Abstract
Introduction
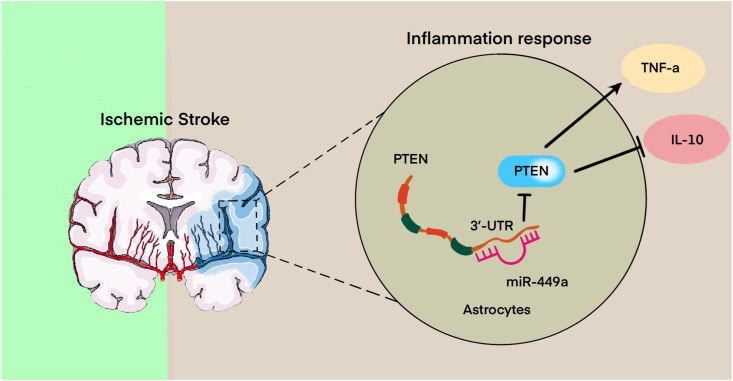
Ischemic stroke (IS) is a common and severe neurological disorder and is associated with high rates of mortality and morbidity. Inflammatory reaction in astrocytes is one of the important pathological factors of stroke. Improved understanding of the underlying molecular mechanisms should aid better treatment of the disease. This study aimed to test our hypothesis that a miR-499a played an important role in the inflammatory response in astrocytes induced by IS targeting phosphatase and tensin homologue deleted on chromosome 10 (PTEN).
Methods
This study was comprised of two models: oxygen-glucose deprivation (OGD) and reoxygenation model. Quantitative real-time PCR (qRT-PCR) and Western blot were used to examine gene expression levels, and MTT assay analysis were used to examine cell states. The relationships between miR-499a and PTEN were confirmed by luciferase reporter assay.
Results
MiR-499a was robustly downregulated with OGD induced injury in astrocytes. Forced transient expression of miR-499a in OGD astrocytes nearly completely reversed the inflammatory response. Knockdown of miR-499a by its specific inhibitor in healthy astrocytes induced the inflammatory response resembling those produced by OGD. On the other hand, PTEN was markedly upregulated in OGD astrocytes, which was reciprocal to the expression of miR-499a. PTEN was experimentally validated as a direct target gene for miR-499a. Overexpression of PTEN was able to induce an inflammatory response of astrocytes. Moreover, PTEN siRNA counteracted the inflammatory response induced by OGD.
Conclusions
Taken together, our findings indicate miR-499a as an important factor to prevent inflammatory response and suggest miR-499a as a new molecule for the treatment of IS. The present study also demonstrated the relationship between miR-499a and PTEN, with PTEN as a downstream signaling mediator of miR-499a in the inflammatory response of astrocytes induced by IS.
Keywords: miR-499a, PTEN, Stroke
1. Introduction
Ischemic stroke (IS) is the second-highest cause of death globally and a leading cause of disability [1]. Astrocytes are the most numerous glial cell types of brain, play important roles in acute cerebrovascular disease [2]. Astrocytes can provide support to neurons under ischemia-hypoxic and secrete a series of pro-inflammatory and anti-inflammatory cytokines in the central nervous system (CNS) [3]. Ischemic inflammation mediated by astrocytes is a vital contributing factor in acute cerebrovascular disease. Cerebrovascular injury astrocytes stimulate the release of pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and anti-inflammation cytokines interleukin-10 (IL-10) [4,5].
An increasing body of evidence indicates that microRNAs (miRNAs) serve as an important layer of the regulatory network in cerebrovascular disease including IS and intracerebral hemorrhage (ICH). MiR-499a associated with autoimmune thyroid diseases and the upregulated expression of miR-499a induced by pregnancy-related complications in placental tissue may cause later onset of cardiovascular and cerebrovascular diseases of offspring [6,7]. In our pilot study aiming to investigate the role of miR-499a in stroke, we found miR-499a was substantially downregulated. However, the role of miR-499a in astrocytes has not been studied. Recent studies showed that phosphatase and tensin homologue deleted on chromosome 10 (PTEN) as a target gene of miR-499a [8]. Moreover, PTEN and other miRNAs are reportedly important in IS [[9], [10], [11], [12]]. For instance, Zhong et al., demonstrated that the expression of miR-144 is negatively correlated with that of PTEN. While inhibition of miR-144 overexpression could induce the increased expression of PTEN in vitro [10]. Furthermore, miR-144 can indirectly inhibit the expression of Caspase3 by acting on PTEN, thereby reducing apoptosis and inflammation, which subsequently reduces the brain damage caused by ischemia-reperfusion. In additional, PTEN antagonizes the PI3K/Akt signaling pathway in IS [11]. MiRNA/PTEN/PI3K/Akt signaling pathway is the most important pathway involved in neuroprotection and plays a key role in the post-ischemic survival of neurons [[11], [12], [13]]. Zheng et al. demonstrated that miR-130a prevented cerebral ischemia-reperfusion brain damage by mediating the PTEN/PI3K/Akt axis, which provided a novel idea for the target therapy of IS [11]. In other study, Peng et al. indicated that PTEN was a direct target of miR-221 in human umbilical vein endothelial cells (HUVECs) [12]. In addition, miR-221 upregulation significantly inhibited PTEN expression and enhanced the phosphorylation of PI3K/Akt in HUVECs. In the result, miRNA-221 participates in IS by modulating endothelial cell function and angiogenesis by regulating the PTEN/PI3K/AKT pathway. In addition, PTEN is capable of inhibiting Akt/mTOR signaling pathway in IS [14]. Through interfering with these pathways, PTEN regulates cell growth and survival, proliferation, migration, the expression of pro-inflammatory cytokines and chemokines, and angiogenesis. Increasing evidence has pointed PTEN plays an important role in regulating neuronal differentiation and synaptogenesis [15,16].
Based on these facts, we hypothesized that miR-499a might be involved in IS through targeting PTEN. This study was designed to examine this notion in Oxygen-glucose deprivation (OGD) and reoxygenation model.
2. Methods
2.1. Cell cultures and treatment
Primary culture astrocytes were isolated from the brain tissues of 1–3-day-old rat pups. Briefly, the mesencephalic tissues were minced and digested in 0.1% trypsin for 30 min at 37 °C. After being washed with PBS, the cells were resuspended in Dulbecco modified eagle medium (DMEM, Gibco, Rockville, MD, USA) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA).
The cells were passaged at 6 days, and astrocytes were purified from the primary mixed cultures by three to four repetitions of trypsinization and replating. All experimental procedures were approved by the Institutional Animal Care and Use.
2.2. Committee of Harbin Medical University
2.2.1. OGD and reoxygenation model
After washing twice, astrocytes were immersed in 1-ml deoxygenated custom DMEM without glucose and FBS (GIBCO, CA, USA). Then, they were placed inside an incubator (Thermo scientific, Waltham, MA, USA) for 2/4/6 h with a premixed gas (1% O2, 94% N2, 5% CO2). After that, cells were immersed in normal DMEM containing 10% FBS and transferred to a CO2 incubator (95% air and 5% CO2) for 24 h.
2.3. Cell viability assays
The cells were cultured in 96-well plates, and each well was seeded with 4 × 103 cells. After treatment with astrocytes and negative control, the viability of the cancer cells was detected with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Roche; Switzerland). 20 μl of MTT solution (5 mg/ml) was added to each well, and the mixtures were incubated for 4 h at 37 °C. Then, 150 μl of dimethyl sulfoxide (DMSO) was added to the wells. The absorbance was measured using an ELISA plate reader at 490 nm. A high absorbance indicated a greater viability. For the cell viability assay, we removed MTT solution before addition of DMSO.
2.4. 4. Quantitative real-time PCR (qRT-PCR)
Total RNA samples were extracted from cultured astrocytes using TRIZOL reagent (Invitrogen, USA). To detect mRNA expression levels of miR-499a and PTEN, real-time PCR was carried out on ABI 7500 fast real-time PCR system (Applied Biosystems, USA) with SYBR Green I (Applied Biosystems, USA). GAPDH was used as an internal control. miR-499a level was quantified by the mirVana qRT-PCR miRNA detection kit (Ambion, USA), according to the manufacturer's protocols and as previously described. U6 was used as an internal control for miRNA quantification.
2.5. Western blot
The concentration of proteins from astrocytes was determined with BCA Protein Assay Kit. The samples were subjected to electrophoresis in 10% SDS-PAGE and then transferred to nitrocellulose filter membrane. The membrane was blocked in 5% skim milk for 2 h at 25 °C, then incubated with the primary antibodies against PTEN (1:1000; Abcam, USA) and GAPDH (1:10000; Proteintech, USA), respectively, at 4 °C overnight. After washed three times/10 min, the membrane was incubated with the fluorescence-conjugated secondary antibody (Invitrogen, USA) at dilution 1:10000 for 1 h. Western blot bands were quantified by using Odyssey infrared imaging system (LI-COR; Lincoln, NE).
2.6. MiRNA, siRNA and plasmid transfection
MiR-499a mimic, miRNA-negative control (NC), and miR-499a antisense inhibitor (AMO-499a) were synthesized by RIBOBIO (Guangzhou, China). PTEN siRNA was synthesized by GenePharma (Shanghai, China). PTEN overexpression plasmid was synthesized by GeneChem (Shanghai, China). Astrocytes were transfected with miR-499a, NC, AMO-499a, siRNA or plasmid using X-treme (Roche Switzerland) following the manufacturer's protocol.
2.7. Luciferase activity assay
To generate reporter vectors bearing miRNA-binding sites, the 3′-untranslated region (3′-UTR) of PTEN and its mutant variant were synthesized by Sangon (Shanghai, China). The construct was inserted into the multiple cloning sites downstream of the luciferase gene (Hind III and Sac I sites) in the pMIR-REPORT luciferase miRNA expression reporter vector (Ambion, USA). For luciferase assay, 0.1 μg of luciferase reporters containing 3′-UTR was co-transfected with NC, miR-499a, or AMO-499a and 10 ng of PRL-TK (TK-driven Renilla luciferase expression vector) into HEK-293 cells using X-treme gene siRNA transfection reagent (Roche, Switzerland), according to the manufacturer's instructions. Luciferase activity was measured 48 h after transfection with a dual luciferase reporter assay kit (Promega, USA).
2.8. Enzyme-linked immunosorbent assay
After 24 h reoxygenation, we collected the medium from astrocytes. Release of the pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and anti-inflammatory cytokines IL-10 from the cellular supernatant was performed using specific enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, UK) according to manufacturers’ guidelines.
2.9. Statistical analysis
All the data are presented as means ± SEM. The statistical comparisons among multiple groups were performed using analysis of variance (ANOVA). If significant effects were indicated by ANOVA, a t-test using the Bonferroni correction or a Dunnett's test was used to evaluate the significance of the differences between the individual means. Otherwise, the data were compared by Student's t-test. A two-tailed difference with p < 0.05 was considered statistically significant. The data were analyzed using GraphPad Prism 5.0.
3. Results
3.1. Downregulation of miR-499a correlates with OGD-induced injury in astrocytes
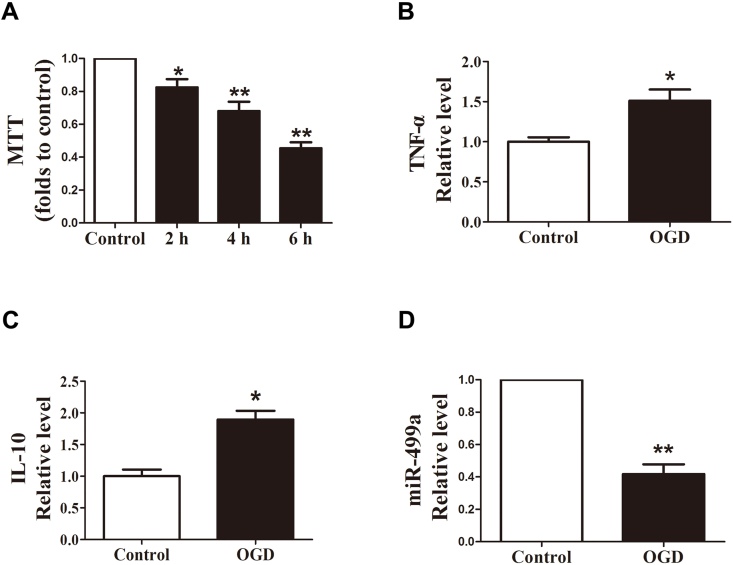
Inflammation response was crucial for ischemic injury. Astrocytes were subjected to OGD for 2/4/6 h and persisted in reoxygenation for 24 h. The cell viability was evaluated by MTT assay. As Fig. 1A shown OGD-induced remarkable decrease of cell viability compared with the control group. Enzyme-linked immunosorbent assay was performed to evaluate the release of pro-inflammatory cytokines TNF-α and anti-inflammatory cytokines IL-10 from the cellular supernatant. The results shown OGD group had significantly higher expressions of pro-inflammatory mediator TNF-α and anti-inflammatory mediator IL-10 (Fig. 1B and C). Meanwhile, to understand if miR-499a is involved in the inflammatory response, we carried out real-time RT-PCR to detect miR-499a expression level in response to OGD. The miR-499a level was found substantially suppressed (Fig. 1 D).
Fig. 1.
miR-499a was upregulated in OGD astrocytes. (A) OGD-induced decrease of cellular viability of astrocytes. *p < 0.05 and **p < 0.01 vs. control; n = 6. (B) OGD-induced increased release of TNF-α in astrocytes. *p < 0.05 vs. control; n = 5. (C) OGD-induced increased release of IL-10 in astrocytes. *p < 0.05 vs. control; n = 5. (D) OGD downregulated the expression of miR-499a in astrocytes. **p < 0.01 vs. control; n = 6.
3.2. MiR-499a restrain the inflammatory response induced by OGD in astrocytes
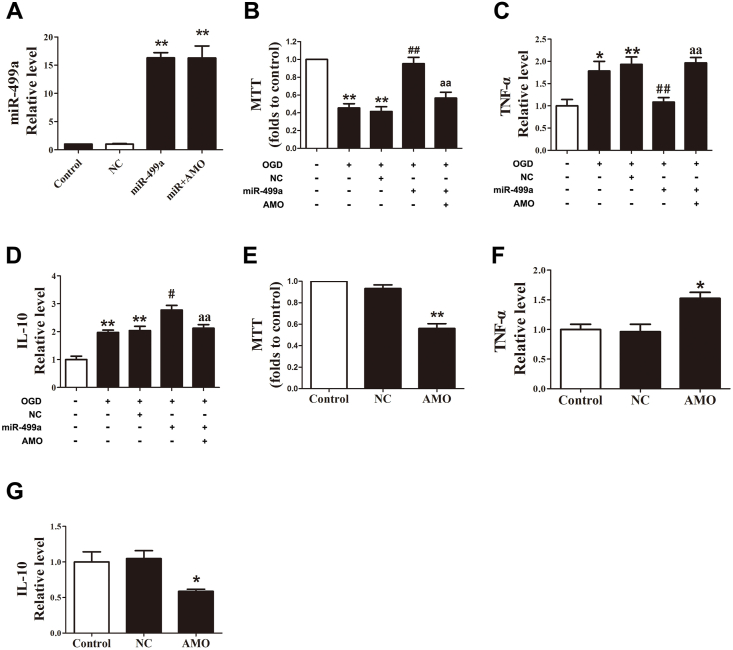
Downregulation of miR-499a in astrocytes subjected to OGD suggests the OGD-induced inflammatory response might be related to this miRNA. To examine this hypothesis, we transfected miR-499a mimic, miR-499a inhibitors (anti-miR-499a anti-sense oligodeoxyribonucleotide, AMO-499a) or NC into astrocytes in OGD states. Transfected efficiency was verified by a significant increase in miR-499a mimic alone or co-transfection of miR-499a mimic and AMO-499a (Fig. 2A). MTT assay showed that after OGD, the astrocytes transfected with miR-499a had a substantially elevated cellular viability compared with those transfected with NC. The co-transfection of miR-499 mimic and AMO-499a markedly decreased cell viability relative to the transfection of miR-499 mimic alone (Fig. 2B). As shown in Fig. 2C and D, miR-499a reversed the increased pro-inflammatory mediator TNF-α and enhanced the increased anti-inflammatory mediator IL-10 induced by OGD. Moreover, this effect of miR-499a was reduced by co-transfected miR-499a and AMO-499a (Fig. 2C and D). To assess whether knockdown of miR-499a offers a pro-inflammation effect, we compared the cellular viability and release of inflammatory cytokines in astrocytes with or without AMO-499a transfection. AMO-499a alone induced the decrease of cell viability and IL-10 and an increase of TNF-α (Fig. 2E–G).
Fig. 2.
miR-499a restrains the inflammation induced by OGD in astrocytes. (A) The successful transfection of miR-499a was verified. **p < 0.01 vs. NC; n = 6. (B) OGD-induced decrease of cellular viability of astrocytes was abrogated by miR-499a. The co-transfection of miR-499a and AMO-499a reversed the effect of miR-499a. **p < 0.01 vs. control, **p < 0.01 vs. (OGD + NC), **p < 0.01 vs. (OGD + miR-499a); n = 6. (C) miR-499a downregulated TNF-α in OGD astrocytes but was increased by co-transfect with AMO-499a. *p < 0.05, **p < 0.01 vs. control, **p < 0.01 vs. (OGD + NC), **p < 0.01 vs. (OGD + miR-499a); n = 5. (D) miR-499a upregulated IL-10 in OGD astrocytes but was reduced by co-transfected miR-499a and AMO-499a. **p < 0.01 vs. control, *p < 0.05 vs. (OGD + NC), **p < 0.01 vs. (OGD + miR-499a); n = 5. (E) AMO-499a suppressed astrocytes viability. **p < 0.01 vs. NC; n = 6. (F) AMO-499a increased TNF-α. *p < 0.05 vs. NC; n = 5. (G) AMO-499a decreased IL-10. **p < 0.01 vs. NC; n = 5.
3.3. PTEN as a direct target of miR-499a is upregulated in OGD treatment astrocytes
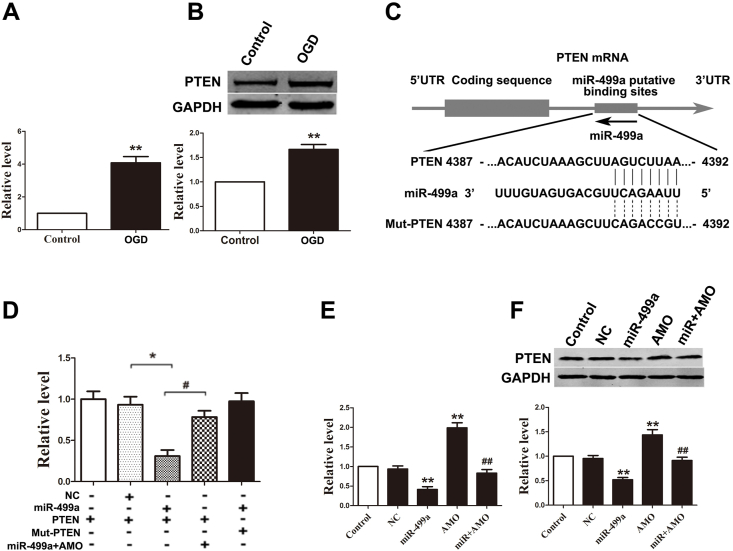
PTEN is a tumor suppressor that is a dual-specificity phosphatase and plays an important role in neuronal death in neural cells [17]. PTEN deletion prevents ischemic brain injury and promotes neuroprotective effects [18,19]. A more recent study revealed that bisperoxovanadium (Bpv), a specific inhibitor of PTEN's phosphatase activity, could modulate inflammatory production in cerebral ischemia and reperfusion injury [16]. In agreement with these previous studies, we observed significant increases of PTEN at both mRNA and protein levels in OGD astrocytes (Fig. 3A and B). The inverse correlation between miR-499a and PTEN in OGD astrocytes suggests a targeting relationship between them. Our computational analysis using TargetScan miRNA database predicted PTEN is a potential target of miR-499a. To validate this prediction, we constructed luciferase reporter vectors containing a segment of the 3-UTR of PTEN (PTEN) and a mutated 3′-UTR of PTEN (Mut-PTEN) (Fig. 3C). The constructs were co-transfected with miR-499a mimic into HEK293 cells. As shown in Fig. 3D, miR-499a inhibited the luciferase activity of the PTEN reporter, whereas no effect was observed with Mut-PTEN. Furthermore, both mRNA and protein levels of PTEN were downregulated by miR-499a in astrocytes, which was reversed by the co-transfection of AMO-499a (Fig. 3E and F).
Fig. 3.
PTEN is a direct target of miR-499a and its expression is upregulated by OGD. (A and B) Both mRNA and protein levels of PTEN were upregulated in OGD astrocytes. **p < 0.01 vs. control; n = 5. (C) The binding site for miR-499a in the 3′-UTR of PTEN gene, as predicted by TargetScan algorithm. The mutant sequences were equivalent to wild-type with the exception of mutation at the 3′ end of the target site. (D) Luciferase activities were analyzed in HEK293 cells 48 h after transfection. The data were presented as mean ± SEM from four separate experiments; *p < 0.05 and *p < 0.05. (E, F) Regulation of mRNA and protein levels of PTEN by miR-499a in astrocytes. **p < 0.01 vs. NC, **p < 0.01 vs. miR-499a; n = 5.
3.4. PTEN concerned with the inflammatory response of astrocytes
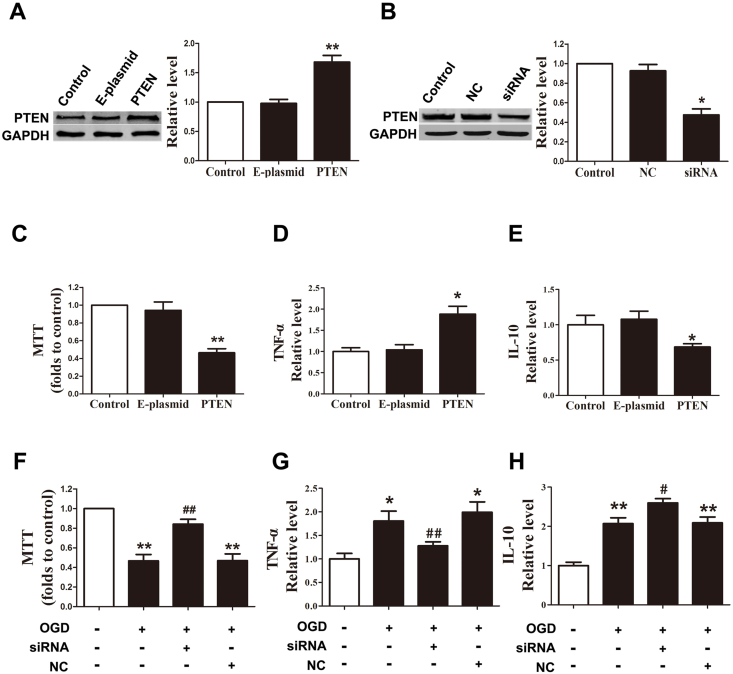
The above-described results suggested miR-499a might prevent the inflammatory response of astrocytes by targeting PTEN. Our subsequent data indeed provided evidence in support of this hypothesis. We employed both gain- and loss-of-function approaches to look at the effects of PTEN on the inflammatory response of astrocytes. PTEN protein expression level was increased by the PTEN plasmid and decreased by the siRNA of PTEN (Fig. 4A and B). As depicted in Fig.4C to E, overexpression of PTEN in astrocytes transfected with PTEN-carrying plasmid (Fig. 4A) suppressed the cellular viability and promoted inflammatory response by upregulating TNF-α and down-regulating IL-10. However, downregulation PTEN with PTEN siRNA (Fig. 4B) suppressed the OGD injury as indicated by the improved cell viability, decreased TNF-α, and increased IL-10 compared with NC astrocytes in OGD state (Fig. 4F–H) (see Fig. 5).
Fig. 4.
miR-499a protected astrocytes at OGD state by targeting PTEN. (A) PTEN-carrying plasmid increased the protein level of PTEN. **p < 0.01 vs. empty plasmid (E-plasmid); n = 5. (B) PTEN siRNA inhibited the expression of PTEN. *p < 0.05 vs. NC; n = 5. (C) PTEN decreased the astrocytes viability. **p < 0.01 vs. E-plasmid; n = 6. (D) PTEN up-regulated TNF-α in astrocytes. *p < 0.05 vs. E-plasmid; n = 5. (E) PTEN down-regulated IL-10 in astrocytes. *p < 0.05 vs. E-plasmid; n = 5. (F) Silence of PTEN by siRNA upregulated the cell viability in OGD astrocytes. **p < 0.01 vs. control, **p < 0.01 vs. (OGD + NC); n = 6. (G) PTEN siRNA decreased TNF-α in OGD astrocytes. *p < 0.05 vs. control, **p < 0.01 vs. (OGD + NC); n = 6. (H) PTEN siRNA increased IL-10 in OGD astrocytes. **p < 0.01 vs. control, **p < 0.01 vs. (OGD + NC); n = 6.
Fig. 5.
Schematic diagram of the implicated signaling pathway relating to the anti-inflammatory effects of miR-499a by inhibits PTEN expression through targeting its 3ʹ-UTR on ischemic stroke (IS). The upregulated miR-499a induces production of pro-inflammatory cytokines including IL-6 and TNF-α.
4. Discussion
To date, over 2000 human miRNAs have been identified (miRBase: the microRNA database). Though they are theoretically predicted to target thousands of protein-coding genes involving a wide spectrum of biological and pathophysiological processes, experimental characterization of their functions has been limited to a small portion of these known miRNAs. In the past years, a number of miRNAs have been reported to participate in stroke. These findings have greatly advanced our understanding of the molecular mechanisms for and opened up a new opportunity for therapeutic intervention of this pathological entity. Nonetheless, given a large number of miRNAs expressed in astrocytes, our knowledge about miRNAs regulation of IS remains far from complete. Here we identified miR-499a as a new anti-inflammation miRNA in astrocytes; forced transient expression of miR-499a in OGD astrocytes nearly completely reversed the inflammatory response whereas knockdown of miR-499a by its specific inhibitor in healthy astrocytes induced the inflammatory response resembling those produced by OGD.
Several lines of evidence were generated in the present study for the role of miR-499a as an anti-inflammation miRNA. First, miR-499a was found remarkably downregulated in OGD astrocytes. Second, forced transient expression of miR-499a in astrocyte nearly completely reversed the changes in cell viability and expression of inflammatory cytokines induced by OGD. Third, knockdown of miR-499a by its specific inhibitor in healthy astrocytes induced the phenotypes resembling those produced by OGD. Finally, we observed that PTEN, which has been previously characterized as a pro-inflammation signaling molecule, was markedly upregulated in OGD astrocytes at least partially as a result of abnormal downregulation of miR-499a as it was validated as a direct target gene for miR-499a [16].
To the best of our knowledge, our study is the first to identify the role of miR-499a in stroke. However, miR-499a plays an important role in multiple diseases. One study aims to investigate gestational hypertension, preeclampsia and intrauterine growth restriction induce dysregulation of cardiovascular and cerebrovascular disease-associated miRNAs in maternal whole peripheral blood observed a trend to downregulation inappropriate pregnancy-related complications, suggesting the potential role of miR-499a in such a condition. However, the function of miR-499a was not investigated in this study [20]. Xu Z et al., demonstrated a serum-based miRNA expression profile for atherosclerotic coronary artery disease (CAD) patients, potentially revealing a previously undocumented mechanism for cell proliferation and migration mediated by miR-499a, and might provide novel insights into the role of circulating miRNAs in atherosclerosis pathogenesis [21]. Another study observed miR499a was significantly decreased in acute myocardial infarction (AMI) samples validating its use as a reliable biomarker for AMI during postmortem examination [22].
MiRNAs have recent emerged as important epigenetic modulators of gene expression and there is much evidence to suggest that miRNAs are involved in inflammatory. MiR-155 contributes to enhanced resistance to apoptosis in monocytes from patients with rheumatoid arthritis [23]. MiR-322 inhibits inflammatory cytokine expression and promotes cell proliferation in LPS-stimulated murine macrophages by targeting nuclear factor-κB (NF-κB) [24]. Downregulated expression of miR-124 in pediatric intestinal failure patients modulates macrophages activation by inhibiting Signal transducer and activator of transcription 3 (STAT3) and acetylcholinesterase (AChE) [25]. They showed intestinal macrophages increasingly expressed the AChE and STAT3 in intestinal failure patients when compared with controls. The inhibitors against STAT3 and AChE significantly suppressed the lipopolysaccharides-induced IL-6 and TNF-α production in macrophages. Additionally, miRNAs were proved to be one regulator of inflammation in various CNS pathologies. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer's disease triple transgenic mouse model through targeting Suppressor of Cytokine Signaling-1 (SOCS-1), a negative regulator of inflammatory gene response [26]. A set of miRNAs such as miR-146, miR-142, and miR-27a have been implicated in epileptogenesis [[27], [28], [29]]. The expression of astrocytes miR-146a, an inflammation-associated microRNA, was upregulated in experimental and human temporal lobe epilepsy [30]. Further, it has been shown that in astrocytes miR-21 was significantly upregulated in stroke possibly playing a role in neuroinflammation and in extracellular vesicles leads to neurotoxicity via toll-like receptor 7 (TLR7) signaling in simian immunodeficiency virus (SIV) - associated neurological disease [31]. Moreover, Yin-Feng Dong group found that OGD significantly downregulated miR-7 and upregulated Herpud2 along with significant elevations of pro-inflammatory TNF-α and IL-1β in astrocytes. Correspondingly, pretreatment with nicorandil could remarkably reverse these changes and thereby attenuate inflammatory responses and astrocytic damages [2]. These studies showed that targeting such miRNAs might be identified as a potential therapeutic strategy to treat various CNS.
Another important finding in this study is that PTEN likely mediated the anti-inflammatory action of miR-499a. Four lines of evidence were obtained for this conclusion. First, expression of PTEN was increased in OGD astrocytes, which was reciprocal to the change of expression of miR-499a. Second, overexpression of miR-499a significantly repressed PTEN expression at both mRNA and protein levels. Third, overexpression of PTEN in astrocytes promoted inflammatory responses. Finally, the silence of PTEN counteracted the inflammatory responses induced by OGD. While previous studies have documented the direct involvement of PTEN in inflammation [16,32]. For instance, Qin et al. demonstrated that glycine treatment was shown to upregulate miR-26 b, which led to PTEN downregulation followed by Akt activation, resulting in inhibition of neuronal death subarachnoid hemorrhage (SAH) model in vitro and in vivo [33]. Furthermore, glycine treatment suppressed SAH-induced M1 microglial polarization and thereby inflammation. Li et al. indicated that miR-23a directly targeted PTEN and suppresses its expression in vitro and in vivo of traumatic brain injury (TBI) model [34]. The authors demonstrated that the upregulation of miR-23a could improve the neurological outcome after TBI by inhibiting neurons apoptosis and inflammatory response via reactivating PTEN/AKT/mTOR signaling pathway. Our results established a mechanistic link between miR-499a and stroke with PTEN serving as a mediator of the anti-inflammatory signal of miR-499a.
5. Conclusions
Collectively, we presented the first evidence that miR-499a prevents astrocytes mediated inflammatory response in IS by targeting PTEN. In light of the fact that overexpression of miR-499a was able to reverse the inflammatory alterations, together with the observation that miR-499a was abnormally downregulated, it may be speculated that miR-499a is a new molecular for the treatment of IS and other possible CNS pathologies associated with miR-499a as well.
Sources of funding
None.
Conflicts of Interest/Disclosures
The authors have no conflicts of interest to disclose.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
CRediT authorship contribution statement
Xiaoxiang Guan: Conceptualization, Writing – original draft, Writing – review & editing. Yiwei Zhang: Conceptualization, Writing – original draft, Writing – review & editing. Ilgiz Gareev: Conceptualization, Writing – original draft, Writing – review & editing. Ozal Beylerli: Conceptualization, Writing – original draft, Writing – review & editing. Xinyuan Li: Data curation, Methodology. Guitian Lu: Data curation, Methodology, Validation, Software. Lin Lv: Data curation, Methodology, Validation, Software, Resources. Xin Hai: Conceptualization, Project administration, Funding acquisition, Supervision.
References
- 1.Campbell B.C.V., De Silva D.A., Macleod M.R., Coutts S.B., Schwamm L.H., Davis S.M., Donnan G.A. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 2.Dong Y.F., Chen Z.Z., Zhao Z., Yang D.D., Yan H., Ji J., Sun X.L. Potential role of microRNA-7 in theanti-neuroinflammation effects of nicorandil in astrocytes induced by oxygen-glucose deprivation. J. Neuroinflammation. 2016;13(1):60. doi: 10.1186/s12974-016-0527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhury G.R., Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol. Dis. 2016;85:234–244. doi: 10.1016/j.nbd.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amantea D, Micieli G, Tassorelli C, Cuartero MI, Ballesteros I, Certo M, Moro MA, Lizasoain I, Bagetta G. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front. Neurosci. 9:147. doi: 10.3389/fnins.2015.0014. [DOI] [PMC free article] [PubMed]
- 5.Lee J.H., Wei Z.Z., Cao W., Won S., Gu X., Winter M., Dix T.A., Wei L., Yu S.P. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol. Dis. 2016;96:248–260. doi: 10.1016/j.nbd.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai T., Li J., An X., Yan N., Li D., Jiang Y., Wang W., Shi L., Qin Q., Song R., Wang G., Jiang W., Zhang J.A. Polymorphisms in mir499a and mir125a gene are associated with autoimmune thyroid diseases. Mol. Cell. Endocrinol. 2017;440:106–115. doi: 10.1016/j.mce.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Hromadnikova I., Kotlabova K., Hympanova L., Krofta L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PloS One. 2015;10(9) doi: 10.1371/journal.pone.0138383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q., Feng K., Xia L., Wang C., Zhu J. Combined use of serum miR-499a-5p and CA199 increases the diagnostic sensitivity of pancreatic cancer. Clin. Lab. 2019;65(11) doi: 10.7754/Clin.Lab.2019.190416. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Wang Q., Zhao X., Shao L., Liu G., Zheng X., Xie L., Zhang Y., Sun C., Xu R. YTHDC1 mitigates ischemic stroke by promoting Akt phosphorylation through destabilizing PTEN mRNA. Cell Death Dis. 2020;11(11):977. doi: 10.1038/s41419-020-03186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong S.J., Cui M.M., Gao Y.T., Cao X.Y., Chen B., Wen X.R. MicroRNA‐144 promotes remote limb ischemic preconditioning-mediated neuroprotection against ischemic stroke via PTEN/Akt pathway. Acta Neurol. Belg. 2021;121(1):95–106. doi: 10.1007/s13760-020-01500-5. [DOI] [PubMed] [Google Scholar]
- 11.Zheng T., Shi Y., Zhang J., Peng J., Zhang X., Chen K., Chen Y., Liu L. MiR-130a exerts neuroprotective effects against ischemic stroke through PTEN/PI3K/AKT pathway. Biomed. Pharmacother. 2019;117:109117. doi: 10.1016/j.biopha.2019.109117. [DOI] [PubMed] [Google Scholar]
- 12.Peng H., Yang H., Xiang X., Li S. ΜicroRNA-221 participates in cerebral ischemic stroke by modulating endothelial cell function by regulating the PTEN/PI3K/AKT pathway. Exp Ther Med. 2020;19(1):443–450. doi: 10.3892/etm.2019.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghafouri-Fard S., Abak A., Shoorei H., Mohaqiq M., Majidpoor J., Sayad A., Taheri M. Regulatory role of microRNAs on PTEN signaling. Biomed. Pharmacother. 2021;133:110986. doi: 10.1016/j.biopha.2020.110986. [DOI] [PubMed] [Google Scholar]
- 14.Yan Y.T., Li S.D., Li C., Xiong Y.X., Lu X.H., Zhou X.F., Yang L.Q., Pu L.J., Luo H.Y. Panax notoginsenoside saponins Rb1 regulates the expressions of Akt/mTOR/PTEN signals in the hippocampus after focal cerebral ischemia in rats. Behav. Brain Res. 2018;345:83–92. doi: 10.1016/j.bbr.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.E., Lim M.S., Park J.H., Park C.H., Koh H.C. PTEN promotes dopaminergic neuronal differentiation through regulation of ERK-dependent inhibition of S6K signaling in human neural stem cells. Stem Cells Transl Med. 2016;5(10):1319–1329. doi: 10.5966/sctm.2015-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao L.L., Hao D.L., Mao X.W., Xu Y.F., Huang T.T., Wu B.N., Wang L.H. Neuroprotective effects of bisperoxovanadium on cerebral ischemia by inflammation inhibition. Neurosci. Lett. 2015;602:120–125. doi: 10.1016/j.neulet.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 17.Kang J., Li Z., Zhi Z., Wang S., Xu G. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019;26:491–503. doi: 10.1038/s41434-019-0101-8. [DOI] [PubMed] [Google Scholar]
- 18.Shi G.D., OuYang Y.P., Shi J.G., Liu Y., Yuan W., Jia L.S. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochemical and biophysical research communications. 2011;404:941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 19.Baek S.H., Lee J.H., Ko J.H., Lee H., Nam D., Lee S.G., Yang W.M., Um J.Y., Lee J., Kim S.H., Shim B.S., Ahn K.S. Ginkgetin blocks constitutive STAT3 activation and induces apoptosis through induction of SHP-1 and PTEN tyrosine phosphatases. Phytother Res. 2016;30(4):567–576. doi: 10.1002/ptr.5557. [DOI] [PubMed] [Google Scholar]
- 20.Hromadnikova I., Kotlabova K., Hympanova L., Krofta L. Gestational hypertension, preeclampsia and intrauterine growth restriction induce dysregulation of cardiovascular and cerebrovascular disease associated microRNAs in maternal whole peripheral blood. Thromb. Res. 2016;137:126–140. doi: 10.1016/j.thromres.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Han Y, Liu J, Jiang F, Hu H, Wang Y, Liu Q, Gong Y, Li X. MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Sci. Rep., 12276; doi: 10.1038/srep12276. [DOI] [PMC free article] [PubMed]
- 22.Kakimoto Y., Kamiguchi H., Ochiai E., Satoh F., Osawa M. MicroRNA stability in postmortem FFPE tissues: quantitative analysis using autoptic samples from acute myocardial infarction patients. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0129338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajasekhar M., Olsson A.M., Steel K.J., Georgouli M., Ranasinghe U., Brender Read C., Frederiksen K.S., Taams L.S. MicroRNA-155 contributes to enhanced resistance to apoptosis in monocytes from patients with rheumatoid arthritis. J. Autoimmun. 2017;79:53–62. doi: 10.1016/j.jaut.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K., Song F., Lu X., Chen W., Huang C., Li L., Liang D., Cao S., Dai H. MicroRNA-322 inhibits inflammatory cytokine expression and promotes cell proliferation in LPS-stimulated murine macrophages by targeting NF-κB1 (p50) Biosci. Rep. 2017;37(1) doi: 10.1042/BSR20160239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y.T., Wang J., Lu W., Cao Y., Cai W. Downregulated expression of microRNA-124 in pediatric intestinal failure patients modulates macrophages activation by inhibiting STAT3 and AChE. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guedes J.R., Custodia C.M., Silva R.J., de Almeida L.P., Pedroso de Lima M.C., Cardoso A.L. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer's disease triple transgenic mouse model. Hum. Mol. Genet. 2014;23(23):6286–6301. doi: 10.1093/hmg/ddu348. [DOI] [PubMed] [Google Scholar]
- 27.Kretschmann A., Danis B., Andonovic L., Abnaof K., van Rikxoort M., Siegel F., Mazzuferi M., Godard P., Hanon E., Frohlich H., Kaminski R.M., Foerch P., Pfeifer A. Different MicroRNA profiles in chronic epilepsy versus acute seizure mouse models. J. Mol. Neurosci. 2015;55:466–479. doi: 10.1007/s12031-014-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez-Mateos E.M., Bray I., Sanz-Rodriguez A., Engel T., McKiernan R.C., Mouri G., Tanaka K., Sano T., Saugstad J.A., Simon R.P., Stallings R.L., Henshall D.C. miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. The American journal of pathology. Am. J. Pathol. 2011;179(5):2519–2532. doi: 10.1016/j.ajpath.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorter J.A., Iyer A., White I., Colzi A., van Vliet E.A., Sisodiya S., Aronica E. Hippocampal subregion-specific microRNA expression during epileptogenesis in experimental temporal lobe epilepsy. Neurobiol. Dis. 2014;62:508–520. doi: 10.1016/j.nbd.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Aronica E., Fluiter K., Iyer A., Zurolo E., Vreijling J., van Vliet E.A., Baayen J.C., Gorter J.A. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur. J. Neurosci. 2010;31(6):1100–1107. doi: 10.1111/j.1460-9568.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- 31.Yelamanchili S.V., Lamberty B.G., Rennard D.A., Morsey B.M., Hochfelder C.G., Meays B.M., Levy E., Fox H.S. MiR-21 in extracellular vesicles leads to neurotoxicity via TLR7 signaling in SIV neurological disease. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kweon H.J., Yu S.Y., Kim D.I., Suh B.C. Differential regulation of proton-sensitive ion channels by phospholipids: a comparative study between ASICs and TRPV1. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0122014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin X., Akter F., Qin L., Xie Q., Liao X., Liu R., Wu X., Cheng N., Shao L., Xiong X., Liu R., Wan Q., Wu S. MicroRNA-26b/PTEN signaling pathway mediates glycine-induced neuroprotection in SAH injury. Neurochem. Res. 2019;44(11):2658–2669. doi: 10.1007/s11064-019-02886-2. [DOI] [PubMed] [Google Scholar]
- 34.Li Z., Xu R., Zhu X., Li Y., Wang Y., Xu W. MicroRNA-23a-3p improves traumatic brain injury through modulating the neurological apoptosis and inflammation response in mice. Cell Cycle. 2020;19(1):24–38. doi: 10.1080/15384101.2019.1691763. [DOI] [PMC free article] [PubMed] [Google Scholar]