Abstract
Amniotic membrane is attracting attention as a new material for regenerative medicine. We herein report that the culture of primary rat hepatocytes on human amniotic membrane maintained their morphology and their production of albumin for at least two months. Human amniotic membrane was collected during planned cesarean section and kept frozen until usage. Primary rat hepatocytes were plated on human amniotic membrane. Hepatocytes accumulated as colonies on amniotic membrane, and their rat albumin level was maintained for two months. Their three-dimensional structure on extracellular matrix, which is abundant in amniotic membranes might influence the maintenance of the hepatocyte-specific function.
Keywords: Human amniotic membrane, Rat hepatocyte, Albumin synthesis
Abbreviations: AM, amniotic membrane; DMSO, dimethyl sulfoxide; EGF, epidermal growth factor; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; HBV, hepatitis-B virus; HCV, hepatitis-C virus; HGF, hepatocyte growth factor; HIV, human immunodeficiency virus; HTLV-1, human T-cell leukemia virus type 1; LT, liver transplantation; PBS, phosphate-buffered saline
Highlights
-
•
Long-term primary culture of rat hepatocyte on the human amniotic membrane was successful.
-
•
Albumin production from primary isolated hepatocytes was maintained for the long term.
-
•
Amniotic membrane provided the situation of 3D structure for isolated rat hepatocyte.
1. Introduction
Although metabolic liver diseases are relatively rare, they have the potential to progress to cirrhosis and metabolic abnormalities can cause serious conditions, the acute exacerbation of which often has a poor prognosis, and which necessitate liver transplantation (LT). LT is the only established treatment for such conditions. However, donor scarcity is a worldwide problem [1]. To solve the lack of deceased organ donation, living donor liver transplantation has been performed and accepted in Asian countries. The living donor might be exposed to risks after donation, even when the donor is healthy. Clinical trials and basic research on hepatocyte transplantation have been conducted to investigate alternatives to LT; however, they have not reached the stage of clinical application. Hepatocytes might have highly proliferative cells in the liver, but they hardly proliferate under culture conditions, and can only be cultured for approximately one week under normal culture conditions. Thus, long-term culture and subculture from isolated hepatocytes is not possible. This is one of the reasons why the clinical application of research on hepatocyte transplantation has not progressed.
Recent studies have reported the usefulness of the amniotic membrane (AM) in regenerative medicine [2]. The AM is a thin, translucent membrane that covers the uterus and the innermost layer of the placenta and surrounds the fetal side of the placenta and the umbilical cord. It is composed of amniotic epithelial cells, an underlying basement membrane, and a compact layer that is rich in collagen (Fig. 1). The amniotic membrane contains pluripotent stem cells [3], and the amniotic membrane cells differentiate into various cells, including pancreatic islet cells, nerve cells, hepatocytes, chondrocytes, and myocardial cells [[2], [3], [4], [5], [6]]. However, most of the amniotic membrane is discarded after childbirth without any clinical application. In addition, the amniotic membrane contains extracellular matrix [7], which serves as a scaffold for culturing cells and which contains various factors, including epidermal growth factor (EGF) and hepatocyte growth factor (HGF) [8,9]. It is highly promising as a substrate which is helpful for cell culturing because it produces growth factors. Recent studies have shown that the amniotic membrane is associated with immunologic tolerance, cells isolated from amniotic and chorionic membranes do not elicit an allogeneic or xenogeneic immune response [2,10]. In the clinical setting, hepatocyte transplantation can be performed for patients with small-for-size graft livers. When a graft is from marginal donor such as an old donor, fatty liver is not appropriate for a recipient, on these occasion hepatocytes isolated from the marginal donor could be cultured on AM for a long-term function. These hepatocytes sheet transplantation into patients with small-for-size graft syndrome can then be performed as temporary support until the liver regenerated. We preliminary reported the temporary functional support of layered hepatocytes sheets on the liver surface [11].
Fig. 1.
The structure of amniotic membrane. (a) Schema of amniotic membrane which is composed of amniotic epithelial cells, an underlying basement membrane, and a compact layer. (b) The microscopic findings of amniotic membrane.
The aim of this study was to investigate whether the albumin-producing ability of rat hepatocytes could be maintained for a long period using human amniotic membrane as a culture medium.
2. Methods
2.1. Amniotic membrane collection and cryopreservation
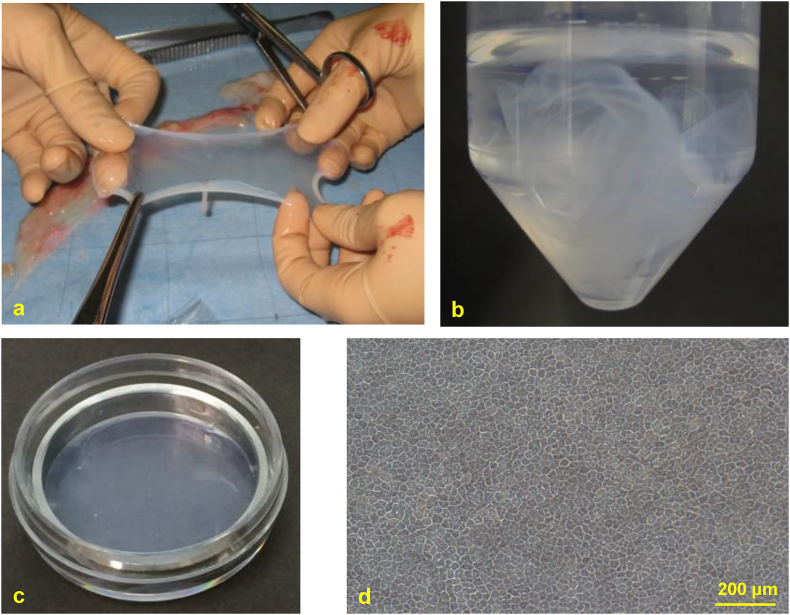
The protocol for the preparation of human amniotic membrane followed that of Kyoto Prefectural University of Medicine, Kyoto, Japan [12]. Placentae from patients who delivered by cesarean section, and who gave their written informed consent, were obtained with the approval of the local ethics committee. This study adheres to the tenets of the Declaration of Helsinki. All placentae were derived from planned cesarean sections at term. Patients with hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), or human T-cell leukemia virus type 1 (HTLV-1) infection or emergency cesarean sections were excluded. Placentae were transported in PBS (GIBCO®) supplemented with 100 μg/mL panimycin. When the AM was peeled off by blunt dissection and separated from the chorionic membrane (Fig. 2a), the compact layer-side, which contacted with the chorionic membrane, was identified and marked by suturing. After several washing steps in PBS, AM was cut into five centimeter square. AM was dipped into 0.5 mol/L-, 1.0 mol/L-, and 1.5 mol/L-concentrations of DMSO for every 5 min. AM was then divided into plastic vials and cryopreserved at −80 °C (Fig. 2b). When used for culture, AM was thawed at room temperature, washed three times with saline, and then washed with saline containing 100 μg/mL panimycin, which was used for culture. This study about the harvest and the preparation of human amniotic membrane was approved by the Institutional Review Board of each institution. The approval ID in Nagasaki University was 13042603-3.
Fig. 2.
Harvesting and storage of amniotic membrane (AM). (a) AM stripped from the chorionic membrane. (b) AM was cut into small pieces, divided into plastic vials. (c) cryopreservation of AM at −80 °C. AMs fixed on dishes using a metal ring. (d) Morphological feature of amniotic epithelial cells was clearly observed in the dish.
2.2. Live/dead staining viability
To assess the viability of amniotic epithelial cells, AM was subjected to Live/Dead staining [13] before cryopreservation and when thawed for use in the hepatocyte cultures (one to three months after cryopreservation), respectively.
2.3. Primary rat hepatocyte isolation
Ethical approval for primary rat hepatocyte isolation was obtained from the Animal Care and Use Committee of Nagasaki University and was performed in accordance with relevant guidelines and regulations. Primary rat hepatocytes were isolated from the whole liver of an adult Wistar rat (male, 7–8 weeks old) by liver perfusion using 130 U/mL collagenase (Wako Pure Chemical, Osaka, Japan) [14]. To enrich viable hepatocytes, the cell suspension in 40% Percoll Plus solution (GE Healthcare, Tokyo, Japan) was centrifuged at 50×g for 20 min at 41 °C. Cell viability was determined by the trypan blue exclusion test, and suspensions with >90% viable cells were used in this study. The medium for isolation was Dulbecco's modified Eagle's medium (Wako Pure Chemical) supplemented with 10% FBS, 10 mM 4-(2-hydroxyethyl)-1- piperazine ethanesulfonic acid, 2 mM l-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (all from Invitrogen, Carlsbad, CA, USA) [15].
2.4. Primary culture of rat hepatocytes on human AMs
Six culture conditions were performed in this study (Table 1): hepatocytes cultured on the epithelial cell-side (E) or the compact layer-side (Co) of AM, a non-coated dish (NC), a collagen-coated dish (CC), a Matrigel dish (M) (dish coated with extracellular matrix; Corning® BioCoat™) [16], and AM without hepatocytes (AM). At one day before isolation of primary hepatocytes, AMs were fixed on dishes using a metal ring (Fig. 2c). Amniotic epithelial cells can be clearly observed by this method (Fig. 2d). Primary rat hepatocytes were plated at a density of 5.21 × 104 cells/cm2 (5.00 × 105 cells/35-mm dish). Hepatocytes were cultured with 2 mL of Hepato-STIM Culture Medium (Corning Glass Works, Corning, NY, USA) supplemented with 10% FBS, 2 mML-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin.
Table 1.
Characteristics of the cultures.
| Hepatocyte | AM | n | |
|---|---|---|---|
| Epithelial cell-side (E) | (+) | (+) | 10 |
| Compact layer-side (Co) | (+) | (+) | 10 |
| Non-coated dish (NC) | (+) | (−) | 8 |
| Collagen-coated dish (CC) | (+) | (−) | 8 |
| Matrigel (M) | (+) | (−) | 5 |
| AM only (AM) | (−) | (+) | 5 |
This medium was changed 24 h after hepatocyte inoculation and every two days thereafter. Samples of media were collected on days 3, 5, 7, 11, 15, 21, and 29. Cell culture was only prolonged in groups E and Co, and the samples were collected on days 35, 43, and 57 (Fig. 3). All samples were stored at −20°Cuntil the time of the assay.
Fig. 3.
Schematic diagram of primary rat hepatocyte culture. The isolated primary rat hepatocyte was plated on each dish. Samples of media were collected on days 3, 5, 7, 11, 15, 21, and 29. In group E and Co, samples collected on days 35, 43, and 57.
2.5. Histopathologic analysis
Hepatocytes were incubated with fresh medium that was changed every two days during incubation. At each medium exchange, the morphological findings of hepatocytes in dishes were captured by an image analyzer (confocal laser scanning microscope; Olympus Corporation, Tokyo, Japan).
2.6. Albumin and HGF assays
The concentrations of rat albumin in the culture medium were determined by an ELISA. Goat to Rat Albumin (Catalog 55727, 4 pg/mL) and peroxidase-conjugated sheep anti-rat albumin (Catalog 55776, 10 μg/mL) antibodies were used to detect rat albumin (both from MP Biomedicals, LLC-Cappel products, Irvine, CA, USA). The HGF concentration was measured using DuoSet ELISA Development Systems (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
2.7. Statistical analysis
Data are presented from at least 4 time points from two independent cell preparations. All statistical analyses were performed with the GraphPad Prism 7 software program for Windows (GraphPad, San Diego, CA). The analyses were performed using non-parametric methods: the significance of differences between the groups was evaluated with the Mann–Whitney U-test. P values of <0.05 were considered to indicate statistical significance.
3. Results
3.1. Live/dead staining viability
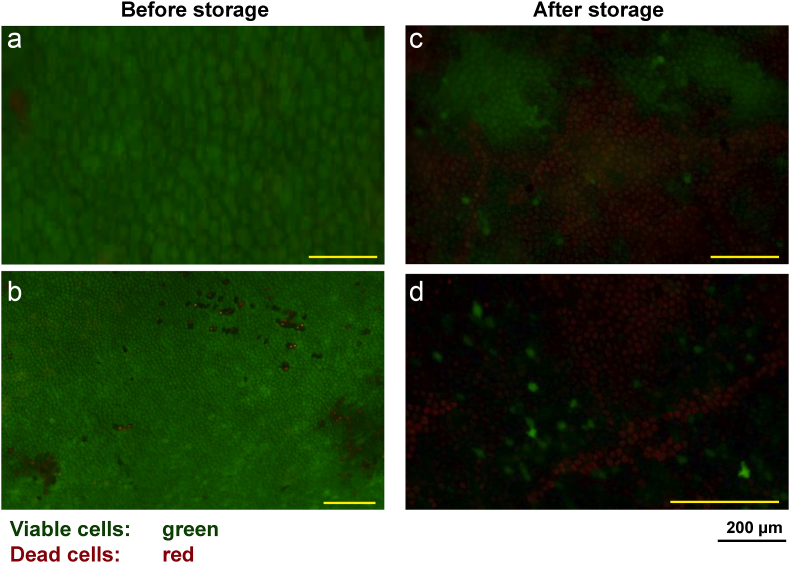
The viability of the amniotic cells on the amniotic membrane was 80–90% before cryopreservation. However, the viability of the amniotic cells was approximately 20% at one to three months after preservation (Fig. 4).
Fig. 4.
Live/Dead staining viability. The viable cells were stained green, whereas dead cells were stained red. (a, b) Left figures showed the viability of the amniotic cells on the amniotic membrane was 80–90% before storage. (c, d) Right figures showed that the viability of the amniotic cells was approximately 20% at one to three months after storage.
3.2. Morphology of hepatocytes on the amniotic membrane
In comparison to the control groups without AM, the hepatocytes in groups E and Co appeared to be slightly smaller and thicker, and seemed to have a nearly spherical morphology. This tendency was more pronounced in cultures in group E (Fig. 5a). On day 7, most hepatocytes in the control group died and were replaced by fibroblasts, whereas hepatocytes in groups E and Co remained agglomerated and some normal cultured hepatocytes were also present. The aggregation of hepatocytes was also more pronounced in group E (Fig. 5b).
Fig. 5.
Cell morphology of rat primary hepatocytes. (a) On day 3, the hepatocytes appeared to be smaller and thicker, and seemed to have a nearly spherical morphology on the amniotic membrane. This tendency was more pronounced in cultures in group E. (b) Hepatocytes could not seen in the NC and CC group, whereas hepatocytes remained as aggregated in group E and Co (b).
3.3. The changes in the rat albumin concentration
The changes in the rat albumin concentration are shown in Fig. 6. In the control groups without amniotic membrane, the albumin concentration decreased from day 6 and became almost unmeasurable on day 14. The albumin concentration showed the same tendency in Group M. In contrast, the albumin concentration on day 2 was maintained until day 56 in Group Co and E. In the comparison between groups E and Co, the value was always significantly higher in group Co. Albumin was not measured in group AM.
Fig. 6.
Production of rat albumin. Albumin production of primary rat hepatocyte was maintained at 2 months on human amniotic membrane. The production of albumin was not maintained in primary rat hepatocytes cultured on Matrigel.
3.4. The changes in the human HGF concentration (Fig. 7).
Fig. 7.
Changes in human HGF. The HGF concentration gradually decreased in each condition.
The HGF concentration was highest in group AM, followed by the E group and Co. No HGF was detected in control groups without AM. The HGF concentration gradually decreased to approximately 1/3 of the initial concentration on day 28.
4. Discussion
Several studies have reported that the AM is useful as a substrate for cell culture [9]. To the best of our knowledge, this is the first study to report to describe culturing of rat hepatocytes on human AM. We found that the use of AM as a culture substrate allowed hepatocytes to remain more spherical and for cell agglomeration to be maintained, and the albumin-producing ability of hepatocytes was maintained for at least two months on AM.
We proposed several hypotheses and conducted experiments to investigate the reasons for these results. The first hypothesis is that HGF produced by amniotic epithelial cells may maintain the hepatocyte function. It has already been reported that amniotic epithelial cells produce HGF and other growth factors [8,9]. It is known that HGF promotes the proliferation of hepatocytes. It was also reported that HGF promotes the synthesis of albumin by hepatocytes [17]. However, in this study, we used AMs that had been cryopreserved. The viability of amniotic epithelial cells was 80–90% before cryopreservation, but decreased to approximately 20% after cryopreservation. HGF in the culture supernatant also showed a constant downward trend during culturing, and we considered that viable amniotic epithelial cells did not continue to produce HGF during hepatocyte culture. The HGF concentration decreased to 0.5 ng/mL on day 28. The above-mentioned study reported that at least 1.0 ng/mL of HGF was needed to promote the synthesis of albumin by hepatocytes [17]. The primary matured hepatocytes on AM would not have a proliferation ability such as that induced by a high expression of Ki-67 during long-term incubation as we found that primary matured hepatocyte had a reduced expression of Ki-67, EGF, and fibroblast growth factor (FGF) in vitro [18]. Litwiniuk et al. reported that high amounts of EGF and TGF-β were detected in the amnion samples an in vitro study; however, the viability of amniotic epithelial cells decreased to approximately 20% after cryopreservation. In our study, we hypothesized that primary mature hepatocytes on an amnion membrane would aggregate spontaneously resembling a 3D structure such as a spheroid morphology. This might aid in the maintenance of albumin production on AM for long-term culture [19].
Our second hypothesis was that extracellular matrix, which is abundant in the dense layer of amniotic epithelium, helps to maintain the hepatocyte function. Several studies reported that the albumin synthesis capacity was maintained hepatocytes cultured on Matrigel-coated dishes [20]. To prove this hypothesis, we performed the same experiment using Matrigel, a dish coated with type IV collagen (a type of extracellular matrix); however, the albumin-producing capacity in Matrigel was diminished within two weeks, as it was in control groups without AM. This indicates that the albumin production capacity cannot be maintained simply by coating with extracellular matrix.
The comparison between groups E and Co revealed that the hepatocytes in group E aggregated and formed a cell mass. It has been reported that hepatocytes aggregate and take the form of spheroids to maintain their hepatocyte-specific function [21]. From this point of view, aggregated hepatocytes in group E seemed preferable; however, the albumin concentration was always significantly higher in group Co from day 2 until the end of culture on day 56. Considering that there was a significant difference on day 2, it was difficult for the aggregated hepatocytes to adhere to amniotic epithelial cells, which may have reduced the amount of hepatocytes that could be stably cultured. The secretion of alpha1-antitrypsin in primary culture of rat hepatocytes was similar to the production of albumin with parallel concentration. Therefore, additional function of hepatocytes such as secretion of alpha1-antitrypsin might have been maintained for three months on the plate [22]. Further experiments are needed to prove this hypothesis.
In this study, hepatocytes were cultured on amniotic membranes fixed by a metal ring that matched the size of the dish. This enabled amniotic epithelial cells to be clearly observed during culturing. Initially, hepatocytes were seeded directly on the non-fixed AM. Consequently, the hepatocytes did not adhere well to the AM and it was difficult to observe them with an optical microscope due to a lack of focus. By fixing the AM to the dish with a metal ring, the amnion could be held in place and the cell morphology could be easily observed during culturing. This technique can be useful when thin membranes are used as substrates for cell culture. In addition, rat hepatocytes spontaneously aggregated under long-term culture in epithelial cell-side (E) and compact layer-side (Co) groups in Fig. 5b. We reported that 3D-cultured chemically induced liver progenitor cells produced more albumin than 2D-cultured cells [23]. Although ESC/iPSC-derived 3D structures were reportedly established in several studies, those systems either involved culture in a gel-embedding system with long culture duration or by coculture with other cells [24,25]. This phenomenon might be occurred in isolated rat hepatocyte on AM. AM might provide the situation of 3D structure for isolated rat hepatocyte in this study.
5. Conclusion
The present study suggests that the amniotic membrane may be useful as a culture substrate for hepatocytes.
6. Financial support
This study was supported by the Japan Society for the Promotion of Science KAKENHI Grant Number 16K19901.
Declaration of competing interest
All authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Kyoichiro Maekawa, Email: minomushi0418@yahoo.co.jp.
Koji Natsuda, Email: no_rain_no_rainbow1018@yahoo.co.jp.
Masaaki Hidaka, Email: mahidaka@nagasaki-u.ac.jp.
Masafumi Uematsu, Email: uematsu-ngs@umin.ac.jp.
Akihiko Soyama, Email: soyama@nagasaki-u.ac.jp.
Takanobu Hara, Email: harataka@nagasaki-u.ac.jp.
Mitsuhisa Takatsuki, Email: mtaka1@med.u-ryukyu.ac.jp.
Kazuhiro Nagai, Email: agwkn@nagasaki-u.ac.jp.
Kiyonori Miura, Email: kiyonori@nagasaki-u.ac.jp.
Susumu Eguchi, Email: sueguchi@nagasaki-u.ac.jp.
References
- 1.Soyama A., Eguchi S., Egawa H. Liver transplantation in Japan. Liver Transplant. 2016;22(10):1401–1407. doi: 10.1002/lt.24502. Epub 2016/06/28. [DOI] [PubMed] [Google Scholar]
- 2.Parolini O., Alviano F., Bagnara G.P., Bilic G., Bühring H.J., Evangelista M. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cell. 2008;26(2):300–311. doi: 10.1634/stemcells.2007-0594. Epub 2007/11/03. [DOI] [PubMed] [Google Scholar]
- 3.Umezawa A., Hasegawa A., Inoue M., Tanuma-Takahashi A., Kajiwara K., Makino H. Amnion-derived cells as a reliable resource for next-generation regenerative medicine. Placenta. 2019;84:50–56. doi: 10.1016/j.placenta.2019.06.381. Epub 2019/07/06. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q.W., Liu Q.Y., Li J.Y., Wei L., Ren K.K., Zhang X.C. Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure. Stem Cell Res Ther. 2018;9(1):321. doi: 10.1186/s13287-018-1063-2. Epub 2018/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marongiu F., Gramignoli R., Dorko K., Miki T., Ranade A.R., Paola Serra M. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53(5):1719–1729. doi: 10.1002/hep.24255. Epub 2011/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miki T., Lehmann T., Cai H., Stolz D.B., Strom S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cell. 2005;23(10):1549–1559. doi: 10.1634/stemcells.2004-0357. Epub 2005/08/06. [DOI] [PubMed] [Google Scholar]
- 7.Leal-Marin S., Thomas K., Nicola H., Olena P., Carsten F., Martin B. Human Amniotic Membrane: a review on tissue engineering, application, and storage. J Biomed Mater Res B Appl Biomater. 2021;109(8):1198–1215. doi: 10.1002/jbm.b.34782. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi N.J., Inatomi T.J., Sotozono C.J., Fullwood N.J., Quantock A.J., Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20(3):173–177. Epub 2000/03/01. [PubMed] [Google Scholar]
- 9.Niknejad H., Peirovi H., Jorjani M., Ahmadiani A., Ghanavi J., Seifalian A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. Epub 2008/05/01. [DOI] [PubMed] [Google Scholar]
- 10.Banas R.A., Trumpower C., Bentlejewski C., Marshall V., Sing G., Zeevi A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 2008;69(6):321–328. doi: 10.1016/j.humimm.2008.04.007. Epub 2008/06/24. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto D., Sakai Y., Huang Y., Yamasaki C., Tateno C., Hasegawa E. Functional changes of cocultured hepatocyte sheets subjected to continuous liver regeneration stimulation in cDNA-uPA/SCID mouse: differences in transplantation sites. Regen Ther. 2021 Mar 18;18:7–11. doi: 10.1016/j.reth.2021.02.004. eCollection 2021 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koizumi N., Fullwood N.J., Bairaktaris G., Inatomi T., Kinoshita S., Quantock A.J. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41(9):2506–2513. Epub 2000/08/11. [PubMed] [Google Scholar]
- 13.Boulos L., Prévost M., Barbeau B., Coallier J., Desjardins R. LIVE/DEAD BacLight : application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J Microbiol Methods. 1999;37(1):77–86. doi: 10.1016/s0167-7012(99)00048-2. Epub 1999/07/08. [DOI] [PubMed] [Google Scholar]
- 14.Seglen P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. Epub 1976/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Sakai Y., Koike M., Kawahara D., Hasegawa H., Murai T., Yamanouchi K. Controlled cell morphology and liver-specific function of engineered primary hepatocytes by fibroblast layer cell densities. J Biosci Bioeng. 2018;126(2):249–257. doi: 10.1016/j.jbiosc.2018.02.006. Epub 2018/03/10. [DOI] [PubMed] [Google Scholar]
- 16.Hughes C.S., Postovit L.M., Lajoie G.A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10(9):1886–1890. doi: 10.1002/pmic.200900758. Epub 2010/02/18. [DOI] [PubMed] [Google Scholar]
- 17.Yamaoka M., Hirata K., Ogata I., Tomiya T., Nagoshi S., Mochida S. Enhancement of albumin production by hepatocyte growth factor in rat hepatocytes: distinction in mode of action from stimulation of DNA synthesis. Liver. 1998;18(1):52–59. doi: 10.1111/j.1600-0676.1998.tb00127.x. Epub 1998/04/21. [DOI] [PubMed] [Google Scholar]
- 18.Sakai Y., Yamanouchi K., Ohashi K., Koike M., Utoh R., Hasegawa E. Vascularized subcutaneous human liver tissue from engineered hepatocyte/fibroblast sheets in mice. Biomaterials. 2015 Oct;65:66–75. doi: 10.1016/j.biomaterials.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 19.Litwiniuk M., Radowicka M., Krejner A., Grzela T. The influence of amniotic membrane extracts on cell growth depends on the part of membrane and childbirth mode selected: a proof-of-concept study. J Wound Care. 2017 Aug 2;26(8):498–503. doi: 10.12968/jowc.2017.26.8.498. [DOI] [PubMed] [Google Scholar]
- 20.Shih H.M., Towle H.C. Matrigel treatment of primary hepatocytes following DNA transfection enhances responsiveness to extracellular stimuli. Biotechniques. 1995;18(5):813–814. 6. Epub 1995/05/01. [PubMed] [Google Scholar]
- 21.Bell C.C., Hendriks D.F., Moro S.M., Ellis E., Walsh J., Renblom A. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep. 2016;6:25187. doi: 10.1038/srep25187. Epub 2016/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross V., Geiger T., Tran-Thi T.A., Gauthier F., Heinrich P.C. Biosynthesis and secretion of alpha 1-antitrypsin in primary cultures of rat hepatocytes. Characterization of differently glycosylated intracellular and extracellular forms. Eur J Biochem. 1982 Dec 15;129(2):317–323. doi: 10.1111/j.1432-1033.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y., Sakai Y., Hara T., Katsuda T., Ochiya T., Adachi T. Development of bifunctional three-dimensional cysts from chemically induced liver progenitors. Stem Cell Int. 2019 Sep 3;2019:3975689. doi: 10.1155/2019/3975689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anzai K., Chikada H., Tsuruya T., Ida K., Kagawa T., Inagaki Y. Foetal hepatic pro-genitor cells assume a cholangiocytic cell phenotype during two-dimensional pre-culture. Sci Rep. 2016;6(1) doi: 10.1038/srep28283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dianat N., Dubois-Pot-Schneider H., Steichen C., Desterke C., Leclerc P., Taveux A. Gen-eration of functional cholangiocyte-like cells from human plu-ripotent stem cells and HepaRG cells. Hepatology. 2014;60(2):700–714. doi: 10.1002/hep.27165. [DOI] [PMC free article] [PubMed] [Google Scholar]