Abstract
Chromosomal rearrangements involving the c‐ros oncogene 1 (ROS1) are identified in approximately 1% of non‐small cell lung cancer (NSCLC) patients. Crizotinib is the first tyrosine kinase inhibitor (TKI) against ROS1‐rearranged NSCLC. G2032R, a secondary resistant mutation, is observed in 41% of patients treated with crizotinib. Entrectinib, a TKI against neurotrophic tropomyosin receptor kinase, is reportedly efficacious against ROS1‐rearranged NSCLC. However, ROS1‐G2032R is resistant to entrectinib both in vitro and in vivo. We report an 85‐year‐old female patient with ROS1‐rearranged NSCLC, who developed drug‐induced interstitial lung disease (DI‐ILD) 2 months after crizotinib treatment, and was treated with prednisolone followed by entrectinib. Entrectinib treatment resulted in stable disease with a marginal response after a partial response to crizotinib. Entrectinib treatment following crizotinib cessation due to DI‐ILD was efficacious, which suggested that ROS1‐G2032R gatekeeper mutation, frequently observed in crizotinib‐resistant disease, was absent.
Keywords: crizotinib, c‐ros oncogene 1, drug‐induced interstitial lung disease, entrectinib, non‐small cell lung cancer
We report a patient with c‐ros oncogene 1 (ROS1)‐rearranged non‐small cell lung cancer, who developed drug‐induced interstitial lung disease 2 months after crizotinib treatment, and was sequentially treated with entrectinib, resulting in a stable disease with a marginal response.
INTRODUCTION
Chromosomal rearrangements involving the c‐ros oncogene 1 (ROS1) are identified in approximately 1% of non‐small cell lung cancer (NSCLC) patients. Crizotinib is the first tyrosine kinase inhibitor (TKI) against ROS1‐rearranged NSCLC. ROS1‐G2032R is a secondary mutation frequently observed in crizotinib‐resistant disease.1, 2 Entrectinib, a TKI against neurotrophic tropomyosin receptor kinase (NTRK), is reportedly efficacious as a first‐line treatment for ROS1‐rearranged NSCLC.3 ROS1‐G2032R is resistant to entrectinib.4 This hindered the sequential treatment with entrectinib in patients whose disease progressed during crizotinib treatment.
We report a patient with ROS1‐rearranged NSCLC, who developed drug‐induced interstitial lung disease (DI‐ILD) 2 months after crizotinib treatment, and was sequentially treated with entrectinib, resulting in a stable disease with a marginal response.
CASE REPORT
An 85‐year‐old female patient was referred to our department for the evaluation of a mass in the right upper lobe (Figures 1A and 2A [same as 1A], 2D) on chest computed tomography (CT) before lumbar spine surgery at the Department of Orthopedics. The patient was a non‐smoker with a medical history of dyslipidaemia and hypertension, and a family history of gastric cancer. Histological examination via fibreoptic bronchoscopy revealed an adenocarcinoma, harbouring ROS1 rearrangement, with multiple brain metastases (Figure 1B). The clinical stage was cT1cN3M1c (BRA), stage IVB. The ROS1 rearrangement was diagnosed by next‐generation sequencing (Oncomine Dx Target Test Multi‐CDx system, Life Technologies Corporation Japan).
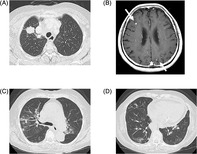
FIGURE 1.
Chest computed tomography (CT) obtained before the treatment (A) demonstrated a mass located at S1b of the right upper lobe. Brain magnetic resonance imaging with contrast enhancement at baseline (B) exhibited multiple brain metastases (arrows) in bilateral cerebral hemispheres. Chest CT obtained on day 115 (C, D) demonstrated non‐segmental ground‐glass opacities, consolidation and reticular shadow, suggesting drug‐induced interstitial lung disease
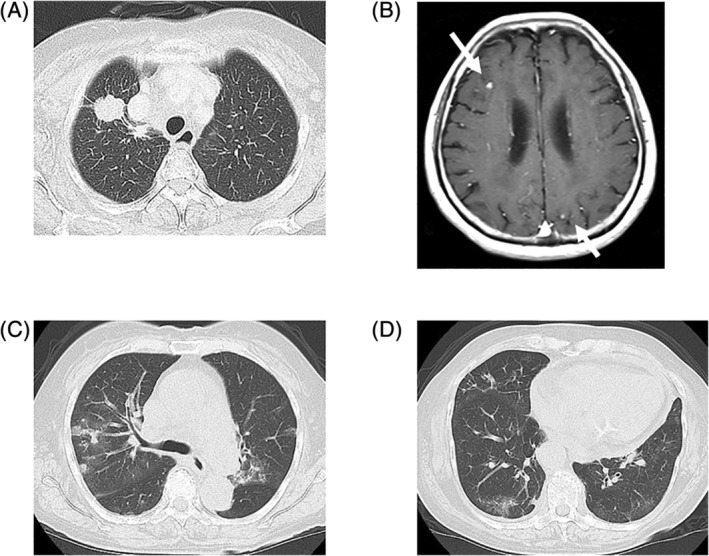
FIGURE 2.
Chest computed tomography obtained before the treatment in axial (A) and coronal (D) slices demonstrated a primary site in the right upper lobe. The shrinkage of the primary site with a partial response, first recorded on day 33 after crizotinib initiation, was maintained after 115 days in axial (B) and coronal (E) slices, with non‐segmental ground‐glass opacities and consolidation in bilateral lung lobes (arrows). The primary site exhibited further shrinkage with marginal response 28 days after entrectinib initiation, with the improvement of drug‐induced interstitial lung disease in axial (C) and coronal (F) slices
She was initially treated with crizotinib 250 mg twice daily. The primary tumour resulted in a partial response (PR) on day 33 after crizotinib administration, and brain metastases exhibited a complete response without any radiotherapy on day 38. She developed crizotinib‐induced nausea and vomiting on day 8 (grade 1 by Common Terminology Criteria for Adverse Events [CTCAE] ver. 5). On day 57, this worsened to CTCAE grade 2. The dose was then reduced to 250 mg daily. DI‐ILD due to crizotinib was documented on chest CT on day 115 (Figure 1C,D), when the PR of the primary tumour was maintained (Figure 2B,E). Thereafter, crizotinib was discontinued, and treatment with prednisolone at 30 mg/day was initiated and tapered to 10 mg/day in 10 days, which was continued to prevent the flare of DI‐ILD. DI‐ILD responded well to prednisolone, and entrectinib was introduced as the second‐line treatment 15 days after crizotinib cessation. She lost weight during crizotinib treatment. Her height, body weight and body surface area (BSA), calculated using the Fujimoto formula at the time of entrectinib initiation, were 148 cm, 56 kg and 1.43 m2, respectively. Infants receive entrectinib at 300 mg/m2. Based on this, the patient received entrectinib at a dose of 400 mg once daily to account for her age, BSA and small physique. Tumour shrinkage with a marginal response was observed on day 28 after entrectinib initiation (Figure 2C,F). The patient has continued receiving treatment 6 months after entrectinib initiation without any adverse events.
DISCUSSION
The discovery of driver oncogenes and the development of relevant TKIs have revolutionized the treatment strategy for NSCLC.
ROS1 is a rare driver oncogene located on chromosome 6q22. It is structurally similar to anaplastic lymphoma kinase (ALK). Similar to ALK‐positive NSCLC, ROS1‐rearranged NSCLC is sensitive to pemetrexed.1 Crizotinib and entrectinib have been approved by the Food and Drug Administration (United States), European Medicines Agency (European Union) and Pharmaceuticals and Medical Devices Agency (Japan). The application of which ROS1‐TKI to be administered as a first‐line therapy has not been established. Crizotinib has been evaluated in one phase I trial5 and four phase II trials.6, 7, 8, 9 It was consistently efficacious against ROS1‐rearranged NSCLC with an objective response ratio (ORR) ranging 67%–72%, and a median progression‐free survival (mPFS) ranging 15.9–19.2 months. Entrectinib has been evaluated in a combined analysis of two phase I trials and one phase II trial, with an ORR of 77% and mPFS of 19.0 months.3
The treatment of elderly patients with NSCLC harbouring ROS1 rearrangement with multiple brain metastases involves two major issues: (1) the management of brain metastases and (2) subsequent treatment following disease progression during the first‐line ROS1‐TKI treatment.
Brain metastases frequently occur in ROS1‐rearranged NSCLC at the time of diagnosis and treatment course. Central nervous system (CNS) activity is an essential factor for ROS1‐TKIs. Many TKIs are strong substrates of P‐glycoprotein (P‐gp), an efflux transporter that actively transports drugs out of the blood–brain barrier (BBB). This leads to poor CNS efficacy. As crizotinib is a potent substrate for P‐gp, it has a poor CNS penetration rate, causing ineffective control of CNS metastases, including carcinomatous meningitis. Entrectinib was formerly designed as an NTRK‐TKI with satisfactory CNS penetration and sustained CNS exposure due to it being a weak P‐gp substrate.10 Entrectinib was a viable first‐line ROS1‐TKI drug in cases with CNS metastases. Crizotinib was effective against radiologically detectable brain metastases, wherein the BBB was damaged, enabling a more accessible drug delivery route than carcinomatous meningitis. Crizotinib was effective against brain metastases in the current case.
Resistance to the first‐line ROS1‐TKI arises secondary to on‐target mechanisms through secondary mutations in the ROS1 kinase domain, occurring in approximately 50%–60% of cases, and off‐target mechanisms, including bypass signalling track or phenotypic changes.1 Among secondary gatekeeper mutations, ROS1‐G2032R was the most frequently observed mutation in 41% of patients treated with crizotinib.2 As ROS1‐G2032R was resistant to entrectinib both in vitro and in vivo,4 sequential treatment with entrectinib may be ineffective in approximately 40% of cases, wherein the disease progressed during crizotinib treatment. Next‐generation ROS1‐TKIs, including taletrectinib, are developed to overcome ROS1‐G2032R gatekeeper mutation.4
In the current case, entrectinib was administered following crizotinib, not due to disease progression, but because of crizotinib cessation after developing DI‐ILD. Therefore, sequential entrectinib administration could be effective in patients who have low risk of ROS1‐G2032R development, typically in cases without disease progression during crizotinib administration.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Mai Tanimura designed the work and wrote the manuscript. Nobutaka Kataoka was a treating physician. Nobutaka Kataoka, Yusuke Kunimatsu and Rei Tsutsumi collected data. Izumi Sato, Takayuki Nakano and Keiko Tanimura took the initiative in the analysis and interpretation of the clinical course. All authors participated in the discussion. Takayuki Takeda supervised and revised the manuscript.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this case report and accompanying images.
Tanimura M, Kataoka N, Kunimatsu Y, Tsutsumi R, Sato I, Nakano T, et al. Entrectinib for ROS1‐rearranged non‐small cell lung cancer after crizotinib‐induced interstitial lung disease: A case report. Respirology Case Reports. 2021;9:e0857. 10.1002/rcr2.857
Associate Editor: Tracy Leong
REFERENCES
- 1.Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol. 2017;12:1611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gainor JF, Tseng D, Yoda S, Dagogo‐Jack I, Friboulet L, Lin JJ, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1‐positive non‐small‐cell lung cancer. JCO Precis Oncol. 2017;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dziadziuszko R, Krebs MG, De Braud F, Siena S, Drilon A, Doebele RC, et al. Updated integrated analysis of the efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion‐positive non‐small‐cell lung cancer. J Clin Oncol. 2021;39:1253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katayama R, Gong B, Togashi N, Miyamoto M, Kiga M, Iwasaki S, et al. The new‐generation selective ROS1/NTRK inhibitor DS‐6051b overcomes crizotinib resistant ROS1‐G2032R mutation in preclinical models. Nat Commun. 2019;10:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371:1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, et al. Phase II study of crizotinib in East Asian patients with ROS1‐positive advanced non‐small‐cell lung cancer. J Clin Oncol. 2018;36:1405–11. [DOI] [PubMed] [Google Scholar]
- 7.Michels S, Massutí B, Schildhaus HU, Franklin J, Sebastian M, Felip E, et al. Safety and efficacy of crizotinib in patients with advanced or metastatic ROS1‐rearranged lung cancer (EUCROSS): a European phase II clinical trial. J Thorac Oncol. 2019;14:1266–76. [DOI] [PubMed] [Google Scholar]
- 8.Landi L, Chiari R, Tiseo M, D'Incà F, Dazzi C, Chella A, et al. Crizotinib in MET‐deregulated or ROS1‐rearranged pretreated non‐small cell lung cancer (METROS): a phase II, prospective, multicenter, two‐arms trial. Clin Cancer Res. 2019;25:7312–9. [DOI] [PubMed] [Google Scholar]
- 9.Moro‐Sibilot D, Cozic N, Pérol M, Mazières J, Otto J, Souquet PJ, et al. Crizotinib in c‐MET‐ or ROS1‐positive NSCLC: results of AcSé phase II trial. Ann Oncol. 2019;30:1985–91. [DOI] [PubMed] [Google Scholar]
- 10.Facchinetti F, Friboulet L. Profile of entrectinib and its potential in the treatment of ROS1‐positive NSCLC: evidence to date. Lung Cancer (Auckl). 2019;10:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


