Abstract
Objectives
Most electronically delivered lifestyle interventions are labor intensive, requiring logging onto websites and manually recording activity and diet. Cumbersome technology and lack of a human coach may have contributed to the limitations of prior interventions. In response, the current program of research created a comprehensive electronically delivered lifestyle intervention using a user‐friendly, interactive, smartphone app‐based model, and evaluated it in a randomized controlled trial.
Methods
Twenty‐eight adults, body mass index 25–42 kg/m2, with smartphones and sedentary jobs, were randomized to the intervention, along with conventional outpatient weight‐management visits every 3 months, or to a wait‐listed control group that received only weight‐management visits. The intervention included wearable activity trackers, smartscales, food photography logs, physician‐driven app‐based behavioral coaching, and peer support via the app. The prespecified primary outcome was a comparison of change in weight in kilograms, in the intervention versus control group at 6 months.
Results
At 6 months, the intervention group experienced a statistically significant weight change of −7.16 ± 1.78 kg (mean ± SE, 95% CI −11.05 to −3.26, p < 0.01), which differed from the weight change in controls by −4.16 ± 2.01 kg (95% CI −8.29 to −0.02, p < 0.05, prespecified primary outcome). Weight change in the control group was −3.00 ± 1.05 kg (95% CI −5.27 to −0.73, p < 0.05). Waist circumference and hemoglobin A1c significantly improved (intervention vs. control: p < 0.01, p < 0.05, respectively, prespecified secondary outcomes). Weight change in the intervention group correlated with numbers of food photographs participants shared (rho = −0.86, p < 0.01), numbers of their text messages (rho = −0.80, p < 0.01), number of times and days each participant stepped on the smartscale (rho = −0.73, p < 0.01; rho = −0.608, p < 0.05, respectively), and mean daily step counts (rho = −0.55, p < 0.05).
Conclusion
This app‐based electronically delivered lifestyle intervention produced statistically significant, clinically meaningful weight loss and improved metabolic health. Engagement with the intervention correlated strongly with weight loss. Given the limited sample size, larger and longer studies of this intervention are needed.
Keywords: lifestyle intervention, smartphone app, weight loss
1. INTRODUCTION
There is an urgent need to create realistic interventions capable of achieving sustained weight loss to reduce the heightened cardiovascular and other health risks associated with increasing weight.1 , 2 The problem has become more acute as increases in mean daily energy intake have occurred in tandem with reductions in energy expenditure at work, with the widespread transition to an information (digital) economy.2 , 3
Lifestyle interventions using conventional in‐person approaches produce weight loss and improve health, but can be difficult to implement and sustain.4 , 5 High‐intensity comprehensive weight‐loss interventions, which include self‐monitoring and extensive personalized feedback, are effective at producing weight loss of up to 5 kg at 6–12 months, but these interventions are too costly in time and personnel to be implemented in typical resource‐limited primary‐care practices. On the other hand, low‐ to moderate‐intensity lifestyle interventions for weight loss are more affordable but have not been shown to be effective.6 Along similar lines, a review of randomized controlled trials (RCTs) found that behavioral weight‐loss interventions by primary‐care physicians have achieved only very small declines in body weight over 12 months in patients with obesity or who are overweight.7, 8 In contrast, clinically meaningful weight loss typically requires high‐intensity comprehensive lifestyle interventions provided in individual or group sessions by a trained interventionist, with ≥14 face‐to‐face sessions in 6 months.6 A comprehensive lifestyle intervention that combines dietary, physical activity, and behavioral components is key to achieve and maintain weight loss.6 While conventional high‐intensity comprehensive approaches can produce clinically meaningful weight losses of 7%–9%, access and adherence to such programs are limited by barriers such as program availability, patient proximity, transportation concerns, and time constraints on healthcare providers and patients.3 , 9 In addition, few studies of lifestyle interventions have been successful at producing lasting weight loss, owing to poor long‐term compliance, with typical annual attrition rates of over 30%.10, 11, 12
There is a growing interest in the use of electronically delivered lifestyle interventions for weight loss (e.g., by Internet or telephone). Electronically delivered programs may reduce many of the barriers, listed above, that are characteristic of conventional lifestyle interventions.9 Unfortunately, trial data so far of weight‐loss interventions that involved diet and activity tracking via apps have shown mixed results, with some studies of technology‐based interventions showing no statistically significant difference in weight loss between the study groups13 , 14 or were less effective than expected, that is, showing paradoxically less weight loss in the group receiving the technology‐based intervention or using wearable activity trackers.15 Some emerging evidence supports the efficacy of electronically delivered lifestyle interventions, provided that they incorporate personalized feedback by a trained coach,6 , 9 , 16 a feature that adds expense and time commitment, that is, two key barriers seen with conventional high‐intensity interventions.9 Even so, only a few Internet‐based interventions to date have produced clinically significant weight losses of 5% or greater.9 , 16 For example, one short‐term web‐based weight‐loss intervention was shown to be effective over 12 weeks in primary‐care patients with obesity.16 Evidence from an RCT showed that 38.2% of participants in an intervention group that received exclusively remote support achieved 5% weight loss or greater after 24 months, compared with 41.4% of participants who received in‐person along with remote support, suggesting that electronically delivered lifestyle interventions can be effective tools for weight loss.17 Nevertheless, electronically delivered weight‐loss interventions have generally produced less weight loss than conventional high‐intensity interventions involving face‐to‐face counseling.6 , 9 To date, most electronically delivered lifestyle interventions have relied primarily on labor‐intensive features, such as email, logging onto websites for educational materials, and/or manually recording physical activity and dietary data.18, 19, 20
The current study was based on the hypothesis that small benefits or adverse effects in previous studies of electronically delivered lifestyle interventions for weight loss could be attributed, at least in part, to the cumbersome nature of the applications (apps) and wearables from even just a few years ago, as well as the absence of regular physician support through the apps. Thus, a key objective was to create a comprehensive electronically delivered lifestyle intervention that could be implemented entirely on a user‐friendly, interactive smartphone app‐based platform, to improve adherence, sustainability, and efficacy. The United States has a high smartphone penetration rate, with 69.6% of the population using a smartphone in 2018.21 This figure represents a significant increase from 2012, when the country's penetration rate stood at just under 40%.21 Modern smartphone apps can be designed, chosen, or modified to avoid tedious features, such as logging on to websites and manual tracking of weight, activity, and caloric intake.22 Here, the user‐friendly, interactive, smartphone app‐based lifestyle intervention was designed to incorporate the three core aspects of a comprehensive lifestyle intervention (diet, physical activity, and behavioral strategies)6 and allowed for convenient monitoring with limited burden on the participants and the professional coach. Accordingly, the intervention included easy access to smartphone app‐based professional coaching (physician support) for feedback, peer‐to‐peer support (group chat in a social network on the app), along with automated quantitative tracking of weight (smartscales) and physical activity (wearable three‐axis accelerometers), as well as a food photography log (Smart Food Diary™) to monitor diet. The app‐based lifestyle intervention was added to conventional outpatient weight management visits that occurred every 3 months. An endpoint at 6 months was chosen because noncompliance is often evident by then.23 Objective parameters of retention and engagement with the intervention were assessed as well. The experimental objective of this study was to determine the effectiveness of the app‐based lifestyle intervention in adults with obesity or who are overweight in a 6‐month prospective randomized controlled clinical trial. Thus, the trial design tested if this convenient, interactive smartphone app‐based weight‐loss intervention would produce clinically meaningful improvements in weight and metabolic health, with high user retention and engagement.
2. METHODS
2.1. Study design
The prospective RCT was conducted at Temple University Hospital, a tertiary‐care academic medical center in Philadelphia, Pennsylvania, United States, from November 2016 to December 2017. Informed consent was obtained from all participants. There was no monetary compensation for study participation, nor could participants randomized to the intervention group keep the smartscales or wearable accelerometers after completing the 6‐month program. Study sponsors were the Temple University Department of Medicine and the Obesity Treatment Foundation. No commercial entity donated any funds, goods, or services to this research. The trial, including prespecified primary and secondary outcomes listed below, was registered at ClinicalTrials.gov (NCT02742662). The study was approved by the Temple Institutional Review Board.
2.2. Study participants
Participants were recruited by flyer advertisements and email listservs at Temple University Hospital and its affiliated hospital sites. Inclusion and exclusion criteria are shown in Table S1. In brief, key inclusion criteria were age 18–65 years, body mass index (BMI) 25–42 kg/m2, employment in a sedentary job as assessed below, ownership and daily use of a smartphone before enrollment, ability to engage in moderate‐intensity exercise, willingness to track and share data on food and physical activity with other participants in the intervention group, and ability to comply with study requirements, such as attending study visits. Key exclusion criteria were diabetes mellitus, psychiatric diagnoses such as anxiety disorders or clinical depression under treatment, eating disorders, use of weight‐loss medications, and other serious comorbid conditions listed in Table S1. At the time of screening, details on the nature of each participant's job, including the number of hours spent seated during the day, were obtained. Participants' jobs were considered sedentary if they involved sitting for a majority of the workday. Sedentary jobs are a major contributor to obesity,2 , 3 and this evaluation was aimed at keeping the baseline physical activity levels comparable among study participants. Comorbid conditions and psychiatric diagnoses were assessed based on self‐reported history. Participants underwent preliminary screening over the telephone followed by an in‐person screening visit that included a physical exam, a comprehensive metabolic panel, hemoglobin A1c level (HgbA1c), and a thyroid‐stimulating hormone level. Participants underwent an overnight dexamethasone suppression test if they had signs suggestive of Cushing's syndrome such as hirsutism or striae. A total of 247 participants were screened for eligibility, of whom 186 did not meet inclusion/exclusion criteria. The most common reasons for exclusion were BMI out of range in 22.2%, psychiatric disease in 11.5%, musculoskeletal problems in 9.7%, and diabetes mellitus in 6.4%.
2.3. Study intervention
The new electronically delivered lifestyle intervention is a smartphone app‐based monitoring and coaching system designed to enhance behavior modification with the goal of increasing energy expenditure and reducing energy intake. The intervention was designed to objectively track physical activity, weight, and diet as automatically and seamlessly as possible. The informed consent form explicitly stated that data on physical activity and diet will be viewable within the apps by the professional coach and by study peers. Consistent with the goal of encouraging social support, daily weights were specifically excluded from the data shared among the participants. Daily weights were shared only with the coach.
The app‐based electronically delivered lifestyle intervention used new, small, convenient wearable devices and a networking system to connect users with each other and with the professional coach. The professional coach throughout the study was an Obesity Medicine Board‐certified Endocrinologist (CLV). Subjects randomized to the intervention group received two “smart” devices—a wrist‐worn three‐axis accelerometer to monitor physical activity (Fitbit Charge Heart Rate™) and a smartscale (Fitbit Aria™) at the baseline visit, which was 40–50 min in duration. At this visit, participants in the intervention group were logged onto three apps: the Fitbit™ app that links to the activity tracker and smartscale, and commercially available messaging and photo‐sharing apps. Research staff gave participants basic instructions on how to use the devices and apps. Daily weights were tracked from the smartscales, which were Wi‐Fi enabled and synced to automatically send weights to each individual participant's smartphone for self‐monitoring and to the study database for monitoring by the professional coach. Participants in the intervention group were instructed to step on the smartscale every morning, without clothing, right after arising from bed and while still fasted. The app was programmed to automatically send out a reminder to each user to motivate him or her to meet the target for physical activity for that day, based on continuous activity data obtained from the wearable activity tracker. Participants in the intervention group were instructed to wear the activity tracker as close as possible to 24 h per day, 7 days per week, and any day with <500 recorded steps indicated a tracking problem. Participants were also instructed to keep the device charged and received automated private reminders via the app to charge their trackers if the battery was low. Research staff monitoring the study database would send additional reminders to charge trackers if needed.
Early lifestyle modification programs prescribed low‐fat, high‐carbohydrate diets.3 , 24 Research over the past 15 years, however, has shown that a variety of dietary approaches, including low‐carbohydrate high‐protein, Mediterranean‐style, and low‐glycemic‐load diets can aid weight loss, if they facilitate the achievement of caloric deficits.3 , 6 Therefore, in the current study, each participant was allowed to choose a diet plan from a selection of low glycemic index, low fat, low carbohydrate, or Mediterranean style. Diet plans were formulated using academic resources and guidelines and were tailored to be shared in an online format via the app.6 , 7 An individualized recommended caloric intake was calculated on the first day of the intervention, with the goal of producing 5% weight loss in the next 3 months by using each participant's baseline and projected increase in physical activity and each participant's baseline data (age, sex, height, and weight).25 As soon as a participant met the initial 5% weight‐loss target, he or she was given a new goal of additional 5% weight loss. To conveniently track intake, participants were instructed to enable the photo‐sharing app to allow them to take and share photographs of their meals using their smartphones as an easy, quick, qualitative alternative to cumbersome, inaccurate, traditional dietary logs.2 , 26 Food photographs were shared with all the other participants in the intervention group and with the professional coach, forming the Smart Food Diary™. The Smart Food Diary™ assisted with behavior modification by enabling the individual, the professional coach, and other participants to observe food intake of all participants in a visually appealing, organized manner, and allowed the coach to provide positive reinforcement with “stars” on healthy meals. The hypothesis was that sharing photographs of meals with other participants and the coach would enhance self‐awareness of dietary intake, feelings of accountability, and motivation.
Peer social networking occurred via the smartphone app that connected participants in the intervention group with each other. Participants could observe each other's physical activity levels and food photographs. Thus, participants had access to an online peer‐based social support network as they progressed through the study. Remote professional coaching by the physician consisted of feedback delivered via group and private messaging using shared activity data, shared food photography logs, daily weights captured from smartscales, and a virtual reward system for behavior change, SmartReward™, that consisted of competitions on levels of physical activity and dietary adherence, with emoticons as well as convenient icons to signal approval with a single click, without having to type anything (“likes”). During the course of the 6‐month intervention, the professional coach sent out tailored text messages that provided advice on strategies for behavior modification, such as stimulus control, goal setting, and problem solving via group messaging on the app. Coaching messages were sent out to the group based on physician judgment and did not involve a preset protocol for frequency of delivery. Participants entered competitions and received virtual rewards via the app in the form of “trophy” emoticons for maintaining physical activity targets and “stars” for food photographs that indicated dietary adherence. Thus, the intervention was designed to encourage participants to stay well connected with the professional coach and with each other during the study.
2.4. Study procedures
Participants who met the eligibility criteria and consented were assigned by block randomization, block size of two, using the Randomizer for Clinical Trial Medsharing software, to receive the app‐based lifestyle intervention, or to a wait‐listed control group. The control group was wait‐listed to receive the smart lifestyle intervention after the initial 6‐month period, but only if the trial met its prespecified primary outcome. Both the intervention and the control group received conventional outpatient weight‐management visits at baseline and at 3 and 6 months. The outpatient weight‐management visits for both groups were performed in the Section of Endocrinology, Diabetes, & Metabolism by the same physician who performed the coaching. The visits included a diet plan, goals for physical activity and weight, and a standard, but minimal, amount of behavioral feedback, all of which were printed on the after‐visit summary for the patient, following routine practice in our weight‐loss clinic.6 All participants had weight, blood pressure, and waist circumference measured at baseline and at the 3‐ and 6‐month visits. In our clinic, the weight was measured by a medical assistant, who was not purposefully blinded to the study condition, using a single designated research smartscale (Fitbit Aria™), which was the same type of scale that participants in the intervention group used to monitor daily weights. Waist circumference was measured in a standing position, midway between the lower rib margin and the iliac crest. Laboratory tests were performed at baseline and at 6 months. Insulin resistance for handling glucose was calculated using the homeostatic model assessment (HOMA‐IR).
2.5. Prespecified primary and secondary outcomes
Prespecified primary outcome: comparison of the changes in body weight in kilograms, from baseline to 6 months, in the intervention group versus the control group. Prespecified secondary outcomes: comparison of the changes in waist circumference, systolic and diastolic blood pressure, HOMA‐IR, HgbA1c, and plasma concentrations of triglycerides, from baseline to 6 months, in the intervention group versus the control group; and comparison of the changes in body weight, from baseline to 3 months, in the intervention group versus the control group.
2.6. Statistical analyses
Sample size to adequately power the clinical trial was estimated using published data from a previous 6‐month technology‐based behavioral weight‐loss interventional study that included physical activity monitoring and a stated goal of ≥5% weight loss.27 Calculations based on this prior work indicated that 1 standard deviation (SD) for weight loss at 6 months corresponds to approximately 5.96 kg of body weight, which would be a clinically meaningful difference. The original design of the current study had 80% power to detect 1 SD difference from zero in the prespecified primary outcome with 16 subjects in each group, with a two‐sided significance level of 0.05. Once the actual sample sizes (n = 13 and n = 15) were finalized, revised calculations indicated that the study had 75.1% power to detect an effect size of 1 SD in the primary outcome between the two groups.
Data are given as mean ± SE for continuous, normally distributed parameters. Baseline characteristics and all primary and secondary outcomes that involved comparisons between the two study groups were analyzed by the unpaired Student's t‐test for continuous, normally distributed parameters and by the chi‐squared test for categorical parameters. Within each group, changes in continuous variables from baseline to 3 or 6 months were analyzed by the paired Student's t‐test. Spearman's rank correlation coefficients were calculated to test relations of weight change with quantitative parameters that indicated several different aspects of participant engagement (numbers of food photographs, numbers of text messages in the group chat, two assessments of smartscale use, and mean daily step counts). All statistical tests were two‐sided with p < 0.05 considered significant. A modification of the standard intention‐to‐treat approach, described immediately below, was used to compare weight change at 6 months between the two study groups.
After completing our screening procedures, 31 participants in total were randomized, 15 to the intervention, and 16 to the wait‐listed control group. After randomization, two participants assigned to the intervention arm were not reachable for the study and thus did not have any baseline measurements and could not begin any intervention. An additional participant from the control group developed clinical depression requiring medication and was excluded before starting the study. Therefore, the analysis included all participants who were seen for the baseline visit (n = 28 total). An additional participant from the control group was lost to follow‐up after the baseline visit but was included in the analysis as last observation carried forward. Thus, statistical analyses were performed on all 28 randomized participants with baseline measurements for the prespecified primary outcome of difference in weight change at 6 months between the two study groups, and the prespecified secondary outcomes of differences in changes in waist circumference and in systolic and diastolic blood pressure, between the two study groups. For the prespecified secondary outcomes of differences in changes in HOMA‐IR, HgbA1c, and plasma concentrations of triglycerides, all of which involved laboratory analyses, a per‐protocol analysis was performed by including participants who had blood drawn at baseline and within 7 days of trial completion 6 months later. This study design placed a premium on precise timing of the 6‐month laboratory values, because the wait‐listed controls began the app‐based electronically delivered lifestyle intervention at the 6‐month mark, and many of those secondary parameters are known to improve within just days of increased activity and decreased caloric intake. Study retention and several different aspects of engagement, listed above, were measured to test feasibility and acceptance of the intervention.
3. RESULTS
3.1. Baseline characteristics of study participants
Baseline characteristics are shown in Table 1. In the intervention group, participants were 85% female, age 40.15 ± 3.72 years (means ± SE, n = 13), initial weight 94.13 ± 3.4 kg, and BMI 34.46 ± 1.24 kg/m2. In the control group, participants were 87% female, age 45.93 ± 3.29 years, initial weight 92.25 ± 4.37 kg, and BMI 34.35 ± 1.47 kg/m2 (n = 15). Two participants in each group met criteria for the metabolic syndrome. There were no significant differences in body weight, gender or ethnic distributions, average age, or any other baseline clinical characteristics between the intervention and control groups (Table 1).
TABLE 1.
Baseline characteristics for the intervention and control groups
| Baseline | Intervention (n = 13) | Control (n = 15) | All subjects (n = 28) | Intervention versus Control (95% CI) | p‐Value |
|---|---|---|---|---|---|
| Age | 40.15 ± 3.72 (32.03–48.27) | 45.93 ± 3.29 (38.87–52.99) | 43.25 ± 2.48 (38.14–52.99) | −5.77 ± 4.95 (−15.95–43.9) | 0.25 |
| Gender | |||||
| Male | 2 (15%) | 2 (13%) | 4 (14%) | 0.88 | |
| Female | 11 (85%) | 13 (87%) | 24 (86%) | ||
| Race/ethnicity | |||||
| African American | 4 (30%) | 5 (33%) | 9 (32%) | 0.55 | |
| Caucasian | 7 (54%) | 5 (33%) | 12 (43%) | ||
| Hispanic | 1 (8%) | 4 (27%) | 5 (18%) | ||
| Asian origin | 1 (8%) | 1 (7%) | 2 (7%) | ||
| Weight (kg) | 94.13 ± 3.40 (86.71–101.56) | 92.25 ± 4.37 (82.87–101.62) | 93.12 ± 2.78 (87.41–98.83) | 1.88 ± 5.67 (−9.77–13.54) | 0.74 |
| BMI (kg/m2) | 34.46 ± 1.24 (31.74–37.19) | 34.35 ± 1.47 (31.19–37.52) | 34.40 ± 0.96 (32.43–36.38) | 0.11 ± 1.96 (−3.93–4.15) | 0.96 |
| WC (cm) | 103.79 ± 2.60 (98.06–109.42) | 101.93 ± 2.21 (97.18–106.21 | 102.77 ± 8.85 (99.34–106.69) | 1.81 ± 3.40 (−5.17–8.80) | 0.60 |
| SBP (mm Hg) | 123.07 ± 3.20 (116.10–130.05) | 122.87 ± 3.91 (114.46–131.27) | 122.96 ± 2.52 (117.78–128.14) | 0.21 ± 5.16 (−10.40–10.82) | 0.97 |
| DBP (mm Hg) | 80.07 ± 2.77 (75.11–85.03) | 77.0 ± 2.18 (72.31–81.68) | 78.42 ± 1.57 (75.19–81.65) | 3.07 ± 3.16 (−3.42–9.57) | 0.34 |
| HOMA‐IR | 2.55 ± 0.42 (1.57–3.53) | 2.61 ± 0.50 (1.42–3.80) | 2.58 ± 0.31 (1.91–3.25) | −0.05 ± 0.65 (−1.45–1.33) | 0.93 |
| HgbA1c (%) | 5.54 ± 0.08 (5.35–5.72) | 5.53 ± 0.08 (5.34–5.73) | 5.54 ± 0.05 (5.42–5.65) | 0.006 ± 0.11 (−0.23–0.25) | 0.95 |
| Triglycerides (mg/dl) | 90.66 ± 10.51 (66.42–114.91) | 100.50 ± 16.61 (61.21–139.78) | 95.29 ± 9.35 (75.45–115.13) | −9.83 ± 19.19 (−50.75–31.08) | 0.62 |
Note: Shown are means ± SEMs (95% CI). The unpaired two‐tailed t‐test was used to compare continuous, normally distributed parameters between the study groups. Categorical parameters (sex, race/ethnicity) were compared using the χ2 test. P < 0.05 was considered significant. Two participants in each study group met criteria for the metabolic syndrome.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HgbA1c, hemoglobin A1c; SBP, systolic blood pressure; WC; waist circumference.
3.2. Prespecified primary and secondary outcomes with exploratory analyses
From baseline to 6 months, the intervention group underwent a clinically and statistically significant change in body weight of −7.16 ± 1.78 kg (95% CI −11.05 to −3.26, p < 0.01), which differed from the weight change in the control group by −4.16 ± 2.01 kg (95% CI −8.29 to −0.02, p < 0.05, prespecified primary outcome) (Figures 1 and 2). To explore these results, additional analyses of body weight were performed that were not prespecified in our study design. The percentage change in body weight in the intervention group was −7.92% ± 2.15% (95% CI −12.6 to −3.23, p < 0.01). The control group showed a statistically significant, but clinically inadequate, weight change from baseline to 6 months of −3.00 ± 1.05 kg (95% CI −5.27 to −0.73, p < 0.05) and a % weight change of −3.15% ± 1.20% (95% CI −5.73 to −0.56 p < 0.05) (Figure 2 and Table 2). Individual changes in body weight from baseline to 6 months for each participant in the two groups are shown in Figure 3. Of note, 8 participants out of 13 (61.5%) in the intervention group lost ≥5% of their body weight (vertical arrows in Figure 3A), compared with only four controls out of 15 (26.7%; vertical arrows in Figure 3B). Achievement of ≥10% weight loss showed a similar pattern: five participants in the intervention group (38.5%) met this threshold, versus only one of the controls (6.7%).
FIGURE 1.
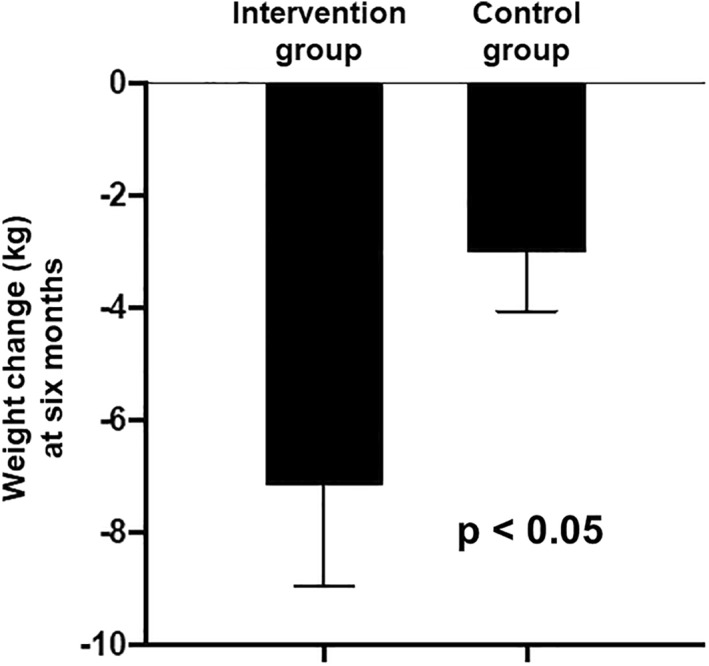
Prespecified primary outcome: comparison of the changes in body weight in kg, from baseline to 6 months, in the intervention group versus the control group. Values are mean ± SE. n = 13 intervention, n = 15 control, p = 0.0488 by the unpaired two‐tailed t‐test
FIGURE 2.
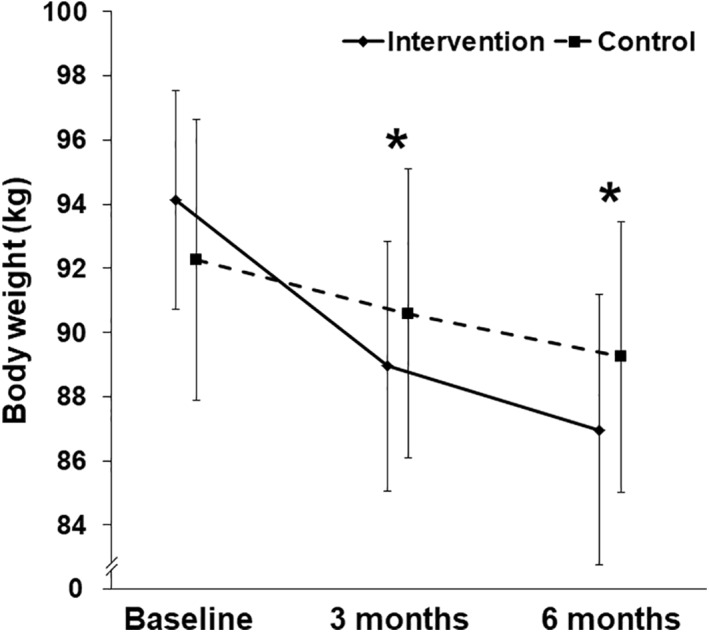
Body weights in the intervention and control groups over the 6 months of the study. Values are mean ± SE. *p < 0.05 for the comparisons of the changes in body weight at 3 months (prespecified secondary outcome) and at 6 months (prespecified primary outcome) between the two treatment groups (unpaired two‐tailed t‐test). Body weights at baseline were not statistically distinguishable between the two groups (Table 1). n = 13 intervention, n = 15 control. Calculated p‐values can be found in the legend to Figure 1 and in Tables 1 and 2
TABLE 2.
Prespecified primary and secondary outcomes with exploratory analyses
| Intervention (n = 13) | Control (n = 15) | Intervention versus control | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 months data | Value at 6 months | Change from baseline to 6 months | p | Value at 6 months | Change from baseline to 6 months | p | Change from baseline to 6 months | p |
| Prespecified primary outcome, with exploratory analyses | ||||||||
| Weight (kg) | 86.97 ± 4.22 (77.76 – 96.18) | −7.16 ± 1.78 (−11.05 – −3.26) | 0.0017 | 89.24 ± 4.21 (80.21 – 98.28) | −3.00 ± 1.05 (−5.27 – − 0.73) | 0.0131 | −4.16 ± 2.01 (−8.29 – −0.02) | 0.0488* |
| % Change in body weight | −7.92 ± 2.15 (−12.60 – −3.23) | 0.0031 | −3.15 ± 1.20 (−5.73 – − 0.56) | 0.0203 | −4.76 ± 2.38 (−9.66 – 0.12) | 0.0558 | ||
| Prespecified secondary outcomes, with exploratory analyses | ||||||||
| WC (cm) | 96.17 ± 2.76 (90.14 – 102.21) | −7.57 ± 1.65 (−11.17 – −3.96) | 0.0006 | 100.07 ± 2.47 (94.76 – 105.38) | −1.86 ± 1.25 (−4.55 – 0.83) | 0.1601 | −5.70 ± 2.04 (−9.91 – −1.50) | 0.0097* |
| SBP (mm Hg) | 128.30 ± 4.81 (117.81–138.80) | 5.23 ± 5.56 (−6.89 – 17.35) | 0.3657 | 123.73 ± 2.74 (117.83 – 129.62) | 0.86 ± 3.71 (−7.09 – 8.83) | 0.8188 | 4.36 ± 6.53 (−9.06 – 17.79) | 0.51 |
| DBP (mm Hg) | 77.30 ± 1.93 (73.08 – 81.52) | −2.76 ± 2.21 (−7.60 – 2.06) | 0.2359 | 77.46 ± 2.61 (71.86 –83.06) | 0.46 ± 2.49 (−4.87 – 5.81) | 0.8541 | −3.23 ± 3.38 (−10.18 – 3.71) | 0.3476 |
| HOMA‐IR | 1.68 ± 0.35 (0.86 –2.51) | −0.86+ ± 0.41 [n = 9] (−1.82 –0.09) | 0.0713 | 1.66 ± 0.35 (0.81 – 2.50) | −0.95 ± 0.67 [n = 8] (−2.55 – 0.65) | 0.2037 | 0.08 ± 0.77 (−1.57 – 1.73) | 0.9159 |
| HgbA1c (%) | 5.35 ± 0.08 (5.16 –5.54) | −0.18 ± 0.03 [n = 9] (−0.27 – −0.09) | 0.0013 | 5.60 ± 0.09 (5.38 – 5.81) | 0.06 ± 0.08 [n = 8] (−0.13 – 0.26) | 0.4830 | −0.25 ± 0.08 (−0.44 – −0.06) | 0.0131* |
| Triglycerides (mg/dl) | 82.66 ± 7.98 (64.25 –101.08) | −8.00 ± 6.11 [n = 9] (−22.11 –6.11) | 0.2274 | 83.75 ± 8.89 (62.70 – 104.79) | −16.75 ± 18.56 [n = 8] (−60.65 – 27.15) | 0.3969 | 8.75 ± 18.60 (−30.91 – 48.41) | 0.6450 |
| 3 months data | Value at 3 months | Change from baseline to 3 months | p | Value at 3 months | Change from baseline to 3 months | p | Change from baseline to 3 months | p |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | 88.96 ± 3.89 (80.47 –97.44) | −5.17 ± 1.35 (−8.11 – −2.23) | 0.0024 | 90.58 ± 4.50 (80.91 – 100.24) | −1.66 ± 0.74 (−3.26 – −0.07) | 0.0412 | −3.50 ± 1.48 (−6.56 – −0.44) | 0.0263* |
Note: Shown are means ± SEMs (95% CI). The prespecified primary outcome was the comparison of the changes in body weight in kg, from baseline to 6 months, in the intervention group versus the control group. The prespecified secondary outcomes were comparisons between the intervention group and the control group in their changes from baseline to 6 months in WC, SBP, DBP, insulin resistance for handling glucose (HOMA‐IR), HgbA1c, and plasma triglyceride concentrations, as well as their changes from baseline to 3 months in body weight in kg. Statistical comparisons between the two study groups were performed with the unpaired two‐tailed t‐test. Comparisons within each study group of values at 6 or 3 months versus baseline were performed with the paired two‐tailed t‐test. p < 0.05 was considered significant (*).
Abbreviations: DBP, diastolic blood pressure; HgbA1c, hemoglobin A1c; SBP, systolic blood pressure; WC; waist circumference.
FIGURE 3.
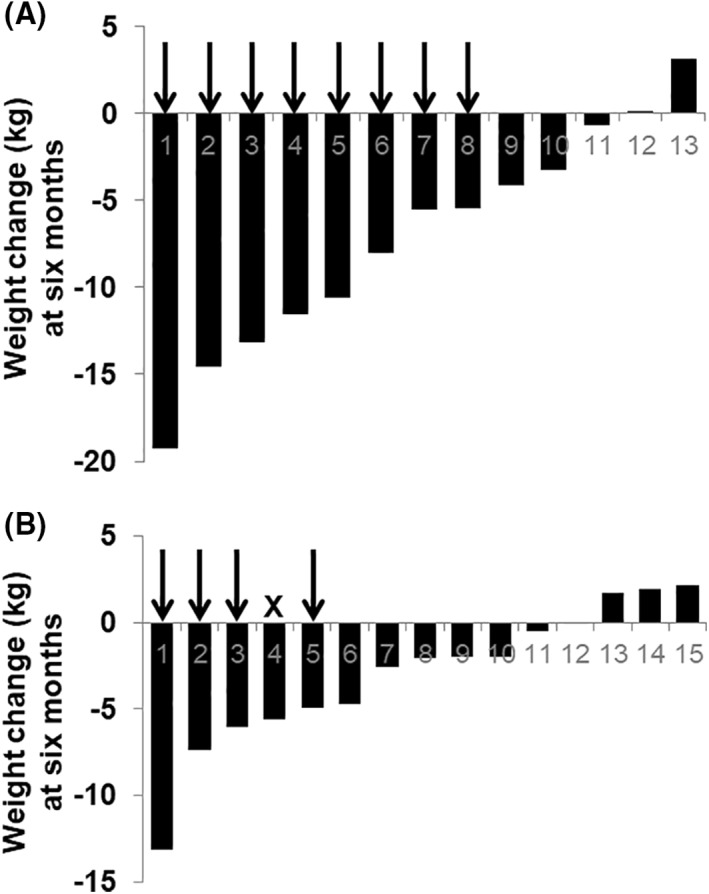
Weight change in kilograms from baseline to 6 months for each participant in the intervention group (panel A) and in the control group (panel B). The vertical arrows in each panel indicate participants who achieved ≥5% weight loss, a standard threshold for a clinically meaningful change. “X” denotes a participant who achieved <5% weight loss
Regarding the prespecified secondary outcomes, waist circumference significantly improved by −5.70 ± 2.04 cm (95% CI −9.91 to −1.50 p < 0.01), intervention versus control (Figure 4A and Table 2). Waist circumference data by gender are available in Table S2. HgbA1c significantly improved by −0.25 ± 0.08% (95% CI −0.44 to −0.06 p < 0.05), per‐protocol analysis, intervention (n = 9), versus control (n = 8) (Figure 4B and Table 2). There was no statistically significant difference in the changes in systolic or diastolic blood pressure, HOMA‐IR, or plasma triglyceride concentrations between the two study groups at 6 months (Table 2).
FIGURE 4.
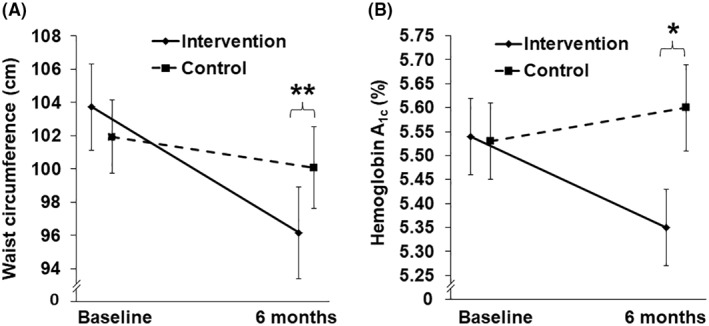
Changes in waist circumference (panel A) and hemoglobin A1c (panel B) over 6 months. Values are mean ± SE. **Changes in waist circumference at 6 months, intervention versus control, p = 0.0097. n = 13 intervention, n = 15 control. *Changes in hemoglobin A1c at 6 months, intervention versus control, p = 0.0131. n = 9 intervention, n = 8 control
3.3. Subject retention and engagement during the intervention
All 13 participants who began the intervention completed the entire 6 months of the intervention and engaged in all of its key components, that is, wearing the activity tracker, sharing food photographs, using their smartscales, and staying connected with the coach and other participants on the apps (data on the participants' degree of engagement are analyzed immediately below). All 13 participants attended each of the three in‐person study visits. Thus, 100% retention of study participants who initiated the intervention indicates that the program was well received and acceptable. Study participants reported no adverse events.
Changes in weight of each participant in the intervention group were statistically associated with objective measurements of several different aspects of engagement in the app‐based intervention. Attention to caloric intake, assessed by the total number of food photographs sent via the app (“shared”), was the strongest correlate with weight change (Figure 5A: Spearman's rho = −0.87, p < 0.01), followed closely by engagement with the app‐based coaching model within the social network, assessed by the total number of text messages sent by each participant (Figure 5B: rho = −0.81 , p < 0.01). The number of food photographs shared by each participant was 0.41 ± 0.12 (mean ± SE) per day, and quantitative data for each participant can be seen along the x‐axis of Figure 5A and in Table S3. The number of messages each participant sent in the group chat was 0.36 ± 0.09 per day (see Figure 5B and Table S3 for individual data). Weight change also correlated significantly with weight‐monitoring behaviors, assessed by the total number of times each participant stepped on the smartscale (Figure 5C: rho = −0.74, p < 0.01), and the number of unique days each participant checked his or her weight on the smartscale (Figure 5D: rho = −0.61, p < 0.05). The number of times each subject stepped on his or her smartscale per day was 0.77 ± 0.11, and the proportion of days that each participant stepped on the smartscale at least once (daily weighing rate) was 0.47 ± 0.06. Consistent with known effects of physical activity on energy balance, weight change significantly correlated with mean daily accelerometer‐tracked step counts (Figure 5E: rho = −0.55, p < 0.05).
FIGURE 5.
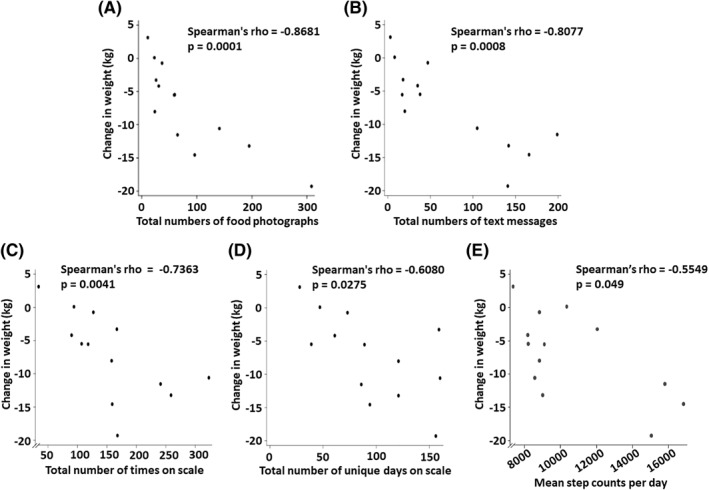
Correlations of each participant's weight change in the intervention group with objective indicators of different aspects of each participant's engagement with the app‐based lifestyle intervention over the 6 months of the study. Shown are correlations of weight change with total number of food photographs sent via the app (“shared”) (A), total number of text messages sent (B), total number of times on the smartscale (C), total number of unique days when the smartscale was used at least once (D), and average daily step counts (E). Displayed are Spearman's rank correlation coefficients (rho) and the corresponding p‐values. In panel A, one participant achieved a weight change of −5.48 kg and shared 60 food photographs, and another participant had a weight change of −5.53 kg and shared 59 food photographs, and so their data points mostly overlap on the graph; these numerical data are given in Table S3
Changes in waist circumference in the intervention group also correlated with the number of food photographs shared (Figure 6A: rho = −0.59, p < 0.05), the total number of text messages that each participant sent to the group chat (Figure 6B: rho = −0.61, p < 0.05), and each participant's mean daily step counts (Figure 6C: rho = −0.57, p < 0.05). The distributions of diet plans chosen by participants in each study group are shown in Table S4.
FIGURE 6.
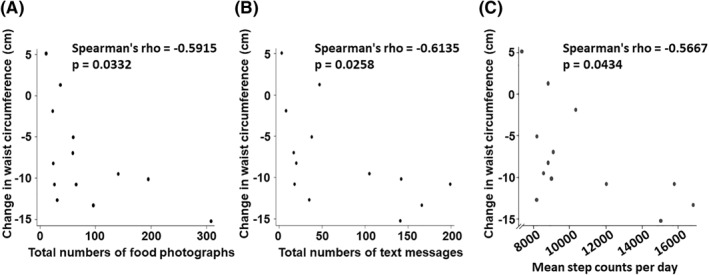
Correlations of each participant's change in waist circumference in the intervention group with objective indicators of different aspects of each participant's engagement with the app‐based lifestyle intervention over the 6 months of the study. Shown are correlations of changes in waist circumference with total number of food photographs shared (A), total number of text messages sent (B), and average daily step counts (C). Displayed are Spearman's rank correlation coefficients (rho) and the corresponding p‐values
Regarding professional coaching, messages from the physician were sent out to the entire intervention group on 140 out of 185 unique days, which was approximately 75% of days during the study intervention. There were 22 competitions over the course of the intervention, that is, nearly 1 per week. Thus, the intervention successfully encouraged participants to stay well connected with the professional coach and with each other during the study, and several quantitative indicators of individual engagement correlated with individual changes in waist circumference and strongly correlated with changes in body weight.
4. DISCUSSION
This study demonstrated that the user‐friendly, interactive, app‐based electronically delivered lifestyle intervention met the prespecified primary outcome of reducing weight over control, as well as prespecified secondary outcomes of reducing waist circumference and HgbA1c. The intervention required a substantial commitment of time and effort by the participants, who received no financial compensation and, once the study was completed, could not keep any devices provided by the study. Thus, unusually high participant retention and engagement support the feasibility of this intervention. In this study, participants in the intervention group lost an average of 7.16 kg at 6 months, corresponding to a nearly 8% weight loss. A 5% lower body weight has been shown to reduce the risk of type 2 diabetes mellitus by 9% in women and 11% in men.28 The degree of weight loss achieved with this electronically delivered lifestyle intervention was similar to the weight‐loss trend at the 6‐month time point in the Diabetes Prevention Program (DPP), reported as a reduction of 6.79 kg.29 The current intervention also produced significant reductions in waist circumference, with a mean change of −7.57 cm, amounting to −7.23%. For every 5% less waist circumference, the risk of developing type 2 diabetes mellitus has been reported to drop by 21% in women and 23% in men.28 Accordingly, we also saw a decrease in HgbA1c in the intervention group compared with controls. Thus, this electronically delivered lifestyle intervention produced significant metabolic health benefits.
Several components of the current intervention distinguish it from previous technology‐based programs. First, prior studies of app‐based lifestyle interventions that did not produce a statistically significant difference in weight loss between the study groups had used monitoring apps but without any human interface.13 A basis for the design of the current intervention was that feedback from a human coach may be required to produce meaningful behavioral changes. Second, studies reporting that their smartphone‐based interventions did not enhance weight loss had included social networking with counselors and study participants but, unlike our approach, did not include feedback from the monitoring app.14 Third, studies of wearable activity trackers, such as the IDEA clinical trial,15 concluded that devices that monitor and provide feedback on physical activity actually detract from standard behavioral weight‐loss approaches. The IDEA trial reported that the addition of a wearable technology device to a standard behavioral intervention resulted in less weight loss over 24 months15 Of note, the wearable activity trackers in that trial were too large to be placed on the wrist (product dimensions 3.8 × 8.8 × 6.3 cm), requiring upper arm placement instead, and were worn for an average of only 4 h a day, and therefore would not reflect the convenience of the more contemporary wrist‐worn devices used in the present study. In addition, the IDEA trial did not initiate use of the wearable device at the onset of the intervention, a factor that might have impeded how readily the participants adopted and used the technology.
The use of online social networks for weight management is still in its early stages. Only a few studies have shown promise, with the optimal use of electronically delivered lifestyle interventions and their eventual efficacy for weight loss remaining undetermined.18 , 30 Accordingly, a systematic review of weight‐loss interventions delivered by online social networks found that only one study reported a clinically meaningful weight loss of ≥5%.31 Moreover, electronically delivered lifestyle interventions have yet to be standardized: a systematic review of technology‐assisted interventions for weight loss found that prior studies used various forms of personnel, technology, and interventional strategies for behavioral change.18 Trials most frequently utilized medical doctors (44%), web‐based applications (63%), and self‐monitoring (81%). Interventions that included clinician‐guiding software or feedback from personnel appeared to promote more weight loss than fully automated interventions.18 While there was some evidence of benefit, studies often did not utilize pragmatic methodology and rarely provided publicly available technology, thereby limiting scalability of the interventions.18
Another component of the current intervention that distinguishes it from previous weight‐loss programs is the way participants in the intervention group were assisted in setting weight‐loss goals. Goal setting for weight management has been shown to be effective in other studies of electronically delivered lifestyle interventions.32 Previous weight‐loss studies have shown numerous health benefits from weight loss of 7%–10%.24 , 33 , 34 The DPP set a fixed, study‐wide weight‐loss goal of 7% of initial body weight, based on previous weight‐loss trials and epidemiologic data supporting this level of weight loss as effective in reducing diabetes risk.24 , 29 In the Look AHEAD trial, each study center was expected to obtain a mean loss ≥7% of initial weight during the first year, although individual participants were each given a fixed goal of losing 10% or more of initial body weight.34 After 1 year, the participants had lost a mean of 8.5% of initial weight.35 Published evidence does not demonstrate that setting smaller goals causes more weight loss.36 Therefore, the approach in the current study to goal setting was different from these prior approaches in that here, the aim was to personalize, and thereby maximize, each participant's weight loss. This approach thereby avoided a fixed study‐wide weight‐loss goal, but instead set an individualized, moving target: each participant was constantly given a new weight target along with a virtual reward once he or she achieved the previous goal. It is possible that DPP participants might have lost more weight if a more stringent goal or a moving goal had been set. The current study did not limit each participant's weight loss goal to 7%, and so the intervention was able to enhance individual weight‐loss efforts beyond that number.
The current study was designed to provide ongoing feedback, both through the automated technology interface and through technologically facilitated interactions with the professional coach. The current study incorporated a human interface into an electronically delivered lifestyle intervention via incorporation of professional and peer coaching, which presumably enhanced the effectiveness of the intervention. In addition, the act of daily weight monitoring and tracking was made as seamless as possible, based on prior literature that frequent weighing aids weight loss.37 As noted above, the data in Figure 5C,D and Table S3 show that the participants in the intervention group adhered to this health behavior. Further studies would be needed to determine how much of the treatment effect here was driven by each of these distinguishing features, particularly professional coaching and peer support, the method for setting individualized moving weight‐loss targets, the self‐monitoring apps, and the convenient, user‐friendly wearable devices and smartscales. Thus, while self‐monitoring apps and wearable devices may enhance effectiveness of a weight‐loss intervention, their efficacy in the current study was tested exclusively in the setting of professional and peer support delivered via an interactive, user‐friendly, convenient system. In another prior study, a fully automated behavioral intervention for diabetes prevention, delivered via email, Internet, and automated phone calls to participants with prediabetes, produced a mean difference in HgbA1c reduction of only 0.08% in the intervention versus control groups.38 Several factors may be responsible for the greater reduction in HgbA1c achieved in the current study, including the Smart Food Diary™, physician‐driven professional coaching, and modern, convenient wearable activity trackers.
The current electronically delivered lifestyle intervention allowed easy access to app‐based behavioral coaching from a physician, who then directed the social networking interface among study participants. By staying connected on the app with the physician‐coach, participants possibly had increased conscientiousness, motivation, and diligence with the behavior‐modification strategies. The participants' awareness of being “tracked” by their doctor would serve to reinforce their new healthy habits over the 6‐month time frame. While automated systems that monitor and encourage behavioral changes can improve adherence to electronic health interventions, human support may enhance adherence to a significantly greater degree.39 Therefore, the current intervention was designed to include clear, process‐oriented expectations that each participant was involved in determining.40 Reciprocity in the relationship, through which the participant derived clear benefits, was explicit in the coaching model. Human support increases adherence through supportive accountability to a coach who is seen as trustworthy, benevolent, and having expertise.40 People may respond more positively to accountability demands from a coach who is perceived as legitimate.41 Moreover, the therapeutic bond is an important predictor of outcome in distance treatments, particularly when those treatments focus on providing behavioral training, a key component of our smartphone app‐based lifestyle intervention.42 Therefore, it was decided to have just one physician, with expertise in weight management and electronic health interventions, to serve as the professional coach throughout the study. Participants derived moral support from being connected to the coach and to each other within the social network. The novel SmartReward™ system increased participants' competitiveness, which furthered their motivation.
Major strengths of this study include its prospective randomized controlled design; achievement of the prespecified primary outcome; clinically and statistically significant weight loss in the intervention group but not clinically adequate weight loss in the control group; a large effect size in the primary outcome and in key secondary outcomes of changes in waist circumference and HgbA1c; measurement of HgbA1c levels by clinical laboratory, not by in‐home HgbA1c kits, thereby increasing reliability; frequent objective measures of participants' body weights with smartscales that automatically transmit readings, thereby reducing chances of erroneous or omitted weight readings from manual data entry; use of the food photography log that avoided much of the inaccuracy and tediousness associated with conventional food diaries; frequent objective measures of physical activity that were also automatically transmitted and recorded; the user‐friendly interactive design of the intervention; a high rate of retention of participants who began the intervention, in contrast to most weight‐loss studies, which report high rates of attrition10, 11, 12; and objective measures that show high rates of participant engagement. Importantly, there were no statistically significant differences in key baseline characteristics, such as body weight, ethnicity, or age, between the two treatment groups.
The major limitation of the study was a small sample size. A future goal will be to replicate the study with a larger n. Although there were unusually high rates of retention overall, two participants in the intervention group were lost to follow up before the baseline visit, and two participants in the control group were also lost from the study (one developed clinical depression requiring medication and the other was lost to follow‐up after the baseline visit). Loss of participants, who are often nonadherers, can affect results. By necessity, this was an un‐blinded study, and the wait‐listed control group was aware of the precise nature of the intervention because all participants gave consent to participate before randomization. Thus, some controls may have initiated components of the intervention on their own, before completing the full 6 months. As noted above, one participant dropped out of the control group; misgivings about treatment assignment may have played a role in this decision, because this participant reported enrolling in a weight‐loss program that included weight‐loss medications. Because the same physician was the lead investigator and also delivered the coaching during the outpatient weight management visits to both study groups and performed coaching via the smartphone, there could have been some confounding. On the other hand, the control group also lost a statistically significant amount of weight at 6 months, and the degree of their weight loss is entirely consistent with prior literature on the efficacy of conventional coaching.
This study's recruiting method via flyers and emails, rather than by physician referrals, required an active step by potential participants to approach research staff. Thus, the study purposefully enrolled from a self‐selected sample of participants who may have entered the trial more motivated to change their lifestyle than the average patient with obesity. Selecting participants who were in the preparation phase of behavior change43 increased the likelihood of success of a lifestyle intervention—although it also increased the likelihood of weight loss in our control group. Participants with BMI >42 kg/m2, joint disease, type 2 diabetes mellitus, and/or psychiatric disease, such as anxiety disorders or clinical depression, were excluded from this study. Thus, the study population was not fully representative of the entire population with obesity, in which arthritis, type 2 diabetes, and clinical depression are prevalent. The patient demographic was predominantly female, which is common in behavioral weight‐loss interventions. Some participants had delays in laboratory testing, and because the wait‐listed control group began to receive the app‐based electronically delivered lifestyle intervention promptly at the 6‐month mark, we had to exclude these delayed laboratory measurements from our analyses. Thus, the sample size for the prespecified secondary outcomes of HgbA1c, HOMA‐IR, and plasma triglycerides was reduced.
Because both the professional coaching through the app and the in‐person weight management visits were physician‐driven, the cost of scaling up this exact intervention would be high. on cost‐effectiveness and efficacy of this intervention using alternatives to physician support, such as nurse practitioners and nutritionists, may be required to allow scalability. Further studies will also be needed to determine which individual components of this electronically delivered lifestyle intervention, such as remote coaching, competitions, food photographs, or activity tracking, produced the most effect and whether some components should be omitted, reduced, or intensified. Modification of the intensiveness of the intervention may increase its sustainability for a longer period.
5. CONCLUSIONS
This app‐based electronically delivered lifestyle intervention met the prespecified primary outcome of clinically meaningful and statistically significant weight loss at 6 months over control. Consistent with the magnitude of weight loss, the intervention improved metabolic health, as assessed by reductions in waist circumference and HgbA1c. Weight loss in the intervention group strongly correlated with several specific aspects of the participants' engagement in technology‐based coaching—namely, their attention to caloric intake, engagement with the social network, weight‐monitoring behaviors, and physical activity. Moreover, unusually high participant retention and engagement support the feasibility of the intervention. Thus, weight loss comparable to the DPP goal of 7% may become more broadly achievable.
This app‐based electronically delivered lifestyle intervention could become a useful tool to promote weight loss, improve metabolic health, and possibly prevent type 2 diabetes. Given the small sample size of this trial, larger and longer studies of the lifestyle intervention in different demographic groups will be valuable next steps. Importantly, this intervention produced a large, beneficial effect compared with other electronically delivered lifestyle interventions. Qualitative analyses of each component of this weight‐loss intervention will be required to understand the reasons for the successful outcome and to guide potential improvements.
CONFLICT OF INTERESTS
Kevin Jon Williams reports an ownership interest in Hygieia, Inc., and in Gemphire Therapeutics, Inc., and recently served on the Medical and Scientific Advisory Board of Gemphire Therapeutics, Inc. The other authors have no conflict of interest to declare. Portions of this work were presented at the American Diabetes Association Scientific Sessions in 2018.
Supporting information
TABLE S1
TABLE S2
TABLE S3
TABLE S4
ACKNOWLEDGMENT
Temple University Department of Medicine Junior Faculty Research Development Award (C. L. Vaz) and the Obesity Treatment Foundation (C. L. Vaz). No commercial entity donated any funds, goods, or services to this research.
Vaz CL, Carnes N, Pousti B, Zhao H, Williams KJ. A randomized controlled trial of an innovative, user‐friendly, interactive smartphone app‐based lifestyle intervention for weight loss. Obes Sci Pract. 2021;7(5):555‐568. doi: 10.1002/osp4.503
REFERENCES
- 1.Pi‐Sunyer X. The medical risks of obesity. PGM (Postgrad Med). 2009;121:21‐33. 10.3810/pgm.2009.11.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: clinical harm, molecular mechanisms, and a way forward. Atherosclerosis. 2016;247:225‐282. 10.1016/j.atherosclerosis.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Tronieri JS, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020;75(2):235‐251. 10.1037/amp0000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Admiraal WM, Vlaar EM, Nierkens V, et al. Intensive lifestyle intervention in general practice to prevent type 2 diabetes among 18 to 60‐year‐old South Asians: 1‐year effects on the weight status and metabolic profile of participants in a randomized controlled trial. PLoS One. 2013;8(7):e68605. 10.1371/journal.pone.0068605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann‐Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P. Behavioural weight management review group. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta‐analysis. Obes Rev. 2014;15(11):920‐932. 10.1111/obr.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen MD, Ryan DH, Apovian CM, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2013;63(25 Pt B):2985‐3023. 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines formedical care of patients with obesity. Endocr Pract. 2016;22(3_Suppl):1‐203. 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 8.Booth HP, Prevost TA, Wright AJ, Gulliford MC. Effectiveness of behavioural weight loss interventions delivered in a primary care setting: a systematic review and meta‐analysis. Fam Pract. 2014;31(6):643‐653. 10.1093/fampra/cmu064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey‐Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med. 2010;51(2):123‐128. 10.1016/j.ypmed.2010.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Publ Health. 2015;105(9):e54‐e59. 10.2105/AJPH.2015.302773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalle Grave R, Calugi S, Compare A, et al. Weight loss expectations and attrition in treatment‐seeking obese women. Obes Facts. 2015;8:311–318. 10.1159/000441366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo O, Ferretti VVV, Ferraris C, et al. Is drop‐out from obesity treatment a predictable and preventable event? Nutr J 2014;13:13. 10.1186/1475-2891-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BY Laing, Mangione CM, Tseng C‐H, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med. 2014;161(10_Suppl):S5‐S12. 10.7326/M13-3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner‐McGrievy G, Tate D. Tweets, apps, and pods: results of the 6‐month mobile pounds off digitally (mobile POD) randomized weight‐loss intervention among adults. J Med Internet Res. 2011;13(4):e120. 10.2196/jmir.1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long‐term weight loss. J Am Med Assoc. 2016;316(11):1161‐1171. 10.1001/jama.2016.12858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web‐based weight loss in primary care: a randomized controlled trial. Obes. 2010;18(2):308‐313. 10.1038/oby.2009.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel LJ, Clark JM, Yeh H‐C, et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959‐1968. 10.1056/NEJMoa1108660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine DM, Savarimuthu S, Squires A, Nicholson J, Jay M. Technology‐assisted weight loss interventions in primary care: a systematic review. J Gen Intern Med. 2015;30(1):107‐117. 10.1007/s11606-014-2987-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JK, Stephens J, Patel A. Technology‐assisted weight management interventions: systematic review of clinical trials. Telemed e‐Health. 2014;20(12):1103‐1120. 10.1089/tmj.2014.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grock S, Ku J‐h, Kim J, Moin T. A review of technology‐assisted interventions for diabetes prevention. Curr Diabetes Rep. 2017;17(11):107. 10.1007/s11892-017-0948-2 [DOI] [PubMed] [Google Scholar]
- 21.O'Dea, S. Smartphone penetration in the U.S. as share of population 2010‐2021. https://www.statista.com/statistics/201183/forecast‐of‐smartphone‐penetration‐in‐the‐us/ [Google Scholar]
- 22.Pellegrini C, Pfammatter A, Conroy D, Spring B. Smartphone applications to support weight loss: current perspectives. Ahct. 2015;1:13‐22. 10.2147/AHCT.S57844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertogh EM, Schuit AJ, Peeters PHM, Monninkhof EM. Noncompliance in lifestyle intervention studies: the instrumental variable method provides insight into the bias. J Clin Epidemiol. 2010;63(8):900‐906. 10.1016/j.jclinepi.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 24.Diabetes Prevention Program (DPP) Research Group . The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165‐2171. 10.2337/diacare.25.12.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826‐837. 10.1016/S0140-6736(11)60812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhurandhar NV, Schoeller D, Schoeller D, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39:1109‐1113. 10.1038/ijo.2014.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leahey TM, Wing RR. A randomized controlled pilot study testing three types of health coaches for obesity treatment: professional, peer, and mentor. Obesity. 2013;21(5):928‐934. 10.1002/oby.20271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labraña AM, Durán E, Martínez MA, et al. Menor peso corporal, de índice de masa corporal y de perímetro de cintura se asocian a una disminución en factores de riesgo cardiovascular en población chilena: findings from the Chilean health surveyç. Rev Med Chile. 2017;145(5):585‐594. 10.4067/S0034-98872017000500005 [DOI] [PubMed] [Google Scholar]
- 29.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102‐2107. 10.2337/dc06-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall AK, Cole‐Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Publ Health. 2015;36:393‐415. 10.1146/annurev-publhealth-031914-122855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis EA, Szabo‐Reed AN, Ptomey LT, et al. Do weight management interventions delivered by online social networks effectively improve body weight, body composition, and chronic disease risk factors? A systematic review. J Telemed Telecare. 2017;23(2):263‐272. 10.1177/1357633X16630846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Hara BJ, Gale J, McGill B, et al. Weight‐related goal setting in a telephone‐based preventive health‐coaching program: demonstration of effectiveness. Am J Health Promot. 2017;31(6):491‐501. 10.1177/0890117116660776 [DOI] [PubMed] [Google Scholar]
- 33.Guare JC, Wing RR, Grant A. Comparison of obese NIDDM and nondiabetic women: short‐ and long‐term weight loss. Obes Res. 1995;3:329‐335. 10.1002/j.1550-8528.1995.tb00158.x [DOI] [PubMed] [Google Scholar]
- 34.The Look AHEAD Research Group . The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737‐752. 10.1038/oby.2006.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Look AHEAD Research Group . Eight‐year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity. 2014;22:5‐13. 10.1002/oby.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durant NH, Joseph RP, Affuso OH, Dutton GR, Robertson HT, Allison DB. Empirical evidence does not support an association between less ambitious pre‐treatment goals and better treatment outcomes: a meta‐analysis. Obes Rev. 2013;14(7):532‐540. 10.1111/obr.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg DM, Bennett GG, Askew S, Tate DF. Weighing every day matters: daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Dietetics. 2015;115(4):511‐518. 10.1016/j.jand.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240. 10.2196/jmir.4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beun RJ, Brinkman W‐P, Fitrianie S, et al. Improving adherence in automated e‐coaching. In: Meschtscherjakov A, De Ruyter B, Fuchsberger V, Murer M, Tscheligi M, eds. Persuasive Technology. Cham: Springer International Publishing; 2016:276‐287. [Google Scholar]
- 40.Mohr DC, Cuijpers P, Lehman K. Supportive Accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. 10.2196/jmir.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Toorn J, Tyler TR, Jost JT. More than fair: outcome dependence, system justification, and the perceived legitimacy of authority figures. J Exp Soc Psychol. 2011;47:127‐138.doi. 10.1016/j.jesp.2010.09.003 [DOI] [Google Scholar]
- 42.Taber BJ, Leibert TW, Agaskar VR. Relationships among client‐therapist personality congruence, working alliance, and therapeutic outcome. Psychotherapy. 2011;48(4):376‐380. 10.1037/a0022066 [DOI] [PubMed] [Google Scholar]
- 43.Prochaska JO, Norcross JC, DiClemente CC. Applying the stages of change. Psychother Aust. 2013;19(2):177‐181. 10.1093/med:psych/9780199845491.003.0034 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1
TABLE S2
TABLE S3
TABLE S4


