Abstract
Hematopoietic stem and progenitor cells (HSPCs) are frequently located around the bone marrow (BM) vasculature. These so-called perivascular niches regulate HSC function both in health and disease, but they have been poorly studied in humans due to the scarcity of models integrating complete human vascular structures. Herein, we propose the stromal vascular fraction (SVF) derived from human adipose tissue as a cell source to vascularize 3D osteoblastic BM niches engineered in perfusion bioreactors. We show that SVF cells form self-assembled capillary structures, composed by endothelial and perivascular cells, that add to the osteogenic matrix secreted by BM mesenchymal stromal cells in these engineered niches. In comparison to avascular osteoblastic niches, vascularized BM niches better maintain immunophenotypically-defined cord blood (CB) HSCs without affecting cell proliferation. In contrast, HSPCs cultured in vascularized BM niches showed increased CFU-granulocyte-erythrocyte-monocyte-megakaryocyte (CFU-GEMM) numbers. The vascularization also contributed to better preserve osteogenic gene expression in the niche, demonstrating that niche vascularization has an influence on both hematopoietic and stromal compartments. In summary, we have engineered a fully humanized and vascularized 3D BM tissue to model native human endosteal perivascular niches and revealed functional implications of this vascularization in sustaining undifferentiated CB HSPCs. This system provides a unique modular platform to explore hemato-vascular interactions in human healthy/pathological hematopoiesis.
Keywords: Hematopoietic stem cell, engineered 3D niches, human hematopoiesis, bone marrow microenvironment, perivascular niche
Introduction
Hematopoietic stem and progenitor cells (HSPCs) reside in 3D specialized compartments in the bone marrow (BM) and are responsible for sustaining the lifelong blood cell production. These so-called BM microenvironments or niches are composed of multiple cellular and molecular signals that critically modulate HSPC function and thus, both steady-state and stress hematopoiesis.1–3 In this context, the BM is a highly vascularized tissue and cumulative evidences have revealed that HSPCs are frequently found in perivascular locations, where their functions are regulated by both endothelial and perivascular cells.4–6
However, human BM stem cell niches are poorly understood due to the obvious difficulties to access and manipulate human BM in normal conditions.7 Therefore, existing knowledge is based on either “humanized” mouse models or 3D biomimetics in vitro models.8 Multiple bioengineering approaches have been developed to generate humanized environments in vivo, some of them including human vascular structures9–12 and exploited to study human healthy HSPCs13–15 (broadly reviewed by Bessy et al.16). Nevertheless, there are concerns regarding the contribution of the murine vasculature to these humanized niches upon implantation, and the impact of conditioning regimens (sometimes used to facilitate the engraftment of human cells) on the human vascular structures.17 In contrast, 3D in vitro culture systems with engineered perivascular niches offer a model to study human hematopoiesis in long-term controlled settings, but they often rely on the use of Human Umbilical Vein Endothelial cells (HUVECs) to construct the capillary networks, requiring thus an additional source of perivascular cells (normally BM-MSCs) and complex medium compositions with angiogenic factors.18–22
We previously developed a 3D angiogenic niche that bypasses this constraint by culturing the stromal vascular fraction (SVF) cells from human adipose tissue on collagen scaffolds and inside perfusion bioreactors.23 The SVF is a heterogenous mix of pericytes, mesenchymal stromal cells (MSCs), mature endothelial cells and endothelial progenitor cells24,25 that reproducibly promotes the formation of vascular structures (endothelium + perivascular cells) and the release of angiogenic factors in vitro.26 Indeed, SVF cells can undergo osteoblastic differentiation when exposed to typical osteogenic culture conditions in vitro27 and can be used to generate osteogenic-vasculogenic grafts for bone regeneration upon in vivo implantation.10,28 However, MSCs are well-known to be tissue specific cells and exhibit differences in their immunophenotype, differentiation potential, transcriptome, epigenome, proteome, and immunomodulatory activity.29–31 Therefore, BM-MSCs should be the preferred source to engineer biomimetic niches recapitulating features from native BM. In this regard, we reported a 3D engineered human BM analog capable to sustain and expand cord blood (CB) HSPCs. This approach includes ceramic-based, bone-mimicking scaffolds functionalized with osteogenic matrix secreted by BM-MSC cultured in osteoblastic differentiation conditions within perfusion bioreactors.32,33 Unfortunately, the vascular network was completely absent in this system.
In the current study we first hypothesized that SVF cells can be added as vasculogenic cell source to increase the complexity of the afore mentioned 3D engineered human BM analog and mimic the native perivascular niches in perfusion bioreactors. Then, we investigated the potential of these 3D vascularized BM niches (engineered with BM-MSC + SVF cells) to maintain and differentiate CB HSPCs in comparison to avascular osteoblastic niches (engineered only with BM-MSCs).
Methods
Human primary cells
CB cells, human BM aspirates and human adipose tissue were collected from healthy donors at the University Hospital Basel. Informed consent was obtained preoperatively and the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz, ref. 78/07) approved the protocol. All experiments were compliant with EU recommendations.
Engineering of BM niches in perfusion bioreactors
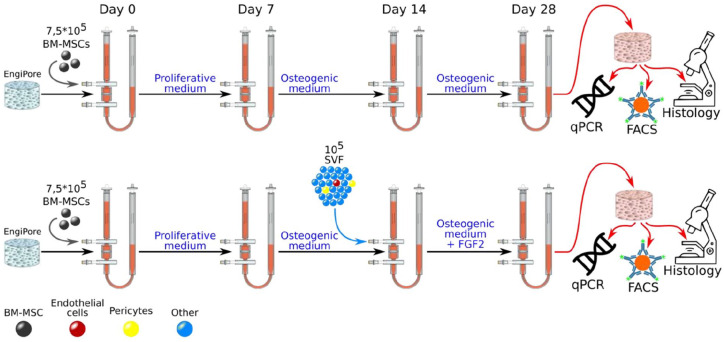
Osteoblastic avascular BM niches were generated using BM-MSCs (isolated as described in Online Supplemental Methods) as previously described.32 Briefly, hydroxyapatite scaffolds (EngiPore®, Finceramica-Faenza, Faenza, Italy) of 4 mm height and 8 mm diameter were seeded directly in perfusion bioreactor with 7.5 × 105 BM-MSCs for 24 h at a superficial velocity of 2800 μL/min. After the cell seeding phase, the superficial velocity was reduced to 280 μL/min for the perfusion culture of BM-MSCs. Cells were cultured for 1 week in proliferative medium (PM), consisting of CM supplemented with 100 nM dexamethasone (Sigma; cat# D4902), 0.1 mM ascorbic acid-2-phosphate (Sigma; cat# A92902) and 5 ng/mL FGF-2 (Miltenyi Biotec; cat# 130-093-838), followed by 3 weeks of Osteogenic Medium (OM) consisting of CM supplemented with 10 nM dexamethasone, 10 mM β-glycerophosphate, and 0.1 mM ascorbic acid-2-phosphate. Vascularized BM niches were generated by seeding 105 SVF cells (isolated as described in Online Supplemental Methods) in osteoblastic BM niches for 24 h at a superficial velocity of 2800 μL/min after 1 week of osteogenic differentiation (day 14). After the cell seeding phase, the superficial velocity was reduced to 280 μL/min for perfusion coculture for two more weeks in OM supplemented with 5 ng/mL FGF-2 (See Figure 1). Culture medium was changed every third day. At the end of the culture (day 28), mature engineered niches were processed for gene expression (Q-PCR), FACS and histological analyses (further described in Online Supplemental Methods). Alternatively, engineered niches were seeded with CB CD34+ HSPCs for a 1-week coculture followed by endpoint analyses.
Figure 1.
Experimental design illustrating the generation of engineered niches within perfusion bioreactors. Avascular osteoblastic BM niches (upper row) were engineered by seeding bone marrow mesenchymal stromal cells (BM-MSCs) in hydroxyapatite-based EngiPore scaffolds. Cells were first cultured for 7 days in proliferative medium and then for another 21 days in osteogenic medium (total 28 days). To engineer vascularized BM niches (bottom row), stromal vascular fraction (SVF) cells were seeded in niches engineered with BM-MSCs after 1 week of osteogenic differentiation (day 14) and cultured for 2 weeks in osteogenic medium plus FGF2 (total 28 days). See materials and methods for detailed mediums composition. At final analysis, engineered niches were harvested and processed for Q-PCR, flow activated cell sorting (FACS) and histology.
Culturing CB HSPCs in engineered BM niches
Human CB CD34+ HSPC were extracted and isolated as indicated in Online Supplemental Methods. 7 × 104 CD34+ cells were resuspended in Serum Free Expansion Medium (SFEM, Stemcell Technologies), supplemented with the following cytokines: SCF (10 ng/mL; cat# 130-093-991), FLT3-ligand (10 ng/mL; cat# 130-093-854); TPO (10 ng/mL; cat# 130-094-011) (all from Miltenyi Biotec.), and seeded in mature engineered niches for 24 h at a superficial velocity of 3000 μL/min. After the cell seeding phase, the superficial velocity was reduced to 300 μL/min for perfusion of the 1-week coculture. Culture medium was changed twice a week, during which the retrieved medium was harvested and spun down (300 g, 5 min) to collect cells in suspension. The pelleted cells were resuspended in fresh medium and injected back in the bioreactor.
Endpoint analyses of engineered BM niches with CB HSPCs
Floating and loosely attached cells were firstly harvested from the medium contained in the bioreactors tubes (supernatant; SN). For histological analyses, engineered niches were extracted from the bioreactor chamber and fixed in PFA 2% (Thermoscientific; cat# 28908) for 24 h. In order to obtain a single cell suspension for Q-PCR, FACS or CFU-C assays, cells were harvested from engineered niches after enzymatic digestion (0.3% collagenase II, Thermofischer; cat# 17101015) and trypsinization (0.05% Trypsin-EDTA, Gibco; cat# 25300-054) treatments (further detailed in Online Supplemental Methods).
Results
SVF cells generate self-assembled vascular structures in engineered BM niches
Our previously validated BM analog was engineered by seeding BM-MSCs in hydroxyapatite 3D scaffolds (EngiPore) within perfusion bioreactors and expanding them for 7 days before inducing the osteogenic differentiation for another 21 days.32 This approach allowed us to generate mature niches recapitulating features from osteoblastic microenvironments in a total of 28 days culture period (Figure 1, upper row). In order to assess the capacity of adipose tissue-derived SVF cells to vascularize these 3D osteoblastic microenvironments and mimic native endosteal perivascular niches, we introduced SVF cells in the bioreactor system after 1 week of osteogenic differentiation, when cells are already embedded in an osteogenic matrix (day 14) (Figure 1, bottom row). We hypothesized that 105 SVF cells would contain enough vascular cells to vascularized BM niches and preserve the ratio endothelial/stromal cells found in the native tissue. At the end of the culture period, niches engineered with only BM-MSCs or BM-MSCs together with SVF cells were harvested from the perfusion bioreactors for characterization by whole-mount immunofluorescent stainings, flow activated cell sorting (FACS) and gene expression analyses (Figure 1).
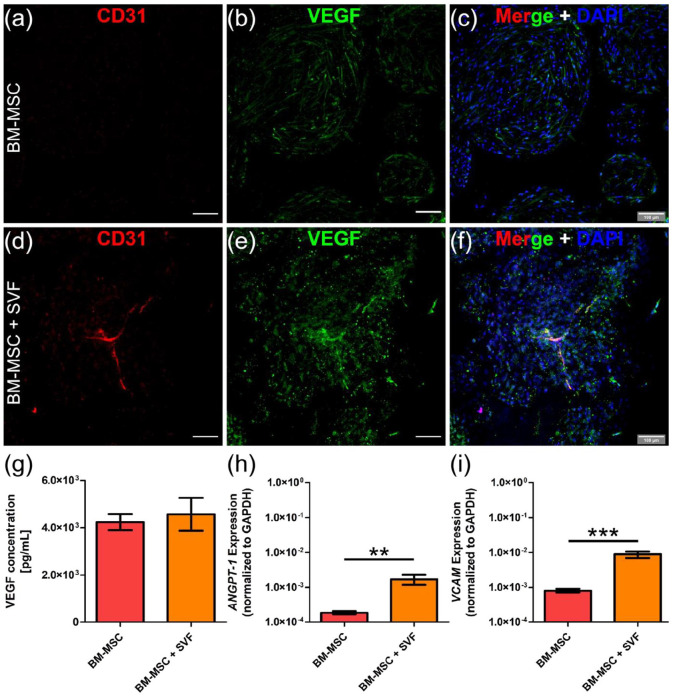
Immunofluorescent stainings for CD31 and Vascular Endothelial Growth Factor (VEGF) revealed the presence of capillary-like vascular structures in those niches containing SVF cells. Interestingly, while CD31 labeled endothelial cells, VEGF was expressed by all stromal cells (Figure 2(a)–(f)), suggesting than BM-MSCs could also provide angiogenic signals and, indirectly, contribute to the vascularization process. Indeed, we did not detect differences in VEGF protein content between both engineered niches (Figure 2(g)). In order to evaluate changes at gene expression level, we extracted RNA from these engineered niches and performed Q-PCR. As expected, vascular-related genes such as Angiopoietin 1 (ANGPT-1) and Vascular Cell Adhesion Molecule 1 (VCAM1) were upregulated in those niches with SVF cells (Figure 2(h) and (i)).
Figure 2.
Vascular structures are found only in engineered niches including SVF cells. (a–f) Whole-mount immunostaining for CD31 (red) and VEGF (green) in niches engineered with only BM-MSCs (a–c) or BM-MSCs + SVF cells (d–f). (c, f) Nuclei are labeled with DAPI (blue). Scale bar, 100 µm. (g) VEGF protein content in engineered niches. (h, i) Q-PCR data showing ANGPT-1 and VCAM1 gene expression in engineered niches. Data are plotted as means ± standard deviations; n = 4–12; **p < 0.01, ***p < 0.001. Unpaired two-tailed t tests.
SVF cells-induced vascularization does not compromise the osteoblastic nature of engineered BM niches
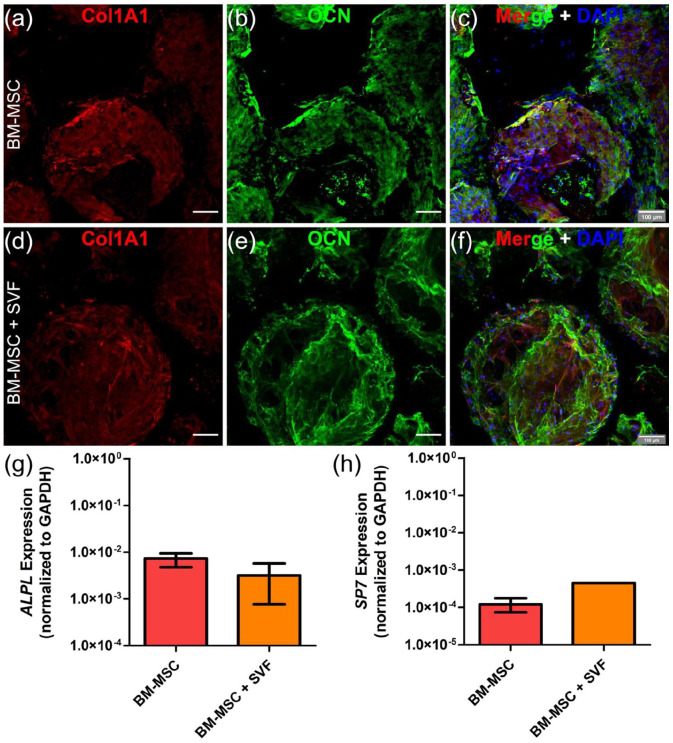
To evaluate the potential impact that vascularization might have on the extracellular matrix secreted by osteogenically-differentiated BM-MSCs, we performed immunostainings for Osteocalcin (OCN) and Collagen type 1 (COL1A1). We did not observe differences between niches engineered with only BM-MSCs and BM-MSCs + SVF cells (Figure 3(a)–(f)). At transcriptional level, similar expression of early osteoblastic genes such as Alkaline Phosphatase (ALPL) and Osterix (SP7) was measured (Figure 3(g) and (h)). Together, these findings suggest that SVF cells-induced vascularization does not alter the osteogenic nature of the engineered niche.
Figure 3.
SVF-induced vascularization does not compromise niche osteogenic matrix. (a–f) Whole-mount immunostaining for COL1A1 (red) and OCN (green) in niches engineered with only BM-MSCs (a–c) or BM-MSCs + SVF cells (d–f). (c, f) Nuclei are labeled with DAPI (blue). Scale bar, 100 µm. (g, h) Q-PCR data showing ALPL and SP7 gene expression in engineered niches. Data are plotted as means ± standard deviations; n = 4–12. Unpaired 2-tailed t tests.
Engineered BM niches vascularized with SVF cells do not contain hematopoietic stem and progenitor cells (HSPCs)
The SVF fraction is a heterogenous mix of cell populations that contains an important fraction of hematopoietic cells (Supplemental Figure S1) (CD45+).10 Since we planned to assess the functionality of vascularized BM niches by co-culturing cord blood (CB) HSPCs, we first investigated if any SVF-derived hematopoietic cells remain after the culture period required to generate these niches.
Taking advantage of flow activated cell sorting (FACS) analysis, we only detected residual and unspecific CD45 expression in mature niches engineered only with BM-MSCs or in combination with SVF cells (Supplemental Figure S2(a) and (b)). Moreover, we were unable to detect CD34+CD38− HSPCs in either case (Supplemental Figure S2(c)).
Vascularized BM niches favor the maintenance of undifferentiated CB HSPCs
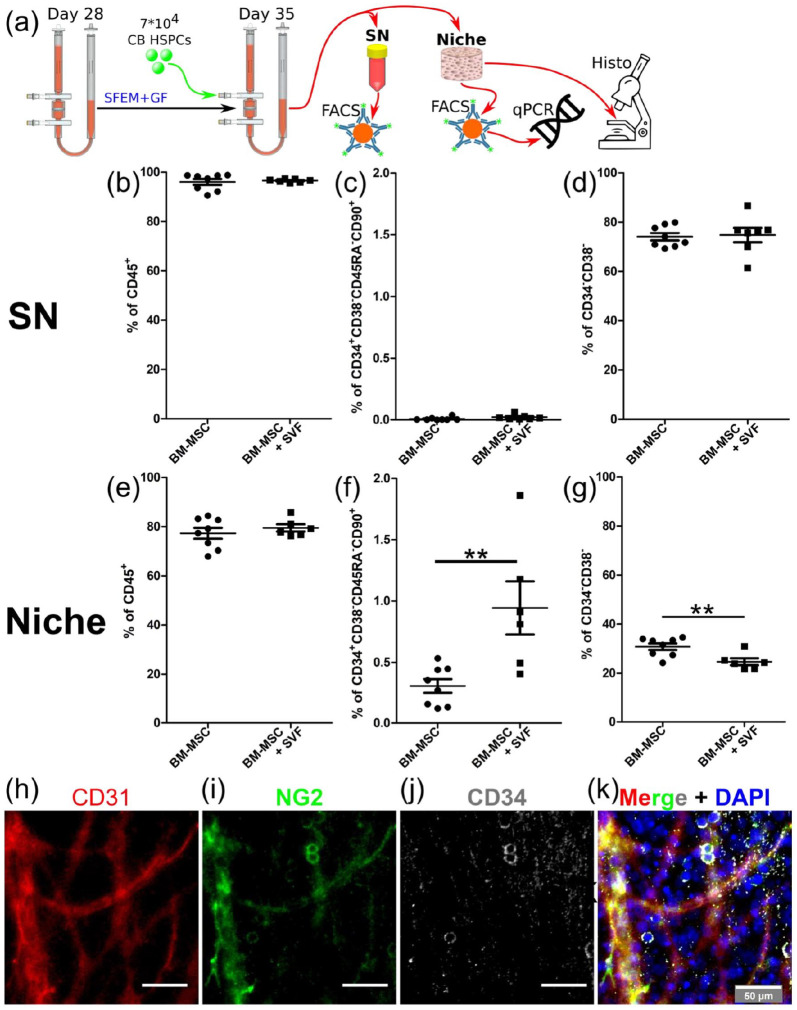
Once demonstrated we can engineer 3D vascularized BM niches combining BM-MSCs with adipose tissue-derived SVF cells, we assessed the capacity of these niches to sustain the culture of CB HSPCs. For this purpose, we seeded 7 × 104 CB-derived CD34+ cells pooled from 10 different donors in engineered niches and cultured them for 1 week in serum-free medium (SFM) supplemented with low concentration of hematopoietic cytokines (10 ng/mL thrombopoietin (TPO), stem cell factor (SCF), and Fms-related tyrosine kinase 3 ligand (Flt3-L) (Figure 4(a)). At the endpoint analysis, the liquid phase of the bioreactor (supernatant compartment) was collected to analyze cell populations released from the niche (stromal/ECM compartment) by FACS. The engineered niches were harvested from the bioreactor chamber and either fixed for histological analysis or digested and processed for FACS to quantify the different stromal/hematopoietic cell populations (Figure 4(a) and Supplemental Figure S4).
Figure 4.
Vascularized BM niches preserve undifferentiated CB HSCs better than avascular BM niches. (a) Experimental setup depicting the 1-week culture of CB HSPCs in engineered niches and the different read-outs performed in the supernatant (SN) and niche compartments. (b–g) Percentage of (b, e) CD45+ cells. (c–f) CD34+CD38−CD45RA−CD90+ HSCs and (d, g) CD34−CD38− mature cells retrieved from the SN and niche compartments at final analysis. (h–k) Whole-mount immunostaining for CD31 (red), NG2 (green) and CD34 (gray) in BM niches vascularized with SVF cells. (k) Nuclei are labeled with DAPI (blue). Scale bar, 50 µm, and (b–g) data are plotted as means ± standard deviations; n = 6–8. **p < 0.01. Unpaired two-tailed t tests.
Similarly to avascular niches engineered only with BM-MSCs,32 3D vascularized BM niches exhibited a functional compartmentalization in which only hematopoietic CD45+ cells were released to the supernatant phase, while stromal cells remained in the niche compartment (80% hematopoietic cells and 20% stromal cells in this fraction) (Figure 4(b) and (e)). Interestingly, the immunophenotypic HSC fraction (CD34+CD38-CD45RA-CD90+) was approximately 50-fold more abundant in the niche compartment (Figure 4(c) and (f)), whereas mature hematopoietic cells (CD34−CD38−) proportion was about double in the supernatant compartment (Figure 4(d) and (g)). This compartmentalization recapitulates the cellular spatial distribution found in native BM stem cell niches, where HSPCs remain mostly anchored to their niches and mature cells traffic in/out the BM. Indeed, our immunofluorescent stainings showed the distribution of some CD34+ cells around vascular structures composed by CD31+ endothelial cells and NG2+ perivascular cells in 3D vascularized BM niches (Figure 4(h)–(k) and Supplemental Figure S5).
Interestingly, vascularized BM niches (BM-MSC + SVF condition) better preserved immunophenotypically-defined HSCs (CD34+CD38−CD45RA−CD90+) in comparison to avascular niches (BM-MSC condition) (Figure 4(f)). Simultaneously, the percentages of multipotent progenitors (MPPs; CD34+CD38−CD45RA−CD90−), multilymphoid progenitors (MLP; CD34+CD38−CD45RA+CD90−), committed progenitors (CD34+/−CD38+) and mature cells (CD34−CD38−) decreased in the niche compartment (Figure 4(g) and Supplemental Figures S3 and S4), suggesting that the vascular component negatively regulates CB HSPC differentiation in our engineered niches.
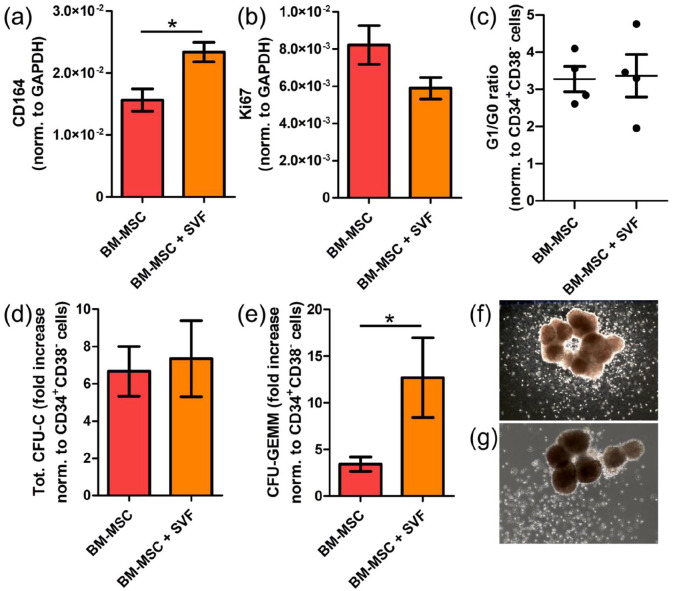
To further characterize HSPCs cultured in vascularized BM niches, we isolated them after 1-week culture and performed gene expression analysis in combination with cell cycle analysis and colony forming-units in culture (CFU-C) assays to measure, respectively, cell proliferation and differentiation. In agreement with the FACS data, HSPCs cultured in vascularized BM niches exhibited higher expression of CD164 (endolyn), a membrane-associated sialomucin recently reported to be a reliable marker for the earliest branches of HSPCs specification34 (Figure 5(a)). Although the expression of the proliferative maker Ki67 trended to decrease (Figure 5(b)), cell cycle analyses revealed no significant differences between HSPCs cultured in avascular or vascularized BM niches (Figure 5(c)). Therefore, our data suggest that vascularized BM niches do not alter HSPC proliferation in comparison to avascular ones.
Figure 5.
Vascularized BM niches does not impact the proliferation of CB HSCs, but affect their differentiation potential. (a, b) Q-PCR data showing CD164 and Ki67 gene expression in HSCs sorted from engineered BM niches. (c) Hoechst 42/Ki67 staining of HSCs sorted after 1-week coculture in engineered niches. Ratio G1/G0 is plotted as an indicator of the proliferative status. (d, e) Fold increase in (d) total colony forming-units in culture (CFU-C) and (e) colony forming-units granulocytes-erythrocytes-monocytes-megakaryocytes (CFU-GEMM) normalized to uncultured CD34+CD38− cells. (f, g) Representative images showing CFU-GMM from (f) avascular and (g) vascularized niches. (a–e) Data are plotted as means ± standard deviations; n = 4; *p < 0.05. Unpaired two-tailed t tests.
Since FACS immunophenotypic data showed accumulation of undifferentiated HSCs in combination with decreased multipotent and more committed progenitors in vascularized BM niches (Figure 4(d)–(g) and Supplemental Figures S3 and S4), we hypothesized that this might reflect an effect on HSPC differentiation potential. Although we could not see any difference in the total amount of CFU-C derived from HSPCs cultured in both niches (Figure 5(d)), we revealed increased CFU-granulocyte-erythrocyte-monocyte-megakaryocyte (CFU-GEMM) activity in those CB HSPCs isolated from vascularized BM niches (Figure 5(e)).Those CFU-GEMM were similar in size to those obtained from CB HSPCs cultured in avascular niches (Figure 5(f) and (g)). In contrast, we did not observe differences in burst forming-units erythroid (BFU-E) or CFU-granulocyte-monocytes (CFU-GM) (Supplemental Figure S6). Collectively, these results reveal an important contribution of vascular cells preserving undifferentiated HSPCs in engineered 3D BM niches.
SVF-induced vascularization protects the osteogenic potential of BM engineered niches in time
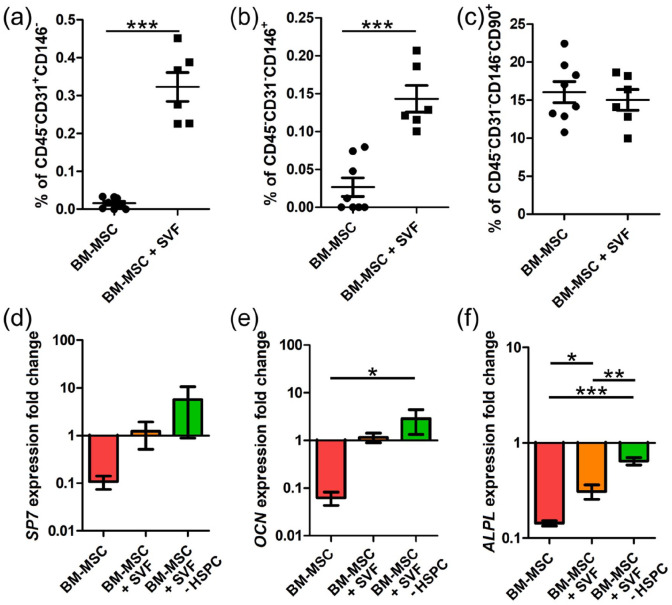
Once demonstrated that 3D vascularized BM niches can decisively influence the function of CB HSCs in vitro, we explored if vascular cells have an impact on the preservation of the niche. We had already shown that the addition of vascular cells did not alter the osteogenic matrix during the generation of the niche (Figure 3). We now evaluated the changes after the coculture with CB HSCs. As expected, when we looked into the stromal fraction (CD45- cells), we revealed a significant enrichment in endothelial cells (CD45-CD31+CD146-) and pericytes (CD45−CD31−CD146+) in vascularized BM niches, which confirms the maintenance of vascular cells after the coculture with CB HSPCs (Figure 6(a) and (b) and Supplemental Figure S4). In contrast, we did not see any quantitative difference in the abundance of mesenchymal stromal cells (CD45-CD31-CD146- CD90+) (Figure 6(c)).
Figure 6.
SVF-induced vascularization contributes to maintain the osteogenic potential of engineered BM niches. (a–c) Percentage of (a) CD45−CD31+CD146− endothelial cells and (b) CD45−CD31−CD146+ perivascular cells and (c) CD45−CD31−CD146−CD90+ mesenchymal stromal cells in engineered niches after 1-week coculture with CB HSPCs. (d–f) Fold change gene expression of SP7, OCN, and ALPL in sorted MSCs after the 1-week coculture with/without CB HSPCs. Data are plotted as means ± standard deviations; n = 6–8. *p < 0.05, **p < 0.01, ***p < 0.001. (a–c) Unpaired two-tailed t tests. (d–f) One-way ANOVA with Tukey’s multiple comparison tests.
To unravel the potential influence of vascular cells on BM-MSCs after the 1-week co-culture with CB HSPCs, we sorted MSCs from engineered niches before and after 1-week coculture with CB CD34+ cells, and then measured the expression of osteogenic genes. In order to dissect the contribution linked to hematopoietic cells from that one derived from SVF cells, we also sorted MSCs from vascularized BM niches that were also cultured for a week in HSC medium, but in absence of CB CD34+ cells.
Surprisingly, we observed that the expression of osteogenic genes such as ALPL, SP7 and OCN was 10-fold reduced in MSCs isolated from avascular BM niches after the 1-week coculture (Figure 6(d)–(f)). However, MSCs isolated from vascularized BM niches (BM-MSC + SVF condition) maintained the expression of SP7 and OCN, and the decrease in ALPL expression was significantly lower. Since we were unable to see differences between those MSCs isolated from vascularized niches seeded with or without CB CD34+ cells (Figure 6(d)–(f)), we concluded that this protective effect was not directly linked to CB HSPCs but to the vascularization. These results suggest that niche vascularization contributes to stabilize the osteogenic potential of engineered niches after the 1-week coculture with CB HSPCs.
Discussion
In this study we provide evidence that SVF cells from human adipose tissue can be used as vasculogenic cell source to generate capillary-like vascular structures that add to the osteogenic matrix secreted by BM mesenchymal stromal cells in engineered osteoblastic niches. The resulting vascularized BM niches improved the preservation of undifferentiated CB HSPCs and contributed to maintaining the osteoblastic gene signature of the niche. Thus, our strategy enabled to model perivascular endosteal BM niches in a fully humanized setting at a larger fidelity than achieved to date.
Different studies have shown that human perivascular niches can be engineered in vitro using HUVEC. Some of these approaches have been developed to study hematopoietic cell trafficking and thrombopoiesis mechanisms,35 but they often rely on the addition of MSCs, which under specific culture conditions can serve as perivascular cells.20,22 This often requires complex medium compositions to match the different cell types requirements.36 Furthermore, these approaches lack the osteoblastic cells typically found in native human BM.22 In this study, we propose a unique primary cell source (human adipose tissue-derived SVF cells) to vascularize osteoblastic BM niches (previously engineered only with BM-MSCs) with capillaries composed by both endothelial and perivascular cells. Furthermore, our approach does not require extra supplementation with angiogenic cytokines or specific medium for vascular cells.
To our knowledge, only a recent study successfully managed to engineer a primary 3D tissue including at the same time both osteoblastic and perivascular cells, which was also tested to sustain CD34+ HSPCs.37 Despite recapitulating multiple features from human BM niches, this approach was based on static cultures in Matrigel, which contains murine-derived proteins. Moreover, although useful to study HSPC proliferation and differentiation, that model offers limited possibility to study HSPC trafficking. In this regard, we engineered vascularized BM niches within perfusion bioreactors with different compartments: a liquid phase (supernatant) and the niche itself contained in the bioreactor chamber (see Figure 1). Since both compartments are in communication, HSPCs can freely transit and mimic the in vivo scenario, where HSPCs constantly circulate between the bloodstream and their BM niches. In agreement with the physiological scenario, our results revealed that primitive HSCs are retained in vascularized BM niches, while more mature cells are preferentially released to the supernatant fraction.
Multiple studies reported the maintenance of primitive and quiescent murine HSCs in perivascular niches,1 independently of the spatial region that these niches occupy in the BM (e.g. around sinusoids in central BM or in endosteal vessels in osteoblastic-enriched areas close to the bone surface).6,38 Here we show that engineered 3D BM niches vascularized with SVF cells mimic human perivascular niches maintaining primitive CB HSCs better than avascular niches. However, we have only explored the vascularization of an osteoblastic environment generating capillary-like structures that resemble arteriolar vessels. Therefore, our current model cannot recapitulate the features of human perisinusoidal niches in central BM. In this regard, future models should consider the diversity of vascular structures and incorporate both arteriolar and sinusoidal vessels, possibly using alternative vasculogenic cell sources.
We also demonstrated that SVF-induced vascularization does not only influence HSC activity but also the osteogenic matrix. We observed that niches engineered with BM-MSCs tend to lose some osteogenic features over culture time. This reduction was abrogated in vascularized BM niches independently of the presence of hematopoietic cells in the system, suggesting that vascularization might have a positive influence keeping the osteogenic profile of stromal cells in engineered niches. These results support previous reports indicating a mutual positive influence of endothelial cells and BM-MSCs,39–41 which now can be further extended in the presence of HSPCs. Further studies are now required to investigate the contribution of non-vascular SVF cells to the process of vascularization of engineered BM niches.
In conclusion, we propose a modular bioengineering approach in which human adipose tissue derived-SVF cells can be added to the system as a single cell source component in order to generate functional and vascularized endosteal BM niches. Following the same modular principle, various niche components (e.g. HSPCs, osteoblasts, vasculature, nerves, immune cells. . .) can be used as “building blocks” in different combinations to deconstruct and reconstruct multiple human BM microenvironments. These might be used as controlled models to address specific questions concerning HSC niche biology in pathophysiological conditions.
Supplemental Material
Supplemental material, sj-docx-1-tej-10.1177_20417314211044855 for Engineering of fully humanized and vascularized 3D bone marrow niches sustaining undifferentiated human cord blood hematopoietic stem and progenitor cells by Gordian Born, Marina Nikolova, Arnaud Scherberich, Barbara Treutlein, Andrés García-García and Ivan Martin in Journal of Tissue Engineering
Acknowledgments
We thank Manuele G. Muraro and all members of the Tissue Engineering group for advice and support, DBM facilities for assistance and support.
Footnotes
Author contributions: G.B. designed research, performed and analyzed experiments, prepared figures and drafted the manuscript. M.N. A.S. and B.T provided key reagents and insightful discussion. A.G-G. designed research, performed and analyzed experiments, supervised and wrote the manuscript. I.M. designed research, supervised and wrote the manuscript. All authors edited the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was received from the Swiss National Science Foundation (Grant 31003A_179259) (to I.M.). This work was also supported by the Swiss National Science Foundation as part of the NCCR Molecular Systems Engineering (51NF40-141825) (to B.T. and I.M.).
ORCID iDs: Andrés García-García  https://orcid.org/0000-0002-8797-649X
https://orcid.org/0000-0002-8797-649X
Ivan Martin  https://orcid.org/0000-0001-6493-0432
https://orcid.org/0000-0001-6493-0432
Supplemental material: Supplemental material for this article is available online.
References
- 1.Morrison SJ, Scadden DT.The bone marrow niche for haematopoietic stem cells. Nature 2014; 505(7483): 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinho S, Frenette PS.Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 2019; 20(5): 303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466(7308): 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulais PE, Frenette PS.Making sense of hematopoietic stem cell niches. Blood 2015; 125(17): 2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramasamy SK, Kusumbe AP, Itkin T, et al. Regulation of hematopoiesis and osteogenesis by blood vessel-derived signals. Annu Rev Cell Dev Biol 2016; 32: 649–675. [DOI] [PubMed] [Google Scholar]
- 6.Itkin T, Gur-Cohen S, Spencer JA, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 2016; 532(7599): 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doulatov S, Notta F, Laurenti E, et al. Hematopoiesis: a human perspective. Cell Stem Cell 2012; 10(2): 120–136. [DOI] [PubMed] [Google Scholar]
- 8.Abarrategi A, Mian SA, Passaro D, et al. Modeling the human bone marrow niche in mice: from host bone marrow engraftment to bioengineering approaches. J Exp Med 2018; 215(3): 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koike N, Fukumura D, Gralla O, et al. Tissue engineering: creation of long-lasting blood vessels. Nature 2004; 428(6979): 138–139. [DOI] [PubMed] [Google Scholar]
- 10.Scherberich A, Galli R, Jaquiery C, et al. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells 2007; 25(7): 1823–1829. [DOI] [PubMed] [Google Scholar]
- 11.McFadden TM, Duffy GP, Allen AB, et al. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen-glycosaminoglycan scaffold in vivo. Acta Biomater 2013; 9(12): 9303–9316. [DOI] [PubMed] [Google Scholar]
- 12.Lin RZ, Lee CN, Moreno-Luna R, et al. Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nat Biomed Eng 2017; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Jacamo R, Shi YX, et al. Human extramedullary bone marrow in mice: a novel in vivo model of genetically controlled hematopoietic microenvironment. Blood 2012; 119(21): 4971–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abarrategi A, Foster K, Hamilton A, et al. Versatile humanized niche model enables study of normal and malignant human hematopoiesis. J Clin Invest 2017; 127(2): 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passaro D, Abarrategi A, Foster K, et al. Bioengineering of humanized bone marrow microenvironments in mouse and their visualization by live imaging. J Vis Exp 2017; 126: 55914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bessy T, Itkin T, Passaro D.Bioengineering the bone marrow vascular niche. Front Cell Dev Biol 2021; 9: 645496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommerkamp P, Mercier FE, Wilkinson AC, et al. Engineering human hematopoietic environments through ossicle and bioreactor technologies exploitation. Exp Hematol 2021; 94: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson MR, Ghoshal D, Mejías JC, et al. A multi-niche microvascularized human bone marrow (hBM) on-a-chip elucidates key roles of the endosteal niche in hBM physiology. Biomaterials 2021; 270: 120683. [DOI] [PubMed] [Google Scholar]
- 19.Atlas Y, Gorin C, Novais A, et al. Microvascular maturation by mesenchymal stem cells in vitro improves blood perfusion in implanted tissue constructs. Biomaterials 2021; 268: 120594. [DOI] [PubMed] [Google Scholar]
- 20.Barnhouse V, Petrikas N, Crosby C, et al. Perivascular secretome influences hematopoietic stem cell maintenance in a gelatin hydrogel. Ann Biomed Eng 2021; 49(2): 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blache U, Vallmajo-Martin Q, Horton ER, et al. Notch-inducing hydrogels reveal a perivascular switch of mesenchymal stem cell fate. EMBO Rep 2018; 19(8): e45964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marturano-Kruik A, Nava MM, Yeager K, et al. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc Natl Acad Sci U S A 2018; 115: 1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerino G, Gaudiello E, Muraro MG, et al. Engineering of an angiogenic niche by perfusion culture of adipose-derived stromal vascular fraction cells. Sci Rep 2017; 7(1): 14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser JK, Wulur I, Alfonso Z, et al. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006; 24(4): 150–154. [DOI] [PubMed] [Google Scholar]
- 25.Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004; 110(3): 349–355. [DOI] [PubMed] [Google Scholar]
- 26.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 2004; 109(5): 656–663. [DOI] [PubMed] [Google Scholar]
- 27.Hattori H, Sato M, Masuoka K, et al. Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs 2004; 178(1): 2–12. [DOI] [PubMed] [Google Scholar]
- 28.Güven S, Mehrkens A, Saxer F, et al. Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials 2011; 32(25): 5801–5809. [DOI] [PubMed] [Google Scholar]
- 29.Strioga M, Viswanathan S, Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012; 21(14): 2724–2752. [DOI] [PubMed] [Google Scholar]
- 30.Kozlowska U, Krawczenko A, Futoma K, et al. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells 2019; 11(6): 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinisch A, Etchart N, Thomas D, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood 2015; 125(2): 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgine PE, Klein T, Paczulla AM, et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc Natl Acad Sci U S A 2018; 115(25): E5688–E5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourgine PE, Martin I, Schroeder T.Engineering human bone marrow proxies. Cell Stem Cell 2018; 22(3): 298–301. [DOI] [PubMed] [Google Scholar]
- 34.Pellin D, Loperfido M, Baricordi C, et al. A comprehensive single cell transcriptional landscape of human hematopoietic progenitors. Nat Commun 2019; 10(1): 2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotha SS, Hayes BJ, Phong KT, et al. Engineering a multicellular vascular niche to model hematopoietic cell trafficking. Stem Cell Res 2018; 9(1): 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K, Silva EA, Mooney DJ.Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011; 8(55): 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braham MVJ, Li Yim ASP, Garcia Mateos J, et al. A human hematopoietic niche model supporting hematopoietic stem and progenitor cells In vitro. Adv Healthc Mater 2019; 8(10): e1801444. [DOI] [PubMed] [Google Scholar]
- 38.Acar M, Kocherlakota KS, Murphy MM, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015; 526(7571): 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue Y, Xing Z, Hellem S, et al. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online 2009; 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Liu H, He Y, et al. Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway. Stem Cell Res Ther 2020; 11(1): 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genova T, Petrillo S, Zicola E, et al. The crosstalk between osteo differentiating stem cells and endothelial cells promotes angiogenesis and bone formation. Front Physiol 2019; 10: 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tej-10.1177_20417314211044855 for Engineering of fully humanized and vascularized 3D bone marrow niches sustaining undifferentiated human cord blood hematopoietic stem and progenitor cells by Gordian Born, Marina Nikolova, Arnaud Scherberich, Barbara Treutlein, Andrés García-García and Ivan Martin in Journal of Tissue Engineering