Abstract
Patient: Female, 66-year-old
Final Diagnosis: Lemierre’s syndrome
Symptoms: Chills • neck pain • odynophagia • rigors • trismus
Medication: —
Clinical Procedure: Computed tomography
Specialty: Cardiology • Hematology • Infectious Diseases • General and Internal Medicine
Objective:
Unusual clinical course
Background:
Lemierre’s syndrome (LS), a potentially fatal condition, is characterized by thrombophlebitis of a head or neck vein secondary to a head or neck infection, most commonly involving Fusobacterium necrophorum. Its association with polycythemia vera (PV) is not well reported despite the predisposition to thrombogenesis.
Case Report:
We present the case of a 66-year-old woman with a known history of polycythemia vera (PV) who presented with 4 days of worsening right-sided neck pain and odynophagia. The physical examination revealed poor oral dentition, mild erythema of the posterior pharyngeal mucosa, and non-erythematous tonsils without exudate. A computed tomography with i.v. contrast of the neck revealed complete thrombosis of the right internal jugular vein (IJV). Treatment was initiated with i.v. antibiotics and anticoagulation, with symptoms improving rapidly within 24 h. She was eventually discharged on apixaban and clindamycin and was encouraged to follow up with her hematologist.
Conclusions:
PV predisposes patients to a hyper-viscous and prothrombotic state, which may warrant a stronger suspicion of Lemierre’s syndrome. In addition, lack of aspirin use for prophylaxis of thrombosis and undiagnosed oral infection are factors to consider when assessing risk factors for Lemierre’s syndrome in PV patients.
Keywords: Lemierre Syndrome, Polycythemia Vera, Thrombophlebitis
Background
Lemierre’s syndrome (LS) is a rare condition with an incidence of around 1 in 1 000 000, classically described as a thrombophlebitis of the internal jugular vein (IJV) secondary to an oropharyngeal infection. It is usually seen among the adolescent and young adult populations [1]. A rare and often initially overlooked disease, LS can have mortality rates of over 90% if left untreated [2]. Even treated cases have a mortality rate of 5–18% in the advanced stages [1]. It is not yet known whether there are any predisposing conditions that can make a person susceptible to acquiring this disease. As our case shows, one such condition could be polycythemia vera (PV). It is a neoplastic proliferation of red blood cells, known to cause hyperviscosity of the blood along with thrombocytosis, and is associated with an increased risk of arterial and venous thrombosis [3–5]. In PV, thrombotic events manifest most commonly as deep vein thrombosis (27.8%), acute coronary syndrome (27.8%), and stroke (24.5%) [6]. To the best of our knowledge, this report is the first to demonstrate LS in a polycythemia vera patient.
Case Report
A 66-year-old woman with a past medical history of paroxysmal atrial fibrillation (PAF) and PV presented to the Emergency Department (ED) for worsening right-sided neck pain for 4 days, associated with intermittent rigors and chills. The pain was also accompanied with trismus and odynophagia, as a result of which she was unable to eat or drink. The patient denied any recent fevers, trauma, or dental procedures. One day prior to presentation, she was seen by an otolaryngologist, who had prescribed antibiotics in hopes of treating a suspected dental infection. The patient also reported that she was not compliant with her aspirin (prescribed by a hematologist as a measure against thrombosis in PV) or apixaban (for PAF) and was managing her PV exclusively by therapeutic phlebotomy every 3–4 months. On arrival to the ED, she was afebrile, with other vital signs within normal limits. On the initial encounter, a physical examination revealed a nontoxic-appearing middle aged woman who was alert and oriented to time, place, and person. Inspection of the neck showed a swelling, noted just below the right mandibular angle, with associated erythema of the skin. On palpation, tenderness was elicited over the entire right sternocleidomastoid region, but no palpable lymphadenopathy, fluctuance, or abscess was discovered. Trismus was noted on the affected side, with inability to fully open the mouth due to pain. Examination of the oral cavity revealed poor oral dentition, multiple caries, and mild erythema of the posterior pharyngeal mucosa, with non-erythematous tonsils, without any exudate. Results of pulmonary and cardiovascular examinations were unremarkable.
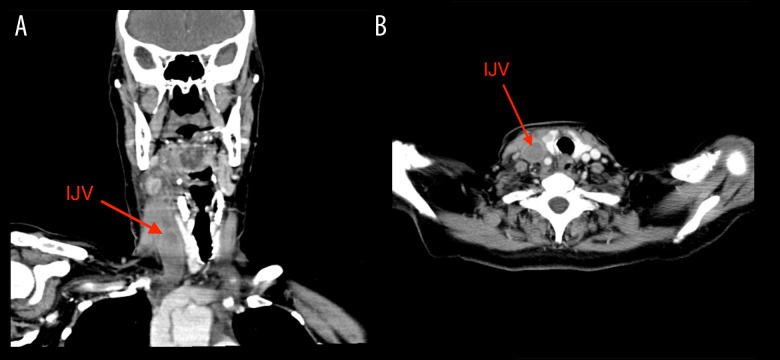
Her initial complete blood count revealed a total white blood cell count of 21 500/mm3, with a differential count showing 81.1% neutrophils, hemoglobin of 13.9 g/dL, hematocrit 48.1%, and platelet count of 381 000/uL (Table 1). The comprehensive metabolic panel showed electrolytes and kidney function were within normal reference ranges (Table 1). Throat and blood cultures did not grow any organisms. Computed tomography with i.v. contrast of the neck showed complete thrombosis of the right internal jugular vein, extending into the superior portion of the SVC, with partial occlusion of the superior segment of the superior vena cava at the junction of the right subclavian and right internal jugular veins (Figure 1).
Table 1.
BMP and CBC results for patient with reference values.
| Lab test | Result | Reference range |
|---|---|---|
| Sodium | 131 | 135–149 mmol/L |
| Potassium | 4.5 | 3.4–4.8 mmol/L |
| Chloride | 98 | 93–105 mmol/L |
| CO2 | 27 | 23–32 mmol/L |
| Glucose | 95 | 59–140 mg/dL |
| BUN | 11.14 | 7–21 mg/dL |
| Creatinine | 0.9 | 0.3–1.1 mg/dL |
| Calcium | 8.6 | 8.2–10.1 mg/dL |
| Anion gap | 6 | 3–11 mmol/L |
| PT | 14 | 10.5–12.8 s |
| PTT | 30.5 | 24.5–32.3 s |
| WBC | 21.5 | 4.8–10.8 K/UL |
| RBC | 7.15 | 4.20–5.40 M/DL |
| HGB | 13.9 | 12.0–16.0 GM/DL |
| HCT | 48.1 | 37.0–47.0% |
| MCV | 67.3 | 81.0–99.0 FL |
| MCH | 19.4 | 27.0–31.0 PG |
| MCHC | 28.9 | 33.0–37.0% |
| RDW | 21.1 | 11.4–16.4% |
| Platelet count | 381 | 150–450 K/UL |
| Neutrophil count | 81.1 | 37.9–70.5% |
| Lymphocyte count | 7.4 | 19.8–47.7% |
| Monocyte count | 6.6 | 2.7–11.7% |
| Eosinophil count | 2.1 | 0.3–5.9% |
| Basophil count | 2 | 0.1–2.4% |
BUN – blood urea nitrogen; PT – prothrombin time; PTT – partial thromboplastin time; WBC – total white blood cell count; RBC – total red blood cell count; HGB – hemoglobin count; HCT – hematocrit; MCV – mean corpuscular volume; MCH – mean corpuscular hemoglobin; MCHC – mean corpuscular hemoglobin concentration; RDW – red cell distribution width.
Figure 1.
(A) CT imaging of complete thrombosis of the right internal jugular vein. Coronal images from CT neck with i.v. contrast showing complete thrombosis of the right internal jugular vein (IJV) (red arrows). There is also significant edema on the right side of the neck causing a leftward shift of neck structures. (B) CT imaging of complete thrombosis of the right internal jugular vein. Axial images from CT neck with i.v. contrast showing complete thrombosis of the right internal jugular vein (IJV) (red arrows).
Keeping in mind the clinical presentation coupled with the laboratory and imaging data, the patient was diagnosed with Lemierre’s syndrome and was admitted for treatment with i.v. antibiotics and anticoagulation. She was treated with therapeutic doses of enoxaparin and also received i.v. metronidazole and ceftriaxone. During the course of her stay, she remained afebrile and her symptoms improved rapidly, with excellent resolution of her swelling within 24 h and return of ability to chew and swallow adequately. She was eventually discharged on apixaban and clindamycin and was encouraged to maintain compliance with her medications and to follow up regularly with her hematologist. On speaking to the patient 3 months after discharge, we learned that she was advised by both her hematologist and cardiologist (for PAF) to continue apixaban therapy indefinitely to prevent the possibility of further thrombotic events resulting from PV or PAF.
Discussion
Conventionally, clinical diagnosis of LS is based on 3 criteria: (1) history of a head or neck infection, (2) thrombosis or thrombophlebitis of a head or neck vein, or metastatic lesions, and (3) isolation of Fusobacterium necrophorum as the causative pathogen. However, all 3 components of the criteria are not required for diagnosis, as there is still ongoing debate about whether an oropharyngeal infection is essential to diagnose LS. Also, isolation of Fusobacterium or demonstration of thrombus in the IJV is not necessary for diagnosis, as the former is a difficult organism to isolate or grow and other bacteria are also known to cause LS [7]. The thrombus is often small and easily missed and may not persist throughout the clinical course [7]. In conclusion, presence of either imaging evidence of thrombosis of a head or neck vein or isolation of Fusobacterium necrophorum from an oro-dental source should warrant a presumptive diagnosis of LS. Although LS has a low incidence, it is imperative for physicians to recognize the condition and begin immediate treatment, since untreated cases are very likely to be fatal.
Interestingly, our patient was not in the age group in which LS is usually seen (19–22 years) [1]. Additionally, although she had poor orodental hygiene, she did not have any obvious neck infection on clinical exam or on CT imaging. She was also afebrile, with sterile throat and blood cultures. However, it is difficult to completely rule out oral infection since she was already on antibiotics for 1 day before presenting to us. Given this clinical profile, it is reasonable to say that she had an interesting presentation of LS, which makes us wonder if it is important to consider the role of polycythemia vera as a risk factor in her development of LS. There is evidence of a 20–50% chance of thrombotic events in PV patients [8], but to the best of our knowledge there are no reported cases of LS or IJV thrombosis with PV.
Her predisposition to thrombosis in the setting of PV may also be due to certain risk factors specific to PV, such as age >60 years old and with elevated hematocrit [9]. PV patients can reduce the risk of thrombosis by keeping the hematocrit below 45% [9], but our patient’s hematocrit upon admission was 48.1%. Another factor, leukocytosis, also poses a major risk for thrombosis in PV patients [9,10], and our patient’s WBC count was initially 21 500 and reached a maximum of 30 300 on day 2 of hospitalization.
Low-dose aspirin has been shown to decrease the risk of thrombosis among PV patients [11]. It is also noteworthy that our patient discontinued her low-dose aspirin for 2 weeks before this event, subjecting her to a hypercoagulable state. An analysis of this patient’s many risk factors is summarized in Figure 2.
Figure 2.
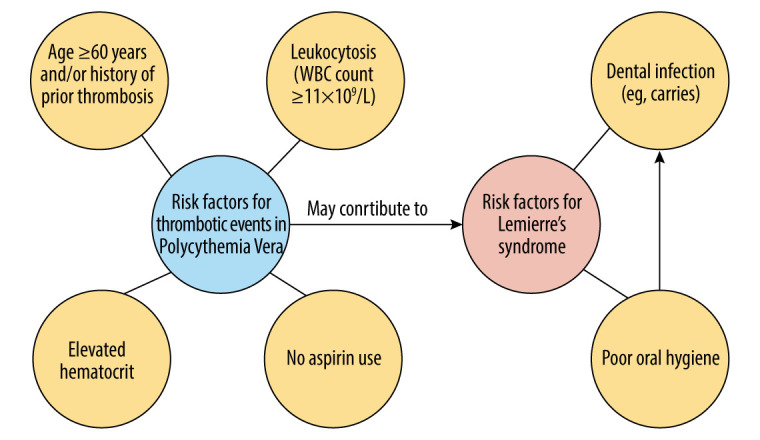
Analysis of risk factors for this patient with PV and LS. Analysis of risk factors for this patient with PV and LS.
Standard management of LS includes prompt empiric therapy with an i.v. beta-lactamase-resistant beta lactam antibiotic for coverage of F. necrophorum and oral Streptococci [11]. Possible therapies include i.v. ceftriaxone and metronidazole, or piperacillin-tazobactam. If a patient is allergic to beta-lactams, clindamycin or metronidazole may be used [1]. Clinicians should refer to susceptibility data if available and culture results for antibiotic selection. Patients should continue antibiotic treatment for 6 weeks. If the patient develops complications such as an abscess, respiratory distress, metastasis, or extension into the mediastinum or cerebrum, then surgery should be considered [1]. It is somewhat controversial whether anticoagulant therapy should be started, mainly due to risk of thromboembolization from the primary thrombus, although anticoagulants may reduce the risk of recurrence and extension of the thrombus [7]. Anticoagulation is essential in cases where there is cerebral sinus thrombosis, large or bilateral clots, or if no improvement is seen within 72 h of treatment initiation [1]. Recently, the trend has shifted to a more liberal approach, as all LS patients are recommended to be anticoagulated.
A recent meta-analysis by Adedeji et al reported that anticoagulation among LS patients is both safe and efficacious with DOACs (direct oral anticoagulants) for a suggested duration of 6–12 weeks [12]. However, among all cases included in the meta-analyses, the initial anticoagulation therapy was parenteral (heparin, Lovenox, or fondaparinux) while the patient was in the hospital [12].
Conclusions
PV predisposes patients to a hyper-viscous and prothrombotic state, which may warrant a stronger suspicion of Lemierre’s disease in symptomatic presentations. In addition, lack of aspirin use for prophylaxis of thrombosis and undiagnosed oral infection are factors to consider when assessing risk factors for Lemierre’s syndrome in PV patients. However, further research is needed to confirm this suspicion, which may be a challenge given the rarity of LS.
Footnotes
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Allen BW, Anjum F, Bentley TP. Lemierre syndrome. StatPearls; Published December 4, 2020. Accessed May 16, 2021 https//www.ncbi.nlm.nih.gov/books/NBK499846/ [PubMed] [Google Scholar]
- 2.Coultas JA, Bodasing N, Horrocks P, Cadwgan A. Lemierre’s syndrome: Recognising a typical presentation of a rare condition. Case Rep Infect Dis. 2015;2015:797415. doi: 10.1155/2015/797415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021–23. doi: 10.1182/blood-2014-07-591610. [DOI] [PubMed] [Google Scholar]
- 4.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–32. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 5.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 6.Kaifie A, Kirschner M, Wolf D, et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): Analysis from the German SAL-MPN-registry. J Hematol Oncol. 2016;9:18. doi: 10.1186/s13045-016-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valerio L, Corsi G, Sebastian T, Barco S. Lemierre syndrome: Current evidence and rationale of the Bacteria-Associated Thrombosis, Thrombophlebitis and Lemierre syndrome (BATTLE) registry. Thromb Res. 2020;196:494–99. doi: 10.1016/j.thromres.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Pearson TC. The risk of thrombosis in essential thrombocythemia and polycythemia vera. Semin Oncol. 2002;29(3 Suppl. 10):16–21. doi: 10.1053/sonc.2002.33756. [DOI] [PubMed] [Google Scholar]
- 9.Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019;98(5):1071–82. doi: 10.1007/s00277-019-03625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2006;109(6):2446–52. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 11.Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114–24. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 12.Adedeji A, Chukwura O, Obafemi T, et al. Anticoagulation strategies in the management of Lemierre syndrome: A systematic review of the literature. Ann Pharmacother. 2021;55(5):658–65. doi: 10.1177/1060028020957620. [DOI] [PubMed] [Google Scholar]