Abstract
Background
New-onset atrial fibrillation (AF) in critically ill patients is associated with adverse outcomes. In non-critical settings, the circadian variation in paroxysmal AF is of significant interest; however, circadian variation in critically ill patients with new-onset AF has not been thoroughly studied. This study aimed to examine the association between AF onset time and in-hospital mortality.
Methods
This was a secondary analysis of a prospective multicenter observational study enrolling adult critically ill patients. According to AF onset time, patients were divided into nighttime (0:00–7:59), daytime (8:00–15:59), and evening (16:00–23:59). We conducted a multiple logistic regression analysis to assess the potential association between AF onset time and in-hospital mortality. We also assessed the distribution of AF onset, crude in-hospital mortality, and adjusted in-hospital mortality according to bihourly intervals.
Results
Of 423 patients, in-hospital mortality was 26%. During nighttime, 135 patients (32%) developed new-onset AF. AF emerged during daytime for 141 (33%) and during evening for 147 (35%). Daytime AF was significantly associated with an increased risk of in-hospital mortality (adjusted OR: 1.92; 95% CI: 1.07–3.44; p = 0.030). Bihourly interval analysis showed that adjusted in-hospital mortality was unevenly distributed and bimodal with troughs between 6:00 and 7:59 and between 18:00 and 19:59. A similar trend was seen in the distribution of the number of new-onset AF.
Conclusions
We found that the bihourly adjusted in-hospital mortality was distributed in a bimodal fashion. Further research is needed to determine the causes of the diurnal variation and its impact on patient outcomes.
Keywords: New-onset atrial fibrillation, Onset time, Critical illness, In-hospital mortality
1. Introduction
New-onset atrial fibrillation (AF) commonly occurs in critically ill patients, with a reported incidence varying from 4.5 to 15% among the general intensive care unit (ICU) population[1], [2]. Several studies have reported that new-onset AF is significantly associated with prolonged ICU stay and increased mortality [1], [2], [3], [4].
As well as other cardiovascular diseases, such as acute myocardial infarction [5], [6] and out-of-hospital cardiac arrest[7], the circadian variation in paroxysmal AF in the non-critically ill setting has been of considerable interest[8], [9]. Although not necessarily in agreement, many studies have reported that there is a peak of AF onset during non-daytime[8], [9], [10], [11], [12]. These studies lead to a better understanding of the role of autonomic nervous system in shaping the circadian variation in AF onset[13], [14]. Furthermore, daytime onset and non-daytime onset AF are thought to be caused by different autonomic trigger patterns (adrenergic and vagal triggers, respectively) [13], [14], [15]. Different antiarrhythmic drugs are recommended for different trigger patterns[16] and a previous study suggested that mismatch of autonomic trigger patterns and antiarrhythmic drugs may worsen paroxysmal AF to persistent or permanent AF[17], leading to increased thromboembolic events. Therefore, onset time could be one of the important factors that should be taken into account when considering the treatment of AF in non-critically ill setting.
On the other hand, because AF in the ICU is considered to be caused not only by preexisting factors prior to ICU admission but also by ICU-related factors such as the severity of critical illness, inflammation, autonomic dysfunction, vasopressors and mechanical ventilation[2], [3], [18], the circadian variation of AF onset in the ICU is expected to be different from that in the non-critically ill setting. However, this possibility has not been thoroughly studied[19]. It has also not been examined whether AF onset time in the ICU is as important a factor influencing outcomes as it is in the non-critically ill setting.
The purpose of this study was to examine the relationship between onset time of new-onset AF and in-hospital mortality among critically ill patients.
2. Methods
2.1. Study design and setting
The AFTER-ICU study included adult critically ill patients (18 years old or older) with new-onset AF during their ICU stay. AF was defined as an arrhythmia with irregular R-R intervals without obvious P waves or with F waves that lasted more than 5 min or with recurrent episodes within 5 min, as confirmed using 12–lead electrocardiograms or continuous 3–lead electrocardiograms. Patients who developed AF in the emergency room, operating room, or general wards prior to ICU admission were not included. Patients meeting any of the following criteria were excluded: (1) under 18 years old; (2) admission to the ICU after cardiac surgery or cardiac arrest; (3) ICU stay<24 h; (4) previous AF history; (5) use of a pacemaker at AF onset; (6) withholding or withdrawal of medical therapy; or (67) declinature of enrollment. The Institutional Review Board at each participating hospital approved the protocol which included an opt-out policy for the patient or his or her proxy. The detail of the AFTER-ICU study has been described in the previous report[20], [21].
2.2. Participants
For the current study, we included all patients by the AFTER-ICU study. Those with the following missing data were excluded: AF onset time, age, Acute Physiology, and Chronic Health Evaluation (APACHE) II score at ICU admission, vasopressor use, mechanical ventilator, renal replacement therapy, infection at initial AF onset, and in-hospital mortality. We handled these variables as primary exposure, factors adjusting multivariate logistic regression analyses or primary outcome as below.
2.3. Data collection
The following data were collected: ICU admission time, age, sex, body mass index, APACHE II score at ICU admission, patient category at ICU admission, organ category at ICU admission, pre-existing condition (ischemic heart disease, congestive heart failure, hypertension, diabetes mellitus, stroke or transient ischemia attack, chronic hemodialysis), previous medication (calcium-channel blockers, β-blocking agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, antidiabetic agents, antiarrhythmic drugs), AF onset time, heart rate and mean arterial pressure just before AF onset, heart rate and mean arterial pressure at AF onset, Glasgow Coma Scale, antiarrhythmic drug, vasopressors, inotropes, sedative drugs (midazolam, propofol, dexmedetomidine), mechanical ventilation, renal replacement therapy, Sequential Organ Failure Assessment score, infection, sepsis, septic shock, the most recent laboratory data obtained within 24 h before the initial AF onset (lactate, white blood cells, C-reactive protein (CRP), sodium concentration, potassium concentration, magnesium concentration), interventions for the initial AF (antiarrhythmic drugs, electrical cardioversion), anticoagulants within seven days of the onset of AF or during the ICU stay, restoration of sinus rhythm after AF onset during the ICU stay, AF within seven days of the onset of AF or during the ICU stay, AF rhythm at ICU discharge, ICU length of stay, hospital length of stay, ICU mortality, in-hospital mortality, and stroke or transient ischemic attack after AF onset.
2.4. Exposure and outcome measures
According to the AF onset time, patients were divided into three groups at 8-hour intervals: nighttime (0:00 to 7:59), daytime (8:00 to 15:59), and evening (16:00 to 23:59). This categorization was determined based on previous reports[22], [23]. The primary outcome was in-hospital mortality.
2.5. Statistical analysis
Continuous variables are expressed as median with interquartile range and were compared using Kruskal-Wallis tests. Categorical variables are expressed as a number with percentages and were compared using chi-square tests or Fisher’s exact tests. A Bonferroni correction was used for multiple comparisons, if needed.
We compared patients’ characteristics, interventions, and outcomes between the three groups. To examine the association of AF onset time at 8-hour intervals considering the nighttime group as the reference with in-hospital mortality, we performed a multivariate logistic regression analysis adjusted for age, APACHE II score, patient category, vasopressor use, mechanical ventilator, renal replacement therapy, and infection at initial AF onset. These factors were essentially the same as those used in the previous study[20], and only patient category was added to account for the possibility that it might be related to the primary exposure in this study.
A two-sided P-value of<0.05 was considered statistically significant. Missing data were not replaced or estimated. All statistical analyses were performed using R (version 4.0.3).
2.6. Sensitivity analyses
We performed three sensitivity analyses. First, although we determined the eight-hourly division based on the previous studies and daily work schedules[22], [23], there was no fixed time classification method for examining circadian rhythms and other classification methods such as the following are naturally possible; another 8-hour division (20:00 to 3:59, 4:00 to 11:59, and 12:00 to 19:59), a 6-hour division (0:00 to 5:59, 6:00 to 11:59, 12:00 to 17:59, and 18:00 to 23:59), and a 12-hour division (19:00 to 6:59 and 7:00 to 18:59) [24], [25]. We performed multivariate logistic regression analyses adjusted the same factors in the main analysis to examine the association of AF onset time divided by these splitting methods with in-hospital mortality. Second, because septic shock was more frequent in the daytime group, we performed a multivariate analysis in which infection was replaced by septic shock in the primary analysis. Third, since there was a possibility that the rough classification, according to their AF onset time, would obscure the actual diurnal variation, we assessed the distribution of AF onset, crude in-hospital mortality, and adjusted in-hospital mortality according to bihourly intervals. Adjusted in-hospital mortality was calculated using multivariate logistic regression analysis adjusted for the same covariates as the main analysis. Furthermore, since the distribution of this bihourly adjusted in-hospital mortality seemed to be bimodal, to assess the periodicity, we also calculated the adjusted in-hospital mortality by another multivariate logistic regression analysis adjusted for angle-transformed onset time in addition to the same covariates as the main analysis. One day was modeled as two cycles. The fit of the two models was assessed by Akaike’s Information Criterion (AIC).
3. Results
A total of 423 patients were registered into the AFTER-ICU study. No patients met the exclusion criteria. One hundred thirty-five patients (32%) developed new-onset AF during nighttime, 141 patients (33%) during the daytime, and the remaining 147 patients (35%) during the evening.
Baseline characteristics between the three groups were almost similar (Table 1) except that the daytime group was less frequently treated with β-blocking agents before ICU admission than the nighttime group. Table 2 shows patient conditions and laboratory data around AF onset. The proportion of patients with septic shock differed significantly between the three groups, and seemed highest in the daytime group. However, multiple comparison tests showed no significant difference between any two groups. CRP in the daytime group was significantly higher than that in the other two groups.
Table 1.
Baseline characteristics: AF onset during evening and nighttime vs. daytime.
| Variables | Total n = 423 |
Nighttime n = 135 |
Daytime n = 141 |
Evening n = 147 |
p value a | Missing data | |
|---|---|---|---|---|---|---|---|
| ICU admission time | 0.614 | 0 | |||||
| Nighttime, n (%) | 82 (19) | 29 (22) | 28 (20) | 25 (17) | |||
| Daytime, n (%) | 151 (36) | 47 (35) | 55 (39) | 49 (33) | |||
| Evening, n (%) | 190 (45) | 59 (44) | 58 (41) | 73 (45) | |||
| Age, years | 75 [67, 81] | 75 [68, 82] | 74 [65, 81] | 75 [67, 81] | 0.455 | 0 | |
| Male sex, n (%) | 286 (68) | 94 (70) | 95 (67) | 97 (66) | 0.806 | 0 | |
| Body mass index, kg/m2 | 22.5 [19.5, 25.0] |
22.9 [19.8, 25.0] |
22.6 [19.5, 25.3] |
22.0 [19.1, 24.8] |
0.312 | 1 | |
| APACHE II score | 23 [18, 29] | 23 [18, 29] | 23 [19, 27] | 24 [17, 32] | 0.621 | 0 | |
| Patient category | 0.542 | 0 | |||||
| Non-scheduled surgery, n (%) | 95 (23) | 28 (21) | 28 (20) | 39 (27) | |||
| Scheduled surgery, n (%) | 61 (14) | 23 (17) | 19 (14) | 19 (13) | |||
| Medical, n (%) | 267 (63) | 84 (62) | 94 (67) | 89 (61) | |||
| Disease category | 0.334 | 0 | |||||
| Cardiovascular disorder | 55 (13) | 21 (16) | 17 (12) | 17 (12) | |||
| Respiratory disorder | 110 (26) | 31 (23) | 40 (28) | 39 (27) | |||
| Gastrointestinal disorder | 118 (28) | 41 (30) | 31 (22) | 46 (31) | |||
| Neurologic disorder | 24 (5.7) | 5 (3.7) | 12 (8.5) | 7 (4.8) | |||
| Trauma | 26 (6.1) | 8 (5.9) | 9 (6.4) | 9 (6.1) | |||
| Metabolic disorder | 13 (3.1) | 2 (1.5) | 5 (3.5) | 6 (4.1) | |||
| Hematologic disorder | 9 (2.1) | 3 (2.2) | 5 (3.5) | 1 (0.7) | |||
| Urogenital disorder | 21 (5.0) | 5 (3.7) | 6 (4.3) | 10 (6.8) | |||
| Musculoskeletal or skin disorder | 20 (4.7) | 5 (3.7) | 9 (6.4) | 6 (4.1) | |||
| Others | 27 (6.4) | 14 (10) | 7 (5.0) | 6 (4.1) | |||
| Pre-existing condition | |||||||
| Ischemic heart disease, n (%) | 43 (10) | 12 (8.9) | 13 (9.2) | 18 (12) | 0.584 | 0 | |
| Congestive heart failure, n (%) | 43 (10) | 15 (11) | 9 (6.4) | 19 (13) | 0.168 | 0 | |
| Hypertension, n (%) | 199 (47) | 63 (47) | 60 (43) | 76 (52) | 0.297 | 0 | |
| Diabetes mellitus, n (%) | 112 (27) | 34 (25) | 36 (26) | 42 (29) | 0.774 | 0 | |
| Stroke or TIA, n (%) | 45 (11) | 17 (13) | 15 (11) | 13 (8.8) | 0.594 | 0 | |
| Chronic hemolysis, n (%) | 24 (5.7) | 6 (4.4) | 8 (5.7) | 10 (6.8) | 0.694 | 0 | |
| Previous medication | |||||||
| Calcium-channel blockers, n (%) | 141 (33) | 42 (31) | 40 (28) | 59 (40) | 0.085 | 0 | |
| β-Blocking agents, n (%) | 56 (13) | 25 (19) | 10 (7.1) b | 21 (14) | 0.018 | 0 | |
| ACE inhibitors, n (%) | 22 (5.2) | 6 (4.4) | 7 (5.0) | 9 (6.1) | 0.808 | 0 | |
| ARBs, n (%) | 89 (21) | 25 (19) | 31 (22) | 33 (22) | 0.681 | 0 | |
| Antidiabetic agents, n (%) | 97 (23) | 29 (22) | 32 (23) | 36 (25) | 0.832 | 0 | |
| Antiarrhythmic drugs, n (%) | 5 (1.2) | 2 (1.5) | 2 (1.4) | 1 (0.7) | 0.784 | 0 | |
AF atrial fibrillation, ICU intensive care unit, APACHE II Acute Physiology and Chronic Health Evaluation II, TIA transient ischemic attack, ACE angiotensin converting enzyme, ARBs angiotensin II receptor blockers
Kruskal-Wallis tests for continuous variables, and chi-square tests or Fisher’s exact tests for categorical variables between the three groups
p < 0.05 vs. nighttime group after Bonferroni correction
Table 2.
Patient conditions and laboratory data: AF onset during evening and nighttime vs. daytime.
| Variables | Total n = 423 |
Nighttime n = 135 |
Daytime n = 141 |
Evening n = 147 |
p value a | Missing data | ||
|---|---|---|---|---|---|---|---|---|
| From ICU admission to AF onset, days | 1.6 [0.7, 3.0] | 1.5 [0.6, 3.2] | 1.7 [0.8, 4.0] | 1.4 [0.4, 2.9] | 0.130 | 0 | ||
| Heart rate before AF onset, beats per min | 95 [83, 107] | 92 [82, 103] | 97 [86, 109] | 96 [82, 107] | 0.180 | 0 | ||
| MAP before AF onset, mmHg | 80 [71, 92] | 80 [69, 89] | 79 [70, 91] | 84 [73, 94] | 0.120 | 0 | ||
| Heart rate after AF onset, beats per min | 130 [112, 148] | 128 [111, 146] | 133 [115, 150] | 131 [109, 148] | 0.413 | 0 | ||
| MAP after AF onset, mmHg | 76 [64, 89] | 77 [63, 87] | 74.00 [63, 89] | 79 [67, 93] | 0.090 | 0 | ||
| Glasgow Coma Scale after AF onset | 14 [12, 15] | 14 [12, 15] | 14 [12, 15] | 15 [12, 15] | 0.744 | 0 | ||
| Antiarrhythmic drug at AF onset, n (%) | 40 (10) | 14 (10) | 14 (10) | 12 (8) | 0.796 | 0 | ||
| Vasopressors at AF onset, n (%) | 192 (45) | 59 (44) | 72 (51) | 61 (42) | 0.236 | 0 | ||
| Noradrenaline, n (%) | 182 (43) | 56 (41) | 68 (48) | 58 (39) | 0.293 | 0 | ||
| Noradrenaline, μg/kg/min b | 0.12 [0.05, 0.22] |
0.10 [0.05, 0.20] |
0.15 [0.05, 0.28] |
0.15 [0.06, 0.22] |
0.405 | 0 | ||
| Adrenaline, n (%) | 14 (3) | 5 (4) | 4 (3) | 5 (3) | 0.944 | 0 | ||
| Adrenaline, μg/kg/min b | 0.05 [0.03, 0.25] |
0.07 [0.03, 0.30] |
0.10 [0.04, 0.17] |
0.05 [0.04, 0.05] |
0.960 | 0 | ||
| Dopamine, n (%) | 32 (8) | 7 (5) | 11 (8) | 14 (10) | 0.385 | 0 | ||
| Dopamine, μg/kg/min b | 3.4 [2.1, 4.9] | 3.0 [2.4, 5.3] | 2.6 [1.5, 4.0] | 3.8 [3.0, 4.8] | 0.401 | 0 | ||
| Vasopressin, n (%) | 39 (9) | 11 (8) | 12 (9) | 16 (11) | 0.685 | 0 | ||
| Inotropes at AF onset, n (%) | 52 (12) | 20 (15) | 20 (14) | 12 (8) | 0.166 | 0 | ||
| Dobutamine, n (%) | 41 (10) | 17 (13) | 16 (11) | 8 (5) | 0.092 | 0 | ||
| PDE inhibitors, n (%) | 12 (3) | 4 (3) | 4 (3) | 4 (3) | 1.000 | 0 | ||
| Sedative drug at AF onset, n (%) | 181 (43) | 67 (50) | 49 (35) | 65 (44) | 0.040 | 0 | ||
| Midazolam at AF onset, n (%) | 47 (11) | 16 (12) | 13 (9) | 18 (12) | 0.678 | 0 | ||
| Propofol at AF onset, n (%) | 87 (21) | 32 (24) | 24 (17) | 31 (21) | 0.382 | 0 | ||
| Dexmedetomidine, n (%) | 84 (20) | 31 (23) | 24 (17) | 29 (20) | 0.465 | 0 | ||
| MV at AF onset, n (%) | 255 (60) | 81 (60) | 81 (57) | 93 (63) | 0.599 | 0 | ||
| RRT at AF onset, n (%) | 104 (25) | 29 (22) | 33 (23) | 42 (29) | 0.356 | 0 | ||
| SOFA score at AF onset | 7 [5, 10] | 7 [5, 10] | 8 [5, 10] | 7 [4, 10] | 0.662 | 11 | ||
| Infection at AF onset, n (%) | 295 (70) | 92 (68) | 105 (75) | 98 (67) | 0.314 | 0 | ||
| Sepsis at AF onsetc, n (%) | 286 (69) | 90 (67) | 100 (74) | 96 (68) | 0.354 | 11 | ||
| Septic shock at AF onsetd, n (%) | 76 (18) | 18 (13) | 34 (25) | 24 (17) | 0.036 | 11 | ||
| Lactate, mmol/L | 1.6 [1.1, 2.4] | 1.5 [1.1, 2.1] | 1.7 [1.1, 2.6] | 1.6 [1.2, 2.4] | 0.325 | 5 | ||
| White blood cells, 103/μL | 10 [7, 15] | 10 [7, 14] | 10 [6, 15] | 11 [7, 16] | 0.515 | 3 | ||
| C-reactive protein, mg/dL | 13.1 [5.0, 23.6] | 10.8 [4.3, 20.2] | 18.4 [7.1, 26.5] e | 12.4 [3.5, 22.7] f | 0.001 | 11 | ||
| Sodium concentration, mmol/L | 140 [137, 143] | 140 [137, 143] | 140 [137, 144] | 139 [136, 142] | 0.065 | 3 | ||
| Potassium concentration, mmol/L | 4.0 [3.7, 4.6] | 4.0 [3.7, 4.5] | 4 0.0 [3.7, 4.5] | 4.1 [3.7, 4.7] | 0.590 | 3 | ||
| Magnesium concentration, mg/dL | 2.0 [1.8, 2.4] | 2.0 [1.8, 2.5] | 2.1 [1.9, 2.5] | 2.0 [1.8, 2.3] | 0.555 | 190 | ||
AF atrial fibrillation, ICU intensive care unit, MAP Mean arterial pressure, MV mechanical ventilation, RRT renal replacement therapy, SOFA Sequential Organ Failure Assessment.
Kruskal-Wallis tests for continuous variables, and chi-square tests or Fisher’s exact tests for categorical variables between the three groups.
Mean dose only for the patients administrated.
Patients with infections who have a SOFA score of 2 or higher at the initial AF onset were considered sepsis.
Patients with infections who have a SOFA score of 2 or higher, were treated with vasopressors, and have a lactate level of 2 mmol/l or higher at the initial AF onset were considered septic shock.
p < 0.05 vs. nighttime group after Bonferroni correction.
p < 0.05 vs. daytime group after Bonferroni correction.
Interventions for new-onset AF and outcomes are shown in Table 3. There were no significant differences in antiarrhythmic drugs, electrical cardioversion, anticoagulant, and any outcomes between the three groups.
Table 3.
Interventions and Outcomes: AF onset during evening and nighttime vs. daytime.
| Total n = 423 |
Nighttime n = 135 |
Daytime n = 141 |
Evening n = 147 |
p value a | Missing data | |
|---|---|---|---|---|---|---|
| Variables | ||||||
| Antiarrhythmic drugsb, n (%) | 296 (70) | 93 (69) | 100 (71) | 103 (70) | 0.934 | 0 |
| Electrical cardioversionc, n (%) | 65 (15) | 26 (19) | 20 (14) | 19 (13) | 0.302 | 0 |
| Anticoagulantsd, n (%) | 173 (41) | 61 (45) | 60 (43) | 52 (35) | 0.218 | 0 |
| SR after AF onset during ICU stay, n (%) | 380 (90) | 118 (87) | 128 (91) | 134 (91) | 0.525 | 0 |
| AF recurrence, n (%) | 89 (21) | 34 (25) | 21 (15) | 34 (23) | 0.083 | 0 |
| AF at ICU discharge, n (%) | 62 (17) | 19 (16) | 19 (16) | 24 (18) | 0.861 | 0 |
| ICU length of staye, days | 5.6 [2.6, 11.2] | 5.4 [2.5, 10.4] | 5.2 [2.8, 10.1] | 5.9 [2.7, 12.2] | 0.780 | 0 |
| Hospital length of stayf, days | 26 [13, 49] | 31 [13, 55] | 25 [11, 51] | 23 [13, 46] | 0.355 | 0 |
| ICU mortality, n (%) | 54 (13) | 14 (10) | 25 (18) | 15 (10) | 0.096 | 0 |
| Hospital mortality, n (%) | 112 (26) | 29 (23) | 45 (32) | 38 (26) | 0.142 | 0 |
| Stroke after AF onset, n (%) | 19 (4.5) | 6 (4.4) | 5 (3.5) | 8 (5.4) | 0.767 | 0 |
AF atrial fibrillation, ICU intensive care unit, SR sinus rhythm.
Kruskal-Wallis tests for continuous variables, and chi-square tests or Fisher’s exact tests for categorical variables between the three groups.
Antiarrhythmic drugs includes any calcium-channel blockers, beta blocking agents, amiodarone, pilsicainide, magnesium sulfate, digoxin and others.
During AF from the initial AF onset.
Within seven days of the onset of AF or during ICU stay, whichever is shorter.
Length from the initial onset of AF to ICU discharge.
Length from the initial onset of AF to hospital discharge.
The results of the multivariate logistic regression analysis are summarized in Table 4. With reference to the nighttime group, the daytime group was significantly associated with an increased risk of in-hospital mortality (adjusted odds ratio: 1.92; 95% confidence interval: 1.07–3.44; p = 0.030). However, no specific time period was found to be significantly associated with in-hospital mortality in the other splitting methods (Additional file 1: Table S1).
Table 4.
Multivariate logistic regression analysis for in-hospital mortality.
| Covariates | Adjusted OR (95% CI) | p value |
|---|---|---|
| AF onset time | ||
| ighttime | Reference | – |
| Daytime | 1.92 (1.07–3.44) | 0.030 |
| Evening | 1.10 (0.60–2.00) | 0.764 |
| Age, years | 1.01 (0.99–1.03) | 0.270 |
| APACHE II score, point | 1.06 (1.02–1.09) | 0.001 |
| Patient category | ||
| Scheduled surgery | Reference | – |
| Non-scheduled surgery | 1.07 (0.40–2.85) | 0.890 |
| Medical | 1.22 (0.48–3.12) | 0.675 |
| Mechanical ventilation at AF onset | 2.49 (1.38–4.48) | 0.002 |
| Renal replacement therapy at AF onset | 2.37 (1.39–4.03) | 0.002 |
| Vasopressors at AF onset | 0.93 (0.56–1.56) | 0.787 |
| Infection at AF onset | 0.98 (0.52–1.83) | 0.944 |
AF atrial fibrillation, APACHE II Acute Physiology and Chronic Health Evaluation II.
Additional file 3: Table S2 shows the results of the multivariate logistic regression analysis with septic shock as a co-variable. Although the daytime group lost the statistical significance, there is a similar trend to the main analysis that the daytime group was associated with an increased risk of in-hospital mortality (adjusted odds ratio: 1.78; 95% confidence interval: 0.98–3.23; p = 0.059).
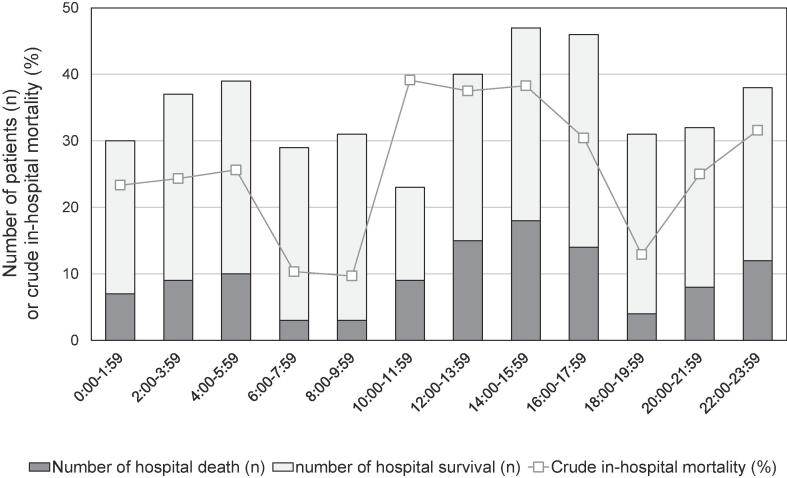
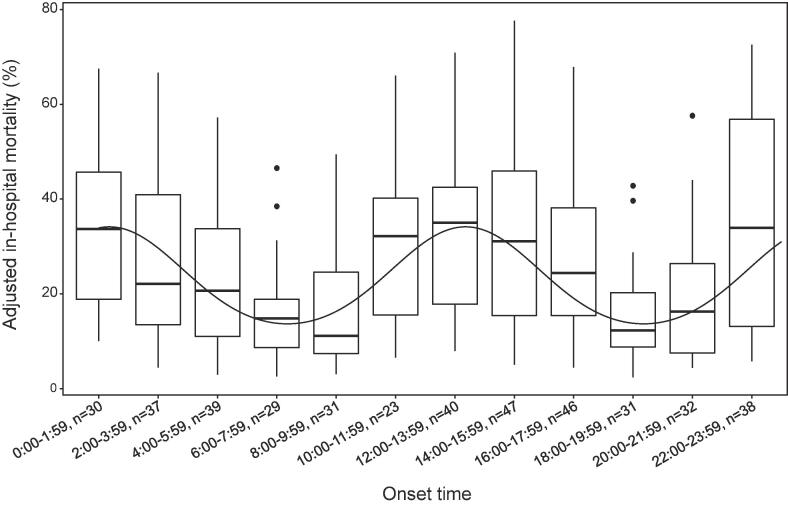
Fig. 1 shows distributions of AF onset and crude in-hospital mortality across the bihourly intervals. The number of patients in each time period ranged from 23 to 47 and crude in-hospital mortality ranged from 9.7% to 39.1%. Bihourly adjusted mortality was computed with the same covariates as in the main analysis is shown in Additional file 3: Figure S1. It was unevenly distributed (Kruskal-Wallis test, p = 0.032) and seemed to be bimodal. The distribution and prediction curve of bihourly adjusted mortality calculated from the model that incorporated periodicity are shown in Fig. 2. Two troughs were observed between 6:00 and 7:59 and between 18:00 and 19:59. By taking into account the periodicity, the AIC decreased from 450 to 443.
Fig. 1.
Circadian distribution of the AF onset and crude in-hospital mortality.
Fig. 2.
Circadian distribution of adjusted in-hospital mortality calculated with considering periodicity. The median is indicated by the black lines, and the 25th and 75th percentiles by the hinges, maximum value or 1.5 times of the interquartile range as the bars, and values beyond as points. The overlaid curve shows the predicted curve from the multivariate logistic regression model that took periodicity into account.
4. Discussion
4.1. Key findings
In this secondary analysis of the multicenter prospective cohort study, we assessed an association of AF onset time with in-hospital mortality among the general ICU population. In our primary analysis, new-onset AF occurring during between 8:00 to 15:59 was significantly associated with an increased risk of in-hospital mortality. In addition, bihourly interval analysis showed that adjusted in-hospital mortality was not uniformly distributed and appeared to be bimodal with troughs between 6:00 and 7:59 and between 18:00 and 19:59. A similar pattern was seen in the distribution of the numbers of new-onset AF cases.
4.2. Relationship with previous studies
There is only one previous study examining the circadian variation in AF onset among critically ill patients[19]. In that study, although a clear diurnal distribution was not observed, there were two peaks between 8:00 and 10:00, and around midnight. These periods were not consistent with those observed in our study. This discrepancy may be due to differences in the study population. More than half of the patients in the previous study were post-cardiac surgery and post-cardiac arrest, but our study did not include these types of patients. There are also several studies to assess the circadian variation in AF onset among non-critically ill patients. Although not necessarily in agreement, many studies reported that there is a peak of AF onset during non-daytime.[8], [9], [10], [11], [12]. According to the most extensive observational study, including more than 3,000 patients, a double peak in AF onset at about 8:00 and 20:00 was observed[9]. These periods are generally consistent with the trough periods of AF onset in our study. Although we could not investigate the reason for the exact opposite, it might be partly due to autonomic dysfunction. The autonomic nervous system plays an important role in shaping the circadian variation in paroxysmal AF onset among non-critically ill patients[13], [14]. Theoretically, autonomic dysfunction could disrupt the original circadian variation of AF. Conversely, new-onset AF occurring during a time period when AF would be less likely to occur in the non-critically ill setting may be itself a sign of more intense autonomic dysfunction leading to an increased risk of mortality[26], [27]. In fact, we found that new-onset AF that occurred during the trough periods in the previous study[9] showed high adjusted in-hospital mortality. However, we did not directly measure any indicators of autonomic dysfunction, such as heart rate variability[28]. We only estimated autonomic dysfunction based on the deviation from the circadian variation of AF onset in the non-critically ill setting formed by the autonomic nervous system. In addition, critically ill patients receive many interventions such as mechanical ventilators and vasopressors. ICU-related factors other than autonomic dysfunction may have contributed to the differences in diurnal variations. Our findings should be validated by future studies.
Our main eight-hour interval analysis showed that AF onset during daytime was significantly associated with an increased risk of in-hospital mortality. As already mentioned, paroxysmal AF among non-critically ill patients is less likely to occur during daytime. Autonomic dysfunction may also explain this result. Furthermore, we found that the daytime group had higher CRP. Inflammation is a well-known predictor of AF in non-critical and septic setting[4], [29]. At the same time, although multiple comparisons showed no significant difference between any of the two groups, there were more patients in the daytime group with septic shock. Septic shock is considered to be a high-risk population for new-onset AF[2], [4]. A previous study suggested that inflammation and septic shock may also be linked with autonomic dysfunction[30]. These variables may be associated not only with AF onset, but also with increased mortality after AF onset. The main analysis could not rule out the possibility that septic shock led the greatest impact on hospital mortality. However, our sensitivity analysis with septic shock as a covariate showed that, although statistically not significant, there seemed to be a similar trend to the main analysis that AF occurring during daytime was associated with an increased risk of in hospital mortality. Further studies considering septic shock are needed to determine the relationship between daytime AF onset and mortality. On the other hand, our sensitivity analysis using the other splitting methods showed that no specific time period was found to be significant. This may be because these divisions obscure the periodic diurnal variations shown in Fig. 2, and only the main analysis captured them well. In the main analysis, 8:00 to 15:59 included the peak of the adjusted mortality rate and the time period around it. On the other hand, 0:00 to 7:59 (the control group) included the declining period and the trough. This contrast may have contributed to detect the significant difference. Further studies are needed to confirm our study findings.
4.3. Significance and implications
We found that the bihourly adjusted in-hospital mortality was distributed in a bimodal fashion and that a similar trend was observed in the distribution of the AF onset. Furthermore, AF occurring between 8:00 and 15:59 was associated with an increased risk of in-hospital mortality compared with AF between 0:00 and 7:59. Since autonomic dysfunction, reported to be associated with poor outcomes in critically ill patients[26], [31], may be one of the causes, onset time should be noted as a predictive marker for in-hospital mortality. Theoretically, although active avoidance of drugs causing autonomic dysfunction (e.g., catecholamines[3]) and the use of drugs maintaining of autonomic balance (e.g., statins[32]) could improve clinical outcomes, further studies are needed.
4.4. Strengths and limitations
The strengths of our study are that we used the well-managed prospective multicenter study data with few missing values and that this is the first report on the association between AF onset time and in-hospital mortality.
However, this study also has several limitations. First, this was a secondary analysis of a multicenter prospective cohort study. Although we performed the multivariate logistic regression analyses, selection biases, and uncontrolled confounding factors could have existed. Furthermore, we could not mention the causal relationship due to the nature of the observational study. Second, as already mentioned, we did not directly measure autonomic dysfunction, and the mechanism of the relationship between AF onset time and mortality is unknown. Third, we could not consider the influence of healthcare provider staffing. However, compared to nighttime, daytime is usually less vulnerable. Our result that daytime AF onset was associated with an increased risk of in-hospital mortality might be underestimated, but would not be overestimated due to this limitation. Fourth, although the main analysis found the relationship between daytime AF onset and in-hospital mortality, our sensitivity analysis showed that no specific time period was found to be significant. Larger prospective studies are needed to determine if there are specific time periods associated with mortality. Fifth, there were no specific management protocols for new-onset AF in this study. Treatment strategies depended crucially on each physician or each institution so that it may have led to additional biases. Sixth, we did not collect several of the factors that may be associated with new-onset AF, such as hypokalemia, hypomagnesemia, hypovolemia, and use of particular types of vasopressors, echocardiographic data, patient-ventilator asynchrony, detailed information for infection, analgesics or neurological disorders, and nursing care timing. Seventh, as in several studies of new-onset AF in the ICU[4], [33], [34], the detection of AF in this study was done by bedside medical staffs, which may have led to problems in the precision of detecting AF. A retrospective observational study using an automated system reported that there were many cases of new-onset AF overlooked by physicians, but at the same time, these were not associated with in-hospital mortality[35]. Our results should be validated by future studies using an automated system. Moreover, we did not evaluate long-term outcomes. Finally, the AFTER-ICU study was carried out only in Japan, so it is unknown whether our findings can be extrapolated to other regions.
5. Conclusions
We found that the bihourly adjusted in-hospital mortality was distributed in a bimodal fashion and that a similar trend was observed in the distribution of the number of AF onset. Further research is needed to determine the causes of the diurnal variation and its impact on patient outcome.
6. Ethics approval and consent to participate
The Institutional Review Board at each participating hospital approved the protocol which included an opt-out policy for the patient or his or her proxy.
7. Consent for publication
Not applicable.
8. Availability of data and material
The datasets used in the current study are available from the corresponding author on reasonable request.
9. Authors’ contributions
TO has full access to all the data in the study and takes responsibility for the integrity of the data. Study concept and design: TO, TY, SU, YS. Acquisition of data: TY, SU. Analysis and interpretation of data: TO, TY, SU, YS. Drafting of the manuscript: TO. Critical revision of the manuscript for important intellectual content: TO, TY, SU, YS. All authors read and approved the final manuscript.
Funding
This research was supported by The Jikei University Research Fund. The funder had no role in the design and conduct of the study; data collection, management, analysis, and interpretation; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Any potential conflicts of interest, including related consultancies, shareholdings and funding grants
All the authors declare that they have no competing interests.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The AFTER-ICU study group: Hokkaido university: Tomonao Yoshida; Iwate Prefectural Central hospital: Hiroshi Nashiki; Saitama Red Cross Hospital: Hajime Suzuki; Steel Memorial Muroran Hospital: Hiroshi Takahashi; Japanese Red Cross Musashino Hospital: Yuki Kishihara; Shonan Kamakura General Hospital: Shinya Nagasaki; Jichi Medical University Hospital: Shinshu Katayama; JA Hiroshima General Hospital: Masaaki Sakuraya; Japanese Red Cross Maebashi Hospital: Takayuki Ogura; Nara Medical University: Satoki Inoue; Dokkyo Medical University: Masatoshi Uchida; National Hospital Organization Tokyo Medical Center: Yuka Osaki; Kurashiki Central Hospital: Akira Kuriyama, Hiromasa Irie; Hiroshima University: Michihito Kyo; Wakayama Medical University: Nozomu Shima; Hirosaki University Hospital: Junichi Saito; Okinawa Chubu Hospital: Izumi Nakayama; Fujita Medical University: Naruhiro Jingushi; Kyoto Medical Center: Kei Nishiyama; Tokyo Medical and Dental University: Takahiro Masuda; Shiga University of Medical Science: Yasuyuki Tsujita; Aichi Medical University: Masatoshi Okumura; Nagasaki University: Haruka Inoue; Shizuoka General Hospital: Yoshitaka Aoki; National Hospital Organization Nagoya Medical Center: Takashiro Kondo; Yokohama City Minato Red Cross Hospital: Isao Nagata; Kyorin University: Takashi Igarashi; Nippon Medical School Chiba Hokusoh Hospital: Nobuyuki Saito; Tottori University: Masato Nakasone. We would like to thank all our colleagues in the AFTER-ICU study participating hospitals who performed the extensive data entry. This research was supported by The Jikei University Research Fund.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100880.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Yoshida T., Fujii T., Uchino S., Takinami M. Epidemiology, prevention, and treatment of new-onset atrial fibrillation in critically ill: a systematic review. J. Intensive Care. 2015;3:19. doi: 10.1186/s40560-015-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetterslev M., Haase N., Hassager C., Belley-Cote E.P., McIntyre W.F., An Y., Shen J., Cavalcanti A.B., Zampieri F.G., Guimaraes H.P., Granholm A., Perner A., Møller M.H. New-onset atrial fibrillation in adult critically ill patients: a scoping review. Intensive Care Med. 2019;45(7):928–938. doi: 10.1007/s00134-019-05633-x. [DOI] [PubMed] [Google Scholar]
- 3.Bosch N.A., Cimini J., Walkey A.J. Atrial Fibrillation in the ICU. Chest. 2018;154(6):1424–1434. doi: 10.1016/j.chest.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein Klouwenberg P.M.C., Frencken J.F., Kuipers S., Ong D.S.Y., Peelen L.M., van Vught L.A., Schultz M.J., van der Poll T., Bonten M.J., Cremer O.L. Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am. J. Respir. Crit. Care Med. 2017;195(2):205–211. doi: 10.1164/rccm.201603-0618OC. [DOI] [PubMed] [Google Scholar]
- 5.Muller J.E., Stone P.H., Turi Z.G., Rutherford J.D., Czeisler C.A., Parker C., Poole W.K., Passamani E., Roberts R., Robertson T., Sobel B.E., Willerson J.T., Braunwald E. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 1985;313(21):1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Hall M, Dondo TB, Wilkinson C, Ludman P, DeBelder M, et al. Association between time of hospitalization with acute myocardial infarction and in-hospital mortality. Eur Heart J. 2019;40:1214–21. [DOI] [PubMed]
- 7.Muller J.E., Ludmer P.L., Willich S.N., Tofler G.H., Aylmer G., Klangos I., Stone P.H. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75(1):131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 8.Rostagno C., Taddei T., Paladini B., Modesti P.A., Utari P., Bertini G. The onset of symptomatic atrial fibrillation and paroxysmal supraventricular tachycardia is characterized by different circadian rhythms. Am. J. Cardiol. 1993;71(5):453–455. doi: 10.1016/0002-9149(93)90454-k. [DOI] [PubMed] [Google Scholar]
- 9.Viskin S. Circadian variation of symptomatic paroxysmal atrial fibrillation. Data from almost 10000 episodes. Eur. Heart J. 1999;20(19):1429–1434. doi: 10.1053/euhj.1999.1632. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T., Murakawa Y., Sezaki K., Inoue M., Hayami N., Shuzui Y., Omata M. Circadian variation of paroxysmal atrial fibrillation. Circulation. 1997;96(5):1537–1541. doi: 10.1161/01.cir.96.5.1537. [DOI] [PubMed] [Google Scholar]
- 11.Gillis A.M., Connolly S.J., Dubuc M., Yee R., Lacomb P., Philippon F. Circadian variation of paroxysmal atrial fibrillation. PA3 Investigators. Atrial Pacing Peri-ablation for Prevention of Atrial Fibrillation Trial. Am. J. Cardiol. 2001;87(794–8):A8. doi: 10.1016/s0002-9149(00)01509-5. [DOI] [PubMed] [Google Scholar]
- 12.Deguchi Y, Amino M, Adachi K, Matsuzaki A, Iwata O, Yoshioka K, et al. Circadian Distribution of Paroxysmal Atrial Fibrillation in Patients with and without Structural Heart Disease in Untreated State. Ann Noninvasive Electrocardiol. 2009;14:280–9. [DOI] [PMC free article] [PubMed]
- 13.Shen M.J., Choi E.-K., Tan A.Y., Lin S.-F., Fishbein M.C., Chen L.S., Chen P.-S. Neural mechanisms of atrial arrhythmias. Nat. Rev. Cardiol. 2012;9(1):30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 14.Chen P.-S., Chen L.S., Fishbein M.C., Lin S.-F., Nattel S. Role of the Autonomic Nervous System in Atrial Fibrillation: Pathophysiology and Therapy. Circ. Res. 2014;114(9):1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter A., Frontera A., Bond R., Duncan E., Thomas G. Vagal atrial fibrillation: What is it and should we treat it? Int. J. Cardiol. 2015;201:415–421. doi: 10.1016/j.ijcard.2015.08.108. [DOI] [PubMed] [Google Scholar]
- 16.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 17.de Vos C.B., Nieuwlaat R., Crijns H.J.G.M., Camm A.J., LeHeuzey J.-Y., Kirchhof C.J., Capucci A., Breithardt G., Vardas P.E., Pisters R., Tieleman R.G. Autonomic trigger patterns and anti-arrhythmic treatment of paroxysmal atrial fibrillation: data from the Euro Heart Survey. Eur. Heart J. 2008;29(5):632–639. doi: 10.1093/eurheartj/ehn025. [DOI] [PubMed] [Google Scholar]
- 18.Vieillard-Baron A., Boyd J. Non-antiarrhythmic interventions in new onset and paroxysmal sepsis-related atrial fibrillation. Intensive Care Med. 2018;44(1):94–97. doi: 10.1007/s00134-017-4986-7. [DOI] [PubMed] [Google Scholar]
- 19.Delle Karth G., Reinelt P., Buberl A., Geppert A., Huelsmann M., Berger R., Heinz G. Circadian variation in ventricular tachycardia and atrial fibrillation in a medical-cardiological ICU. Intensive Care Med. 2003;29(6):963–968. doi: 10.1007/s00134-003-1735-x. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T., Uchino S., Sasabuchi Y., Hagiwara Y., Yoshida T., Nashiki H., Suzuki H., Takahashi H., Kishihara Y., Nagasaki S., Okazaki T., Katayama S., Sakuraya M., Ogura T., Inoue S., Uchida M., Osaki Y., Kuriyama A., Irie H., Kyo M., Shima N., Saito J., Nakayama I., Jingushi N., Nishiyama K., Masuda T., Tsujita Y., Okumura M., Inoue H., Aoki Y., Kondo T., Nagata I., Igarashi T., Saito N., Nakasone M. Prognostic impact of sustained new-onset atrial fibrillation in critically ill patients. Intensive Care Med. 2020;46(1):27–35. doi: 10.1007/s00134-019-05822-8. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T., Uchino S., Sasabuchi Y. Clinical course after identification of new-onset atrial fibrillation in critically ill patients: The AFTER-ICU study. J. Crit. Care. 2020;59:136–142. doi: 10.1016/j.jcrc.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Jones D., Bellomo R., Bates S., Warrillow S., Goldsmith D., Hart G. Patient monitoring and the timing of cardiac arrests and medical emergency team calls in a teaching hospital. Intensive Care Med. 2006;32:1352–1356. doi: 10.1007/s00134-006-0263-x. [DOI] [PubMed] [Google Scholar]
- 23.Hansen C.M., Wissenberg M., Weeke P., Ruwald M.H., Lamberts M., Lippert F.K., Gislason G.H., Nielsen S.L., Køber L., Torp-Pedersen C., Folke F. Automated External Defibrillators Inaccessible to More Than Half of Nearby Cardiac Arrests in Public Locations During Evening, Nighttime, and Weekends. Circulation. 2013;128(20):2224–2231. doi: 10.1161/CIRCULATIONAHA.113.003066. [DOI] [PubMed] [Google Scholar]
- 24.Wallace D.J., Angus D.C., Barnato A.E., Kramer A.A., Kahn J.M. Nighttime Intensivist Staffing and Mortality among Critically Ill Patients. N. Engl. J. Med. 2012;366(22):2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerlin M.P., Small D.S., Cooney E., Fuchs B.D., Bellini L.M., Mikkelsen M.E., Schweickert W.D., Bakhru R.N., Gabler N.B., Harhay M.O., Hansen-Flaschen J., Halpern S.D. A Randomized Trial of Nighttime Physician Staffing in an Intensive Care Unit. N. Engl. J. Med. 2013;368(23):2201–2209. doi: 10.1056/NEJMoa1302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt H., Müller-Werdan U., Hoffmann T., Francis D.P., Piepoli M.F., Rauchhaus M., Prondzinsky R., Loppnow H., Buerke M., Hoyer D., Werdan K. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups*. Crit. Care Med. 2005;33(9):1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt H., Hoyer D., Wilhelm J., Söffker G., Heinroth K., Hottenrott K., Said S.M., Buerke M., Müller-Werdan U., Werdan K. The Alteration of Autonomic Function in Multiple Organ Dysfunction Syndrome. Crit. Care Clin. 2008;24(1):149–163. doi: 10.1016/j.ccc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt HB, Werdan K, Müller-Werdan U. Autonomic dysfunction in the ICU patient: Curr Opin Crit Care. 2001;7:314–22. [DOI] [PubMed]
- 29.Aviles R.J., Martin D.O., Apperson-Hansen C., Houghtaling P.L., Rautaharju P., Kronmal R.A., Tracy R.P., Van Wagoner D.R., Psaty B.M., Lauer M.S., Chung M.K. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation. 2003;108(24):3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 30.Admiraal M.M., Gilmore E.J., Van Putten M.J.A.M., Zaveri H.P., Hirsch L.J., Gaspard N. Disruption of Brain-Heart Coupling in Sepsis. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2017;34(5):413–420. doi: 10.1097/WNP.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt H., Hoyer D., Hennen R., Heinroth K., Rauchhaus M., Prondzinsky R., Hottenrott K., Buerke M., Müller-Werdan U., Werdan K. Autonomic dysfunction predicts both 1- and 2-month mortality in middle-aged patients with multiple organ dysfunction syndrome*. Crit. Care Med. 2008;36(3):967–970. doi: 10.1097/CCM.0B013E3181653263. [DOI] [PubMed] [Google Scholar]
- 32.Millar P.J., Floras J.S. Statins and the autonomic nervous system. Clin. Sci. 2014;126:401–415. doi: 10.1042/CS20130332. [DOI] [PubMed] [Google Scholar]
- 33.Liu W.C., Lin W.Y., Lin C.S., Huang H.B., Lin T.C., Cheng S.M., Yang S.P., Lin J.C., Lin W.S. Prognostic impact of restored sinus rhythm in patients with sepsis and new-onset atrial fibrillation. Crit. Care. 2016;20(1) doi: 10.1186/s13054-016-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida T., Uchino S., Yokota T., Fujii T., Uezono S., Takinami M. The impact of sustained new-onset atrial fibrillation on mortality and stroke incidence in critically ill patients: A retrospective cohort study. J. Crit. Care. 2018;44:267–272. doi: 10.1016/j.jcrc.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 35.Moss T.J., Calland J.F., Enfield K.B., Gomez-Manjarres D.C., Ruminski C., DiMarco J.P., Lake D.E., Moorman J.R. New-Onset Atrial Fibrillation in the Critically Ill*. Crit. Care Med. 2017;45(5):790–797. doi: 10.1097/CCM.0000000000002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.