Abstract
Pelvic hydatid bone disease is a rare and debilitating condition. Patients often present with symptoms and signs when the disease process is advanced and curative resection is not possible. We present a case of destructive bone hydatid disease affecting the left iliac bone. A 45-year-old woman presented initially 5 years ago with a left pelvic mass to the gynaecology department. Computed tomography (CT) scan done at that time showed a large pelvic, left iliac fossa cystic mass with the destruction of the left iliac bone. Extension of the cystic mass transversed the iliac bone into the posterior soft tissue. Percutaneous biopsy taken showed hydatid cystic disease. The patient was planned for surgery and, however, was lost to follow-up. Four years later, she presented with a history of worsening left pelvic pain with an enlarging, left pelvic mass, and another mass in the posterior gluteal area. In addition, CT imaging showed extensive left iliac bone destruction with posterior soft tissue extension to the gluteus muscle. A multidisciplinary team concluded that complete excision would not result in cure. Thus, complete iliac wing bone reconstruction was not an option in this patient. Instead, palliative measures were deemed in the patient’s best interest to control disease progression and relieve painful pressure-related symptoms from the hydatid cystic mass. The patient received preoperative albendazole and underwent an extraperitoneal debulking of the soft tissue hydatid infiltration and debridement of bony fragments from left iliac bone destruction. Postoperatively, the patient did well, and her main complaint of pain related to the cystic mass pressure had improved significantly.
Keywords: echinococcus granulosus, bone hydatid disease, hydatid pelvic bone disease
Introduction
Human hydatid disease is caused by the tapeworm Echinococcus granulosus, causing cystic hydatid disease.1–5 Humans act as intermediate hosts and are infected by faecal oral spread, particularly when in contact with dogs infected with echinococcosis, who then shed the tapeworm eggs in their faeces.3,5–7 Hydatid disease commonly affects the liver (75%), followed by the lungs (15%).7–8 Bony hydatid disease is rare, accounting for only 0.5–4% of cases.1,4,5 Patients with bony hydatid disease often present with symptoms only once the disease process is advanced.1,8 Management options in bony hydatid disease are limited to the use of anthelmintic therapy with surgical intervention.5 Cure, however, is rarely possible as extensive bony destruction is usually present at the time of diagnosis.3,5 Here, we present a case of hydatid bone disease causing left iliac bone destruction with posterior soft tissue extension.
Case report
A 45-year-old woman with no underlying comorbidities presented to our unit with progressively worsening left-sided pelvic pain, especially when walking.
At the first presentation to our unit 5 years ago, she had been initially referred to us by our gynaecology colleagues after a workup for a long-standing history of pelvic pain revealed a 7.5 × 6.7 × 8.8 cm [transverse (TV) × anteroposterior (AP) × craniocaudal (CC)] multiloculated cystic lesion in the left iliac fossa on computed tomography (CT) of the abdomen and pelvis (Figure 1). The lesion extended posteriorly, involving the gluteus medius muscle and notable destruction of the left iliac wing bone. Percutaneous biopsy of the cystic mass revealed histopathology consistent with hydatid disease. Therefore, a tentative diagnosis of primary pelvic bone hydatid disease was made. Preoperative albendazole was given, and it was decided to proceed with surgical exploration to excise the cystic lesion. Unfortunately, due to poor social circumstances, the patient defaulted on follow-up.
Figure 1.

Axial view of contrast-enhanced CT of the pelvis showing a large left, multicystic hydatid lesion with iliac bone destruction and extension to posterior soft tissue.
CT, computed tomography.
She now re-presented to our department 4 years later with worsening left-sided pelvic pain impeding her ability to weight-bear. She had also noted that her initial left pelvic mass had increased in size compared with 4 years ago and that there was another enlarging mass noted over the left posterior gluteal area. Physical examination revealed a 10 × 14 cm fixed, nontender, firm mass in the left iliac fossa. In addition, another 5 × 5 cm was found in the left superior gluteal region. Laboratory workup, including CA125, was unremarkable. Repeat CT showed a large thick-walled multicystic lesion in the left lower pelvis extending posteriorly into the gluteus muscle (Figure 2). Imaging also revealed significant lytic change within the left iliac bone resulting in extensive bony destruction of the left iliac wing and involvement of the sacroiliac joint (Figure 3).
Figure 2.

Coronal view of contrast-enhanced CT of the abdomen and pelvis showing extent of (a) left pelvic, iliac bone destruction and (b) large pelvic, multicystic hydatid lesion causing compression on adjacent organs.
CT, computed tomography.
Figure 3.
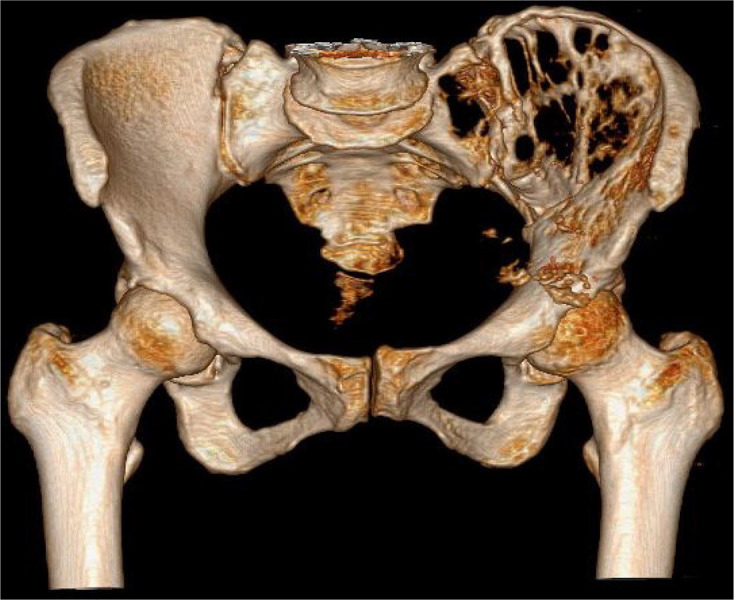
Preoperative CT 3D construction of left iliac bone destruction with sacroiliac joint involvement.
CT, computed tomography; 3D, 3-dimensional.
A decision was made at the multidisciplinary team meeting, including an orthopaedic surgeon and social worker, to surgically explore and debulk the lesion to relieve painful pressure effects of the hydatid, cystic mass and prevent further disease progression. An orthopaedic consultation advised that major pelvic bone reconstruction would not be possible. Due to the extensive left iliac wing destruction and involvement of the sacroiliac joint, complete excision would not be possible without causing significant disability to the patient postoperatively.
After a 14-day course of preoperative albendazole therapy, she was taken to theatre. A left anterior iliac crest incision was made. Operative findings revealed a large extraperitoneal multicystic lesion containing thick hydatid sand and multiple daughter cysts. Sterile swabs soaked in scolicidal agent (0.1% sodium hypochlorite solution) were placed around the cystic operative field (Figure 4). Extraperitoneal excision of the multicystic lesion was performed. The extraperitoneal cavity and bony fragments from the pelvic wing that were visualised intraoperatively were also debrided (Figure 5). The cystic cavity was then irrigated with a scolicidal agent, followed by saline. The cavity was closed with closed suction drains left in place. Histopathological analysis confirmed the diagnosis of the left pelvic bone and soft tissue hydatid disease.
Figure 4.

Intraoperative photograph showing one of many daughter cysts within the extraperitoneal cavity and scolicidal soaked swabs around the operative site.
Figure 5.

Intraoperative photograph showing the bony fragments within the cavity resulting from extensive left, iliac bone destruction.
The postoperative period was uneventful. A small discharging sinus at the incision site settled with gentle saline irrigation. On follow-up, a month later, her pain symptoms had largely resolved, and she was walking with much less difficulty and able to weight-bear. She continued albendazole therapy for 6 months postsurgery and remained asymptomatic throughout.
Discussion
Hydatid disease, caused by the larvae of the parasitic tapeworm E granulosus, may occur in various locations in the body.1,2,4
The life cycle of E granulosus has canines as the most common definitive host. Eggs released in their stool are then ingested by sheep and cattle, that is, the intermediate host. Humans are accidental hosts due to the ingestion of parasitic eggs from contaminated food or water.1,4,5 Once in the human circulation, the scolex has to pass the filters of the lungs and liver before being carried to the bone. This explains why the liver (75%) and lungs (15%)7, 8 account for the majority of cases, and bone involvement (0.5–4%) is much rarer.1,5
Where bone is involved, the vertebrae are most commonly affected (40–50%).2,5,8 When vertebral hydatid bone involvement is present, patients may complain of chronic back pain on history. Patients may also present with signs of spinal cord compression syndromes.2 Involvement of the bony pelvis accounts for only 15–20% of all bony hydatid cases, posing a diagnostic challenge to the treating physician.5,9,10 Bone hydatid disease symptoms may include localised pain and discomfort due to bony destruction.4 Other presenting complaints may include pressure symptoms, swelling, sinus formation and pathological fractures.1,3 In pelvic bone involvement, patients may complain of a gluteal swelling, as in our case. This occurs due to cold abscess migration, where the abscess may cross from inside to outside the pelvis through the sciatic notch. This may lead to compressive symptoms of the sciatic and femoral nerves.10,11 Signs and symptoms of bone hydatid disease are commonly vague and nonspecific, leading to diagnostic uncertainty on patient presentation.1,8 If symptoms are present, they usually manifest very late in the disease process, often once bone destruction has already taken place, as in our patient.1,8
Hydatid infiltration of the bone occurs primarily through haematogenous dissemination.3,10–12 The hydatid cystic structure in liver and lungs differs from the cystic structure found in bone disease. Hydatid cysts affecting the liver, lungs or both consist of an inner germinal layer that is then surrounded by an acellular, outer laminated layer.7,13,14 The inner germinal layer and surrounding outer, laminated layer are the true wall structures of the cyst and are typically referred to as the endocyst.15 The germinal layer is responsible for the production of small vesicles called brood capsules that divide via asexual division and produce many protoscolices.8,13 A third layer, the pericyst, is formed by the host’s immune response to the hydatid cyst infection by producing a granulomatous, fibrous, adventitial layer.7,13–15 In bone involvement, the characteristic pericyst formation does not occur, allowing the proliferation of parasitic microvesicles through the bone canals.1,3,6 This results in multiple parasitic microvesicles replacing the normal bone tissue. With further growth and expansion, the parasite may breach the bone cortex with subsequent spread of the disease to surrounding tissues in the pelvis, as was in our case.1,6 Eventual bony destruction occurs through three pathological mechanisms, namely,1 compression of the expansive cyst on surrounding tissue leads to pressure-induced bone atrophy,2 obstruction of bone nutrient vessels leads to ischaemia and3 osteoclast proliferation occurring around the site of bony compression.9–12
Hydatid disease should be included in the differential diagnosis for any cystic lesion, especially in endemic areas. Although blood tests may show eosinophilia and serum echinococcosis serology may be suggestive, further imaging is required to make the diagnosis. Blood serum antibody testing with the use of IHA (indirect haemagglutination), CIE (counter immunoelectrophoresis), ELISA (enzyme-linked immunosorbent assay) and gold labelled antibody may be used in the diagnosis of human echinococcosis.6 Pamir et al. 2002 noted that in abdominal hydatid disease, the sensitivity for IHA, complement fixation and ELISA tests is approximately 80–100% with a specificity of 88–96%. In extrahepatic disease, however, the sensitivity of these tests reduces to about 25–56%, limiting the use of serology to aid in the diagnosis of bone hydatid disease.2,8 Our patient had a weak positive titre of 64 on echinococcus IHA serology testing.
While ultrasound is the diagnostic tool of choice for visceral cystic echinococcosis, x-rays, CT and magnetic resonance imaging (MRI) are commonly used to diagnose hydatid bone disease.3,6 X-rays may show nonspecific features which may look similar to tuberculosis of the spine or primary bone tumours, including the appearance of cortical thinning with an expansile lucent lesion.4–6,10 In the pelvis, iliac bone involvement can have the characteristic ‘waffle-appearance’, which pertains to a sizable area of osteolysis.1,9,10 The CT imaging in bone hydatid disease may reveal a cystic lesion representing an ovoid appearance with ‘double-layered arcuate calcifications’.1,6 The CT imaging may also assist with detecting cortical thinning and the presence of an underlying pathological fracture.10 As with our case, extra- and endopelvic cystic collections can be seen with the use of CT.12 The MRI can provide further imaging of soft tissue extension and bony lesions, specifically spinal involvement.3,8,10,12 The MRI of the pelvis may show an expansive cystic lesion(s) causing destruction of the sacrum.6 A differential diagnosis of cystic lesions other than sacral hydatid disease include a schwannoma, meningocele, giant cell tumour or chordoma.16 Hydatid cysts can be distinguished on MRI T2W image sequence where both parent and daughter cysts show high-intensity signal.6,16 Furthermore, MRI may be useful for monitoring patients postoperatively and those suspected of having a local recurrence of bony hydatid disease.10,12
Treatment of bony hydatid disease requires both medical and surgical management.1,3,8,12,17,18 While the role of albendazole is well documented in visceral hydatid disease, its use in hydatid bone disease is less well defined.4,17 Medical management with albendazole aims to reduce cyst size before surgery and sterilise cysts before surgery in case of accidental spillage of cyst contents.1,3,12 According to the World Health Organization (WHO) guidelines, monotherapy with albendazole is the recommended anthelmintic drug of choice for visceral disease.17,19 Regarding bone hydatid disease, the literature is limited regarding the choice and duration of anthelmintic drug therapy.17 Steinmetz et al.17 reported that albendazole is the first drug of choice for hydatid bone disease. There is, however, no consensus on the duration of therapy and the role of other anthelmintic drugs used in combination with albendazole.5 Praziquantel (40 mg/kg/once a week) in combination with albendazole has been described in a limited number of descriptive studies in the use of therapy for intra-abdominal hydatid disease.20 Bonifacino et al. looked at patients with secondary disseminated hydatid disease or with severe bone involvement and concluded that the role of praziquantel use in these severe cases required further appraise.20,21 Although it has been suggested that praziquantel used in combination with albendazole may have synergistic effects resulting in increased levels of albendazole,19,22 there is limited evidence on whether this dual therapy is superior compared with albendazole alone.20 Gdoura et al.3 described a protocol with albendazole administered at a dose of 400 mg, orally, twice daily 2 weeks before surgery and approximately 6 months postsurgery. A similar protocol was used in our patient.
Surgical management of pelvic bone hydatid disease is notoriously challenging. The aim is to eradicate all the hydatid cysts with complete excision; this is, however, rarely possible.5,6,12 There is often extensive bony destruction, be it of the pelvis, vertebrae and/ or long bones, at the time of index presentation.3,5 Pelvic iliac wing destruction can be approached with a hemipelvectomy with a possible reconstruction of the hemipelvis after that.1,12 The use of scolicidal agent wash intraoperatively, postcyst excision, has been described to prevent a recurrence.9–11 Complete excision and eradication of the disease process, however, are rarely possible, especially when the sacroiliac and hip joints are involved.1,5,12 Complete excision, in this case, would lead to significant patient disability.1,5 Even when complete excision is attempted, frequent recurrence of the disease has been reported, and surgery to palliate symptoms is more frequently performed.1,6
Our patient had extensive bony destruction of the left pelvic wing with the involvement of the left sacroiliac joint. This precluded complete resection in our patient, and we opted to debulk the pelvic mass that had been causing pressure-related pain symptoms for the patient.
Conclusion
Pelvic hydatid bone disease presents a diagnostic and therapeutic challenge. Vague pelvic symptoms and obscure radiological appearance of bony involvement may delay presentation and diagnosis. Physicians must have a high index of suspicion, especially in endemic populations. Management is complex and involves both medical and surgical treatment. Due to the high incidence of recurrence and the debilitative nature of surgery for hydatid disease of the pelvic bone, measures to debulk the enlarged cysts are often the treatment of choice.
Acknowledgments
The authors thank Drs KK Kotze and HM Van der Westhuizen who assisted with the initiation of writing the case report as well as the idea for the unique title name of the report – Lytic Parasitic.
Footnotes
Author contributions: AG reviewed the literature and drafted the manuscript with input and images from JJ and PRB. JJ and PRB reviewed and edited the manuscript. All authors issued final approval for the version to be submitted for publication.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for the anonymised information and the accompanying images to be published in this article.
ORCID iDs: Avaan Govindasamy  https://orcid.org/0000-0003-1189-0021
https://orcid.org/0000-0003-1189-0021
Jeff John  https://orcid.org/0000-0002-6139-810X
https://orcid.org/0000-0002-6139-810X
Contributor Information
Avaan Govindasamy, Division of General Surgery, Department of Surgery, Frere Hospital and Walter Sisulu University, East London 5200, South Africa.
Pushpa Raj Bhattarai, Division of General Surgery, Department of Surgery, Frere Hospital and Walter Sisulu University, East London, South Africa.
Jeff John, Division of Urology, Department of Surgery, Frere Hospital and Walter Sisulu University, East London, South Africa.
References
- 1.Inayat F, Rana RE, Azam S, et al. Pelvic bone hydatidosis: a dangerous crippling disease. Cureus 2019; 11: e4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pamir MN, Özduman K, Elmaci I. Spinal hydatid disease. Spinal Cord 2002; 40: 153–160. [DOI] [PubMed] [Google Scholar]
- 3.Gdoura F, Trigui M, Zribi W, et al. Pelvic bone hydatidosis. Orthop Traumatol Surg Res 2010; 96: 85–89. [DOI] [PubMed] [Google Scholar]
- 4.Chrispinus Siteti M. Update on human bone hydatid disease. Sci J Clin Med 2015; 4: 10–17. [Google Scholar]
- 5.Monge-Maillo B, Samperio MO, Pérez-Molina JA, et al. Osseous cystic echinococcosis: a case series study at a referral unit in Spain. PLoS Negl Trop Dis 2019; 13: e0007006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song XH, Ding LW, Wen H. Bone hydatid disease. Postgrad Med J 2007; 83: 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pakala T, Molina M, Wu GY. Hepatic echinococcal cysts: a review. J Clin Transl Hepatol 2016; 4: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laubscher M, Hardcastle P, Naudé P, et al. Hydatid disease of bone. SA Orthop J 2008; 7: 44–48. [Google Scholar]
- 9.Bhatnagar N, Kishan H, Sura S, et al. Pelvic hydatid disease: a case report and review of literature. J Orthop Case Rep 2017; 7: 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papanikolaou A. Osseous hydatid disease. Trans R Soc Trop Med Hyg 2008; 102: 233–238. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Gupta A, Choudhary R, et al. Pelvic echinococcosis presenting as a mass in the gluteal region: a case report. Infez Med 2019; 27: 456–460. [PubMed] [Google Scholar]
- 12.Najib A, Rhanim A, Lamrani MO, et al. Hydatidosis of the pelvic bone: case report and review of the literature. Eur J Orthop Surg Traumatol 2012; 22(Suppl. 1): 239–244. [DOI] [PubMed] [Google Scholar]
- 13. Bhutani N, Kajal P. Hepatic echinococcosis: a review. Ann Med Surg. doi: 10.1016/j.amsu.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golzari SEJ, Sokouti M. Pericyst: the outermost layer of hydatid cyst. World J Gastroenterol 2014; 20: 1377–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedrosa I, Saíz A, Arrazola J, et al. Hydatid disease: radiologic and pathologic features and complications. Radiographics 2000; 20: 795–817. [DOI] [PubMed] [Google Scholar]
- 16.Segura-Trepichio M, Montoza-Nuñez JM, Candela-Zaplana D, et al. Primary sacral hydatid cyst mimicking a neurogenic tumor in chronic low back pain: case report and review of the literature. J Neurosci Rural Pract 2016; 7: S112–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinmetz S, Racloz G, Stern R, et al. Treatment challenges associated with bone echinococcosis. J Antimicrob Chemother 2014; 69: 821–826. [DOI] [PubMed] [Google Scholar]
- 18.Martínez Herrera A, Cuenca J, Herrero L. Hydatidosis of the pelvis and hip. Int Orthop 2001; 25: 302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114: 1–16. [DOI] [PubMed] [Google Scholar]
- 20.Bygott JM, Chiodini PL. Praziquantel: neglected drug? Ineffective treatment? Or therapeutic choice in cystic hydatid disease? Acta Trop 2009; 111: 95–101. [DOI] [PubMed] [Google Scholar]
- 21.Bonifacino R, Carter SD, Craig PS, et al. Assessment of the immunological surveillance value of humoral and lymphocyte assays in severe human cystic echinococcosis. Trans R Soc Trop Med Hyg 2000; 94: 97–102. [DOI] [PubMed] [Google Scholar]
- 22.Cobo F, Yarnoz C, Sesma B, et al. Albendazole plus praziquantel versus albendazole alone as a pre-operative treatment in intra-abdominal hydatidosis caused by Echinococcus granulosus. Trop Med Int Health 1998; 3: 462–466. [DOI] [PubMed] [Google Scholar]


