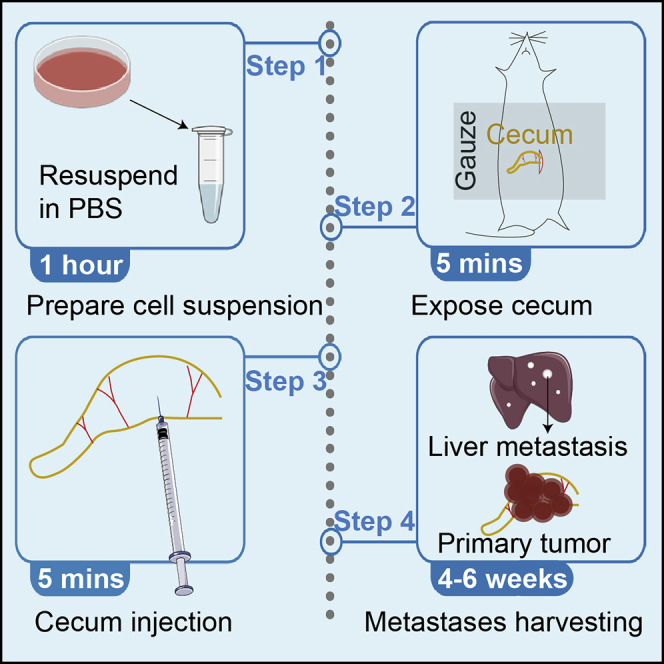
Summary
This protocol describes the generation of a mouse colorectal cancer (CRC) model to study the mechanism of CRC growth and metastasis. Compared to existing protocols, this protocol is mainly to improve the incidence of metastasis. The major advantage of this model is that it mimics the process of clinical CRC metastasis. Thus, it can be used to study different stages of CRC progression and to identify molecular markers or therapeutic targets. The limitation is the difficulty of performing the operation.
For complete details on the use and execution of this protocol, please refer to Zhang et al. (2021).
Subject areas: Cancer, Model Organisms
Graphical abstract
Highlights
-
•
CRC orthotopic mouse model recapitulates human CRC progression including metastasis
-
•
CRC orthotopic mouse model generates paired primary CRC tumor and metastases
-
•
CRC orthotopic mouse model is a useful tool to study CRC tumor microenvironment
This protocol describes the generation of a mouse colorectal cancer (CRC) model to study the mechanism of CRC growth and metastasis. Compared to existing protocols, this protocol is mainly to improve the incidence of metastasis. The major advantage of this model is that it mimics the process of clinical CRC metastasis. Thus, it can be used to study different stages of CRC progression and to identify molecular markers or therapeutic targets. The limitation is the difficulty of performing the operation.
Before you begin
Mouse maintenance and experimental procedures were approved by the Biomedical Research Ethics Committee of the Institute of Biophysics, Chinese Academy of Sciences.
6–8 weeks old female mice are used in this protocol.
Preparation of mouse surgery
Timing: 1 h
-
1.
Label and weigh the mice before surgery.
-
2.
Prepare a set of autoclaved and dried items for each mouse one day before surgery: 2 gauzes; 10 cotton swabs; 1 scissors; 2 forceps; 1 needle holders.
Preparation of cell suspension for orthotopic injection
Timing: 1 h, on the day for surgery.
-
3.
Prewarm PBS, 0.25% trypsin-EDTA and RPMI 1640 medium at water bath at 37°C before use.
-
4.
When HCT116, CRC57 or CT26 cells reach 70%–80% confluence in a 10 cm-dish, dissociate the cells using trypsin-EDTA. Recommend passage numbers less than 20.
-
5.
Transfer the cell suspension to a 15 mL polypropylene tube.
-
6.
Centrifuge the tube at 200 g for 3 min at 4°C.
-
7.
Aspirate supernatant and wash the cell pellet with 8 mL PBS and count the cells.
-
8.
Resuspend the cell pellet with PBS at 4×107 cells/mL and transfer to a 1.5 mL tube.
Note: Prepare at least 20% more injection volume in the case of the loss during resuspending the cells or with leakage during the injection. Inject 50 μL cell suspension per mouse.
-
9.
Keep the cell suspension on ice.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| PBS (no calcium, no magnesium) | Thermo Fisher Scientific | Cat# 10010023 |
| Trypsin-EDTA (0.25%), phenol red | Gibco | Cat# 25200072 |
| RPMI 1640 medium | Gibco | Cat# C11875500BT |
| Tribromoethanol | Sigma | Cat# T48402 |
| Bupivacaine | ADAMAS | Cat# 23486A |
| Dexamethasone | Sigma | Cat# D4902 |
| 70% Ethanol | Mint | N/A |
| Iodine | Mint | N/A |
| Sterile saline | SHIMEN | N/A |
| Tert-amyl alcohol | Sigma | Cat# 152463 |
| Experimental models: Cell lines | ||
| HCT116 | ATCC | Cat# CCL-247 |
| CRC57 | Gift form David Hsu at Duke University | N/A |
| CT26 | ATCC | Cat# CRL-2638 |
| Experimental models: Organisms/strains | ||
| Mouse: NCG | GemPharmatech | Cat# T001475 |
| Mouse: BALB/c | GemPharmatech | Cat# N000020 |
| Other | ||
| 15 mL Polypropylene tube | Corning | Cat# 430052 |
| Scissors | Sanyou | Cat# 042000 |
| Forceps | Sanyou | Cat# 044570 |
| Needle holders | Sanyou | Cat# 014014 |
| Cotton swabs | N/A | N/A |
| Suture (pre-autoclaved container) | Jinhuan Medical | Cat# CR631 |
| Autoclave bag | Medicom | Cat# 88025 |
| Tape | Deli | Cat# 30670 |
| Heat pad | N/A | N/A |
| Insulin syringe, 29 gauge | BD | Cat# 328421 |
| Loctite 406 glue | N/A | N/A |
Materials and equipment
| Reagent | Final concentration | Stock concentration | Amount |
|---|---|---|---|
| Tribromoethanol | 0.02 g/mL in sterile saline | 1.6 g/mL in Tert-amyl alcohol | 0.2 mL/10g body weight |
| Bupivacaine | 0.00125 g/mL in sterile saline | 0.0084 g/mL in sterile saline | 100 μL per mouse |
| Dexamethasone | 0.1 mg/mL in sterile PBS | 1 mg/mL in sterile PBS | 50 μL/20 g body weight |
Store stock solution at 4°C for tribromoethanol for 1 year, and −20°C for bupivacaine and dexamethasone for 6 months.
Step-by-step method details
Setup surgical platform
Timing: 40 min
-
1.
Turn on UV light of the biological safety cabinet and sterile surgical platform for 30 min.
-
2.
Place heat pad in the middle of the cabinet. Turn on the heat by plugging into the outlet. Ensure it is working. Cover the heat pad with another sterile field piece and fix it using tape.
-
3.
Open autoclaved toolbox and lay out the tools onto sterile surgery field.
CRITICAL: avoid touching them with contaminated gloves.
-
4.
Open suture and lay out it onto sterile surgery field.
-
5.
Prepare tubes with iodine and 70% ethanol.
-
6.
Place other required materials in the biological safety cabinet (Figure 1).
Figure 1.
Surgical area
(A–L) (A) Sterile 1 mL syringe, (B) Tape, (C) Razor, (D) 70% ethanol, (E) Iodine, (F) Sterile gauze, (G) Forceps, (H) Scissors, (I) Sterile Cotton swabs, (J) 29-gauge insulin syringe, (K) Needle holders, (L) Suture (pre- autoclaved container)
Mouse setup
Timing: 10 min
-
7.
Prepare a spare cage for holding mice after surgery.
-
8.
Setup a heat pad for warming up the mouse cage. Half of the post-surgery cage should be placed on the heat pad, so mouse can warm up. Make sure heat pad is functional.
-
9.
Weigh the mouse.
-
10.
Anesthetize the mouse by intraperitoneal injection of 0.2 mL/10 g body weight tribromoethanol solution. Monitor respiratory and heart rate of the mouse. Assess level of anesthesia by pedal reflex (firm toe pinch). The mouse should fall asleep within 5 min.
-
11.
Transfer the mouse to the sterile platform above the heat pad. Fix the mouse in position by taping down the limbs.
-
12.
Apply the ophthalmic ointment to prevent retina damage.
Mouse surgery
Timing: 10 min
-
13.
Shave the mouse hair around the surgery site.
-
14.
Clean and sterilize the surgery area using sterile cotton swabs with iodine and 70% ethanol, consecutively. It should be done in a spiral motion, from the middle to the outside and the process should be repeated 3 times.
-
15.
Place sterile gauze around surgery site to prevent contamination (Figure 2).
-
16.
Subcutaneously inject the mouse with 100 μL 0.125% bupivacaine for analgesia.
-
17.
Using forceps and surgical scissors, open the outer skin layer. Make a smaller incision into the inner muscle layer (Figure 3).
CRITICAL: avoid hurting blood vessels or organs.
-
18.
Identify and expose the cecum using forceps and cotton swabs. Do not use forceps to drag tissue.
Note: Cecum is usually located at the right side of stomach (Figure 4).
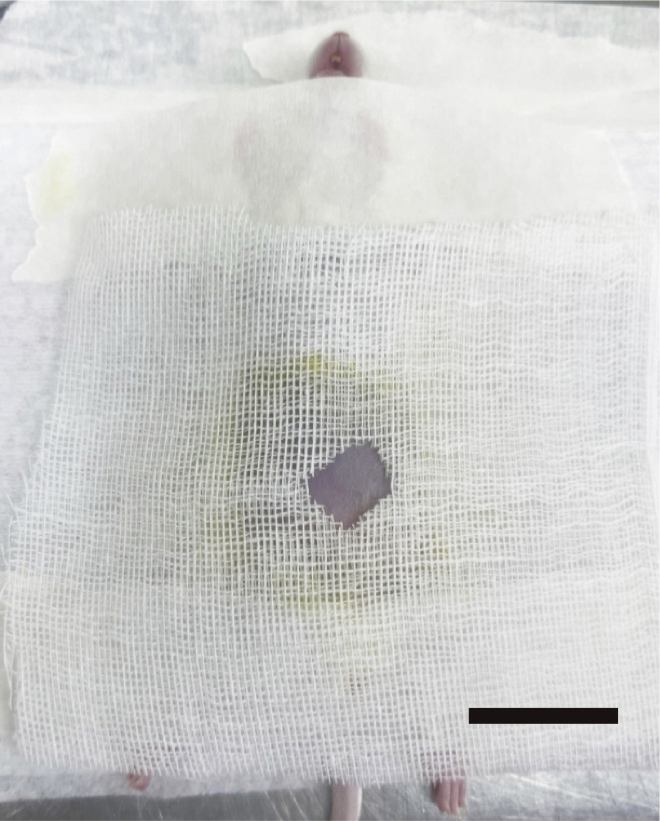
Figure 2.
Mice with sterile gauze, scale bar, 1.5 cm
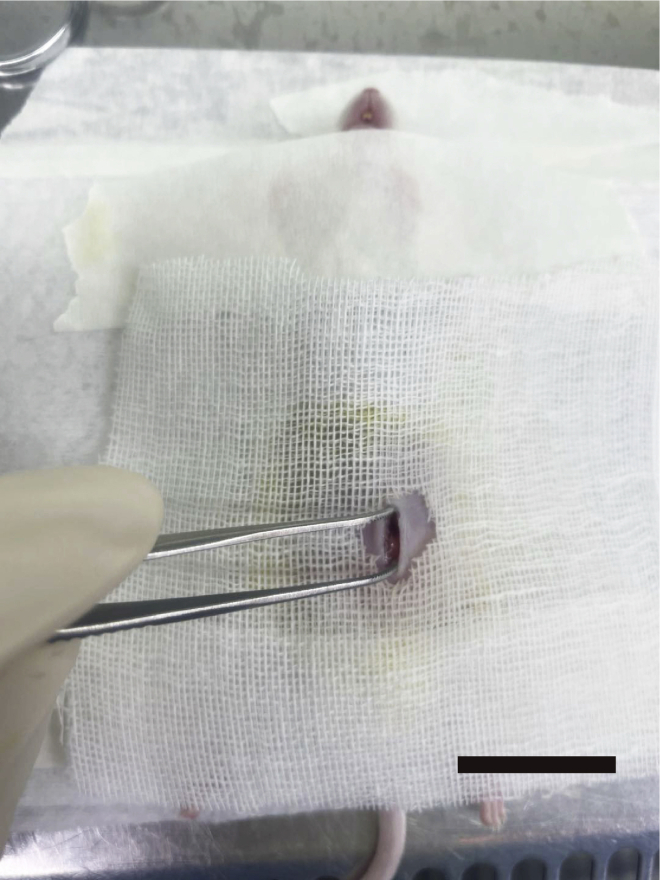
Figure 3.
Open the abdomen, scale bar, 1.6 cm
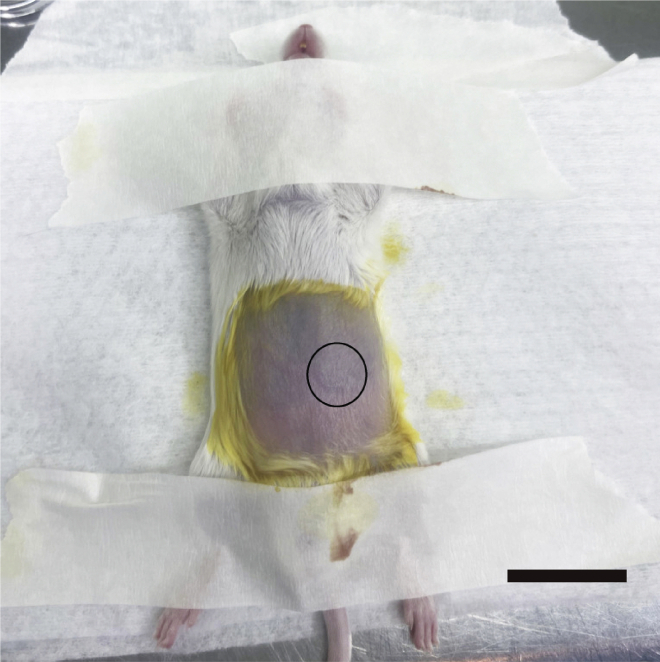
Figure 4.
Cecum location, scale bar, 1.5 cm
Note: If the cecum is dry, use 1 mL syringe with sterile saline to sustain tissue hydration.
-
19.
Withdraw 50 μL of the cell suspension with a 29-gauge insulin syringe.
Note: Keep the cell suspension on ice and mix upside down before injection.
-
20.
Fix the injection site by using cotton swabs.
Note: Do not injure vessels in submucosa of cecum or mesentery. Injection site should be between the blood vessels (Figure 5).
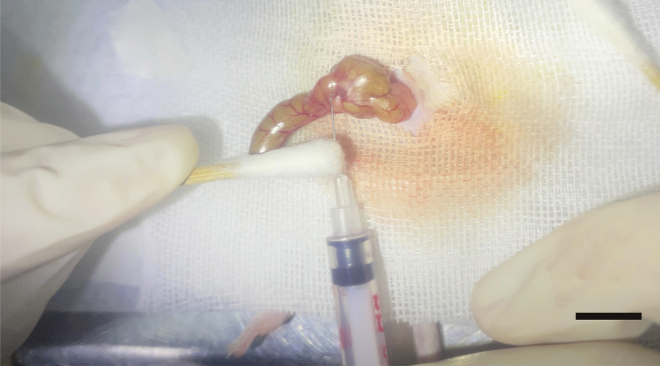
Figure 5.
Cecum injection, scale bar, 1 cm
-
21.
Gently insert the 29-gauge needle of the insulin syringe, approximately 1 mm, into the sub serosal layer.
Note: Face a needle tip upward and insert as shallow as possible.
-
22.
Slowly inject 50 μL of the cell suspension into the semitransparent sub serosal layer. Sub serosal swelling will be observed if injection is performed successfully.
Note: This procedure requires surgical skill and practice to obtain reproducible results.
-
23.
Check the injection site for leakage and bleeding (Kasashima et al., 2021).
-
24.
Use sterile suture and needle holder to close the inner muscle layer and the skin layer using discontinuous knots.
-
25.
Use Loctite 406 glue to close the skin over the suture, to prevent it breaking apart.
-
26.
Inject mouse with 50 μL/20 g mouse dexamethasone (at 0.1 mg/mL) to avoid surgery-caused inflammation.
-
27.
Put the mouse on heat pad.
-
28.
Record mouse data in record sheet found in surgery logbooks.
After surgery
Timing: 4–6 weeks
-
29.
If more mice are being used, replace the sterile surgical tools onto sterile pad.
-
30.
Wait until mice recover from anesthesia. Put mice back and make sure that the mice have a water bottle and wet food.
-
31.
Inject mice with 50 μL dexamethasone every day for the next 3 days to avoid surgery-caused inflammation.
-
32.
Weigh the mice and assess their health until the experiment is finished.
Expected outcomes
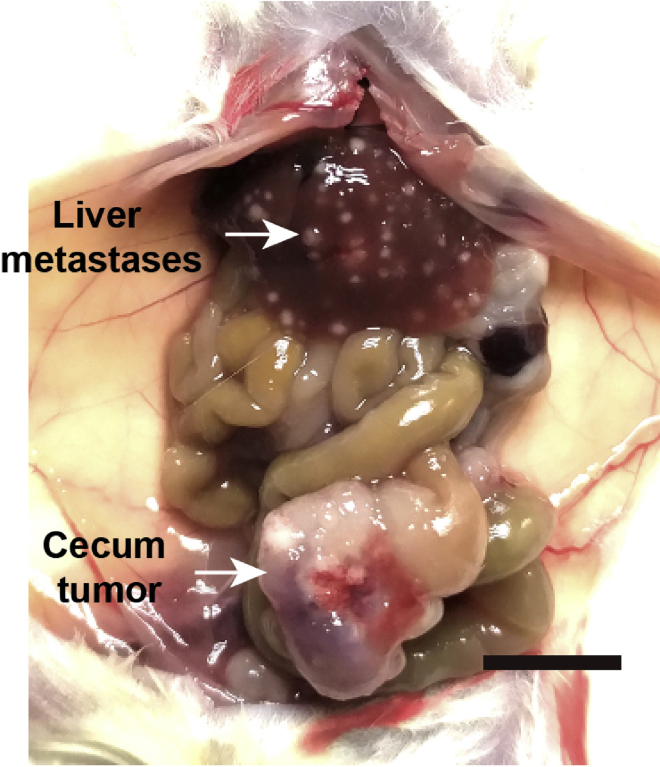
In approximately 4–6 weeks the mice will be moribund. Sacrifice all mice to assess tumors in cecum and metastases in liver (Figures 6 and 7).
Figure 6.
Tumors in cecum and liver, scale bar, 1 cm
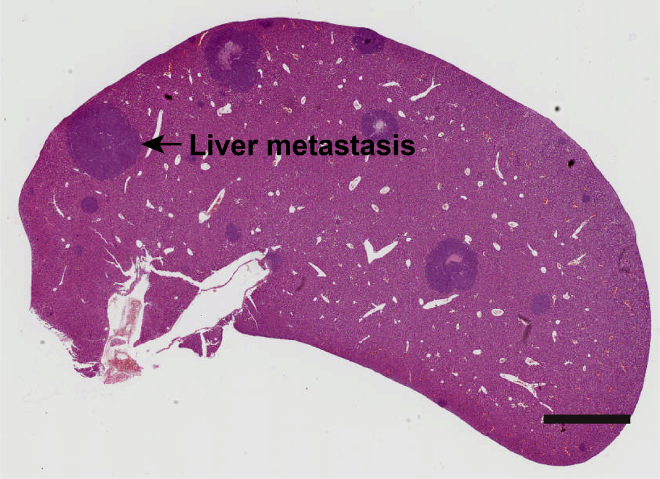
Figure 7.
Hematoxylin and eosin staining show metastatic tumors in liver (Zhang et al., 2021), scale bar, 0.3 cm
Quantification and statistical analysis
-
1.
Create an excel file to record data of each mouse. Data to be recorded should include sex, date of birth, age, and body weight before surgery, body weight at the endpoint.
-
2.
At the endpoint, weight of tumor, the number of liver metastases, photo of primary tumor and liver should be recorded.
Limitations
In addition to orthotopic CRC model, splenic injection and tail vein injection are used to study CRC metastasis as well. The advantage of orthotopic CRC model is that the model is to mimic human CRC development process and can study both primary CRC growth, invasion, and liver metastasis. The limitation of the model mainly lies in its difficulty for operation. If cecum injection leaks, tumor cell growth occurs intraperitoneally. Furthermore, the development of liver metastasis using orthotopic CRC model differs when different cell lines and mice strain are used.
This model covers most stages of human tumor growth and metastasis, including primary tumor growth, invasion, and liver metastasis, similar to CRC progression in patients. In our experiments, ten out of ten mice developed liver metastasis when CRC57 or HCT116 were injected, while four out of ten mice had liver metastasis when CT26 were injected, ten out of ten mice that die from tumors develop liver metastases. The main reason for the high metastasis rate compared to other models is the different microenvironment of tumor growth.
Troubleshooting
Problem 1
Can’t find cecum (Step 18).
Potential solution
Open abdomen at bottom right (Figure 4).
Problem 2
Leakage from the injection site (step 22).
Potential solution
Inject cell suspension as slowly as possible and hold the syringe for a while. If still leakage, reduce the injection volume may help.
Problem 3
Sub-serosal swelling in cecum cannot be seen (step 22).
Potential solution
Cecum injection is too deep. Face a needle tip upwards and insert as shallowly as possible (Kasashima et al., 2021).
Problem 4
Mice die of infection several days after the surgery (step 32).
Potential solution
Because NCG mice are severely immunodeficient, whole process must be strictly sterilized.
Problem 5
No metastases are observed (step 32).
Potential solution
Sacrifice mice too early. Make sure most mice got moribund before sacrifice.
Make sure the cells are well cultured and haven’t been passaged too many times.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Pengcheng Bu (bupc@ibp.ac.cn).
Materials availability
This study did not generate new materials.
Acknowledgments
Research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29040100), the Chinese Ministry of Science and Technology (2017YFA0504103), and the National Natural Science Foundation of China (31771513, 81972797, 81921003, 81870393, 81571563, and 82000812).
Author contributions
L.Z. and P.B. set up the experiments and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate datasets.
References
- Kasashima H., Duran A., Cid-Diaz T., Muta Y., Kinoshita H., Batlle E., Diaz-Meco M.T., Moscat J. Mouse model of colorectal cancer: orthotopic co- implantation of tumor and stroma cells in cecum and rectum. STAR Protoc. 2021;2:100297. doi: 10.1016/j.xpro.2021.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu Z., Yan H., Wang W., Wu Z., Zhang F., Zhang Q., Shi G., Du J., Cai H. Creatine promotes cancer metastasis through activation of Smad2/3. Cell Metab. 2021;33:1111–1123 e1114. doi: 10.1016/j.cmet.2021.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate datasets.