Abstract
Introduction
Extranodal lymphomas are commonly encountered in the gastrointestinal tract but lymphomas of colon and rectum are rare. Non-Hodgkin lymphoma is the most common type of colonic lymphoma and represents less than 0.5% of colorectal neoplasms. Chemotherapeutical agents are gateway to disease remission and sometimes cure in most patients but surgery may be necessary in emergent situations.
Case presentation
A 77-year-old male patient presented with abdominal discomfort, constipation, and obstructive defecation symptoms. Radiological imaging revealed a mass in the sigmoid colon extending towards the rectum. Colonoscopy was performed and biopsy of a nearly 10 cm ulcerovegetative lesion was obtained. Histological examination following biopsy revealed it to be a diffuse large B-cell lymphoma of the sigmoid colon. There was no indication for surgery and the patient was referred to medical oncology clinic for chemotherapy treatment.
Discussion
Non-Hodgkin lymphoma is a lymphoproliferative disorder with the diffuse large B cell lymphoma (DLBCL) being the most common subtype. The DLBCL subtype is rarely observed in the colon and rectum. Chromosomal abnormalities are involved in the pathophysiology and gene rearrangements lead to adjustments in lymphocyte function and differentiation.
Conclusion
In this case report, we present a rare presentation of a Non-Hodgkin lymphoma presenting in the sigmoid colon. The disease can present with nonspecific symptoms and various imaging modalities along with histopathological evaluation is necessary for the correct subtyping of lymphoma. Chemoradiotherapy is key for treatment, and surgery is usually reserved for cases of obstruction, perforation, or bleeding.
Keywords: Diffuse large B-cell lymphoma, Primary colonic lymphoma, Colonic obstruction
Highlights
-
•
Non-Hodgkin lymphoma is the most common type of colonic lymphoma.
-
•
Extranodal lymphomas of the colon and rectum are rare.
-
•
Histopathological evaluation is important for subtyping of lymphoma.
-
•
Chemotherapy is the main form of treatment but sometimes surgery is required.
1. Introduction
Primary lymphoma of the colon and rectum is a very rare disease accounting for less than 0.5% of all colorectal neoplasms combined [1], [2]. Extranodal lymphomas are most frequently encountered in the gastrointestinal tract, with non-Hodgkin lymphoma being the most common subtype and stomach being the most common location [3], [4], [5]. Less commonly, they can be observed in the small bowel, colon, and rectum. Non-specific symptoms necessitate extensive laboratory and imaging work-up and prompt diagnosis is crucial in order to establish patient-tailored management of the tumor with chemotherapy, radiotherapy, and surgery.
2. Case presentation
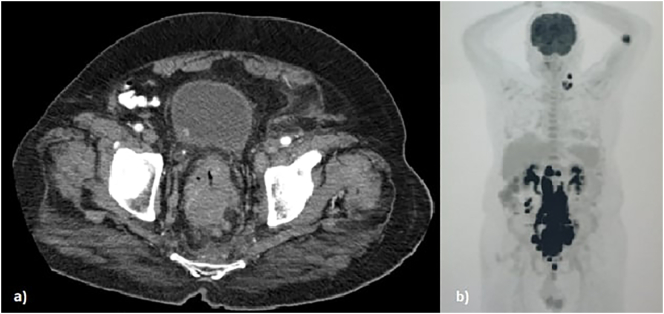
A 77-year-old male patient presented to the clinic with a two-month history of abdominal discomfort, constipation, and difficulty during defecation. History of the patient was unremarkable except for a mild form of depression treated with escitalopram. Family history was not notable and there were no history of genetic disease. Physical examination was unremarkable. Laboratory tests were remarkable for anemia (Hb 10,4 g/dl), leukopenia (WBC 3,8 103/μl), lymphopenia (0,6 103/μl), and a high CRP (18,55 mg/l). Colonoscopy showed a partially-obstructing, ulcerovegetative wall-thickening nearly 10 cm in size extending from the sigmoid colon towards the rectum. Sigmoid diverticula were also observed. The abdominal computerized tomography (CT) showed a mass protruding inside the lumen and conglomerated lymph nodes in the rectosigmoid region (Fig. 1). Multiple conglomerated lymph nodes were observed starting from the level of renal arteries, filling the para-aortic and aorto-caval space, and extending down towards the left iliac region adjacent to the rectum. Biopsy of the tumor cells showed large cells with round and irregular nuclei with dark chromatin and distinct central nucleoli. Immunohistochemistry staining of the atypical cells resulted in CD30 (−), CD20 (+), CD10 (−), Bcl-6 (+), MUM1 (+), Bcl-2 (+) and c-MYC (−). EBV-RNA transcript was not observed with in-situ hybridization technique. The Ki-67 proliferative index was high (50–70%). The pathological diagnosis was diffuse large B-cell lymphoma (DLBCL) of non-germinal center type (Figs. 3–6). Positron emission tomography-computerized tomography (PET/CT) showed an increased uptake of 18F-Fluorodeoxyglucose (FDG) in the sigmoid colon extending towards the rectum along with many adjacent lymph node stations such as para-aortic, para-caval, interaortocaval, retrocrural, pelvic, and bilateral common iliac nodes (Fig. 2). Radioactive tracer uptake was also seen in left supraclavicular and left cervical lymph nodes. The patient did not require surgery (i.e., resection, or a diverting colostomy) and was referred to the hematology clinic where rituximab, cyclophosphamide, adriamycin, vincristine, and methylprednisolone treatment regimen was initiated. After four cycles of chemotherapy, the interim PET/CT showed total (or near total) regression in the left cervical and supraclavicular hypermetabolic lymph nodes and multiple abdominopelvic hypermetabolic lymph nodes. The paraaortocaval and left external iliac lymph nodes showed minimal FDG uptake indicating a residual increase. The hypermetabolic lesions that were previously observed in the sigmoid colon, rectum, and the anal canal showed a near total regression and only a residual FDG uptake in the sigmoid colonic wall. The patient continues his follow-up with the hematology clinic to complete his chemotherapy. This case has been reported in line with the SCARE 2020 criteria [6].
Fig. 1.
A) The abdominal CT scan showing a mass protruding and nearly obstructing inside the bowel lumen, B) PET scan showing primary tumor location with multiple infra- and supra-diaphragmatic lymph node involvement.
Fig. 2.
A) Normal colonic mucosa and diffuse tumoral infiltration in lamina propria (H&E, 20× magnification), B) atypical lymphoid cells with irregular round nuclei, dark chromatin, distinct central nucleoli (H&E, 400× magnification), C) Mum-1 positivity suggesting a non-germinal center B-cell DLBCL subtype (ABC method 400× magnification), D) atypical lymphoid cells staining positive for CD20 (ABC method 400× magnification).
3. Discussion
Non-Hodgkin lymphoma is a malignant neoplasm categorized under lymphoproliferative disorders originating from B lymphocytes, T lymphocytes or natural killer cells [7], [10]. The mature B lymphocyte neoplasms can be further subcategorized, with DLBCL being the most common subtype and the most common type of Non-Hodgkin lymphoma worldwide [8], [9].
Non-Hodgkin lymphomas of the colon and rectum present itself at a median age of 55 (range: 23–86 years), and tends to have a male predominance [11]. Risk factors implicated in the development of gastrointestinal lymphoma include human immunodeficiency virus, celiac disease, immunosuppression, and inflammatory bowel disease [18], [19]. Chromosomal abnormalities in band 3q27 and activation of proto-oncogenes are involved in the pathophysiology of colorectal lymphomas; specifically the Bcl-6 gene rearrangement is involved in 35% of the cases in DLBCL [12]. The Bcl-6 gene has key roles in the formation of germinal centers, adjusting lymphocyte function and lymphocyte differentiation [13].
Tumor cells of this patient included atypical large mononuclear lymphoid cells with irregular round nuclei, dark chromatin, and distinct central nucleoli. The atypical lymphoid cells were strongly positive for immunohistochemistry with CD20. Nuclear and cytoplasmic features along with CD20 positivity were diagnostic for DLBCL in this patient. Using the Hans algorithm, immunohistochemistry staining of the tumor cells in this patient resulted in CD10 (−), Bcl-6 (+) and Mum-1 (+), leading to a diagnosis of non-germinal center B cell type DLBCL [20].
Treatment strategies available for DLBCL include chemotherapy, radiotherapy, surgery, or their combinations. The use of anthracyclines has become gold standard and a cornerstone in treating Non-Hodgkin lymphoma. These include cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) [11]. The anti-CD20 monoclonal antibody rituximab can be added to this anthracycline-based combination and has been found to be effective in leading to complete remission in most patients and cure in more than half of patients [14], [15]. This addition has been shown to improve outcome and prolong progression-free, event-free, disease-free, and overall survival in patients diagnosed with DLBCL [12], [16], [17] There is an increasing trend towards the use of anthracycline and monoclonal antibody combination in majority of the cases and surgery is reserved for emergent situations such as obstruction, perforation, or bleeding [21].
The role of surgery in treating colonic lymphomas is still debatable. Some authors suggest surgery as the initial form of treatment despite the clinical stage of the tumor since they believe such action prevents complications and increase the chances of cure with or without adjuvant chemotherapy [22], [23]. However, others believe that colonic lymphoma is a systemic disease and suggest that surgery should be reserved for emergency situations such as hemorrhage, perforation, or obstruction [24], [25]. They accept that systemic chemotherapy as the main standard treatment for colonic lymphoma. In a study performed by Cai et al. where primary colonic lymphomas were identified from the SEER database from the dates 1973 to 2011, 2050 of 3342 patients (61,3%) underwent surgical intervention indicating that surgery is quite frequent once a diagnosis is established [26]. Efficacy of surgery also seems to be site-dependent. Surgical intervention has a positive effect on survival outcomes in right-sided colonic lymphomas (p < 0.001) compared to left-sided and rectal lymphomas (p = 0.197 and p = 0.036, respectively) [26]. This study has shown that patients who underwent surgery showed significant improvement in survival (113 months versus 74 months, adjusted HR = 0.69, 95% CI: 0.59–0.81).
4. Conclusion
Non-Hodgkin lymphoma of the colon and rectum is a rare disease presenting with nonspecific symptoms. Imaging modalities can aid in locating and staging the tumor but histopathological examination is key for diagnosis. Combination of rituximab with anthracyclines have been proven to be effective in treating primary colorectal lymphomas and has been shown to improve survival in various studies.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
None.
Ethical approval
None.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Research registration
None.
Guarantor
Ergin Erginoz.
CRediT authorship contribution statement
Ergin Erginoz: Case report concept, writing the paper. Ahmet Askar: Case report interpretation. Gokce Hande Cavus: Case report concept and interpretation. Mehmet Velidedeoglu: Case report concept, reviewing.
Declaration of competing interest
The authors have no conflicts of interest to declare.
References
- 1.Skube S.J., Arsoniadis E.G., Sulciner M.L. Colorectal lymphoma: a contemporary case series. Dis. Colon Rectum. 2019 Jun;62(6):694–702. doi: 10.1097/DCR.0000000000001373. [DOI] [PubMed] [Google Scholar]
- 2.Ardakani V.J., Rashidian N., Adman A.A. Rectal lymphoma: report of a rare case and review of literature. Acta Med. Iran. 2014 May 25;52(10):791–794. [PubMed] [Google Scholar]
- 3.Pascual M., Sánchez-González B., García M. Primary lymphoma of the colon. Rev. Esp. Enferm. Dig. 2013 Feb;105(2):74–78. doi: 10.4321/s1130-01082013000200003. [DOI] [PubMed] [Google Scholar]
- 4.Tauro L.F., Furtado H.W., Aithala P.S. Primary lymphoma of the colon. Saudi J. Gastroenterol. 2009 Oct-Dec;15(4):279–282. doi: 10.4103/1319-3767.56095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Times M. Colorectal lymphoma. Clin. Colon Rectal Surg. 2011;24(3):135–141. doi: 10.1055/s-0031-1285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.for the SCARE Group. Agha R.A., Franchi T., Sohrabi C., Mathew G. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Sapkota S., Shaikh H. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2021 Jan. Non-Hodgkin lymphoma. [Updated 2020 Dec 14]https://www.ncbi.nlm.nih.gov/books/NBK559328/ [PubMed] [Google Scholar]
- 8.Padala S.A., Kallam A. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2021 Jan. Diffuse large B cell lymphoma. [Updated 2020 May 14]https://www.ncbi.nlm.nih.gov/books/NBK557796/ [PubMed] [Google Scholar]
- 9.Li S., Young K.H., Medeiros L.J. Diffuse large B-cell lymphoma. Pathology. 2018 Jan;50(1):74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Olszewska-Szopa M., Wróbel T. Gastrointestinal non-hodgkin lymphomas. Adv. Clin. Exp. Med. 2019 Aug;28(8):1119–1124. doi: 10.17219/acem/94068. [DOI] [PubMed] [Google Scholar]
- 11.Chang S.C. Clinical features and management of primary colonic lymphoma. Formosan J. Surg. 2012;45:73–77. [Google Scholar]
- 12.Sharma B., Pavelock N., Antoine M. Primary diffuse large B-cell lymphoma of the descending colon. Am. J. Med. Sci. 2019 Aug;358(2):164–167. doi: 10.1016/j.amjms.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Jardin F., Ruminy P., Bastard C. The BCL6 proto-oncogene: a leading role during germinal center development and lymphomagenesis. Pathol. Biol. (Paris) 2007 Feb;55(1):73–83. doi: 10.1016/j.patbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Testoni M., Zucca E., Young K.H. Genetic lesions in diffuse large B-cell lymphomas. Ann. Oncol. 2015 Jun;26(6):1069–1080. doi: 10.1093/annonc/mdv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luminari S., Montanini A., Federico M. Anthracyclines: a cornerstone in the management of non-Hodgkin's lymphoma. Hematol. Rep. 2011;3(3s) doi: 10.4081/hr.2011.s3.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison V.A. Evolution of R-CHOP therapy for older patients with diffuse large B-cell lymphoma. Expert. Rev. Anticancer. Ther. 2008;8:1651–1658. doi: 10.1586/14737140.8.10.1651. [DOI] [PubMed] [Google Scholar]
- 17.Stanojevic G.Z., Nestorovic M.D., Brankovic B.R. Primary colorectal lymphoma: an overview. World J. Gastrointest. Oncol. 2011;3(1):14–18. doi: 10.4251/wjgo.v3.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghimire P., Wu G.Y., Zhu L. Primary gastrointestinal lymphoma. World J. Gastroenterol. 2011;17(6):697–707. doi: 10.3748/wjg.v17.i6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engels E.A. Infectious agents as causes of non-hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2007 Mar;16(3):401–404. doi: 10.1158/1055-9965.EPI-06-1056. [DOI] [PubMed] [Google Scholar]
- 20.Hans C.P., Weisenburger D.D., Greiner T.C. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004 Jan 1;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 21.Pandey M., Swain J., Iyer H.M. Primary lymphoma of the colon: report of two cases and review of literature. World J. Surg. Oncol. 2019 Jan 15;17(1):18. doi: 10.1186/s12957-018-1548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drolet S., Maclean A.R., Stewart D.A. Primary colorectal lymphoma-clinical outcomes in a population-based series. J. Gastrointest. Surg. 2011 Oct;15(10):1851–1857. doi: 10.1007/s11605-011-1572-0. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y.L., Lin J.K., Liang W.Y. Surgical resection combined with chemotherapy can help achieve better outcomes in patients with primary colonic lymphoma. J. Surg. Oncol. 2011 Sep 1;104(3):265–268. doi: 10.1002/jso.21927. [DOI] [PubMed] [Google Scholar]
- 24.Koniaris L.G., Drugas G., Katzman P.J. Management of gastrointestinal lymphoma. J. Am. Coll. Surg. 2003 Jul;197(1):127–141. doi: 10.1016/S1072-7515(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 25.Cai S., Cannizzo F., Jr., Dunn B.K.M. The role of surgical intervention in non-Hodgkin’s lymphoma of the colon and rectum. Am. J. Surg. 2007 Mar;193(3):409–412. doi: 10.1016/j.amjsurg.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y.B., Chen H.Y., He J.J. The role of surgical intervention in primary colorectal lymphoma: a SEER population-based analysis. Oncotarget. 2016;7(44):72263–72275. doi: 10.18632/oncotarget.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]