Introduction
The pioneering of new medications promises improved disease management for patients, but invites a multitude of adverse reactions. The recent advent and increased use of biologic agents, such as denosumab, has been accompanied by a growing number of autoimmune events. Denosumab is a human monoclonal antibody targeting the receptor activator of nuclear factor κΒ ligand (RANKL), a key protein in osteoclast-mediated bone resorption. It is indicated for postmenopausal osteoporosis with a high risk of fracture and for skeletal complications of malignancy.1 Evidence on denosumab's safety and associated reactions is scarce due to its recent approval for clinical use. The FREEDOM trial details the most common adverse events as severe infection, cutaneous effects, and osteonecrosis of the jaw.2 Less common events, such as alopecia areata (AA) and lichen planus (LP), are mentioned in case reports, suggesting denosumab's role in autoimmunity.3,4 AA is an autoimmune disorder characterized by a reversible hair loss that preserves the hair follicle. AA can manifest as a single patch, conjoined patches, or multiple and separated patches of hair loss.5 LP is a cutaneous inflammatory condition suspected to be caused by autoimmunity. LP lesions appear as violaceous polygonal papules and typically appear on the flexural region of the wrists and ankles.6 We review a case of AA and LP attributed to denosumab therapy with remission after cessation of the biologic drug.
Case report
A 71-year-old African American woman with a medical history of hypertension and osteoporosis presented with a 2-month onset of rapidly progressive hair loss and multiple skin lesions. These findings occurred 2 months after the patient received a 60-mg subcutaneous dose of denosumab for her osteoporosis that was resistant to bisphosphonates, calcium, and vitamin D. She experienced rapidly progressive, patchy, nonscarring hair loss and pruritic, plaque-like skin lesions on the right tibia and wrist. Hair loss continued for another 2 months before the patient visited the dermatology clinic. She denied a personal or familial history of AA and LP. She also denied recent travel, infection, new medications, and major life stressors. Physical examination found sharply demarcated patches of hair loss limited to 50% of the patient's scalp, more significant in the crown, vertex, and occipital areas. Skin biopsy of the scalp was not performed as clinical and trichoscopic findings revealed features of AA. A scaly violaceous polygonal papule was located on the volar aspect of the patient's wrist (Fig 1). A shave biopsy of the right wrist was performed with histologic analysis (Fig 2). The findings of her laboratory examination, including an electrolyte panel, complete blood cell count, antinuclear antibodies, hepatitis C, and hepatitis B, were normal. The patient's second dose of denosumab injection was held due to severe symptoms related to LP. On the initial presentation, 4 months after injection with denosumab in November 2019, the patient was started on topical 0.05% augmented betamethasone twice daily for AA and LP. A follow-up in December 2019 revealed the resolution of lichenoid papules and hair regrowth, with complete recovery in June 2020 (Fig 3).
Fig 1.
Scaly polygonal lichenoid papule on the right wrist.
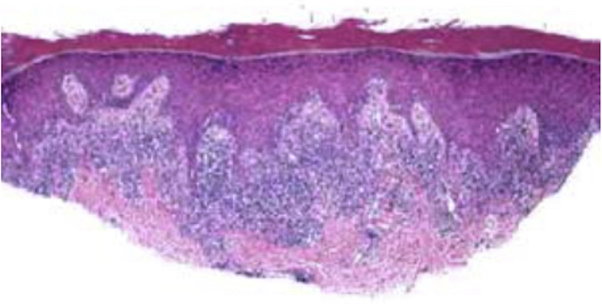
Fig 2.
The histopathology of lichen planus on the right wrist: acanthosis, hypergranulosis, hyperkeratosis, and a sawtooth pattern of the rete ridges architecture. A band-like lymphocytic infiltrate with basal keratinocyte injury and cytoid body formation can be observed.
Fig 3.
A, Widespread alopecia areata of the scalp 4 months after denosumab injection. B, Hair regrowth 11 months after denosumab injection.
Discussion
Autoimmune induction of AA is characterized as a rare side effect of denosumab therapy for osteoporosis.4 Denosumab is a monoclonal antibody against RANKL, inhibiting osteoclast maturation and bone resorption. We presented a case of a patient in whom AA and LP developed after she received denosumab for osteoporosis. The patient had no previous history of autoimmune disease and no previous eruptions of LP or AA. Although there have been other reports of AA after initiating denosumab therapy, we discussed a case of a patient with AA and concomitant LP that was reversible upon cessation of denosumab.
Because AA is mostly a self-limiting disease, it is possible that the remission of this patient's hair loss was spontaneous and unrelated to the discontinuation of denosumab. However, the temporal association between discontinuing denosumab before complete remission of AA and LP led us to believe that spontaneous remission is less likely.
In addition to modulating bone resorption, the interaction between RANKL and receptor activator of nuclear factor κΒ (RANK) plays a role in regulating immunity. We hypothesized that there is a link between the induction of LP and AA and the use of denosumab, based on previous reports describing lichenoid eruptions, and instances of alopecia within weeks of starting denosumab. Our hypothesis alludes to the involvement of the RANKL-RANK axis in central and peripheral tolerance.
Central tolerance refers to the maturation of T cells undergoing positive and negative selection in the thymus during embryogenesis. Negative selection in the thymic medulla relies on medullary thymic epithelial cells (mTECs) displaying self-antigens to negatively select autoreactive T cells. Self-antigens presented by mTECs are made via the expression of the autoimmune regulator gene. mTEC progenitor cells interact with RANKL-expressing lymphoid tissue inducer cells, ensuring the autoimmune regulator gene expression. Mice deficient for RANK or RANKL lack the autoimmune regulator gene and mTEC in the embryonic thymus. Although the requirement of RANKL-RANK is more important during embryonic mTEC development, it still plays an important role in maintaining adult mTECs.7
Peripheral tolerance refers to the processes occurring outside the primary lymphoid tissues that prevent autoimmunity, relying on antigen-presenting cells and regulatory T cells. Antigen-presenting cells are immune cells, such as dendritic cells (DCs) in the skin, responsible for presenting self or foreign antigen to T cells for maturation.7 This maturation process is necessary for the specification of the immune reaction. RANKL in the epidermis binds to RANK on DCs, priming them to maintain peripheral T cells. This RANKL-RANK interaction prolongs the survival of DCs, increases the number of regulatory T cells, and destroys autoreactive T cells in the periphery via Fas-mediated apoptosis.7, 8, 9 To sufficiently suppress autoimmunity, DCs must survive long enough for a proper interaction with T cells. The T cell–DC interaction involves several costimulatory signals, such as RANKL on T cells interacting with RANK on DCs. This interaction enhances the specification of the immune response.7 These costimulatory signals ensure the survival of DC by upregulating antiapoptotic proteins such as BCL-xL and BCL2.8
We have not been able to find previous cases of AA and concomitant LP with the resolution of symptoms after discontinuation of denosumab. Surveillance of adverse events following administration of biologic medications is essential for maintaining patient safety.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Deeks E.D. Denosumab: a review in postmenopausal osteoporosis. Drugs Aging. 2018;35(2):163–173. doi: 10.1007/s40266-018-0525-7. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli R., Reginster J.Y. Adverse drug reactions to osteoporosis treatments. Expert Rev Clin Pharmacol. 2011;4(5):593–604. doi: 10.1586/ecp.11.42. [DOI] [PubMed] [Google Scholar]
- 3.Cachia M., Betts A., Aquilina S. Denosumab and lichen planus: two cases. Clin Exp Dermatol. 2021;46(2):356–357. doi: 10.1111/ced.14378. [DOI] [PubMed] [Google Scholar]
- 4.Lyakhovitsky A., Oshinsky S., Gilboa S., Barzilai A. Alopecia areata after denosumab treatment for osteoporosis. JAAD Case Rep. 2016;2(4):298–300. doi: 10.1016/j.jdcr.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratt C.H., King L.E., Jr., Messenger A.G., Christiano A.M., Sundberg J.P. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi: 10.1038/nrdp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusari A., Ahluwalia J. Lichen planus. N Engl J Med. 2018;379(6):567. doi: 10.1056/NEJMicm1802078. [DOI] [PubMed] [Google Scholar]
- 7.Walsh M.C., Choi Y. Regulation of T cell-associated tissues and T cell activation by RANKL-RANK-OPG. J Bone Miner Metab. 2021;39(1):54–63. doi: 10.1007/s00774-020-01178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izawa T., Ishimaru N., Moriyama K., Kohashi M., Arakaki R., Hayashi Y. Crosstalk between RANKL and Fas signaling in dendritic cells controls immune tolerance. Blood. 2007;110(1):242–250. doi: 10.1182/blood-2006-11-059980. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama T., Shinzawa M., Akiyama N. RANKL-RANK interaction in immune regulatory systems. World J Orthop. 2012;3(9):142–150. doi: 10.5312/wjo.v3.i9.142. [DOI] [PMC free article] [PubMed] [Google Scholar]