Abstract
Since its discovery, spike timing-dependent synaptic plasticity (STDP) has been thought to be a primary mechanism underlying the brain’s ability to learn and to form new memories. However, despite the enormous interest in both the experimental and theoretical neuroscience communities in activity-dependent plasticity, it is still unclear whether plasticity rules inferred from in vitro experiments apply to in vivo conditions. Among the multiple reasons why plasticity rules in vivo might differ significantly from in vitro studies is that extracellular calcium concentration use in most studies is higher than concentrations estimated in vivo. STDP, like many forms of long-term synaptic plasticity, strongly depends on intracellular calcium influx for its induction. Here, we discuss the importance of considering physiological levels of extracellular calcium concentration to study functional plasticity.
Keywords: synaptic plasticity, synapse, STDP, hippocampus, learning, memory, calcium
Introduction
Spike timing-dependent plasticity (STDP) is a form of long-term synaptic modification thought to constitute a mechanism underlying formation of new memories. The polarity of synaptic modifications is controlled by the relative timing between pre- and post-synaptic action potentials (APs; Dan and Poo, 2004; Feldman, 2012). Following the Konorski-Hebb principle (Konorski, 1948; Hebb, 1949), timing-dependent long-term synaptic potentiation (t-LTP) in hippocampal and neocortical pyramidal neurons, results from the temporal conjunction of synaptic activity followed by one or more backpropagating APs in the post-synaptic cell (Gustafsson and Wigström, 1986; Markram et al., 1997; Bi and Poo, 1998; Debanne et al., 1998; Feldman, 2000). In contrast, following Stent principle (Stent, 1973), timing-dependent synaptic depression (t-LTD) is induced when synaptic activity is repeatedly preceded by one of more backpropagating action potentials (Debanne et al., 1994, 1996a, 1998; Markram et al., 1997; Bi and Poo, 1998; Feldman, 2000). It is important to note that t-LTP and t-LTD have been reported in early studies when two synaptic inputs, namely a weak input producing a subthreshold response and a strong input producing an action potential were paired with positive or negative delays (Baranyi and Fehér, 1981; Levy and Steward, 1983; Stanton and Sejnowski, 1989).
STDP and Calcium
At excitatory synapses, the amplitude of post-synaptic calcium influx determines the orientation of plasticity towards synaptic potentiation or depression (Artola et al., 1990). The better demonstration for that is provided by the fact that buffering post-synaptic calcium with BAPTA prevents the induction of both LTP and LTD (Debanne et al., 1994; Nevian and Sakmann, 2006). In a similar way, uncaging of calcium in CA1 pyramidal cells selectively induces LTP or LTD depending on the magnitude of calcium influx (Yang et al., 1999). In Hebbian STDP, the correlation between an EPSP and the backpropagated action potential (bAP) corresponding to a pre-before-post pairing that leads to t-LTP, induces large calcium entry (Koester and Sakmann, 1998). In contrast, post-before-pre pairing that induces t-LTD, comparatively produces a weaker calcium entry (Koester and Sakmann, 1998). Induction of t-LTP involves several mechanisms: (1) removal of the magnesium block from the NMDA receptor (Kampa et al., 2004); (2) inactivation of A-type current and activation of sodium channels to improve signal propagation (Hoffman et al., 1997; Stuart and Häusser, 2001); and (3) AMPA receptor depolarization to boost NMDA receptor calcium signal (Fuenzalida et al., 2010; Holbro et al., 2010). However, the NMDA receptor is the major player as its blockade (by perfusion of MK801 in the post-synaptic neuron for example) prevents LTP induction. Expression of t-LTP requires kinases activation such as CaMKII to phosphorylate AMPA and NMDA receptors thereby increasing their conductance (Otmakhova et al., 2002; Lisman et al., 2012) as well as the insertion of new AMPA receptors (Malinow and Malenka, 2002). In t-LTD, low calcium entry leads to inactivation of NMDA receptors by activation of phosphatases (Rosenmund et al., 1995). These two forms of plasticity which are dependent on post-synaptic NMDA receptors are found in the hippocampus at CA3-CA1 synapses of rodents (Nishiyama et al., 2000; Andrade-Talavera et al., 2016) or in the layer II/III of the cortex of rodents (Froemke et al., 2006). Some forms of LTD are also dependent on the presynaptic NMDA receptor. In the cortex or hippocampus, perfusion of MK801 in the pre-synaptic neurons prevents LTD but not LTP (Sjöström et al., 2003; Rodríguez-Moreno and Paulsen, 2008; Banerjee et al., 2009; Andrade-Talavera et al., 2016). Other forms of NMDA receptor-independent LTD expressed at hippocampal CA3-CA1 and cortical L4-L2/3 synapses, requires metabotropic glutamate receptors (mGluRs), voltage-dependent calcium channels, cannabinoid receptors and astrocytic signaling (Normann et al., 2000; Bender, 2006). In these forms of LTD, production of a retrograde messenger, the endocannabinoids (eCBs), will decrease the probability of pre-synaptic release (Bender, 2006; Chevaleyre et al., 2006). However, this simplistic view is now challenged by several studies that do not necessarily observe a correlation between calcium entry and plasticity. In layer II/III of the cortex, the same rise in post-synaptic calcium can lead to LTP or LTD (Nevian and Sakmann, 2006) and a broadening of the action potential that induce a larger calcium influx may surprisingly facilitate LTD (Zhou et al., 2005).
NMDA Spikes
STDP relies heavily on the bAP which cannot propagate too deeply into the dendritic tree (Spruston, 2008). As a result, distal synapses require a local source of depolarization for t-LTP. Thus, dendritic NMDA receptors are an important source of calcium (Schiller and Schiller, 2001). Several studies have now shown that they are necessary for the induction of t-LTP. In the hippocampus, at mossy fibers-CA3 synapses, t-LTP can only be induced when NMDA spikes are triggered (Brandalise et al., 2016). In the cortex, at layer II/III-V distal synapses, dendritic depolarization can switch plasticity between LTD and LTP (Sjöström and Häusser, 2006). In addition, distal synapses can be cooperative. In CA1 pyramidal cells, synaptic cooperativity is observed at distal but not proximal dendritic locations following repetitive subthreshold activation of small spine clusters (Weber et al., 2016). Recently, in layer 5 pyramidal neurons, it was shown that synaptic cooperativity disrupts t-LTD and extends the temporal window for the induction of t-LTP (Tazerart et al., 2020).
Mathematical Models of STDP Based on Calcium
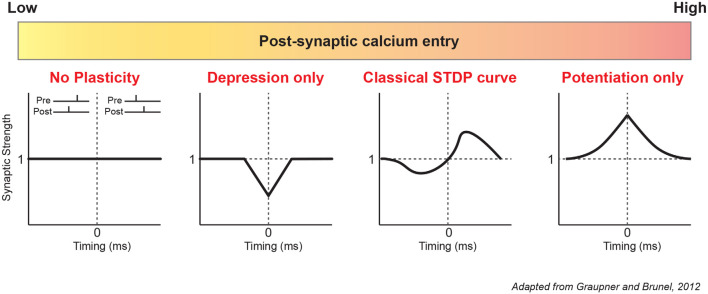
Synaptic plasticity models have been first theorized by John Lisman in 1989. According to this pioneering work, high post-synaptic calcium influx represents a condition favorable to LTP induction because protein kinases are preferentially activated whereas low to moderate calcium influx induces LTD because protein phosphatases are selectively activated. Most recent mathematical models of STDP incorporate the biochemical pathways described above to link post-synaptic calcium and plasticity (Karmarkar and Buonomano, 2002; Shouval et al., 2002; Graupner and Brunel, 2010). One of the first models to investigate the role of extracellular calcium in STDP was the one developed by Graupner and Brunel (2012). By variation of the extracellular calcium, and therefore postsynaptic calcium entry, they showed that a multitude of STDP curves could be obtained in response to a simple pre-post (1:1) or post-pre protocol (1:1; Figure 1). In the most extreme cases, only LTD or LTP could be observed respectively for a very weak or very strong calcium influx. For intermediate influx, the classical curve was found with a window of t-LTD (Δt ≤ 0 ms), a window of t-LTP (Δt ≥ 0 ms) and sometimes a second window of depression for longer delays (Δt = 20–30 ms). The concentration of extracellular calcium is consequently important for the orientation of plasticity but also for its maintenance. Time scales of memory maintenance can be extended by lowering extracellular calcium concentration to in vivo levels (Higgins et al., 2014). Spontaneous synaptic activity is known to erase memory induced by STDP in the tadpole visual system (Zhou et al., 2003). Similar behavior has been reproduced in silico with a calcium-based model (Higgins et al., 2014). In the presence of background synaptic noise, synaptic changes induced by STDP disappear in a few minutes with a high concentration of calcium, whereas they last 1 hour with a physiological concentration.
Figure 1.
Diversity of modeled STDP curves based on calcium. According to the modeling of Graupner and Brunel (2012), post-synaptic calcium is a major determinant of plasticity. From left to right: low calcium entry causes no plasticity or only depression (t-LTD). Intermediate calcium entry produces the classic STDP curve (Hebbian STDP), with a depression window (t-LTD) for negative delays and a potentiation window (t-LTP) for positive delays. High calcium entry results in only potentiation for all delays. Note that these results were obtained with a simple 1:1 protocol (1 presynaptic, 1 postsynaptic stimulation) with a low pairing frequency of 1 Hz and 60 repetitions. STDP, Spike Timing-Dependent Plasticity.
Role of External Calcium Level
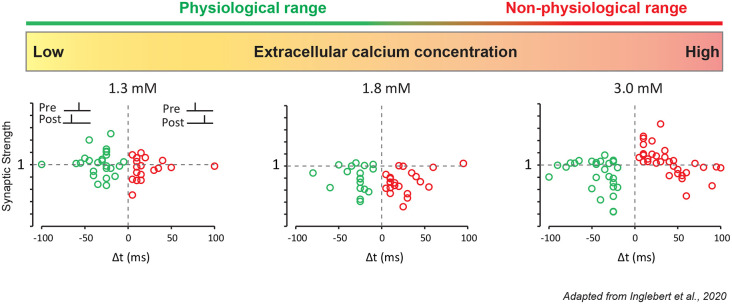
Most, if not all, in vitro studies of STDP have used non-physiological extracellular calcium concentrations ([Ca2+]e), commonly between 2 and 3 mM (Table 1). But physiological [Ca2+]e is about 2 to 3 times lower, i.e., ranging from 1.3–1.8 mM in young rodent (Jones and Keep, 1988; Silver and Erecińska, 1990; Ding et al., 2016). In fact, past studies already showed discrepancies regarding STDP induced in vitro. The same protocol in the hippocampus could lead to a radically different STDP curve (Wittenberg and Wang, 2006; Campanac and Debanne, 2008) and the only obvious difference was the concentration of extracellular calcium used (2 vs. 3 mM). Recently, it has been confirmed experimentally that the overall calcium concentration has a significant effect on plasticity (Inglebert et al., 2020). At CA3-CA1 synapses, while the Ca2+/Mg2+ ratio is kept unchanged, Hebbian STDP is found for [Ca2+]e = 3 mM while no plasticity is observed for [Ca2+]e = 1.3 mM and only LTD for positive and negative timing for [Ca2+]e = 1.8 mM (Figure 2). But adjusting the protocol by increasing the pairing frequency from 0.3 to 5–10 Hz, or the number of postsynaptic APs from 1 to 3–4, allows to restore classical Hebbian STDP (Inglebert et al., 2020). These results obtained in young animals need to be confirmed by further studies in the adult and encourage a reexamination of STDP under physiological conditions.
Table 1.
Selected publications on Spike Timing-Dependent Plasticity.
| Reference | Synapses | Induction protocol | Plasticity observed | [Ca2+]e |
|---|---|---|---|---|
| Debanne et al. (1994) | CA3-CA1 | 50–100 pairings @ 0.3 Hz + Postsynaptic burst | LTD (Δt < 0 ms) | 2.8 mM |
| Bi and Poo (1998) | Hippocampal | 60 pairings @ 1 Hz + Postsynaptic depolarization | LTP (Δt > 0 ms) and LTD (Δt < 0 ms) | 3 mM |
| neurons | ||||
| Markram et al. (1997) | L5-L5 | 10 pairings @ 20 Hz (2:2 or 5:5 or 10:10) | LTP (Δt > 0 ms) | 2 mM |
| Sjöström et al. (2001) | L5-L5 | 15 pairings @ 40 Hz (1:5) | LTP (Δt > 0 ms) | 2.5 mM |
| Froemke et al. (2006) | L2/3 | 60–100 pairings @ 0.2 Hz (1:1) | LTP (Δt > 0 ms) and LTD (Δt < 0 ms | 2.5 mM |
| Froemke et al. (2006) | L2/3 | 30–40 pairings @ 0.2 Hz (5:5) | No Plasticity, LTD or LTP depending on burst frequency | 2.5 mM |
| Wittenberg and Wang (2006) | CA3-CA1 | 70–100 pairings @ 0.1–0.5 Hz (1 :1) | LTD only (Δt > 0 ms and Δt < 0 ms) | 2 mM |
| Wittenberg and Wang (2006) | CA3-CA1 | 100 pairings @ 5 Hz (1:2) | LTD (Δt < 0 ms) and LTP (Δt > 0 ms) | 2 mM |
| Nishiyama et al. (2000) | CA3-CA1 | Train of stimuli at 5 Hz + postsynaptic Spike | LTP (Δt > 0 ms) and LTD (Δt < 0 ms)* | 2–2.6 mM |
| Campanac and Debanne (2008) | CA3-CA1 | 100 pairings @ 0.3 Hz for LTP | LTD (Δt < 0 ms) and LTP (Δt > 0 ms) | 3 mM |
| 150 pairings @ 0.3 Hz for LTD (1:1) | ||||
| Pawlak and Kerr (2008) | Corticostriatal | 60 pairings @ 0.1 Hz (1:1) | LTD (Δt < 0 ms) and LTP (Δt > 0 ms) | 2.5 mM |
| pathway | ||||
| Mishra et al. (2016) | CA3-CA3 | 300 pairings @ 1 Hz (1:1) | LTP only (Δt > 0 ms and Δt < 0 ms) | 2 mM |
| Inglebert et al. (2020) | CA3-CA1 | 100 pairings @ 0.3 Hz for LTP | No Plasticity or LTD only | 1.3–1.8 mM |
| 150 pairing @ 0.3 Hz for LTD (1:1) |
This table summarizes the plasticity observed in several studies as a function of extracellular calcium concentration. The brain region studied and the induction protocol used are also indicated. The number of repetitions, the pairing frequency and number of pre/post stimulation are specified. For example, 5:5 indicates five presynaptic and five postsynaptic stimulations. *A second LTD window is visible around Δt = 20 ms. STDP, Spike Timing-Dependent Plasticity.
Figure 2.
STDP under various external calcium concentrations. CA1 pyramidal neurons were recorded in a whole-cell configuration. Pre-synaptic stimulation was evoked by a stimulation electrode placed in the Schaffer collaterals. These results were obtained with a simple 1:1 protocol (1 presynaptic, 1 postsynaptic stimulation) with a low pairing frequency of 0.3 Hz and 100 repetitions for t-LTP, and 150 repetitions for t-LTD. In 1.3 mM extracellular calcium, no plasticity is induced. In 1.8 mM extracellular calcium, pre-post and post-pre protocols leads to t-LTD. In 3 mM extracellular calcium, pre-post protocol leads to t-LTP and post-pre protocol leads to t-LTD.
Reevaluation of STDP Rules
Multiple STDP Rules
The findings of Inglebert et al. invite a reexamination of plasticity at many synapses. For example, at CA3-CA3 synapses where only LTP is observed (Mishra et al., 2016), it is likely that this would not be the same in physiological calcium. Hebbian STDP (t-LTP for positive timings and t-LTD for negative timings) is found predominantly at excitatory synapses. Despite being ubiquitous, STDP curve can take many different shapes. Anti-Hebbian STDP (t-LTP for negative timings or t-LTD for positive timings) is found largely at inhibitory synapses. In particular, the striatum, which is composed principally of inhibitory neurons, has many different STDP curves depending on the neuron considered (Fino and Venance, 2010). For example, interneurons that expressed Nitric Oxide synthase, present a window of LTP for a positive timing around Δt = +50 ms. In the dorsal cochlear nucleus (DCN), pre-before-post protocol induced LTD in cartwheel cells (Tzounopoulos et al., 2004). Would all these results persist in physiological calcium or plasticity has been overestimated?
Dendritic Calcium Spikes in Physiological Calcium
Among the factors that could be potentially affected by physiological calcium, the occurrence of dendritic calcium spikes or NMDA spikes that have been shown to be critical in LTP induction (Kampa et al., 2006; Brandalise et al., 2016) could be greatly reduced. A possibility is that in physiological calcium, a larger number of synaptic inputs would be required to trigger an NMDA spike. Alternatively, the spatial extent of the NMDA spike could be reduced in physiological calcium rending induction of plasticity more difficult. Further studies will be required to test these possibilities.
Calcium Micro-Domains in Physiological Calcium
Calcium micro-domains are supposed to play a critical role in STDP (Mihalas, 2011). A major consequence of reducing external calcium concentration to physiological values is a great reduction of the size of calcium micro-domains. As a consequence, the calcium-sensitive effector might be disconnected from the source of calcium, thus accounting for the observed reduction in plasticity (Inglebert et al., 2020). Additional studies will be, however, required to test further these hypotheses.
Presynaptic Aspects of STDP in Physiological Calcium
Neocortical STDP relies heavily on presynaptic glutamate release and presynaptic firing rate (Markram et al., 1997; Sjöström et al., 2001, 2003). However, it is now clear that spontaneously released vesicles and evoked transmission use distinct mechanisms (Kavalali, 2015; Abrahamsson et al., 2017). Therefore, physiological calcium could have distinct consequences on evoked and spontaneous release. At many synapses, evoked glutamate release is controlled by pre-synaptic Ca2+ entry through voltage-dependent calcium channels (VDCC) and [Ca2+]e (Südhof, 2012). Reduced [Ca2+]e is associated with decreased synaptic transmission (Borst and Sakmann, 1996; Debanne et al., 1996b; Hardingham et al., 2006). On the contrary, spontaneous release is poorly sensitive to fluctuations in [Ca2+]e and is not triggered by Ca2+ entry via VDCC (Scanziani et al., 1992; Vyleta and Smith, 2011). Interestingly, neocortical presynaptic NMDA receptors regulate both spontaneous and evoked release by distinct molecular pathways (Abrahamsson et al., 2017; Bouvier et al., 2018), are required for neocortical t-LTD (Sjöström et al., 2003) and for hippocampal t-LTD (Andrade-Talavera et al., 2016). The work of Inglebert et al. (2020) focused on the post-synaptic calcium hypothesis but further studies are needed to explore pre-synaptic long-term plasticity.
Towards Standardization of Induction Protocols
All studies use different protocols (Table 1). The number of repetitions, the pairing frequency, the somato-dendritic distance of the inputs or the number of postsynaptic potentials are different among studies. Therefore, it is often difficult to compare the different results obtained by each study. In physiological calcium (i.e 1.3 mM), it appears that t-LTP and t-LTD requires a greater frequency of pairing (>5 Hz) or a greater number of postsynaptic APs (>3) even with a large number of repetitions (100 or 150; Inglebert et al., 2020). A better understanding of the rules of STDP induction in vitro under physiological conditions will allow a more robust application in vivo.
Implication for In vivo Exploration of STDP
In opposition to in vitro studies, and by definition, in vivo studies are inherently in a physiological calcium concentration. First demonstration of STDP in vivo was performed in the retinotectal pathway of Xenopus (Zhang et al., 1998). t-LTP and t-LTD were observed but were not robust and easily abolished by hyperpolarizations or spontaneous activities, a limitation recently highlighted by a biophysical model of STDP (Higgins et al., 2014). The use of a non-physiological concentration of calcium may lead to an underestimation of the time scales of memory maintenance as background activities is an important factor for limiting plasticity. Furthermore, most in vitro studies are performed in juvenile rodent while in vivo studies are performed in older animals. This may constitute an additional limitation to the transposition of the results observed in vitro. Several studies suggest that the capacity to induce t-LTD decreases with age (Banerjee et al., 2009; Verhoog et al., 2013) although a recent study has shown t-LTD at cortical layer V synapses in adult mice following pre-before-post protocol (Louth et al., 2021). Similarly, induction of t-LTD by a STDP protocol in the somato-sensory cortex of adult rats by pairing postsynaptic spikes and subthreshold whisker deflection is relatively frequent whereas induction of t-LTP in the same preparation is rare (Jacob et al., 2007). t-LTD disappeared rapidly after a few minutes (5–10 min) and t-LTP was sporadic following pre-before-post pairings (Jacob et al., 2007). As already suggested, STDP in older animals may require protocols that produce stronger depolarization (Meredith et al., 2003). In concordance with this idea, electrical stimulation of afferent input at high frequency paired with post-synaptic burst produced robust t-LTP in cat visual cortex (Frégnac et al., 2010). It is important to note that most of the results observed in vivo are obtained in the anesthetized animal. Although there are variations in extracellular calcium concentration of about 0.2 mM between awake and anesthetized animals (Ding et al., 2016), they are not sufficient to produce a major effect on the induction and maintenance of plasticity. An attractive explanation could be that in anesthetized animals, neuromodulation is largely depressed.
Importance of Neuromodulation
As demonstrated by Inglebert et al. fine tuning of pre- and postsynaptic activity can restore t-LTD and t-LTP in physiological extracellular calcium condition. But would it be possible to restore classic plasticity rules under regular patterns of activity? The key component could be neuromodulation. Interestingly, replay of activity from place-cells with overlapping firing field in hippocampal slices induced t-LTP only in the presence of Carbachol, a cholinergic agonist (Isaac et al., 2009). Many studies have now shown the effects of various neuromodulators on STDP. One of the most explored is dopamine (DA). It is involved in learning and reward processes (Schultz, 1997; Suri and Schultz, 1999). The activation of the D1 receptor (D1-R) has been shown to increase temporal window for t-LTP and to allow induction of t-LTP with fewer spike pairs at glutamatergic synapses of hippocampal neurons (Zhang et al., 2009). At CA3-CA1 synapses, D1-R activation switches t-LTD into t-LTP (Brzosko et al., 2015). In the prefrontal cortex, DA application allow t-LTP induction (He et al., 2015). The effects of the D1-R are widespread (Neve et al., 2004), but many of them could explain the reasons for promoting LTP. They could facilitate signal propagation by inhibiting A-type current (Hamilton et al., 2010; Edelmann and Lessmann, 2011; Yang and Dani, 2014) or simply increased intracellular calcium (Lezcano and Bergson, 2002). D2 receptors (D2-R) are also involved in t-LTP and t-LTD. In lateral amygdala, D2-R gates LTP induction by suppressing feedforward inhibition (Bissière et al., 2003). In some cases, a synergy is observed between the two receptors. In layer V of the prefrontal cortex, D1-R and D2-R co-activation enables the induction of t-LTP at extended timing interval (Xu and Yao, 2010). Noradrenaline (NA) is also involved in memory formation. Activation of β-adrenergic receptors (β-R) increases intracellular calcium (Seol et al., 2007) and facilitate bAP by inhibition of A-type current (Yuan et al., 2002) or SK channels (Faber et al., 2008). At CA3-CA1 synapses, β-R activation increases the temporal window induction for t-LTP (Lin et al., 2003). In layer II/III of visual cortex, co-activation of β-R and α-adrenergic receptor (α-R) are required for bidirectional STDP in fast-spiking and somatostatin interneurons. β-R activation promoted t-LTP whereas α-R activation induced t-LTD (Huang et al., 2013, 2014). Neuromodulation could be seen as the necessary factor for the induction of t-LTP and t-LTD in physiological calcium without tuning pre- and postsynaptic activity.
Conclusion
Use of Physiological Calcium Levels for Studying Short-Term Synaptic Plasticity
The use of physiological external calcium concentration not only modulates the learning rules for long-term synaptic plasticity but it also enhances context-dependent synaptic plasticity. Analog-digital modulation of action potential-evoked synaptic transmission lies on modification of spike shape, by either broadening the axonal spike (Shu et al., 2006; Kole et al., 2007) or by modulating its amplitude (Rama et al., 2015; Zbili et al., 2020). Because transmitter release is almost maximal in high calcium, conditions that enhance release are somehow difficult to reach. Indeed, switching to physiological calcium concentration (i.e., 1.3 mM) was found to significantly enhance spike amplitude-dependent synaptic plasticity (Rama et al., 2015; Zbili et al., 2020).
Use of Physiological Calcium Levels for Studying Intrinsic Plasticity
Hebbian plasticity and Intrinsic plasticity are closely linked and are synergistically modified (Debanne et al., 2019). Generally, t-LTP is associated with an increase in excitability and t-LTD with a reduced excitability (Ganguly et al., 2000; Li et al., 2004). Interestingly, recordings using physiological calcium show a significant increase excitability of CA1 pyramidal neurons: lowered firing threshold, increased spontaneous firing and more depolarized resting membrane potential (Bjorefeldt et al., 2018). Dendritic integration or EPSP-Spike coupling is also modified with synaptic changes (Campanac and Debanne, 2008). Generally, LTP is accompanied by an increase in the probability of emitting an AP for the same synaptic input (EPSP-spike potentiation) whereas LTD is accompanied by a decrease in the probability of emitting an AP (EPSP-spike depression). This plasticity of the output, without modification of the input, result partially from postsynaptic changes in voltage dependent channels such as IH or IA (Daoudal and Debanne, 2003). EPSP-Spike coupling modification is also conditioned by changes in inhibitory synaptic transmission. Intriguingly, recording in physiological calcium in hippocampus have revealed that excitatory-inhibitory balance was disrupted and disynaptic inhibition was strongly decreased (Aivar et al., 2014).
Author Contributions
YI and DD wrote the article and YI built the figures. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. M. Russier for critically reading a preliminary version of the manuscript.
Funding
This work was supported by Agence Nationale de la Recherche (ANR-14-NEUC-0004 and ANR-14-CE13-003 to DD) and Fondation pour la Recherche Médicale (FRM, FDT2017-0437059 to YI).
References
- Abrahamsson T., Chou C. Y. C., Li S. Y., Mancino A., Costa R. P., Brock J. A., et al. (2017). Differential regulation of evoked and spontaneous release by presynaptic NMDA receptors. Neuron 96, 839–855.e5. 10.1016/j.neuron.2017.09.030 [DOI] [PubMed] [Google Scholar]
- Aivar P., Valero M., Bellistri E., Prida L. M. D. L. (2014). Extracellular calcium controls the expression of two different forms of ripple-like hippocampal oscillations. J. Neurosci. 34, 2989–3004. 10.1523/JNEUROSCI.2826-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Talavera Y., Duque-Feria P., Paulsen O., Rodríguez-Moreno A. (2016). Presynaptic spike timing-dependent long-term depression in the mouse hippocampus. Cereb. Cortex 26, 3637–3654. 10.1093/cercor/bhw172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A., Bröcher S., Singer W. (1990). Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 347, 69–72. 10.1038/347069a0 [DOI] [PubMed] [Google Scholar]
- Banerjee A., Meredith R. M., Rodríguez-Moreno A., Mierau S. B., Auberson Y. P., Paulsen O. (2009). Double dissociation of spike timing-dependent potentiation and depression by subunit-preferring NMDA receptor antagonists in mouse barrel cortex. Cereb. Cortex 19, 2959–2969. 10.1093/cercor/bhp067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi A., Fehér O. (1981). Synaptic facilitation requires paired activation of convergent pathways in the neocortex. Nature 290, 413–415. 10.1038/290413a0 [DOI] [PubMed] [Google Scholar]
- Bender V. A. (2006). Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 26, 4166–4177. 10.1523/JNEUROSCI.0176-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G., Poo M. (1998). Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength and postsynaptic cell type. J. Neurosci. 18, 10464–10472. 10.1523/JNEUROSCI.18-24-10464.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S., Humeau Y., Lüthi A. (2003). Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat. Neurosci. 6, 587–592. 10.1038/nn1058 [DOI] [PubMed] [Google Scholar]
- Bjorefeldt A., Illes S., Zetterberg H., Hanse E. (2018). Neuromodulation via the cerebrospinal fluid: insights from recent in vitro studies. Front. Neural Circuits 12:5. 10.3389/fncir.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J. G. G., Sakmann B. (1996). Calcium influx and transmitter release in a fast CNS synapse. Nature 383, 431–434. 10.1038/383431a0 [DOI] [PubMed] [Google Scholar]
- Bouvier G., Larsen R. S., Rodríguez-Moreno A., Paulsen O., Sjöström P. J. (2018). Towards resolving the presynaptic NMDA receptor debate. Curr. Opin. Neurobiol. 51, 1–7. 10.1016/j.conb.2017.12.020 [DOI] [PubMed] [Google Scholar]
- Brandalise F., Carta S., Helmchen F., Lisman J., Gerber U. (2016). Dendritic NMDA spikes are necessary for timing-dependent associative LTP in CA3 pyramidal cells. Nat. Commun. 7:13480. 10.1038/ncomms13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzosko Z., Schultz W., Paulsen O. (2015). Retroactive modulation of spike timing-dependent plasticity by dopamine. eLife 4:e09685. 10.7554/eLife.09685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanac E., Debanne D. (2008). Spike timing-dependent plasticity: a learning rule for dendritic integration in rat CA1 pyramidal neurons. J. Physiol. 586, 779–793. 10.1113/jphysiol.2007.147017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V., Takahashi K. A., Castillo P. E. (2006). Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 29, 37–76. 10.1146/annurev.neuro.29.051605.112834 [DOI] [PubMed] [Google Scholar]
- Dan Y., Poo M.-M. (2004). Spike timing-dependent plasticity of neural circuits. Neuron 44, 23–30. 10.1016/j.neuron.2004.09.007 [DOI] [PubMed] [Google Scholar]
- Daoudal G., Debanne D. (2003). Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn. Mem. 10, 456–465. 10.1101/lm.64103 [DOI] [PubMed] [Google Scholar]
- Debanne D., Gähwiler B. H., Thompson S. M. (1994). Asynchronous pre-and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proc. Natl. Acad. Sci. U S A 91, 1148–1152. 10.1073/pnas.91.3.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D., Gähwiler B. H., Thompson S. M. (1996a). Cooperative interactions in the induction of long-term potentiation and depression of synaptic excitation between hippocampal CA3-CA1 cell pairs in vitro. Proc. Natl. Acad. Sci. U S A 93, 11225–11230. 10.1073/pnas.93.20.11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D., Guérineau N. C., Gähwiler B. H., Thompson S. M. (1996b). Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J. Physiol. 491, 163–176. 10.1113/jphysiol.1996.sp021204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D., Gähwiler B. H., Thompson S. M. (1998). Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J. Physiol. 507, 237–247. 10.1111/j.1469-7793.1998.237bu.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D., Inglebert Y., Russier M. (2019). Plasticity of intrinsic neuronal excitability. Curr. Opin. Neurobiol. 54, 73–82. 10.1016/j.conb.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Ding F., O’Donnell J., Xu Q., Kang N., Goldman N., Nedergaard M. (2016). Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352, 550–555. 10.1126/science.aad4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann E., Lessmann V. (2011). Dopamine modulates spike timing-dependent plasticity and action potential properties in CA1 pyramidal neurons of acute rat hippocampal slices. Front. Synaptic Neurosci. 3:6. 10.3389/fnsyn.2011.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber E. S. L., Delaney A. J., Power J. M., Sedlak P. L., Crane J. W., Sah P. (2008). Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J. Neurosci. 28, 10803–10813. 10.1523/JNEUROSCI.1796-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. E. (2000). Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron 27, 45–56. 10.1016/s0896-6273(00)00008-8 [DOI] [PubMed] [Google Scholar]
- Feldman D. E. (2012). The spike-timing dependence of plasticity. Neuron 75, 556–571. 10.1016/j.neuron.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E., Venance L. (2010). Spike-timing dependent plasticity in the striatum. Front. Synaptic Neurosci. 2:6. 10.3389/fnsyn.2010.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y., Pananceau M., René A., Huguet N., Marre O., Levy M., et al. (2010). A re-examination of hebbian-covariance rules and spike timing-dependent plasticity in cat visual cortex in vivo. Front. Synaptic Neurosci. 2:147. 10.3389/fnsyn.2010.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke R. C., Tsay I. A., Raad M., Long J. D., Dan Y. (2006). Contribution of individual spikes in burst-induced long-term synaptic modification. J. Neurophysiol. 95, 1620–1629. 10.1152/jn.00910.2005 [DOI] [PubMed] [Google Scholar]
- Fuenzalida M., Fernández de Sevilla D., Couve A., Buño W. (2010). Role of AMPA and NMDA receptors and back-propagating action potentials in spike timing-dependent plasticity. J. Neurophysiol. 103, 47–54. 10.1152/jn.00416.2009 [DOI] [PubMed] [Google Scholar]
- Ganguly K., Kiss L., Poo M. (2000). Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat. Neurosci. 3, 1018–1026. 10.1038/79838 [DOI] [PubMed] [Google Scholar]
- Graupner M., Brunel N. (2010). Mechanisms of induction and maintenance of spike-timing dependent plasticity in biophysical synapse models. Front. Comput. Neurosci. 4:136. 10.3389/fncom.2010.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner M., Brunel N. (2012). Calcium-based plasticity model explains sensitivity of synaptic changes to spike pattern, rate and dendritic location. Proc. Natl. Acad. Sci. U S A 109, 3991–3996. 10.1073/pnas.1109359109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. (1986). Hippocampal long-lasting potentiation produced by pairing single volleys and brief conditioning tetani evoked in separate afferents. J. Neurosci. 6, 1575–1582. 10.1523/JNEUROSCI.06-06-01575.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. J., Wheatley B. M., Sinclair D. B., Bachmann M., Larkum M. E., Colmers W. F. (2010). Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc. Natl. Acad. Sci. U S A 107, 18185–18190. 10.1073/pnas.1011558107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N. R., Bannister N. J., Read J. C. A., Fox K. D., Hardingham G. E., Jack J. J. B. (2006). Extracellular calcium regulates postsynaptic efficacy through group 1 metabotropic glutamate receptors. J. Neurosci. 26, 6337–6345. 10.1523/JNEUROSCI.5128-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Huertas M., Hong S. Z., Tie X., Hell J. W., Shouval H., et al. (2015). Distinct eligibility traces for LTP and LTD in cortical synapses. Neuron 88, 528–538. 10.1016/j.neuron.2015.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. O. (1949). The Organization of Behavior; A Neuropsychological Theory . Oxford, England: Wiley. [Google Scholar]
- Higgins D., Graupner M., Brunel N. (2014). Memory maintenance in synapses with calcium-based plasticity in the presence of background activity. PLoS Comput. Biol. 10:e1003834. 10.1371/journal.pcbi.1003834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D. A., Magee J. C., Colbert C. M., Johnston D. (1997). K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875. 10.1038/43119 [DOI] [PubMed] [Google Scholar]
- Holbro N., Grunditz A., Wiegert J. S., Oertner T. G. (2010). AMPA receptors gate spine Ca(2+) transients and spike-timing-dependent potentiation. Proc. Natl. Acad. Sci. U S A 107, 15975–15980. 10.1073/pnas.1004562107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Huganir R. L., Kirkwood A. (2013). Adrenergic gating of Hebbian spike-timing-dependent plasticity in cortical interneurons. J. Neurosci. 33, 13171–13178. 10.1523/JNEUROSCI.5741-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Rozas C., Treviño M., Contreras J., Yang S., Song L., et al. (2014). Associative hebbian synaptic plasticity in primate visual cortex. J. Neurosci. 34, 7575–7579. 10.1523/JNEUROSCI.0983-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglebert Y., Aljadeff J., Brunel N., Debanne D. (2020). Synaptic plasticity rules with physiological calcium levels. Proc. Natl. Acad. Sci. U S A 117, 33639–33648. 10.1073/pnas.2013663117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. T. R., Buchanan K. A., Muller R. U., Mellor J. R. (2009). Hippocampal place cell firing patterns can induce long-term synaptic plasticity in vitro. J. Neurosci. 29, 6840–6850. 10.1523/JNEUROSCI.0731-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob V., Brasier D. J., Erchova I., Feldman D., Shulz D. E. (2007). Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat. J. Neurosci. 27, 1271–1284. 10.1523/JNEUROSCI.4264-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Keep R. F. (1988). Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J. Physiol. 402, 579–593. 10.1113/jphysiol.1988.sp017223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa B. M., Clements J., Jonas P., Stuart G. J. (2004). Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J. Physiol. 556, 337–345. 10.1113/jphysiol.2003.058842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa B. M., Letzkus J. J., Stuart G. J. (2006). Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J. Physiol. 574, 283–290. 10.1113/jphysiol.2006.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar U. R., Buonomano D. V. (2002). Model of spike-timing dependent plasticity: one or two coincidence detectors. J. Neurophysiol. 88, 507–513. 10.1152/jn.2002.88.1.507 [DOI] [PubMed] [Google Scholar]
- Kavalali E. T. (2015). The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 16, 5–16. 10.1038/nrn3875 [DOI] [PubMed] [Google Scholar]
- Koester H. J., Sakmann B. (1998). Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc. Natl. Acad. Sci. U S A 95, 9596–9601. 10.1073/pnas.95.16.9596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole M. H. P., Letzkus J. J., Stuart G. J. (2007). Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55, 633–647. 10.1016/j.neuron.2007.07.031 [DOI] [PubMed] [Google Scholar]
- Konorski J. (1948). Conditioned Reflexes and Neuron Organization. New York, NY: Cambridge University Press. [Google Scholar]
- Levy W. B., Steward O. (1983). Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience 8, 791–797. 10.1016/0306-4522(83)90010-6 [DOI] [PubMed] [Google Scholar]
- Lezcano N., Bergson C. (2002). D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J. Neurophysiol. 87, 2167–2175. 10.1152/jn.00541.2001 [DOI] [PubMed] [Google Scholar]
- Li C., Lu J., Wu C., Duan S., Poo M. (2004). Bidirectional modification of presynaptic neuronal excitability accompanying spike timing-dependent synaptic plasticity. Neuron 41, 257–268. 10.1016/s0896-6273(03)00847-x [DOI] [PubMed] [Google Scholar]
- Lin Y.-W., Min M.-Y., Chiu T.-H., Yang H.-W. (2003). Enhancement of associative long-term potentiation by activation of β-adrenergic receptors at CA1 synapses in rat hippocampal slices. J. Neurosci. 23, 4173–4181. 10.1523/JNEUROSCI.23-10-04173.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., Yasuda R., Raghavachari S. (2012). Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182. 10.1038/nrn3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louth E. L., Jørgensen R. L., Korshoej A. R., Sørensen J. C. H., Capogna M. (2021). Dopaminergic neuromodulation of spike timing dependent plasticity in mature adult rodent and human cortical neurons. Front. Cell. Neurosci. 15:135. 10.3389/fncel.2021.668980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Malenka R. C. (2002). AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126. 10.1146/annurev.neuro.25.112701.142758 [DOI] [PubMed] [Google Scholar]
- Markram H., Lübke J., Frotscher M., Sakmann B. (1997). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215. 10.1126/science.275.5297.213 [DOI] [PubMed] [Google Scholar]
- Meredith R. M., Floyer-Lea A. M., Paulsen O. (2003). Maturation of long-term potentiation induction rules in rodent hippocampus: role of GABAergic inhibition. J. Neurosci. 23, 11142–11146. 10.1523/JNEUROSCI.23-35-11142.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalas S. (2011). Calcium messenger heterogeneity: a possible signal for spike timing-dependent plasticity. Front. Comput. Neurosci. 4:158. 10.3389/fncom.2010.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R. K., Kim S., Guzman S. J., Jonas P. (2016). Symmetric spike timing-dependent plasticity at CA3-CA3 synapses optimizes storage and recall in autoassociative networks. Nat. Commun. 7:11552. 10.1038/ncomms11552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve K. A., Seamans J. K., Trantham-Davidson H. (2004). Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 24, 165–205. 10.1081/rrs-200029981 [DOI] [PubMed] [Google Scholar]
- Nevian T., Sakmann B. (2006). Spine Ca2+ signaling in spike-timing-dependent plasticity. J. Neurosci. 26, 11001–11013. 10.1523/JNEUROSCI.1749-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M., Hong K., Mikoshiba K., Poo M. M., Kato K. (2000). Calcium stores regulate the polarity and input specificity of synaptic modification. Nature 408, 584–588. 10.1038/35046067 [DOI] [PubMed] [Google Scholar]
- Normann C., Peckys D., Schulze C. H., Walden J., Jonas P., Bischofberger J. (2000). Associative long-term depression in the hippocampus is dependent on postsynaptic N-type Ca2+ Channels. J. Neurosci. 20, 8290–8297. 10.1523/JNEUROSCI.20-22-08290.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova N. A., Otmakhov N., Lisman J. E. (2002). Pathway-specific properties of AMPA and NMDA-mediated transmission in CA1 hippocampal pyramidal cells. J. Neurosci. 22, 1199–1207. 10.1523/JNEUROSCI.22-04-01199.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V., Kerr J. N. D. (2008). Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 28, 2435–2446. 10.1523/JNEUROSCI.4402-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama S., Zbili M., Bialowas A., Fronzaroli-Molinieres L., Ankri N., Carlier E., et al. (2015). Presynaptic hyperpolarization induces a fast analogue modulation of spike-evoked transmission mediated by axonal sodium channels. Nat. Commun. 6:10163. 10.1038/ncomms10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Moreno A., Paulsen O. (2008). Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat. Neurosci. 11, 744–745. 10.1038/nn.2125 [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Feltz A., Westbrook G. L. (1995). Calcium-dependent inactivation of synaptic NMDA receptors in hippocampal neurons. J. Neurophysiol. 73, 427–430. 10.1152/jn.1995.73.1.427 [DOI] [PubMed] [Google Scholar]
- Scanziani M., Capogna M., Gähwiler B. H., Thompson S. M. (1992). Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron 9, 919–927. 10.1016/0896-6273(92)90244-8 [DOI] [PubMed] [Google Scholar]
- Schiller J., Schiller Y. (2001). NMDA receptor-mediated dendritic spikes and coincident signal amplification. Curr. Opin. Neurobiol. 11, 343–348. 10.1016/s0959-4388(00)00217-8 [DOI] [PubMed] [Google Scholar]
- Schultz W. (1997). Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol. 7, 191–197. 10.1016/s0959-4388(97)80007-4 [DOI] [PubMed] [Google Scholar]
- Seol G. H., Ziburkus J., Huang S., Song L., Kim I. T., Takamiya K., et al. (2007). Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron 55, 919–929. 10.1016/j.neuron.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval H. Z., Bear M. F., Cooper L. N. (2002). A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc. Natl. Acad. Sci. U S A 99, 10831–10836. 10.1073/pnas.152343099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., Hasenstaub A., Duque A., Yu Y., McCormick D. A. (2006). Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature 441, 761–765. 10.1038/nature04720 [DOI] [PubMed] [Google Scholar]
- Silver I. A., Erecińska M. (1990). Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J. Gen. Physiol. 95, 837–866. 10.1085/jgp.95.5.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström P. J., Häusser M. (2006). A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron 51, 227–238. 10.1016/j.neuron.2006.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström P. J., Turrigiano G. G., Nelson S. B. (2001). Rate, timing and cooperativity jointly determine cortical synaptic plasticity. Neuron 32, 1149–1164. 10.1016/s0896-6273(01)00542-6 [DOI] [PubMed] [Google Scholar]
- Sjöström P. J., Turrigiano G. G., Nelson S. B. (2003). Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39, 641–654. 10.1016/s0896-6273(03)00476-8 [DOI] [PubMed] [Google Scholar]
- Spruston N. (2008). Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 9, 206–221. 10.1038/nrn2286 [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sejnowski T. J. (1989). Associative long-term depression in the hippocampus induced by hebbian covariance. Nature 339, 215–218. 10.1038/339215a0 [DOI] [PubMed] [Google Scholar]
- Stent G. S. (1973). A physiological mechanism for hebb’s postulate of learning. Proc. Natl. Acad. Sci. U S A 70, 997–1001. 10.1073/pnas.70.4.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. J., Häusser M. (2001). Dendritic coincidence detection of EPSPs and action potentials. Nat. Neurosci. 4, 63–71. 10.1038/82910 [DOI] [PubMed] [Google Scholar]
- Südhof T. C. (2012). The presynaptic active zone. Neuron 75, 11–25. 10.1016/j.neuron.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri R. E., Schultz W. (1999). A neural network model with dopamine-like reinforcement signal that learns a spatial delayed response task. Neuroscience 91, 871–890. 10.1016/s0306-4522(98)00697-6 [DOI] [PubMed] [Google Scholar]
- Tazerart S., Mitchell D. E., Miranda-Rottmann S., Araya R. (2020). A spike-timing-dependent plasticity rule for dendritic spines. Nat. Commun. 11:4276. 10.1038/s41467-020-17861-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T., Kim Y., Oertel D., Trussell L. O. (2004). Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat. Neurosci. 7, 719–725. 10.1038/nn1272 [DOI] [PubMed] [Google Scholar]
- Verhoog M. B., Goriounova N. A., Obermayer J., Stroeder J., Hjorth J. J. J., Testa-Silva G., et al. (2013). Mechanisms underlying the rules for associative plasticity at adult human neocortical synapses. J. Neurosci. 33, 17197–17208. 10.1523/JNEUROSCI.3158-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyleta N. P., Smith S. M. (2011). Spontaneous glutamate release is independent of calcium influx and tonically activated by the calcium-sensing receptor. J. Neurosci. 31, 4593–4606. 10.1523/JNEUROSCI.6398-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. P., Andrásfalvy B. K., Polito M., Magó Á., Ujfalussy B. B., Makara J. K. (2016). Location-dependent synaptic plasticity rules by dendritic spine cooperativity. Nat. Commun. 7:11380. 10.1038/ncomms11380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg G. M., Wang S. S.-H. (2006). Malleability of spike-timing-dependent plasticity at the CA3-CA1 synapse. J. Neurosci. 26, 6610–6617. 10.1523/JNEUROSCI.5388-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T.-X., Yao W.-D. (2010). D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc. Natl. Acad. Sci. U S A 107, 16366–16371. 10.1073/pnas.1004108107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Dani J. A. (2014). Dopamine D1 and D5 receptors modulate spike timing-dependent plasticity at medial perforant path to dentate granule cell synapses. J. Neurosci. 34, 15888–15897. 10.1523/JNEUROSCI.2400-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-N., Tang Y.-G., Zucker R. S. (1999). Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J. Neurophysiol. 81, 781–787. 10.1152/jn.1999.81.2.781 [DOI] [PubMed] [Google Scholar]
- Yuan L.-L., Adams J. P., Swank M., Sweatt J. D., Johnston D. (2002). Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J. Neurosci. 22, 4860–4868. 10.1523/JNEUROSCI.22-12-04860.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbili M., Rama S., Yger P., Inglebert Y., Boumedine-Guignon N., Fronzaroli-Moliniere L., et al. (2020). Axonal Na+ channels detect and transmit levels of input synchrony in local brain circuits. Sci. Adv. 6:eaay4313. 10.1126/sciadv.aay4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-C., Lau P.-M., Bi G.-Q. (2009). Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc. Natl. Acad. Sci. U S A 106, 13028–13033. 10.1073/pnas.0900546106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. I., Tao H. W., Holt C. E., Harris W. A., Poo M. (1998). A critical window for cooperation and competition among developing retinotectal synapses. Nature 395, 37–44. 10.1038/25665 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Tao H. W., Poo M. (2003). Reversal and stabilization of synaptic modifications in a developing visual system. Science 300, 1953–1957. 10.1126/science.1082212 [DOI] [PubMed] [Google Scholar]
- Zhou Y.-D., Acker C. D., Netoff T. I., Sen K., White J. A. (2005). Increasing Ca2+ transients by broadening postsynaptic action potentials enhances timing-dependent synaptic depression. Proc. Natl. Acad. Sci. U S A 102, 19121–19125. 10.1073/pnas.0509856103 [DOI] [PMC free article] [PubMed] [Google Scholar]