Abstract
Nanoparticle characterization and in vitro data on the effects of combined PARP inhibition and DNA damage by chemoradiation are shown. This data accompanies the research article “Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer” (DuRoss et al., 2021) Additional characterization of the physiochemical properties of nanoscale metal organic frameworks (nMOFs) comprised of hafnium and 1,4-dicarboxybenzene (Hf-BDC) loaded with temozolomide (TMZ) and talazoparib (Tal) are presented. Toxicity data of the drug-loaded nMOF coated with fucoidan (TT@Hf-BDC-Fuco) in colorectal cancer cells, CT-26, from alamarBlue-based chemoradiation experiments are shown. Experimental methods for the nanoparticle characterization and cell-based assays of the nMOF formulation are presented.
Keywords: Radiation therapy, Nanomedicine, DNA Damage, MOF, PARP
Specifications Table
| Subject | Cancer Research |
| Specific subject area | Tumor targeted nanoscale metal organic frameworks |
| Type of data | Image Graph Figure Table |
| How data were acquired | Images were acquired by transmission electron microscopy (TEM). Hydrodynamic size measurements were measured with dynamic light scattering (DLS) methods. Surface modification of the nanoparticles was evaluated by Fourier transform infrared spectroscopy (FTIR). Cell toxicity was measured by alamarBlue assay. |
| Data format | Raw Analyzed |
| Parameters for data collection | Physiochemical properties of the polymer-coated drug-loaded nanoscale metal organic frameworks (nMOFs) were collected from colloidal solutions analysed by TEM, DLS and FTIR. The toxicity of the drug combination temozolomide (TMZ) and talazoparib (Tal) was assessed in CT26 cells by measuring viability. |
| Description of data collection | The synthesis and characterization of a nMOF formulation was performed. In vitro data of the effects of this formulation which combines PARP inhibition and DNA damage by chemoradiation were collected. |
| Data source location | Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR, USA |
| Data accessibility | With the article |
| Related research article | DuRoss, A.N., Landry, M.R., Thomas, C.R., Neufeld, M.J., Sun. C., Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Letters. https://doi.org/10.1016/j.canlet.2020.11.021 |
Value of the Data
-
•
The data can be useful to develop formulations of temozolomide/talazoparib for treating colorectal cancer.
-
•
The data are useful for formulation scientists examining novel carriers for chemoradiation.
-
•
The data provides insight on the physiochemical properties of the metal organic framework drug formulation for further development of this therapeutic platform.
-
•
The data on the in vitro effects of the nMOF formulation can be used in the engineering of a nanomedicine-based approach to chemoradiation for cancer.
1. Data Description
The data in this article shows the characterization of a formulation of temozolomide (TMZ) and talazoparib (Tal). in hafnium and 1,4-dicarboxybenzene (Hf-BDC) nanoscale metal organic frameworks (nMOFs) coated with fucoidan (TT@Hf-BDC-Fuco) by transmission electron microscopy (TEM, Fig. 1), dynamic light scattering (DLS, Fig. 2) and Fourier-transform infrared spectroscopy (FTIR, Fig. 3). Individual data files for hydrodynamic size measurements are included in supplementary materials (Appendix A). Cell viability measurements by alamarBlue assay were performed to evaluate the effects of the nMOF formulations and dextran controls on colorectal cancer cells both with and without concurrent radiotherapy (Table 1).
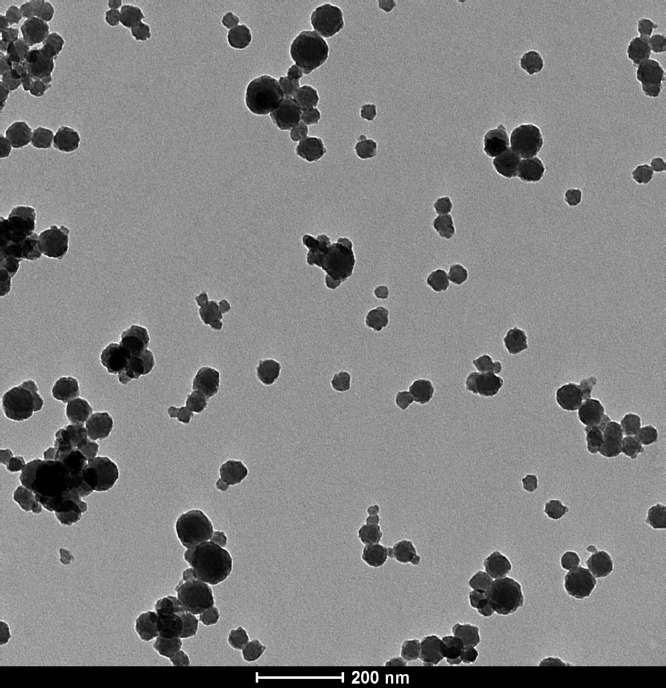
Fig. 1.
Transmission electron microscopy image of fucoidan-coated Hf-BDC nMOFs.
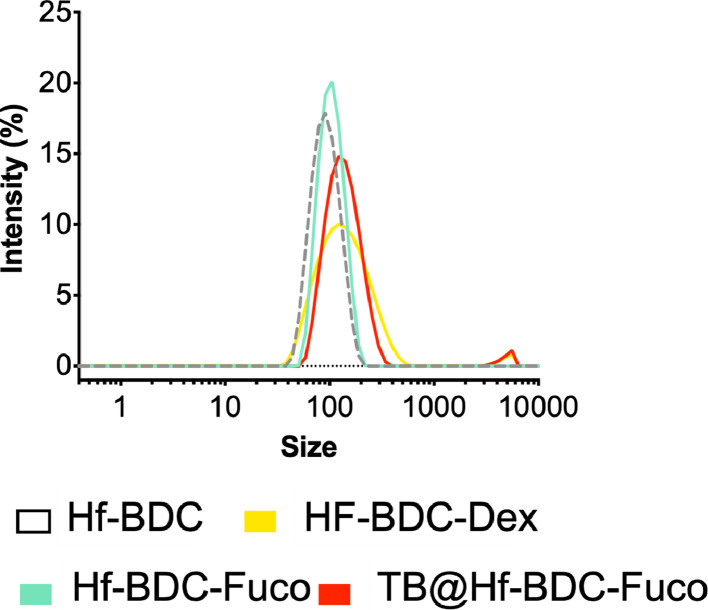
Fig. 2.
Hydrodynamic size by intensity as measured by DLS.
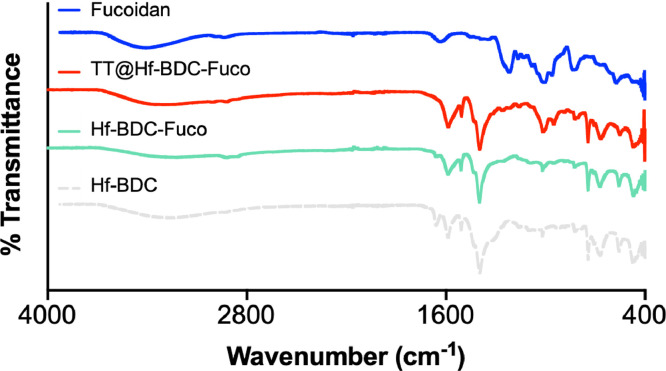
Fig. 3.
FTIR spectra of free Fucoidan and nMOF formulations.
Table 1.
Percentages of cell viability in CT-26 cells after exposure to a variety of nMOF formulations. Drug concentrations for each drug-loaded nMOF treatment are listed at the top of each column.
| Tal (µM) | 4.80E+00 | 2.40E+00 | 1.20E+00 | 6.00E-01 | 3.00E-01 | 1.50E-01 | 7.50E-02 | 3.70E-02 |
| TMZ (µM) | 4.80E+00 | 2.40E+00 | 1.20E+00 | 6.00E-01 | 3.00E-01 | 1.50E-01 | 7.50E-02 | 3.70E-02 |
| TMZ + Tal (µM) | 9.60E+00 | 4.80E+00 | 2.40E+00 | 1.20E+00 | 6.00E-01 | 3.00E-01 | 1.50E-01 | 7.50E-02 |
| TT@HF-BDC Replicate 1 | 76% | 82% | 87% | 87% | 90% | 90% | 92% | 98% |
| TT@HF-BDC Replicate 2 | 69% | 76% | 77% | 81% | 84% | 82% | 89% | 94% |
| TT@HF-BDC-Dex Replicate 1 | 76% | 79% | 78% | 81% | 85% | 85% | 91% | 97% |
| TT@HF-BDC-Dex Replicate 2 | 78% | 78% | 80% | 79% | 81% | 77% | 90% | 94% |
| TT@HF-BDC-Fuco Replicate 1 | 72% | 60% | 64% | 55% | 65% | 76% | 80% | 85% |
| TT@HF-BDC-Fuco Replicate 2 | 51% | 42% | 54% | 46% | 62% | 68% | 73% | 78% |
| TT@HF-BDC + 2 Gy Replicate 1 | 65% | 55% | 79% | 73% | 72% | 75% | 76% | 81% |
| TT@HF-BDC + 2 Gy Replicate 2 | 65% | 51% | 64% | 57% | 64% | 76% | 71% | 69% |
| TT@HF-BDC-Dex + 2 Gy Replicate 1 | 57% | 46% | 65% | 65% | 67% | 67% | 68% | 81% |
| TT@HF-BDC-Dex + 2 Gy Replicate 2 | 43% | 41% | 47% | 51% | 62% | 57% | 70% | 86% |
| TT@HF-BDC-Fuco + 2 Gy Replicate 1 | 44% | 39% | 55% | 48% | 49% | 49% | 49% | 65% |
| TT@HF-BDC-Fuco + 2 Gy Replicate 2 | 34% | 33% | 34% | 38% | 37% | 38% | 37% | 46% |
2. Experimental Design, Materials and Methods
2.1. Materials
Murine CT26.wt cells were purchased from ATCC and maintained in RPMI, at 37 °C and 5% CO2. Media was supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin. Talazoparib and temozolomide were purchased from MedChemExpress (Monmouth Junction, NJ, USA). Phosphate buffered saline (PBS) 1X was purchased from Corning Inc. (Corning, NY, USA). RPMI 1640 was purchased from Thermo Fisher Scientific (Carlsbad, CA, USA). Fucoidan (Fucus vesiculosus >95%) and dextran sulfate sodium salt (MW 7,000-20,000) were purchased from Sigma Aldrich (St. Louis, MO, USA). Glacial acetic acid, terephthalic acid (1,4-benzenedicarboxylic acid -BDC), DMF, and hafnium (IV) chloride were purchased from Fischer Scientific. Additional information on the nMOF can be found in the accompanying manuscript [1] and prior report on the synthesis of Hf-BDC nMOFs [2].
2.2. Characterization of Hf-BDC nMOFs
Dynamic light scattering was performed using a Malvern Nano ZSP (Malvern Panalytical, Malvern, UK) to determine the hydrodynamic size and polydispersity. TEM images were obtained with a Technai with iCORR (FEI, Hillsboro, OR). The TEM samples were prepared by drop casting a colloidal nMOF solution onto the copper surface of Ted Pella Formvar/carbon-backed grids (Redding, CA, USA). Grids were submitted to the OHSU Multiscale Microscopy Core for imaging. Infrared spectroscopy was carried out using a Nicolet iS5 spectrophotometer (ThermoFisher, Waltham, MA). Freshly prepared NP solutions were lyophilized, and the dry powder was compressed into a diamond filament. Total internal reflectance measurements were used to acquire the spectrum for each formulation at 32X resolution between 4000 and 400 cm–1.
2.3. In vitro cytotoxicity
The toxicity of Hf-BDC formulations were investigated in CT26.wt cells. 4 × 103 cells were plated per well and allowed to settle overnight. After settling, cells were dosed with various concentrations of Hf-BDC-Fuco and allowed to incubate at 37 °C for 24 h. At 24 h, cells were irradiated with 2 Gy (or control plate was left unirradiated) and incubated for an additional 24 h before running the alamarBlue assay. To evaluate the ability of dual-drug loading to potentiate the radiosensitivity of CT26.wt cells, nMOF with various coatings (uncoated, dextran, fucoidan) were loaded with 1:1 TMZ:Tal. Cells were plated at 4 × 103 cells per well in two 96-well plates and allowed to settle for 24 h. To understand the impact of the drug combination, nMOF concentration was maintained across all treatments (15 µg/mL) while total drug concentration was evaluated across a range (9.57 µM - 0.07 µM total drug in half dilutions) 24 h after plating. Drug-loaded nMOF was spiked into each well and empty nMOF was added at the correct concentration so all wells had the same final nMOF concentration. One day later, one of the plates was irradiated with 2 Gy. Finally, after another 24 h later, cells were incubated with alamarBlue to determine viability.
Ethics Statement
All animal studies were approved by and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Oregon Health and Sciences University (OHSU) and the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
CRediT authorship contribution statement
Allison N. DuRoss: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Madeleine R. Landry: Validation, Investigation, Writing – review & editing. Charles R. Thomas: Conceptualization, Writing – review & editing, Supervision. Megan J. Neufeld: Methodology, Resources, Writing – review & editing. Conroy Sun: Conceptualization, Methodology, Formal analysis, Resources, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors (AND, MJN, and CS) declare that a patent is pending on the technology described in this manuscript. MRL and CRT declare that they have no know competing financial interests or personal relationships which have or could be perceived to have influenced the work reported in this article.
Acknowledgments
The authors thank the Histology, Multiscale Microscopy, and Advanced Light Microscopy Cores at Oregon Health & Sciences University for their assistance. This work was supported by the NIH 1R35GM119839-01 and Oregon State University College of Pharmacy Start-up Funds.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2021.107394.
Appendix. Supplementary materials
References
- 1.DuRoss A.N., Landry M.R., Thomas C.R., Neufeld M.J., Sun C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021;500:208–219. doi: 10.1016/j.canlet.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neufeld M.J., DuRoss A.N., Landry M.R., Winter H., Goforth A.M., Sun C. Co-delivery of PARP and PI3K inhibitors by nanoscale metal–organic frameworks for enhanced tumor chemoradiation. Nano Res. 2019;12:3003–3017. doi: 10.1007/s12274-019-2544-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.