Abstract
Despite significant progress, metastatic urothelial cancer remains an incurable condition with a limited life expectancy. Platinum-based chemotherapy is still the mainstay of treatment for metastatic disease, but immunotherapy, antibody drug conjugates, and targeted agents have shown encouraging results in several recent practice changing trials. In this review, we discuss the standard of care, recent therapeutic advances, ongoing clinical trials, and future perspectives in metastatic urothelial carcinoma.
Keywords: antibody-drug conjugate, bladder cancer, FGFR, immunotherapy, immune checkpoint inhibitors, metastatic urothelial carcinoma
Introduction
According to global cancer statistics, both the incidence and mortality from bladder cancer have been rising over the past few decades. In 2020, there were 573,278 new cases and 212,536 deaths.1 At presentation, approximately 70% of bladder cancers are classified as non-muscle invasive (NMIBC), involving only the innermost layer of the bladder wall. About 25% are muscle-invasive (MIBC), involving the muscle and deeper layers of the bladder wall but still confined to the bladder. In the remaining 5% of cases, the cancer has already spread to nearby tissues, lymph nodes, or to distant metastatic sites such as the lung and bone. Although only 5% of patients are metastatic at presentation, nearly 50% of patients with MIBC, undergoing curative-intent treatment, will eventually relapse and develop metastatic disease.2,3
Survival in the metastatic setting is 12–15 months with cisplatin-based combination chemothrapy, but only 3–6 months if left untreated.2 More recently, with the advent of immunotherapy, antibody-drug conjugates, and targeted agents, the treatment landscape has changed significantly, with overall survival now approaching 2 years.4 The aim of this review is to discuss the current treatment options, ongoing clinical trials, and future perspectives for the management of metastatic urothelial carcinoma.
Methods
We conducted an extensive literature research using PubMed/Medline databases, Scopus, Science direct, Google scholar, ASCO abstracts, and Clinicaltrials.gov. Key words used for the search included bladder cancer, urothelial carcinoma, transitional cell carcinoma, oligometastases, oligoprogression, molecular biology, chemotherapy, immunotherapy, immune-oncology, checkpoint inhibitors, antibody-drug conjugates, Nectin-4, Trop-2 and FGFR inhibitors.
Molecular characterization of urothelial carcinoma
Two distinct pathways have been implicated in the pathogenesis of urothelial carcinoma (UC): papillary and non-papillary UC, which also corresponds to nonaggressive and aggressive forms of the disease. The first arises from tissue hyperplasia and is characterized by fibroblast growth factor receptor 3 (FGFR3) gene mutations. It presents with genetic stability and minimal genetic alterations. The latter arises from severe dysplasia, normally exhibits alterations in the tumor suppressor genes p53 and RB transcriptional corepressor 1 (RB1), is genetically unstable, and often develops multiple chromosomal aberrations during the course of the disease, including loss of heterozygosity at chromosome 9.5 As expected, non-papillary cancers have a worse prognosis and usually present with higher grade tumors and muscle invasive disease.6
The Cancer Genome Atlas (TCGA), which genetically characterized MIBCs, reported that urothelial carcinomas have one of the highest somatic mutation rates (median 5.5 per megabase): similar to that seen in both non-small cell lung cancers and melanoma. These somatic mutations may result in neo-antigens appearing on the cell surface, which can be targeted by the immune system and may explain, in part, why these cancers preferentially respond to novel immunotherapy-based approaches.7 In addition to a high mutation rate, the specific genes in which the mutations occur, and types of mutations may also be important. Some of the genomic abnormalities detected by the TCGA analysis were found in the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mTOR pathway, cyclin dependent kinase inhibitor 2a (CDKN2A)/cyclin dependent kinase 4 (CDK4)/cyclin D1 (CCND1), receptor tyrosine kinase (RTK)/rat sarcoma virus (RAS) pathways, Erb-B2 receptor tyrosine kinase 2 (ERBB2) (Her-2), ERBB3, and fibroblast growth factor receptor 3 (FGFR3); some of which already represent important therapeutic targets in this disease.8
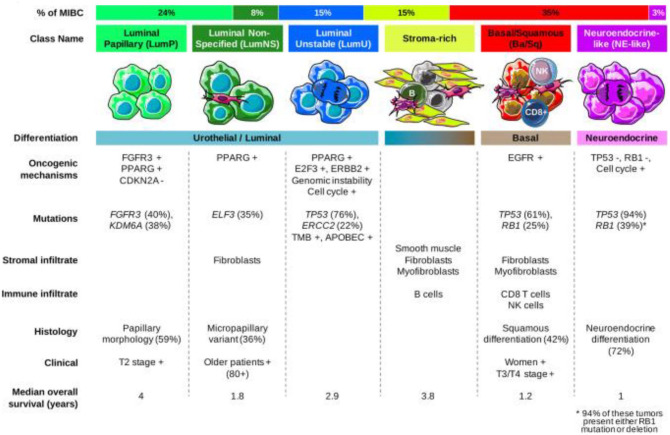
Based on in-depth gene expression profiling, MIBC can now be classified into different molecular subtypes. At least six proposed molecular classifications are described.7,9–13 Aiming for a unified consensus, a recently published analysis has suggested six biologically relevant molecular classes, labeled respectively as: luminal papillary, luminal non-specified, luminal unstable, stroma-rich, basal/squamous, and neuroendocrine-like (Figure 1).14 This simplified classification still needs clinical validation, but supports precision medicine, by connecting molecular findings and clinical findings and by identifying biomarkers which might improve patient management.14
Figure 1.
Bladder cancer molecular classification.
Immunohistochemical analysis has allowed identification of several key biomarkers. Programmed death-ligand 1 (PD-L1) expression, for example, ranges from 20% to 72% in patients with metastatic urothelial carcinoma (mUC).15 Other important biomarkers include nectin-4, which was found to be positive in almost all cases of mUC;16 and Trop-2, which was also found to be widely expressed in up to 83% of cases.17
First line treatment
Chemotherapy and platinum eligibility
Cisplatin-based chemotherapy remains the preferred frontline treatment in mUC, with response rates ranging from 49% to 72%, and overall survival (OS) of 14–15 months, varying according to the chosen regimen.18,19 Despite encouraging response rates (RRs), durability is an issue and most patients will experience disease progression. In addition, a significant proportion of patients will be deemed ineligible for cisplatin, according to the Galsky criteria. These criteria include an Eastern Cooperative Oncology Group performance status (ECOG PS) of ⩾ 2, creatinine clearance (CrCl) less than 60 ml/min, grade ⩾ 2 hearing loss, grade ⩾ 2 neuropathy, and/or New York Heart Association Class ⩾ III heart failure.20 In patients not eligible for cisplatin, carboplatin-based regimens represent an alternative treatment option, but have lower RRs and shorter OS (9 months) compared to cisplatin-based regimens.21 Another emerging option would be to split the cisplatin dose over 2 days, for patients with CrCl ranging from 40 to 60 ml/min. In a single arm trial this has been shown to be a feasible approach; however, this has not yet been compared head-to-head against either standard cisplatin or carboplatin-based regimens.22,23
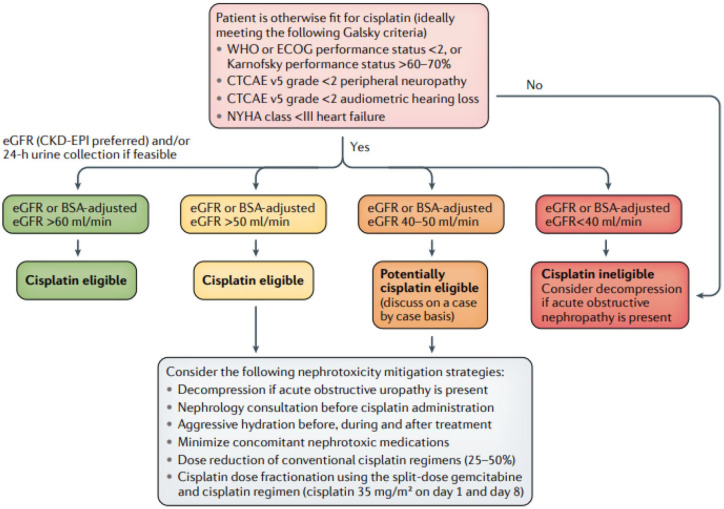
Determining eligibility for cisplatin, one of the most active drugs in this disease, is therefore a major consideration in advanced UC. In MIBC, Jiang et al. have recently proposed a new algorithm that emphasizes a multidisciplinary approach to guide treatment decisions in order to optimize the use of cisplatin-based chemotherapy in the curative neoadjuvant setting24 (Figure 2). Aspects of this new algorithm can also be applied to the metastatic setting, to maximize the number of patients ultimately receiving cisplatin-based therapy. Many experts, for example, will offer cisplatin-based chemotherapy to patients with a CrCl threshold of 50 ml/min or higher, even in the metastatic setting, given the superiority of cisplatin over all other agents. Achieving a response and avoiding progression on front-line therapy is even more critical now, with the recent approval of maintenance immunotherapy with avelumab precisely in patients not progressing on front-line chemotherapy.
Figure 2.
Proposed algorithm for determining eligibility for neoadjuvant cisplatin-based chemotherapy in patients with MIBC.24
MIBC, muscle invasive bladder cancer.
Immunotherapy for cisplatin ineligible patients
In patients with locally advanced or mUC, who are not eligible for cisplatin-based chemotherapy and whose tumors express PD-L1, or patients who are not eligible for any platinum-based regimen regardless of PD-L1 status, two immune checkpoint inhibitors (ICI), atezolizumab and pembrolizumab, had received accelerated US Food and Drug administration (FDA) approval, pending further discussions about the confirmatory trials for these agents in this setting. More recently, the FDA converted the accelerated approval for Pembrolizumab in patients not eligible to receive platinum-based chemotherapy, into a full approval.25–27
Atezolizumab, an anti PD-L1, was studied in the IMVIGOR 210 single arm, 2-cohort phase II trial. Cohort 1 enrolled treatment-naive, cisplatin-ineligible patients. The objective response rate (ORR) was 23% in the overall populatio and OS was close to 16 months. When stratified by PD-L1 subgroups, the ORR was 21% in IC0, 21% in IC1, 24% in IC1/2/3, and 28% in IC 2/3 patients, detected by the Ventana method (SP142 antibody).25
Pembrolizumab, an anti PD-1, was studied in KEYNOTE 052, another single arm phase II study. Long term outcomes, reported at a follow up of 5 years, showed an ORR of 28.9% in the entire cohort, including 9.5% complete responses (CR), and an OS of 11.3 months. As expected, higher responses were seen in patients with a combined positive score (CPS) ⩾ 10%, but low or absent PD-L1 did not preclude responses, therefore justifying its use for patients unfit for chemotherapy, especially taking into consideration the durability of responses observed in the study26,27 (Table 1).
Table 1.
First line immune checkpoint inhibitors.
| Pembrolizumab | Atezolizumab | |
|---|---|---|
| Phase | Phase II (Keynote-052) | Phase II (IMvigor 210, Cohort 1) |
| Patients | 370 | 119 |
| Dosing | 200 mg every 3 weeks | 1200 mg every 3 weeks |
| ORR | 28.9% (9.5% CR) | 23% (9% CR) |
| Duration of response | 39.4% responses ongoing at ⩾48 months | 70% responses ongoing at 17.2 months |
| Median OS | 11.3 months | 15.9 months |
| Median PFS | 2 months | 2.7 months |
| Rate of grade 3/4 treatment-related AEs (%) | 19 | 16 |
AE, adverse events; CR, complete response; ORR, objective response rate; OS, overall survival; PFS, progression free survival
Combined chemotherapy and immune checkpoint inhibitors
Based on the documented benefit of both chemotherapy and the ICIs in mUC, several efforts are underway evaluating a combined approach in the frontline setting. Additional rationale for this approach comes from the fact that cisplatin may even increase PD-L1 expression, potentially triggering resistance to ICI, that could be overcome by combining both strategies upfront. In addition, given the aggressive nature of this disease, some patients may never receive second line therapy, so an upfront strategy may also overcome this issue.28 Unfortunately, the results so far have not been as promising as expected, raising the issue that more intensive treatment, in mUC is not always better. Some of the reasons for this could be that chemotherapy and immunotherapy are targeting a similar population of cells, or that chemotherapy and immunotherapy are antagonistic on some level.
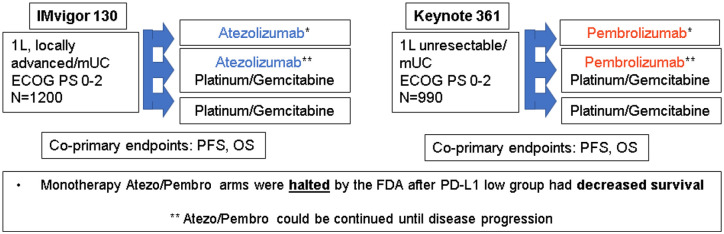
IMvigor 130
IMvigor 130 was a phase III study for patients with mUC, eligible for platinum-based chemotherapy. Patients were randomized to one of three arms: A, atezolizumab plus gemcitabine/platinum B, atezolizumab alone, or C, placebo plus platinum/gemcitabine. It should be noted that patients in arm B were allowed to continue on atezolizumab as maintenance therapy after the chemotherapy, and regardless of the response to chemotherapy. Final PFS results significantly favored atezolizumab plus chemotherapy [8.2 versus 6.3 months hazard ratio (HR) 0.82, 95% confidence interval (CI) 0.70–0.96; p = 0.007), but interim OS data did not show a significant improvement as it did not reach the pre-specified threshold for p-value (16 versus 13.4 months, HR 0.83, 95% CI 0.69–1.00; p = 0.027). Similarly, OS comparisons between arms B and C also showed no statistically significant difference, but numerically favored atezolizumab in the PD-L1 IC2/3 subgroup (not estimable versus 17.8 months, HR 0.68, 95% CI 0.43–1.08) (Table 2). Although the ORR favored the combination, there was no clear evidence of synergy. Another interesting consideration is that a larger effect size was observed when adding atezolizumab to cisplatin, as opposed to the carboplatin regimens, but possible imbalances in baseline factors complicates the interpretation of these results. Longer follow-up is needed to better understand the possible interaction of different chemotherapy regimens with immunotherapy and the effect on survival outcomes.29
Table 2.
Outcomes of chemotherapy and immune checkpoint inhibitors combination.
| Imvigor 130 | Keynote 361 | |||||
|---|---|---|---|---|---|---|
| Atezolizumab + chemotherapy | Chemotherapy + placebo | p-value | Pembrolizumab + chemotherapy | Chemotherapy alone | p-value | |
| mPFS | 8.2 months (6.5–8.3) | 6.3 months (6.2–7.0) | 0.007 | 8.3 months (7.5–8.5) | 7.1 months (6.4–7.9) | 0.0033 |
| mOS | 16 months (13.9–18.9) | 13.4 months (12–15.2) | 0.023 | 15.6 months (12.1–17.9) | 14.3 months (12.3–16.7) | 0.0407 |
| ORR (%) | 47 | 44 | – | 54.7 | 45 | – |
mOS, median overall survival; mPFs, median progression-free survival; ORR, overall response rate.
Keynote 361
The Keynote-361 study had a very similar design to IMvigor 130 (Figure 3). Patients were randomized to pembrolizumab plus platinum/gemcitabine, pembrolizumab alone, or platinum/gemcitabine alone, and again were allowed to continue pembrolizumab as maintenance after the chemotherapy was completed and regardless of response. When compared to chemotherapy alone, the combination of pembrolizumab and chemotherapy had a numerically better median PFS (8.3 versus 7.1 months, HR 0.78, 0.65–0.93, p = 0.0033) and OS (17 versus 14.3 months, HR 0.86, 0.72–1.02, p = 0.0407), but did not reach the significance threshold for p-value, required for the study to be declared positive. Again, ORR in the combination arm did not suggest synergy30 (Table 2).
Figure 3.
Design of first line combination trials: chemotherapy and immune checkpoint inhibitors.
FDA Warnings
Based on significantly decreased survival observed in the immunotherapy alone arms on both of these trials compared to chemotherapy, the FDA issued a warning that single agent immunotherapy should only be used in patients who are not eligible for cisplatin-based therapy and have PD-L1 expression, or in patients not eligible for any platinum-based regimens regardless of PDL1 expression. Given the widespread uptake of immunotherapy and patients’ strong desire to avoid chemotherapy, the FDA warnings are very important and serve to highlight why phase III randomized trials remain critical in evaluating novel therapeutic approaches in this disease.
DANUBE
Combination ICI (anti PDL1 and anti CTLA4) was also evaluated in the frontline DANUBE study. Patients were randomized to durvalumab (D), durvalumab plus tremelimumab (D+T) or standard of care chemotherapy. The co-primary endpoints were OS in the high PD-L1 population when comparing durvalumab versus chemotherapy and OS in the intention to treat (ITT) population when comparing D+T versus chemotherapy. Interestingly, an exploratory secondary endpoint demonstrated that in the PDL1 population, the arm receiving D+T had improved OS versus the chemotherapy arm (17.9 versus 12.1 months; HR: 0.74, 0.59–0.93) and showed comparable ORR (47 versus 48%), which may warrant further study.31
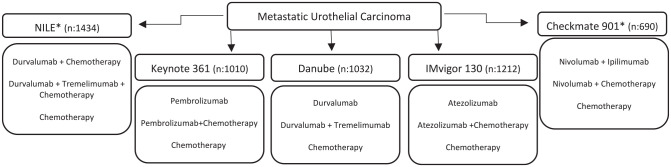
Ongoing First Line Combination Studies
The NILE phase III study (Clinicaltrial.gov identifier: NCT03682068) is also evaluating the role of D+T, but this time, in combination with chemotherapy. The CheckMate 901 study (Clinicaltrial.gov identifier: NCT03036098) also has a similar design, assessing the efficacy and safety of nivolumab plus chemotherapy and nivolumab plus ipilimumab versus chemotherapy alone. Both studies have recently completed enrollment, and their results will hopefully provide further insight into the efficacy of immunotherapy combinations, especially when compared to chemotherapy in patients with high PD-L1 status (Figure 4).
Figure 4.
Trials with chemotherapy and immune checkpoint inhibitors combination.
*Completed enrolment, awaiting results.
Maintenance immunotherapy
Maintenance strategies are already considered standard of care for other advanced solid tumors, including lung and colorectal cancers. Switch maintenance approaches differ from continuation maintenance as it avoids superimposed toxicity. By using different mechanisms of action, it may be possible to target subpopulations of tumor cells that are resistant to first line chemotherapy, thus delaying disease progression.32 Furthermore, chemotherapies may increase antitumor immune activity in many ways, including the depletion of immunosuppressive cells, such as myeloid derived suppressor cells (gemcitabine); and increasing tumor antigen expression and presentation (e.g. cisplatin, gemcitabine, or paclitaxel), thereby increasing tumor cells sensitivity to immune-mediated lysis.32 The cancer immunoediting conceptual framework may help support the rationale for ICI maintenance after chemotherapy: tumor growth or the ‘escape phase’ occurs when the immune system’s capacity to control tumor proliferation is exceeded. Chemotherapy can help the immune system to regain control of the situation, preventing further tumor growth and entering an ‘equilibrium phase’. Chemotherapy may also help by decreasing the tumor burden in the so called ‘elimination phase’. As we know, these effects are transitory in the majority of patients until resistance mechanisms arise. Administration of maintenance immunotherapy may enhance immune system activity, prolong the equilibrium phase, delay disease progression, and extend survival.32,33
Following that premise, the JAVELIN Bladder 100 trial was designed to assess the role of maintenance avelumab, an anti PD-L1, in patients who had not experienced disease progression after a first line platinum-based chemotherapy. The co-primary endpoints were OS in all randomized patients and in the PD-L1 positive population, and the results were practice changing. The median OS among all comers was 21.4 months in the maintenance avelumab arm versus 14.3 months favoring the experimental in the best supportive care arm (HR: 0.69, 95% CI, 0.54–0.92; p = 0.001), and when looking at the PD-L1 positive population the survival again favored the avelumab maintenance arm (not estimated versus 17.1 months, HR: 0.56, 95% CI, 0.40–0.79, p < 0.001). Interestingly, longer survival was observed in both arms, avelumab and control, when patients had high PD-L1, suggesting that in the maintenance setting using the specific assay used in the JAVELIN trial (SP263, Ventana Medical Systems), PD-L1 expression may represent a prognostic marker.4 A subsequent subgroup analysis of the Javelin study aimed to specifically evaluate the predictive utility of several emerging biomarkers, including PD-L1 protein expression (either in the tumor cell or immune cell), tumor mutational burden, and gene expression signatures, but none of them, either alone or in combination, optimally predicted OS benefit with avelumab.34 Another exploratory analysis reported that OS and PFS were longer with avelumab maintenance versus best supportive care alone, irrespective of the selected chemotherapy regimen (carboplatin or cisplatin), and irrespective of disease response (stable disease, complete, or partial responses).35
Another maintenance study trial, HCRN GU 14-182, also explored the switch maintenance strategy. This was a phase II trial with a very similar design to the JAVELIN trial, and randomized patients to either pembrolizumab or placebo after platinum-based chemotherapy, excluding patients with progressive disease. However, unlike the JAVELIN trial, patients were offered the possibility to crossover at disease progression, characterizing the placebo arm as a treatment break. The primary endpoint of the study was PFS, and it reached statistical significant difference favoring the maintenance arm (5.4 × 3.0 months, HR of 0.65, log-rank p value 0.04), but the OS, a secondary endpoint, was not significantly different between the two arms (22 versus 18.7 months, HR: 0.91; 95% CI, 0.52–1.59).36
Despite crossover not being allowed in the JAVELIN trial, the proportion of patients who ended up receiving subsequent immunotherapy at disease progression was similar to those who crossed over to pembrolizumab in the HCRN GU 14-182 study, so that would not be a definitive explanation for the discrepancy in the OS results. What may have contributed is the fact that HCRN GU trial, unlike the JAVELIN trial, was mainly conducted in academic centers in the US, where there was access to a variety of subsequent treatment modalities, including access to many clinical trials.4,36 There was also a slightly higher use of cisplatin-based regimens in the HCRN GU trial, compared to the JAVELIN trial, which may also have improved outcomes in the control arm, cross-trial comparisons notwithstanding.
Comparing the JAVELIN trial4 with trials like IMvigor 13024 and Keynote 36125, where ICIs were also given in the maintenance phase, it is important to note that there was no benefit in OS, despite patients in the combination arm also receiving maintenance ICI. As previously discussed, no significant survival difference was achieved when immunotherapy was combined with chemotherapy as opposed to the positive results achieved with the maintenance strategy. One possible explanation for this may be attributed to patient selection, as the ones with progressive disease after chemotherapy were excluded from the JAVELIN trial. Another key question is regarding the optimal interval from the end of chemotherapy to starting on maintenance immunotherapy, based on the speculated immunosuppressive effect of chemotherapy. In the JAVELIN trial, avelumab was started 4–10 weeks after the last chemotherapy cycle, and so a post hoc analysis of survival according to the treatment free-interval could also be helpful in understanding the optimal time to start maintenance after chemotherapy within the 4–10 week window.37 Finally, the proportion of patients receiving cisplatin-based chemotherapy, as opposed to carboplatin, was higher in the JAVELIN trial, when compared to both IMvigor 130 and Keynote-361, which may also have played a role in the discrepant survival results.
Second line treatment
Immune checkpoint inhibitors
In patients with disease progression during or after platinum-based chemotherapy, immunotherapy is currently the standard of care. The efficacy of five different anti PD-1 and PD-L1 antibodies have been documented in the second line setting, but pembrolizumab is the only ICI to receive full FDA approval, based on the results of the Phase III Keynote 045 study. Keynote-045 randomized 542 patients to receive either pembrolizumab or investigator’s choice of chemotherapy, in the second line setting. The primary endpoint was met. The median OS was significantly improved in the pembrolizumab arm compared to chemotherapy (10.1 versus 7.3 months, HR 0.70, 95% CI 0.57–0.85).38
Atezolizumab received accelerated FDA approval based on the single arm, phase II study, IMvigor 210, which showed an ORR of 15% and a median OS of 7.9 months in all patients.39 The IMvigor 211 was a randomized phase III study that had a very similar design to the Keynote-045 and tested atezolizumab in the same setting. The primary endpoint was OS, which was tested hierarchically, in pre-specified populations according to PD-L1 expression. In the IC2/3 population (n = 234), OS was not significantly different between patients in the atezolizumab and chemotherapy groups (11.1 versus 10.6 months, HR 0.87, 95% CI 0.63–1.21; p = 0.41); therefore, as the study missed its primary endpoint, the FDA indication was withdrawn. The explanation for the discrepancies between IMvigor 211 and Keynote-045 are not fully understood. But, differences in the PD-L1 assay used and the fact that unlike in the IMvigor 211 study, the Keynote-045 trial assessed PD-L1 expression on both immune and tumor cells, might have played a decisive role.40
Nivolumab (Checkmate 275),41 avelumab (JAVELIN solid tumor)42, and durvalumab43 have also received FDA accelerated approval in previously treated patients with locally advanced or metastatic disease based on their phase I/II studies. Recently, however, the FDA indication for both atezolizumab and durvalumab in the platinum-refractory setting has been withdrawn, the latter based on the negative findings of the confirmatory DANUBE study. Study details are described on Table 2.
The combination of nivolumab and ipilimumab has also been tested in CheckMate 032 trial but has not been approved for this indication. This was a multi-cohort phase II study that randomly assigned patients to nivolumab 3 mg/kg single agent (N3), ipilimumab 1 mg/kg plus nivolumab 3 mg/kg (I1N3) or ipilimumab 3 mg/kg plus nivolumab 1 mg/kg (I3N1), and documented a higher ORR for the I3N1 arm, of 38%, versus 26 and 27% for the N3 and I1N3 groups, respectively. The median OS was 15.3 months, versus 9.9 and 7.4 months.44
Differences in clinical outcomes between anti-PD-L1 and anti PD-1 drugs are not clearly established but results from a systematic review and meta-analysis of 19 randomized clinical trials involving more than 11,000 patients have shown significant OS advantage favoring anti PD-1.45 In urothelial carcinomas, PD-1 appears to have an advantage over anti PD-L1 in the second line setting, but in the maintenance setting, it seems to be the opposite. Reasons for this are still lacking elucidation, but may be associated with different assays, cutoff values, and types of cells where PDL1 expression is tested (tumor versus immune cells) (Table 3).
Table 3.
Indirect comparison between immune checkpoint inhibitors approved in second line.
| Drug | Phase | Patients | ORR (%) | mOS |
|---|---|---|---|---|
| Pembrolizumab | III | 542 | 21.1 | 10.1 months |
| Atezolizumab* | III | 931 | 13.4 | 11.1 months |
| Atezolizumab* | II | 316 | 15 | 7.9 months |
| Nivolumab | II | 265 | 20.7 | 8.7 months |
| Avelumab | I | 249 | 17 | 6.5 months |
| Durvalumab* | I/II | 182 | 17.8 | 18.2 months |
FDA indication for second line treatment in mUC was withdrawn in 2021.
mOS, median overall survival; ORR, overall response rate.
Fibroblast growth factor receptor inhibitors
Fibroblast growth factor receptor inhibitors (FGFRs) (1–4) are tyrosine kinase receptors, that when activated by their specific ligands, induce kinase activation, leading to the initiation of important intracellular processes, that includes cell proliferation, differentiation, growth, and survival. Therefore, as expected, some alterations in FGFR may cause constitutive FGFR signaling contributing to oncogenesis, including of urothelial lineage.46 Targeting these receptors has, therefore, become an area of growing interest. In mUC, susceptible alterations are detected in approximately 15–20% of patients.47
Erdafininib is an oral potent tyrosine kinase inhibitor of FGFR 1-4, and currently the only drug of its class approved for locally advanced or mUC progressing on platinum-based chemotherapy. Interestingly, not all FGFR alterations are targeted by erdafinitib. For that reason, erdafitinib is only approved in patients with susceptible FGFR3 gene mutations (R248Cs, S249C, G370C, Y373C) or FGFR2/3 gene fusions (FGFR3-TACC3, FGFR3 BAIAP2L1, FGFR2-BICC1, FGFR2-CASP7), and whose disease has progressed during or following platinum-based chemotherapy, with prior immunotherapy also being allowed.48 This approval was based on the pivotal phase II BLC2001 trial,46 conducted in 99 patients harboring the aforementioned alterations in FGFR 2 and 3. The confirmed RR was 40% (95% CI, 31–50); an additional 39% had stable disease as best response. With a median follow-up of 11.2 months, the median PFS was 5.5 months (95% CI, 4.2–6.0), and the median OS was 13.8 months (95% CI, 9.8 to not reached).47 The most common treatment related adverse events (TRAE) were hyperphosphatemia (77% any grade), stomatitis (58%), diarrhea (51%), dry eye and vision problems (47%) dry mouth (46%), hand-foot syndrome (23%), onycholysis (18%), paronychia (17%), and nail dystrophy (16%). TRAEs ⩾ G3 were reported in 46% of the patients, requiring treatment discontinuation in 13%.47
Despite being approved for the second line setting, erdafinitib use is mostly reserved for third line, after progression on immunotherapy. There is a suggestion that FGFR alterations are more prevalent in luminal papillary tumors, which have reduced T-cell infiltration, possibly indicating a lower sensitivity to immunotherapy.7,49 However, retrospective data from IMVigor 210 and CheckMate 275 indicates responses to ICI are seen, regardless of the FGFR status.49,50 Available data at this point does not allow any conclusions regarding the FGFR mutation status as a biomarker for resistance to ICIs. The results of the ongoing phase III THOR trial, investigating the role of erdafinitib compared to chemotherapy or pembrolizumab in patients with mUC with FGFR gene alterations, will hopefully be able to answer how to best sequence these drugs (Clinicaltrials.gov identifier: NCT03390504).
Subsequent lines of therapy
Another option in the post chemotherapy and checkpoint inhibitor refractory setting is the antibody drug conjugate enfortumab-vedotin (EV).
Antibody-drug conjugate (ADC)
Enfortumab-Vedotin
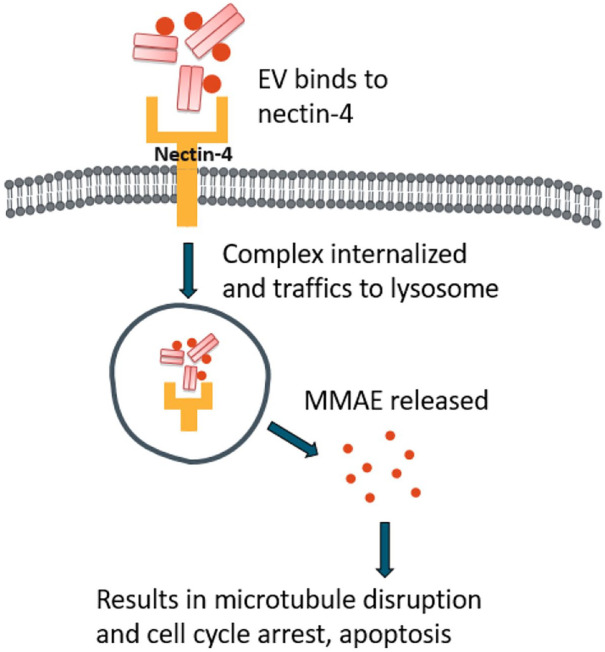
EVs is a monoclonal ADC, directed against nectin-4, a protein that is highly expressed in urothelial carcinoma.16 EV, conjugated with a microtubule-disrupting agent (monomethyl auristatin E or MMAE), selectively binds to nectin-4, resulting in internalization of the ADC-nectin-4 complex and release of the MMAE inside the cell. This will ultimately lead to microtubule disruption and apoptotic cell death (Figure 5).51
Figure 5.
Mechanism of action of enfortumab-vedotin.
Accelerated FDA approval for EV in patients with mUC was given based on the results of the EV-201 phase II trial. The confirmed ORR was 44% (95% CI, 35.1–53.2%), and included 12% CR. The median PFS and OS were respectively 5.8 months (95% CI, 4.9–7.5 months) and 11.7 months (95% CI, 9.1 months to not reached).16 The most common side effects associated with EV were fatigue (50% all grade), alopecia (49% all grade), decreased appetite (44% all grade), dysgeusia (40% all grade), and peripheral sensory neuropathy (40% all grade).16
The results of the randomized phase III study, EV-301, have also been recently reported. Patients with locally advanced or mUC who had previously received a platinum-based chemotherapy and an ICI were randomized to receive either EV or single agent chemotherapy (n = 608). OS, the primary endpoint, was significantly longer in the EV arm (12.8 versus 8.9 months; HR 0.70: 95% CI 0.56–0.89; p = 0.001), and mPFS, a key secondary endpoint, was also prolonged (5.55 versus 3.71 months; HR 0.62; 95% CI, 0.51–0.75; p < 0.001), with no significant differences in AEs between treatment arms. A survival benefit was observed across most of the pre-specified subgroups, including patients with liver metastasis.52
EV is also currently being investigated in earlier settings of mUC, supported by comparable ORR to carboplatin-based chemotherapy in the first line setting,16,21 and its liver metabolism, not requiring dose adjustments for kidney dysfunction. Preliminary results of the EV-103, a phase Ib/II study, showed that the combination of EV plus pembrolizumab resulted in an ORR of 73%, in previously untreated patients, ineligible for cisplatin.53
Sacituzumab Govitecan
Another ADC that has activity against solid tumors, including mUC, is sacituzumab govitecan (SG). This is a humanized anti-trophoblast cell-surface antigen 2 (Trop 2) antibody, conjugated with SN-38, an active metabolite of irinotecan that inhibits the nuclear topoisomerase 1, inducing double-stranded DNA breaks and, ultimately, cell death.54,55
TROPHY-U-01 is a multicohort, phase II trial, investigating the role of SG in patients with mUC whose disease has progressed after platinum-based chemotherapy and ICI. The final results of cohort 1 of this study were recently published56 and confirmed an ORR of 27% (31/113; 95% CI 19.5–36.6) with 6 CRs. The median PFS was 5.4 months (95% CI 3.5–7.2) and median OS 10.9 months (95% CI 9.0–12.8). The most common TRAEs were diarrhea (65% all grade, 10% ⩾G3), followed by, nausea (58% all grade), fatigue (50%), alopecia (47%), and neutropenia (46% all grade, 34% ⩾G3). Only 6% had to discontinue due to TRAEs and treatment related death was observed in 1 patient. Based on the encouraging data from the phase II trial, FDA granted SG accelerated approval on April 13, 2021. A phase III trial (Clinicaltrial.gov identifier: TROPiCS-04-NCT04527991) is current underway, comparing SG to single agent chemotherapy in the post platinum, post ICI setting
As previously described, SG and EV have different mechanisms of action and different toxicity profiles, therefore making it feasible for both of them to be used in the same patient throughout the disease course. The ideal sequencing approach would, however, need to be validated in prospective trials.
Single agent chemotherapy
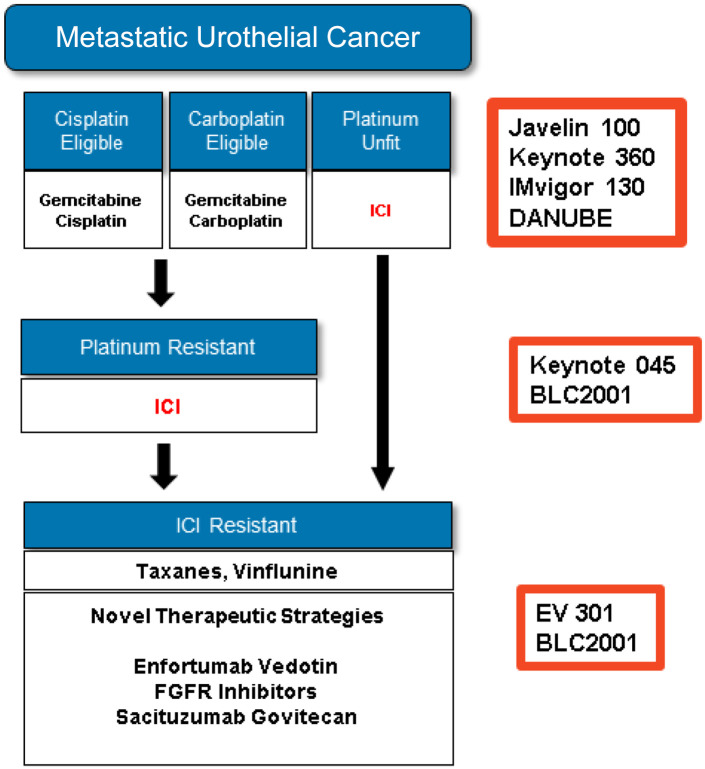
Many chemotherapy options have been tested for mUC with some response after platinum-based treatment, most of them in small single arm trials, including docetaxel, gemcitabine, ifosfamide, and pemetrexed57–60 (Figure 6). There are, however, two randomized trials worthy of attention: a phase III study, comparing vinflunine to best supportive care (BSC) alone,60 and a phase II trial, comparing paclitaxel to nab-paclitaxel.62
Figure 6.
Management algorithm.
Vinflunine plus BSC was compared to BSC alone in a phase III trial with 370 patients. In the intention-to-treat population, the survival analysis numerically favored the vinflunine arm, but failed to demonstrate statistically significant difference across treatment arms (6.9 × 4.6 months, HR:0.88; 95% CI 0.69–1.12, p = 0.287), probably due to imbalances in performance status between the two arms. An ORR of 9% significantly favored the experimental arm (p = 0.006).61 Based on these results, vinflunine has been approved for second line treatment in Europe, but is not approved in other parts of the world.
Nab-paclitaxel and paclitaxel were compared in a randomized phase II trial, conducted across Canada and Australia, which included 199 patients after disease progression on platinum-based chemotherapy. There was no significant difference in PFS, OS, or ORR between treatment arms. The median PFS with nab-paclitaxel was 3.4 versus 3.0 months (HR 0.92; 90% CI, 0.68–1.23; p = 0.31), the median OS was 7.5 versus 8.8 months (HR, 0.95; 90% CI, 0.70–1.30, p = 0.40), and the ORR was 22% for nab-paclitaxel versus 25% for paclitaxel (p = 0.97). Interestingly, more G3/4 TRAEs were observed in the nab-paclitaxel arm (66 versus 46%, p = 0.009), but the incidence of peripheral neuropathy was similar. Paclitaxel is a well-known and safe drug and is relatively inexpensive, with RRs observed in a quarter of the patients; it therefore remains a potential option in further lines of treatment.62 In jurisdictions without access to novel therapies, paclitaxel remains a viable option in the post-platinum setting, with RRs at least similar to that seen with second line immunotherapy.
Managing oligometastatic disease and the role for metastasis directed treatment
With the development of novel imaging modalities, like positron-emission tomography (PET) scans, the detection rate of distant metastasis has improved when compared to conventional techniques,63 leading to the emergence of a new disease state entitled oligometastatic disease (OM). Definitions may vary according to the tumor site, but it is generally described as ‘a solitary or a few detectable metastatic lesions of a small size that are generally confined to a single or a few organs’.62 The number of metastatic lesions that would fit the definition normally varies between 3 and 5, but remains an area of controversy.64,65 This entity has increasingly gained attention due to the availability of new therapeutic options that are safe and non-invasive/minimally invasive, which may lead to long-term survival and, in some cases, cure.64
A retrospective study by Ogihara et al, showed that patients with OM urothelial cancer had better survival when compared to non-OM group (53.3 vs. 16.1%, p < 0.001), and are more likely to respond to chemotherapy, suggesting that OM foci hold the same characteristics as tumor cells from the primary.64 Based on that premise, of a more favorable tumor biology in OM disease, a hypothesis that the outcomes would be favorable regardless of metastasis directed treatment has to be considered.66,67
A meta-analysis of 17 articles between 1990 and 2015, including 412 patients, demonstrated a mean time to recurrence of 14.2 months after metastasectomy, with OS ranging from 2 to 60 months. It also reported an improvement in OS in patients treated surgically, when compared to the ones undergoing systemic treatment only (HR 0.63; 95% CI, 0.49–0.81); despite this, most of the studies were retrospective and non-randomized, possibly associated with serious patient selection bias.67 The role of consolidative radiation after a partial response to chemotherapy was studied in a trial including 22 patients with mUC, and demonstrated a PFS and OS of 13 and 29 months, respectively. A total of 8 patients (36%) in this study were alive and disease-free after 6 years, of which 6 had limited regional nodal disease and 2 had distant disease (single mediastinal lymph node and lung nodule).68
To date, based on the limited evidence, there is still no consensus on how to optimally approach patients with OM urothelial carcinomas, and discussion in multidisciplinary tumor boards are important, especially for patients with chemo-sensitive, low volume disease, confined to lungs or lymph nodes, where the evidence for integrating metastasis-directed treatment is stronger.66 An interesting trial is currently recruiting patients with metastatic bladder cancer with no more than 3 residual metastatic lesions after first line chemotherapy for consolidative radiotherapy (Clinicaltrial.gov identifier: BLAD-RAD01- NCT0448554). Several other trials assessing the role of radiation for oligometastatic tumor are also underway, 4 of them allowing inclusion of patients with mUC (Clinicaltrial.gov identifiers: NCT03543696, NCT03599765, NCT03862911, NCT03721341).
Future perspectives
Unprecedented improvements have been made in the treatment of mUC in the last few years, with a number of new treatment options becoming available for this patient population. Along with the recent exciting advances, there are a number of important topics that warrant further discussion. These include the importance of PD-L1 status as a prognostic or predictive marker, the assay used for analysis, predictive and prognostic biomarkers, and the reason why combining chemotherapy and immunotherapy in the first line setting has not demonstrated the expected efficacy.
A recent meta-analysis, compiling results of 27 studies, with over 4 thousand patients, suggested that PDL1 expression on either tumor cells (TCs) or tumor infiltrating immune cells (ICs) and combined positive score (CPS) was indeed associated with significantly better ORR to ICI, although not translated into better OS. The pooled survival analysis showed that PD-L1 expression on ICs was an independent predictor of OS.15 There are currently 4 distinct assays available for PD-L1 assessment (Dako 22-08, Dako 22C3, Ventana SP142, and SP263), which raise questions regarding interchangeability and comparability among them, highlighting the need for prospective harmonization studies to optimize determination of PD-L1 positivity.69 A study conducted by Mauji et al.70 assessed the PD-L1 expression of 139 patients with mUC using all commercially available antibodies. They detected a substantial concordance of 80–90% among the antibodies. However, given the limited discriminative value of these assays in accurately predicting outcomes in mUC, the results of this study may still have limited effect on overall patient selection for ICIs.
The search for predictive and prognostic biomarkers in urothelial carcinoma continues. PD-L1 status has been shown to predict response in some trials and is required if ICI is being considered in the front-line cisplatin-ineligible setting20,21, but ICI response has also been observed in PD-L1 negative disease.15 The lack of standardization to assess PD-L1 expression hinders its use as a reliable biomarker. Moreover, PD-L1 is a dynamic protein, with variable expression over time.71 Another possible biomarker is tumor mutational burden (TMB), already shown to predict response to ICI in patients with lung cancer and melanoma.72,73 Subgroup analysis of the IMvigor 210 and Checkmate 275 trials demonstrated a positive correlation of TMB and both response to ICI and survival,25,45,71 even when adjusted for PD-L1 expression, but issues regarding optimal assessment of TMB are yet to be resolved60. The role of DNA damage repair (DDR) genes has also been studied as possible biomarkers in predicting platinum sensitivity in a retrospective study by Teo et al.,75 and documented a significant improvement in both PFS (9.3 versus 6.0 months, p = 0.007) and OS (23.7 versus 13.0 months, p = 0.006) in patients with DDR gene alterations.
Platinum-based chemotherapy rechallenge has not yet been tested in prospective trials, but due to a lack of options in further lines of treatment, it has been attempted in some instances, especially if the patient has a good performance status. A small French case series reported unexpected responses to cisplatin rechallenge after ICI in patients deemed refractory to a platinum regimen. A total of 12 patients were retrospectively assessed and results showed 1 (8.3%) CR, 7 (58.4%) PR, 1 (8.3%) stable disease, and 3 (25%) progressive disease, suggesting that maybe ICIs can restore platinum sensitivity.76 Rechallenge with a different checkpoint inhibitor combined or not with another agent after progression on immunotherapy has also been proposed as a possible treatment strategy, but to date there are insufficient data to support a formal recommendation, and prospective trials are needed to investigate this promising idea.77
Treatment re-challenge with ICIs has also gained attention as a promising strategy, demonstrating encouraging efficacy and safety in a recent systematic review, where the ORR was 22–36%, the disease control rate was 40–64%, and the mOS was 13.4–20.6 months, with <10% grade ⩾3 irAEs.78 Among the 22 studies identified for this meta-analysis, only 1 included patients with urothelial carcinoma.79 An interesting prospective trial is currently recruiting patients with mUC, with disease progression on a prior PD1/PDL1, for atezolizumab plus chemotherapy and will hopefully add important information on the topic of retreatment (Clinicaltrial.gov identifier: NCT03737123). Another phase II study has been recruiting patients with mUC refractory or ineligible to cisplatin to investigate intermittent ICI dosing, where patients with an initial disease response of ⩾10% will discontinue the ICI until they experience a ⩾20% disease progression, at which point the ICI will be restarted (Clinicaltrial.gov identifier: NCT04322643).
Conclusions
Important advances in metastatic urothelial carcinoma have been made in the past few years as a result of our better understanding of molecular biology and genomic characterization as well as large scale multidisciplinary collaborations and patient engagement. The treatment landscape has been rapidly changing, based on the positive results of many recent trials on immunotherapy, targeted agents, and antibody-drug conjugate, resulting in significant OS improvement. At the same time, we also continue to learn from well conducted negative trials. A number of studies evaluating promising therapeutic strategies are still ongoing and will hopefully provide information for some important unanswered questions and further guide treatment sequencing in advanced urothelial carcinoma (Tables 4 and 5).
Table 4.
Recruiting trials in the first line setting for advanced urothelial carcinoma.
| Study name/ID | Investigational drug | Phase | Primary end point |
|---|---|---|---|
| EV-301 (Clinicaltrial.gov identifier: NCT04223856) |
Enfortumab-vedotin | III | OS, PFS |
| LEAP-011 (NCT03898180) | Sacituzumab-govitecan | III | OS, PFS |
| NCT03967977 | Tislelizumab | III | OS |
| NCT04486781 | sEphB4-HAS + pembrolizumab | II | ORR |
| NCT04601857 | Futibatinib + pembrolizumab | II | ORR |
| AUREA (NCT04602078) |
Atezolizumab + split dose cisplatin/gemcitabine | II | ORR |
| NCT04264936 | RC48-ADC and JS001 | Ib/II | Adverse events and maximal tolerated dose |
| NCT03534804 | Cabozantinib + pembrolizumab | II | ORR |
| FORT-2 (NCT03473756) | Rogaratinib + atezolizumab | II | Dose-limiting toxicity, TRAE, PFS |
| NCT03237780 | Eribulin mesylate + atezolizumab | II | ORR, TRAE, OTR |
| GCISAVE (NCT03324282) | Avelumab + chemotherapy | II | ORR, proportion of severe toxicity |
| NCT03272217 | Atezolizumab + Bevacizumab | II | OS |
OS, overall survival; ORR, objective tumor response; PFS, progression free survival; TRAE, treatment related adverse events.
Table 5.
Recruiting trials in the second or further lines for advanced urothelial carcinoma.
| Study name/ID | Investigational drug | Phase | Primary end point |
|---|---|---|---|
| TROPiCS-04 (Clinicaltrial.gov identifier: NCT04527991) | Sacituzumab govitecan | III | OS |
| NCT03448718 | Olaparib | II | ORR |
| NCT04383067 | Adoptative cell therapy | II | OTR |
| NCT03513952 | Atezolizumab + recombinant human IL-7 | II | ORR |
| NCT03557918 | Tremelimumab | II | ORR |
| NCT03547973 | Sacituzumab govitecan | II | ORR |
| CabUC NCT04066595 | Cabozantinib | II | ORR |
| NCT03676946 | ZKAB001 (PD-L1 antibody) | I/II | DLT |
| NCT04562311 | Chidamide + immunotherapy | II | ORR |
| NCT03854474 | Tazemetostat | I/II | ORR |
| NCT03744793 | Pemetrexede + avelumab | II | ORR |
| NCT04073602 | Recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate | II | ORR |
| AVETAX-NCT03575013 | Avelumab + taxane | Ib | DLT/ORR |
| NCT03915405 | KHK2455 (IDO inhibitor) + avelumab | I | TRAE |
| NCT03606174 | Sitravatinib + immunotherapy | II | ORR |
| NCT02717156 | sEphB4-HAS + pembrolizumab | II | TRAE/ORR |
| NCT03375307 | NCT03375307 | II | ORR |
| NCT04045613 | Derazantinib + atezolizumab | Ib/II | ORR/recommended phase II dose |
| NCT04349280 | Bintrafusp Alfa | IB | ORR |
| NCT04492293 | ICP-192 (FGFR inhibitor) | II | ORR |
| NCT03473743 | Erdafinitib + cetrelimab | I/II | DLT/ORR/TRAE |
| NCT03606174 | Sitravatinib + PD-(L)1 inhibitor | II | ORR |
DLT, dose limiting toxicity; OTR, objective tumor response; ORR, objective response rate; TRAE, treatment related adverse event.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Carlos Stecca  https://orcid.org/0000-0001-9103-9119
https://orcid.org/0000-0001-9103-9119
Contributor Information
Carlos Stecca, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, Toronto, ON, Canada.
Osama Abdeljalil, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, Toronto, ON, Canada.
Srikala S. Sridhar, Professor, University of Toronto, Medical Oncologist, Princess Margaret Cancer Center, Chair, GU Medical Oncologists of Canada, 7-625 -700 University Avenue, Toronto, ON M5G 2M9, Canada.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–3077. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Overview of bladder cancer, https://www.cancer.org/content/dam/CRC/PDF/Public/8557.00.pdf (accessed 23 March, 2020).
- 4.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020; 383: 1218–1230. [DOI] [PubMed] [Google Scholar]
- 5.Seront E, Machiels JP.Molecular biology and targeted therapies for urothelial carcinoma. Cancer Treat Rev 2015; 41: 341–353. [DOI] [PubMed] [Google Scholar]
- 6.Santos L, Pereira S, Leite RP, et al. Chromosome instability and progression in urothelial cell carcinoma of the bladder. Acta Oncol 2003; 42: 169–173. [DOI] [PubMed] [Google Scholar]
- 7.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2018; 174: 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014; 507: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014; 111: 3110–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebouissou S, Bernard-Pierrot I, de Reyniès A, et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med 2014; 6: 244ra91. [DOI] [PubMed] [Google Scholar]
- 11.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014; 25: 152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzouka NA, Eriksson P, Rovira C, et al. A validation and extended description of the Lund taxonomy for urothelial carcinoma using the TCGA cohort. Sci Rep 2018; 8: 3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mo Q, Nikolos F, Chen F, et al. Prognostic power of a tumor differentiation gene signature for bladder urothelial carcinomas [published correction appears in J Natl Cancer Inst 2019; 111: 1236]. J Natl Cancer Inst 2018; 110: 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamoun A, de Reyniès A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol 2020; 77: 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding X, Chen Q, Yang Z, et al. Clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: a meta-analysis. Cancer Manag Res 2019; 11: 4171–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019; 37: 2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab govitecan, a novel antibody--drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer 2016; 14: e75–e79. [DOI] [PubMed] [Google Scholar]
- 18.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23: 4602–4608. [DOI] [PubMed] [Google Scholar]
- 19.Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006; 42: 50–54. [DOI] [PubMed] [Google Scholar]
- 20.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol 2011; 29: 2432–2438. [DOI] [PubMed] [Google Scholar]
- 21.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012; 30: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain SA, Palmer DH, Lloyd B, et al. A study of split-dose cisplatin-based neo-adjuvant chemotherapy in muscle-invasive bladder cancer. Oncol Lett 2012; 3: 855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von der Maase H, Andersen L, Crinò L, et al. Weekly gemcitabine and cisplatin combination therapy in patients with transitional cell carcinoma of the urothelium: a phase II clinical trial. Ann Oncol 1999; 10: 1461–1465. [DOI] [PubMed] [Google Scholar]
- 24.Jiang DM, Gupta S, Kitchlu A, et al. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat Rev Urol 2021; 18: 104–114. [DOI] [PubMed] [Google Scholar]
- 25.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial [published correction appears in Lancet 2017; 390: 848]. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017; 18: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell PH, Balar AV, Vuky J, et al. First-line pembrolizumab (pembro) in cisplatin-ineligible patients with advanced urothelial cancer (UC): response and survival results up to five years from the KEYNOTE-052 phase 2 study. J Clin Oncol 2021; 39(Suppl. 15): 4508. [Google Scholar]
- 28.Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020; 395: 1547–1557. [DOI] [PubMed] [Google Scholar]
- 29.Tsai TF, Lin JF, Lin YC, et al. Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway [published correction appears in Biosci Rep 2020; 40: BSR-20190362_COR]. Biosci Rep 2019; 39: BSR20190362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powles T, Gschwend JE, Loriot Y, et al. Phase 3 KEYNOTE-361 trial: pembrolizumab (pembro) with or without chemotherapy versus chemotherapy alone in advanced urothelial cancer. J Clin Oncol 2017; 35(Suppl. 15): TPS4590. [Google Scholar]
- 31.Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial [published correction appears in Lancet Oncol 2021; 22: e5]. Lancet Oncol 2020; 21: 1574–1588. [DOI] [PubMed] [Google Scholar]
- 32.Grivas P, Monk BJ, Petrylak D, et al. Immune checkpoint inhibitors as switch or continuation maintenance therapy in solid tumors: rationale and current state. Target Oncol 2019; 14: 505–525. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber RD, Old LJ, Smyth MJ.Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 34.Sridhar S.Avelumab first-line (1L) maintenance + best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma (UC): association between clinical outcomes and exploratory biomarkers. Presented at ESMO Virtual Congress, Lugano, Switzerland, 19-21September2020. [Google Scholar]
- 35.Grivas P. Avelumab 1L maintenance + best supportive care (BSC) vs BSC alone with 1L chemotherapy for advanced urothelial carcinoma: subgroup analyses from JAVELIN Bladder 100. Presented at ESMO Virtual Congress, Lugano, Switzerland, 19-21September2020. [Google Scholar]
- 36.Galsky MD, Mortazavi A, Milowsky MI, et al. Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J Clin Oncol 2020; 38: 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buti S, Bersanelli M.Avelumab maintenance for urothelial carcinoma. N Engl J Med 2020; 383: 2482–2483. [DOI] [PubMed] [Google Scholar]
- 38.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial [published correction appears in Lancet 2018; 392: 1402]. Lancet 2018; 391: 748–757. [DOI] [PubMed] [Google Scholar]
- 41.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017; 18: 312–322. [DOI] [PubMed] [Google Scholar]
- 42.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial [published correction appears in Lancet Oncol 2018; 19: e335]. Lancet Oncol 2018; 19: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 2017; 3: e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma P, Siefker-Radtke A, de Braud F, et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results [published correction appears in J Clin Oncol 2019; 37: 2094]. J Clin Oncol 2019; 37: 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan J, Cui L, Zhao X, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol 2020; 6: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haugsten EM, Wiedlocha A, Olsnes S, et al. Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res 2010; 8: 1439–1452. [DOI] [PubMed] [Google Scholar]
- 47.Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019; 381: 338–348. [DOI] [PubMed] [Google Scholar]
- 48.Janssen Products. Balversa (package insert). Horshan, PA: Janssen Products, 2019. [Google Scholar]
- 49.Sharma V, Vanidassane I.Erdafitinib in urothelial carcinoma. N Engl J Med 2019; 381: 1593–1594. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Gong Y, Saci A, et al. Fibroblast growth factor receptor 3 alterations and response to PD-1/PD-L1 blockade in patients with metastatic urothelial cancer. Eur Urol 2019; 76: 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol 2020; 38: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021; 384: 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg JE, Flaig TW, Friedlander TW, et al. Study EV-103: preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2020; 38(Suppl. 6): 441. [Google Scholar]
- 54.Starodub AN, Ocean AJ, Shah MA, et al. First-in-human trial of a novel anti-trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res 2015; 21: 3870–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alt M, Stecca C, Tobin S, et al. Enfortumab Vedotin in urothelial cancer. Ther Adv Urol 2020; 12: 1756287220980192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol. 2021; 39(22): 2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997; 15: 1853–1857. [DOI] [PubMed] [Google Scholar]
- 58.Lorusso V, Pollera CF, Antimi M, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer 1998; 34: 1208–1212. [DOI] [PubMed] [Google Scholar]
- 59.Witte RS, Elson P, Bono B, et al. Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol 1997; 15: 589–593. [DOI] [PubMed] [Google Scholar]
- 60.Sweeney CJ, Roth BJ, Kabbinavar FF, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol 2006; 24: 3451–3457. [DOI] [PubMed] [Google Scholar]
- 61.Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract [published correction appears in J Clin Oncol. 2010; 28: 182]. J Clin Oncol 2009; 27: 4454–4461. [DOI] [PubMed] [Google Scholar]
- 62.Sridhar SS, Blais N, Tran B, et al. Efficacy and safety of nab-paclitaxel vs paclitaxel on survival in patients with platinum-refractory metastatic urothelial cancer: the Canadian Cancer Trials Group BL.12 Randomized Clinical Trial. JAMA Oncol 2020; 6: 1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SK.Role of PET/CT in muscle-invasive bladder cancer. Transl Androl Urol 2020; 9: 2908–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogihara K, Kikuchi E, Watanabe K, et al. Can urologists introduce the concept of “oligometastasis” for metastatic bladder cancer after total cystectomy? Oncotarget 2017; 8: 111819–111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019; 393: 2051–2058. [DOI] [PubMed] [Google Scholar]
- 66.Reyes DK, Pienta KJ.The biology and treatment of oligometastatic cancer. Oncotarget 2015; 6: 8491–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel V, Collazo Lorduy A, Stern A, et al. Survival after metastasectomy for metastatic urothelial carcinoma: a systematic review and meta-analysis. Bladder Cancer 2017; 3: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah S, Zhang CA, Hancock S, et al. Consolidative radiotherapy in metastatic urothelial cancer. Clin Genitourin Cancer 2017; 15: 685–688. [DOI] [PubMed] [Google Scholar]
- 69.Eckstein M, Cimadamore A, Hartmann A, et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med 2019; 7: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rijnders M, van der Veldt AAM, Zuiverloon TCM, et al. PD-L1 antibody comparison in urothelial carcinoma. Eur Urol 2019; 75: 538–540. [DOI] [PubMed] [Google Scholar]
- 71.Mendiratta P, Grivas P.Emerging biomarkers and targeted therapies in urothelial carcinoma. Ann Transl Med 2018; 6: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non– small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371: 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yarchoan M, Hopkins A, Jaffee EM.Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377: 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teo MY, Bambury RM, Zabor EC, et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res 2017; 23: 3610–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gravis G, Billon E, Baldini C, et al. Unexpected response to cisplatin rechallenge after immune checkpoint inhibitors in patients with metastatic urothelial carcinoma refractory to platinum regimen. Eur J Cancer 2018; 104: 236–238. [DOI] [PubMed] [Google Scholar]
- 77.Saleh K, Khalifeh-Saleh N, Kourie HR.Is it possible to rechallenge with PD-1/PD-L1 inhibitors after progression?. Immunotherapy 2018; 10: 345–347. [DOI] [PubMed] [Google Scholar]
- 78.Yang K, Li J, Sun Z, et al. Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review. Ther Adv Med Oncol 2020; 12: 1758835920975353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Niglio SA, Girardi DD, Mortazavi A, et al. Ipilimumab challenge/re-challenge in metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors treated with cabozantinib+nivolumab (CaboNivo) or cabozantinib+ nivolumab+ipilimumab (CaboNivoIpi). J Clin Oncol 2020; 38(Suppl. 15): 5039. [DOI] [PMC free article] [PubMed] [Google Scholar]