of the novel antibiotics in the pipeline. We found that the “novel” antibiotics were often based on previously known molecules or pre-existing antimicrobial agents. With regard to the spectra of activity, there are a limited number of new antibiotics against high priority organisms such as multi-drug-resistant P. aeruginosa, and carbapenem-resistant Enterobacteriaceae. These antibiotics include: plazomicin [21], eravacycline [22, 23], imipenem–cilastatin + relebactam [14–18], Sulbactam/Diazabicyclooctane [70] and Cefiderocol [33–36]. This is an important deficit as the acute shortage is particularly challenging for the “priority organisms”. The issue of the need for new antimicrobial agents for priority organisms had been on the radar of the World Health Organization (WHO) for some time. The WHO priority list includes: 1) three “critical” organisms (A. baumannii, carbapenem-resistant P. aeruginosa, carbapenem-resistant and 3rd-generation cephalosporin-resistant Enterobacteriaceae); 2) six “high priority” organisms (Enterococcus faecium, S. aureus (vancomycin-resistant, methicillin-resistant, vancomycin intermediate and resistant), clarithromycin-resistant Helicobacter pylori, fluoroquinolone-resistant Campylobacter, fluoroquinolone-resistant Salmonella spp., 3rd-generation cephalosporin-resistant fluoroquinolone-resistant N. gonorrhoeae; and 3) three “medium priority” organisms (penicillin-non-susceptible S. pneumoniae, ampicillin-resistant H. influenzae, fluoroquinolone-resistant Shigella spp.) [74]. There is still a need to have further studies addressing these priority organisms as suggested by the WHO especially multi-drug-resistant tuberculosis and Gram-negative bacteria [75]. Of the included studies, few studies addressed healthcare-associated pneumonia. However, the burden of drug-resistant bacteria is high among these types of infections. This is an added issue to the clinical trial for the therapy of MDRO or pan-drug-resistant organism therapy. One of the issues of such trials is the use of strict definitions and the need to include one source of infection such as bloodstream infection [76]. Another difficulty is the difficulty in recruiting patients with MDRO. One study showed that out of the 2100 screened patients only 37 patients were randomized [77]. It had been suggested that such trials should include observational studies that are planned and executed in a way to reduce bias in the search of therapy for MDRO [76]. How scientists could run such observational studies giving the different predictors of mortality including the site of infection, gender, comorbidities and the implicated organism is an important question to address [78].
The strength of our review is the thorough and comprehensive searches of platforms, clinical trial registries, databases and pharmaceutical company websites. The review is limited by the heterogenicity of included studies and difficulties in the quantification of comparator arms. The regulatory requirements for the registration of antibiotics vary between the US and the EU and some of the drug approval status may quickly change. However, the review highlights a pipeline paucity of these essential drugs. This problem may be further enhanced by the current COVID-19 pandemic where there may be lack of economic support for developing new agents reinforcing the need for international cooperation and coordination [79]. There is also a concern regarding the increased costs of these new antimicrobial agents for the treatment of MDR-organisms. In one study, the estimated cost for the treatment of methicillin-resistant S. aureus bacteremia is 6–60 times the cost of older antibiotics and that for carbapenem-resistant Enterobacterales or MDR P. aeruginosa or carbapenem-resistant A. baumannii is 2–20 times that of the older medications [80]. Clearly, there must be incentives for the industry to develop novel antibiotics and R&D incentive strategies ranging from single rewards to complex international models are needed to push future development. There is no alternative—when the pipeline runs dry, MDR-organisms will rule the world.
Declarations
Conflict of interest
All the authors have no conflicts of interest.
Footnotes
The original online version of this article was revised: Modifications have been made to text (Section Omadacycline) and also to Table 1. Full information regarding the corrections made can be found in the erratum/correction for this article.
Change history
3/10/2022
A Correction to this paper has been published: 10.1007/s15010-022-01776-0
References
- 1.Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Tawfiq JA, Stephens G, Memish ZA. Inappropriate antimicrobial use and potential solutions: a Middle Eastern perspective. Expert Rev Anti Infect Ther. 2010;8:765–774. doi: 10.1586/eri.10.56. [DOI] [PubMed] [Google Scholar]
- 3.Towse A, Hoyle CK, Goodall J, Hirsch M, Mestre-Ferrandiz J, Rex JH. Time for a change in how new antibiotics are reimbursed: development of an insurance framework for funding new antibiotics based on a policy of risk mitigation. Health Policy. 2017;121:1025–1030. doi: 10.1016/J.HEALTHPOL.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 4.FDA. Novel drug approvals for 2017 n.d. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017. Accessed 20 Nov 2020.
- 5.Medicines Agency E. European Medicines Agency decision P/0148/2018 of 18 May 2018 on the review of a granted waiver for delafloxacin (EMEA-001080-PIP01–10). n.d.
- 6.Jorgensen SCJ, Mercuro NJ, Davis SL, Rybak MJ. Delafloxacin: place in therapy and review of microbiologic, clinical and pharmacologic properties. Infect Dis Ther. 2018;7:197–217. doi: 10.1007/s40121-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullman J, Gardovskis J, Farley B, Sun E, Quintas M, Lawrence L, et al. Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: A Phase 3, double-blind, randomized study. J Antimicrob Chemother. 2017;72:3471–3480. doi: 10.1093/jac/dkx329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Riordan W, McManus A, Teras J, Poromanski I, Cruz-Saldariagga M, Quintas M, et al. A Comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a phase 3, multinational, double-blind, randomized study. Clin Infect Dis. 2018;67:657–666. doi: 10.1093/cid/ciy165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horcajada JP, Salata RA, Álvarez-Sala R, Nitu FM, Lawrence L, Quintas M, et al. A phase 3 study to compare delafloxacin with moxifloxacin for the treatment of adults with community-acquired bacterial pneumonia (Define-CABP) Open Forum Infect Dis. 2020 doi: 10.1093/ofid/ofz514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon S. Meropenem/vaborbactam: a review in complicated urinary tract infections. Drugs. 2018;78:1259–1270. doi: 10.1007/s40265-018-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaye KS, Bhowmick T, Metallidis S, Bleasdale SC, Sagan OS, Stus V, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection the TANGO I randomized clinical trial. JAMA. 2018;319:788–799. doi: 10.1001/jama.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melinta Therapeutics I. A study of Meropenem-Vaborbactam versus Piperacillin/Tazobactam in participants with hospital-acquired and ventilator-associated bacterial pneumonia—full text view—ClinicalTrials.gov 2016. https://clinicaltrials.gov/ct2/show/NCT03006679. Accessed 28 Nov 2020.
- 14.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, et al. Imipenem-relebactam and meropenem–vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs. 2018;78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 15.Kaye K, File T, Boucher HW, Brown M, Aggrey A, Khan I, et al. 1339. Results for the supplemental microbiological modified intent-to-treat (SmMITT) population of the RESTORE-IMI 1 trial of imipenem/cilastatin/relebactam (IMI/REL) vs. imipenem/cilastatin plus colistin (IMI+CST) in patients with Imipenem-nonsusceptible (NS) bacterial infections. Open Forum Infect Dis. 2018;5:S409–S409. doi: 10.1093/ofid/ofy210.1171. [DOI] [Google Scholar]
- 16.Brown ML, Motsch J, Kaye KS, File TM, Boucher HW, Vendetti N, et al. Evaluation of renal safety between imipenem/relebactam and colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections in the randomized, Phase 3 RESTORE-IMI 1 Study. Open Forum Infect Dis. 2020 doi: 10.1093/ofid/ofaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaye KS, Boucher HW, Brown ML, Aggrey A, Khan I, Joeng HK, et al. Comparison of treatment outcomes between analysis populations in the RESTORE-IMI 1 phase 3 trial of imipenem-cilastatin-relebactam versus colistin plus imipenem-cilastatin in patients with imipenem-nonsusceptible bacterial infections. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.02203-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Titov I, Wunderink RG, Roquilly A, Rodríguez Gonzalez D, David-Wang A, Boucher HW, et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 Study) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaeer KM, Zmarlicka MT, Chahine EB, Piccicacco N, Cho JC. Plazomicin: a next-generation aminoglycoside. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:77–93. doi: 10.1002/phar.2203. [DOI] [PubMed] [Google Scholar]
- 20.Cloutier DJ, Komirenko AS, Cebrik DS, Keepers TR, Krause KM, Connolly LE, et al. Plazomicin vs. meropenem for complicated urinary tract infection (cUTI) and acute pyelonephritis (AP): diagnosis-specific results from the phase 3 EPIC study. Open Forum Infect Dis. 2017;4:S532–S532. doi: 10.1093/ofid/ofx163.1385. [DOI] [Google Scholar]
- 21.McKinnell JA, Connolly LE, Pushkin R, Jubb AM, O’Keeffe B, Serio AW, et al. Improved outcomes with plazomicin (PLZ) compared with colistin (CST) in patients with bloodstream infections (BSI) caused by carbapenem-resistant enterobacteriaceae (CRE): results from the CARE study. Open Forum Infect Dis. 2017;4:S531–S531. doi: 10.1093/ofid/ofx163.1383. [DOI] [Google Scholar]
- 22.Sutcliffe JA, O’Brien W, Fyfe C, Grossman TH. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother. 2013;57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis. 2019;69:921–929. doi: 10.1093/cid/ciy1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial a randomized clinical trial. JAMA Surg. 2017;152:224–232. doi: 10.1001/jamasurg.2016.4237. [DOI] [PubMed] [Google Scholar]
- 25.Cho JC, Childs-Kean LM, Zmarlicka MT, Crotty MP. Return of the tetracyclines: omadacycline, a novel aminomethylcycline antimicrobial. Drugs Today. 2018;54:209–217. doi: 10.1358/dot.2018.54.3.2800620. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorganic Med Chem. 2016;24:6409–6419. doi: 10.1016/j.bmc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Chambers HF. Omadacycline—the newest tetracycline. N Engl J Med. 2019;380:588–589. doi: 10.1056/nejme1900188. [DOI] [PubMed] [Google Scholar]
- 28.Stets R, Popescu M, Gonong JR, Mitha I, Nseir W, Madej A, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med. 2019;380:517–527. doi: 10.1056/nejmoa1800201. [DOI] [PubMed] [Google Scholar]
- 29.O’Riordan W, Green S, Overcash JS, Puljiz I, Metallidis S, Gardovskis J, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med. 2019;380:528–538. doi: 10.1056/nejmoa1800170. [DOI] [PubMed] [Google Scholar]
- 30.Veve MP, Wagner JL. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018;38:935–946. doi: 10.1002/phar.2166. [DOI] [PubMed] [Google Scholar]
- 31.File TM, Goldberg L, Das A, Sweeney C, Saviski J, Gelone SP, et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the phase III lefamulin evaluation against pneumonia (LEAP 1) trial. Clin Infect Dis. 2019;69:1856–1867. doi: 10.1093/cid/ciz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander E, Goldberg L, Das A, Moran GJ, Sandrock C, Gasink LB, et al. LB6. Oral lefamulin is safe and effective in the treatment of adults with community-acquired bacterial pneumonia (CABP): results of lefamulin evaluation against pneumonia (LEAP 2) study. Open Forum Infect Dis. 2018;5:S761–S761. doi: 10.1093/ofid/ofy229.2180. [DOI] [Google Scholar]
- 33.Kish T. New antibiotics in development target highly resistant gram-negative organisms. Pharm Ther. 2018;43:116–120. [PMC free article] [PubMed] [Google Scholar]
- 34.Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother. 2018 doi: 10.1128/AAC.02163-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wunderink RG, Matsunaga Y, Ariyasu M, Clevenbergh P, Echols R, Kaye KS, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021;21:213–225. doi: 10.1016/S1473-3099(20)30731-3. [DOI] [PubMed] [Google Scholar]
- 36.Shionogi. Study of S-649266 or best available therapy for the treatment of severe infections caused by carbapenem-resistant gram-negative pathogens—full text view—ClinicalTrials.gov 2016. https://clinicaltrials.gov/ct2/show/NCT02714595. Accessed 13 Dec 2020.
- 37.Jacobs MR, Bajaksouzian S, Windau A, Appelbaum PC, Patel MV, Gupte SV, et al. In vitro activity of the new quinolone WCK 771 against staphylococci. Antimicrob Agents Chemother. 2004;48:3338–3342. doi: 10.1128/AAC.48.9.3338-3342.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatia A, Mastim M, Shah M, Gutte R, Joshi P, Kumbhar D, et al. Efficacy and safety of a novel broad-spectrum anti-MRSA agent levonadifloxacin compared with linezolid for acute bacterial skin and skin structure infections: a phase 3, openlabel, randomized study. J Assoc Physicians India. 2020;68:30–36. [PubMed] [Google Scholar]
- 39.Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 40.Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893–902. doi: 10.1056/nejmoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang DB, Strader CD, MacDonald JS, VanArendonk M, Peck R, Holland T. An updated review of iclaprim: a potent and rapidly bactericidal antibiotic for the treatment of skin and skin structure infections and nosocomial pneumonia caused by gram-positive including multidrug-resistant bacteria. Open Forum Infect Dis. 2018;5:ofy003. doi: 10.1093/ofid/ofy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbas M, Paul M, Huttner A. New and improved? A review of novel antibiotics for Gram-positive bacteria. Clin Microbiol Infect. 2017;23:697–703. doi: 10.1016/j.cmi.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Huang DB, Corey GR, Holland TL, Lodise T, O’Riordan W, Wilcox MH, et al. Pooled analysis of the phase 3 REVIVE trials: randomised, double-blind studies to evaluate the safety and efficacy of iclaprim versus vancomycin for treatment of acute bacterial skin and skin-structure infections. Int J Antimicrob Agents. 2018;52:233–240. doi: 10.1016/j.ijantimicag.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Noviello S, Ralph Corey G, Holland TL, Lodise T, O’Riordan W, Wilcox MH, et al. A pooled analysis of patients with wound infections in the Phase 3 REVIVE trials: randomized, double-blind studies to evaluate the safety and efficacy of iclaprim versus vancomycin for treatment of acute bacterial skin and skin structure infections. J Med Microbiol. 2020;69:625–630. doi: 10.1099/jmm.0.001177. [DOI] [PubMed] [Google Scholar]
- 45.Karlowsky JA, Adam HJ, Baxter MR, Denisuik AJ, Lagacé-Wiens PRS, Walkty AJ, et al. In vitro activity of sulopenem, an Oral Penem, against urinary isolates of Escherichia coli. Antimicrob Agents Chemother. 2019 doi: 10.1128/AAC.01832-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puttagunta S, Aronin S, Huband M, Flamm RK, Dunne M. 1363. Sulopenem activity against enterobacteriaceae isolates from patients with urinary tract infection or intra-abdominal infection. Open Forum Infect Dis. 2018;5:S417–S417. doi: 10.1093/ofid/ofy210.1194. [DOI] [Google Scholar]
- 47.Iterum Therapeutics IL. Oral sulopenem-etzadroxil/probenecid versus ciprofloxacin for uncomplicated urinary tract infection in adult women—full text view—ClinicalTrials.gov 2017. https://clinicaltrials.gov/ct2/show/NCT03354598. Accessed 14 Dec 2020.
- 48.Iterum Therapeutics IL. Sulopenem followed by sulopenem-etzadroxil/probenecid vs ertapenem followed by cipro for complicated UTI in adults—full text view—ClinicalTrials.gov. 2017 n.d. https://clinicaltrials.gov/ct2/show/NCT03357614. Accessed 14 Dec 2020.
- 49.Iterum Therapeutics IL. Sulopenem versus ertapenem for complicated intra-abdominal infection (cIAI)—full text view—ClinicalTrials.gov 2017. https://clinicaltrials.gov/ct2/show/NCT03358576. Accessed 14 Dec 2020.
- 50.Wu J, Cao G, Wu H, Chen Y, Guo B, Wu X, et al. Evaluation of the effect of contezolid (MRX-I) on the corrected QT interval in a randomized, double-blind, placebo- And positive-controlled crossover study in healthy Chinese volunteers. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.02158-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MicuRx reports favorable results of phase 3 trial of contezolid in China n.d. https://www.ns-healthcare.com/news/micurx-contezolid-china/. Accessed 8 Sept 2021.
- 52.Donald BJ, Surani S, Deol HS, Mbadugha UJ, Udeani G. Spotlight on solithromycin in the treatment of community-acquired bacterial pneumonia: design, development, and potential place in therapy. Drug Des Dev Ther. 2017;11:3559–3566. doi: 10.2147/DDDT.S119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buege MJ, Brown JE, Aitken SL. Solithromycin: a novel ketolide antibiotic. Am J Heal Pharm. 2017;74:875–887. doi: 10.2146/ajhp160934. [DOI] [PubMed] [Google Scholar]
- 54.Barrera CM, Mykietiuk A, Metev H, Nitu MF, Karimjee N, Doreski PA, et al. Efficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: a global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial (SOLITAIRE-ORAL) Lancet Infect Dis. 2016;16:421–430. doi: 10.1016/S1473-3099(16)00017-7. [DOI] [PubMed] [Google Scholar]
- 55.File TM, Rewerska B, Vucinić-Mihailović V, Gonong JRV, Das AF, Keedy K, et al. SOLITAIRE-IV: a randomized, double-blind, multicenter study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumonia. Clin Infect Dis. 2016;63:1007–1016. doi: 10.1093/cid/ciw490. [DOI] [PubMed] [Google Scholar]
- 56.Chen MY, McNulty A, Avery A, Whiley D, Tabrizi SN, Hardy D, et al. Solithromycin versus ceftriaxone plus azithromycin for the treatment of uncomplicated genital gonorrhoea (SOLITAIRE-U): a randomised phase 3 non-inferiority trial. Lancet Infect Dis. 2019;19:833–842. doi: 10.1016/S1473-3099(19)30116-1. [DOI] [PubMed] [Google Scholar]
- 57.Crandon JL, Nicolau DP. In vivo activities of simulated human doses of cefepime and cefepime-AAI101 against multidrug-resistant gram-negative enterobacteriaceae. Antimicrob Agents Chemother. 2015;59:2688–2694. doi: 10.1128/AAC.00033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crandon JL, Nicolau DP. In vitro activity of cefepime/AAI101 and comparators against cefepime non-susceptible enterobacteriaceae. Pathogens. 2015;4:620–625. doi: 10.3390/pathogens4030620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allecra. Safety and efficacy study of cefepime-AAI101 in the treatment of complicated urinary tract infections—full text view—ClinicalTrials.gov 2018. https://clinicaltrials.gov/ct2/show/NCT03687255. Accessed 15 Dec 2020.
- 60.Vickers RJ, Tillotson GS, Nathan R, Hazan S, Pullman J, Lucasti C, et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, active-controlled, non-inferiority study. Lancet Infect Dis. 2017;17:735–744. doi: 10.1016/S1473-3099(17)30235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinebrunner N, Stremmel W, Weiss KH. Ridinilazole—a novel antibiotic for treatment of Clostridium difficile infection. J Thorac Dis. 2018;10:118–120. doi: 10.21037/jtd.2017.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Summit Therapeutics. A study of ridinilazole (SMT19969) compared with fidaxomicin for the treatment of Clostridium difficile infection (CDI)—full text view—ClinicalTrials.gov 2016. https://clinicaltrials.gov/ct2/show/NCT02784002. Accessed 15 Dec 2020.
- 63.Summit Therapeutics. Comparison of ridinilazole versus vancomycin treatment for Clostridium difficile infection—full text view—ClinicalTrials.gov 2018. https://clinicaltrials.gov/ct2/show/NCT03595553. Accessed 15 Dec 2020.
- 64.Taylor SN, Morris DH, Avery AK, Workowski KA, Batteiger BE, Tiffany CA, et al. Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a phase 2, randomized, doseranging, single-oral dose evaluation. Clin Infect Dis. 2018;67:504–512. doi: 10.1093/cid/ciy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’riordan W, Tiffany C, Scangarella-Oman N, Perry C, Hossain M, Ashton T, et al. Efficacy, safety, and tolerability of gepotidacin (GSK2140944) in the treatment of patients with suspected or confirmed gram-positive acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2017 doi: 10.1128/AAC.02095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.GlaxoSmithKline. A dose-ranging study evaluating the efficacy, safety, and tolerability of GSK2140944 in the treatment of uncomplicated urogenital gonorrhea caused by Neisseria gonorrhoeae—full text view—ClinicalTrials.gov 2014. https://clinicaltrials.gov/ct2/show/NCT02294682. Accessed 15 Dec 2020.
- 67.GlaxoSmithKline. Dose-ranging study of GSK2140944 in the treatment of subjects with suspected or confirmed gram-positive acute bacterial skin and skin structure infections—full text view—ClinicalTrials.gov 2014. https://clinicaltrials.gov/ct2/show/NCT02045797. Accessed 15 Dec 2020.
- 68.GlaxoSmithKline. A study to evaluate efficacy and safety of gepotidacin in the treatment of uncomplicated urinary tract infection (UTI)—full text view—ClinicalTrials.gov 2019. https://clinicaltrials.gov/ct2/show/NCT04020341. Accessed 15 Dec 2020.
- 69.Barnes MD, Kumar V, Bethel CR, Moussa SH, O’donnell J, Rutter JD, et al. Targeting multidrug-resistant Acinetobacter spp.: sulbactam and the diazabicyclooctenone β-lactamase inhibitor etx2514 as a novel therapeutic agent. MBio. 2019;10:1–15. doi: 10.1128/MBIO.00159-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Entasis Therapeutics. Study to evaluate the efficacy and safety of intravenous sulbactam-ETX2514 in the treatment of patients with infections caused by Acinetobacter baumannii–calcoaceticus complex—full text view—ClinicalTrials.gov 2019. https://clinicaltrials.gov/ct2/show/NCT03894046. Accessed 15 Dec 2020.
- 71.Kern G, Palmer T, Ehmann DE, Shapiro AB, Andrews B, Basarab GS, et al. Inhibition of Neisseria gonorrhoeae type II topoisomerases by the novel spiropyrimidinetrione AZD0914. J Biol Chem. 2015;290:20984–20994. doi: 10.1074/jbc.M115.663534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.National Institute of Allergy and Infectious Diseases (NIAID). Randomized, open-label phase 2 study of oral AZD0914 in the treatment of gonorrhea—full text view—ClinicalTrials.gov 2014. https://clinicaltrials.gov/ct2/show/NCT02257918. Accessed 15 Dec 2020.
- 73.VenatoRx Pharmaceuticals I. Safety and efficacy study of cefepime/VNRX-5133 in patients with complicated urinary tract infections—full text view—ClinicalTrials.gov 2019. https://clinicaltrials.gov/ct2/show/NCT03840148. Accessed 15 Dec 2020.
- 74.Tacconelli E, Magrini N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Organ Mund La Salud. 2017. pp. 1–7. http://www.cdc.gov/drugresistance/threat-report-2013/. Accessed 20 Feb 2021.
- 75.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 76.M P, L S. Clinical research designs to study treatment effects for multidrug-resistant bacteria. Clin Microbiol Infect 2019;25:929–31. 10.1016/J.CMI.2019.05.004. [DOI] [PubMed]
- 77.JA M, JP D, GH T, LE C, I F, A S, et al. Plazomicin for infections caused by carbapenem-resistant enterobacteriaceae. N Engl J Med 2019;380:791–3. 10.1056/NEJMC1807634. [DOI] [PubMed]
- 78.M P, V S, E M, G K, E R, L L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010;54:4851–63. 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed]
- 79.Cama J, Leszczynski R, Tang PK, Khalid A, Lok V, Dowson CG, et al. To push or to pull? In a post-COVID world, supporting and incentivizing antimicrobial drug development must become a governmental priority. ACS Infect Dis. 2021 doi: 10.1021/acsinfecdis.0c00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yahav D, Shepshelovich D, Tau N. Cost analysis of new antibiotics to treat multidrug-resistant bacterial infections: mind the gap. Infect Dis Ther. 2021 doi: 10.1007/s40121-021-00412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]