Abstract
In recent years, markers research has focused on the structural components of cartilage matrix. Specifically, a second generation of degradation markers has been developed against type II collagen neoepitopes generated by specific enzymes. A particular effort has been made to measure the degradation of minor collagens III and X of the cartilage matrix. However, because clinical data, including longitudinal controlled studies, are very scarce, it remains unclear whether they will be useful as an alternative to or in combination with current more established collagen biological markers to assess patients with osteoarthritis (OA). In addition, new approaches using high-throughput technologies allowed to detect new types of markers and improve the knowledge about the metabolic changes linked to OA. The relative advances coming from phenotype research are a first attempt to classify the heterogeneity of OA, and several markers could improve the phenotype characterization. These phenotypes could improve the selection of patients in clinical trials limiting the size of the studies by selecting patients with OA characteristics corresponding to the metabolic pathway targeted by the molecules evaluated. In addition, the inclusion of rapid progressors only in clinical trials would facilitate the demonstration of efficacy of the investigative drug to reduce joint degradation. The combination of selective biochemical markers appears as a promising and cost-effective approach to fulfill this unmet clinical need. Among the various potential roles of biomarkers in OA, their ability to monitor drug efficacy is probably one of the most important, in association with clinical and imaging parameters. Biochemical markers have the unique property to detect changes in joint tissue metabolism within a few weeks.
Keywords: osteoarthritis, biomarkers, micro-RNA, synovial fluid, molecular phenotypes, disease progression, treatment response, treatment efficacy
Osteoarthritis (OA) is the most common joint disease in the elderly, affecting 250 million people worldwide.1 It is a major social and economic burden, with a medical cost of OA accounting for 1–2.5% of the gross domestic product of the high-income countries.2 OA is a multifactorial disease of unknown aetiology. It is considered a discontinuous phasic disease3 with slow progression altering all tissues of the affected joint. The knee is the most common site of OA with a prevalence ranging from 30% to 40% in the older population in North America followed by the hand and the hip.4 Age is the most obvious risk factor for OA as a result of cumulative exposure to various risk factors. Person-level risk factors include female sex, dietary factors and obesity, whereas joint-level risk factors associated with OA are joint loading and injury, joint shape and malalignment.5 More recently, low-grade systemic inflammation appeared as a risk factor.6
From decades, the development of treatments against OA has been slowed down by at least four sticking points. First, the diagnosis is commonly based on symptoms and usually confirmed by X-ray imaging of the joint. These criteria are somehow subjective, and when diagnosis is established, joint damage may already be significant. Thus, there is a clear need to identify quantifiable parameters, sensitive enough to detect metabolic/structural alterations early in the disease. Second, the wide range of risk factors indicate that OA is an heterogeneous pathology characterized by a diversity of clinical phenotypes that are underpinned by a number of molecular mechanisms, the endotypes. The ability to select patients with OA corresponding to the metabolic pathway(s) dysregulated and targeted by the drug tested may help to improve the demonstration of drug efficacy. Third, only a minority of patients enrolled in the OA population cohorts will progress fastly. The ability to identify at the beginning of the clinical trial, rapid progressors may increase the probability to demonstrate the efficacy of the tested drug. Fourth, the current technologies available to assess treatment response on the joint structure, radiography and magnetic resonance imaging (MRI), have both limitations including poor sensitivity and limited availability, respectively. Having access to biological parameters that can detect the changes in joint tissue metabolism within a few months and that predict the long-term clinical benefit of the drugs may thus accelerate the development of effective therapies.
The biomarkers which are molecules and/or fragments arising from the turnover of joint tissues and released into synovial fluid (SF), blood, and eventually urine have unique properties of dynamic changes, high sensitivity and easy measurement, features that may overcome some of the limitations of the current methods for OA assessment.
The objectives of this article are 1/ to describe the biochemical characteristics of conventional and more recent biological markers and 2/ analyze their associations with clinical and imaging data to assess whether they can address the current shortcomings in OA management.
Methods
In this descriptive review, we have chosen to focus our analysis mainly on soluble biochemical markers of OA. We based our selection on the manual screen of published peer-reviewed articles in English language only. The following broad search terms were included: osteoarthritis, biomarkers, collagen, cartilage, diagnosis and prognosis.
Conventional biological markers of joint tissue turnover
Conventional markers are biochemical indices which have been developed with usual biochemistry technologies (see Figure 1). Generally, the exact structure and the biological processes of their release from the tissue are not well defined. The main conventional markers have been described in detail and classified into five categories: burden of disease, investigative, prognostic, efficacy, and diagnostic, the so-called BIPED classification.7 They can be classified as markers of anabolism or catabolism even if this distinction is not always totally accurate. Cartilage turnover has been extensively studied through the formation and degradation of type II collagen which is the main organic component of the cartilage matrix. The type II collagen formation was previously evaluated with the measurement of blood levels of the propeptides at the C- and N-termini (PIICP and PIINP, respectively). These propeptides are cleaved by specific proteases when procollagen is converted to mature type II collagen. PIINP exists in two splicing alternative forms which differ by the presence (PIIANP) or absence (PIIBNP) of a 69 amino acid sequence coded by exon 2. An assay has been developed for the measurement of serum PIIANP, usually restricted to embryogenesis but reexpressed in OA cartilage.8 On the contrary, PIIBNP is mainly expressed during the formation of type II collagen in healthy adults. A second generation of serum PIIBNP assay was developed in 20149 using the electrochemiluminescence immunoassay (ECLIA) format showing an increase of sensitivity (7 times more) compared with conventional enzyme-linked immunosorbent assay (ELISA) for detecting patients with knee OA.10 Although PIIANP and PIIBNP are supposed to reflect the same metabolic process, this study found no significant correlation between PIIANP and PIIBNP when assessed in the same samples from patients with OA. Blood levels of both of them were however decreased in patients compared to healthy controls.
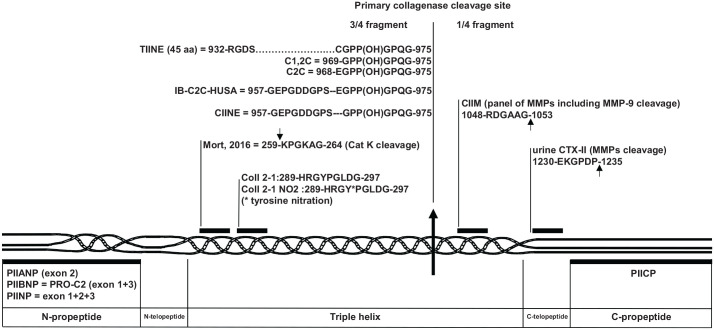
Figure 1.
Schematic localization of epitopes used as biochemical markers in human type II procollagen sequence with the numbering according to UniProtKB accession n° P02452. The arrows indicate the site of cleavage leading to the neoepitopes.
Protein nitration is a prominent feature of the inflammatory processes in the joint. Deberg et al.11,12 developed two immunoassays recognizing a peptide of nine amino acids (Coll2-1) or its nitrated form (Coll2-1 NO2). The serum levels of both peptides were significantly increased in patients with OA and rheumatoid arthritis (RA), and the ratio Coll2-1/Coll2-1 NO2 was higher in RA than in OA patients.
Finally, urinary CTX-II, a matrix metalloproteinase (MMP)-derived type II collagen fragment, is currently the most promising biological OA marker, which has been evaluated in a variety of clinical studies. It has been associated with the presence, incidence and progression of OA and with bone marrow lesions, the extend of the osteophytes and the level of pain.13
The post-translational modifications of proteins are also interesting biological markers. These include the advanced glycation end products (AGEs) such as pentosidine and the isomerization of type I collagen reflecting the aging process of collagen matrix.14
A local inflammation is often observed in OA. The conventional biological marker of systemic inflammation, serum high-sensitive C-reactive protein (hsCRP) has been shown to be elevated in the early stages of OA, but its high correlation with body mass index (BMI), a major clinical risk factor of OA, obscures its independent association with incident knee OA.15
New biological markers of joint tissue remodeling
Protein-derived biological markers
Type II collagen biomarkers
More recently, studies have been undertaken to identify the enzymes involved in the formation of the collagen neoepitopes used as second-generation biomarkers (see Figure 1). The key enzymatic event is the cleavage by the collagenases of the collagen molecule located in the three-fourth length of the triple helix where the helix appeared to be less stable. Several assays have been developed to measure the three-fourth fragment C-terminal neoepitope with a relative specificity for type II collagen (TIINE, C2C, and C1,2C).7 In 2012, Takahashi et al.16 indicated that the recognition of the specific antibody was affected by the hydroxylation of proline-971. In the OA joints, the content of hydroxyproline in newly synthesized cartilage is higher than in the normal cartilage suggesting that the measured levels of this neoepitope reflected in part the level of hydroxylation and not only the level of type II degradation. Therefore, the scientists have subsequently developed the sandwich CIINE assay recognizing the C-terminal neoepitope of collagenase-cleaved type I, II, and III collagens with similar affinity for the hydroxylated and nonhydroxylated forms. Poole et al.17 in 2016 published results with human urine sandwich assay IB-C2C-HUSA consisting in a sequence which is very similar to that of CIINE for the capture antibody, except for the amino acid E in position −8. Finally, Mort et al.18 in 2016 produced an antibody against a neoepitope generated by the cathepsin K cleavage of type II collagen, although it has not yet been used in a human assay.
On the C-terminal side of the initial collagenase cleavage, the CIIM assay has been developed in 2011 by Bay-Jensen et al.19 This neoepitope corresponds to the C-terminal end of a 36-amino acid peptide obtained by proteomics methods using mass spectrometry.20 It has been detected in vitro after human articular cartilage digestion by a panel of MMPs and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5 suggesting that it may be more abundant than other fragments shed and therefore measurable in serum. The CIIM and CTX-II are differently distributed in cartilage from OA patients suggesting that they could reflect different pathological processes. Serum CIIM levels were significantly higher in patients with mild to severe OA than in healthy controls.19
Finally, a serum version of the CTX-II assay was developed using an ECLIA sandwich format.21 The serum CTX-II levels, however, were not correlated with those of urine CTX-II in a study including 227 individuals with knee OA. Moreover, these two assays showed an opposite association with knee pain and stiffness.
Type III collagen biomarkers
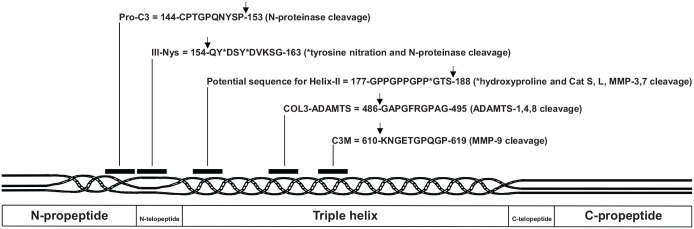
Bay-Jensen et al.22 in 2018 developed the COL3-ADAMTS assay measuring human type III collagen fragments derived from the activity of ADAMTS (see Figure 2). The epitope is located in the triple helix. The serum levels of COL3-ADAMTS were modestly correlated (r < 0.2) with the patient pain score. This correlation is independent of the radiographic disease severity confirming that joint X-ray structure abnormalities and pain are only modestly associated in OA.23 In 261 patients with primary OA, the low levels of serum COL3-ADAMTS were predictive of symptomatic radiographic OA with an odds ratio of 1.8 suggesting that COL3-ADAMTS could be useful for the identification of patients with early and potentially progressive OA.22
Figure 2.
Schematic localization of epitopes used as biochemical markers in human type III procollagen sequence with numbering according to UniProtKB accession n° P02461. The arrows indicate the sites of cleavage leading to the neoepitopes.
Three other biological markers were also previously developed to measure the type III collagen metabolism. The first one is the competitive Pro-C3 assay measuring type III collagen formation and corresponding to the C-terminal end of the N-propeptide after N-proteinase cleavage.24 No serum data are currently available in patients with OA.
The second one is the competitive C3M assay against an MMP-9 neoepitope located in the triple helix.25 It is released from the synovial membrane under inflammatory conditions26 and originally developed for the evaluation of lung and liver fibrosis in both serum and urine.27,28 Low serum C3M levels were predictive of symptomatic radiographic OA with an odds ratio of 2.5.22
Finally, the IIINys assay was developed to recognize type III collagen fragments in the N-telopeptide region in which the tyrosine residues have been post-translationally nitrated by nitric oxide (NO). Because type III collagen is a major constituent of the synovial membrane and that NO is an important mediator of oxidative damage, this assay is believed to reflect synovial tissue inflammation. The levels of serum IIINys were measured in 87 patients with painful knee OA and in 40 sex- and age-matched healthy controls. The levels of serum IIINys were 1.5-fold higher in patients with knee OA than in healthy controls and they correlated with CRP values. Interestingly the levels of serum IIINys were also markedly elevated (+207%) in patients with RA and the IIINys epitope detected by immunohistochemistry was highly expressed by the inflammed synovial tissue.29,30
Helix-II marker
The Helix-II is a biological marker that was initially developed to measure the degradation of the helical portion of human type II collagen. The antibody used to detect Helix-II fragment was based on the published sequence of human type II collagen that was available at the time of its development. Several years later, it was demonstrated—by direct sequencing of human cartilage type II collagen—that the previously published sequence was incorrect,31 therefore making the specificity of Helix-II for type II collagen questionable. The presence of 4-hydroxyproline is a key point for antibody recognition as demonstrated by Charni-Ben Tabassi et al.29 in 2005. Thus, a potential target for the Helix-II antibody is type III collagen because the GTS sequence and the 4-hydroxyproline at the P* site of 183-GPP*GTS-188 are both present in the sequence of human type III collagen. Our preliminary unpublished results indicate that the Helix-II antibody recognizes the human type III collagen sequence 183-GPP*GTS-188 (with a P* for 4-hydroxyproline), only when it is cleaved, indicating that it is a new proteolytic neoepitope for type III collagen (see Figure 2, potential sequence location). It should be pointed out that type III collagen is present in a significant amount in human cartilage matrix, its contribution to the pool of collagen molecules increasing in OA cartilage.32
From a biological point of view, however, a body of experimental data indicate that the measurement of Helix-II is a valuable marker to assess joint tissue alterations in arthritis. The urinary Helix-II levels markedly increased in 90 patients with OA of the knee (+56%) and in 89 patients with early RA (+123%) compared with 162 healthy sex- and age-matched controls.33 The serum Helix-II levels were decreased by a median of 18% (p = 0.0015) as early as 1 month after initiating anti-tumor necrosis factor (TNF) (etanercept) treatment in 29 patients with spondyloarthropathy (AS), reaching a median decrease of −33.4% (p = 0.0079) at month 12.34 It is also a very sensitive indicator of the clinical response of patients with RA treated with the anti-interleukin (IL)-6 receptor antibody tociluzimab.35 Interestingly, in patients with early RA or OA of the hip, the combined measurement of urinary Helix-II and urinary CTX-II was more effective than either marker alone to identify the patients with a rapidly destructive disease.33,36 Such independent relationship is likely to be due to the different localization of Helix-II and CTX-II in OA cartilage matrix37 and the different patterns of catabolic enzymes involved in their generation from human cartilage collagen.38 In addition, because the type III collagen is present both in synovial tissue (which constitutes the major organic component) and cartilage tissue (see above), this makes Helix-II a unique integrated biological marker of joint tissue turnover.
Type X collagen biomarkers
Type X collagen is a nonfibrillar collagen with a short triple helix, the half of the length of type I, II, and III triple helix with a globular domain NC1 at the C-terminal end and a noncollagenous domain NC2 at the amino end (see Figure 3). It is a well-established marker for hypertrophic chondrocyte differentiation with overexpression in human OA cartilage. It is found in the deep zone close to the tidemark.39 The degradation of type X collagen generates several C-terminal fragments containing the NC1 domain and variable portions of the collagenous region (several sites of cleavage in Figure 3).
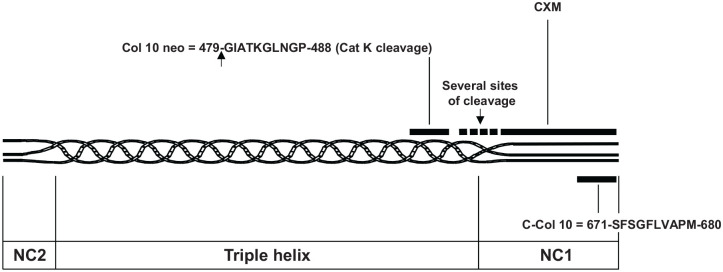
Figure 3.
Schematic localization of epitopes used as biochemical markers in human type X procollagen sequence with numbering according to UniProtKB accession n° Q03692. The arrows indicate the sites of cleavage leading to the neoepitopes. NC1 and NC2 = non-collagenous domain 1 and 2 respectively.
In 2014, He et al.40 developed a competitive assay against the last 10 amino acids of the NC1 domain called C-Col 10. In a cohort of 271 patients with knee OA, they showed significant higher levels in the serum of patients with Kellgren–Lawrence (KL) score 2 compared with patients without radiological OA. The level of serum C-Col 10 was also significantly correlated with serum levels of C2M indicating a close relationship between chondrocyte hypertrophy and articular cartilage degradation. More recently, Coghlan et al.41 used slow off-rate modified aptamers (SOMAmers) technology to select high-affinity SOMAmers for the NC1 region of type X collagen, CXM assay, although no clinical data are yet available in OA. Finally, a neoepitope within the helix portion of type X collagen (Col-10 neo) generated by cathepsin K was identified in the urine of patients with OA based on gas chromatography and mass spectrometry technology. In a small study including 142 symptomatic knee OA, 34 RA and 20 controls, the plasma levels of Col-10 neo were found to be statistically higher in patients with OA than in patients with RA and controls.42
To sum up, in the last years, a second generation of degradation markers has been developed against type II collagen neoepitopes generated by specific enzymes and a special effort has been made to measure the degradation of the minor collagen types III and X of the cartilage matrix. However, because the clinical data, including longitudinal controlled studies, are very scarce, it remains unclear whether they will be useful as an alternative to or in combination with current more established collagen biological markers to assess patients with OA.
Total cartilage oligomeric matrix protein (COMP), D-COMP, and COMP neoepitope
COMP is a noncollagenous protein of articular cartilage. The serum COMP levels have been associated with the diagnosis, burden of disease and prognosis of OA disease according to the BIPED classification.7 Kluzek et al.43 in 2015 demonstrated that serum COMP levels were predictive of incidence of painful OA and structural changes in 593 women without baseline OA followed for 20 years. D-COMP is a desaminated form of COMP protein. The serum levels of D-COMP were associated with the severity of hip OA but not with knee OA. In contrast, the serum levels of total COMP were associated with knee but not hip OA suggesting that D-COMP may be joint specific,44 for yet unclear reasons. Finally, in 2017, Lorenzo et al. developed two automated assays against total COMP and COMP neoepitope-S77. The ratio of these two assays could distinguish between progressors and nonprogressors for patients with RA.45
Active enzymes as biomarkers
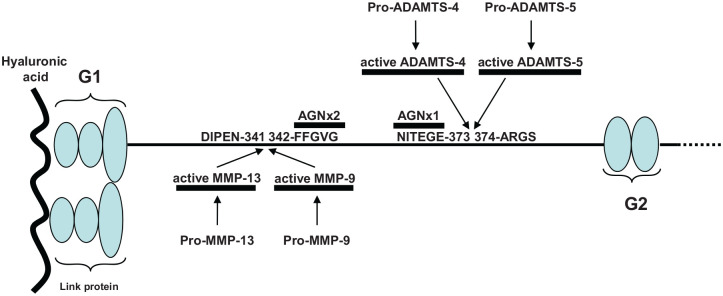
During the last years, several assays have been developed to measure the concentrations of the active enzymes potentially involved in the OA disease, particularly the active forms of cathepsin K and MMP-3 (see Figure 4).46,47 Both enzymes are synthesized as proenzymes with a signal peptide cleaved during the activation process and generating proteolytic neoepitopes recognized by specific antibody. With the same approach, the team of Dr Bay-Jensen (Nordic Biosciences, Denmark) focused on the active enzymes able to degrade the aggrecan molecules in the interglobular domain. They developed specific assays for the active forms of ADAMTS-4, ADAMTS-5, MMP-3, and MMP-9.48 They have previously developed assays against the aggrecan neoepitopes NITEGE (AGNx1) and FFGV (AGNx2) generated by ADAMTS-4/5 and MMPs, respectively.49,50
Figure 4.
Schematic localization of epitopes used as biochemical markers in the interglobular domain of human aggrecan sequence with numbering according to UniProtKB accession n° P16112. and enzymes involved in its degradation. The arrows indicate the sites of cleavage leading to the neoepitopes. G1 = globular domain 1; G2 = globular domain 2.
Because enzymes are widely distributed in the body, it is expected that such markers will lack specificity for the joint tissues when measured in the serum. In addition, because the circulating concentration of active enzymes is very low, the sensitivity of such biomarker is likely to be limited. Clearly, clinical data on the associations of active enzyme concentration and OA are needed.
Periostin (POSTN) as an OA biomarker
POSTN is a secreted protein synthesized by osteoblasts and chondrocytes participating in the cross talk between subchondral bone and cartilage facilitated by alteration of the tidemark in OA disease. In a prospective study in postmenopausal women, serum levels of human POSTN at baseline were significantly lower in those with prevalent knee OA and in OA progressors than in controls. For each increase of one POSTN quartile, the risk of progression decreased by 0.82 after adjustment for age and for OA at the other anatomical sites.51 In a second study, plasma POSTN levels were not significantly different between patients with knee OA and controls, but this study was cross-sectional and POSTN was evaluated with a different assay.52
In summary, during the last three decades, a large number of markers derived from proteins or fragments thereof have been developed. However, only a few have been evaluated in several well-characterized cohorts. To analyze their potential clinical utility in the management of OA, these new assays need to be improved technically, especially in terms of robustness and reproducibility. Then, these assays can be distributed widely to be evaluated independently by several laboratories and clinically validated in larger prospective studies.
MicroRNAs
Epigenetic is a field of genetics where variations in the cell phenotype are considered to result from external factors that regulate gene activity. MicroRNAs (miRNAs) could play a key role in OA because several of them are regulators of genes involved in cartilage development, homeostasis and OA pathology.53 Several in vitro and in vivo studies have demonstrated miR involvements in the OA onset and progression, and until now, 46 miRNAs have been reported to be associated with OA.54,55 For example, our team recently showed that serum miR-146a-5p was increased in postmenopausal women suffering from mild to moderate OA compared with healthy controls from the same population-based cohort. Importantly, serum miR-186 is also increased in those women who will develop radiographic knee OA over the next 4 years, therefore it could have the ability to detect preclinical knee OA.56 Last year, Skrzypa et al.57 observed that miR-146-5p was significantly upregulated in the cartilage of patients with OA and miR-146-5p serum levels were increased compared with healthy controls, confirming the clinical utility of this miR as a biomarker for OA management. In 2020, Chao et al.58 showed that the synovial fluid levels of miR-140 and miR-199 were downregulated in the patients with OA compared with controls and that they were negatively correlated with the progression of OA disease. This indicates that miR-140 and miR-199 might affect the expression levels of relevant metalloprotease and cytokines by regulating MMP-3 and IL-1β mRNA expression. Finally, the long noncoding RNA (lncRNA; length of more than 200 nucleotides) can act as a miRNAs sponge and regulates their expression. It was possible to predict the level of pain in patients with OA based on the expression profiles of eight lncRNAs with an abnormal expression pattern.59
To sum up, epigenetics has significantly expanded our knowledge on the molecular mechanisms involved in the pathogenesis of OA disorders. Some miR signatures have been established in a few small-size studies, but the replication in larger studies is awaiting. More research, including replication data in large prospective longitudinal studies that establish this novel type of biomarker as a valuable tool to improve the prognosis of OA, is required.
Measurements of biological markers in SFs: opportunities and challenges
Several studies have analyzed the association between the SF levels of biological markers and OA. They mostly involved inflammatory parameters which have shown a positive correlation with disease severity evaluated by X-ray of the targeted joint.60–75 Interestingly, such associations could not be observed when the same markers were measured in the serum, suggesting that SF measurements may be more sensitive than systemic assessments likely due to the lower specificity for articular metabolism.
The undercarboxylated matrix Gla protein (ucMGP) belonging to the vitamin K–dependent family showed a negative correlation with the OA severity when measured in the SF, and the serum levels were decreased in the patients with knee OA.76 Conversely, the SF levels of the upper zone of growth plate and cartilage matrix associated (UCMA) protein were positively correlated with the OA severity, in the agreement with the increased serum levels.77
SF levels of markers have also been evaluated to OA in longitudinal studies. In 132 patients with knee OA, the TSG-6 activity levels at baseline, a hyaluronan-binding protein associated with inflammation, were predictive of progression at 3 years.78 Ritter et al.79 in a study of 173 patients with knee OA used selected reaction monitoring (SRM) to detect peptides from clusterin and lubricin in the SF and plasma which were predictive of OA progression assessed by radiographs over 30 months. This year, the SF levels of elastase and transforming growth factor (TGF)-β1 have been reported to predict the progression of OA assessed by total joint replacement (TJR) in 39 patients with knee OA. These data suggest that there may be a synergetic role between neutrophil and macrophage populations in the pathogenesis of OA.80
Finally, in recent years, the proteomic and metabolomic analyses were conducted in small studies enrolling at best only a few dozen OA patients. These studies highlighted several peptides or metabolites in SF to be modified in patients with OA.79,81–88
In conclusion, SF measurements of biological markers make feasible a more direct assessment of joint tissue metabolism than serum/urinary assessments. This seems to be particularly interesting for inflammatory cytokines/chemokines which are parameters that are not joint tissue specific and thus lack sensitivity when measured into the circulation. However, SF analyses are somehow challenging because this requires an invasive procedure which is not accepted by all patients and difficult to be applied in large OA studies, including clinical OA trials of novel therapies. In addition, the reproducibility of these measurements is also limited because standardizing collection of SF and adjustments for variability of SF volume is difficult. Consequently, it appears that SF may be particularly useful in small early phase studies to investigate the mechanisms of action of novel therapies and give some indications of their biological efficacy.
Biomarkers to segregate OA patients in molecular endotypes
During the last years, an important advance in the characterization of OA disease was to try to classify patients into several clinical phenotypes. A phenotype corresponds to a group of patients with common observable characteristics of a disease such as morphology, development, biochemical or physiological properties, or behavior.89 Theoretically, a phenotype is underpinned by a specific endotype, that is, the dysregulation of a molecular pathway explaining its observable properties. However, because OA is a complex disease, a phenotype can be the clinical result of several dysregulated pathways. Currently, there is no consensus on the number and definition of endotypes and phenotypes identified by the different research groups working on this topic.89–91
Inflammation phenotype
The inflammation phenotype represents between 16% and 30% of patients with knee OA according to the studies.90 The inflammation in OA is a relatively low-grade process in comparison with degree observed in RA or spondyloarthritides. A meta-analysis of 32 studies showed that the systemic inflammation evaluated with serum hsCRP was modestly but significantly higher in patients with OA compared with controls with a significant heterogeneity between studies92 suggesting that CRP has a limited value in monitoring inflammation in OA. In contrast, C-reative protein metabolite (CRPM) is a degraded product of CRP released by MMP and supposed to reflect the chronic inflammation in contrast to the acute phase reaction measured by circulating CRP. Siebuhr et al.25 in 2014 found in a cohort of 342 patients with SKOA that serum CRP selected a subpopulation of patients with an acute inflammed OA (18%) which was different from patients identified with high serum CRPM levels (19%) with only a small overlap of 18% between the two groups. Consequently, one may speculate that only a fraction of patients presenting with an inflammation phenotype may benefit from anti-inflammatory drugs. In the Cohort Hip and Cohort Knee (CHECK) cohort of patients with an early OA phenotype and pain in the knee and/or the hip followed for 10 years, principal component analysis showed that serum C3M and serum CRPM levels may reflect different distinct inflammatory domains compared with serum C1M (a marker measuring type I collagen degradation), erythrocyte sedimentation rate, and hsCRP.93
Finally, in a study including 99 patients with erosive and non-erosive hand OA, Fioravanti et al.94 in 2018 found higher serum visfatin levels in erosive hand OA compared with nonerosive hand OA. Visfatin has proinflammatory and immunomodulation functions contributing to cartilage degeneration.
Metabolic syndrome phenotype
In 55 patients with knee OA, it were shown that a set of MMPs (1, 7, 8, 9, 10, and 12) measured in the SF were significantly increased in patients with diabetes compared with OA patients without diabetes or healthy controls.95 Another study including 952 middle-aged women with knee and hand OA demonstrated that metabolic syndrome (MS) was strongly associated with painful interphalangeal hand OA but not with knee OA once BMI was taking into consideration. This result suggested that the specific metabolic pathways may affect different joints.96
Sex and stage of OA phenotype
Using liquid chromatography–mass spectrometry technology to analyze the post-mortem SF metabolome of 75 healthy, early or late donors with OA, Carlson et al. highlighted stage-dependent phenotypes driving differences in symptoms. Two phenotypes for early OA were identified with increased inflammation or structural deterioration, and two in late OA, with inflammation associated with oxidative stress and structural degradation products.87 Significant sex-dependent differences in OA cytokine profiles from plasma may also reflect distinct pathogenesis of knee OA.97
Bone phenotype
Some evidence suggests that subchondral bone changes occur with OA progression.98 By using radiographic features to identify OA phenotypes, Kinds et al.99 classified 24% of 417 patients with knee or hip OA from the CHECK cohort as belonging to the bone-specific phenotype. In a study enrolling 149 patients with a symptomatic and radiographic OA phenotype, it was shown that the, alpha-CTX-I epitope, a marker of degradation of newly synthsized type I collagen, was localized in the areas of subchondral bone tissue with high turnover, while urinary levels were associated with increased subchondral bone turnover measured by bone scintigraphy and progression of osteophyte and joint space narrowing.100 In the same study, the CTX-II epitope was localized at the bone–cartilage interface and urinary CTX-II levels were associated with joint space narrowing. The coupling of cartilage and bone metabolisms suggests that the patients who will benefit the most from treatments targeting the bone metabolism may include those with a high bone turnover status.
Pain phenotype
Pain is a predominant symptom in OA. Observational studies suggested that pain is associated with a number of structural features, mainly the presence of bone marrow lesions and synovitis.101 However, cartilage loss and pain are poorly correlated102 even although recent data suggest that pain and joint destruction seem to be linked to some cartilage metabolites generated by the ADAMTS-5 enzyme.103 Later studies suggested that at least two different profiles of soluble markers are associated with pain. In 109 patients with symptomatic knee OA, SF TNFα levels were associated with the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index pain score and the pain at rest, whereas SF IL-6 and IL-8 were correlated with the pain on movement.104 Levels of CTX-II measured either in the SF or urine samples were associated with the radiographic severity but not with knee pain. On the contrary, a post hoc analysis of 1241 patients with radiographic OA and presence of pain showed that urinary CTX-II was associated with weight-bearing pain but not with non-weight-bearing pain.105 Finally, the serum COL3/ADAMTS was associated with the pain score in a study including 261 patients with different degrees of knee pain based on their VAS (visual analog scale) pain score.22
Several other phenotypes have been suggested, but the involvement of current soluble markers to highlight them seems more limited. The effects of a potential drug would be easier to investigate if the clinical trial included specifically patients with a phenotype corresponding to the molecular pathway, their endotype, targeted by the drug. This approach is rarely used for the development of new molecules, only 6.4% of clinical trials all disciplines combined used such strategy. However it was shown in the same survey that using biomarkers to select patients raises the likelihood of approval from Phase I to commercialisation by 50% (15.9% vs 7.6% with no biomarker selection).106 For OA, the success of such strategy is highly dependent on the collaboration between the pharmaceutical laboratories and the biomarker companies to customize the development of specific assay(s) reflecting the metabolic pathway(s) targeted by the tested molecule.
Clinical uses of biomarkers in OA
Prediction of disease progression
A prognostic marker is a baseline characteristic that predicts the risk of disease progression concerning a predefined clinical endpoint.
Early-stage OA
Serum COMP, hyaluronic acid (HA), and type I collagen cross-linked N-telopeptides (NTX-I; a marker of bone resorption) have been reported to be increased in early OA in some, but not all, studies.7,107,108 Several studies enrolled patients without radiographic evidence of OA to study an early stage of OA even if ‘early’ is incorrect due to the large period of silent OA. Legrand et al.109 developed diagnostic algorithms to select 28 patients with an early OA and found that plasma glucosepane, a lysine–arginine protein cross-linking product, was increased in early-stage OA (+38%) and by sixfold in advanced OA compared with healthy controls. In 225 patients with early knee OA, mass spectrometry analysis associated with a machine learning analysis identified a combination of plasma glycated, oxidized, and nitrated amino acids which when combined with urinary hydroxyproline and anti-cyclic citrullinated peptide (CCP) status was able to detect patients with early OA.88 In a study including 47 preradiographic OA patients, CCL3, a chemokine promoting macrophage migration, could be a potential serum biomarker for knee OA with the ability to detect preradiographic OA patients, although there was a significant difference of age between early OA patients and healthy individuals.110 Liem et al.111 in 2020 showed that urinary CTX-II may be useful in early diagnosis of OA in symptomatic patients without radiographic evidence of OA. In the same study using the Osteoarthritis Initiative (OAI) database, Coll2-1NO2, CS846, COMP, and urinary CTX-II, when combined, provided additional predictive power over and above the established demographics predictors of OA such as age, sex, BMI, and race. Lazzarini et al.112 in 2017 also used a machine learning method to construct five predictive models for knee OA incidence in overweight or obese women, including serum C2M, urinary Coll2-1 NO2, and serum C1M. In 2019, a specific panel of serum autoantibodies was detected at baseline in 327 participants developing an incident radiographic knee OA during a 96-month follow-up period.113 Recently, 52 clinical, biological and high-resolution radiomic markers were combined using machine learning algorithms to detect temporomandibular joint (TMJ) OA status. Interestingly, although the expression levels of individual serum and saliva protein markers were similar between controls and TMJ OA patients, the integrative prediction model had an accuracy of 0.823 illustrating the importance of combining markers of various origins to improve the diagnosis of OA.114
OA progression
In our previous review paper published in 2012, we concluded that among the markers developed at that time, serum HA was consistently associated with the radiologic progression in both knee and hip OA.7 Urinary CTX-II and serum COMP appeared also to predict progression when the studies included a relatively large sample size with a precise assessment of progression. In addition, the combination of two biomarkers, urinary CTX-II and serum PIIANP reflecting different pathways of type II collagen metabolism, was more effective for predicting disease progression than one marker alone in knee or hip OA.115
Markers of type I collagen
Urinary alpha-CTX-I, a marker of newly formed type I collagen matrix degradation, predicted knee OA progression in a cohort of 149 participants with symptomatic and radiographic knee OA followed for 3 years. Urinary levels of alpha-CTX-I correlated with joint space narrowing and osteophyte score whereas urinary CTX-II was associated with osteophyte progression only.100 In 2013, 128 patients with chronic knee complaints were followed-up for over 6 years. Serum PINP, a marker of collagen type I collagen formation, had a predictive value for knee OA progression, especially for progressive osteophytosis.116 However, Teirlinck et al.117 in a review of 57 papers found no significant association between radiological progression and CTX-I, COMP, NTX-I, PINP or PIINP.
Markers of type II collagen
A large study enrolling 3582 individuals from three cohorts concluded that serum C2M and urinary CTX-II levels were associated with the risk of progression and the incidence of knee OA.13 In 2016, Poole et al.17 conducted a study with 253 subjects having knee pain. They showed that the IB-C2C-HUSA levels in urine at baseline were higher in progressors compared with nonprogressors and were associated with progression of cartilage degradation over the next 3 years. This year, Luo et al. in a study enrolling 253 patients with knee OA participating in a randomized clinical trial testing the efficacy of calcitonin showed that patients with low levels of baseline serum/plasma PRO-C2 were more likely to progress compared with those with high levels. Thus, low cartilage formation based on PRO-C2 serum levels appears to be a quantifiable OA endotype associated with the OA progression and the response to calcitonin treatment.10
Noncollagenic proteins and metabolic mediators
Serum COMP levels appeared as a useful indicator of patients at risk of rapid progression in a study enrolling 150 patients with knee OA.118 A meta-analysis conducted by Hao et al.119 confirmed that serum COMP has a potential utility in predicting OA progression. In serum, a new assay for aggrecan ARGS fragments which was more precise and reproducible than previous versions was developed. Using this new test, it was shown that low levels of serum ARGS provided an odds ratio of 3 for the identification of fast radiographic progressors over 2 years in a cohort including 145 patients with knee OA.120 The marker of tissue inflammation, serum CRPM, predicted the risk of OA progression independently of urinary CTX-II and serum COMP in 1335 participants of the Rotterdam study.121 A panel of inflammatory markers have been evaluated in plasma in a 2-year prospective analysis of radiographic progression including 183 patients with SKOA. Plasma levels of IL-1Ra were modestly associated with the severity and the progression of SKOA independently of other risk factors.122 In 2018, Huang et al.123 showed in a study of 431 patients with knee OA that the baseline and time-integrated concentrations of plasma lipopolysaccharide and soluble Toll-like receptor 4 (TLR4) were associated with knee OA progression over 16–18 months.
Combination of soluble markers
In 69 plasma samples from patients with knee OA, surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF) analysis identified three potential markers, apolipoprotein C-I, C-III, and an N-terminal truncated form of transthyretin, with peak intensities significantly different between progressors and nonprogressors.124 In the plasma of 173 patients with knee OA, SRM assays were developed to measure tryptic peptides representative of 23 proteins. Peptides from clusterin, lumican, and lubricin showed significant associations with joint space narrowing after age and sex adjustment.79 In 2017, Kraus et al. found that eight biomarkers significantly predicted pain and structural worsening of OA in 194 patients with knee OA followed during 48 months participating in the multicenter national institutes of health (NHI)-funded OAI study. The most predictive model included the time-integrated concentrations of urinary CTX-II, serum HA, and serum NTX-I.125 A study enrolling 44 overweight and obese adults from the IDEA cohort followed for 18 months found a metabolic profile including glycolate, hippurate, and trigonelline that was able to discriminate between progressors and nonprogressors suggesting that metabolite measurements could be useful to predict progression.126 In 2019, Hsueh et al.80 showed that SF levels of elastase and TGF-β1 alone or combined strongly predicted the risk of OA progression in a small study including 39 patients followed for 3 years. Finally, in serum, an autoantibody signature was discovered at baseline in 327 individuals developing incident radiographic knee OA during a 96-month follow-up period.113
Combination of soluble markers with markers from others approaches
In a study including three independent cohorts and a total of 339 patients, plasma IL-1b, TNF-α and Cox-2 mRNA in peripheral blood leukocytes predicted higher risk for radiographic progression evaluated by joint space narrowing.127 This year, Hunter et al. investigated the optimal combination of MRI, radiographic, and biochemical biomarkers to predict knee OA progression in a cohort of 600 participants with at least one knee with a frequent pain and a KL grade 1, 2 or 3 at baseline. They found that a model including 24-month changes of urinary NTX-I and selected MRI markers (effusion-synovitis, meniscal morphology, cartilage damage, central medial femoral cartilage thickness, medial tibial cartilage volume, lateral patellofemoral bone area) together with a radiographic marker (horizontal trabecular bone texture) and pain progression at 48 months was the most effective. Although urinary CTX-II was the strongest biochemical predictor in univariate analysis in this cohort, it did not contribute significantly to prediction models including MRI markers suggesting a collinearity with these two diagnostic markers.128 Such combined approach confirmed results of a previous study testing different models to predict moderate to severe OA progression over 8 years. Adding MRI biomarkers significantly improved the prognostic ability compared with clinical and radiographic characteristics only.129 A machine learning approach in a study of 600 individuals with knee OA found that among the 76 baseline parameters tested including demographic, imaging, and biochemical variables, baseline variables contributing to progression at 48 months included bone marrow lesions, osteophytes, medial extrusion and urinary CTX-II.130 A very interesting approach was conducted by Sofat et al. in a group of 130 participants with advanced and mild knee OA. They followed up the pain rather than the structural evolution and showed that urinary CTX-II combined with MRI parameters can be used to track the progression in painful knee OA.131 Finally, Martel-Pelletier investigators using an automated machine learning patient and sex-based model identified three baseline serum biomarkers [ratios CRP/monocyte chemoattractant protein-1 ratios CRP/-1 and leptin/MCP-1] and two clinical risk factors (age and BMI) as the most important variables for the prediction of strutural progression.132,133
Biomarkers to predict TJR
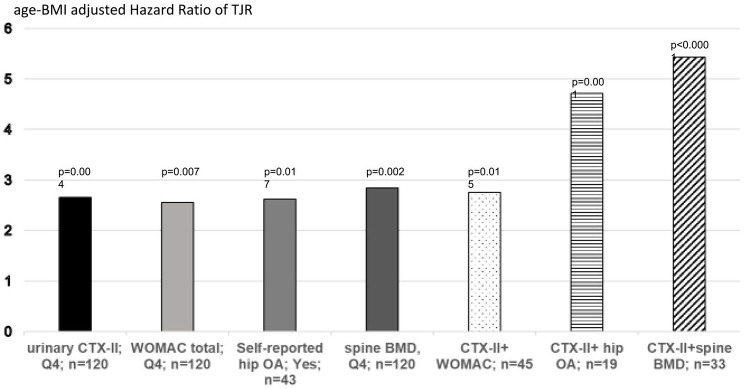
In 2019, Bjerre-Bastos et al. showed in a cohort of 676 patients with knee OA that high baseline serum CTX-I was significantly associated with a 3.4 times higher risk of arthroplasty of the knee or hip but did not reach significance for the risk of knee arthroplasty alone. In this study, the urinary CTX-II levels at baseline were associated with increased risk (3.08) of undergoing replacement surgery of the knee or hip during the 2-year study period.134 The anti-Nerve growth factor (NGF) antibody tanezumab is associated with a strong inhibition of pain in preclinical and clinical studies but, when combined with chronic nonsteroidal anti-inflammatory drug (NSAID) treatment, there is a small subset of patients that has a high risk of rapid progressive OA (RPOA).135 In a randomized placebo-controlled clinical study of tanezumab including 174 patients who experienced TJR and 321 who did not, Arends et al.136 in 2017 found two biomarker combinations that can identify patients who remained free of TJR. Conversely, Karsdal et al.137 in 2019 identified two biomarker phenotypes by classification and regression tree (CART) analysis detecting patients with a significant higher risk of developing RPOA in a clinical trial of tanezumab although the number of patients with RPOA was very limited. Clearly, such biomarker phenotypes should be validated in larger clinical studies. In a prospective population-based study including 478 apparently healthy postmenopausal women from the OFELY cohort followed for a median of 17.8 years, urinary CTX-II levels were an independent risk factor of TJR; women with high levels of CTX-II (above the 95 percentile of healthy premenopausal women) having a risk multiplied by 2.45 compared to all other subjects after adjustment for age, BMI, hip bone mineral density (BMD), and the WOMAC Index.138 Its predictive value was of the similar magnitude to the WOMAC score, self-reported hip OA and spine BMD. Interestingly, women with both a high level of CTX-II and self-reported hip OA or spine BMD had a 4.75- and 5.4-fold higher risk of TJR than the other women, respectively (Figure 5). The value of urinary CTX-II to predict TJR was confirmed in a study enrolling 1255 patients with knee OA participating in two randomized clinical trials when combined with age, sex, BMI, and KL grade.139
Figure 5.
Age-BMI adjusted Hazard Ratio (HR) of baseline risk factors and combination for predicting the risk of incident total joint replacement (TJR) in postmenopausal women. Women were categorized in quartile of CTX-II, WOMAC score, spine BMD or by the prevalence of self-reported hip OA. The HR for incident TJR and p values for levels in the highest quartile (Q4) vs 3 lowest quartiles (or presence vs absence of hip OA) was calculated by COX analysis. The number of subjects at risk for each parameter is mentioned.
Nonsoluble biological markers
Soluble biochemical markers are not the only biomarkers to be useful in OA management. The genomic (IL-1β, ASPN, COMP gene expression, and mitochondrial DNA variants) and imaging approaches [bone area and three-dimensional (3D) shape, subchondral bone texture, trabecular bone texture, cartilage thickness, quantitative MRI and effusion-synovitis and infrapatellar fat pad intensity signal] have brought attractive data to detect OA progressors.140–148 Finally, the preradiographic structural pathology can be detected by MRI parameters including ligamentous degeneration, effusion/synovitis, and meniscal pathology.149
Currently, the patients included in clinical trials are selected on conventional clinical and radiographic baseline features. These inclusion criteria are unable to distinguish with enough accuracy rapidly from slowly progressive OA patients. The rapid progressors constitute the optimal target population to demonstrate the effect of a treatment aimed at slowing the progression of the OA disease in a reasonable time frame. This implies that investigators have to include a large number of patients to demonstrate a significant difference in joint space narrowing between the active and placebo groups because the proportion of OA patients with radiographic progression over a 2-year interval ranging from 6% to 20%.150 The combination of soluble biomarkers together with other approaches may thus represent a promising and cost-effective strategy to fulfill this unmet clinical need. This selection strategy needs, however, to be validated in adequately prospective studies in which patients are selected according to a multiparameter algorithm before randomization in the clinical trial.
Prediction of treatment response
Because not all patients respond to a particular therapy and/or will present with safety issues, it would be highly valuable to identify, before initiating a treatment, which patients are likely to benefit from the drug or are at a higher risk of deleterious effects. Although some clinical and radiological parameters have been shown to have some predictive value, clearly they are not sensitive and specific enough to fulfill this role. Thus, the biological markers have been suggested to have a complementary utility. However, there is no therapy available that has been shown to effectively decrease bone or cartilage deterioration or reverse any of the existing structural defects in properly and replicated clinical trials. Consequently, this hypothesis is currently difficult to test. There is, however, some evidences from retrospective analyses of prospective clinical trials suggesting that biochemical markers of joint tissue turnover may help. From a theoretical point of view, classifying patients in different endotypes could segregate patients who are more likely to respond to a given drug, for example, the patients with high bone turnover for an antiresorptive agent. Meanwhile, such a hypothesis has been evaluated by analyzing retrospectively the relationships between preoperative levels of biomarker and clinical efficacy of TJR. For example, a recent study showed that among 754 patients with OA undergoing TJR, those with increased serum COMP levels had a larger decrease of the WOMAC stiffness index compared with those with lower values.151 In terms of safety issues, a recent exploratory retrospective analysis of the anti-NGF tanezumab trials showed that a combination of some biomarkers may be useful to identify patients with knee OA who are at a high risk of rapidly destructive OA and/or TJR.137
Monitor treatment efficacy
Among the various potential roles of biomarkers in OA, their ability to monitor drug efficacy is probably one of the most important, in association with clinical and imaging parameters. Biochemical markers have the unique property to detect changes in joint tissue metabolism within a few weeks. The issue is then whether the early changes in biochemical markers are predictive of long-term effects of treatment on joint structure, for example, changes in X-ray joint space width/MRI cartilage volume or TJR. As indicated previously, in the absence of approved structural OA treatment, there is no prospective longitudinal study that could evaluate this hypothesis. Conversely, several retrospective, often not randomized, nor properly controlled, studies showed that some biological markers decrease within a few weeks/months with various therapies, including glucosamine (serum CTX-II and YKL-40),152 clodronate (serum COMP),153 and alendronate (urinary CTX-II and NTX-I).154 More recently, the efficacy of novel therapies tested in randomized placebo-controlled clinical studies is becoming available. For example, phase II and phase III studies using the antiresorptive agents, risedronate,155 calcitonin,156 and strontium ranelate,157 showed an early significant decrease of urinary CTX-II, despite any significant demonstrable effects on X-ray progression, except for strontium ranelate on spinal OA (although evaluated retrospectively in a population of postmenopausal with osteoporosis). Whether this discrepancy between the biochemical response and the lack of structural efficacy is due to a true inability of markers to predict progression with these treatments, inadequate inclusion criteria to recruit subjects with endotypes susceptible to respond to such treatment (see above) or a poor sensitivity of the imaging techniques used to assess progression within the duration of the study remains unclear. Fibroblast growth factor (FGF)-18 (sprifermin), a potent cartilage anabolic agent, has shown favorable effects on cartilage tissues assessed by MRI.158–160 Although the effects of FGF-18 on biochemical markers in clinical studies are not yet available, this compound has shown to be highly efficient in increasing PIIBNP, a biological marker of type II collagen synthesis, in an in vitro system of human cartilage explants.161,162 One of the largest randomized placebo-controlled studies analyzing the relationships between the changes of biomarkers and the changes in joint space width by radiography are the phase III trials of the OA risedronate program. Although the overall effect of the study on joint structure was negative, it was shown that among subjects randomized to risedronate, those who started the treatment with an increased level of urinary CTX-II and who normalized this marker after 6 months, there was a significant 40% lower risk of radiological progression at 24 months compared with patients who did not normalize urinary CTX-II.163 Thus, monitoring the early changes in markers may help to predict long-term structural efficacy, an hypothesis that needs to be confirmed in prospective studies with an effective drug.
The analysis of above reviewed clinical data shows that biochemical markers (most probably combined with other approaches in sophisticated models) may become a cornerstone in evaluating the efficacy of novel treatments. However, this concept is currently based on partial and inconclusive data. We do have multiple marker assays available, may be too many. The lack of treatments developed on strong biological bases with substantial clinical efficacy evaluated in several clinical trials is currently the main factor which impairs the efficacy of this approach. In the meantime, what we could do in the OA biomarker research community is to improve assay performances which need to be cross-validated in different laboratories in order to identify a panel of selected promising biological markers to be ready for their use as efficacy endpoints when the drug(s) will become available.
Conclusion and research agenda
It is increasingly difficult to synthesize the data on OA biological markers because of the large heterogeneity in reporting data, including various types of markers, with results being somehow inconsistent from study to study. This situation illustrates a lack of coordination between the different players in this research area. To advance this field and establish a validated and regulatory-approved predictive model, it is critical to have a global and multidisciplinary approach.
The most important topics to drive the future research can be based on the gaps in the performances of current markers and the needs arising from the clinical studies and clinicians. These may include the following:
- from a biological endpoint, increase the basic research to discover the critical metabolic pathways responsible for OA progression. Those should then constitute the basis of both drug and biomarker target identification.
- improve the technical performance of the assays and making them available worldwide to allow cross-validation studies.
- increase the use of ‘omics’ technologies to discover and characterize new markers and combine them with the more traditional markers to improve the diagnosis of OA and the monitoring of treatment efficacy.
- promote partnerships between the academic and private actors to assess the utility of markers and test the predictive models in large and well-characterized prospective studies.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jean-Charles Rousseau  https://orcid.org/0000-0002-3755-2891
https://orcid.org/0000-0002-3755-2891
Contributor Information
Jean-Charles Rousseau, INSERM Unit 1033, Pavillon F, Hôpital E. Herriot, 5 Place d’Arsonval, 69437 Lyon Cedex 03, France; Biochemical Marker Assay Laboratory for Clinical Research (PMO-Lab), Lyon, France; INSERM 1033, Lyon, France.
Roland Chapurlat, Biochemical Marker Assay Laboratory for Clinical Research (PMO-Lab), Lyon, France; INSERM UMR 1033, Lyon, France; Université de Lyon, Lyon, France; Hôpital Edouard Herriot, Hospice Civils de Lyon, Lyon, France.
Patrick Garnero, Biochemical Marker Assay Laboratory for Clinical Research (PMO-Lab), Lyon, France; INSERM UMR 1033, Lyon, France.
References
- 1.Hunter DJ, Bierma-Zeinstra S.Osteoarthritis. Lancet 2019; 393: 1745–1759. [DOI] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 3.Sharif M, Kirwan JR, Elson CJ, et al. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum 2004; 50: 2479–2488. [DOI] [PubMed] [Google Scholar]
- 4.Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014; 73: 1659–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen KD, Golightly YM.State of the evidence. Curr Opin Rheumatol 2015; 27: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sellam J, Berenbaum F.Is osteoarthritis a metabolic disease? Joint Bone Spine 2013; 80: 568–573. [DOI] [PubMed] [Google Scholar]
- 7.Rousseau J, Garnero P.Biological markers in osteoarthritis. Bone 2012; 51: 265–277. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau JC, Zhu Y, Miossec P, et al. Serum levels of type IIA procollagen amino terminal propeptide (PIIANP) are decreased in patients with knee osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 2004; 12: 440–447. [DOI] [PubMed] [Google Scholar]
- 9.Gudmann NS, Wang J, Hoielt S, et al. Cartilage turnover reflected by metabolic processing of type II collagen: a novel marker of anabolic function in chondrocytes. Int J Mol Sci 2014; 15: 18789–18803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo Y, He Y, Reker D, et al. A novel high sensitivity type ii collagen blood-based biomarker, PRO-C2, for assessment of cartilage formation. Int J Mol Sci 2018; 19: 3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deberg M, Labasse A, Christgau S, et al. New serum biochemical markers (Coll 2-1 and Coll 2-1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 2005; 13: 258–265. [DOI] [PubMed] [Google Scholar]
- 12.Deberg MA, Labasse AH, Collette J, et al. One-year increase of Coll 2-1, a new marker of type II collagen degradation, in urine is highly predictive of radiological OA progression. Osteoarthritis Cartilage 2005; 13: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 13.Valdes AM, Meulenbelt I, Chassaing E, et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage 2014; 22: 683–689. [DOI] [PubMed] [Google Scholar]
- 14.Pavelka K, Forejtova S, Olejarova M, et al. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthritis Cartilage 2004; 12: 277–283. [DOI] [PubMed] [Google Scholar]
- 15.Sowers M, Jannausch M, Stein E, et al. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage 2002; 10: 595–601. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, Naito S, Onoda J, et al. Development of a novel immunoassay for the measurement of type II collagen neoepitope generated by collagenase cleavage. Clin Chim Acta 2012; 413: 1591–1599. [DOI] [PubMed] [Google Scholar]
- 17.Poole AR, Ha N, Bourdon S, et al. Ability of a urine assay of type II collagen cleavage by collagenases to detect early onset and progression of articular cartilage degeneration: results from a population-based cohort study. J Rheumatol 2016; 43: 1864–1870. [DOI] [PubMed] [Google Scholar]
- 18.Mort JS, Beaudry F, Theroux K, et al. Early cathepsin K degradation of type II collagen in vitro and in vivo in articular cartilage. Osteoarthritis Cartilage 2016; 24: 1461–1469. [DOI] [PubMed] [Google Scholar]
- 19.Bay-Jensen AC, Liu Q, Byrjalsen I, et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM—increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin Biochem 2011; 44: 423–429. [DOI] [PubMed] [Google Scholar]
- 20.Zhen EY, Brittain IJ, Laska DA, et al. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum 2008; 58: 2420–2431. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, B-JA, Karsdal MA, Qvist P, et al. Serum CTX-II does not measure the same as urinary CTX-II. Osteoarthritis Cartilage 2018; 26: S179. [Google Scholar]
- 22.Bay-Jensen AC, Kjelgaard-Petersen CF, Petersen KK, et al. Aggrecanase degradation of type III collagen is associated with clinical knee pain. Clin Biochem 2018; 58: 37–43. [DOI] [PubMed] [Google Scholar]
- 23.Arendt-Nielsen L.Joint pain: more to it than just structural damage. Pain 2017; 158(Suppl. 1): S66–S73. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen MJ, Nedergaard AF, Sun S, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res 2013; 5: 303–315. [PMC free article] [PubMed] [Google Scholar]
- 25.Siebuhr AS, Petersen KK, Arendt-Nielsen L, et al. Identification and characterisation of osteoarthritis patients with inflammation derived tissue turnover. Osteoarthritis Cartilage 2014; 22: 44–50. [DOI] [PubMed] [Google Scholar]
- 26.Kjelgaard-Petersen C, Siebuhr AS, Christiansen T, et al. Synovitis biomarkers: ex vivo characterization of three biomarkers for identification of inflammatory osteoarthritis. Biomarkers 2015; 20: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassiliadis E, Veidal SS, Barascuk N, et al. Measurement of matrix metalloproteinase 9-mediated collagen type III degradation fragment as a marker of skin fibrosis. BMC Dermatol 2011; 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veidal SS, Vassiliadis E, Barascuk N, et al. Matrix metalloproteinase-9-mediated type III collagen degradation as a novel serological biochemical marker for liver fibrogenesis. Liver Int 2010; 30: 1293–1304. [DOI] [PubMed] [Google Scholar]
- 29.Charni-Ben Tabassi N, Richardot P, Toh L, et al. Circulating nitrated N-telopeptide of type III collagen (IIINys) as a biochemical marker of oxidative-related synovial tissue metabolism in rheumatoid arthritis. Ann Rheum Dis 2009; 68: 451–452. [DOI] [PubMed] [Google Scholar]
- 30.Richardot P, Charni-Ben Tabassi N, Toh L, et al. Nitrated type III collagen as a biological marker of nitric oxide-mediated synovial tissue metabolism in osteoarthritis. Osteoarthritis Cartilage 2009; 17: 1362–1367. [DOI] [PubMed] [Google Scholar]
- 31.Eyre DR, Weis MA.The Helix-II epitope: a cautionary tale from a cartilage biomarker based on an invalid collagen sequence. Osteoarthritis Cartilage 2009; 17: 423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseininia S, Weis MA, Rai J, et al. Evidence for enhanced collagen type III deposition focally in the territorial matrix of osteoarthritic hip articular cartilage. Osteoarthritis Cartilage 2016; 24: 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charni N, Juillet F, Garnero P.Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 2005; 52: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 34.Briot K, Roux C, Gossec L, et al. Effects of etanercept on serum biochemical markers of cartilage metabolism in patients with spondyloarthropathy. J Rheumatol 2008; 35: 310–314. [PubMed] [Google Scholar]
- 35.Garnero P, Thompson E, Woodworth T, et al. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum 2010; 62: 33–43. [DOI] [PubMed] [Google Scholar]
- 36.Garnero P, Charni N, Juillet F, et al. Increased urinary type II collagen helical and C telopeptide levels are independently associated with a rapidly destructive hip osteoarthritis. Ann Rheum Dis 2006; 65: 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bay-Jensen AC, Andersen TL, Charni-Ben Tabassi N, et al. Biochemical markers of type II collagen breakdown and synthesis are positioned at specific sites in human osteoarthritic knee cartilage. Osteoarthritis Cartilage 2008; 16: 615–623. [DOI] [PubMed] [Google Scholar]
- 38.Charni-Ben Tabassi N, Desmarais S, Bay-Jensen AC, et al. The type II collagen fragments Helix-II and CTX-II reveal different enzymatic pathways of human cartilage collagen degradation. Osteoarthritis Cartilage 2008; 16: 1183–1191. [DOI] [PubMed] [Google Scholar]
- 39.Girkontaite I, Frischholz S, Lammi P, et al. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol 1996; 15: 231–238. [DOI] [PubMed] [Google Scholar]
- 40.He Y, Siebuhr AS, Brandt-Hansen NU, et al. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Musculoskelet Disord 2014; 15: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coghlan RF, Oberdorf JA, Sienko S, et al. A degradation fragment of type X collagen is a real-time marker for bone growth velocity. Sci Transl Med 2017; 9: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Manon-Jensen T, Arendt-Nielsen L, et al. Potential diagnostic value of a type X collagen neo-epitope biomarker for knee osteoarthritis. Osteoarthritis Cartilage 2019; 27: 611–620. [DOI] [PubMed] [Google Scholar]
- 43.Kluzek S, Bay-Jensen AC, Judge A, et al. Serum cartilage oligomeric matrix protein and development of radiographic and painful knee osteoarthritis. A community-based cohort of middle-aged women. Biomarkers 2015; 20: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catterall JB, Hsueh MF, Stabler TV, et al. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem 2012; 287: 4640–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenzo P, Aspberg A, Saxne T, et al. Quantification of cartilage oligomeric matrix protein (COMP) and a COMP neoepitope in synovial fluid of patients with different joint disorders by novel automated assays. Osteoarthritis Cartilage 2017; 25: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 46.Sun S, Karsdal MA, Bay-Jensen AC, et al. The development and characterization of an ELISA specifically detecting the active form of cathepsin K. Clin Biochem 2013; 46: 1601–1606. [DOI] [PubMed] [Google Scholar]
- 47.Sun S, Bay-Jensen AC, Karsdal MA, et al. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet Disord 2014; 15: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Zheng Q, Jiang M, et al. The effect of protease inhibitors on the induction of osteoarthritis-related biomarkers in bovine full-depth cartilage explants. PLoS ONE 2015; 10: e0122700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumer EU, Sondergaard BC, Rousseau JC, et al. MMP and non-MMP-mediated release of aggrecan and its fragments from articular cartilage: a comparative study of three different aggrecan and glycosaminoglycan assays. Osteoarthritis Cartilage 2007; 15: 212–221. [DOI] [PubMed] [Google Scholar]
- 50.Wang B, Chen P, Jensen AC, et al. Suppression of MMP activity in bovine cartilage explants cultures has little if any effect on the release of aggrecanase-derived aggrecan fragments. BMC Res Notes 2009; 2: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rousseau JC, Sornay-Rendu E, Bertholon C, et al. Serum periostin is associated with prevalent knee osteoarthritis and disease incidence/progression in women: the OFELY study. Osteoarthritis Cartilage 2015; 23: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 52.Honsawek S, Wilairatana V, Udomsinprasert W, et al. Association of plasma and synovial fluid periostin with radiographic knee osteoarthritis: cross-sectional study. Joint Bone Spine 2015; 82: 352–355. [DOI] [PubMed] [Google Scholar]
- 53.Malemud CJ.MicroRNAs and osteoarthritis. Cells 2018; 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trachana V, Ntoumou E, Anastasopoulou L, et al. Studying microRNAs in osteoarthritis: critical overview of different analytical approaches. Mech Ageing Dev 2018; 171: 15–23. [DOI] [PubMed] [Google Scholar]
- 55.Cong L, Zhu Y, Tu G.A bioinformatic analysis of microRNAs role in osteoarthritis. Osteoarthritis Cartilage 2017; 25: 1362–1371. [DOI] [PubMed] [Google Scholar]
- 56.Rousseau JC, Millet M, Croset M, et al. Association of circulating microRNAs with prevalent and incident knee osteoarthritis in women: the OFELY study. Arthritis Res Ther 2020; 22: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skrzypa M, Szala D, Gablo N, et al. miRNA-146a-5p is upregulated in serum and cartilage samples of patients with osteoarthritis. Pol Przegl Chir 2019; 91: 1–5. [DOI] [PubMed] [Google Scholar]
- 58.Chao Y, Zhang L, Zhang X, et al. Expression of MiR-140 and MiR-199 in synovia and its correlation with the progression of knee osteoarthritis. Med Sci Monit 2020; 26: e918174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Lin Y, Bai Y, et al. A long noncoding RNA (lncRNA)-associated competing endogenous RNA (ceRNA) network identifies eight lncRNA biomarkers in patients with osteoarthritis of the knee. Med Sci Monit 2019; 25: 2058–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou Y, Li Y, Lu L, et al. Correlation of fractalkine concentrations in serum and synovial fluid with the radiographic severity of knee osteoarthritis. Ann Clin Biochem 2013; 50: 571–575. [DOI] [PubMed] [Google Scholar]
- 61.Song YZ, Guan J, Wang HJ, et al. Possible involvement of serum and synovial fluid resistin in knee osteoarthritis: cartilage damage, clinical, and radiological links. J Clin Lab Anal 2016; 30: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nugzar O, Zandman-Goddard G, Oz H, et al. The role of ferritin and adiponectin as predictors of cartilage damage assessed by arthroscopy in patients with symptomatic knee osteoarthritis. Best Pract Res Clin Rheumatol 2018; 32: 662–668. [DOI] [PubMed] [Google Scholar]
- 63.Huang K, Du G, Li L, et al. Association of Chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers 2012; 17: 16–20. [DOI] [PubMed] [Google Scholar]
- 64.Xu Q, Sun XC, Shang XP, et al. Association of CXCL12 levels in synovial fluid with the radiographic severity of knee osteoarthritis. J Investig Med 2012; 60: 898–901. [DOI] [PubMed] [Google Scholar]
- 65.Snelling SJ, Bas S, Puskas GJ, et al. Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype. PLoS ONE 2017; 12: e0175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saetan N, Honsawek S, Tanavalee A, et al. Association of plasma and synovial fluid interferon-gamma inducible protein-10 with radiographic severity in knee osteoarthritis. Clin Biochem 2011; 44: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 67.Rubenhagen R, Schuttrumpf JP, Sturmer KM, et al. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop 2012; 83: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monibi F, Roller BL, Stoker A, et al. Identification of synovial fluid biomarkers for knee osteoarthritis and correlation with radiographic assessment. J Knee Surg 2016; 29: 242–247. [DOI] [PubMed] [Google Scholar]
- 69.Li B, Zhang YL, Yu SY.Synovial fluid eotaxin-1 levels may reflect disease progression in primary knee osteoarthritis among elderly Han Chinese: a cross-sectional study. Cartilage 2019; 10: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koh SM, Chan CK, Teo SH, et al. Elevated plasma and synovial fluid interleukin-8 and interleukin-18 may be associated with the pathogenesis of knee osteoarthritis. Knee 2020; 27: 26–35. [DOI] [PubMed] [Google Scholar]
- 71.Guan J, Li Y, Ding LB, et al. Relationship between serum and synovial fluid CCL20 concentrations with disease severity in primary knee osteoarthritis. J Musculoskelet Neuronal Interact 2019; 19: 326–332. [PMC free article] [PubMed] [Google Scholar]
- 72.He W, Wang M, Wang Y, et al. Plasma and synovial fluid CXCL12 levels are correlated with disease severity in patients with knee osteoarthritis. J Arthroplasty 2016; 31: 373–377. [DOI] [PubMed] [Google Scholar]
- 73.Gao F, Tian J, Pan H, et al. Association of CCL13 levels in serum and synovial fluid with the radiographic severity of knee osteoarthritis. J Investig Med 2015; 63: 545–547. [DOI] [PubMed] [Google Scholar]
- 74.Bournazou E, Samuels J, Zhou H, et al. Vascular adhesion protein-1 (VAP-1) as predictor of radiographic severity in symptomatic knee osteoarthritis in the New York University Cohort. Int J Mol Sci 2019; 20: 2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou SJ, Sun ZX, Liu J.Neopterin concentrations in synovial fluid may reflect disease severity in patients with osteoarthritis. Scand J Clin Lab Invest 2013; 73: 344–348. [DOI] [PubMed] [Google Scholar]
- 76.Bing W, Feng L.Attenuate synovial fluid uncarboxylated matrix gla-protein (ucMGP) concentrations are linked with radiographic progression in knee psteoarthritis. Adv Clin Exp Med 2015; 24: 1013–1017. [DOI] [PubMed] [Google Scholar]
- 77.Okuyan HM, Terzi MY, Ozcan O, et al. Association of UCMA levels in serum and synovial fluid with severity of knee osteoarthritis. Int J Rheum Dis 2019; 22: 1884–1890. [DOI] [PubMed] [Google Scholar]
- 78.Wisniewski HG, Colon E, Liublinska V, et al. TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthritis Cartilage 2014; 22: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ritter SY, Collins J, Krastins B, et al. Mass spectrometry assays of plasma biomarkers to predict radiographic progression of knee osteoarthritis. Arthritis Res Ther 2014; 16: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsueh MF, Zhang X, Wellman SS, et al. Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol 2021; 73: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ritter SY, Subbaiah R, Bebek G, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum 2013; 65: 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan X, Huang L, Chen J, et al. Analysis of synovial fluid in knee joint of osteoarthritis:5 proteome patterns of joint inflammation based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Int Orthop 2012; 36: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mickiewicz B, Kelly JJ, Ludwig TE, et al. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J Orthop Res 2015; 33: 1631–1638. [DOI] [PubMed] [Google Scholar]
- 84.Mateos J, Lourido L, Fernandez-Puente P, et al. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC-MALDI TOF/TOF. J Proteomics 2012; 75: 2869–2878. [DOI] [PubMed] [Google Scholar]
- 85.Liao W, Li Z, Wang H, et al. Proteomic analysis of synovial fluid: insight into the pathogenesis of knee osteoarthritis. Int Orthop 2013; 37: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlson AK, Rawle RA, Adams E, et al. Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochem Biophys Res Commun 2018; 499: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carlson AK, Rawle RA, Wallace CW, et al. Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmed U, Anwar A, Savage RS, et al. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res Ther 2016; 18: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mobasheri A, Saarakkala S, Finnila M, et al. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 2019; 8: 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dell’Isola A, Allan R, Smith SL, et al. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2016; 17: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deveza LA, Nelson AE, Loeser RF.Phenotypes of osteoarthritis: current state and future implications. Clin Exp Rheumatol 2019; 37 Suppl. 120(5): 64–72. [PMC free article] [PubMed] [Google Scholar]
- 92.Jin X, Beguerie JR, Zhang W, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 703–710. [DOI] [PubMed] [Google Scholar]
- 93.Bay-Jensen ACKM, Ladel C, van Spil WE. C1M, C2M, C3M, PRO-C2 and CRPM in serum reflect different potential pathogenetic domains of osteoarthritis, data from CHECK. Osteoarthritis Cartilage 2018; 26(Suppl.): S179. [Google Scholar]
- 94.Fioravanti A, Cheleschi S, De Palma A, et al. Can adipokines serum levels be used as biomarkers of hand osteoarthritis. Biomarkers 2018; 23: 265–270. [DOI] [PubMed] [Google Scholar]
- 95.Luo S, Shi Q, Chen J, et al. Expression and significance of MMPs in synovial fluid, serum and PBMC culture supernatant stimulated by lps in osteoarthritis patients with or without diabetes. Exp Clin Endocrinol Diabetes 2019; 127: 195–202. [DOI] [PubMed] [Google Scholar]
- 96.Sanchez-Santos MT, Judge A, Gulati M, et al. Association of metabolic syndrome with knee and hand osteoarthritis: a community-based study of women. Semin Arthritis Rheum 2019; 48: 791–798. [DOI] [PubMed] [Google Scholar]
- 97.Kisand K, Tamm AE, Lintrop M, et al. New insights into the natural course of knee osteoarthritis: early regulation of cytokines and growth factors, with emphasis on sex-dependent angiogenesis and tissue remodeling. Osteoarthritis Cartilage 2018; 26: 1045–1054. [DOI] [PubMed] [Google Scholar]
- 98.Karsdal MA, Bay-Jensen AC, Lories RJ, et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments. Ann Rheum Dis 2014; 73: 336–348. [DOI] [PubMed] [Google Scholar]
- 99.Kinds MB, Marijnissen AC, Viergever MA, et al. Identifying phenotypes of knee osteoarthritis by separate quantitative radiographic features may improve patient selection for more targeted treatment. J Rheumatol 2013; 40: 891–902. [DOI] [PubMed] [Google Scholar]
- 100.Huebner JL, Bay-Jensen AC, Huffman KM, et al. Alpha C-telopeptide of type I collagen is associated with subchondral bone turnover and predicts progression of joint space narrowing and osteophytes in osteoarthritis. Arthritis Rheumatol 2014; 66: 2440–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Neill TW, Felson DT.Mechanisms of osteoarthritis (OA) pain. Curr Osteoporos Rep 2018; 16: 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Phan CM, Link TM, Blumenkrantz G, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. Eur Radiol 2006; 16: 608–618. [DOI] [PubMed] [Google Scholar]
- 103.Sharma N, Drobinski P, Kayed A, et al. Inflammation and joint destruction may be linked to the generation of cartilage metabolites of ADAMTS-5 through activation of toll-like receptors. Osteoarthritis Cartilage 2020; 28: 658–668. [DOI] [PubMed] [Google Scholar]
- 104.Leung YY, Huebner JL, Haaland B, et al. Synovial fluid pro-inflammatory profile differs according to the characteristics of knee pain. Osteoarthritis Cartilage 2017; 25: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 105.Bihlet AR, Byrjalsen I, Bay-Jensen AC, et al. Associations between biomarkers of bone and cartilage turnover, gender, pain categories and radiographic severity in knee osteoarthritis. Arthritis Res Ther 2019; 21: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomas D, Micklus A, LaFever S, et al. Clinical development success rate and contributing factors 2011-2020, 2021, https://pharmaintelligence.informa.com/~/media/informa-shop-window/pharma/2021/files/reports/2021-clinical-development-success-rates-2011-2020-v17.pdf
- 107.Ren G, Krawetz RJ.Biochemical markers for the early identification of osteoarthritis: systematic review and meta-analysis. Mol Diagn Ther 2018; 22: 671–682. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J.Meta-analysis of serum C-reactive protein and cartilage oligomeric matrix protein levels as biomarkers for clinical knee osteoarthritis. BMC Musculoskelet Disord 2018; 19: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Legrand C, Ahmed U, Anwar A, et al. Glycation marker glucosepane increases with the progression of osteoarthritis and correlates with morphological and functional changes of cartilage in vivo. Arthritis Res Ther 2018; 20: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao XY, Yang ZB, Zhang ZJ, et al. CCL3 serves as a potential plasma biomarker in knee degeneration (osteoarthritis). Osteoarthritis Cartilage 2015; 23: 1405–1411. [DOI] [PubMed] [Google Scholar]
- 111.Liem Y, Judge A, Kirwan J, et al. Multivariable logistic and linear regression models for identification of clinically useful biomarkers for osteoarthritis. Sci Rep 2020; 10: 11328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lazzarini N, Runhaar J, Bay-Jensen AC, et al. A machine learning approach for the identification of new biomarkers for knee osteoarthritis development in overweight and obese women. Osteoarthritis Cartilage 2017; 25: 2014–2021. [DOI] [PubMed] [Google Scholar]
- 113.Camacho-Encina M, Balboa-Barreiro V, Rego-Perez I, et al. Discovery of an autoantibody signature for the early diagnosis of knee osteoarthritis: data from the Osteoarthritis Initiative. Ann Rheum Dis 2019; 78: 1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bianchi J, de Oliveira Ruellas AC, Goncalves JR, et al. Osteoarthritis of the Temporomandibular Joint can be diagnosed earlier using biomarkers and machine learning. Sci Rep 2020; 10: 8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garnero P, Ayral X, Rousseau JC, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum 2002; 46: 2613–2624. [DOI] [PubMed] [Google Scholar]
- 116.Kumm J, Tamm A, Lintrop M, et al. Diagnostic and prognostic value of bone biomarkers in progressive knee osteoarthritis: a 6-year follow-up study in middle-aged subjects. Osteoarthritis Cartilage 2013; 21: 815–822. [DOI] [PubMed] [Google Scholar]
- 117.Teirlinck CH, Dorleijn DMJ, Bos PK, et al. Prognostic factors for progression of osteoarthritis of the hip: a systematic review. Arthritis Res Ther 2019; 21: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verma P, Dalal K.Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J Orthop Res 2013; 31: 999–1006. [DOI] [PubMed] [Google Scholar]
- 119.Hao HQ, Zhang JF, He QQ, et al. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2019; 27: 726–736. [DOI] [PubMed] [Google Scholar]
- 120.He YJK, Siebuhr AS, Karsdal MA, et al. Development of a highly sensitive chemiluminescence immunoassay for quantification of aggrecanase-generated ARGS aggrecan fragments in serum. Osteoarthritis Cartilage 2021; 3: 100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saberi Hosnijeh F, Siebuhr AS, Uitterlinden AG, et al. Association between biomarkers of tissue inflammation and progression of osteoarthritis: evidence from the Rotterdam study cohort. Arthritis Res Ther 2016; 18: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Attur M, Statnikov A, Samuels J, et al. Plasma levels of interleukin-1 receptor antagonist (IL1Ra) predict radiographic progression of symptomatic knee osteoarthritis. Osteoarthritis Cartilage 2015; 23: 1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang ZY, Perry E, Huebner JL, et al. Biomarkers of inflammation—LBP and TLR- predict progression of knee osteoarthritis in the DOXY clinical trial. Osteoarthritis Cartilage 2018; 26: 1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takinami Y, Yoshimatsu S, Uchiumi T, et al. Identification of potential prognostic markers for knee osteoarthritis by serum proteomic analysis. Biomark Insights 2013; 8: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kraus VB, Collins JE, Hargrove D, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 2017; 76: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Loeser RF, Collins JA, Diekman BO.Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016; 12: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Attur M, Krasnokutsky S, Statnikov A, et al. Low-grade inflammation in symptomatic knee osteoarthritis: prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol 2015; 67: 2905–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hunter DJ, Deveza LA, Collins JE, et al. Multivariable modeling of biomarker data from the phase 1 Foundation for the NIH Osteoarthritis Biomarkers Consortium. Arthritis Care Res (Hoboken). Epub ahead of print 9January2021. DOI: 10.1002/acr.24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Joseph GB, Nevitt MC, McCulloch CE, et al. Associations between molecular biomarkers and MR-based cartilage composition and knee joint morphology: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2018; 26: 1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nelson AE, Fang F, Arbeeva L, et al. A machine learning approach to knee osteoarthritis phenotyping: data from the FNIH Biomarkers Consortium. Osteoarthritis Cartilage 2019; 27: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sofat N, Ejindu V, Heron C, et al. Biomarkers in painful symptomatic knee OA demonstrate that MRI assessed joint damage and type II collagen degradation products are linked to disease progression. Front Neurosci 2019; 13: 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martel-Pelletier J, Tardif G, Rousseau Trepanier J, et al. The ratio adipsin/MCP-1 is strongly associated with structural changes and CRP/MCP-1 with symptoms in obese knee osteoarthritis subjects: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2019; 27: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 133.Bonakdari H, Jamshidi A, Pelletier JP, et al. A warning machine learning algorithm for early knee osteoarthritis structural progressor patient screening. Ther Adv Musculoskelet Dis 2021; 13: 1759720X21993254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bjerre-Bastos J, B-JA, Karsdal M, Byrjalsen JR, et al. Biomarkers of bone and cartilage turnover CTX-I and CTX-II predict total joint replacements in osteoarthritis. Osteoarthritis Cartilage 2019; 27: S31–S32. [Google Scholar]
- 135.Hochberg MC.Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015; 23(Suppl. 1): S18–S21. [DOI] [PubMed] [Google Scholar]
- 136.Arends R, Karsdal MA, Verburg KM, et al. Identification of serological biomarker profiles associated with total joint replacement in osteoarthritis patients. Osteoarthritis Cartilage 2017; 25: 866–877. [DOI] [PubMed] [Google Scholar]
- 137.Karsdal MA, Verburg KM, West CR, et al. Serological biomarker profiles of rapidly progressive osteoarthritis in Tanezumab-treated patients. Osteoarthritis Cartilage 2019; 27: 484–492. [DOI] [PubMed] [Google Scholar]
- 138.Garnero P, Sornay-Rendu E, Chapurlat R.The cartilage degradation marker, urinary CTX-II, is associated with the risk of incident total joint replacement in postmenopausal women. A 18 year evaluation of the OFELY prospective cohort. Osteoarthritis Cartilage 2020; 28: 468–474. [DOI] [PubMed] [Google Scholar]
- 139.Bihlet AR, Bjerre-Bastos JJ, Andersen JR, et al. Clinical and biochemical factors associated with risk of total joint replacement and radiographic progression in osteoarthritis: data from two phase III clinical trials. Semin Arthritis Rheum 2020; 50: 1374–1381. [DOI] [PubMed] [Google Scholar]
- 140.Attur M, Belitskaya-Levy I, Oh C, et al. Increased interleukin-1beta gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum 2011; 63: 1908–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mishra A, Awasthi S, Raj S, et al. Identifying the role of ASPN and COMP genes in knee osteoarthritis development. J Orthop Surg Res 2019; 14: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fernandez-Moreno M, Soto-Hermida A, Vazquez-Mosquera ME, et al. A replication study and meta-analysis of mitochondrial DNA variants in the radiographic progression of knee osteoarthritis. Rheumatology (Oxford) 2017; 56: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hunter D, Nevitt M, Lynch J, et al. Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 2016; 75: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 144.MacKay JW, Kapoor G, Driban JB, et al. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study. Eur Radiol 2018; 28: 4687–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kraus VB, Collins JE, Charles HC, et al. Predictive validity of radiographic trabecular bone texture in knee osteoarthritis: the Osteoarthritis Research Society International/Foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthritis Rheumatol 2018; 70: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eckstein F, Collins JE, Nevitt MC, et al. Brief report: cartilage thickness change as an imaging biomarker of knee osteoarthritis progression: data from the foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthritis Rheumatol 2015; 67: 3184–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gallo MC, Wyatt C, Pedoia V, et al. T1rho and T2 relaxation times are associated with progression of hip osteoarthritis. Osteoarthritis Cartilage 2016; 24: 1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davis JE, Ward RJ, MacKay JW, et al. Effusion-synovitis and infrapatellar fat pad signal intensity alteration differentiate accelerated knee osteoarthritis. Rheumatology (Oxford) 2019; 58: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harkey MS, Davis JE, Lu B, et al. Early pre-radiographic structural pathology precedes the onset of accelerated knee osteoarthritis. BMC Musculoskelet Disord 2019; 20: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Attur M, Krasnokutsky-Samuels S, Samuels J, et al. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol 2013; 25: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Riegger J, Rehm M, Buchele G, et al. Serum cartilage oligomeric matrix protein in late-stage osteoarthritis: association with clinical features, renal function, and cardiovascular biomarkers. J Clin Med 2020; 9: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang P, Song J, Qian D.CTX-II and YKL-40 in early diagnosis and treatment evaluation of osteoarthritis. Exp Ther Med 2019; 17: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saviola G, Abdi-Ali L, Povino MR, et al. Intramuscular clodronate in erosive osteoarthritis of the hand is effective on pain and reduces serum COMP: a randomized pilot trial-The ER.O.D.E. study (ERosive Osteoarthritis and Disodium-clodronate Evaluation). Clin Rheumatol 2017; 36: 2343–2350. [DOI] [PubMed] [Google Scholar]
- 154.Nishii T, Tamura S, Shiomi T, et al. Alendronate treatment for hip osteoarthritis: prospective randomized 2-year trial. Clin Rheumatol 2013; 32: 1759–1766. [DOI] [PubMed] [Google Scholar]
- 155.Bingham CO, III, Buckland-Wright JC, Garnero P, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum 2006; 54: 3494–3507. [DOI] [PubMed] [Google Scholar]
- 156.Karsdal MA, Byrjalsen I, Alexandersen P, et al. Treatment of symptomatic knee osteoarthritis with oral salmon calcitonin: results from two phase 3 trials. Osteoarthritis Cartilage 2015; 23: 532–543. [DOI] [PubMed] [Google Scholar]
- 157.Alexandersen P, Karsdal MA, Byrjalsen I, et al. Strontium ranelate effect in postmenopausal women with different clinical levels of osteoarthritis. Climacteric 2011; 14: 236–243. [DOI] [PubMed] [Google Scholar]
- 158.Gigout A, Guehring H, Froemel D, et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis Cartilage 2017; 25: 1858–1867. [DOI] [PubMed] [Google Scholar]
- 159.Eckstein F, Kraines JL, Aydemir A, et al. Intra-articular sprifermin reduces cartilage loss in addition to increasing cartilage gain independent of location in the femorotibial joint: post-hoc analysis of a randomised, placebo-controlled phase II clinical trial. Ann Rheum Dis 2020; 79: 525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hochberg MC, Guermazi A, Guehring H, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA 2019; 322: 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reker D, Kjelgaard-Petersen CF, Siebuhr AS, et al. Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo. J Transl Med 2017; 15: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reker D, Siebuhr AS, Thudium CS, et al. Sprifermin (rhFGF18) versus vehicle induces a biphasic process of extracellular matrix remodeling in human knee OA articular cartilage ex vivo. Sci Rep 2020; 10: 6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garnero P, Aronstein WS, Cohen SB, et al. Relationships between biochemical markers of bone and cartilage degradation with radiological progression in patients with knee osteoarthritis receiving risedronate: the Knee Osteoarthritis Structural Arthritis randomized clinical trial. Osteoarthritis Cartilage 2008; 16: 660–666. [DOI] [PubMed] [Google Scholar]