Abstract
32D cells, a murine myeloid cell line, rapidly undergo apoptosis upon withdrawal of interleukin-3 (IL-3) supplement in culture. We found that 32D cells, if infected by several species of human mycoplasmas that rapidly activated NF-κB, would live and continue to grow in IL-3-depleted culture. Mycoplasma-infected cells showed no evidence of autocrine production of IL-3. Pyrrolidine dithiocarbamate (PDTC) blocked activation of NF-κB and led to prominent cell death. Heat-killed mycoplasmas or mycoplasmal membrane preparations alone could support continued growth of 32D cells in culture without IL-3 supplement for a substantial period of time. However, upon removal of heat-inactivated mycoplasmas, 32D cells quickly became apoptotic. In comparison, live Mycoplasma fermentans or M. penetrans infection for 4 to 5 weeks induced malignant transformation of 32D cells. Transformed 32D cells grew autonomously and no longer required support of growth-stimulating factors including IL-3 and mycoplasmas. The transformed 32D cells quickly formed tumors when injected into nude mice. Karyotyping showed that development of chromosomal changes and trisomy 19 was often associated with malignant transformation and tumorigenicity of 32D cells. Mycoplasmal infections apparently affected the fidelity of genomic transmission in cell division as well as checkpoints coordinating the progression of cell cycle events.
Mycoplasmas are a heterogeneous group of the smallest organisms capable of self-replication. Mycoplasmas can cause a wide variety of diseases in animals (28). Some mycoplasmas cause respiratory or urogenital diseases in humans (18, 29), but others chronically colonize our respiratory and urogenital tracts without apparent clinical significance. In this respect, wall-free mycoplasmas are among the few prokaryotes that can grow in close interaction with mammalian cells, often silently for a long period of time. However, prolonged interactions with mycoplasmas with seemingly low virulence could, through a gradual and progressive course, significantly affect many biological properties of mammalian cells.
Using a murine embryonic (C3H) cell system, we demonstrated that chronic infection by mycoplasmas induced chromosomal instability as well as malignant transformation of mammalian cells. This mycoplasma-mediated oncogenic process had a long latency and demonstrated distinct multistage progression (30). Overexpression of H-ras and c-myc oncogenes was found to be closely associated with both the initial reversible and the subsequent irreversible states of the mycoplasma-mediated transformation in C3H cells (36). We have developed a new paradigm for neoplastic processes based on our in vitro studies. We hypothesize that chronic infection or colonization by certain mycoplasmas may gradually induce malignant transformation and promote tumorous growth of mammalian cells.
It is important to note that previous studies reported isolation of mycoplasmas from human leukemic bone marrow (1, 7, 10, 14, 21). A majority of the mycoplasma population isolated was identified as Mycoplasma fermentans. Furthermore, experimental inoculation of M. fermentans induced leukemoid disease with myeloproliferative changes in mice (20). However, the mycoplasma oncogenesis hypothesis failed to advance because although mycoplasma was isolated most frequently from patients with leukemia, the same mycoplasma could also be found in nonleukemic children or adults (19). Decades later, our understanding of chronic infections, cancer latency, and cancer-associated microbes has changed significantly (3, 4, 23). The previously described evidence of latent mycoplasmal infection in bone marrow and our findings that chronic infections by mycoplasmas could be associated with a unique form of pathogenesis including cell transformation (37) prompted us to reexamine the mycoplasmal effects on malignant transformation of hematopoietic cells. Growth of murine myeloid (32D) cells depends on the continuous induction of interleukin-3 (IL-3) (12, 13). Differing from other IL-3-dependent cells such as FD cells, 32D cells remain under strict regulation by the growth signaling of IL-3 and rarely undergo spontaneous transformation or become IL-3 independent. Withdrawal of IL-3 supplement in culture rapidly induces the 32D cells to undergo apoptosis, and more than 80% of the cells die within 4 to 5 days. The growth of 32D cells is regulated closely by growth factor signaling, which provides an ideal model system to study the transforming effects of mycoplasmas.
Remarkably, we found that infections by several species of human mycoplasmas, but not all species tested, would effectively prevent 32D cells from undergoing apoptosis in culture without IL-3 supplement. The mycoplasma-infected 32D cells continued to grow without the induction of IL-3 growth signaling. Moreover, after a period of 4 to 5 weeks of infection by M. fermentans or M. penetrans, 32D cells gradually underwent malignant transformation and no longer required the continued presence of mycoplasmas for growth in the IL-3-free culture. These 32D cells grew autonomously and became highly tumorigenic when injected into nude mice. This in vitro model system allowed us to explore mycoplasma-mediated molecular mechanisms that rescue cells from apoptosis and induce continuous cell growth. We also studied the machinery that could lead to malignant transformation in 32D cells chronically infected by mycoplasmas.
MATERIALS AND METHODS
Mycoplasmas and cell culture.
M. fermentans PG18 (ATCC 19989), incognitus (previously isolated from our laboratory [17]), and A25 (isolated from a patient with AIDS and to be reported separately), M. penetrans GTU-54 (previously isolated from our laboratory [16]), M. salivarium ATCC 23064, M. genitalium ATCC 33530, M. pneumoniae ATCC 15531, M. orale ATCC 23714, and M. pirum (kindly provided by J. G. Tully, National Institute of Allergy and Infectious Diseases, National Institutes of Health) were grown aerobically in SP-4 broth medium. The 32D cell line (kindly provided by Jaclyn H. Pierce, National Cancer Institute, National Institutes of Health) is an IL-3-dependent, nontumorigenic cell line that has an undifferentiated myeloid phenotype and a normal diploid karyotype. The cell line was maintained in RPMI culture medium containing 15% fetal calf serum and 5% WEHI-3B conditioned medium (also provided by Jaclyn H. Pierce).
Infection of 32D cells with mycoplasmas.
32D cells were transferred to culture medium free of IL-3 then inoculated with various species of Mycoplasma at a ratio of 1,000 color change units/cell. To determine the growth kinetics of 32D cells in cultures infected with mycoplasmas, cell cultures were initiated at 2 × 105 cells/ml. Viable cells stained with trypan blue were examined and counted every 2 to 3 days in a hemocytometer. Cell densities of cultures were adjusted to 2 × 105 cells/ml when subcultured.
Heat inactivation of mycoplasmas.
Mycoplasmas were cultured in SP-4 medium to log growth phase. After a culture sample was taken for titration, mycoplasma cultures were incubated in a water bath at 70°C for 30 min. Heat-treated mycoplasmas were tested by culture to ensure complete inactivation of the organisms and centrifuged in a microcentrifuge at 12,000 rpm for 20 min. Mycoplasma pellets were then suspended in RPMI 1640 medium at 1/10 of the original volume. These suspensions of heat-killed mycoplasmas were kept frozen at −70°C until needed. The amount of heat-inactivated mycoplasmas added to the cell cultures equaled to 1,000 color change units/ml.
Preparation of nuclear proteins.
Nuclear proteins were prepared by the method of Schreiber et al. (27). Typically, 5 × 106 to 1 × 107 32D cells were washed with 10 ml of Tris-buffered saline and pelleted. The pellet was resuspended in 1 ml of Tris-buffered saline and pelleted again by spinning for 15 s in a microcentrifuge. Tris-buffered saline was removed, and the cell pellet was resuspended in 0.8 ml of cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, 10 mM leupeptin, 1.5 mM pepstatin). The cells were incubated on ice for 15 min, after which 50 μl of a 10% solution of Nonidet NP-40 was added and the tube vigorously vortexed for 10 s. The homogenate was centrifuged for 30 s in a microcentrifuge, and the supernatant was removed. The nuclear pellet was resuspended in 100 μl of ice-cold buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 10 mM leupeptin, and 1.5 mM pepstatin), and the tube was vigorously rocked at 4°C for 15 min on a shaking platform. The nuclear extract was centrifuged for 5 min in a microcentrifuge at 4°C, and the supernatant was frozen in aliquots at −70°C. Protein determination was performed by using a Bio-Rad DC protein assay kit.
EMSA.
Electrophoretic mobility shift assay (EMSA) was performed as described by Vincenti et al. (32). A double-stranded oligonucleotide containing a mouse tumor necrosis factor alpha κB enhancer located at −510 bp from the start of transcription to the tumor necrosis factor alpha gene was used for the binding assay. The sequences of the two strands of the oligonucleotide with an NF-κB binding site were 5′-CAA ACA GGG GGC TTT CCC TCC TC-3′ and 3′-GTT TGT CCC CCG AAA GGG AGG AG-5′; those of the oligonucleotide with an AP-1 binding site were 5′-CGC TTG ATG ACT CAG CCG GAA-3′ and 3′-GCG AAC TAC TGA GTC GGC CTT-5′. In the assay, a 32P-labeled oligonucleotide fragment (100,000 cpm) was mixed with 2 μg of nuclear protein in a total volume of 20 μl of 25 mM HEPES (pH 7.9)–0.5 mM EDTA–0.5 mM DTT–0.1 M NaCl–10% glycerol–1 μg of bovine serum albumin–2 μg of poly(dI-dC). After 30 min of incubation at room temperature, the reaction mixtures were loaded onto 6% polyacrylamide gels in 0.5× Tris-borate-EDTA. The gels were prerun for 1 h at 150 V prior to the actual run of 2.5 h at the same voltage. After electrophoresis, the gels were dried and exposed for autoradiography. Quantitation of protein-bound oligonucleotide bands was done using Storm 860 scanner with ImageQuant version 4.2 software (Molecular Dynamics).
Eradication of mycoplasmas in cell cultures.
To eradicate mycoplasmas from the infected 32D cell cultures, the cultures were treated with ciprofloxacin (10 μg/ml) for 3 to 4 weeks. Growth responses of the infected cells to treatment were examined every 2 to 3 days by counting viable cell numbers in the cultures. The cell density was adjusted to 2 × 105 cells/ml when subculture was needed. To determine if mycoplasmas were successfully eradicated in the cell cultures, samples of cell culture supernatants were cultured in the SP-4 broth medium for isolating mycoplasmas (5). Additionally, DNAs were isolated from the cell cultures and assayed for the presence of mycoplasmal DNA by PCR using primers specific for M. fermentans and M. penetrans (34) or specific 16S rRNA genes (11).
Tumorigenicity in nude mice.
Normal, infected, and transformed 32D cells were harvested from cultures and suspended in a small volume (less than 1 ml) of phosphate-buffered saline (PBS). About 5 × 106 cells in 0.2 ml PBS were injected subcutaneously into each nude mouse (6 to 8 weeks old; Harlan-Sprague Dawley). The animals were carefully monitored for 1 year for tumor formation.
Karyotype analysis.
Actively growing 32D cell cultures were incubated in the presence of colcemid (0.05 μg/ml) for 1 h to accumulate metaphase cells. Cells were suspended in hypotonic solution (0.075 M KCl) for 10 to 15 min; cell pellets were fixed in methanol-acetic acid mixture (3:1 vol/vol) and then washed three times in the fresh fixative. Chromosome preparations were stained with Giemsa staining solution or by the trypsin-Giemsa banding method (35).
RESULTS
Mycoplasmal infections induce continued growth of 32D cells in IL-3-deprived culture.
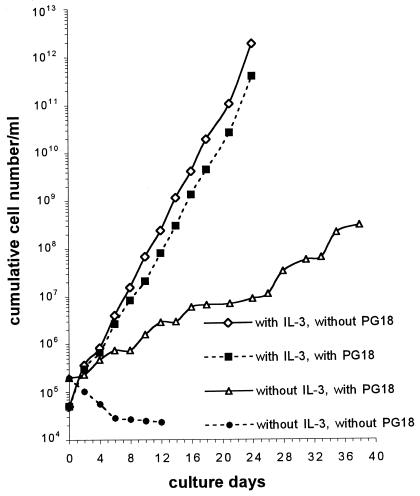
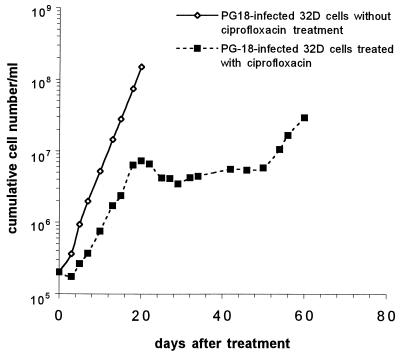
Growth of 32D cells was strictly dependent on IL-3. Removal of IL-3 from the culture caused immediate growth arrest and apoptosis of 32D cells; about 75% of the cells died by day 4. Surprisingly, infections by mycoplasmas effectively prevent 32D cells from undergoing apoptosis in the IL-3-deprived culture. As a typical example, Fig. 1 shows that infection by M. fermentans PG18 rescued 32D cells from apoptosis and induced continued cell growth in the IL-3-free culture. In this study, all mycoplasma-infected 32D cells that continued to proliferate were maintained in culture without IL-3 supplement for more than 3 months. We examined nine different strains and species of human mycoplasmas for the ability to prevent apoptosis and induce continued growth of 32D cells in the IL-3-deprived culture. In addition to M. fermentans PG18, infections by M. fermentans incognitus and A25, M. penetrans GTU-54, M. genitalium, M. orale, and M. pneumoniae were all found to induce continued growth of 32D cells in cultures without IL-3 supplement (Table 1). In contrast, infections by M. salivarium or M. pirum failed to rescue 32D cells from undergoing apoptosis in IL-3-free culture.
FIG. 1.
M. fermentans PG18 infection induces continued cell growth of 32D cells in culture deprived of IL-3. 32D cells initiated at 5 × 104 cells/ml in culture medium containing IL-3 or 2 × 105 cells/ml in culture medium deprived of IL-3 were infected by M. fermentans PG18 at a ratio of 1,000 color change units/cell. To avoid medium depletion and ensure logarithmic growth, infected and noninfected 32D cells cultured in IL-3-containing medium were subcultured with a 1:10 dilution on every 4th day. Infected 32D cells cultured in IL-3-deprived medium were subcultured on days 6, 12, 18, and 28 to maintain a cell density in the range of 5 × 104 to 4 × 105 cells/ml. Viable cells were examined by trypan blue staining and counted in a hemocytometer. Growth curves were plotted based on cumulative cell numbers.
TABLE 1.
Activation of NF-κB, continued cell growth in IL-3-free cultures, and subsequent malignant transformation of 32D cells following mycoplasmal infections
| 32D cells
|
Effect of mycoplasmal infections on 32D cells
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Presence of IL-3 in medium | Infection | NF-κBa (within 2 days) | AP-1b (within 2 days) | Support of continuous cell growth | Eventual cell transformation | NF-κB in transformed cells | AP-1 in transformed cells | Tumorigenicity of transformed cells |
| + | None | − | + | + | − | NAc | NA | − |
| − | None | − | − | − | − | NA | NA | NA |
| − | M. fermentans PG18 | + | + | + | + | − | − | + |
| − | M. fermentans A25 | + | + | + | + | − | − | + |
| − | M. fermentans incognitus | + | + | + | + | − | − | + |
| − | M. penetrans | + | + | + | + | − | + | + |
| − | M. salivarium | − | + | − | − | NA | NA | NA |
| − | M. genitalium | + | + | + | NDd | ND | ND | ND |
| − | M. pneumoniae | + | + | + | ND | ND | ND | ND |
| − | M. orale | + | + | + | ND | ND | ND | ND |
| − | M. pirum | − | + | − | − | NA | NA | NA |
Induction of transcriptional factor NF-κB detected in the nuclear extract of 32D cells.
Binding activity of AP-1 detected in the nuclear extract of 32D cells.
NA, not applicable.
ND, not done.
Mycoplasma-infected 32D cells do not produce IL-3.
One possible explanation that infections by mycoplasmas could induce continued growth of 32D cells in culture without any supplement of IL-3 was that mycoplasma-infected 32D cells were themselves producing IL-3. The newly produced IL-3 would provide autocrine signaling and support growth of 32D cells. Thus, we examined expression of the IL-3 gene in mycoplasma-infected 32D cells. RNA from M. fermentans PG18-infected 32D cells that continued to grow in culture without IL-3 supplement was analyzed for the presence of IL-3 messenger by reverse transcriptase PCR (6). However, after 30 cycles of amplification using an IL-3-specific primer set (sense, 5′-ATG GTT CTT GCC AGC TCT ACC ACC A-3′; antisense, 5′-GAT AAG ACA TTT GAT GGC ATA AAG GA-3′), we could not detect any IL-3 messenger in mycoplasma-infected 32D cells that continued to grow in IL-3-free culture (data not shown). Mycoplasmal infections evidently triggered a replicating machinery that bypassed the IL-3-mediated signal-transducing pathway in rescuing 32D cells from undergoing apoptosis and inducing continued cell growth.
Mycoplasmas induce NF-κB and enhance AP-1 nuclear binding activities in 32D cells.
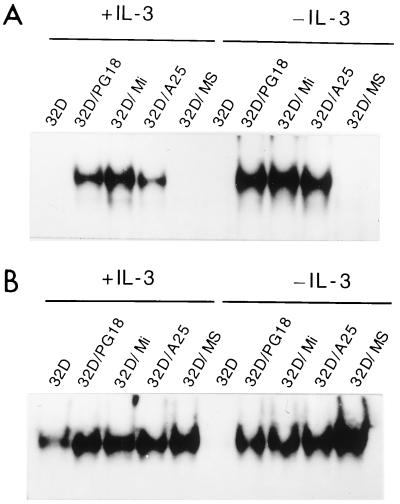
Recently, various membrane preparations from mycoplasmas were found to be potent activators for NF-κB and AP-1 transcription factors in murine macrophages (8, 9, 25, 26). Since NF-κB was known to have marked antiapoptosis functions (2, 31, 33) we examined by EMSA the NF-κB activities in 32D cells before and after mycoplasmal infections. Nuclear extracts of 32D cells grown in culture media supplemented with IL-3 showed little NF-κB activity. Infections by M. fermentans PG18, incognitus, and A25 for 24 h, however, markedly induced NF-κB binding activity in the nuclear extract of 32D cells cultured in medium with or without IL-3 supplement (Fig. 2A). In this study, we also found that infection by M. penetrans, M. pneumoniae, M. genitalium, and M. orale could support continued growth of 32D cells in IL-3 free culture by rapidly inducing NF-κB binding activity in the nuclear extract of 32D cells (Table 1). In comparison, infection by M. salivarium or M. pirum, which failed to support continued growth of 32D cells in IL-3-free culture, could not elicit the same nuclear factor response (Fig. 2A and Table 1).
FIG. 2.
Mycoplasmas induce NF-κB and enhance AP-1 nuclear binding activities in 32D cells. 32D cells were infected with M. fermentans PG18 (PG18), incognitus (Mi), and A25 (A25) or M. salivarium (MS) for 24 h in cultures with or without IL-3. Nuclear extracts prepared from 32D cells with or without mycoplasma infection were analyzed by EMSA for binding to 32P-labeled oligonucleotides with binding sites for NF-κB (A) or AP-1 (B).
There was a basal level of activity for transcription factor AP-1 detected by EMSA in the nuclear extracts of control 32D cells grown in culture supplemented with IL-3. Transferring control 32D cells to IL-3-free culture resulted in a rapid loss of all AP-1 binding activity in the nuclear extracts. As described above, the 32D cells without active AP-1 soon underwent apoptosis and died in a few days. In comparison, 32D cells infected by mycoplasmas for 24 h in IL-3-free culture produced a very high level of AP-1 binding activity (Fig. 2B). In this study, all mycoplasmas tested appeared to have the ability to markedly induce AP-1 activity in 32D cells, including those that failed to support continued growth of 32D cells in IL-3-free culture conditions. For example, 32D cells infected by M. salivarium produced as much of the active form of AP-1 as 32D cells infected by M. fermentans (Fig. 2B).
Interference with induction of NF-κB binding activity in mycoplasma-infected 32D cells results in cell death.
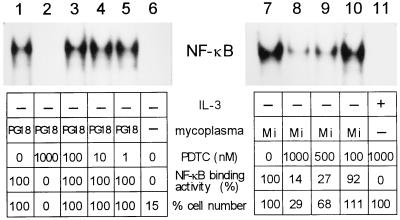
Although infections by mycoplasmas could rapidly activate both NF-κB and AP-1 transcription factors in 32D cells, activation of NF-κB appeared to be more closely correlated with IL-3-independent mycoplasma-induced cell growth. To further explore the role that NF-κB might play in preventing apoptosis and in induction of continued cell growth in the absence of IL-3 signaling, we treated 32D cells with pyrrolidine dithiocarbamate (PDTC; Sigma), a potent and specific inhibitor of NF-κB activation. While pretreatment with 100 nM PDTC had little effect, pretreatment with 1,000 nM completely blocked the activation of NF-κB in 32D cells induced by M. fermentans PG18 infection (Fig. 3). PDTC at the concentration of 100 nM had no effect on growth of 32D cells induced by the mycoplasma. On the other hand, consistent with the lack of NF-κB activation, mycoplasma-infected 32D cells treated with 1,000 nM PDTC failed to grow and quickly died. In a separate study, treatment of M. fermentans incognitus-infected 32D cells with 500 and 1,000 nM PDTC blocked more than 70 and 85% of NF-κB activation, respectively (Fig. 3). Growth of 32D cells induced by infections of the mycoplasma for 2 days was inhibited about 30% by 500 nM and 70% by 1,000 nM PDTC (Fig. 3). PDTC by itself at the concentration of 1,000 nM did not seem toxic or affect the growth of 32D cells supported by IL-3.
FIG. 3.
Interference with induction of NF-κB binding activity results in cell death in mycoplasma-infected 32D cells. 32D cells were treated with PDTC at various concentrations for 1 h prior to transfer to IL-3-free culture medium and infection by M. fermentans PG18 (PG18; lanes 2 to 5) or incognitus (Mi; lanes 8 to 10). As positive controls (lanes 1 and 7), 32D cells without PDTC pretreatment were transferred to IL-3-free culture medium and infected with PG18 or Mi, respectively. As a PDTC control (lane 11), 32D cells were treated with 1,000 nM PDTC for 1 h and then cultured in IL-3-containing medium. As a negative control (lane 6), 32D cells were transferred to IL-3-free culture medium without mycoplasma infection. After 2 days, cell numbers were counted, and nuclear extracts were prepared and examined for NF-κB binding activity. Percent NF-κB binding activity was calculated based on the counts per minute of the protein-bound 32P-labeled oligonucleotide bands quantitated by a Storm 860 scanner. In the PG18-infected group, the activity in lane 1 was used as 100%, and values for lanes 2 to 6 were calculated as percentages of this level. In the Mi-infected group, lane 7 represents 100% activity, and values for lanes 8 to 11 were calculated as percentages of this level. Similarly, percent cell numbers were calculated as percentages of the cell number of PG18-infected, non-PDTC-treated 32D cells (lane 1) in the PG-18-infected group (lanes 2 to 6) and to that of Mi-infected, non-PDTC-treated 32D cells (lane 7) in the Mi-infected group (lanes 8 to 11). When the differences between the PDTC-treated cells and the non-PDTC-treated mycoplasma-infected cells were less than 5%, percent binding activity and percent cell number were designated 100%.
Heat-inactivated mycoplasmas and mycoplasmal lipid-associated membrane proteins (LAMPs) can induce IL-3 independent growth of 32D cells.
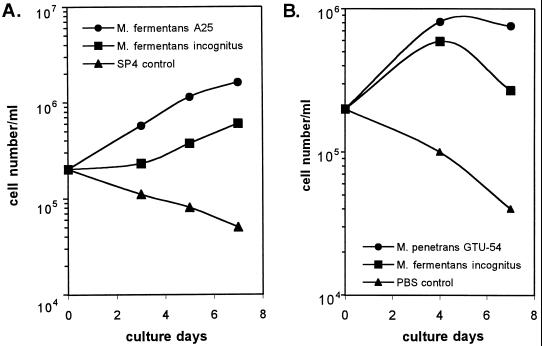
To examine whether infections by live mycoplasmas were required to induce the IL-3-independent growth of 32D cells, we first heat inactivated the mycoplasmas and then examined their ability to induce continued growth of 32D cells in IL-3-free culture. Figure 4A shows that introduction of heat-inactivated M. fermentans incognitus or A25 effectively prevented cell death and induced growth of 32D cells in IL-3-deprived culture. If heat-inactivated mycoplasmas were added whenever the cultures were replenished with fresh medium, 32D cells would continue to proliferate in IL-3 free culture. In this study, the cultures supported by heat-inactivated mycoplasmas were maintained for over 2 months before being terminated. However, transferring these 32D cells into culture with fresh medium without adding the heat-inactivated mycoplasmas quickly resulted in apoptosis and cell death. Continual presence of the heat-inactivated mycoplasmas was evidently required for the continued survival of 32D cells in culture without IL-3 supplements.
FIG. 4.
Heat-inactivated mycoplasmas (A) or mycoplasmal LAMPs (B) induce continued growth of 32D cells in IL-3-deprived culture. M. fermentans A25 and incognitus were heat inactivated by treating the cultures at 70°C for 30 min. Heat-inactivated mycoplasmas, or SP-4 broth medium or PBS used as a control, were added to 32D cells after the cells were transferred to IL-3-deprived cultures. Cell numbers were counted on days 3, 5, and 7. LAMPs prepared from M. penetrans or M. fermentans incognitus or the SP-4 broth medium were added to 32D cells at a concentration of 10 μg of M. penetrans or 4 μg of M. fermentans LAMPs/2 × 105 32D cells/ml after the cells were transferred to IL-3-deprived cultures. Cell numbers were counted on days 4 and 7.
Since the LAMPs of M. fermentans and M. penetrans were potent inducers of NF-κB and AP-1 in murine macrophages (8, 25), we examined if the Triton X-114 preparation of LAMPs could support the IL-3-independent growth of 32D cells. Figure 4B shows that LAMPs prepared from M. fermentans incognitus and M. penetrans rescued 32D cells from apoptosis and induced continued cell growth in culture free of IL-3. Consistent with the earlier finding, heat-killed M. fermentans, M. penetrans, and their LAMPs rapidly induced NF-κB activation in 32D cells (data not shown).
Infection by live mycoplasmas induces transformation of 32D cells.
If continued presence of heat-inactivated mycoplasmas in the IL-3-free culture was required for the continued growth of 32D cells, we wanted to know whether the continued presence of live mycoplasmas was also required for the continued growth of 32D cells in IL-3-free culture. We began to treat a subset of mycoplasma-infected cultures with ciprofloxacin to eradicate the mycoplasmas after 4 to 5 weeks of IL-3-independent mycoplasma-induced cell growth. Figure 5 shows the growth response to ciprofloxacin treatment of 32D cells that had been infected by M. fermentans PG18 for 4 to 5 weeks. Although after 5 days of ciprofloxacin treatment, no viable mycoplasmas could be isolated from the culture, 32D cells continued to grow rapidly for 2 to 3 weeks. As expected, PCR analysis showed that nonviable mycoplasmal organisms were detectable in culture and likely continued to support cell growth during this period. The nonviable organisms, however, were serially diluted to low concentration and were no longer detectable in culture after several subsequent fresh medium replenishments. The increase of cell number in the culture appeared to slow down significantly after 3 weeks. Examination of the culture revealed that many 32D cells were apparently dying while many others continued to grow. After 7 weeks, rapid cell growth resumed in the culture free of IL-3. PCR analyses confirmed the absence of M. fermentans in the continuously growing 32D cell culture without IL-3 supplement. In addition to M. fermentans PG18, we observed similar transformation of strictly IL-3-dependent 32D cells into autonomously growing cells after 4 to 5 weeks of infections by the M. fermentans A25 and incognitus as well as M. penetrans (Table 1). All of these mycoplasmal agents were eradicated from the 32D cell cultures by 3 weeks of antibiotic treatment.
FIG. 5.
Growth response of M. fermentans PG18-infected 32D cells to ciprofloxacin. 32D cells that had been infected with M. fermentans PG18 for 5 to 6 weeks were treated with ciprofloxacin for 3 weeks to eradicate the mycoplasmas in culture. Viable cell numbers in cultures were counted every 2 or 3 days. Cell density was adjusted to 2 × 105 cells/ml when subculture was needed to ensure continual logarithmic growth. Growth curves were plotted based on cumulative cell numbers.
Mycoplasma-transformed 32D cells do not have the active nuclear factors of NF-κB and AP-1.
Our earlier study showed rapid activation of NF-κB by mycoplasmas appeared to be essential in preventing 32D cells from undergoing apoptosis in IL-3-free culture. If the continued presence of live mycoplasmas was no longer required for the continued survival of transformed 32D cells in IL-3-free culture, had 32D cells constitutively activated NF-κB or AP-1 following transformation? When we examined NF-κB and AP-1 binding activities by EMSA in nuclear extracts of these mycoplasma-transformed 32D cells that were growing autonomously, we found no positive binding activity of NF-κB or AP-1 (Table 1). Infections by live mycoplasmas for a few weeks apparently activated a new growth-stimulating pathway, different from those of IL-3 signaling or NF-κB activation, in these autonomously growing cells.
Mycoplasma-transformed 32D cells have abnormal karyotypes.
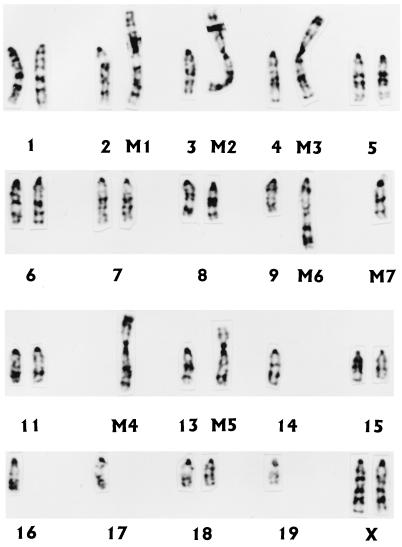
In C3H murine embryonic cells, an irreversible form of malignant transformation induced by chronic infection of M. fermentans or M. penetrans was found to be associated with development of chromosomal changes (30). We examined alteration of chromosomes in 32D cells following mycoplasma infections and particularly in 32D cells that were transformed and grew autonomously in culture free of growth-stimulating mycoplasmas and IL-3 supplement. Karyotypic analysis showed that a great majority of the control IL-3-dependent 32D cells had a total of 34 chromosomes with 7 abnormal chromosomes (Table 2) instead of the normal mouse diploid complement of 40 chromosomes. Five of the seven abnormal chromosomes, M1 [rob(2;17)], M2 [rob(3;10)], M3 [rob(4;12)], M4 [rob(12;16)], and M5 [rob(13;19)], were Robertsonian translocation chromosomes; the other two abnormal chromosomes, M6 and M7, appeared to be derivative chromosomes of complex translocation. The M6 chromosome was derived from translocation between chromosomes 9 and 14 [t(9;14)]; the M7 chromosome was chromosome 10 with a very small piece of additional chromosomal material on its centromere. The additional chromosomal material appeared to be a portion of chromosome 14 that is also involved in the translocation with chromosome 9. The representative karyotype of 32D cells is presented in Fig. 6. Infections by various strains of M. fermentans or M. penetrans for 4 to 5 weeks in culture supplemented with IL-3 caused few chromosomal changes in 32D cells. In contrast, 32D cells infected by these mycoplasmas for 4 to 5 weeks in culture without supplemental IL-3 showed significant chromosomal alteration (Table 2). Nearly 50% (23 of 51) of 32D cells infected by M. fermentans PG18 gained an additional chromosome. Interestingly, half (12 of 23) of the cells that gained an additional chromosome had trisomy 19; the other half had an extra copy of various chromosomes. About 6% (3 of 52) of the 32D cells infected by M. fermentans incognitus for 4 weeks also gained an additional chromosome 19. Most strikingly, more than 95% (52 of 54) of the 32D cells infected by M. penetrans GTU-54 for 3 to 4 weeks in culture free of IL-3 had trisomy 19 (Table 2).
TABLE 2.
Chromosome analysis on 32D cells infected and transformed by M. fermentans and M. penetrans
| Culture | No. of cells with chromosome no.:
|
No. of abnormal chromosomes | ||||
|---|---|---|---|---|---|---|
| 32 | 33 | 34 | 35 | 36 | ||
| 32D cells | 56 | 2 | 7 | |||
| 32D/IL-3+/PG18+ | 2 | 45 | 5 | 7 | ||
| 32D/IL-3−/PG18+ | 1 | 6 | 21 | 23 | 7 | |
| 32D/IL-3−/PG18− | 50 | 11 | 7, 8 | |||
| 32D/IL-3+/Mi+ | 2 | 50 | 7 | |||
| 32D/IL-3−/Mi+ | 1 | 17 | 31 | 3 | 7 | |
| 32D/IL-3−/Mi− | 52 | 2 | 8 | |||
| 32D/IL-3+/Mpe+ | 2 | 48 | 7 | |||
| 32D/IL-3−/Mpe+ | 1 | 52 | 1 | 7 | ||
| 32D/IL-3−/Mpe− | 2 | 50 | 7 | |||
FIG. 6.
Karyotype of control 32D cells. The majority of 32D cells had 34 chromosomes rather than the normal mouse diploid complement of 40 chromosomes. Among the 34 chromosomes, there were 7 abnormal chromosomes, including 5 Robertsonian translocation chromosomes {M1 [rob(2;17)], M2 [rob(3;10)], M3 [rob(4;12)], M4 [rob(12;16)], and M5 [rob(13;19)]} and 2 derivative chromosomes of translocations involving chromosomes 9, 10, and 14 (M6 and M7).
As described earlier, ciprofloxacin treatment to eradicate mycoplasmas from the culture of mycoplasma-infected 32D cells selected population(s) of truly transformed cells, i.e., cells that were capable of growing autonomously without support of IL-3 or continued presence of growth-stimulating mycoplasmas. Karyotypic analysis of the truly transformed cells induced by either M. fermentans or M. penetrans showed that populations of 32D cells with trisomy 19 often prevailed (Table 2). All 32D cells transformed by a period of M. fermentans PG18 infection (4 weeks) and maintained in IL-3-free culture following ciprofloxacin treatment (32D/IL-3−/PG18− culture) either had 34 chromosomes with 8 abnormal chromosomes or 35 chromosomes with an additional copy of chromosome 19. Those cells with 34 chromosomes, in addition to the original 7 abnormal chromosomes, had a new abnormal rob(19;19) chromosome. Thus, both populations of 32D/IL-3−/PG18− cells actually had trisomy 19. All 32D cells transformed by a period of M. fermentans incognitus infection (4 weeks) and maintained in IL-3-free culture following treatment with ciprofloxacin (32D/IL-3−/Mi− culture) had 34 chromosomes. In addition to the original 7 abnormal chromosomes, all of the transformed cells had a new abnormal rob(19;19) chromosome. Thus, they all were trisomy 19. Since more than 95% of 32D cells infected by M. penetrans for 4 to 5 weeks (32D/IL-3−/Mpe+ culture) already had 35 chromosomes with an extra chromosome 19, treatment with ciprofloxacin to eradicate M. penetrans in culture free of IL-3 did not alter the cell karyotype. All of the transformed cells that rapidly prevailed after ciprofloxacin treatment to eradicate mycoplasmas (32D/IL-3−/Mpe− culture) had trisomy 19 (Table 2).
Mycoplasma-transformed 32D cells are highly tumorigenic.
We examined whether the transformed 32D cells induced by mycoplasma infections had become tumorigenic when injected into animals. In this study, 2 × 106 mycoplasma-infected cells, mycoplasma-free transformed cells, or noninfected control cells were inoculated subcutaneously into each of three nude mice. Similar to the IL-3-dependent 32D control cells, cells infected by various strains of M. fermentans for 4 to 5 weeks in culture with or without IL-3 supplement did not form tumors when injected into the animals. As described above, treatment with ciprofloxacin to eradicate M. fermentans from the IL-3-free culture selected populations of truly transformed cells. All cells in the 32D/IL-3−/PG18− and 32D/IL-3−/Mi− cultures had trisomy 19 (Table 2) and formed tumors rapidly in nude mice. Noninfected control cells treated in parallel with ciprofloxacin for 3 weeks did not form tumors when injected into the animals. More than 95% of the cells in the 32D/IL-3−/Mpe+ culture already had trisomy 19 (Table 2), they formed tumors in two out of three nude mice inoculated. At necropsy, tumors formed by the mycoplasma-infected 32D cells in animals were cultured and PCR tested for M. penetrans. No evidence of M. penetrans infection was identified in these tumors. After ciprofloxacin treatment to eradicate M. penetrans, the cells that grew autonomously in the 32D/IL-3−/Mpe− culture rapidly formed tumors in all three animals inoculated. The tumors formed in the animals by the mycoplasma-transformed 32D cells could be regrown easily in cell culture system without IL-3 supplement and could also be passed easily to other animals.
DISCUSSION
IL-3-dependent 32D cells transfected with various oncogenes have served as a model system to study oncogenesis (15). The inherent drawback of this model system is that the cells are transformed artificially by introducing potent transforming genes apart from what naturally transpires. In contrast, our model system using 32D cells examines the transforming effects of infectious agents (mycoplasmas) that are naturally encountered. In this study, we showed that infections by several human mycoplasmas prevented apoptosis and induced continued proliferation of 32D cells in culture without IL-3 supplement. We believe this is the first reported finding that infection by a prokaryote agent replaces the action of a growth factor to which the targeted cells normally respond. Interaction with a mycoplasmal membrane component(s) on the cell surface transmitted a signal(s) that had potent antiapoptotic effects and rescued 32D cells from cell cycle arrest. This mycoplasma-mediated growth-signaling pathway was apparently different from that of IL-3 in supporting continued growth of 32D cells. Activation of previously inactive NF-κB in 32D cells by the mycoplasmas appeared to be closely associated with their ability to rescue these cells from apoptosis in culture deprived of IL-3. Infections by mycoplasmas that markedly enhanced AP-1 activity but did not activate NF-κB failed to support growth of 32D cells in IL-3-free culture. Moreover, blocking activation of NF-κB by an inhibitor led to prominent cell death of 32D cells that were otherwise induced to grow by mycoplasmas. This finding is consistent with recent reports from several laboratories showing that NF-κB appears to mediate survival signals that protect cells from dying of apoptosis (2, 31, 33).
It became clear that rapid activation of NF-κB and induction of continued cell growth in 32D cells did not require infections by live mycoplasmas. Heat-killed mycoplasmas or mycoplasmal membrane preparation LAMPs could effectively activate NF-κB and induce continued growth of 32D cells in IL-3 free culture. The active component that triggered the signaling of NF-κB activation is most likely the lipid moiety of mycoplasmal membrane lipopeptides (8, 9, 25, 26). By continually supplying heat-killed mycoplasmas, we could maintain 32D cells in culture without IL-3 supplement for at least 2 months. Growth of these 32D cells remained dependent on the presence of a mycoplasma-mediated growth signaling(s) for survival. They quickly began to die of apoptosis when transferred to culture without supplement of IL-3 or heat-killed mycoplasmas.
In comparison, infections by live mycoplasmas not only rescued 32D cells from cell cycle arrest and supported continued cell growth in IL-3-free culture but also induced malignant transformation of 32D cells. However, similar to our earlier finding for C3H cells (30), the mycoplasma-mediated cell transformation process would take time and involve a period of latency. In this study, it took more than 4 to 5 weeks of chronic infection by M. fermentans or M. penetrans before some of the 32D cells with autonomous growth ability began to emerge. Initially, the 32D cells that had acquired the unregulated growth property and no longer required the support from IL-3 or mycoplasmas constituted apparently only a small population. Further prolonged infection by the live mycoplasmas in culture could produce more cells with the malignant ability of autonomous growth. Antibiotic eradication of mycoplasmas from these IL-3-free cultures infected by the mycoplasmas effectively selected for a population(s) or clones of transformed cells that were capable of continued growth without the support of growth signaling from either IL-3 or mycoplasmas (Fig. 6). These transformed 32D cells were highly tumorigenic when injected into animals. It is not known whether infections by all mycoplasmas capable of supporting continued growth of 32D cells in IL-3-free culture could subsequently transform 32D cells.
Interestingly, the transformed 32D cells that obtained the malignant property of unregulated growth induced by chronic mycoplasmal infection showed no evidence of NF-κB activation found in the early stage of mycoplasmal infection (Table 1). Infection by the mycoplasmas for 4 to 5 weeks apparently had irreversibly activated an oncogenic process, not involving NF-κB, that was constantly signaling growth to the 32D cells. Previous studies by others and by us showed chronic mycoplasmal infections produced chromosomal instability in mammalian cells (24, 30). Karyotypic analysis revealed development of unregulated cell growth ability and tumorigenic properties of 32D cells following infection by either M. fermentans or M. penetrans was associated with chromosomal changes and trisomy 19. Infection by M. penetrans appeared to be particularly effective in causing trisomy 19 and hence malignant transformation of 32D cells in IL-3-free culture. A great majority (95%) of cells were found to have trisomy 19 after 4 to 5 weeks of M. penetrans infection, without clonal selection by ciprofloxacin treatment (Table 2). Growth of these cells had apparently become unregulated, since they formed tumors in two of three injected nude mice.
Extensive cell cycle studies in recent years helped the development of important concepts of checkpoints and rate-limiting steps in the cell cycle (22). Because cancer is largely a somatic genetic disease, loss of the ability to effectively coordinate the progression of cell cycle events when damage prejudicial to cell division has occurred often leads to development of malignancy. Although the molecular mechanism of mycoplasma-mediated oncogenesis is still not clear, our present study showed infections by M. fermentans and M. penetrans caused infidelity of genomic transmission in cell division. There were apparent aberrations in the machinery of chromosomal segregation as well as cell cycle checkpoint controls in mycoplasma-infected 32D cells. Following a few weeks of infections by either of the mycoplasmas, 32D cells with chromosomal changes and trisomy 19 began to appear and gradually accumulated in culture. It is not known how obtaining additional chromosome 19 was associated with better autonomous growth ability of 32D cells in culture without IL-3 support. Study of overexpression or constitutive activation of various oncogenes in the mycoplasma-transformed 32D cells is in progress.
In a previous study using monolayer culture of murine C3H embryonic cells, we showed that mycoplasmas induced malignant transformation of mammalian cells through chronic persistent infection. The process appeared to be a gradual progression with multiple distinct stages characterized by reversibility or irreversibility of transformation (30). In the present model system, using suspension culture of murine hematopoietic cells, we elucidated two separable avenues of mycoplasmal effects on mammalian cells. The first avenue rapidly activated an antiapoptotic cascade of events exerted through membrane component(s), rescued cells from cell cycle arrest, and induced continued cell growth. The mitogenic effect of mycoplasma-mediated signaling was reversible. The second avenue of effect required infection by live organisms with a latent period, later causing infidelity of genomic transmission in cell division resulting in malignant cell transformation. The effect of the first avenue to induce continued proliferation of cells that would otherwise undergo apoptosis was a prerequisite for subsequent induction of irreversible cell transformation associated with chromosomal changes. Since the mycoplasma-mediated transformation did not require mycoplasmal DNA integration into the host cells (37) or continued presence of the microbes once the genetic changes that lead to unregulated cell growth occurred, it presented a unique form of “hit and run” process.
Finding that human mycoplasmas can render growth factor independence and induce malignant transformation of IL-3-dependent hematopoietic cells in vitro has not only significance in general biology but also great direct clinical implications. In addition to further understanding the molecular mechanisms of mycoplasma-mediated pathway(s) for growth factor independence and oncogenesis, some important questions need to be answered. Can mycoplasmal infections also induce malignant transformation of human blood cells in culture? Is it possible to develop an animal model for mycoplasma-induced malignancies? These important studies will prove to be highly challenging due to the chronic nature of mycoplasma infections and the long latency in oncogenesis in vivo. However, resolving these pieces of the puzzle could fundamentally change the way in which we view many human malignancies.
ACKNOWLEDGMENTS
We thank Douglas J. Wear for critical reading of the manuscript. We also thank Mark Tsai for assistance with Storm860 scanner and Susan Ditty for help with preparation of the manuscript.
This work was supported in part by the American Registry of Pathology.
REFERENCES
- 1.Barile M F, Bodey G P, Snyder J, Riggs D B, Grabowski M W. Isolation of Mycoplasma orale from leukemic bone marrow and blood by direct culture. J Natl Cancer Inst. 1966;36:155–159. [Google Scholar]
- 2.Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 3.Blaser M J, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Investig. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cover T L, Blaser M J. Helicobacter pylori: a bacterial cause of gastritis, peptic ulcer disease, and gastric cancer. ASM News. 1995;61:21–26. [Google Scholar]
- 5.Dawson M S, Hayes M M, Wang R Y, Armstrong D, Kundsin R B, Lo S C. Detection and isolation of Mycoplasma fermentans from urine of human immunodeficiency virus type 1-infected patients. Arch Pathol Lab Med. 1993;117:511–514. [PubMed] [Google Scholar]
- 6.Ehlers S, Mielke M E, Blankenstein T, Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992;149:3016–3022. [PubMed] [Google Scholar]
- 7.Fallon R J, Grist N R, Inman D R, Lemcke R M, Negroni G, Woods D A. Further studies of agents isolated from tissue cultures inoculated with human leukemic bone-marrow. Br Med J. 1965;5458:388–391. [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S-H, Lo S-C. Lipid extract of Mycoplasma penetrans proteinase K-digested lipid-associated membrane proteins rapidly activated NF-κB and activator protein 1. Infect Immun. 1999;67:2951–2956. doi: 10.1128/iai.67.6.2951-2956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia J, Lemercier B, Roman-Roman S, Rawadi G. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kappaB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:34391–34398. doi: 10.1074/jbc.273.51.34391. [DOI] [PubMed] [Google Scholar]
- 10.Grace J R J, Horoszewicz J S, Stim T B, Mirand E A, James C. Mycoplasmas (PPLO) and human leukemia and lymphoma. Cancer. 1965;18:1369–1376. doi: 10.1002/1097-0142(196510)18:10<1369::aid-cncr2820181022>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Grau O, Kovacic R, Griffais R, Montagnier L. Development of a selective and sensitive polymerase chain reaction assay for the detection of Mycoplasma pirum. FEMS Microbiol Lett. 1993;106:327–333. doi: 10.1111/j.1574-6968.1993.tb05984.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenberger J S, Eckner R J, Sakakeeny M, Marks P, Reid D, Nabel G, Hapel A, Ihle J N, Humphries K C. Interleukin 3-dependent hematopoietic progenitor cell lines. Fed Proc. 1983;42:2762–2771. [PubMed] [Google Scholar]
- 13.Greenberger J S, Sakakeeny M A, Humphries R K, Eaves C J, Eckner R J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci USA. 1983;80:2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayflick L, Koprowski H. Direct agar isolation of mycoplasmas from human leukaemic bone marrow. Nature (London) 1965;205:713–714. doi: 10.1038/205713b0. [DOI] [PubMed] [Google Scholar]
- 15.Kruger A, Anderson S M. The v-src oncogene blocks the differentiation of a murine myeloid progenitor cell line and induces a tumorigenic phenotype. Oncogene. 1991;6:245–256. [PubMed] [Google Scholar]
- 16.Lo S C, Hayes M M, Wang R Y, Pierce P F, Kotani H, Shih J W. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet. 1991;338:1415–1418. doi: 10.1016/0140-6736(91)92721-d. [DOI] [PubMed] [Google Scholar]
- 17.Lo S C, Shih J W, Newton III P B, Wong D M, Hayes M M, Benish J R, Wear D J, Wang R Y. Virus-like infectious agent (VLIA) is a novel pathogenic mycoplasma: Mycoplasma incognitus. Am J Trop Med Hyg. 1989;41:586–600. doi: 10.4269/ajtmh.1989.41.586. [DOI] [PubMed] [Google Scholar]
- 18.Loo V G, Richardson S, Quinn P. Isolation of Mycoplasma pneumoniae from pleural fluid. Diagn Microbiol Infect Dis. 1991;14:443–445. doi: 10.1016/0732-8893(91)90071-m. [DOI] [PubMed] [Google Scholar]
- 19.Murphy W H, Bullis C, Dabich L, Heyn R, Zarafonetis C J. Isolation of mycoplasma from leukemic and nonleukemic patients. J Natl Cancer Inst. 1970;45:243–251. [PubMed] [Google Scholar]
- 20.Murphy W H, Bullis C, Ertel I J, Zarafonetis C J. Mycoplasma studies of human leukemia. Ann N Y Acad Sci. 1967;143:544–556. doi: 10.1111/j.1749-6632.1967.tb27701.x. [DOI] [PubMed] [Google Scholar]
- 21.Murphy W H, Furtado D, Plata E. Possible association between leukemia in children and virus-like agents. JAMA. 1965;191:110–115. doi: 10.1001/jama.1965.03080020038012. [DOI] [PubMed] [Google Scholar]
- 22.Nurse P, Masui Y, Hartwell L. Understanding the cell cycle. Nat Med. 1998;4:1103–1106. doi: 10.1038/2594. [DOI] [PubMed] [Google Scholar]
- 23.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 24.Paton G R, Jacobs J P, Perkins F T. Chromosome changes in human diploid-cell cultures infected with Mycoplasma. Nature. 1965;207:43–43. doi: 10.1038/207043a0. [DOI] [PubMed] [Google Scholar]
- 25.Rawadi G, Garcia J, Lemercier B, Roman-Roman S. Signal transduction pathways involved in the activation of NF-kappaB, AP-1, and c-fos by Mycoplasma fermentans membrane lipoproteins in macrophages. J Immunol. 1999;162:2193–2203. [PubMed] [Google Scholar]
- 26.Sacht G, Märten A, Deiters U, Süßmuth R, Jung G, Wingender E, Mulhradt P F. Activation of nuclear factor-κB in macrophages by mycoplasmal lipopeptides. Eur J Immunol. 1998;28:4207–4212. doi: 10.1002/(SICI)1521-4141(199812)28:12<4207::AID-IMMU4207>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simecka J W, Davis J K, Davidson M K, Ross S E, Stadtlander C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Basement J B, Finch L R, Maniloff J, McElhaney R N, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 391–416. [Google Scholar]
- 29.Taylor-Robinson D. Genital mycoplasma infections. Clin Lab Med. 1989;9:501–523. [PubMed] [Google Scholar]
- 30.Tsai S, Wear D J, Shih J W, Lo S C. Mycoplasmas and oncogenesis: persistent infection and multistage malignant transformation. Proc Natl Acad Sci USA. 1995;92:10197–10201. doi: 10.1073/pnas.92.22.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 32.Vincenti M P, Burrell T A, Taffet S M. Regulation of NF-kappa B activity in murine macrophages: effect of bacterial lipopolysaccharide and phorbol ester. J Cell Physiol. 1992;150:204–213. doi: 10.1002/jcp.1041500127. [DOI] [PubMed] [Google Scholar]
- 33.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 34.Wang R Y-H, Lo S-C. PCR detection of Mycoplasma fermentans infection in blood and urine. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology, principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 511–516. [Google Scholar]
- 35.Wurster D H. Mouse chromosomes identified by trypsin-Giemsa (T-G) banding. Cytogenetics. 1972;11:379–387. doi: 10.1159/000130204. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Shih J W, Wear D J, Tsai S, Lo S C. High-level expression of H-ras and c-myc oncogenes in mycoplasma-mediated malignant cell transformation. Proc Soc Exp Biol Med. 1997;214:359–366. doi: 10.3181/00379727-214-44104. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Tsai S, Shih J W-K, Wear D J, Lo S-C. Absence of mycoplasmal gene in malignant mammalian cells transformed by chronic persistent infection of mycoplasmas. Proc Soc Exp Biol Med. 1998;218:82–88. doi: 10.3181/00379727-218-44271. [DOI] [PubMed] [Google Scholar]