Abstract
Aims/Hypothesis
The need for understanding obesity as a chronic disease, its stigmatization, and the lack of actionability related to it demands a new approach. The adiposity‐based chronic disease (ABCD) model is based on adiposity amount, distribution, and function, with a three stage complication‐centric rather than a body mass index (BMI)‐centric approach. The prevalence rates and associated risk factors are presented.
Methods
In total, 2159 participants were randomly selected from Czechia. ABCD was established as BMI ≥ 25 kg/m2 or high body fat percent, or abdominal obesity and then categorized by their adiposity‐based complications: Stage 0: none; Stage 1: mild/moderate; Stage 2: severe.
Results
ABCD prevalence was 62.8%. Stage 0 was 2.3%; Stage 1 was 31.4%; Stage 2 was 29.1%. Comparing with other classifiers, participants in Stage 2 were more likely to have diabetes, hypertension, and metabolic syndrome than those with overweight, obesity, abdominal obesity, and increased fat mass. ABCD showed the highest sensitivity and specificity to detect participants with peripheral artery disease, increased intima media, and vascular disease.
Conclusion/Interpretation
The ABCD model provides a more sensitive approach that facilitates the early detection and stratification of participants at risk compared to traditional classifiers.
Keywords: adiposity, cardiovascular disease, epidemiology, obesity, overweight
1. INTRODUCTION
Complications of abnormal adiposity imposed great burden to healthcare systems globally. High body mass index (BMI) was the sixth leading risk factor of death and disability‐adjusted life year (DALY) in 2017, with 8.4% of total deaths and 5.9% of total DALYs.1 The prevalence of obesity (defined as BMI ≥ 30 kg/m2) continues to grow worldwide,2 and a rapid increase in rural areas is partially responsible for these numbers.3 No country has achieved a general decrease in BMI.4 Causes of high BMI are well understood from genetics and biological predisposition,5 to obesogenic environmental factors, to unhealthy behaviors creating a chronic positive energy balance.6 On the other hand, current efforts to address the modifiable factors have not yet effectively mitigated these drivers. This compromised actionability related to overweight/obesity, compounded by the stigmatization of obesity, contributes to the poor outcomes associated with obesity.7
Understanding obesity as a chronic disease was an important first step to change the management paradigm.8, 9 To extend this advancement, the American Association of Clinical Endocrinologists (AACE) developed a new framework known as adiposity‐based chronic disease (ABCD),10 which broadens the focus beyond simple adiposity amounts per BMI classifications, to abnormal adiposity distribution and function, and then contextualizes based on a complication centric‐approach. This novel approach can potentially increase the detection of patients at risk and through proper codification and reimbursement strategies, improve the precision and implementation of treatment protocols.11 Significantly, in 2019, the European Association for the Study of Obesity (EASO) endorsed the ABCD model,12 reinforcing that the BMI‐centric approach simply does not reflect the complexity of the disease, and that applying ABCD to the clinical assessment may improve diagnostic performance. Unfortunately, the performance of the ABCD model in guiding clinical decision‐making has neither been formally evaluated nor validated. This paper on a European population will be the first to determine the prevalence and CVD risks for each ABCD stage, as well as present comparisons with traditional anthropometrics based on BMI, bioelectrical impedance analysis (BIA), and waist circumference supporting the potential utility of this approach. Additional analysis of this new framework is ongoing in diverse populations, including Brazil, Iran, Unite States, Chile, and Venezuela, as a joint effort to improve the understanding of adiposity‐based complications in diverse environments.
2. METHODS
2.1. Design and population
The study design, sampling, and implementation were described previously.13 In brief, Kardiovize study is a cross‐sectional population‐based study with a random sample of 1% of the adult population of Brno, Czech Republic, and is stratified by sex and gender, age range 25–64 years.13 Brno is the second largest city in the Czech Republic, with 373,327 residents in 2013. Eligibility criteria included permanent residence in Brno and registration with any of the five cooperative health insurance companies operating in the country covering 91.1% of the population.
2.2. Sampling and recruitment
Survey sampling was done in January 2013 with technical assistance from the largest (state‐run) health insurance company using the registries of all health insurance companies. A random stratified sample by age and gender of 3300 persons was adjusted for a response rate of 64.4% (as projected from the Czech post‐MONICA study). Health insurance companies mailed invitation letters with a description of the study ensuring confidentiality. Similar to the post‐Monica study, Kardiovize targeted 1% of the adult urban population between 25 and 64 years old. Because the sample size was not reached, a second random sample was done following the same methodology as the first. For the second invitation, 3077 invitations were mailed. Based on the two samplings with a total of 6377 randomly selected invitees, the overall response rate was 33.9% (Figure 1). No information on non‐respondents was available due to confidentiality restrictions. A total of 2159 individuals signed the informed consent to participate and were enrolled.13 For this analysis, participants with type 1 diabetes were excluded.
FIGURE 1.
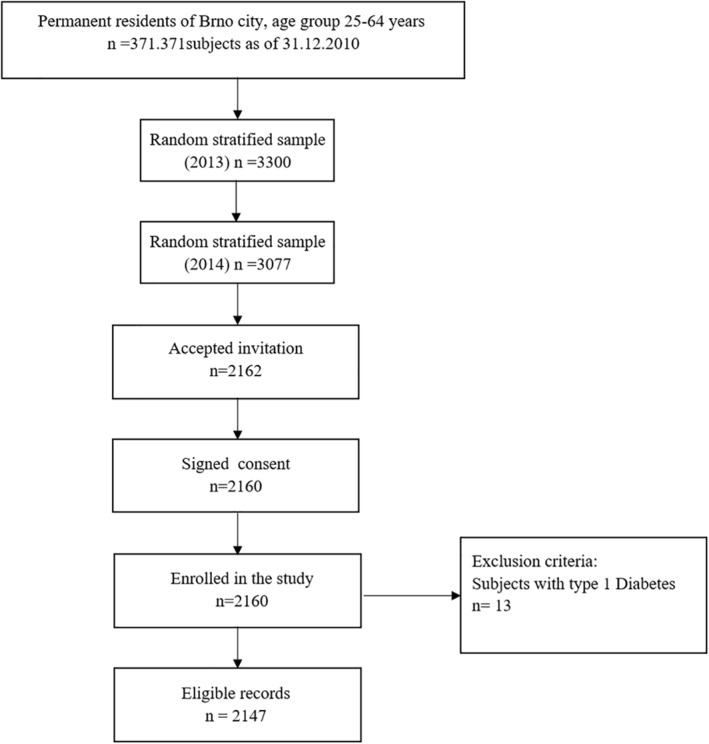
Flow chart of the recruitment, baseline data collection, and selection of participants for the analysis
2.3. Data collection
The baseline health assessment, face‐to‐face health interview, and comprehensive questionnaire were performed by trained nurses and physicians at the International Clinical Research Center (ICRC) of the St. Anne's University Hospital (FNUSA) in Brno; data were collected and entered into the web‐based research electronic data capture (REDCap) database prior to a thorough quality check by the data manager.14 The questionnaire included demographics, socioeconomic status, cardiovascular risk behaviors, and medical history. Laboratory analyses were performed on 12‐h fasting whole blood samples using a Modular SWA P800 analyzer (Roche). Total cholesterol, triglycerides, glucose, and creatinine were analyzed by the enzymatic colorimetric method (Roche Diagnostics GmbH). High‐density lipoprotein cholesterol (HDL‐c) was analyzed with the homogeneous method for direct measurement without precipitation (Sekisui Medical). Low‐density lipoprotein cholesterol (LDL‐c) level was calculated according to the Friedewald equation when triglyceride levels were below 4.5 mmol/L; if the triglyceride level was higher, LDL‐c was analyzed using the homogeneous method for direct measurement (Sekisui Medical). Urinary albumin was analyzed by immunoturbidimetry (Roche Diagnostics GmbH) in a punctual morning urine sample, and the urinary albumin/creatinine ratio was calculated. Blood pressure was measured using an automated office measurement device (BpTRU, model BPM 200; Bp TRU Medical Devices Ltd.).
The anthropometric assessment included height and weight measurements using a medical digital scale with meter (SECA 799; SECA, GmbH and Co. KG) and manual tape measurement of waist, hip, and neck circumference. Weight and body composition analyses were performed using bioelectric impedance analysis (BIA; InBody 370; BIOSPACE Co., Ltd.). The ankle brachial index (ABI) was calculated as the ratio of the highest registered measurements of ankle and brachial blood pressures. Ankle and brachial pressures were measured with the patient lying in the supine position. ABI was measured using VaSera VS‐1500N device (Fukuda Denshi Co., Ltd.). The intima‐media thickness (IMT) ultrasound measurements were obtained with the ESAOTE MyLabClassC ultrasound (ESAOTE S.p.A) using the LA523 4‐13 MHz linear transducer. Both left and right common carotid arteries were measured at 1 cm proximal to their bifurcation. Evaluation of the IMT was performed by semi‐automated ESAOTE MyLabClassC software using patented methods of analyzing RF data from the B‐mode images.15
2.4. Variable and classifier definitions
The diagnosis of ABCD was based on the presence of abnormal adiposity amount, defined as a BMI ≥ 25.0 kg/m2 for Caucasians. Participants were classified as normal weight, overweight, and with obesity according to their BMI: <25.0, 25–29.9, and ≥30 kg/m2, respectively. In participants with BMI <25.0 kg/m2, ABCD was defined as those with BIA as total body fat ˃25% in men and 35% in women, or abnormal adiposity distribution as increased abdominal obesity by waist circumference ≥94 cm in men or ≥80 cm in women.10 Adipose tissue function is based on the validated measurement of biomarkers, such as adipokines; these were not measured in the present study. Participants with ABCD were categorized as Stage 0 when no identifiable adiposity‐based complications were found (cardiometabolic disease stage [CMDS] 0),16 Stage 1 when mild to moderate adiposity‐based complications (CMDS 1) were found, and Stage 2 when severe adiposity‐based complications (CMDS 2–4) were found (Table S1).
Type 2 diabetes was defined as fasting blood glucose ≥126 mg/dl (7.0 mmol/L) or self‐report of diabetes. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic ≥90 mmHg, or personal history of hypertension or use of antihypertensive medication.17 High total cholesterol was defined as total cholesterol ≥5.0 mmol/L. High LDL‐c was categorized according to the global cardiovascular risk calculated by the SCORE and the LDL‐c value: low risk—LDL‐c ≥ 3.0 mmol/L; moderate risk—LDL‐c ≥ 2.6 mmol/L; and high risk—LDL‐c ≥ 1.8 mmol/L.18 Triglycerides were considered elevated if ≥1.7 mmol/L. HDL‐c was considered low if ≤ 1 mmol/L in men or ≤1.2 mmol/L in women. Metabolic syndrome was defined according to the Joint Interim Statement of 2009 as simultaneous presence of three or more of the following components: elevated TG level ≥1.7 mmol/L, or treatment with fibrates or nicotinic acid; low HDL level (<1 mmol/L in men and <1.3 mmol/L in women), or treatment with fibrates or nicotinic acid; previously diagnosed diabetes mellitus, treatment of elevated glucose, or fasting plasma glucose ≥5.6 mmol/L; systolic BP ≥130 mmHg and/or diastolic BP ≥85 mmHg, or treatment of elevated BP; and presence of abdominal obesity, identified as high waist circumference ≥94 cm in men and ≥80 cm in women.19 Vascular disease was defined as a composite of micro‐ and macro‐vascular complications available for the study, as any of the following: (1) personal history of ischemic heart disease, stroke, or claudication; (2) presence of peripheral artery disease, defined as those participants with an ABI < 0.920; (3) carotid IMT thickness increased, defined as those participants with 0.9 mm or more of the maximum measured value of IMT on both carotid17; (4) chronic kidney disease, defined as those with a glomerular filtration rate (GFR) ˂ 60 ml/min/173 m2; or21 (5) microalbuminuria defined as albumin‐to‐creatinine ratio (ACR) between 30 and 300 (μg albumin/mg creatinine) macroalbuminuria as ACR ˃ 300 (μg albumin/mg creatinine).22
Physical activity was assessed using the international questionnaire of physical activity (IPAQ), long version. Participants categorized as “high” where those who participated in vigorous‐intensity activity on at least 3 days and achieving a minimum of at least 1500 MET‐min/week, or seven or more days with any combination of walking, moderate‐intensity, or vigorous‐intensity activities achieving a minimum of at least 3000 MET‐min/week. Participants categorized as “medium” where those who participated in 3 or more days of vigorous activity of at least 20 min/day, or 5 or more days of moderate‐intensity activity or walking of at least 30 min/day, or 5 or more days of any combination of walking, moderate‐intensity, or vigorous‐intensity activities, achieving a minimum of at least 600 MET‐min/week. Participants categorized as “low” where those who did not participate in any of the activities above.23
2.5. Ethics approval
This study protocol complied with the Helsinki declaration and all participants signed the informed consent. The Kardiovize study was approved by the Ethics Committee of St. Anne's University Hospital, Brno, Czech Republic (Ref. Number: 2G/2012).
2.6. Statistical analysis
Analyses were performed using the SPSS software (SPSS, version 25.0, IBM Corp.). The Kolmogorov–Smirnov test was conducted in order to assess the normality of the continuous variables. All variables were non‐normally distributed and therefore reported as median (interquartile range), and their differences were evaluated using the Mann–Whitney U test. Proportions were presented as percentage and 95% confidence intervals (95% CI). Chi‐square test was used to determine different proportions. To determine sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of diverse classifiers and cardiovascular outcomes, crosstab analyses were used. Multinomial regression analysis was used to determine risk factors related to the different adiposity classifiers, adjusting each variable by age and gender.
3. RESULTS
3.1. Participants characteristics
In total, 2147 participants were included, 45.2% men, with a median age of 48.0 (IQR 19.0) years, higher in women (49.0 [20.0]) than men (47.5 [19.0]) (p = 0.019) (Table 1). Men had higher BMI, waist circumference, blood pressure, fasting blood glucose, triglycerides, ABI, and lower HDL‐c than women; women had higher total cholesterol and body fat percent. Men showed higher prevalence of vascular disease and low physical activity. Men also reported higher education level and higher income than women (Table 1).
TABLE 1.
Characteristics of the participants
| Men | Women | Total | p | |
|---|---|---|---|---|
| n (%) | 970 | 1177 | 2147 | |
| Age (years) | 47.5 (19) | 49.0 (20) | 48.0 (19) | 0.019 |
| BMI (kg/m2) | 26.0 (5.0) | 24.0 (6.0) | 25.0 (6.0) | <0.001 |
| Waist circumference (cm) | 96.0 (16.0) | 82.0 (18.0) | 89.0 (21.0) | <0.001 |
| Body fat percent (%) | 21.0 (9.0) | 31.0 (13.0) | 26.0 (14.0) | <0.001 |
| Systolic blood pressure (mmHg) | 121.2 (17.6) | 116.2 (19.8) | 118.8 (19.6) | <0.001 |
| Diastolic blood pressure (mmHg) | 82.2 (12.0) | 77.4 (11.6) | 79.6 (12.6) | <0.001 |
| Fasting blood glucose (mmol/L) | 5.0 (0.7) | 4.8 (0.7) | 4.9 (0.7) | <0.001 |
| Total cholesterol (mmol/L) | 5.0 (1.3) | 5.2 (1.3) | 5.1 (1.3) | 0.003 |
| LDL‐c (mmol/L) | 3.0 (1.2) | 3.0 (1.3) | 3.0 (1.2) | 0.104 |
| HDL‐c (mmol/L) | 1.3 (0.4) | 1.6 (0.5) | 1.5 (0.5) | <0.001 |
| Triglycerides (mmol/L) | 1.2 (0.9) | 0.9 (0.7) | 1.1 (0.8) | <0.001 |
| Carotid intima‐media thickness | 637.0 (198) | 590.5 (186) | 616.5 (153) | <0.001 |
| Ankle brachial index < 0.9 | 1.1 (0.1) | 1.0 (0.1) | 1.1 (0.1) | <0.001 |
| Vascular disease | 8.6 (6.8–10.3) | 5.1 (3.8–6.3) | 6.7 (5.6–7.7) | 0.001 |
| Physical activity level (%) | ||||
| High | 51.0 (47.9–54.2) | 49.2 (46.3–52.0) | 50.0 (47.9–52.1) | <0.001 |
| Moderate | 33.9 (29.4–35.3) | 42.3 (36.4–42.0) | 36.0 (34.0–38.1) | |
| Low | 16.7 (14.5–19.2) | 11.6 (9.9–13.6) | 13.9 (12.5–15.4) | |
| Education level (%) | ||||
| Primary | 21.0 (18.5–23.6) | 19.0 (16.9–21.4) | 19.9 (18.3–21.7) | <0.001 |
| Secondary | 33.9 (31.0–36.9) | 42.3 (39.5–45.1) | 38.5 (36.4–40.5) | |
| Higher | 45.1 (42.0–48.3) | 38.7 (35.9–41.5) | 41.6 (39.4–43.5) | |
| Household income (Euro) (%) | ||||
| High (>1800) | 32.2 (29.3–35.3) | 19.6 (17.4–22.1) | 25.4 (23.5–27.3) | <0.001 |
| Middle (1200–1800) | 34.0 (31.0–37.1) | 29.9 (27.2–32.7) | 31.7 (29.7–33.8) | |
| Low (<1200) | 33.8 (30.8–36.9) | 50.5 (47.5–53.5) | 42.8 (40.7–45.0) | |
Note: Continuous variables are median and IQR. Mann–Whitney U test was used to determine different medians. Proportions are present as percent and 95% confidence intervals. Chi‐square test was used to determine different proportions.
Abbreviations: BMI, body mass index; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol.
3.2. Prevalence of ABCD
The prevalence of ABCD was 62.8%, higher in men (67.2%) than in women (60.3%) (p < 0.001). The prevalence of ABCD by stages was 2.3% (Stage 0), 31.4% (Stage 1), and 29.1% (Stage 2) (Figure 2). The prevalence of Stages 0 and 2 were higher in men, and Stage 1 was higher in women (p < 0.001). The prevalence of Stage 2 increased steadily with age (p < 0.001), while Stage 0 decreased with age.
FIGURE 2.
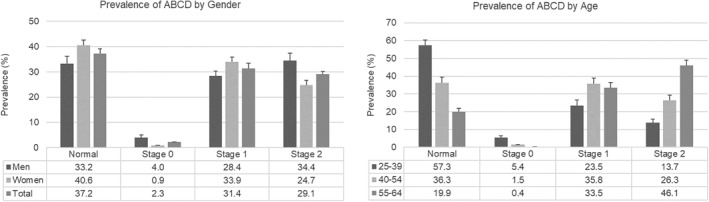
Prevalence of adiposity‐based chronic disease (ABCD). The left graph presents the prevalence of ABCD by gender, showing how the Stages 0 and 2 were higher in men and normal and 1 in women (p < 0.001). The right graph presents the prevalence by age groups, showing how the normal stage decrease steadily with age and stage 2 increase with age (p < 0.001)
3.3. Comparison of prevalence rates between ABCD and other anthropometric classifiers
A higher proportion of participants fulfilled the definition of ABCD (62.8%) than definitions of overweight and obesity based on BMI (34.0% and 18.9%, respectively; 52.9% combined), abdominal obesity (57.5%), and high body fat percentage (29.6%) classifiers (Figure 3). Significantly, the ability of the ABCD definition to detect more participants was observed in both genders, but particularly evident in men, and in all age groups.
FIGURE 3.
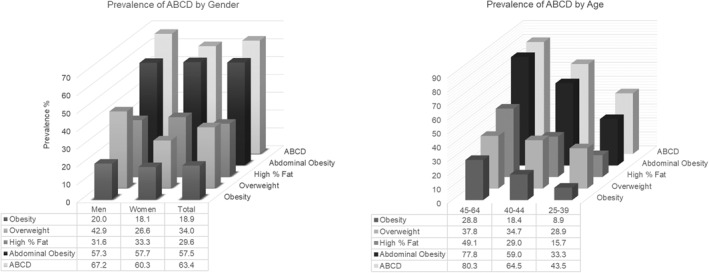
Comparison among the prevalence of adiposity‐based chronic disease (ABCD) and other standard anthropometric measurement. The upper graph presents how the ABCD model detects a higher proportion of participants compared with other definitions in both genders. The lower graph presents how the prevalence changes with age (p < 0.001)
3.4. Risk factors related to the ABCD model and the other classifiers
ABCD was more strongly associated with diverse cardiometabolic risk factors than traditional classifiers for obesity and adiposity. Logistic regression analysis adjusting by age and gender were implemented to assess the associations among cardiometabolic, behavioral, and social risk factors (Table 2). Within ABCD stages, participants in Stage 2 were more likely to have low physical activity, diabetes, hypertension, low HDL‐c, high triglycerides, metabolic syndrome, peripheral arterial disease, high IMT, vascular disease, low education level, and lower income than participants in ABCD Stage 0 or 1. None of those in ABCD Stage 0 or 1 had increased IMT, peripheral arterial disease, or vascular disease.
TABLE 2.
Risk factors related to the different adiposity definitions, adjusting each variable by age and gender using a multinomial regression analysis
| ABCD | BMI categories | Abdominal obesity | High % fat mass | ||||
|---|---|---|---|---|---|---|---|
| Risk factors | Stage 0 | Stage 1 | Stage 2 | Overweight | Obesity | Present | Present |
| Physical activity | |||||||
| High | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Moderate | 0.71 (0.36–1.39) | 1.13 (0.90–1.43) | 1.30 (1.01–1.67) | 1.20 (0.97–1.49) | 1.07 (0.82–1.39) | 1.25 (1.02–1.52) | 1.26 (1.03–1.56) |
| Low | 0.77 (0.31–1.92) | 0.99 (0.70–1.39) | 1.74 (1.24–2.44) | 1.18 (0.87–1.60) | 1.29 (0.91–1.85) | 1.33 (1.00–1.77) | 1.50 (1.13–1.99) |
| Diabetes | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | ‐ | ‐ | 10.2 (4.59–22.5) | 2.94 (1.47–5.89) | 7.73 (3.95–15.1) | 5.23 (2.48–11.0) | 3.41 (2.18–5.33) |
| Hypertension | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | 0.43 (0.15–1.25) | 2.21 (1.71–2.85) | 7.00 (5.37–9.13) | 2.22 (1.77–2.78) | 7.47 (5.64–9.89) | 3.52 (2.84–4.37) | 3.93 (3.18–4.85) |
| High total cholesterol | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | 0.66 (0.35–1.26) | 1.70 (1.36–2.12) | 1.28 (1.01–1.62) | 1.51 (1.23–1.86) | 1.06 (0.83–1.36) | 1.39 (1.15–1.68) | 1.38 (1.13–1.69) |
| High LDL‐c | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | 0.46 (0.23–0.89) | 1.72 (1.34–2.20) | 1.42 (1.09–1.85) | 1.49 (1.18–1.88) | 1.42 (1.06–1.89) | 1.41 (1.15–1.74) | 1.59 (1.26–2.00) |
| Low HDL‐c | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | ‐ | 1.47 (0.93–2.32) | 13.2 (8.83–19.7) | 3.00 (2.13–4.22) | 7.00 (4.90–9.99) | 4.77 (3.38–6.73) | 3.39 (2.59–4.44) |
| High triglycerides | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | ‐ | 1.83 (1.24–2.70) | 14.53 (10.15–20.78) | 3.57 (2.69–4.74) | 4.46 (3.24–6.15) | 4.39 (3.29–5.86) | 3.09 (2.44–3.93) |
| Metabolic syndrome | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | ‐ | 2.34 (0.97–5.64) | 319.5 (146.9–695.0) | 6.69 (4.79–9.35) | 19.3 (13.5–27.6) | 50.7 (26.7–96.3) | 6.76 (5.32–8.60) |
| Peripheral artery disease | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | ‐ | ‐ | 3.70 (1.92–7.13) | 0.87 (0.47–1.63) | 2.08 (1.10–3.92) | 0.98 (0.54–1.78) | 2.26 (1.24–4.11) |
| Increased IMT | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | ‐ | ‐ | 11.50 (1.38–95.96) | 2.38 (0.59–9.54) | 1.72 (0.35–8.35) | 2.86 (0.62–13.15) | 1.01 (0.36–2.85) |
| Vascular disease | |||||||
| Absent | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Present | ‐ | ‐ | 3.93 (2.49–6.18) | 1.47 (0.94–2.29) | 2.42 (1.53–3.83) | 1.51 (1.00–2.28) | 2.13 (1.48–3.07) |
| Education level | |||||||
| Higher | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Secondary | 1.61 (0.88–2.96) | 1.27 (1.00–1.62) | 2.04 (1.56–2.65) | 1.42 (1.14–1.78) | 2.18 (1.64–2.90) | 1.53 (1.24–1.89) | 1.57 (1.25–1.95) |
| Primary | 2.58 (0.17–1.97) | 1.55 (1.14–2.10) | 2.80 (2.03–3.85) | 1.66 (1.25–2.20) | 2.83 (2.03–3.95) | 2.10 (1.61–2.74) | 2.40 (1.86–3.11) |
| Household income (Euros) (%) | |||||||
| High (>1800) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Middle (1200–1800) | 1.93 (0.95–3.92) | 0.92 (0.69–1.22) | 1.11 (0.80–1.53) | 1.23 (0.94–1.62) | 0.97 (0.68–1.37) | 1.01 (0.78–1.29) | 1.07 (0.81–1.41) |
| Low (<1200) | 0.74 (0.29–1.87) | 1.08 (0.81–1.43) | 1.65 (1.21–2.25) | 1.20 (0.92–1.57) | 1.42 (1.03–1.96) | 1.24 (0.97–1.59) | 1.58 (1.22–2.04) |
Note. Logistic regression analysis adjusting each variable by age and gender.
Abbreviations: ABCD, adiposity‐based chronic disease; IMT, intima‐media thickness; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol.
When comparing ABCD Stage 2 with the other classifiers, the diagnose of type 2 diabetes was 3.5x, 1.3x, 2.0x, and 3.0x more likely than those with overweight, obesity, abdominal obesity, and increased fat mass, respectively; the diagnose of hypertension was similar to those with obesity, but 2.2x, 2.0x, and 1.8x more likely than those with overweight, abdominal obesity, and those in with increased fat mass, respectively. A diagnosis with metabolic syndrome was 40 times more likely in participants with ABCD Stage 2 than those with overweight or increased adiposity, 17 times than those with obesity, and 6 times than those with abdominal obesity. Peripheral artery disease was not associated with overweight and abdominal obesity but was associated with ABCD Stage 2, obesity, and increased adiposity. Vascular disease was not associated with overweight. More specifically, participants with ABCD Stage 2 were 1.9, 4.1, and 1.7 times more likely to present peripheral artery disease than those with obesity, abdominal obesity, and increased fat mass, respectively. Increased IMT was only associated with ABCD Stage 2.
Social determinants of health were strongly associated with the different classifiers. Compared with those having a university degree, participants with only a primary education level (grades 1 to 5) were almost three times more likely to present ABCD Stage 2 or obesity, but only two times more likely to present with abdominal obesity or increased adiposity. In those with secondary degree (high school), ABCD Stage 2 and the other classifications were between 42% and 118%, more likely than in those participants with university degree. Compared with participants that reported high household income, those with lower income were 65% more likely to have ABCD Stage 2, 58% more likely to have increased adiposity, and only 42% more likely to have obesity.
The ABCD definition showed the highest sensitivity and specificity to detect participants with cardiovascular complications compared with traditional classifiers. To detect peripheral artery disease, an ABCD diagnosis had a sensitivity of 64.7 followed by abdominal obesity with 54.9, and other definitions <47; ABCD Stage 0 showed the highest specificity with 97.7, and Stage 2 showed the area under the curve (AUC) with 0.68, and highest PPV (Table 3). To detect increased IMT, an ABCD diagnosis had the highest sensitivity with 93.8, followed by abdominal obesity with 87.5, and other definitions <57; ABCD Stage 0 showed the highest specificity with 97.4, and Stage 2 showed the highest AUC with 0.84, and the highest PPV. To detect vascular disease, an ABCD diagnosis showed the highest sensitivity with 77.6, followed also by abdominal obesity with 73.2, and other definitions <54; the highest specificity was also for ABCD Stage 0 with 97.5, and ABCD Stage 2 showed the highest AUC with 0.76.
TABLE 3.
Sensitivity, specificity, PPV, and NPV of diverse definitions associated with cardiovascular outcomes
| Peripheral arterial disease | PPV | NPV | Increased intima‐media thickness | PPV | NPV | Vascular Diseases | PPV | NPV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | Sensitivity | Specificity | AUC | Sensitivity | Specificity | AUC | |||||||
| ABCD present | 64.7 | 36.4 | 0.51 | 2.5 | 97.6 | 93.8 | 35.4 | 0.65 | 2.7 | 99.7 | 77.6 | 37.3 | 0.57 | 8.1 | 95.9 |
| ABCD stage 0 | 0.0 | 97.7 | 0.49 | 0.0 | 97.4 | 0.0 | 97.4 | 0.49 | 0.0 | 98.0 | 0.0 | 97.5 | 0.49 | 0.0 | 93.1 |
| ABCD stage 1 | 0.0 | 67.6 | 0.34 | 0.0 | 96.3 | 0.0 | 65.9 | 0.33 | 0.0 | 97.1 | 0.0 | 66.1 | 0.33 | 0.0 | 90.1 |
| ABCD stage 2 | 64.7 | 72.2 | 0.68 | 5.6 | 98.8 | 93.8 | 73.6 | 0.84 | 6.4 | 99.8 | 77.6 | 74.9 | 0.76 | 18.0 | 97.9 |
| Abdominal obesity | 54.9 | 42.1 | 0.48 | 2.3 | 97.4 | 87.5 | 41.8 | 0.65 | 2.8 | 99.4 | 73.2 | 43.3 | 0.58 | 8.4 | 95.8 |
| High body fat % | 46.9 | 68.6 | 0.58 | 3.5 | 98.2 | 43.8 | 67.9 | 0.56 | 2.5 | 98.4 | 53.3 | 69.7 | 0.61 | 10.8 | 95.6 |
| Overweight | 29.4 | 65.6 | 0.47 | 2.1 | 97.4 | 56.3 | 64.8 | 0.60 | 3.0 | 98.7 | 37.1 | 66.2 | 0.52 | 7.2 | 93.6 |
| Obesity | 29.4 | 81.5 | 0.55 | 3.9 | 97.9 | 25.0 | 81.4 | 0.53 | 2.5 | 98.3 | 34.5 | 82.2 | 0.58 | 12.2 | 94.6 |
Abbreviations: ABCD, adiposity‐based chronic disease; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value.
4. DISCUSSION
The new complication‐centric ABCD framework is more sensitive and specific than the existing BMI‐centric classification models by detecting a higher proportion of participants at risk with better risk‐stratification. Using available metrics, the prevalence of ABCD in adults between 25 and 64 years in a population of Czech Republic was 62.8%; this prevalence increased with age and was higher in men than women. Almost all traditional cardiometabolic risk factors, including hypertension, dyslipidemia, metabolic syndrome, and vascular disease, were more strongly associated with ABCD Stage 2, compared with traditional definitions of obesity by BMI, abdominal obesity by waist circumference, and increased adiposity by BIA.
Compared with the prevalence of overweight (45.2%) and obesity (14.8%) of the European Social Survey in Czech Republic in 2014,24 the prevalence of overweight in this study was lower (34.0%), but obesity was higher (18.9%). This difference could be mainly attributed to the different range of age groups included: the European Social Survey has a large proportion of participants older than 60 years old with a high prevalence of overweight and obesity. The European survey included data from 20 countries,24 and the prevalence of overweight ranged from 31.9% in France to 45.2% in Czech Republic, and the prevalence of obesity ranged from 10.9% in Switzerland to 20.8% in Slovenia. Overall and similar to our study, the prevalence of overweight was higher in men than women and increased steadily with age, with the prevalence for obesity similar between genders.
Understanding obesity as a complex, chronic disease with biological and social drivers, many of which are independent of classically defined unhealthy behaviors,9 is an important departure from the public's oftentimes stigmatized view of this condition. In fact, this new perspective can promote a more concerted management plan,9 , 25 and perhaps a transformed healthcare infrastructure for more effective delivery. The inclusion of the words “chronic disease” in ACBD draws constructive attention to this new paradigm,10 and its unique staging system secures a proactive search for relevant complications. In this study, participants with ABCD Stage 2 were more likely to have obesity‐related complications than traditional classifiers, particularly evident with increased IMT and the diagnosis of metabolic syndrome.
The environmental and sociocultural influence of adiposity and its complications are well described in the obesity transition model proposed by Jaacks et al.,4 identifying four epidemiological stages. Stage 1 is characterized by a rise in the prevalence of obesity in women to above 5%, but lower than 20%, higher than men and children, and a higher prevalence of obesity in higher socioeconomic status (SES) than lower SES. In 1975, countries at Stage 1 were Mexico, Colombia, and Brazil in Latin America; several Middle Eastern countries (Egypt, Turkey, and Iran); Russia; and South Africa. Currently, many countries in south Asia and sub‐Saharan Africa are in this stage. Stage 2 is characterized by a large increase in the prevalence of obesity in adults and smaller increase in children, with a narrowing of the gap between genders and between SES in women. The prevalence of obesity in women at this stage ranges between 25% and 40%. Almost all countries at Stage 1 in 1975 were currently at Stage 2 by 2016. In Stage 3, the differences between genders are decreased and a reversal of the SES differences is observed, with an acceleration in the prevalence of obesity in lower SES surpassing that in high SES. Additionally, there is a more significant increase in the prevalence rate of obesity in children. Stage 3 has been identified currently in the US and European adults. In our study, obesity and ABCD were more prevalent in men than women; however, both conditions were more likely in lower than higher SES, a characteristic commonly observed in the Stage 3 of the obesity transition described above. Stage 4 is a hypothetical reduction on the prevalence of obesity, mainly driven by a reduction in the prevalence in children in high SES, in high‐income countries. Consequently, leaner children will enter adulthood without obesity. Based on this prediction model,4 the Czech population appears to be transitioning from Stage 3 to 4. Hamřík et al.26 recently presented the trend in prevalence of overweight and obesity in children of Czech Republic from 1998 to 2014 (Figure S1), with an increased prevalence rate from 1998 to 2010 and plateau between 2010 and 2014.
Among the limitations, the cross‐sectional design can only demonstrate associations among ABCD stages and cardiometabolic risk factors, and the low response rate limits its representativeness to the entire Czech population. The analysis of adiposity‐based complications in this study was only assessed on the data available and diverse other complications could not be evaluated (e.g., polycystic ovary, fatty liver, sleep apnea). The Kardiovize study is currently following this population cohort and collecting data that can determine the predictive value of ABCD stages for cardiovascular events to further validate the model.
In conclusion, this is the first study specifically evaluating the ABCD model and demonstrates superiority over BMI‐centric models. The ABCD model facilitates early detection, stratification of participants at risk, which do not currently fulfill any of the standard criteria of obesity and often end up overlooked. Since 63.4% of the population evaluated had ABCD, half of them with moderate to high risk for complications (Stage 2), the other half with low risk (Stage 1), and a very small proportion (2.3%) without evidence of risk factors at all (Stage 0), the opportunity is exposed for early, sustainable, and successful prevention. This approach offers tremendous potential for population‐based healthcare systems to reduce the burdens of abnormal adiposity, dysglycemia, and vascular disease, while improving quality of life and through diminished morbidities, the availability of healthcare funding.
CONFLICT OF INTEREST
Dr. Mechanick has received honoraria for lectures and program development from Abbott Nutrition International. The other authors have not conflict of interest.
Supporting information
FIGURE S1
TABLE S1
ACKNOWLEDGMENTS
The authors are grateful to all participants of the study and the team members Jana Jaresova that contributed with coordination of the study and Hana Pernikova, Jana Hruskova, Juraj Jakubik, Alena Zajickova, Maria Skladana, and Anna Pospisilova that contributed with the data collection.
The Kardiovize study was supported by the European Regional Development Fund—Project FNUSAICRC (no. CZ.1.05/1.1.00/02.0123), by project no. LQ1605 from the National Program of Sustainability II (MEYS CR), by project ICRC‐ERA‐Human Bridge (no. 316345) funded by the 7th Framework Program of the European Union, and partly by a grant by the Ministry of Health of the Czech Republic(NT13434‐4/2012), Project ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868).
Gonzalez‐Rivas JP, Mechanick JI, Hernandez JP, et al. Prevalence of adiposity‐based chronic disease in middle‐aged adults from Czech Republic: the Kardiovize study. Obes Sci Pract. 2021;7(5):535‐544. doi: 10.1002/osp4.496
REFERENCES
- 1.Institute for Health Metrics and Evaluation (IHME) . Global Burden Disease (GBD) Compare. https://vizhub.healthdata.org/gbd‐compare/. Accessed February 20, 2020. [Google Scholar]
- 2.Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bixby H, Bentham J, Zhou B, et al. Rising rural body‐mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804‐814. [DOI] [PubMed] [Google Scholar]
- 7.Rubino F, Puhl RM, Cummings DE, Eckel RH, Ryan DH, Mechanick JI, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26:485‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle TK, Dhurandhar EJ, Allison DB. Regarding obesity as a disease: evolving policies and their implications. Endocrinol Metab Clin N Am. 2016;45:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray GA, Kim KK, Wilding JPH, obotWO Federation . Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715–723. [DOI] [PubMed] [Google Scholar]
- 10.Mechanick JI, Hurley DL, Garvey WT. Adiposity‐based chronic disease as a new diagnostic term: The American Association of Clinical Endocrinologists and American College of Endocrinology position statement. Endocr Pract. 2017;23:372–378. [DOI] [PubMed] [Google Scholar]
- 11.Garvey WT, Mechanick JI. Proposal for a scientifically correct and medically actionable disease classification system (ICD) for obesity. Obesity. 2020;28:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obesity facts. 2019;12:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Movsisyan NK, Vinciguerra M, Lopez‐Jimenez F, et al. Kardiovize Brno 2030, a prospective cardiovascular health study in Central Europe: methods, baseline findings and future directions. Eur J Prev Cardiol. 2018;25:54–64. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA. Research electronic data capture (REDCap)‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hruskova J, Maugeri A, Podroužková H, et al. Association of cardiovascular health with epicardial adipose tissue and intima media thickness: the Kardiovize study. J Clin Med. 2018;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garvey WT, Garber AJ, Mechanick JI, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the 2014 advanced framework for a new diagnosis of obesity as a chronic disease. Endocr Pract. 2014;20:977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 18.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. A Joint Interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 20.Miname M, Bensenor IM, Lotufo PA. Different methods of calculating ankle‐brachial index in mid‐elderly men and women: the Brazilian longitudinal study of adult health (ELSA‐Brasil). Braz J Med Biol Res. 2016;49:e5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapter 1: definition and classification of CKD. Kidney Int Suppl. (2011) 2013;3:19–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basi S, Fesler P, Mimran A, Lewis JB. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabets Care. 2008;31:S194–S201. 10.2337/dc08-s249. [DOI] [PubMed] [Google Scholar]
- 23.Wanner M, Probst‐Hensch N, Kriemler S, Meier F, Autenrieth C, Martin BW. Validation of the long international physical activity questionnaire: influence of age and language region. Prev Med Rep. 2016;3:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marques A, Peralta M, Naia A, Loureiro N, de Matos MG. Prevalence of adult overweight and obesity in 20 European countries. Eur J Publ Health. 2014;28:295–300. [DOI] [PubMed] [Google Scholar]
- 25.Nieto‐Martinez R, Gonzalez‐Rivas JP, Ugel E, et al. Application of the AACE/ACE advanced framework for the diagnosis of obesity and cardiometabolic disease staging in a general population from 3 Regions of Venezuela: the VEMSOLS study results. Endocr Pract. 2017;24:6–13. [DOI] [PubMed] [Google Scholar]
- 26.Hamřík Z, Sigmundová D, Pavelka J, Kalman M, Sigmund E. Trends in overweight and obesity in Czech schoolchildren from 1998 to 2014. Cent Eur J Publ Health. 2017;25(Suppl 1):S10–S14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1
TABLE S1


