Abstract
In this article, we consider how social sciences can help us to understand the rising use of antibiotics globally. Drawing on ethnography as a way to research how we are in the world, we explore scholarship that situates antibiotic use in relation to interactions of pathogens, humans, animals and the environment in the context of globalization, changes in agriculture and urbanization. We group this research into three areas: practices, structures and networks. Much of the public health and related social research concerning antimicrobial resistance has focused on antibiotic use as a practice, with research characterizing how antibiotics are used by patients, farmers, fishermen, drug sellers, clinicians and others. Researchers have also positioned antibiotic use as emergent of political-economic structures, shedding light on how working and living conditions, quality of care, hygiene and sanitation foster reliance on antibiotics. A growing body of research sees antibiotics as embedded in networks that, in addition to social and institutional networks, comprise physical, technical and historical connections such as guidelines, supply chains and reporting systems. Taken together, this research emphasizes the multiple ways that antibiotics have become built into daily life. Wider issues, which may be invisible without explication through ethnographic approaches, need to be considered when addressing antibiotic use. Adopting the complementary vantage points of practices, networks and structures can support the diversification of our responses to AMR.
Introduction
Antimicrobial resistance (AMR) is a major challenge to global human and animal health, and a barrier to achieving the Sustainable Development Goals.1 Antibiotic use in human and veterinary medicine, agriculture and aquaculture is a key accelerator to the development of AMR,2,3 and therefore addressing this biological phenomenon becomes a matter of social concern. The inclusion of the social sciences, broadly defined to include anthropology, sociology, geography, history and other disciplines, to conduct research on—and develop multisectoral and multidisciplinary responses to tackle—AMR is increasingly accepted and advocated.4–9
In this review article, we illustrate the value of the social sciences in understanding and addressing antibiotic use and AMR. Social science approaches are often equated with qualitative data collection methods, but they encompass ways of seeing, thinking, writing and doing research that can include both qualitative and quantitative methods. Reflecting this, the field of social research on AMR has expanded in many directions, with innovative studies considering pharmaceuticals, microbes, patients, animals, care providers, policies and much more.10 These studies have contributed to understandings of the interactions of microbes, humans, animals and the environment, and of how these interactions shift with changes in agriculture, urbanization, climate change and globalization. However, this body of work—not always indexed on biomedical literature databases or perhaps written in the technical language of, and informed by the theoretic concerns of, social science disciplines—may not be accessible to the broader AMR community. We seek to begin to remedy this by sharing key approaches and insights from the social sciences that can inform research programmes and policy responses to AMR and antibiotic use.
In this article, we concentrate on ethnographically informed approaches. Rather than limiting ourselves to a disciplinary-oriented remit, as has been productive in the past,11–16 we draw on findings emerging from various social science disciplines. Here, we summarize and develop ideas presented in a recent report, ‘Addressing antibiotic use: insights from social science around the world’ (Figure 1).17 Following a description of ethnographic approaches and the limitations of efforts centred around awareness raising and correcting knowledge deficits, we offer a framework of practices, structures and networks as a tool to support analysis and translate research findings into implementable research and policy recommendations (Figure 2).
Figure 1.
Addressing antibiotic use: insights from social science around the world.
Figure 2.
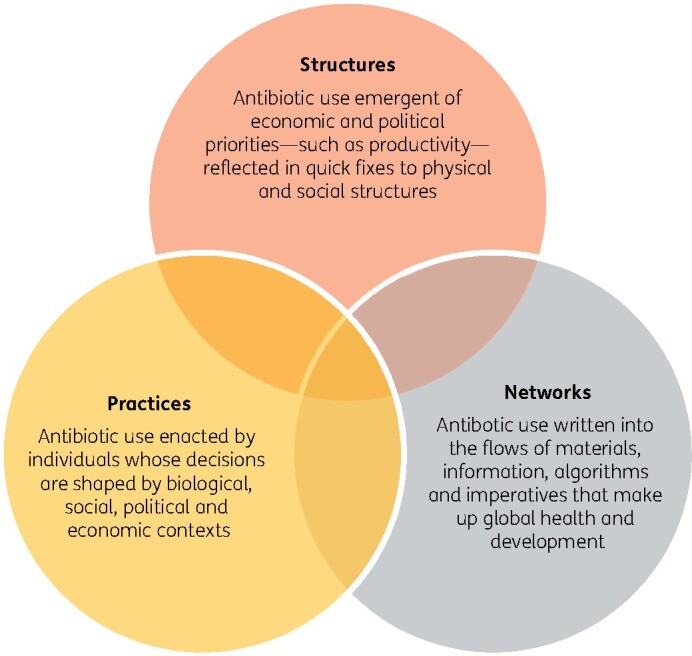
The three overlapping vantage points from which antibiotic use can be understood and that should be considered when seeking to address this problem.17
What do we mean by ethnographic approaches?
In taking a broad definition of ethnographic approaches, we include a range of research that draws on social theory, ‘different lenses through which to look at complicated problems and social issues’ (page 631),18 to help unpick the complex phenomena of antibiotic use. Ethnography—literally ‘people writing’—entails the study of the way people live in the world; their social, material, economic and political arrangements, and the elucidation of what becomes ‘common sense’ in particular places and spaces.19 Participant observation, the cornerstone of ethnographic research, involves immersion in the context of study, with extended interactions with settings and populations a of interest. Studying a setting or group of people over longer time frames facilitates the nuanced and in-depth insights characteristic of ethnographic research.
Increasingly, ethnographic projects are multisited as researchers follow materials, such as antibiotics, ideas and meanings as they move between settings.19,20 However, the central ethnographic interest in developing rich, contextualized understandings necessitates smaller sample sizes than in quantitative studies, whose design is informed by probabilistic sampling theory. From the natural sciences perspective, the integral role of ethnographers in selecting research sites and collecting data—for example, what is tuned into and recorded during fieldwork—might be interpreted as lacking objectivity. However, ethnographic researchers actively reflect on how they influence their research through self-reflexivity, a process of actively engaging with, and reporting on, these issues.21,22
Observation is complemented by methods including interviews, focus groups, surveys and analysis of contemporary and historical documents and discourses. This fosters a rich understanding of the phenomena under consideration and the location of observations in broader temporal and geographical contexts beyond the fieldwork sites. Rather than testing of formal hypotheses determined at the initiation of the project, research activities are relatively unstructured, enabling ethnographers to render visible understandings and interpretations that may differ from conventional biomedical framings, say, through their methodical, detailed and careful data collection and analysis. Comparison—for example by moving between fieldwork sites and/or holding empirical and theoretical data in conversation—remains a key analytical hallmark.23 Ethnographic study findings studies move beyond verbal descriptions, to include explanations and theories arising from this interpretive analysis, with quantification and statistical analyses playing a lesser role.
What these studies share in common, which we group under the ‘ethnographic’ umbrella, are: (i) a commitment to understanding the realities of peoples’ lives, taking their narratives seriously and giving a voice to marginal groups—antibiotic use is not viewed through the dichotomy of ‘appropriate’ or ‘inappropriate’, for example; rather, all antibiotic use is situated in relation to lived realities;24 (2) a commitment to developing a conversation between the emic (insider) and etic (outsider) perspective, providing contrasts in perceptions and understandings that can for example help explain divergent actions with regard to medication and care; and (3) a critical view of existing categories and classifications that are used to order the world, and a reluctance to reproduce these and their ramifications. By unpacking circulating meanings or discourses, ethnographers seek fresh insights into how antibiotic use is socially constructed and how proposed solutions are imagined by scientific, policy and lay communities.
Looking beyond knowledge deficits
The ways by which we investigate antibiotic use and AMR reflect how we conceptualize these phenomena and shape the possibilities for addressing them. Efforts situated within the biomedical tradition presume that the social world can be understood like the natural world: a set of ‘rules’ govern how people use antibiotics, and these rules can be revealed through research. The approach assumes that, when asked, people can articulate and account for their antibiotic use, and therefore surveys and interviews are used to assess knowledge of AMR and antibiotic practices.25
Such studies have a number of in-built assumptions. First, they position antibiotic use as the product of individual decision-making, while overlooking collectively produced understandings of illness, medicine use and healthcare,26 and the practical dimensions of everyday life that determine health actions. The study of individuals, in turn, determines the level at which to intervene. This framing aligns with broader social shifts that have seen the diminishing role of the state in protecting public health and a move towards making individual citizens responsible.27 These studies place knowledge as predictive of antibiotic use, and so ‘inappropriate’ behaviour can be corrected through educating individuals.28 However, such initiatives can fall prey to the assumption that a top-up of knowledge will set in motion behavioural change.26,29 This supposes that target populations have the autonomy to choose their behaviour and prioritize changing antibiotic use over competing interests and contingencies, such as earning a living or other household needs, and institutional and health systems limitations.27,30,31 Below we describe how ethnographical approaches have helped to diversify our understandings of antibiotic use, in part by looking beyond knowledge deficit models of behaviour.16,26,28 We begin with studies that have considered antibiotic use from the practices vantage point.
Practices
Ethnographically informed research on AMR has described how antibiotics are used in practice by patients, their caregivers, farmers, drug sellers, clinicians and others. Rather than solely the result of (a lack of) AMR knowledge, antibiotic use emerges as related to a complex web of social, economic, political and historical conditions, the socio-physical environment and health. Contextualizing antibiotic use enables us to identify behavioural and institutional targets to support changes in practice.32,33
Understanding the everyday lives and livelihoods of those whose antibiotic utilization is of concern has emerged as critical. Following earlier ethnographies that described antibiotic practices,34–37 more recent studies in diverse global settings have shown how antibiotic use practices are shaped by pressures in people’s lives and livelihoods: within primary care in South Africa38 and China; 26,39 hospitals in South Africa and/or India;40–43 and community settings in Bangladesh44,45 and Mozambique.46 Researchers have also considered the logics of antibiotic use in the livelihoods of poultry farmers in Guatemala47 and India;48 Bangladeshi shrimp and prawn farmers;49,50 and dairy farmers in India.51
What emerges from these studies is a nuanced insight into people’s healthcare-seeking behaviour for themselves, their families and their animals that extends beyond the simple binary of whether or not to seek antibiotics.44 For example, ethnography revealed how self-medication with antibiotics is seen as necessary and is widely practiced in the complex therapeutic landscape of multiple practitioners and health systems in Maputo, Mozambique.46 In Bangladesh, medical pluralism, a lack of regulatory infrastructure and perceived consumer demand contribute to ‘inappropriate’ antibiotic use by both qualified and unqualified healthcare providers.45 In both these studies, where formal medical and veterinary services are often inaccessible, self-care and informal healthcare systems emerge as important in alleviating suffering and providing access to antibiotics. Ensuring access to effective antibiotics by engaging with plural health systems, including informal providers, may require novel policy responses beyond regulatory approaches developed in the Global North where biomedical health and veterinary systems dominate.52
Ethnographers have also considered how knowledge of ‘appropriate’ antibiotic use is produced, and the relationships between biomedical understandings and local knowledge systems.25 For example, local patterns of antibiotic prescribing in rural China result from specific knowledge-practice configurations, co-constructed between health professionals and patients, drawing on both biomedicine and Chinese medicine traditions.8 This problematizes unidirectional awareness campaigns seeking to impose biomedical versions of ‘appropriate’ use onto local populations. Rather than seeking to protect antibiotics from misuse, communities could be better equipped with information relevant to their healthcare requirements and provided with improved access to healthcare, enabling them to alleviate suffering while safeguarding antibiotics.
A reoccurring theme in this research is the deployment of antibiotics in a bid to manage uncertainty. Precautionary prescribing has been documented in multiple settings, prompted in part by doctors’ concerns regarding whether patients can afford to return for follow-up appointments.38,53 In rural China, an unpredictable environment characterized by a lack of material resources, diagnostic uncertainty, changing healthcare policies and the necessity to engage in ‘safe practice’, combine to provide the backdrop to antibiotic use.39 The circulation of fake pharmaceuticals, out-of-date medicines and the sale of individual tablets rather than courses all further heighten uncertainty and disrupt treatment protocols.54 In an international project comparing the cultural, ethical and environmental contexts to hospital antibiotic use, the inaccessibility of primary care services and fears of lost earnings through missing work resulted in patients being sicker on hospital admission, thus limiting clinicians’ ability to enact conservative prescribing in Sri Lanka.55 Interventions are needed to support prescribers to manage the uncertainties associated with decisions (not) to provide antibiotics.56 In South Africa, some doctors provide post-dated prescriptions, in the hope that patients will begin to recover before medication is needed.38 Elsewhere, researchers revealed how a stewardship intervention targeting Bangladeshi aquaculturalists inadvertently caused their reliance on antibiotics to increase by ignoring risk profiles associated with local economic and ecological conditions, such as flooding.50 They developed the concept of ‘risk-practices’ to help identify disease reduction opportunities and to avoid the pitfall of a one-size-fits-all response—based on the norms of food production in the Global North—when addressing antibiotic use.50
To summarize, the ‘practices’ group of studies focuses on end-user antibiotic use and emphasizes the importance of understanding the local context when identifying targets for stewardship interventions. Ethnographically informed studies are helping to develop understanding of the complicated and diverse settings in which antibiotic use occurs, on which basis to identify targets for stewardship interventions beyond knowledge deficits. But while immersed in local lived realities, the practice vantage point can struggle to describe the intricacies of what happens beyond the interface of antibiotic use and, therefore, we now consider a second, complementary vantage point.
Structures
A growing number of researchers have considered antibiotic use as a product of the economic and political conditions of modern societies. They propose that tackling reliance on antibiotics requires intervention at levels other than that of the end user. Rather than seeking to ‘fix’ individuals, what if we sought to address the societal structures they are caught up in?
Based on ethnographic research in Uganda and Tanzania, anthropologists elucidated how antibiotics act to ensure the continued productivity in humans and livestock populations, and so are a ‘quick fix’ for illnesses that derive from entrenched problems of inequality, poor sanitation and fractured healthcare systems.57 In India, social scientists described how extreme poverty, social exclusion and inadequate infrastructure exacerbate ‘geographies of vulnerability’, disproportionately exposing poor people to pathogens through their working and living conditions.58 This ‘precarious landscape of disease’ remains largely unaddressed by AMR policy efforts, which also discount the role of pharmaceutical companies in polluting the environment.40 By combining historical and genotyping analyses, researchers traced how, in a fragmented global response to controlling typhoid, antibiotics compensated for weak healthcare, contaminated water and food and poor sanitation in low- and middle-income countries.59 These conditions have fuelled the development of AMR, with a record of neglect by international development and aid initiatives chronicled in the genes of increasingly common forms of drug-resistant typhoid.59
These studies highlight the importance of water, sanitation and hygiene (WASH) infrastructure, particularly in urban informal settlements. Modelling studies indicate that infrastructural improvements reduce the burden of infectious illness and associated antibiotic use, slowing the development and transmission of AMR while also improving maternal and child health.60–62 However, the cost of these improvements is often regarded as prohibitively expensive. Echoing efforts to tackle antibiotic use, infection prevention measures have therefore often been reduced to behavioural changes such as advocating handwashing, interventions with limited impact or that are impossible precisely because of the lack of infrastructure.63,64 Meanwhile evaluation of the effects of improved water and sanitation on drug-resistant infection is surprisingly sparse.65 Future social science-informed research is needed to provide evidence to strengthen WASH infrastructures equitably, and to establish the economic and health costs and co-benefits that extend beyond antibiotic use.
The focus on structures helps us to look beyond patients and healthcare professionals ‘overusing’ antibiotics, and to consider the healthcare system in which they are situated. In many settings, health services are sparse, stock-outs are common, and health workers overworked and under-remunerated. In Russia, a new requirement for a doctor’s prescription to acquire antibiotics caused an unintended increase in prescribing ‘just in case’ by already over-stretched, increasingly inaccessible clinicians, highlighting infrastructural weaknesses in the healthcare system.66 The use of broad-spectrum antibiotics, in contravention to stewardship messaging, offered Sri Lankan hospital clinicians a partial response to missing infrastructures and patient poverty that can only be addressed with huge investment and extensive regulatory and policy interventions.67 An ethnographic study conducted in Bangladeshi public hospitals found adherence to antibiotic use guidelines for diarrhoea patients was hampered by overcrowding, understaffing and lacking hygiene and sanitation.68 In Ghana, a mixed-methods study identified how ‘inappropriate’ antibiotic use was driven by out-of-pocket payments, limiting patients’ contact with the formal health system and the unaffordability of complete antibiotic courses.69 These studies highlight the importance of interventions to enhance access to healthcare insurance, healthcare and affordable medication through universal healthcare coverage, a key structural intervention to tackle AMR.70–72
Ethnographically informed studies have revealed how lives have been made reliant on antibiotics through processes such as modernization, medicalization, urbanization and globalization.73 There is an inherent contradiction between capitalism—with its inbuilt short-term imperatives of productivity and profit—and tackling AMR.74 For example, antibiotics have rendered livestock as predictable units of production in time-sensitive, industrialized livestock farming.75 When confronted with extreme weather events and disease outbreaks, both Thai orange growers and Bangladeshi shrimp producers are forced to resort to ‘desperate measures’ of antibiotic use to protect their crops and businesses.50,76 An ethnographic study of the intensive, industrialized conditions of an American pig farm highlights the tensions around profit, turnover, human and animal health.77 In terms of human workers, healthcare practitioners in urban health clinics in South Africa give antibiotics to vulnerable patients partly in light of their precarious living conditions, and to enable them to stay at work or return there quickly.38
Considering how AMR and/or stewardship efforts might impact people differently can help prevent inadvertently increasing inequality.78 Little attention has been paid to gendered aspects of antibiotic use, for example.79 There are gender differences in pathogenic exposure due to physiology, reproductive and occupational roles.78 Gendered household roles may mean that women have limited input in decision-making and/or access to the economic resources needed to access healthcare, whilst they undertake the majority of caring for children, relatives and animals.80 There are gendered variations in healthcare-seeking behaviour, with social expectations surrounding the unacceptability of ‘strong’ men being ill and seeking help.81 As noted elsewhere, health outcomes including musculoskeletal disorder, reproductive tract infection, injury, psychosocial health and poor nutritional status are all often gendered.82 Research is needed to understand how to better tailor stewardship initiatives and reduce unintended harm in the face of these dynamics, and in the implications for women if antibiotics no longer work as a result of AMR or if their use is restricted.
Given that antibiotics have become a lynchpin in our political-economic systems, simply removing them or educating people about their ‘appropriate’ use is not realistic.73 Instead, AMR-sensitive interventions are needed to address the political and economic imperatives that position antibiotic use as a quick fix.27,57 At the same time, improving working and living conditions, healthcare quality and WASH remains a complex and distant goal.38 In the next section, we reflect on another scale at which to intervene, one perhaps less daunting than these AMR-sensitive structural interventions but not dependent on individual-based responses.
Networks
Individuals may be unaware, or unable, to articulate patterns of antibiotic use, since everyday conditions may be unremarkable or taken for granted. Ethnographic approaches enable the study of the diffuse and prevailing circumstances shaping antibiotic use. The nascent networks grouping draws together ethnographically informed work that elucidates mundane networks of logics, classifications, legacies and flows in which antibiotics are caught up. Rather than being a discrete level of analysis, these networks operate with and are shaped by the practices and structures producing antibiotic use, as described above. The material and meaningful (semiotic) connections between humans and non-humans extend far beyond the moment of antibiotic use, and studies on this help render visible apparatus previously overlooked when considering targets for interventions. Below, we turn to three areas where a networks approach has proved particularly productive: agriculture, circulating discourses around AMR and ‘appropriate’ antibiotic use, and global health architecture. For a fuller discussion of this grouping of research, please refer to the Addressing Antibiotic Use report.17
Adopting a networks vantage point helps elucidate the logics and dynamics underpinning industrialized animal production and the use of antibiotics in this context.83 Webs of people, farm animals, microbes, living conditions, markets, supply chains and regulations make up ‘modern’ farming and aquaculture, from which antibiotic use emerges.50,76,84–86 Understanding these networks assists us to identify alternative means by which to improve animal health and welfare.87
As these studies reveal, interventions are needed to address powerful international corporate interests with the power to mould stewardship responses.75 Historically, policy responses have discounted the structural conditions that necessitate antibiotic deployment along supply chains, thus enabling agribusinesses to comply with regulations without fundamentally altering their organization or strategies of production and profitability.75,88,89 The threat of AMR offers an opportunity to fundamentally reconfigure intensive livestock production and meat consumption.74
In agriculture and beyond, scrutinizing the circulating social scripts—the prevailing language, metaphors, images and understandings—surrounding AMR and ‘appropriate’ antibiotic use aids understanding of how the problem is framed, who is identified as responsible for tackling it, and what the responses might (not) look like.90–92 Narratives of scientific discovery and innovative technology are frequently offered as ways to tackle AMR.93,94 But this account obscures collective responses and the potential role of other framings, including arts, bioethics and the social sciences, in understanding AMR and addressing antibiotic use.92,95,96
In policy documents AMR is conventionally positioned as a threat to economic growth and international security, without attending to how political-economic conditions contribute to antibiotic reliance, the necessity of structural interventions, and the responsibilities of the international pharmaceutical and livestock production industries.29,90–92 Scientific enquiry into environmental AMR is underpinned by assumptions that current levels of pollution will continue and that the solution, therefore, is to mitigate against resulting health risks rather than strengthening WASH infrastructure.97 Diversifying the voices and means used to investigate and represent AMR and antibiotic use will better describe the associated marginalization and injustice across One Health domains.92,96,97
Discourse analysis of written and visual media in the Global North has identified how military framings92,98 and ideas of apocalypse,95,98 migration99 and capitalism100 are all used to explain AMR and our relationship with microbes. A changing understanding of the health importance of human-microbial relations impacts notions of ‘appropriate’ antibiotic use.92,101 The military-inspired framing of the immune system as an army keeping hostile invaders at bay has been challenged as new ways of living with microbes emerge.102–106 In recognizing these potential health benefits and the challenges of living with pathogenic and resistant bacteria, new forms of symbiotic public health and postcolonial, ‘post-colony’ global health are needed.107–110 The coronavirus pandemic, and its multiple ‘waves’, highlights these challenges.111
Consideration of the taken-for-granted backdrop of global health can provide a fresh vantage point from which to address antibiotic use. Unravelling its models, programmes and priorities can elucidate how ideas of ‘appropriate’ antibiotic use have been reached. The forms of technical apparatus—such as clinical guidelines, research methodologies, delivery chains and medical curricula—create channels through which commodities, ideas, knowledge and investments flow, producing situations where antibiotics are present or absent, anticipated or unanticipated.
Global health networks reach through time, space and different locations. Historically informed analysis can help to better understand how the status quo regarding accessing, developing and protecting antibiotics has been reached, and to avoid repeating the mistakes of the past.112–117 Ethnographic studies of patient care pathways and clinic layout reveal how AMR necessitates the reconsideration of hospital design in order to manage the circulation of microbes.118–120 Ethnographers have also been concerned with how knowledge of antibiotic agents, their effects and potential alternatives is produced and translocated.121 This has revealed how antibiotics shape assumptions about what can be known in terms of norms and models of scientific evidence production, for example, the randomized controlled trial.122 As a consequence, the investigation of bacteriophages, living bacteria-eating viruses that could offer a counterfactual to antibiotic use, has been neglected.123
Tackling AMR is bound up in networks of power and control operating within Global Health.110 Forms of colonial health systems persist in healthcare systems today, organized around an abridged form of Western medicine that include antibiotics but with reduced healthcare professional coverage per capita.124 In Zimbabwe, researchers unravelled how the recent global health imperative to protect antibiotics from ‘overuse’ was enacted amidst a legacy of earlier initiatives that built antibiotics into models of care within an essential medicines programme to improve population health.125 The introduction of clinical algorithms and diagnostics—in part, to protect medicines from ‘overuse’—categorizes some patients as undeserving not only of medicines but also of care in these pharmaceuticalized, under-resourced health systems.20,27,126 In such contexts, future research is needed to develop and pilot innovative responses that integrate alternative forms of care.125 Further consideration is also needed to understand how stewardship efforts are enacted amidst development initiatives that seek to support income generation and redress malnutrition through the translocation of intensive forms of livestock production to provide affordable dietary protein.75
Global flows of metrics, data and regulations form networks shaping antibiotic use. Efforts to characterize the distribution of antibiotic use and AMR through metrics typically emanate from influential organizations in the Global North and rely on data extracted from the Global South.52 However, a lack of laboratories, equipment and reporting infrastructures results in datasets centred on the Global North.27 As a result, international policy and stewardship endeavours may have limited resonance or utility in low-resource settings, where frontline clinicians are more interested in the susceptibility of bacteria to their limited stock of antibiotics (i.e. what will work) than their resistance (what will not).52,125 Adopting a networks perspective and working across countries also draws attention to regulatory responses,127 an important, if currently understudied, component of efforts to reduce the global burden of AMR.62
Considering the networks in which antibiotics are entangled offers a novel approach through which to understand antibiotic use, and their careful analysis can identify alternatives and/or render visible previously overlooked targets for stewardship. Understanding the connections between human and non-human components can reveal the subtle and hidden ways these medicines are built into agriculture and global health, for example.
Discussion
In this article, we have collated insights from the growing body of ethnographically informed research into antibiotic use, conducted in diverse settings. These studies take three complementary perspectives from which antibiotic use can be understood: practices, structures and networks. Table 1 summarizes these vantage points and illustrates their use in supporting new ways of thinking and intervening regarding antibiotic use. We propose that this framework be applied to other disciplines to informing future research and support the translation of research insights into policy. Considering structures and networks, in particular, will help to diversify our existing portfolio of responses beyond seeking to change individuals’ behaviour to include more collective and structural responses.
Table 1.
A summary of how the practices, structures and network framework can help to diversify how we understand and address antibiotic use
| Approach | Practices | Structures | Networks |
|---|---|---|---|
| Key message | Antibiotic use practices are determined by wide social and material dimensions that must be addressed. | The structures that antibiotics prop up require investment in order to alter antibiotic use. | Existing public and global health architectures and conventions define antibiotic consumption and must be made visible if antibiotics are to be designed out. |
| Focus of intervening | Interventions change behaviour and practice by understanding and altering the context in which individuals make decisions about antibiotic use. | Interventions modify economic and political conditions to reduce the need for antibiotics. | Interventions redesign networks and tracks that define antibiotic use. |
| Example of interventions | Adjusting practitioner renumeration arrangements, enhancing healthcare accessibility, improving communication between prescribers and patients, providing information on medicines, awareness/education tailored to local understandings of ill health and treatment. | Reduce inequity, prevent infection and support wellbeing by strengthening sanitation and health systems, social safety nets, food security, improved working conditions. | Reconfiguring clinical and veterinary pathways/protocols, strengthening supply chains and aid flows, adjusting accountability frameworks, recognizing the project management orientation of global health, stewardship and rational drug use. |
Adapted from Tompson and Chandler.17
The practices grouping illustrates the strength of ethnographically informed approaches in producing nuanced, in-depth understandings of the local contexts to antibiotic use. These relational practices are influenced by social, economic, political and historical factors that could focus stewardship efforts, helping to broaden current attention on raising awareness of AMR and ‘appropriate’ antibiotic use.
The structures vantage point opens up accountability beyond individual antibiotic users to recognize the roles of political classes, health funders, employers, investors and insurers in enabling the continuation of conditions that lead to infrastructural antibiotic use as a quick fix.28,57,73 A change in the ambition and scale of improvements that we seek to make on people’s lives and living conditions as part of stewardship efforts is needed, for example, by improving access to clean water, sanitation and hygiene. Tackling hunger, poverty and the precarious social conditions in which people experience ill health is increasingly recognized as necessary and yet overlooked means to foster sustained changes in antibiotic use.128–131 Inequality may be reduced by ensuring universal healthcare and strengthening safety nets, enabling ill workers to excuse themselves from the social imperative of being productive in order to recuperate. Insurance schemes to compensate farmers if they lose animals or crops to disease could reduce the pressure to deploy antibiotics as a precautionary measure.
The networks framing of antibiotic use is complementary to, rather than divergent from, the previous vantage points. Studies in this grouping have begun to elucidate the mundane routes through which antibiotics penetrate networks that form the backdrop to our lives. By tracing these networks and revealing roles that would otherwise remain hidden, fresh targets for addressing antibiotic use can be identified. These stretch beyond individuals at the interface of antibiotic use and are perhaps more amendable to change than the long-term political and economic imperatives identified in the structures section.
In addition to describing practices, structures and networks as an analytic framework, we have illustrated the value of incorporating ethnographically informed approaches in multidisciplinary and intersectoral responses to AMR. The importance of interdisciplinary responses to AMR and antibiotic use that are decentralized from the Global North has been noted elsewhere.132–134 To aid such efforts, two international groupings—the International Network for Antimicrobial Resistance Social Science (INAMRSS) and SONAR Global—are seeking to foster collaborations within and beyond the social science community.9,135 SONAR Global has developed online curricula to foster understandings of how the social sciences can help when responding to AMR. For those interested in learning more about social theory, the www.antimicrobialsinsociety.org website offers a curated collection of readings—including journal articles and books—and commentaries explaining relevant insights to understanding AMR and addressing antibiotic use.
Conclusions
Ethnographic approaches concerned with enacted, relational practices and the realities of people’s lives make a valuable contribution to understanding and addressing AMR and antibiotic use. In this article, we have described how social researchers using this approach have addressed antibiotic use from three complementary vantage points: practices, structure and networks. Adopting this framework helps us to see beyond individual-based stewardship approaches and to acknowledge the social structures and networks into which antibiotic use is built. The framework offers a valuable tool to support the translation of research findings into interventions and ensures that these interventions go beyond awareness raising and education campaigns.
Acknowledgements
We gratefully acknowledge our colleagues listed on the AMIS online hub who shared their research insights that contributed to the conceptual development of the framework and reviewed the draft Addressing Antibiotic Use Report.
Funding
This work was supported by The Antimicrobial Resistance Cross Council Initiative supported by the seven research councils in partnership with other funders (grant ref ES/P008100/1). The lead funders for this grant are the Economic and Social Research Council (ESRC), with the Department of Health and Social Care and the Arts and Humanities Research Council (AHRC).
Transparency declarations
None to declare.
References
- 1.So A. Tracking Antimicrobial Resistance in the Sustainable Development Goals. International Institute for Sustainable Development. http://sdg.iisd.org/commentary/guest-articles/tracking-antimicrobial-resistance-in-the-sustainable-development-goals/.
- 2.Holmes A, Moore LS, Sundsfjord A. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 3.Booton RD, Meeyai A, Alhusein N. et al. One Health drivers of antibacterial resistance: quantifying the relative impacts of human, animal and environmental use and transmission. One Health 2021; 12: 100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert H.Social Scientists Needed to Solve the Problem of Antibiotic Overuse. University of Bristol. http://www.bristol.ac.uk/population-health-sciences/projects/amr-champion/news/2015/social-scientists-needed-to-solve-the-problem-of-antibiotic-overuse.html. [DOI] [PubMed] [Google Scholar]
- 5.Smith R.Antimicrobial resistance is a social problem requiring a social solution. BMJ 2015; 350: h2682. [DOI] [PubMed] [Google Scholar]
- 6.Tarrant C.Studying Antimicrobial Resistance: Interdisciplinary Research is Critical, But Challenging. University of Bristol. https://amrchamp.blogs.bristol.ac.uk/2017/07/17/studying-antimicrobial-resistance-interdisciplinary-research-is-critical-but-challenging/. [Google Scholar]
- 7.Rousham EK, Unicomb L, Islam MA.. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: integrating behavioural, epidemiological and One Health approaches. Proc Biol Sci 2018; 285: 20180332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert H.Championing antimicrobial resistance social science research. Impact 2018; issue 4: 14–17.
- 9.Minssen T, Outterson K, Rogers Van Katwyk S. et al. Social, cultural and economic aspects of antimicrobial resistance. Bull World Health Organ 2020; 98: 823–823A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Sheldenkar A, Lwin MO.. A decade of antimicrobial resistance research in social science fields: a scientometric review. Antimicrob Resist Infect Control 2020; 9: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler CIR, Hutchinson C. Antimicrobial Resistance and Anthropology: Research Brief. ESRC AMR Research Champion/University of Bristol, 2016. https://researchonline.lshtm.ac.uk/id/eprint/3418325.
- 12.Donald I.Antimicrobial Resistance and Psychology: Research Brief. ESRC AMR Research Champion/University of Bristol, 2016. https://www.bristol.ac.uk/media-library/sites/social-community-medicine/documents/social-science-and-amr/Psychology&AMR_23082016.pdf. [Google Scholar]
- 13.Elbe S, Rushton S.. Antimicrobial Resistance and International Relations: Research Brief. ESRC AMR Research Champion/University of Bristol, 2016. http://www.bristol.ac.uk/media-library/sites/social-community-medicine/documents/social-science-and-amr/InternationalRelations&AMR_02082016.pdf. [Google Scholar]
- 14.Wood F.Antimicrobial Resistance and Medical Sociology: Research Brief. ESRC AMR Research Champion/University of Bristol, 2016. http://www.bristol.ac.uk/media-library/sites/social-community-medicine/documents/social-science-and-amr/MedicalSociology&AMR21092016.pdf. [Google Scholar]
- 15.Denyer Willis L, Chandler CIR.. Anthropology’s contribution to AMR Control. AMR Control 2018; 4: 114–8. [Google Scholar]
- 16.Lorencatto F, Charani E, Sevdalis N. et al. Driving sustainable change in antimicrobial prescribing practice: how can social and behavioural sciences help? J Antimicrob Chemother 2018; 73: 2613–24. [DOI] [PubMed] [Google Scholar]
- 17.Tompson AC, Chandler CIR.. Addressing Antibiotic Use: Insights from Social Science around the World. London School of Hygiene & Tropical Medicine, 2021. 10.17037/PUBS.04659562. [DOI] [Google Scholar]
- 18.Reeves S, Albert M, Kuper A. et al. Why use theories in qualitative research? BMJ 2008; 337: a949. [DOI] [PubMed] [Google Scholar]
- 19.Hammersley M, Atkinson P.. Ethnography: Principles in Practice, 3rd edn. Routledge, 2007. [Google Scholar]
- 20.Chandler CIR, Hutchinson E, Hutchison C.. Addressing Antimicrobial Resistance through Social Theory: An Anthropologically Oriented Report. London School of Hygiene & Tropical Medicine, 2016. https://researchonline.lshtm.ac.uk/id/eprint/3400500. [Google Scholar]
- 21.Rabinow P, Reflections on Fieldwork in Morocco: Thirtieth Anniversary Edition, with a New Preface by the Author. University of California Press, 2007. [Google Scholar]
- 22.Pope C.Conducting ethnography in medical settings. Med Educ 2005; 39: 1180–7. [DOI] [PubMed] [Google Scholar]
- 23.Kuper A.The comparative method in social anthropology. In: Kuper A, ed. The Social Anthropology of Radcliffe-Brown. Routledge, 2004; 54–69. [Google Scholar]
- 24.Nichter M.Global Health: Why Cultural Perceptions, Social Representations, and Biopolitics Matter. The University of Arizona Press, 2008. [Google Scholar]
- 25.Haenssgen MJ, Charoenboon N, Thavethanutthanawin P. et al. Tales of treatment and new perspectives for global health research on antimicrobial resistance. Med Humanit 2020; doi: 10.1136/medhum-2020-011894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert H, Chen M, Cabral C.. Antimicrobial resistance, inflammatory responses: a comparative analysis of pathogenicities, knowledge hybrids and the semantics of antibiotic use. Palgrave Commun 2019; 5: 85. [Google Scholar]
- 27.Chandler CIR.Current accounts of antimicrobial resistance: stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun 2019; 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Will CM.Editorial: Beyond behavior? Institutions, interactions and inequalities in the response to antimicrobial resistance. Sociol Health Illn 2018; 40: E1–E9. [DOI] [PubMed] [Google Scholar]
- 29.Khan MS, Durrance-Bagale A, Legido-Quigley H. et al. ‘LMICs as reservoirs of AMR’: a comparative analysis of policy discourse on antimicrobial resistance with reference to Pakistan. Health Policy Plan 2019; 34: 178–87. [DOI] [PubMed] [Google Scholar]
- 30.Broom A, Broom J, Kirby E. et al. Antibiotic optimisation in ‘the bush’: local know-how and core-periphery relations. Health Place 2017; 48: 56–62. [DOI] [PubMed] [Google Scholar]
- 31.Haenssgen MJ, Charoenboon N, Khine Zaw Y.. It is time to give social research a voice to tackle antimicrobial resistance? J Antimicrob Chemother 2018; 73: 1112–3. [DOI] [PubMed] [Google Scholar]
- 32.Charani E, Holmes A.. Antibiotic stewardship—twenty years in the making. Antibiotics (Basel) 2019; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haenssgen MJ, Charoenboon N, Zanello G. et al. Antibiotic knowledge, attitudes and practices: new insights from cross-sectional rural health behaviour surveys in low-income and middle-income South-East Asia. BMJ Open 2019; 9: e028224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adome RO, Whyte SR, Hardon A.. Popular Pills: Community Drug Use in Uganda. Het Spinhuis, 1996. [Google Scholar]
- 35.Nichter M.Risk, Vulnerability, and harm reduction: preventing STIs in Southeast Asia by antibiotic prophylaxis, a misguided practice. In: Makhlouf Obermeyer C, ed. Cultural Perspectives on Reproductive Health. Oxford University Press, 2001; 101–27. [Google Scholar]
- 36.Craig D.Antibiotics in market and culture. In: Craig D, ed. Familiar Medicine: Everyday Health Knowledge and Practice in Today's Vietnam. University of Hawai'i Press, 2002; 123–60. [Google Scholar]
- 37.Whyte S, Van der Geest S, Hardon A.. The Social Lives of Medicines. Cambridge University Press, 2003. [Google Scholar]
- 38.Manderson L.Prescribing, care and resistance: antibiotic use in urban South Africa. Humanit Soc Sci Commun 2020; 7: 77. [Google Scholar]
- 39.Chen M, Kadetz P, Cabral C. et al. Prescribing antibiotics in rural China: the influence of capital on clinical realities. Front Sociol 2020; 5: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broom A, Doron A.. Antimicrobial resistance, politics, and practice in India. Qual Health Res 2020; 30: 1684–96. [DOI] [PubMed] [Google Scholar]
- 41.Broom J, Broom A, Kenny K. et al. Antimicrobial overuse in India: a symptom of broader societal issues including resource limitations and financial pressures. Glob Public Health 2021; 16: 1079–87. [DOI] [PubMed] [Google Scholar]
- 42.Bonaconsa C, Mbamalu O, Mendelson M. et al. Visual mapping of team dynamics and communication patterns on surgical ward rounds: an ethnographic study. BMJ Qual Saf 2021; doi: 10.1136/bmjqs-2020-012372. [DOI] [PubMed] [Google Scholar]
- 43.Singh S, Mendelson M, Surendran S. et al. Investigating infection management and antimicrobial stewardship in surgery: a qualitative study from India and South Africa. Clin Microbiol Infect 2021; doi: 10.1016/j.cmi.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Lucas PJ, Uddin MR, Khisa N. et al. Pathways to antibiotics in Bangladesh: a qualitative study investigating how and when households access medicine including antibiotics for humans or animals when they are ill. PLoS One 2019; 14: e0225270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahar P, Unicomb L, Lucas PJ. et al. What contributes to inappropriate antibiotic dispensing among qualified and unqualified healthcare providers in Bangladesh? A qualitative study. BMC Health Serv Res 2020; 20: 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues CF.Self-medication with antibiotics in Maputo, Mozambique: practices, rationales and relationships. Palgrave Commun 2020; 6: 6. [Google Scholar]
- 47.Snively-Martinez AE.Ethnographic decision modeling to understand smallholder antibiotic use for poultry in Guatemala. Med Anthropol 2019; 38: 295–310. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan AS, George MS, Chatterjee P. et al. The social biography of antibiotic use in smallholder dairy farms in India. Antimicrob Resist Infect Control 2018; 7: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinchliffe S, Butcher A, Rahman MM.. The AMR problem: demanding economies, biological margins, and co-producing alternative strategies. Palgrave Commun 2018; 4: 142. [Google Scholar]
- 50.Hinchliffe S, Butcher A, Rahman MM. et al. Production without medicalisation: risk practices and disease in Bangladesh aquaculture. Geogr J 2021; 187: 39–50. [Google Scholar]
- 51.Masud AA, Rousham EK, Islam MA. et al. Drivers of antibiotic use in poultry production in Bangladesh: dependencies and dynamics of a patron-client relationship. Front Vet Sci 2020; 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirchhelle C, Atkinson P, Broom A. et al. Setting the standard: multidisciplinary hallmarks for structural, equitable and tracked antibiotic policy. BMJ Glob Health 2020; 5: e003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearson M, Chandler C.. Knowing antmicrobial resistance in practice: a multi-country qualitative study with human and animal healthcare professionals. Glob Health Action 2019; 12: 1599560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broom J, Broom A, Kirby E.. The drivers of antimicrobial use across institutions, stakeholders and economic settings: a paradigm shift is required for effective optimization. J Antimicrob Chemother 2019; 74: 2803–9. [DOI] [PubMed] [Google Scholar]
- 55.Krockow EM, Tarrant C.. The international dimensions of antimicrobial resistance: contextual factors shape distinct ethical challenges in South Africa, Sri Lanka and the United Kingdom. Bioethics 2019; 33: 756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarrant C, Krockow EM, Nakkawita WMID. et al. Moral and contextual dimensions of “inappropriate” antibiotic prescribing in secondary care: a three-country interview study. Front Sociol 2020; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denyer Willis L, Chandler C.. Quick fix for care, productivity, hygiene and inequality: reframing the entrenched problem of antibiotic overuse. BMJ Glob Health 2019; 4: e001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doron A, Broom A.. The spectre of superbugs: waste, structural violence and antimicrobial resistance in India. Worldwide Waste J Interdiscip Stud 2019; 2: 7. [Google Scholar]
- 59.Kirchhelle C, Dyson ZA, Dougan G.. A biohistorical perspective of typhoid and antimicrobial resistance. Clin Infect Dis 2019; 69: S388–S394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benova L, Cumming O, Campbell OM.. Systematic review and meta-analysis: association between water and sanitation environment and maternal mortality. Trop Med Int Health 2014; 19: 368–87. [DOI] [PubMed] [Google Scholar]
- 61.Araya P, Hug J, Joy G. et al. Review on Antimicrobial Resistance: The Impact of Water and Sanitation on Diarrhoeal Disease Burden and over-Consumption of Antibiotics. London School of Economics and Political Science, 2016. [Google Scholar]
- 62.Collignon P, Beggs JJ, Walsh TR. et al. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health 2018; 2: e398–405. [DOI] [PubMed] [Google Scholar]
- 63.Naikoba S, Hayward A.. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001; 47: 173–80. [DOI] [PubMed] [Google Scholar]
- 64.Cumming O, Arnold BF, Ban R. et al. The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: a consensus statement. BMC Med 2019; 17: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinto JC, Keestra S, Tandon P. et al. WASH and Biosecurity Interventions for Reducing Burdens of Infection, Antibiotic Use and Antimicrobial Resistance in Animal Agricultural Settings: A One Health Mixed Methods Systematic Review. London School of Hygiene and Tropical Medicine/CGIAR/ILRI, 2020. 10.17037/PUBS.04658914. [DOI] [Google Scholar]
- 66.Kamenshchikova A, Fedotova MM, Fedorova OS. et al. Obligatory medical prescription of antibiotics in Russia: navigating formal and informal health-care infrastructures. Sociol Health Illn 2021; 43: 353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarrant C, Colman AM, Jenkins DR. et al. Drivers of broad-spectrum antibiotic overuse across diverse hospital contexts-a qualitative study of prescribers in the UK, Sri Lanka and South Africa. Antibiotics (Basel) 2021; 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biswas D, Hossin R, Rahman M. et al. An ethnographic exploration of diarrheal disease management in public hospitals in Bangladesh: from problems to solutions. Soc Sci Med 2020; 260: 113185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aslam A, Gajdács M, Zin CS. et al. Evidence of the practice of self-medication with antibiotics among the lay public in low- and middle-income countries: a scoping review. Antibiotics (Basel) 2020; 9: 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bloom G, Merrett GB, Wilkinson A. et al. Antimicrobial resistance and universal health coverage. BMJ Glob Health 2017; 2: e000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jonas OB, Irwin A, Berthe F. et al. Drug-Resistant Infections: a Threat to Our Economic Future (Vol. 2): Final Report (English), March 2017. World Bank Group: HNP/Agriculture Global Antimicrobial Resistance Initiative. https://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf. [Google Scholar]
- 72.Tayler E, Gregory R, Bloom G. et al. Universal health coverage: an opportunity to address antimicrobial resistance? Lancet Glob Health 2019; 7: e1480–1. [DOI] [PubMed] [Google Scholar]
- 73.Broom A, Kenny K, Prainsack B. et al. Antimicrobial resistance as a problem of values? Views from three continents. Crit Public Health 2020; 31: 451–63. [Google Scholar]
- 74.De Lima Hutchison C, Knight G, Stabler R. et al. The modern era must end: antibiotic resistance helps us rethink medicine and farming. BMJ. https://blogs.bmj.com/bmj/2018/07/11/the-modern-era-must-end-antibiotic-resistance-helps-us-rethink-medicine-and-farming/. [Google Scholar]
- 75.Kirchhelle C.Pharming animals: a global history of antibiotics in food production (1935–2017). Palgrave Commun 2018; 4: 96. [Google Scholar]
- 76.Urapeepathanapong T, Chawraingern S, de Lima Hutchison C. Antibiotic Angels: Seeing Green in Thailand’s Orange Orchards. Antimicrobials in Society. https://antimicrobialsinsociety.org/commentary/antibiotic-angels-seeing-green-in-thailands-orange-orchards/.
- 77.Blanchette A.Living waste and the labor of toxic health on American factory farms. Med Anthropol Q 2019; 33: 80–100. [DOI] [PubMed] [Google Scholar]
- 78.WHO. Tackling Antimicrobial Resistance (AMR) Together. Working Paper 5.0: Enhancing the Focus on Gender and Equity. 2018. https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-working-papers/tackling-amr-together-working-paper-5-genderandequity-sept2018-en.pdf.
- 79.Saint V.Exploring equity, social determinants of health and gender considerations for antimicrobial resistance. Eur J Public Health 2019; 29 Suppl 4: ckz185.799. [Google Scholar]
- 80.Lohm D, Davis M, Whittaker A. et al. Role crisis, risk and trust in Australian general public narratives about antibiotic use and antimicrobial resistance. Health Risk Soc 2020; 22: 231–48. [Google Scholar]
- 81.Chikovore J, Pai M, Horton KC. et al. Missing men with tuberculosis: the need to address structural influences and implement targeted and multidimensional interventions. BMJ Glob Health 2020; 5: e002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma Waddington H, Cairncross S.. PROTOCOL: water, sanitation and hygiene for reducing childhood mortality in low- and middle-income countries. Campbell Syst Rev 2021; 17: e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bellet C.Change it or perish? Drug resistance and the dynamics of livestock farm practices. J Rural Stud 2018; 63: 57–64. [Google Scholar]
- 84.Fortané N.Veterinarian ‘responsibility’: conflicts of definition and appropriation surrounding the public problem of antimicrobial resistance in France. Palgrave Commun 2019; 5: 67. [Google Scholar]
- 85.Begemann S, Watkins F, Van Hoyweghen I. et al. The governance of UK dairy antibiotic use: industry-led policy in action. Front Vet Sci 2020; 7: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hughes A, Roe E, Hocknell S.. Food supply chains and the antimicrobial resistance challenge: on the framing, accomplishments and limitations of corporate responsibility. Environ Plan A 2021; doi: 10.1177/0308518X211015255. [DOI] [Google Scholar]
- 87.Woods A.Decentring antibiotics: UK responses to the diseases of intensive pig production (ca. 1925-65). Palgrave Commun 2019; 5: 41. [Google Scholar]
- 88.Fortané N. From Nightmare to Promise. Rethinking AMR Narratives. AmAgri, Antimicrobials in Agriculture. https://www.amagri.eu/commentaries/from-nightmare-to-promise-rethinking-amr-narratives.
- 89.Kirchhelle C.Pyrrhic Progress: The History of Antibiotics in Anglo-American Food Production. Rutgers University Press, 2020. [PubMed] [Google Scholar]
- 90.Wernli D, Jorgensen PS, Morel CM. et al. Mapping global policy discourse on antimicrobial resistance. BMJ Glob Health 2017; 2: e000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collins LC, Jaspal R, Nerlich B.. Who or what has agency in the discussion of antimicrobial resistance in UK news media (2010-2015)? A transitivity analysis. Health (London) 2018; 22: 521–40. [DOI] [PubMed] [Google Scholar]
- 92.Walker IF.Beyond the military metaphor. Comparing antimicrobial resistance and the COVID-19 pandemic in the United Kingdom. Med Anthropol Theory 2020; 7: 261–72. [Google Scholar]
- 93.Brown N, Nettleton S.. Bugs in the blog: immunitary moralism in antimicrobial resistance (AMR). Soc Theory Health 2017; 15: 302–22. [Google Scholar]
- 94.Davis M, Lyall B, Whittaker A. et al. A year in the public life of superbugs: news media on antimicrobial resistance and implications for health communications. Soc Sci Med 2020; 256: 113032. [DOI] [PubMed] [Google Scholar]
- 95.Irwin R.Imagining the postantibiotic future: the visual culture of a global health threat. Med Humanit 2020; doi: 10.1136/medhum-2020-011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Will C, Kamenshchikova A.. From universal frames to collective experimentation? Pursuing serious conversations about antimicrobial resistance [version 1; peer review: 2 approved]. Wellcome Open Res 2020; 5: 192. [Google Scholar]
- 97.Helliwell R, Raman S, Morris C.. Environmental imaginaries and the environmental sciences of antimicrobial resistance. Environ Plan E 2020; doi:10.1177%2F2514848620950752. [Google Scholar]
- 98.Nerlich B."The post-antibiotic apocalypse" and the "war on superbugs": catastrophe discourse in microbiology, its rhetorical form and political function. Public Underst Sci 2009; 18: 574–88. discussion 588–90. [DOI] [PubMed] [Google Scholar]
- 99.Brown N, Nettleton S.. There is worse to come: the biopolitics of traumatism in antimicrobial resistance (AMR). Sociol Rev 2017; 65: 493–508. [Google Scholar]
- 100.Brown N, Nettleton S.. Economic imaginaries of the anti-biosis: between ‘economies of resistance’ and the ‘resistance of economies’. Palgrave Commun 2018; 4: 123. [Google Scholar]
- 101.Grondal H.Harmless, friendly and lethal: antibiotic misuse in relation to the unpredictable bacterium Group A streptococcus. Sociol Health Illn 2018; 40: 1127–41. [DOI] [PubMed] [Google Scholar]
- 102.Davis M, Flowers P, Lohm D. et al. Immunity, biopolitics and pandemics: public and individual responses to the threat to life. Bod Soc 2016; 22: 130–54. [Google Scholar]
- 103.Greenhough B, Dwyer A, Grenyer R. et al. Unsettling antibiosis: how might interdisciplinary researchers generate a feeling for the microbiome and to what effect? Palgrave Commun 2018; 4: 149. [Google Scholar]
- 104.Brown N, Biotic politics: immunitary imaginaries in antimicrobial resistance (AMR). In: Brown N, ed. Immunitary Life. Palgrave Macmillan, 2019. [Google Scholar]
- 105.Lorimer J.Hookworms make us human: the microbiome, eco-immunology, and a probiotic turn in Western health care. Med Anthropol Q 2019; 33: 60–79. [DOI] [PubMed] [Google Scholar]
- 106.Brives C.Pluribiosis and the never-ending microgeohistories. In: Brives C, Rest M, Sariola S, eds. With Microbes. Mattering Press, 2021. (in press). [Google Scholar]
- 107.Lorimer J.Parasites, ghosts and mutualists: a relational geography of microbes for global health. Trans Inst Br Geogr 2017; 42: 544–58. [Google Scholar]
- 108.Giraud E, Hadley Kershaw E, Helliwell R. et al. Abundance in the Anthropocene. Sociol Rev 2019; 67: 357–73. [Google Scholar]
- 109.Sariola S, Gilbert SF.. Toward a symbiotic perspective on public health: recognizing the ambivalence of microbes in the Anthropocene. Microorganisms 2020; 8: 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hinchliffe S.Postcolonial global health, post-colony microbes and antimicrobial resistance. Theor Cult Soc 2021; doi: 10.1177/0263276420981606. [DOI] [Google Scholar]
- 111.Hinchliffe S, Manderson L, Moore M.. Planetary healthy publics after COVID-19. Lancet Planet Health 2021; 5: e230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Podolsky SH, Bud R, Gradmann C. et al. History teaches us that confronting antibiotic resistance requires stronger global collective action. J Law Med Ethics 2015; 43 Suppl 3: 27–32. [DOI] [PubMed] [Google Scholar]
- 113.Gradmann C.Re-inventing infectious disease: antibiotic resistance and drug development at the Bayer Company 1945-80. Med Hist 2016; 60: 155–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Landecker H.Antibiotic resistance and the biology of history. Body Soc 2016; 22: 19–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santesmases MJ.The Circulation of Penicillin in Spain: Health, Wealth and Authority. Palgrave Macmillan, 2018. [DOI] [PubMed] [Google Scholar]
- 116.Brazelton MA.The production of penicillin in wartime China and Sino-American definitions of “normal” microbiology. J Mod Chinese Hist 2019; 13: 102–23. [Google Scholar]
- 117.Hobaek B, Lie AK.. Less is more: Norwegian drug regulation, antibiotic policy, and the “need clause”. Milbank Q 2019; 97: 762–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown NGF, Buse C, Lewis A. et al. Pathways, practices and architectures: containing anti-microbial resistance (AMR) in the cystic fibrosis clinic. Health (London) 2021; 25: 196–213. [DOI] [PubMed] [Google Scholar]
- 119.Brown N, Buse C, Lewis A. et al. Air care: an ‘aerography’ of breath, buildings and bugs in the cystic fibrosis clinic. Sociol Health Illn 2020; 42: 972–86. [DOI] [PubMed] [Google Scholar]
- 120.Buse C, Brown N, Nettleton S. et al. Caring through distancing: spatial boundaries and proximities in the cystic fibrosis clinic. Soc Sci Med 2020; 265: 113531. [DOI] [PubMed] [Google Scholar]
- 121.Kochhar R.The virus in the rivers: histories and antibiotic afterlives of the bacteriophage at the sangam in Allahabad. Notes Rec R Soc Lond 2020; 74: 625–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Podolsky SH.Antibiotics and the social history of the controlled clinical trial, 1950-1970. J Hist Med Allied Sci 2010; 65: 327–67. [DOI] [PubMed] [Google Scholar]
- 123.Brives C, Pourraz J.. Phage therapy as a potential solution in the fight against AMR: obstacles and possible futures. Palgrave Commun 2020; 6: 100. [Google Scholar]
- 124.Palanco Lopez P, Chandler CIR.. Histories of Antibiotics: A One Health account of the Arrival of Antimicrobial Drugs to Zimbabwe, Malawi and Uganda. Report for the Improving Human Health Flagship Initiative, Agriculture for Nutrition and Health Research Programme, CGIAR. London School of Hygiene & Tropical Medicine, 2020. 10.17037/PUBS.04658867. [DOI] [Google Scholar]
- 125.Dixon J, Manyau S, Kandiye F. et al. Antibiotics, rational drug use and the architecture of global health in Zimbabwe. Soc Sci Med 2021; 272: 113594. [DOI] [PubMed] [Google Scholar]
- 126.Dixon J, Chandler CIR.. Opening up ‘fever’, closing down medicines. Med Anthropol Theory 2019; 6: 53–79. [Google Scholar]
- 127.Rogers Van Katwyk S, Weldon I, Giubilini A. et al. Making use of existing international legal mechanisms to manage the global antimicrobial commons: identifying legal hooks and institutional mandates. Health Care Anal 2020. doi: 10.1007/s10728-020-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alividza V, Mariano V, Ahmad R. et al. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect Dis Poverty 2018; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haenssgen MJ, Charoenboon N, Xayavong T. et al. Precarity and clinical determinants of healthcare-seeking behaviour and antibiotic use in rural Laos and Thailand. BMJ Glob Health 2020; 5: e003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chaudhuri S, Pradhan R.. Re-examining the notion of irrational antimicrobial prescribing in LMICs. Lancet Infect Dis 2021; 21: 28–9. [DOI] [PubMed] [Google Scholar]
- 131.Glover R, Knight GM, Chandler CIR.. Antimicrobial resistance at the G7. BMJ 2021; 373: n1417. [DOI] [PubMed] [Google Scholar]
- 132.Kamenshchikova A, Wolffs PFG, Hoebe C. et al. Transdisciplinary work against antimicrobial resistance. Lancet Infect Dis 2020; 20: 526–7. [DOI] [PubMed] [Google Scholar]
- 133.Macduff C. CODA AMR: The Contribution of Disciplines from the Arts and Humanities to Addressing Antimicrobial Resistance. The Glasgow School of Art, 2020. http://radar.gsa.ac.uk/7418/1/CODA%20AMR%20final%20report.pdf.
- 134.Veepanattu P, Singh S, Mendelson M. et al. Building resilient and responsive research collaborations to tackle antimicrobial resistance—lessons learnt from India, South Africa, and UK. Int J Infect Dis 2020; 100: 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giles-Vernick T, Kutalek R, Napier D. et al. A new social sciences network for infectious threats. Lancet Infect Dis 2019; 19: 461–3. [DOI] [PubMed] [Google Scholar]
- 136.Vedadhir AA, Rodrigues C, Lambert H.. Social science research contributions to antimicrobial resistance: protocol for a scoping review. Syst Rev 2020; 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]



