Abstract
Context
In December 2020, the US Food and Drug Administration approved a 68Ga-labeled prostate-specific membrane antigen ligand (68Ga-PSMA-11) for positron emission tomography (PET) in patients with suspected prostate cancer (PCa) metastasis who are candidates for initial definitive therapy. 68Ga-PSMA PET is increasingly performed for these patients and is usually combined with computed tomography (CT). In recent years, 68Ga-PSMA PET has been combined with high-resolution magnetic resonance imaging (MRI), which is beneficial for T staging and may further enhance the staging of primary PCa.
Objective
To compare the diagnostic accuracy of 68Ga-PSMA PET/MRI with 68Ga-PSMA PET/CT for staging of primary PCa.
Evidence acquisition
A comprehensive literature search was performed using Embase, PubMed/Medline, Web of Science, Cochrane Library, and Google Scholar up to June 24, 2021 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Risk of bias was assessed using the QUADAS-2 tool.
Evidence synthesis
The search identified 2632 articles, of which 27 were included. The diagnostic accuracy of 68Ga-PSMA PET/MRI, measured as the pooled natural logarithm of diagnostic odds ratio (lnDOR), was 2.27 (95% confidence interval [CI] 1.21–3.32) for detection of extracapsular extension (ECE), 3.50 (95% CI 2.14–4.86) for seminal vesicle invasion (SVI), and 4.73 (95% CI 2.93–6.52) for lymph node metastasis (LNM). For 68Ga-PSMA PET/CT, the analysis showed lnDOR of 2.45 (95% CI 0.75–4.14), 2.94 (95% CI 2.26–3.63), and 2.42 (95% CI 2.07–2.78) for detection of ECE, SVI, and LNM, respectively. The overall risk of bias and applicability concerns were assessed as moderate and low, respectively.
Conclusions
68Ga-PSMA PET/MRI shows high diagnostic accuracy equivalent to that of 68Ga-PSMA PET/CT for detection of ECE, SVI, and LNM in staging of PCa. There is an urgent need for direct comparison of the two diagnostic tests in future research.
Patient summary
The use of radioactively labeled molecules that bind to prostate-specific membrane antigen (68Ga-PSMA) for positron emission tomography (PET) scans combined with either computed tomography (CT) or magnetic resonance imaging (MRI) is increasing for prostate cancer diagnosis. There is a need for direct comparison of the two tests to demonstrate the benefit of 68Ga-PSMA PET/MRI for determining tumor stage in prostate cancer.
Take Home Message
After the recent US Food and Drug Administration approval of 68Ga-labeled prostate-specific membrane antigen ligand (68Ga-PSMA) positron emission tomography (PET) for staging of primary prostate cancer (PCa), it is expected that the use of this imaging modality will increase rapidly. Our review of the literature shows that 68Ga-PSMA PET/magnetic resonance imaging has high diagnostic accuracy equivalent to that of 68Ga-PSMA PET/computed tomography in primary PCa staging. There is an urgent need for direct head-to-head comparison of the two diagnostic tests in future research.
Keywords: Prostate cancer, Primary staging, Diagnostic accuracy, Gallium-68, Positron emission tomography
1. Introduction
Prostate cancer (PCa) is the second most common type of cancer among men and was the cause of 375 304 deaths worldwide in 2020 [1], [2]. Accurate staging of patients with primary PCa is essential for optimal treatment decisions. Although several imaging modalities can be applied for staging of primary PCa, no single modality can currently answer all clinical questions at once. For local tumor staging, in which extracapsular extension (ECE) and seminal vesicle invasion (SVI) are important parameters, prostate magnetic resonance imaging (MRI) is the modality of choice [3]. For evaluation of locoregional lymph node metastasis (LNM) and distant metastases, computed tomography (CT) and bone scintigraphy are traditionally applied, although the sensitivity of these modalities is moderate at 42% and 79%, respectively [4]. As a result, patients often need to undergo multiple imaging modalities before a definitive treatment can be identified.
In the past 5 yr, one of the most successful developments in the field of PCa diagnostics has been positron emission tomography (PET) using a gallium-68 prostate-specific membrane antigen ligand (68Ga-PSMA). PSMA is a type II transmembrane glycoprotein with an intracellular and an extracellular domain [5], [6]. PSMA expression has been observed in benign prostate epithelium and on PCa cells, but it is also expressed on other tissues such as the kidneys, small intestine, and salivary glands. The expression of PSMA on PCa cells is 1000-fold higher than the expression on normal tissues [7] and therefore PSMA is a useful target for imaging of PCa. To date, 68Ga-PSMA PET has mainly been used for detection of recurrent PCa [8]. Owing to its high sensitivity, even at low prostate-specific antigen (PSA) levels in blood, 68Ga-PSMA PET is increasingly used to detect LNM and local recurrence in the biochemical recurrence setting [9], [10]. Although the additional value of 68Ga-PSMA PET for staging at primary diagnosis is still under investigation, 68Ga-PSMA PET appears to outperform conventional imaging modalities in defining N and M stages and shows excellent sensitivity in the initial diagnosis of PCa [8], [11]. The use of 68Ga-PSMA PET is expected to increase rapidly since the recent US Food and Drug Administration (FDA) approval of 68Ga-PSMA-11 for patients with suspected biochemical recurrence and patients with suspected metastasis who are candidates for initial definitive therapy (surgery or radiation therapy) [12].
Traditionally, PET is combined with CT for anatomical correlation and attenuation correction [13]. In recent years, PET/MRI scanners have been introduced in the clinic. This new state-of-the-art hybrid scanner has extended imaging using PET [14]. One of the advantages of MRI over CT is better visualization of soft tissues [15], [16]. 68Ga-PSMA PET/MRI is an all-in-one imaging modality for the staging of primary PCa, as it allows accurate visualization of the local tumor by MRI and sensitive detection of lymph nodes and distant metastases by 68Ga-PSMA PET [17]. Another advantage is the reduction in radiation burden, as replacement of CT by MRI can result in an estimated dose reduction of up to 19.4 mSv per examination [18]. By contrast, PET/MRI is more expensive and image analysis is more complicated and time-consuming than for PET/CT. To determine the diagnostic performance of 68Ga-PSMA PET/MRI for primary PCa staging in comparison to 68Ga-PSMA PET/CT, we performed a systematic review and meta-analysis of the literature.
2. Evidence acquisition
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The research question was the diagnostic accuracy of 68Ga-PSMA PET/MRI and of 68Ga-PSMA PET/CT for staging of primary PCa. Study selection, data extraction, and quality assessment were performed in duplicate by two independent authors (S.W.L. and A.C.d.J.). Discrepancies were resolved via consensus.
2.1. Literature search
A comprehensive search of the Embase, PubMed/Medline, Web of Science, Cochrane Library, and Google Scholar databases for literature published up to June 24, 2021 was performed by our institutional medical library information specialist. The MeSH and search terms used are listed in the Supplementary material.
2.2. Study eligibility
Studies were screened for eligibility, initially based on the title and abstract and subsequently on the full text (Supplementary material). Duplicate articles were removed. Studies were included on the basis of the following criteria: (1) inclusion of patients with primary PCa (primary staging); (2) 68Ga-PSMA PET/MRI and/or 68Ga-PSMA PET/CT as the index test; (3) radical prostatectomy (RP) or lymph node dissection (LND) as the reference standard; (4) diagnostic value reported at the per-patient level; (5) and absolute numbers of true positives (TPs), false positives (FPs), true negatives (TNs), and false negatives (FN) available or calculable from reported data. Studies were excluded according to the following criteria: (1) insufficient data reported; (2) published as a case report, editorial, letter, protocol, review, or conference summary; (3) not published in English; (4) no full text available; (5) not performed in humans; and (6) fewer than ten patients included.
2.3. Data extraction and quality assessment
Data extraction was performed using a self-prepared extraction form (Supplementary material). The following information was extracted from each study: aim of the study, first author, journal, year of publication, study design (prospective or retrospective), number of patients, study population, age, PSA level, index test, reference standard, subgroups, scan protocol, scanner type, tracer, definition of PSMA positivity, type of lesions, sensitivity, specificity, and TP, FN, TN, and FP values. Risk of bias in the studies and the applicability of the studies to the research question were assessed according to QUADAS-2, a tool especially developed for systematic reviews of diagnostic accuracy studies [19].
2.4. Data synthesis and analysis
The diagnostic accuracy of 68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT was based on the sensitivity and specificity of three important characteristics for PCa staging, including the presence of SVI, ECE, and LNM at a per-patient level (not per-lesion level). Sensitivity and specificity were calculated using 2 × 2 contingency tables, with continuity corrections (0.5) added to zero cells to avoid statistical artifacts. Data were presented in forest plots per characteristic (ECE, SVI, and LNM) and per imaging modality (68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT). In addition, weighted crosshair plots and confidence region plots were generated to analyze the data according to the receiver operating characteristic. Publication bias was assessed using the Egger regression test and visualized using funnel plots. All analyses and graphics were performed using R statistical software v4.0.3 with the mada package.
2.5. Analysis of diagnostic accuracy
For assessment of diagnostic accuracy, the diagnostic odds ratio (DOR) was used (ratio of the odds of disease for test positives relative to the odds of disease for test negatives). The DOR value ranges from zero to infinity and a higher value indicates better discriminatory test performance. Meta-analyses of DOR values were performed using a random-effects model to account for heterogeneity. To fit the random-effects model, the DOR values were transformed into natural logarithms (lnDOR). Pooled lnDOR values were presented in forest plots. Owing to a small sample size in several studies, a univariate approach was used for all meta-analyses. In addition, a proportional-hazards model approach was used to assess the diagnostic accuracy. Heterogeneity between studies was assessed using the Cochran Q test and the I2 statistic.
2.6. Reference standard to determine diagnostic accuracy
For calculations of sensitivity, specificity, and lnDOR, results from studies with histopathology (RP or LND) as the reference standard and reporting of TP, TN, FP, and FN were included.
3. Evidence synthesis
3.1. Literature search and study eligibility
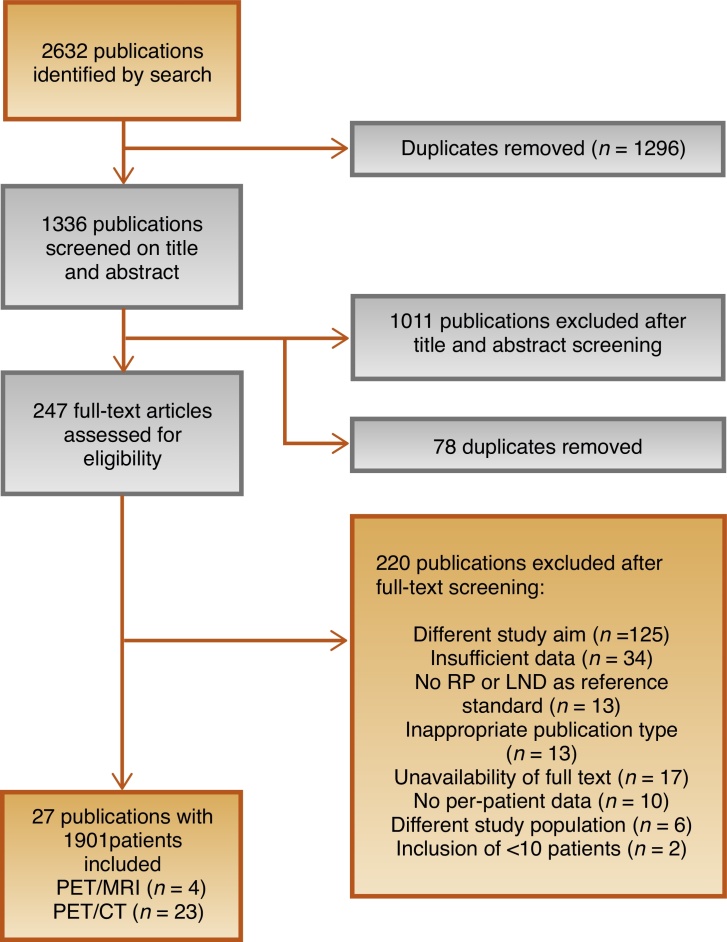
The initial search identified 2632 publications, of which 1296 were removed as duplicates (Fig. 1). After screening of the titles and abstracts, 1011 publications were excluded. During full-text screening, 220 of the 247 remaining studies were excluded because of a different study aim (n = 125), insufficient data (n = 34), no RP or LND as the reference standard (n = 13), inappropriate publication type (n = 13), unavailability of full text (n = 17), no per-patient data (n = 10), different study population (n = 6), or inclusion of fewer than ten patients (n = 2). Finally, 27 publications involving 1901 patients were included in the systematic review.
Fig. 1.
Flowchart of the literature search and study selection. RP = radical prostatectomy; LND = lymph node dissection; PET = positron emission tomography; MRI = magnetic resonance imaging; CT = computed tomography.
3.2. Description of the studies included
68Ga-PSMA PET/MRI was performed in four studies [20], [21], [22], [23] and 68Ga-PSMA PET/CT in 23 studies [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. No direct comparative studies were found. For 68Ga-PSMA PET/MRI, one study included was prospective [21], while the other three studies were retrospective [20], [22], [23]. Two of the 68Ga-PSMA PET/MRI studies only included patients with PCa and eligible for RP [20], [22], while the other two included only patients with high-risk PCa (defined as Gleason score ≥8, PSA > 20 ng/ml, or stage ≥ T2c) [21], [23]. In the 68Ga-PSMA PET/MRI studies, age and PSA ranged from 63 to 68 years and 0.14 to 12.9 ng/ml, respectively (Table 1).
Table 1.
Characteristics of the studies included
| Study | Type | N | Population | Age (yr) a | PSA (ng/ml) a | Imaging modality (index test) |
Reference standard | Tracer dose (MBq) a | Injection time (min) a |
|---|---|---|---|---|---|---|---|---|---|
| Grubmüller 2018 [20] | R | 122 | HP PCa + RP | 64 (59–71)* | 7.6 (5.5–13.4)* | 68Ga-PSMA-11 PET/MRI | RP / LND | 2 MBq/kg | NR |
| Kaufmann 2020 [21] | P | 12 | HP HR PCa + RP | NR | 12.9 (6.2–29.0) | 68Ga-PSMA-11 PET/MRI | RP / LND | 190 ± 40 | 60 |
| Muehlematter 2019 [22] | R | 40 | PCa + RP | 63 ± 6 | 8.12 (NR) | 68Ga-PSMA-11 PET/MRI | RP | 131 ± 18.8 | 60 |
| Thalgott 2018 [23] | R | 73 | HP HR PCa + RP | 68 (63–73)* | 0.14 (0.06–0.35)* | 68Ga-PSMA-11 PET/MRI | RP / LND | 138 (114–156) | 55 (50–67)* |
| Arslan 2020 [37] | R | 39 | HP PCa + RP + LND | 62.47 (45–79) | 0.095 (0.0238–0.59) | 68Ga-PSMA-11 PET/CT | RP / LND | NR | 60 |
| Çelen 2020 [38] | P | 30 | LR, IR, or HR PCa (D’Amico) + RP + LND | 65.07 ± 8.01 (46–82) | 9.49 ± 6.97 (1.3–27) | 68Ga-PSMA-I&T PET/CT | RP / LND | 185 (125–317) | 60 |
| Chen 2020 [39] | R | 54 | HP PCa + RP | 69 (55–84) | 0.133 (0.04–1.10) | 68Ga-PSMA-11 PET/CT | RP | 135.72 (126.2–177.6) | 60 |
| Corona-Montes 2020 [40] | R | 17 | HP HR PCa (+PSA >20 ng/ml and GS ≥ 8 and T3) + RP + LND | 63 (44 − 77) | 9 (6 − 131) | 68Ga-PSMA PET/CT | LND | 2 MBq/kg | 60 |
| Esen 2021 [41] | R | 96 | HP PCa + RP + LND | 65 (61–70)* | 8 (5.5–11.67)* | 68Ga-PSMA-11 PET/CT | LND | 2 MBq/kg (up to 185 MBq) | 45 (WB and prone–pelvic) + 90 (abdominal) |
| Fendler 2016 [24] | R | 21 | HP PCa (+PSA >20 ng/ml and/or GS ≥ 7 and/or bone pain) + RP | 70 (59–80) | 58.7 (3–363) | 68Ga-PSMA-11 PET/CT | RP | 192 ± 48 | 58 ± 12 |
| Franklin 2021 [42] | R | 233 | HP PCa + RP + LND | 68 (48–81) | 7.4 (1.5–72) | 68Ga-PSMA-11 PET/CT | LND | 200 MBq (mean) | 45–60 |
| Frumer 2020 [43] | R | 89 | IR or HR PCa (D’Amico) + RP + LND | 66.9 (64–70)* | 8.5 (5–15)* | 68Ga-PSMA PET/CT | LND | 111–185 | 50–60 |
| Gao 2019 [25] | R | 49 | HP PCa + RP | 69 (55–82) | 15.9 (4.0–72.1) | 68Ga-PSMA-11 PET/CT | RP | 132 (131–178) | 45 |
| Gupta 2017 [26] | R | 12 | HP HR PCa (+PSA >20 ng/ml and GS ≥ 8 and T3) + RP | 62 (46–76) | 55.5 (8.7–200.6) | 68Ga-PSMA-11 PET/CT | LND | 2 MBq/kg | 60 |
| Klingenberg 2021 [44] | R | 177 | HP HR PCa (D’Amico) + RP + LND | NR | NR | 68Ga-PSMA-11 PET/CT | LND | 2,14 MBq/kg | 60 |
| Kopp 2021 [45] | R | 39 | IR PCa (D’Amico) + RP + LND | 64.6 (45.2–77.7) | 6.3 (3.1–9.0) | 68Ga-PSMA PET/CT | LND | NR | NR |
| Kopp 2020 [27] | R | 90 | At least EAU IR PCa + RP | 65 (60–71)* | 7.4 (5.5–12.5)* | 68Ga-PSMA PET/CT | LND | NR (92–242) | 60 |
| Kulkarni 2020 [28] | R | 51 | IR and HR PCa + RP + LND | 66 ± 7 | 0.39 (0.04–0.9) | 68Ga-PSMA-11 PET/CT | LND | NR (111–166) | 60 |
| Öbek 2017 [29] | R | 51 | EAU HR and very HR PCa + RP + LND | 64 ± 6 | 26.5 ± 21.4 | 68Ga-PSMA-11 PET/CT | RP / LND | 166 ± 83 | 45–60 |
| Petersen 2020 [30] | P | 20 | EAU IR or HR PCa + LND + RT | 71 (58–76) | 12.5 (2.8–66.0) | 68Ga-PSMA-11 PET/CT | LND | 2 MBq/kg | 60 ± 9 |
| Simsek 2021 [46] | R | 49 | LR, IR, or HR PCa (D’Amico) + RP + LND | NR | NR | 68Ga-PSMA-11 PET/CT | LND | 185 | 45–60 |
| van Kalmthout 2020 [31] | P | 96 | HP PCa with >10% risk for LNM (MSKCC) + LND | 70 (53–82) | 21.8 (1.7–298.0) | 68Ga-PSMA-11 PET/CT | RP / LND | 1.5 MBq/kg | 60 |
| van Leeuwen 2019 [32] | R | 140 | HP IR or HR PCa (GS ≥ 7, ISUP grade 3) + RP + LND | NR | 9.4 (NR) | 68Ga-PSMA-11 PET/CT | RP / LND | 2 MBq/kg | 45–60 |
| van Leeuwen 2017 [33] | P | 30 | HP IR or HR PCa + >5% risk of LNM (Briganti) + RP + LND | 65 (60–71)* | 8.1 (5.2–10.1) | 68Ga-PSMA-11 PET/CT | RP / LND | NR | 60 |
| von Klot 2017 [34] | R | 21 | PCa + RP | 68 (56–77) | 11.9 ± NR | 68Ga-PSMA-I&T PET/CT | RP | 98 ± 25 | 60 |
| Yaxley 2019 [35] | R | 208 | PCa + RP + LND | 68 (44–80) | 7.6 (1.5–51.0) | 68Ga-PSMA-11 PET/CT | RP / LND | 200 ± NR | 45–60 |
| Zhang 2017 [36] | R | 42 | IR or HR PCa (D’Amico) + RP + LND | 69 (55–82) | 37.3 (7.2–348.0) | 68Ga-PSMA-11 PET/CT | RP / LND | 132 (131–178) | 60 |
R = retrospective; P = prospective; HP = histologically proven; LR = low risk; IR = intermediate risk; HR = high risk; PCa = prostate cancer; RP = radical prostatectomy; LND = lymph node dissection; GS = Gleason score; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen; PET = positron emission tomography; MRI = magnetic resonance imaging; CT = computed tomography; EAU = European Association of Urology; MSKCC = Memorial Sloan Kettering Cancer Center; ISUP = International Society of Urological Pathology; NR = not reported; WB = whole body.
Age, PSA, tracer dose, and injection time are reported according to the original study as mean ± standard deviation, median (range), median (interquartile range)*, or exact dose (MBq/kg).
Of the 68Ga-PSMA PET/CT studies, four were prospective [30], [31], [33], [38] and 19 had a retrospective design. Populations in the 68Ga-PSMA PET/CT studies varied from patients with PCa and eligible for RP to patients with high-risk PCa. Patient age ranged from 62 to 71 yr and PSA from 0.095 to 58.7 ng/ml (Table 1).
3.3. Local tumor staging: identification of ECE and SVI
For local staging, ECE and SVI data could be extracted at the per-patient level from three studies on 68Ga-PSMA PET/MRI, and five 68Ga-PSMA PET/CT studies for ECE and six for SVI (Table 2).
Table 2.
Diagnostic value of the studies for seminal vesicle invasion, extracapsular extension, and lymph node metastases
| Study | N | Seminal vesicle invasion |
Extracapsular extension |
Lymph node metastasis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sns (%) | Spy (%) | PPV (%) | NPV (%) | Sns (%) | Spy (%) | PPV (%) | NPV (%) | Sns (%) | Spy (%) | PPV (%) | NPV (%) | ||
| Grubmüller 2018 [20] | 122 | 94.4 | 95.2 | 85.0 | 98.3 | 66.7 | 91.5 | 73.7 | 88.5 | 68.8 | 100.0 | 100.0 | 92.8 |
| Kaufmann 2020 [21] | 12 | NR | NR | NR | NR | NR | NR | NR | NR | 50.0 | 100.0 | 100.0 | 90.9 |
| Muehlematter 2019 [22] | 40 | 55.0 | 93.6 | 55.0 | 93.6 | 68.8 | 67.0 | 47.1 | 83.3 | NR | NR | NR | NR |
| Thalgott 2018 [23] | 73 | 81.8 | 80.0 | 77.1 | 84.2 | 94.3 | 45.0 | 82.0 | 75.0 | 60.0 | 100.0 | 100.0 | 82.8 |
| Arslan 2020 [37] | 39 | NR | NR | NR | NR | 62.5 | 60.8 | 52.6 | 70.0 | NR | NR | NR | NR |
| Çelen 2020 [38] | 30 | 83.3 | 79.2 | 50.0 | 95.0 | 53.9 | 53.9 | 60.0 | 46.7 | 100.0 | 47.6 | 8.3 | 100.0 |
| Chen 2020 [39] | 54 | 75.0 | 95.0 | 82.0 | 93.0 | 78.0 | 94.0 | 97.0 | 67.0 | NR | NR | NR | NR |
| Corona-Montes 2020 [40] | 17 | NR | NR | NR | NR | NR | NR | NR | NR | 75.0 | 92.3 | 75.0 | 92.3 |
| Esen 2021 [41] | 96 | NR | NR | NR | NR | NR | NR | NR | NR | 53.3 | 98.8 | 88.9 | 92.0 |
| Fendler 2016 [24] | 21 | 72.7 | 100.0 | 100.0 | 76.9 | NR | NR | NR | NR | NR | NR | NR | NR |
| Franklin 2021 [42] | 233 | NR | NR | NR | NR | NR | NR | NR | NR | 48.3 | 92.0 | 66.7 | 84.3 |
| Frumer 2020 [43] | 89 | NR | NR | NR | NR | NR | NR | NR | NR | 25.0 | 94.8 | 42.9 | 89.0 |
| Gao 2019 [25] | 49 | 77.8 | 95.0 | 77.8 | 95.0 | 78.4 | 91.7 | 96.7 | 57.9 | NR | NR | NR | NR |
| Gupta 2017 [26] | 12 | NR | NR | NR | NR | NR | NR | NR | NR | 100.0 | 80.0 | 87.5 | 100.0 |
| Klingenberg 2021 [44] | 177 | NR | NR | NR | NR | NR | NR | NR | NR | 30.6 | 96.5 | 68.8 | 84.5 |
| Kopp 2021 [45] | 39 | NR | NR | NR | NR | NR | NR | NR | NR | 20 | 94.1 | 33.3 | 88.9 |
| Kopp 2020 [27] | 90 | NR | NR | NR | NR | NR | NR | NR | NR | 43.8 | 95.9 | 70.0 | 88.8 |
| Kulkarni 2020 [28] | 51 | NR | NR | NR | NR | NR | NR | NR | NR | 80.0 | 90.3 | 84.2 | 87.5 |
| Öbek 2017 [29] | 51 | NR | NR | NR | NR | NR | NR | NR | NR | 53.3 | 86.1 | 61.5 | 81.6 |
| Petersen 2020 [30] | 20 | NR | NR | NR | NR | NR | NR | NR | NR | 38.5 | 100.0 | 100.0 | 46.7 |
| Simsek 2021 [46] | 49 | NR | NR | NR | NR | NR | NR | NR | NR | 71.4 | 100.0 | 100.0 | 95.4 |
| van Kalmthout 2020 [31] | 96 | NR | NR | NR | NR | NR | NR | NR | NR | 41.5 | 90.9 | 77.3 | 67.6 |
| van Leeuwen 2019 [32] | 140 | 46.5 | 92.8 | 74.1 | 79.6 | NR | NR | NR | NR | 52.9 | 87.6 | 71.1 | 76.5 |
| van Leeuwen 2017 [33] | 30 | NR | NR | NR | NR | NR | NR | NR | NR | 63.6 | 94.7 | 87.5 | 81.8 |
| von Klot 2017 [34] | 21 | 75.0 | 100.0 | 100.0 | 94.4 | 90.0 | 90.9 | 90.0 | 90.9 | NR | NR | NR | NR |
| Yaxley 2019 [35] | 208 | NR | NR | NR | NR | NR | NR | NR | NR | 38.2 | 93.5 | 67.7 | 80.8 |
| Zhang 2017 [36] | 42 | NR | NR | NR | NR | NR | NR | NR | NR | 93.3 | 96.3 | 93.3 | 96.3 |
Sns = sensitivity; Spy = specificity; PPV = positive predictive value; NPV = negative predictive value; NR = not reported.
For ECE, the sensitivity of 68Ga-PSMA PET/MRI ranged from 67.0% to 94.0% and the specificity from 45.0% to 92.0%, in comparison to 31.0% to 98.0% and 29.0% to 99.0%, respectively, for 68Ga-PSMA PET/CT. Analysis of the diagnostic accuracy for ECE (Supplementary material) showed a pooled lnDOR of 2.27 (95% confidence interval [CI] 1.21–3.32) for 68Ga-PSMA PET/MRI and 2.45 (95% CI 0.75–4.14) for 68Ga-PSMA PET/CT.
For SVI, the sensitivity of 68Ga-PSMA PET/MRI ranged from 55.0% to 94.0% and the specificity from 80.0% to 95.0%, in comparison to from 30.0% to 95.0% and from 59.0% to 100.0%, respectively, for 68Ga-PSMA PET/CT (Supplementary material). The diagnostic accuracy for SVI (Supplementary material) showed a pooled lnDOR of 3.50 (95% CI 2.14–4.86) for 68Ga-PSMA PET/MRI and 2.94 (95% CI 2.26–3.63) for 68Ga-PSMA PET/CT (Supplementary material).
3.4. Lymph node staging
For the diagnostic accuracy of lymph node staging, data could be extracted at a per-patient level from three 68Ga-PSMA PET/MRI and 18 68Ga-PSMA PET/CT studies (Table 2). The sensitivity and specificity for detection of LNM ranged from 50.0% to 68.0% and from 95.0%to 99.0%, respectively, for 68Ga-PSMA PET/MRI, and from 6.0% to 99.0% and from 29.0% to 100.0%, respectively, for 68Ga-PSMA PET/CT (Supplementary material). Analysis of the diagnostic accuracy for LNM (Supplementary material) showed a pooled lnDOR of 4.73 (95% CI 2.93–6.52) for 68Ga-PSMA PET/MRI and 2.42 (95% CI 2.07–2.78) for 68Ga-PSMA PET/CT. The lnDOR results are summarized in Table 3.
Table 3.
Summary of the pooled natural log of the diagnostic odds ratio (lnDOR) with 95% confidence interval (CI)
| Diagnosis | Modality | lnDOR (95% CI) |
|---|---|---|
| Extracapsular extension | PET/MRI | 2.27 (1.21–3.32) |
| PET/CT | 2.45 (0.75–4.14) | |
| Seminal vesicle invasion | PET/MRI | 3.50 (2.14–4.86) |
| PET/CT | 2.94 (2.26–3.63) | |
| Lymph node metastasis | PET/MRI | 4.73 (2.93–6.52) |
| PET/CT | 2.42 (2.07–2.78) |
PET = positron emission tomography; MRI = magnetic resonance imaging; CT = computed tomography.
3.5. Quality assessment
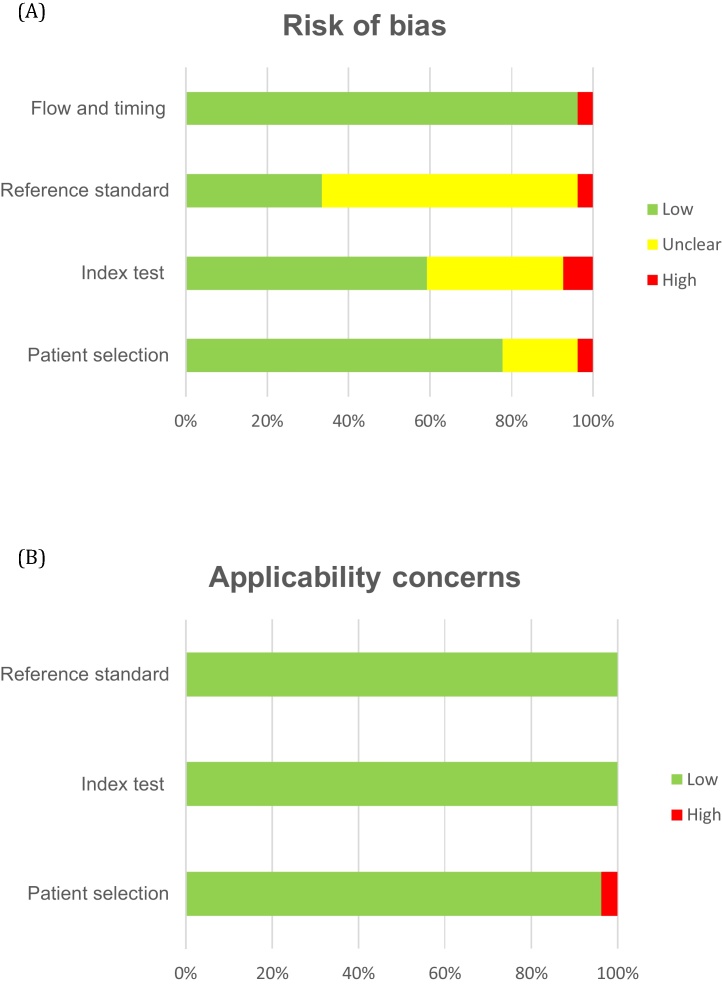
Results for the quality assessment per study are presented in the Supplementary material. The overall risk of bias and the overall applicability concern were assessed as moderate and low, respectively (Fig. 2). One of the four 68Ga-PSMA PET/MRI studies [20] had high risk of bias for the index test and reference standard because the index test was not interpreted blinded to the results of the reference standard and vice versa. For applicability concern, all 68Ga-PSMA PET/MRI studies were scored as low risk. Two of the 27 68Ga-PSMA PET/CT studies [25], [46] were scored as high risk. The first study [25] was scored as high risk for the patient selection domain for both risk of bias and applicability concern because of exclusion of patients with any Gleason score of 5. The second study [46] was scored as high risk for the index test domain because of interpretation of the index test with knowledge of the clinical, histopathological, and imaging data. Five 68Ga-PSMA PET/CT studies [31], [34], [41], [42], [45] were scored as unclear for patient selection as there was no information on whether a consecutive or random sample of patients was included and/or inappropriate exclusions were avoided. Overall for the 68Ga-PSMA PET/CT studies, the risk of bias was assessed as unclear for the index test in nine studies [25], [29], [32], [33], [35], [36], [42], [43], [44] and for the reference standard in 17 studies [26], [27], [28], [30], [31], [32], [34], [35], [36], [37], [38], [39], [41], [42], [44]–46] because it was not reported whether 68Ga-PSMA PET was evaluated without knowledge of the histopathology and vice versa.
Fig. 2.
QUADAS-2: overall risk of bias and applicability concerns for all 27 studies included.
3.6. Publication bias and heterogeneity analysis
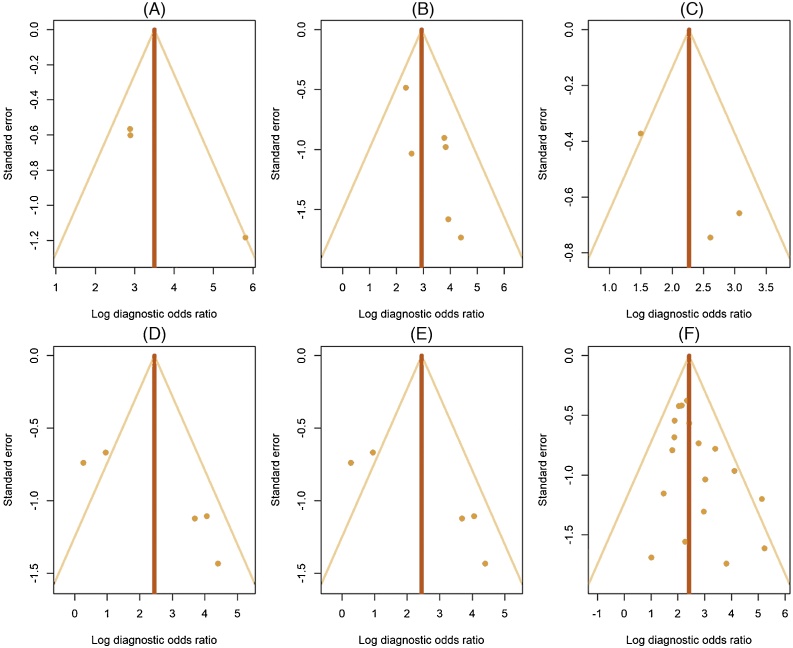
Visual assessment of funnel plots with lnDOR values as outcomes did not show any evidence of publication bias (Fig. 3).
Fig. 3.
Funnel plots of publication bias for detection of seminal vesicle invasion by (A) PET/MRI and (B) PET/CT; extracapsular extension by (C) PET/MRI and (D) PET/CT; and lymph node metastasis by (E) PET/MRI and (F) PET/CT. PET = positron emission tomography; MRI = magnetic resonance imaging; CT = computed tomography.
Overall, the Cochran Q test and I2 statistic showed low heterogeneity between studies within the groups: Q = 1.671 (p = 0.434) and I2 = 0% for ECE on PET/MRI, Q = 4.082 (p = 0.395) and I2 = 2.016% for ECE on PET/CT, Q = 2.954 (p = 0.228) and I2 = 32.284% for SVI on PET/MRI, Q = 4.396 (p = 0.494) and I2 = 0% for SVI on PET/CT, Q = 1.183 (p = 0.553) and I2 = 0% for LNM on PET/MRI, and Q = 17.763 (p = 0.404) and I2 = 4.296% for LNM on PET/CT.
3.7. Discussion
In clinical practice, 68Ga-PSMA PET is increasingly being used for imaging of PCa, mostly in the biochemical recurrence setting. In this meta-analysis, we compared the diagnostic accuracy of 68Ga-PSMA PET/MRI with 68Ga-PSMA PET/CT for primary PCa staging. Four 68Ga-PSMA PET/MRI and 23 68Ga-PSMA PET/CT studies were included to determine the diagnostic accuracy for detection of SVI, ECE, and/or LNM at the per-patient level.
As MRI is the current imaging modality of choice for local staging of PCa [47], it was expected that 68Ga-PSMA PET/MRI would show better diagnostic parameters for detection of ECE and SVI compared to 68Ga-PSMA PET/CT. However, for detection of ECE, analysis of the diagnostic accuracy showed lnDOR values of 2.27 (95% CI 1.21–3.32) for 68Ga-PSMA PET/MRI and 2.45 (95% CI 0.75–4.14) for 68Ga-PSMA PET/CT. These results suggest comparable diagnostic accuracy with wide and overlapping ranges. For detection of SVI, analysis of the diagnostic accuracy showed lnDOR values of 3.50 (95% CI 2.14–4.86) for 68Ga-PSMA PET/MRI and 2.94 (95% CI 2.26–3.63) for 68Ga-PSMA PET/CT. Although the results might suggest better diagnostic accuracy for detection of SVI with 68Ga-PSMA PET/MRI because of the higher upper 95% CI bound (4.86) compared to 68Ga-PSMA PET/CT (3.63), the ranges for the diagnostic accuracy from the studies were in general wide and highly overlapping.
Evangelista et al [48] performed a recent systematic review on the sensitivity and specificity of PET/MRI. The aim was to summarize the diagnostic information provided by PET/MRI for patients with PCa (primary staging and biochemical recurrence) and the authors included studies that used both radiolabeled PSMA and radiolabeled choline in their analysis. For detection of the primary tumor at the per-patient level, they reported pooled sensitivity of 94.9% and specificity of 62.5%. The inclusion of both PSMA and choline means that these results cannot be extrapolated to our data. Higher pooled sensitivity and specificity could be expected for 68Ga-PSMA PET/MRI alone, as it is known that radiolabeled choline is inferior to PSMA. Nevertheless, the results do confirm the diagnostic value of PET/MRI.
For LNM detection, it was expected that PET/MRI and PET/CT would show comparable diagnostic parameters, as the stand-alone 68Ga-PSMA PET has good diagnostic performance for this indication [8]. For LNM identification, analysis of the diagnostic accuracy showed lnDOR values of 4.73 (95% CI 2.93–6.52) for 68Ga-PSMA PET/MRI and 2.42 (95% CI 2.07–2.78) for 68Ga-PSMA PET/CT. The non-overlapping ranges might suggest better LNM detection by 68Ga-PSMA PET/MRI compared to 68Ga-PSMA PET/CT. The difference in diagnostic accuracy could be explained by the superior sensitivity and specificity reported in 68Ga-PSMA PET/MRI studies with fewer patients (n = 165) compared to the 68Ga-PSMA PET/CT studies (n = 1462) [20], [21], [23]. Wu et al [49] recently demonstrated higher diagnostic efficiency of 68Ga-PSMA PET/CT for detection of LNM at primary staging compared to stand-alone MRI for patients with intermediate- or high-risk PCa. The sensitivity of 68Ga-PSMA PET/CT was higher than that of MRI (65% vs 41%) and the specificity was comparable (94% vs 92%). Although these results could not be extrapolated to the current data, they indicate that the MRI component outperforms the CT component for exclusion of PSMA FP lymph nodes. In clinical practice, it can be challenging to distinguish lymph nodes from ganglions on CT. MRI is more accurate for this indication, which might explain the lower number of FP scans.
To evaluate the technical quality of the studies, scan protocols were compared. Among the 68Ga-PSMA PET/MRI studies, all scans were performed after administration of 68Ga-PSMA-11 with activity between 112 and 230 MBq. The time between injection and the scan was between 50 and 71 min and only one study did not specify the time of injection [20]. A Biograph mMR scanner (Siemens) was used three studies and a GE SIGNA T3 scanner in one study [22]. Although the scan protocols in all studies included a whole-body scan and a local scan of the prostate/pelvic area, the MR sequences used differed between studies. All scans were examined by two or more readers blinded to the clinical and histopathological findings, except for one study in which the reader was not blinded to the histopathology [20]. The definition of 68Ga-PSMA–positive lesions was roughly the same among the studies (tracer uptake exceeding that of background tissue) except for one [20], in which lesions were defined as 68Ga-PSMA–positive according to histopathological examination.
Among the 68Ga-PSMA PET/CT studies, most of the scans were performed using 68Ga-PSMA-11 with injected activity between 92 and 240 MBq. Four studies did not specify the PSMA ligand [27], [40], [43], [45] and two studies used 68Ga-PSMA-I&T as the radiotracer [34], [38]. The injection time was between 45 and 97 min before the scan. Different scanner models (uMI, Discovery, Biograph, Ingenuity, Gemini) were used in the 23 studies. All of the scan protocols included a whole-body scan, but the use of contrast enhancement for CT varied. All scans were examined by one or more readers. In 13 studies, readers were blinded to the clinical and/or histopathological findings and in ten studies this was not specified [25], [31], [33], [35], [36], [41], [42], [43], [44], [46]. The definition of 68Ga-PSMA–positive lesions differed among the studies. In most of the studies, lesions were defined as positive if tracer uptake was higher than that of background tissue. In only one study [25], 68Ga-PSMA uptake was correlated with the histopathological results. In another study [30], no protocol-specific criteria were used to define lesions as malignant on 68Ga-PSMA PET/CT, but readers followed the generally accepted current reading criteria. In two studies [35], [42], a lesion defined as nonphysiological/suspicious when moderate or intense 68Ga-PSMA uptake was localized in an anatomical lesion on contrast-enhanced CT. Overall, the differences in imaging protocols, tracers, and scanners between the studies may have affected the results.
As shown by the high number of recent studies, interest in 68Ga-PSMA PET in combination with MRI or CT is increasing among nuclear medicine physicians, urologists, medical oncologists, and radiation oncologists involved in the treatment of PCa. 68Ga-PSMA PET/MRI is an all-in-one imaging modality for the staging of primary PCa, as it allows accurate visualization of the local tumor by MRI and sensitive detection of lymph nodes and distant metastases by 68Ga-PSMA PET [17]. Besides its diagnostic accuracy, PET/MRI also exposes patients to less radiation in comparison to PET/CT as the CT is omitted [16]. Conversely, PET/MRI is more expensive and image analysis is more complicated and time consuming than for PET/CT. However, considering that 68Ga-PSMA PET/CT combined with local MRI is the current standard for primary staging of high-risk PCa, 68Ga-PSMA PET/MRI may be less expensive and more time-efficient. Another consideration is that there are other qualitative parameters, such as patient safety and comfort, that distinguish these two imaging modalities and could determine which is preferred. Furthermore, local experience and availability are important factors when selecting the right imaging modality for each patient.
In addition to differences in imaging protocols, the meta-analysis has several limitations. First, the majority of the patients included had intermediate- or high-risk PCa, which may have led to overestimation of the diagnostic accuracy. Second, 68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT were not performed in the same patient cohorts, which may have affected the results because of the heterogeneous nature of the cohorts and the study designs. Finally, the majority of the studies included in the meta-analysis were retrospective and relatively small in sample size.
In clinical practice, 68Ga-PSMA PET is frequently used, but mainly for detection of PCa biochemical recurrence [50]. With the recent FDA approval of the 68Ga-PSMA-11 ligand, the use of 68Ga-PSMA PET is expected to increase rapidly for staging of primary PCa, especially for patients with suspected metastasis who are candidates for initial definitive therapy (surgery or radiation therapy) [12]. To optimize staging of primary PCa in the clinic, a direct comparison of 68Ga-PSMA PET/MRI with 68Ga-PSMA PET/CT is required.
4. Conclusions
68Ga-PSMA PET/MRI shows high diagnostic accuracy equivalent to that of 68Ga-PSMA PET/CT for detection of ECE, SVI, and LNM in primary PCa staging. The risk of bias and the risk of applicability concern were moderate to low. Future research urgently needs direct head-to-head comparison of the two diagnostic modalities to investigate if either one has additional value in primary PCa staging.
Author contributions: Sui Wai Ling had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ling, de Jong, Schoots, van der Veldt, Brabander.
Acquisition of data: Ling, de Jong.
Analysis and interpretation of data: Ling, de Jong, Nasserinejad.
Drafting of the manuscript: Ling, de Jong.
Critical revision of the manuscript for important intellectual content: Schoots, Nasserinejad, Busstra, van der Veldt, Brabander.
Statistical analysis: Nasserinejad.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: van der Veldt, Brabander.
Other: None.
Financial disclosures: Sui Wai Ling certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors wish to thank Maarten Engel (information specialist) from the Erasmus MC Medical Library for developing and updating the search strategies.
Associate Editor: Jochen Walz
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.09.006.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Global Cancer Observatory. Prostate cancer factsheet. https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf.
- 3.Caglic I., Kovac V., Barrett T. Multiparametric MRI — local staging of prostate cancer and beyond. Radiol Oncol. 2019;53:159–170. doi: 10.2478/raon-2019-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turpin A., Girard E., Baillet C. Imaging for metastasis in prostate cancer: a review of the literature. Front Oncol. 2020;10:55. doi: 10.3389/fonc.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 6.Sweat S.D., Pacelli A., Murphy G.P., Bostwick D.G. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–640. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh A., Heston W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 8.Corfield J., Perera M., Bolton D., Lawrentschuk N. 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol. 2018;36:519–527. doi: 10.1007/s00345-018-2182-1. [DOI] [PubMed] [Google Scholar]
- 9.Perera M., Papa N., Christidis D. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 10.von Eyben F.E., Picchio M., von Eyben R., Rhee H., Bauman G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2018;4:686–693. doi: 10.1016/j.euf.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Satapathy S., Singh H., Kumar R., Mittal B.R. Diagnostic accuracy of 68Ga-PSMA PET/CT for initial detection in patients with suspected prostate cancer: a systematic review and meta-analysis. Am J Roentgenol. 2021;216:599–607. doi: 10.2214/AJR.20.23912. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration . FDA; Silver Spring, MD: 2020. FDA approves first PSMA-targeted PET imaging drug for men with prostate cancer.www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer [Google Scholar]
- 13.Lee T.C., Alessio A.M., Miyaoka R.M., Kinahan P.E. Morphology supporting function: attenuation correction for SPECT/CT, PET/CT, and PET/MR imaging. Q J Nucl Med Mol Imaging. 2016;60:25–39. [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii S., Shimao D., Hara T. Comparison of integrated whole-body PET/MR and PET/CT: Is PET/MR alternative to PET/CT in routine clinical oncology? Ann Nucl Med. 2016;30:225–233. doi: 10.1007/s12149-015-1050-y. [DOI] [PubMed] [Google Scholar]
- 15.Quick H.H., von Gall C., Zeilinger M. Integrated whole-body PET/MR hybrid imaging: clinical experience. Invest Radiol. 2013;48:280–289. doi: 10.1097/RLI.0b013e3182845a08. [DOI] [PubMed] [Google Scholar]
- 16.Singnurkar A., Poon R., Metser U. Comparison of 18F-FDG-PET/CT and 18F-FDG-PET/MR imaging in oncology: a systematic review. Ann Nucl Med. 2017;31:366–378. doi: 10.1007/s12149-017-1164-5. [DOI] [PubMed] [Google Scholar]
- 17.Umutlu L., Beyer T., Grueneisen J.S. Whole-body [18F]-FDG-PET/MRI for oncology: a consensus recommendation. Nuklearmedizin. 2019;58:68–76. doi: 10.1055/a-0830-4453. [DOI] [PubMed] [Google Scholar]
- 18.Brix G., Lechel U., Glatting G. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med. 2005;46:608–613. [PubMed] [Google Scholar]
- 19.Whiting P.F., Rutjes A.W., Westwood M.E. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Grubmüller B., Baltzer P., Hartenbach S. PSMA ligand PET/MRI for primary prostate cancer: staging performance and clinical impact. Clin Cancer Res. 2018;24:6300–6307. doi: 10.1158/1078-0432.CCR-18-0768. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann S., Kruck S., Gatidis S. Simultaneous whole-body PET/MRI with integrated multiparametric MRI for primary staging of high-risk prostate cancer. World J Urol. 2020;38:2513–2521. doi: 10.1007/s00345-019-03066-1. [DOI] [PubMed] [Google Scholar]
- 22.Muehlematter U.J., Burger I.A., Becker A.S. Diagnostic accuracy of multiparametric MRI versus 68Ga-PSMA-11 PET/MRI for extracapsular extension and seminal vesicle invasion in patients with prostate cancer. Radiology. 2019;293:350–358. doi: 10.1148/radiol.2019190687. [DOI] [PubMed] [Google Scholar]
- 23.Thalgott M., Duwel C., Rauscher I. One-stop-shop whole-body 68Ga-PSMA-11 PET/MRI compared with clinical nomograms for preoperative T and N staging of high-risk prostate cancer. J Nucl Med. 2018;59:1850–1856. doi: 10.2967/jnumed.117.207696. [DOI] [PubMed] [Google Scholar]
- 24.Fendler W.P., Schmidt D.F., Wenter V. 68Ga-PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med. 2016;57:1720–1725. doi: 10.2967/jnumed.116.172627. [DOI] [PubMed] [Google Scholar]
- 25.Gao J., Zhang C., Zhang Q. Diagnostic performance of 68Ga-PSMA PET/CT for identification of aggressive cribriform morphology in prostate cancer with whole-mount sections. Eur J Nucl Med Mol Imaging. 2019;46:1531–1541. doi: 10.1007/s00259-019-04320-9. [DOI] [PubMed] [Google Scholar]
- 26.Gupta M., Choudhury P.S., Hazarika D., Rawal S. A comparative study of 68gallium-prostate specific membrane antigen positron emission tomography-computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: an initial experience. World J Nucl Med. 2017;16:186–191. doi: 10.4103/1450-1147.207272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp J., Kopp D., Bernhardt E. Ga-68-PSMA PET/CT based primary staging and histological correlation after extended pelvic lymph node dissection at radical prostatectomy. World J Urol. 2020;38:3085–3090. doi: 10.1007/s00345-020-03131-0. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni S.C., Sundaram P.S., Padma S. In primary lymph nodal staging of patients with high-risk and intermediate-risk prostate cancer, how critical is the role of gallium-68 prostate-specific membrane antigen positron emission tomography-computed tomography? Nucl Med Commun. 2020;41:139–146. doi: 10.1097/MNM.0000000000001110. [DOI] [PubMed] [Google Scholar]
- 29.Öbek C., Doganca T., Demirci E. The accuracy of 68Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1806–1812. doi: 10.1007/s00259-017-3752-y. [DOI] [PubMed] [Google Scholar]
- 30.Petersen L.J., Nielsen J.B., Langkilde N.C. 68Ga-PSMA PET/CT compared with MRI/CT and diffusion-weighted MRI for primary lymph node staging prior to definitive radiotherapy in prostate cancer: a prospective diagnostic test accuracy study. World J Urol. 2020;38:939–948. doi: 10.1007/s00345-019-02846-z. [DOI] [PubMed] [Google Scholar]
- 31.van Kalmthout L.W.M., van Melick H.H.E., Lavalaye J. Prospective validation of gallium-68 prostate specific membrane antigen-positron emission tomography/computerized tomography for primary staging of prostate cancer. J Urol. 2020;203:537–545. doi: 10.1097/JU.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 32.van Leeuwen P.J., Donswijk M., Nandurkar R. Gallium-68-prostate-specific membrane antigen (68Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-risk prostate cancer. BJU Int. 2019;124:62–68. doi: 10.1111/bju.14506. [DOI] [PubMed] [Google Scholar]
- 33.van Leeuwen P.J., Emmett L., Ho B. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119:209–215. doi: 10.1111/bju.13540. [DOI] [PubMed] [Google Scholar]
- 34.von Klot C.A.J., Merseburger A.S., Boker A. Ga-68-PSMA PET/CT imaging predicting intraprostatic tumor extent, extracapsular extension and seminal vesicle invasion prior to radical prostatectomy in patients with prostate cancer. Nucl Med Mol Imaging. 2017;51:314–322. doi: 10.1007/s13139-017-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaxley J.W., Raveenthiran S., Nouhaud F.X. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with 68Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology. J Urol. 2019;201:815–820. doi: 10.1097/JU.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q., Zang S., Zhang C. Comparison of 68Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J Transl Med. 2017;15:230. doi: 10.1186/s12967-017-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arslan A, Karaarslan E, Güner AL, Sağlıcan Y, Tuna MB, Kural AR. Comparing the diagnostic performance of multiparametric prostate MRI versus 68Ga-PSMA PET-CT in the evaluation lymph node involvement and extraprostatic extension. Acad Radiol. In press. 10.1016/j.acra.2020.07.011. [DOI] [PubMed]
- 38.Çelen S., Gültekin A., Özlülerden Y. Comparison of 68Ga-PSMA-I/T PET-CT and multiparametric MRI for locoregional staging of prostate cancer patients: a pilot study. Urol Int. 2020;104:684–691. doi: 10.1159/000509974. [DOI] [PubMed] [Google Scholar]
- 39.Chen M., Zhang Q., Zhang C. Comparison of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) and multi-parametric magnetic resonance imaging (MRI) in the evaluation of tumor extension of primary prostate cancer. Transl Androl Urol. 2020;9:382–390. doi: 10.21037/tau.2020.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corona-Montes VE, González-Cuenca E, Fernández-Noyola G, et al. Primary lymph-node staging with 68Ga-PSMA PET in high-risk prostate cancer: pathologic correlation with extended pelvic lymphadenectomy specimens. Urol Oncol. In press. 10.1016/j.urolonc.2020.10.074. [DOI] [PubMed]
- 41.Esen T., Falay O., Tarim K. 68Ga-PSMA-11 positron emission tomography/computed tomography for primary lymph node staging before radical prostatectomy: central review of imaging and comparison with histopathology of extended lymphadenectomy. Eur Urol Focus. 2021;7:288–293. doi: 10.1016/j.euf.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Franklin A., Yaxley W.J., Raveenthiran S. Histological comparison between predictive value of preoperative 3-T multiparametric MRI and 68Ga-PSMA PET/CT scan for pathological outcomes at radical prostatectomy and pelvic lymph node dissection for prostate cancer. BJU Int. 2021;127:71–79. doi: 10.1111/bju.15134. [DOI] [PubMed] [Google Scholar]
- 43.Frumer M., Milk N., Rinott Mizrahi G. A comparison between 68Ga-labeled prostate-specific membrane antigen-PET/CT and multiparametric MRI for excluding regional metastases prior to radical prostatectomy. Abdom Radiol. 2020;45:4194–4201. doi: 10.1007/s00261-020-02640-1. [DOI] [PubMed] [Google Scholar]
- 44.Klingenberg S., Jochumsen M.R., Ulhøi B.P. 68Ga-PSMA PET/CT for primary lymph node and distant metastasis NM staging of high-risk prostate cancer. J Nucl Med. 2021;62:214–220. doi: 10.2967/jnumed.120.245605. [DOI] [PubMed] [Google Scholar]
- 45.Kopp D, Kopp J, Bernhardt E, et al. Ga-68-prostate-specific membrane antigen positron emission tomography-computed tomography-based primary staging and histological correlation after extended pelvic lymph node dissection in intermediate-risk prostate cancer. Urol Int. In press. 10.1159/000515651. [DOI] [PubMed]
- 46.Simsek D.H., Sanli Y., Engin M.N., Erdem S., Sanli O. Detection of metastases in newly diagnosed prostate cancer by using 68Ga-PSMA PET/CT and its relationship with modified D’Amico risk classification. Eur J Nucl Med Mol Imaging. 2021;48:1639–1649. doi: 10.1007/s00259-020-04995-5. [DOI] [PubMed] [Google Scholar]
- 47.Mottet N., Cornford P., van den Bergh R.C.N. European Association of Urology; Arnhem, The Netherlands: 2021. EAU guidelines: prostate cancer.https://uroweb.org/guideline/prostate-cancer/ [Google Scholar]
- 48.Evangelista L., Zattoni F., Cassarino G. PET/MRI in prostate cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:859–873. doi: 10.1007/s00259-020-05025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H., Xu T., Wang X. Diagnostic performance of 68gallium labelled prostate-specific membrane antigen positron emission tomography/computed tomography and magnetic resonance imaging for staging the prostate cancer with intermediate or high risk prior to radical prostatectomy: a systematic review and meta-analysis. World J Mens Health. 2020;38:208–219. doi: 10.5534/wjmh.180124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fendler W.P., Eiber M., Beheshti M. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. doi: 10.1007/s00259-017-3670-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.