Abstract
Treatment for advanced gastric cancer is challenging. Epidermal growth factor receptor (EGFR) contributes to the proliferation and development of gastric cancer (GC), and its overexpression is associated with unfavorable prognosis in GC. Cetuximab, a monoclonal antibody targeting EGFR, failed to improve the overall survival of gastric cancer patients indicated in phase III randomized trials. Glutamine is a vital nutrient for tumor growth and its metabolism contributes to therapeutic resistance, making glutamine uptake an attractive target for cancer treatment. The aim of the present study was to investigate whether intervention of glutamine uptake could improve the effect of cetuximab on GC. The results of MTT assay showed that by glutamine deprivation or inhibition of glutamine uptake, the viability of gastric carcinoma cells was inhibited more severely than that of human immortal gastric mucosa epithelial cells (GES-1). The expression of the key glutamine transporter alanine-serine-cysteine (ASC) transporter 2 (ASCT2; SLC1A5) was significantly higher in gastric carcinoma tissues and various gastric carcinoma cell lines than in normal gastric tissues and cells, as shown by immunohistochemistry and western blotting, while silencing ASCT2 significantly inhibited the viability and proliferation of gastric carcinoma cells. Consistent with previous studies, it was shown herein by MTT and EdU assays that cetuximab had a weak inhibitory effect on the cell viability of gastric carcinoma cells. However, inhibiting glutamine uptake by blockade of ASCT2 with l-γ-glutamyl-p-nitroanilide (GPNA) significantly enhanced the inhibitory effect of cetuximab on suppressing the proliferation of gastric cancer both in vitro and in vivo. Moreover, combining cetuximab and GPNA induced cell apoptosis considerably in gastric carcinoma cells, as shown by flow cytometry, and had a higher depressing effect on gastric cancer proliferation both in vitro and in vivo, as compared to either treatment alone. The present study suggested that inhibition of glutamine uptake may be a promising strategy for improving the inhibitory efficacy of cetuximab on advanced gastric cancer.
Keywords: gastric cancer, glutamine uptake, ASCT2, EGFR, cetuximab
Introduction
Gastric cancer ranks fifth in global cancer incidence and is the third leading cause of cancer mortality worldwide.1 Gastric cancer is usually at an advanced stage at the time of diagnosis. With chemotherapy as the primary treatment, the prognosis of patients with advanced gastric cancer is poor.2 Biologically targeted treatment is a promising treatment for patients with advanced gastric cancer. At present, trastuzumab against human epidermal growth factor receptor 2 (HER2) is a mature biologically targeted drug for the treatment of gastric cancer with HER2-expression that has achieved an apparent curative effect.3 However, <10% of patients with gastric cancer show a positive expression of HER2. Therefore, most patients with gastric cancer cannot benefit from trastuzumab.4 Some studies have paid attention to the epidermal growth factor receptor (EGFR), another potential target in the treatment of gastric cancer, and explored whether targeting EGFR could benefit more patients with advanced gastric cancer. However, previous phase III randomized trials showed that adding cetuximab, a monoclonal antibody for EGFR, to chemotherapy had no efficacy in improving overall survival or disease control rate in patients with advanced gastric cancer.5 Other strategies for enhancing the efficacy of cetuximab on gastric cancer should be pursued.
Metabolic reprograming drives tumor onset and progression. Blocking cancer metabolism can effectively overcome therapeutic resistance.6 Glutamine is a critical nutrient for cell growth and survival, especially for cancer cells, making targeting glutamine metabolism a potential strategy for cancer therapy.7 A previous study has indicated an active glutamine breakdown for supporting energy in stomach tumor, tissues, as compared with normal tissues.8 Glutamine membrane transporters generally can be recognized by 4 different gene solute carried (SLC) families including SLC1, SLC6, SLC7, and SLC38, which can recognize different amino acids including glutamine as substrates. Among these are transporters of SLC1A5 (alanine-serine-cysteine transporter 2, ASCT2), SLC6A14, and SLC7A5, ASCT2 shares specificity for glutamine and is overexpressed in many tumors.9 ASCT2 is a sodium-dependent neutral amino acid transporter encoded by the SLC1A5 gene, and plays an important role in promoting tumor development by transporting glutamine into cells for energy production, redox homeostasis, macromolecular synthesis, or mTOR signaling activation.10 Many studies have shown that ASCT2 was highly expressed in various types of cancer, including gastric cancer,11-14 and inhibition of ASCT2 significantly suppressed the growth of gastric cancer in patient-derived xenograft mouse models.15 These studies indicated that glutamine played a crucial role in gastric cancer, and ASCT2 that might be an attractive therapeutic target. Of note, ASCT2 can directly associate with EGFR to form a heterotrimeric molecular complex, and ASCT2-EGFR could be co-targeted by cetuximab.16,17 Moreover, our previous study showed that the inhibition of ASCT2 enhanced the efficacy of cetuximab in colorectal cancer.14 Accordingly, we wondered whether intervention of glutamine uptake by inhibiting ASCT2 could also improve the efficacy of cetuximab on gastric cancer, and whether combined targeting against glutamine uptake and EGFR could be an effective therapeutic strategy for advanced gastric cancer.
Materials and Methods
Cell Culture and Transfection
Human immortal gastric mucosa epithelial cell line GES-1, and gastric cancer cell lines BGC803 and MKN45 were obtained from the Cell Bank of Chinese Academy of Sciences and authenticated by the STR method. Cells were cultured in 25 cm2 culture flasks containing 10 ml pre-warmed complete medium RPMI 1640 with 10% fetal bovine serum (FBS) and incubated at 37°C with 5% CO2. Cells were passaged when cells split and reached 80% to 90% confluence. For glutamine deprivation assays, cells were cultured in RPMI 1640 medium without glutamine (Gibco 21870) to achieve the deprived glutamine culture conditions. For silencing SLC1A5, the lentivirus vector GV248 containing specific shRNA (GeneChem, Shanghai, China) was used for stable transduction. The 2 target sequences used in this study were as follows: SLC1A5-sh1 (CTGAGTTGATACAAGTGAA), SLC1A5-sh2 (AGTCCTTGGACTTCGTAAA).
Compounds
l-γ-Glutamyl-p-nitroanilide (GPNA) and Cetuximab (Erbitux™) were purchased from MP Biomedicals and Merck, respectively.
Patient Specimens
Paraffin-embedded samples (n = 30 pairs) and fresh tissues (n = 4 pairs) included gastric carcinoma and para-carcinoma tissues of patients with gastric cancer, were obtained from the Department of Pathology, Nanfang Hospital (Guangzhou, China). These patients were not subjected to any anticancer drug treatments before surgery. Written informed consent was obtained from all patients, and the protocol was approved by the Medical Ethics Committees of Nanfang Hospital.
Gene Expression Analysis
Gene expression of cultured BGC803 cells with or without glutamine derivation was quantified using TaqMan real-time polymerase chain reaction in a LightCycler 480® (Roche Diagnostics, IN, USA) with GAPDH as an internal reference gene to normalize the data. Information for primer sequences for glutamine transporter-associated receptors has been listed in supplemental Table 1.
Western Blotting (WB) and Immunohistochemistry (IHC)
WB and IHC were performed as previously described.18 Briefly, for WB, fresh tissues and treated cells were collected and lysed. Then, the protein was extracted using the Protein Extraction Kit (Beyotime Biotechnology) according to the manufacturer’s protocol. The protein was then loaded in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis for electrophoresis and transferred to a 0.22 μm polyvinylidine difluoride membrane. After blocking, the membrane was incubated with ASCT2 antibody (rabbit monoclonal antibody; cat. no. 8057; Cell Signaling Technology, Inc.) and GAPDH antibody (rabbit polyclonal antibody; cat. no. ab245355; Abcam) overnight at 4°C. Finally, the membrane was incubated with secondary antibody and exposed with a near-infrared imaging system (Odyssey Technologies). The results were quantified using ImageJ software.
For IHC, paraffin-embedded samples were cut into 4-μm sections and rehydrated with ethanol gradient solution. Next, the tissue sections were subjected to antigen repair, endogenous peroxidase activities were eliminated, and blocking was performed before incubation with ASCT2 antibody (rabbit monoclonal antibody; cat. no. 8057; Cell Signaling Technology, Inc). The dilution of the ASCT2 antibody was 1:100. After that, the tissue sections were incubated with secondary antibody and stained with 3,3N-diaminobenzidine tetrahydrochloride. The results were scored as follows: 0 (negative), 1 (low), 2 (moderate), and 3 (high) for staining intensity; 1 (0%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%) for staining area. The IHC score was calculated by multiplying the staining intensity by the staining area. Results with a score of <6 were considered as low expression, and those with a score of ≥6 were considered as high expression.
Cell Viability/Proliferation
Cell viability and proliferation were determined using MTT, clonogenic, and EdU assays. For MTT assay, cells were seeded in 96-well plates at a density of 6000/well and subjected to different treatments for 48 hours. For clonogenic assay, cells were seeded at a density of 2000/well in 6-well plates and cultured in medium with or without glutamine for 2 weeks. For the EdU assay, BGC803 and MKN45 cells were seeded at a density of 6000 cells/well in 96-well plates and treated with PBS, 500 μg/ml cetuximab, 10 mM GPNA or GPNA + cetuximab for 48 hours, respectively.
Glutamine Consumption Assays
To evaluate the glutamine consumption of cells, the level of glutamine in the culture medium was measured with the EnzyChromTM Glutamine Assay Kit (Bioassay Systems, Hayward, CA, USA), according to the manufacturer’s instructions. Briefly, the cell medium was harvested and mixed with the enzyme after deproteinization with an ultracentrifugal filter. The absorbance at 565 nm was measured with a BioTek™ 800TS Absorbance Reader (BioTek) after incubation for 40 minutes in the dark and the concentration of glutamine was calculated by the given kit formula. Then, the glutamine consumption was calculated according to the following equation: Glutamine consumption = (the concentration of glutamine in fresh medium − the concentration of glutamine in cell medium) × the volume of cell medium. The final amount of glutamine consumption was quantified to the content of whole cellular protein and the result was determined as μmol glutamine/mg protein.
ATP Assay
To detect the ATP level of cells, cells were harvested and the ATP content was examined using the ATP Assay kit (Beyotime Institute of Biotechnology), according to the manufacturer’s instructions. The ATP content was quantified to the content of the whole cellular protein and the result was determined as pmol ATP/μg protein.
Lactate Assay
To detect the lactate level of cells, cells were harvested and the lactate concentration was determined by conventional enzymatic methods (Randox, Antrim, UK), according to the manufacturer’s instructions. The lactate content was quantified to the content of the whole cellular protein and the result was determined as nmol lactate/μg protein.
Apoptosis Assay
BGC803 and MKN45 cells were seeded at a density of 200 000/wells in 6-well plates and treated with PBS, 500 μg/ml cetuximab, 10 mM GPNA or GPNA + cetuximab for 48 hours, respectively. Cells were then harvested and washed with PBS, followed by the addition of 500 μl binding buffer, 5 μl Annexin V-FITC, and 5 μl Propidium iodide (Nanjing KeyGen Biotech Co., Ltd.). After 15 minutes of reaction at room temperature in the dark, samples were examined using the flow cytometry system (BD LSR Fortessa).
Mouse Xenograft Model
The animal experiment was approved by the Animal Care and Use Committee of the Nanfang Hospital, Southern Medical University. All animal experiment procedures in the present study were performed according to the National Guidelines for Animal Experimentation. Female BALB/c nude mice aged 4 to 6 weeks were purchased from the Laboratory Animal Center of Southern Medical University. The nude mice were randomly divided into 4 different treatment groups (5 mice per group) and BGC803 cells (~5 × 106) were implanted subcutaneously at the right flanks of mice. The tumor volume was calculated as length × width2/2, and once it reached ~200 mm3, the mice were subjected to the following treatments: Control group, mice received PBS injection; cetuximab group, cetuximab was injected intraperitoneally at a dose of 1 mg/mouse twice a week for 3 weeks; GPNA group, GPNA was injected intraperitoneally at a dose of 50 mg/kg bodyweight every day; combined group, mice were administered treatment with GPNA + cetuximab.
TCGA Analysis
TCGA data for gastric carcinoma was downloaded and analyzed via cBioportal (www.cbioportal.org).
Statistical Analysis
Data in this study are presented as the mean ± standard deviation. SPSS 22.0 software was used for statistical analysis. Differences between groups were examined by Student’s t-test or 1-way ANOVA. P < .05 was considered statistically significant.
Results
Glutamine Deprivation Inhibits Gastric Cancer Cell Viabilities and Proliferation
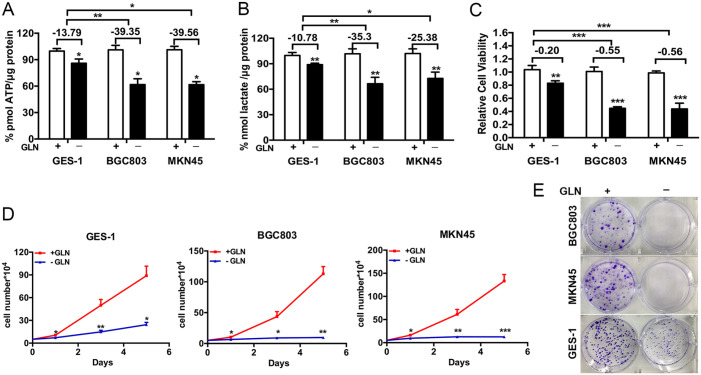
To examine the effect of glutamine on energy production in gastric cancer and non-malignant gastric cells, the cellular ATP and lactate levels were detected after glutamine deprivation. As shown in Figure 1A and B, glutamine deprivation for 48 hours inhibited the production of ATP and lactate in human immortal gastric mucosa epithelial cell line GES-1, and gastric cancer cell lines BGC803 and MKN45, and the inhibitory rate was higher in BGC803 and MKN45 than in GES-1 cells (ATP: 13.79% for GES-1, 39.35% for BGC803, and 39.56% for MKN45, respectively; lactate:10.78% for GES-1, 35.3% for BGC803, and 25.38% for MKN45, respectively). Then, we performed MTT assay to evaluate the effect of glutamine on cell viabilities of gastric cancer cells and non-malignant gastric cells. It was found that the cell viabilities of GES-1, BGC803, and MKN45 were significantly inhibited (0.83 for GES-1, 0.45 for BGC803, and 0.44 for MKN45, respectively), and the inhibitory rate was higher in BGC803 and MKN45 than in GES-1 cells (55% for BGC803, 56% for MKN45, and 20% for GES-1; Figure 1C). Besides, both the cell growth curve and clonogenic assay showed that the proliferation of BGC803 and MKN45 cells was significantly depressed by glutamine deprivation (Figure 1D and E). Similarly, glutamine deprivation also mildly affected the proliferation of GES-1, but not as significantly as the tumor cells (Figure 1D and E). These results suggested an essential role of glutamine in the viability and proliferation of gastric cancer cells.
Figure 1.
Deprivation of glutamine inhibits gastric cancer cell proliferation: (A, B) ATP assay and lactate assay showed deprivation of glutamine for 48 hours decreased the ATP and lactate level of cells, (C) MTT assay showed that glutamine deprivation for 48 hours inhibited cell viabilities and (D, E) Cell growth curves and colony formation assays showed the proliferation of cells after glutamine deprivation.
Student’s t-test.
Abbreviation: GLN, glutamine.
*P < .05. **P < .01. ***P < .001.
Role of Glutamine Transporter ASCT2 in Gastric Cancer
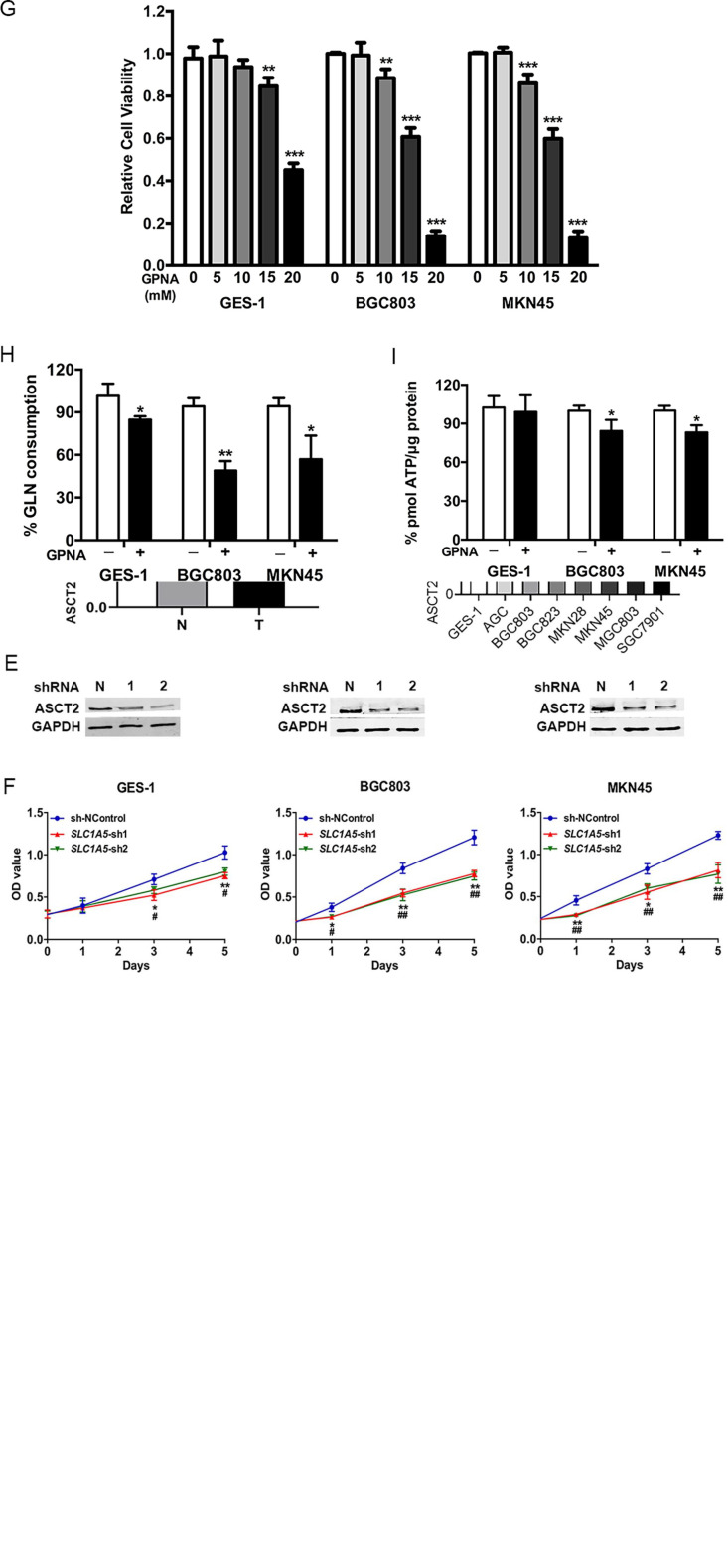
Several transporters including ASCT2, SLC7A5, SLC38A1, SLC38A2, SLC38A5, and SLC6A14 are important for glutamine uptake for cells.9 We found that the mRNA expressions of these transporters were decreased by glutamine deprivation in GC cells, among which SLC1A5, SLC38A1, and SLC38A2 exhibited the best inhibition rate (Supplemental Figure 1A), indicating that glutamine deprivation mainly affected the expression of SLC1A5, SLC38A1, and SLC38A2, but not the other transporters. Besides, through analyzing the TCGA STAD data, we found that all these transporters were more highly expressed in gastric carcinoma tissues than para-carcinoma tissues, and SLC1A5 and SLC6A14 exhibited the most striking expression differences among these transporters (Supplemental Figure 1B and C). In addition, by KM-plotter analysis,19 the roles of SLC1A5 and SLC6A14 in tumor survival were further detected. As a result, high expression of SLC1A5 was correlated with the poor prognosis of gastric cancer (GC), with a hazard ratio of 2.18 (Supplemental Figure 2A). Conversely, high expression of SLC6A14 was related with longer overall survival time in GC (Supplemental Figure 2B). These data suggested that ASCT2 might play a relatively important role in gastric carcinoma, and the role of ASCT2 on GC progression was further detected in the following assays.
IHC and WB were further performed to detect the expression of ASCT2 in gastric carcinoma tissues and cells, as well as relatively healthy gastric tissues and cells. The IHC staining results showed that ASCT2 was mainly expressed in the cell membrane and highly expressed in gastric carcinoma, as compared to the paired para-carcinoma tissues (n = 30; average score, 6.1 vs 2.6; P < 0.001; Figure 2A and B). Consistently, the WB results showed that the ASCT2 expression was increased in gastric carcinoma by 1.8 times, as compared to para-carcinoma tissues (P < 0.001; Figure 2C). In addition, the cellular expression of ASCT2 was also higher in various gastric cancer cell lines, as compared with the immortal gastric GES-1 cell line (Figure 2D). These results confirmed that ASCT2 was highly expressed in gastric cancer, both at the tissue and cellular levels.
Figure 2.
ASCT2 is highly expressed in gastric cancer and functions in supporting gastric cancer cell growth. (A, B) Representative images of IHC and quantitative results of ASCT2 expression in gastric carcinoma and paracarcinoma tissues (magnification, ×200 for the left and ×400 for the right columns). (C, D) WB experiments and corresponding quantitative results of the expression of ASCT2 in gastric carcinoma and paracarcinoma tissues (C) and various gastric cancer cell lines and immortal gastric cell line GES-1(D). (E, F) WB analysis validated that shRNA targeting SLC1A5 successfully inhibited the protein expression of ASCT2 in all 3 cell lines (E), and MTT assay showed the growth rates of SLC1A5-silencing cells were slower than that of control cells (F). (G) MTT assay showed the results of cellular viabilities after treatment with GPNA for 48 hours. (H) Cellular glutamine consumption and (I) ATP level after a 48-hour treatment with 10 mM GPNA.
Student’s t-test.
Abbreviations: N, paracarcinoma tissues; T, gastric carcinoma tissues; SLC1A5, solute carrier 1 family member 5; IHC, immunohistochemistry; WB, western blotting; GPNA, l-γ-glutamyl-p-nitroanilide.
*P < .05. **P < .01. ***P < .001.
Then we detected the role of ASCT2 in cell proliferation and cellular energy production. shRNA was conducted to silence the expression of ASCT2 by targeting the encoding gene SLC1A5. SLC1A5-sh1/2 sequences effectively inhibited the expression of ASCT2 in GES-1, BGC803, and MKN45 cells (Figure 2E). The cell growth curves showed a slower growth rate in SLC1A5-knockdown cells in contrast to cells with normal SLC1A5 expression, and the inhibitory effect on cell growth was relatively stronger in BGC803 and MKN45 than in GES-1 cells (Figure 2F). Next, GPNA, the widely used inhibitor for ASCT2, was used to treat GES-1, BGC803, and MKN45 cells, and it was found that the cell viability of all 3 cell lines was inhibited with the increase in concentration (Figure 2G). However, 48 hours-treatment with 10 mM GPNA succeeded in inhibiting the cell viability of BGC803 and MKN45 (0.88 and 0.86, respectively), but failed to inhibit that of GES-1 (Figure 2G), suggesting that gastric cancer cells were more sensitive to GPNA than immortal gastric cells. After 48 hours of treatment with 10 mM GPNA, the glutamine consumption was significantly decreased in GES-1, BGC803, and MKN45 cells (15%, 49%, and 57%, respectively; Figure 2H); and the ATP level was significantly decreased in BGC803 and MKN45 cells (84% and 83%, respectively), but not in GES-1 cells (Figure 2I), which was consistent with the results in Figure 2G. According to the results, 10 mM GPNA was used for the following experiments with significant inhibitory effects on gastric cancer cells but little effect on immortal gastric cells. These results revealed that ASCT2 was highly expressed in gastric cancer and could be a potential therapeutic target for the inhibition of gastric cancer cell proliferation, while having little effect on noncancerous cells.
Blockade of Glutamine Transporter ASCT2 Enhances the Inhibitory Effect of Cetuximab, and Combined Treatment Significantly Suppresses Gastric Cancer Cell Proliferation
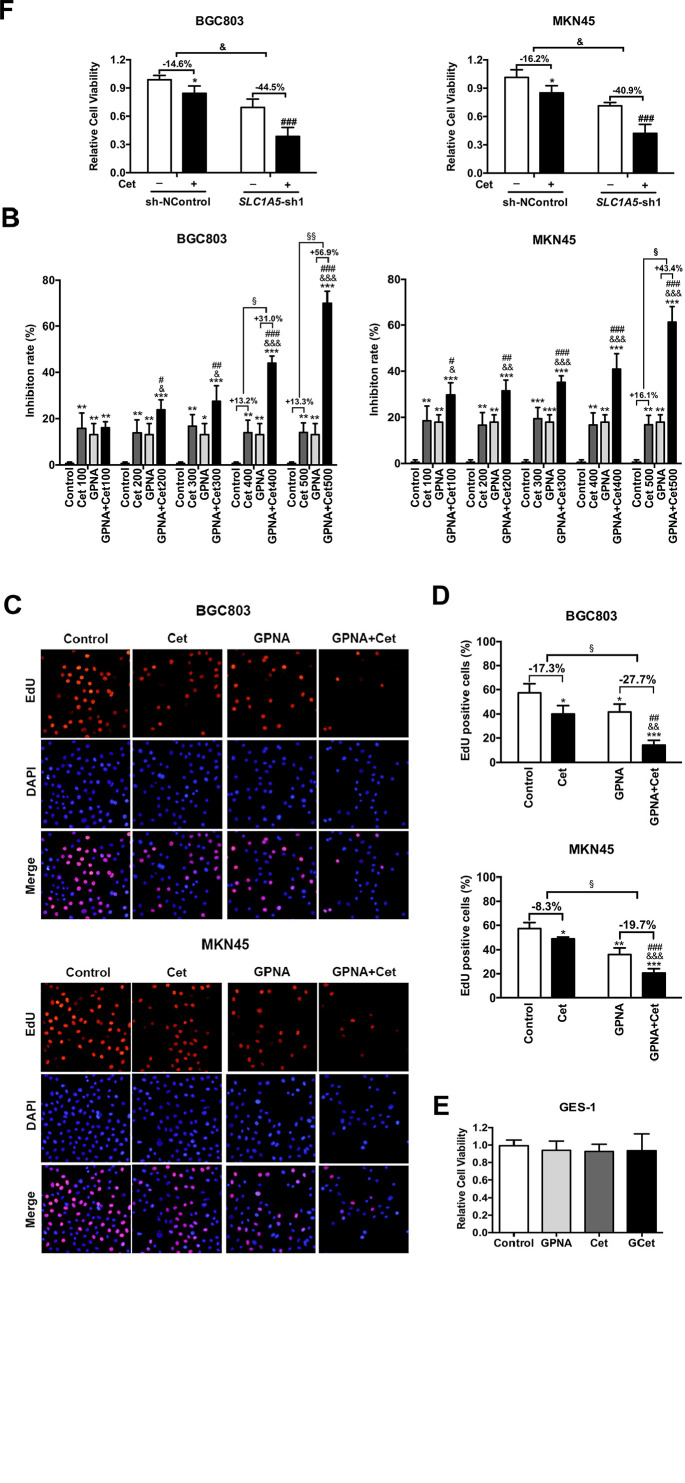
As shown in Figure 3A, cetuximab had a weak impact on inhibiting the viability of BGC803 and MKN45 cells, although the concentration of cetuximab was progressively increased. The inhibition rate of the BGC803 and MKN45 cell viability was increased by combined treatment with cetuximab + 10 mM GPNA, as compared to single treatment (Figure 3B). Moreover, while using GPNA simultaneously, the inhibitory effect of cetuximab was significantly enhanced by 31.0%, 56.9% and 43.4% when the concentration was increased to 400, 500 and 500 μg/ml in BGC803 and MKN45 cells, respectively (Figure 3B). Furthermore, the EdU assay also showed that the proliferation of BGC803 and MKN45 cells was significantly suppressed by treatment with cetuximab, GPNA, and GPNA + cetuximab, and among which, the inhibitory effect of the combination therapy was more significant than that of the monotherapy (Figure 3C). The quantitative results showed that without the GPNA combination, cetuximab reduced the EdU positive cells by only 17.3% and 8.3% in BGC803 and MKN45 cells, respectively. However, when combined with GPNA, cetuximab increased the inhibitory rate to 27.7% and 19.7% in BGC803 and MKN45 cells, respectively (Figure 3D). Interestingly, neither cetuximab alone nor in combination with 10 mM GPNA affected the viability of GES-1 significantly, suggesting that the combined therapy was relatively safe and reliable (Figure 3E). To further state the significance of ASCT2 in affecting the response of gastric cancer to cetuximab, we checked the efficacy of cetuximab on gastric cancer cells when ASCT2 expression was suppressed. The MTT test showed that the cell response to cetuximab was effectively improved when SLC1A5 was silenced in BGC803 and MKN45 cells (Figure 3F). These data indicated that interference with ASCT2 sensitized gastric cancer cells to cetuximab, with modest effect on noncancerous cells.
Figure 3.
Blockade of ASCT2 enhances the inhibitory effect of cetuximab and combined treatment significantly suppresses the proliferation of gastric cancer cells. (A, B) MTT assay showing cellular viability after treatment with cetuximab, GPNA, and cetuximab + GPNA for 48 hours. (C, D) Representative plots of EdU assay and quantitative results of cells after 48 hours of different treatments. (E) Cell viability of GES-1 treated with cetuximab, GPNA, and cetuximab + GPNA for 48 hours. (F) MTT assay proved that targeting SLC5A1 by shRNA significantly enhanced the inhibitory effect of cetuximab on gastric cancer cell lines (BGC803 and MKN45). One-way ANOVA.
Student’s t-test.
Abbreviations: Cet, cetuximab; SLC1A5, solute carrier 1 family member 5; GPNA, l-γ-glutamyl-p-nitroanilide.
*P < .05. **P < .01 and ***P < .001 versus control; #P < .05, ##P < .01 and ###P < .001 versus GPNA; &P < .05, &&P < .01 and &&&P < .001 versus cetuximab. §P < .05 and §§P < .01.
Combined Targeting Against Glutamine Uptake and EGFR Induces Cell Apoptosis in Gastric Cancer
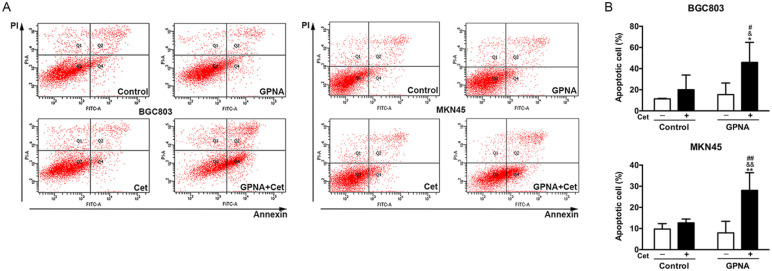
Flow cytometry was used to detect the effect of GPNA and cetuximab on cell apoptosis in gastric cancer. It was found that 48 hours of treatment with 500 μg/ml cetuximab or 10 mM GPNA had no significant effect on the apoptosis of BGC803 and MKN45 cells. However, 48 hours of combined treatment significantly induced apoptosis in BGC803 (46%) and MKN45 (28%) cells, as compared to either control or single treatment (Figure 4A and B). These results further suggested that the combined targeting of glutamine uptake and EGFR could induce cell apoptosis in gastric cancer.
Figure 4.
Combined treatment with GPNA and cetuximab induces gastric cancer cell apoptosis. (A) Representative maps of flow cytometry and quantitative analysis results of BGC803 and MKN45 cells after 48 hours of different treatments. One-way ANOVA.
Abbreviations: Cet, cetuximab; GPNA, l-γ-glutamyl-p-nitroanilide.
*P < .05 and **P < .01 versus control; #P < .05 and ##P < .01 versus GPNA; &P < .05 and &&P < .01 versus cetuximab.
Combined Blockage of Glutamine Uptake and EGFR Significantly Suppressed the Proliferation of Gastric Cancer In Vivo
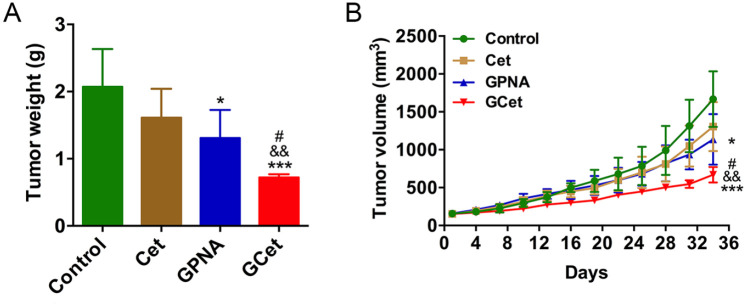
In vivo experiments were performed on mouse xenografts with BGC803 cells to investigate the effect of GPNA, cetuximab, and combined treatment on gastric tumor growth. During procedures, both GPNA and combined treatment successfully reduced the tumor weight and volume, and the combined treatment was better than single treatment in inhibiting tumor growth (0.72 vs 1.31 g for tumor weight; 669 vs 1136 mm3 for tumor volume; Figure 5A and B). In addition, no significant differences in tumor weight and volume were observed between the control and cetuximab groups. By contrast, the tumor weight and volume were significantly reduced by GPNA + cetuximab combination treatment (0.72 vs 1.61 g for tumor weight; 669 vs 1305 mm3 for tumor volume, respectively; Figure 5A and B). These results demonstrated that GPNA suppressed tumor growth and improved the inhibitory effect of cetuximab on gastric cancer, suggesting that combined blockage of glutamine uptake and EGFR significantly suppressed the proliferation of gastric cancer.
Figure 5.
Combined treatment with GPNA and cetuximab significantly suppresses tumor growth in mouse xenografts with BGC803 cells. (A, B) Development of (A) tumor weight and (B) tumor volume in 4 groups with different treatments. One-way ANOVA.
Abbreviations: Cet, cetuximab; GCet, GPNA + cetuximab, respectively; GPNA, l-γ-glutamyl-p-nitroanilide.
*P < .05 and ***P < .001 versus control; #P < .05 versus GPNA; &&P < .01 versus cetuximab.
Discussion
EGFR has an important role in gastric cancer proliferation and development, and its overexpression is associated with an unfavorable prognosis,20 making it an essential target for the treatment of gastric cancer. Cetuximab is a monoclonal antibody that competes with EGF for binding to EGFR and blocks the activation of EGFR.21 Despite its efficacy for the treatment of metastatic colorectal and head and neck cancer, the benefit of cetuximab in progression-free or overall survival for patients with advanced gastric cancer was observed neither in single therapy nor in combined treatment with chemotherapy drugs.22 Similarly, the present study showed that the efficacy of cetuximab on gastric cancer was insufficient in vitro (Figures 3 and 4) and lacking in vivo (Figure 5). Although there are several other monoclonal antibodies targeting EGFR, such as panitumumab and nimotuzumab, which have shown improvements for advanced gastric cancer, the median overall survival remains at <12 months.23 Amplifications, mutations, or overexpression of MET, HER2, or ERBB3 have been found to help the downstream signaling of EGFR remain activated, thereby reducing the tumor sensitivity to EGFR-targeted therapy.24-26 Several drugs targeting these amplifications or overexpression initially re-sensitize tumors to cetuximab, but generally fail due to drug resistance.25-27 Therefore, it is clinically relevant to explore other potential strategies to improve the efficacy of cetuximab on gastric cancer.
Cancer metabolism has been found to play a critical role in tumor growth and therapeutic resistance.6 As compared with targeting against signal transduction molecules, the strategy of directing metabolic molecules cannot easily induce drug resistance in cancer cells, due to the difficulties in compensating for the absence of the critical metabolic pathways.6,28 Therefore, investigating a key metabolic molecule that functions in cancer development and affects the sensitivity of cetuximab would be a promising alternative for treating patients with advanced gastric cancer. As an abundant metabolic nutrient, glutamine is involved in various processes, such as energy formation, macromolecular synthesis, redox homeostasis, and signaling transduction in cancer cells. The versatile features of glutamine metabolism make it an attractive target for clinical intervention.10 Decades ago, researchers discovered that the proliferation of both healthy human gastric mucosal cells and gastric cancer cells was stimulated by glutamine, and gastric cancer cells responded to glutamine at lower concentrations, as compared to healthy human gastric mucosal cells.29 ASCT2/SLC1A5, the key transporter for glutamine, was also found to be highly expressed in several types of cancer and to promote cancer development.12,14,30 Consistent with previous studies,15,31 the present study also showed that glutamine was essential for the growth of gastric cancer (Figure 1), and the expression of ASCT2 was significantly higher in gastric carcinoma than in normal gastric cells and tissues (Figure 2 and Supplemental Figure 1). In addition, the effect of glutamine on gastric carcinoma cells and normal gastric cells was compared, and it was found that gastric carcinoma cells were more sensitive to glutamine deprivation and SLC1A5 inhibition than normal gastric cells (Figures 1 and 2F–I). Both our results and those of other studies suggested that glutamine was an essential nutrient for the growth of gastric cancer, making the glutamine transporter ASCT2 an appealing target for gastric cancer treatment.
In addition to its vital role in promoting cancer development, ASCT2 also plays a role in an anti-cancer response. A study of Jeon et al32 found that knocking down the expression of SLC1A5/SLC38A2 not only inhibited glutamine uptake and breast cancer cell proliferation, but also improved cell response to paclitaxel. Another study showed that the expression of ASCT2 was significantly upregulated in breast cancer cell resistance to aromatase inhibitors, and the inhibition of ASCT2 effectively suppressed the proliferation of aromatase inhibitor-resistant breast cancer cells.33 These studies suggested that ASCT2 regulates the response to chemotherapy drugs.
In addition, ASCT2 was found to affect the efficacy of targeted drugs and to be involved in the function of cetuximab treatment. Tao et al. found that ASCT2 was physically associated with EGFR in a molecular complex, and this association involved the adaptor-related protein complex 1 gamma 1 subunit (AP1G1); however, the biological significance of the ASCT2-EGFR complex remains unclear. In the study of Lu, it was found that the ASCT2-EGFR complex could be targeted by cetuximab, resulting in the downregulation of SLC1A5 through cetuximab-induced EGFR endocytosis, a process independent of the cetuximab-mediated inhibition of EGFR tyrosine kinase, indicating that this mechanism might not involve EGFR signaling. Furthermore, the knockdown of AP1G1 was found to reduce the ASCT2-EGFR association and inhibit the cetuximab-mediated endocytosis of the ASCT2-EGFR complex, decreasing the cellular glutamine uptake.16,17 These findings suggested a new therapeutic strategy to improve cetuximab sensitivity by co-targeting of ASCT2, a process in which the inhibition of the EGFR downstream signaling pathways was unnecessary.16 In our previous study, it was found that ASCT2 was overexpressed in colorectal cancer with cetuximab resistance, and that the inhibition of ASCT2 significantly re-sensitized colorectal cancer to cetuximab.14 Consistently, Zhang et al30 found that the downregulation of ASCT2 improved the response to cetuximab in head and neck squamous cell carcinoma. Our study further showed that the inhibition of ASCT2 improved the response to cetuximab in gastric cancer. All these studies supported the possibility of enhancing the efficacy of cetuximab in gastric cancer.
This study demonstrated that the blockade of glutamine uptake enhanced the inhibitory effect of cetuximab on the proliferation of gastric cancer, and combined treatment further suppressed tumor growth. Combined targeting against glutamine uptake and EGFR may be a promising strategy for the treatment of advanced gastric cancer. However, there were still some limitations in this study. GPNA is not a selective inhibitor of ASCT2 and at least part of the effect of GPNA on gastric cancer cells may be caused by inhibition of other receptors. Thus, we added experiments that using SLC1A5-shRNA to inhibit the expression of ASCT2 and validated the effect of ASCT2 on the proliferation of gastric cancer and its effect on drug efficacy in this work. Besides, several other transporters such as SLC6A14, SLC7A5, SLC38A1, and SLC38A2 can also recognize glutamine as a substrate. As ASCT2 is overexpressed in many tumors including gastric cancer and shares specificity for glutamine,9 and because ASCT2 associates with EGFR,16,17 we did not pay much attention to other transporters but focused on the effect of ASCT2 on glutamine uptake and studied if inhibition of ASCT2 could affect the efficacy of cetuximab on gastric cancer. The associations between other glutamine transporters with cetuximab efficacy are also worthy of further intensive study.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354211045349 for Inhibition of Glutamine Uptake Improves the Efficacy of Cetuximab on Gastric Cancer by Huanrong Ma, Jingjing Wu, Minyu Zhou, Jianhua Wu, Zhenzhen Wu, Li Lin, Na Huang, Wangjun Liao and Li Sun in Integrative Cancer Therapies
Footnotes
Author Contributions: HM, LS, and WL conceived and designed the study. HM and JW performed most of the experiments. MZ and JW helped with the animal studies. ZW and NH contributed to the gene expression experiments. HM and LS analyzed the data and wrote the manuscript. WL revised the manuscript carefully.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant Nos. 81801720 and 81972292); and also supported by the Science and Technology Program of Guangzhou, China (202102020076).
Ethical Approval and Consent to Participate: The present study was approved by the Medical Ethics and Animal Care and Use Committees of the Nanfang Hospital and conducted in accordance with the ethical standards.
ORCID iD: Li Sun  https://orcid.org/0000-0002-0837-7422
https://orcid.org/0000-0002-0837-7422
Availability of Data and Materials: The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PDQ Adult Treatment Editorial Board. Gastric cancer treatment (PDQ®): health professional version. PDQ Cancer Information Summaries [Internet]. National Cancer Institute US:2021;https://www.ncbi.nlm.nih.gov/books/NBK65766/ [Google Scholar]
- 3.Zaanan A, Bouché O, Benhaim L, et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. 2018;50:768-779. [DOI] [PubMed] [Google Scholar]
- 4.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W.HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji L, Gu D, Tan X, Sun H, Chen J.A meta-analysis of clinical trials over regimens with or without cetuximab for advanced gastric cancer patients. J BUON. 2017;22:900-904. [PubMed] [Google Scholar]
- 6.Butler EB, Zhao Y, Muñoz-Pinedo C, Lu J, Tan M.Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance. Cancer Res. 2013;73:2709-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho TM, Cardoso HJ, Figueira MI, Vaz CV, Socorro S.The peculiarities of cancer cell metabolism: a route to metastasization and a target for therapy. Eur J Med Chem. 2019;171:343-363. [DOI] [PubMed] [Google Scholar]
- 8.Hirayama A, Kami K, Sugimoto M, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918-4925. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Zhang N, Tang T, Feng F, Sun H, Qu W.Target the human alanine/serine/cysteine transporter 2(ASCT2): achievement and future for novel cancer therapy. Pharmacol Res. 2020;158:104844. [DOI] [PubMed] [Google Scholar]
- 10.Hensley CT, Wasti AT, DeBerardinis RJ.Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Investig. 2013;123:3678-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Chen M, Tao Z, et al. Effects of targeting SLC1A5 on inhibiting gastric cancer growth and tumor development in vitro and in vivo. Oncotarget. 2017;8:76458-76467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Geldermalsen M, Wang Q, Nagarajah R, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassanein M, Hoeksema MD, Shiota M, et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19:560-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma H, Wu Z, Peng J, et al. Inhibition of SLC1A5 sensitizes colorectal cancer to cetuximab. Int J Cancer. 2018;142:2578-2588. [DOI] [PubMed] [Google Scholar]
- 15.Osanai-Sasakawa A, Hosomi K, Sumitomo Y, et al. An anti-ASCT2 monoclonal antibody suppresses gastric cancer growth by inducing oxidative stress and antibody dependent cellular toxicity in preclinical models. Am J Cancer Res. 2018;8:1499-1513. [PMC free article] [PubMed] [Google Scholar]
- 16.Tao X, Lu Y, Qiu S, Wang Y, Qin J, Fan Z.AP1G1 is involved in cetuximab-mediated downregulation of ASCT2-EGFR complex and sensitization of human head and neck squamous cell carcinoma cells to ROS-induced apoptosis. Cancer Lett. 2017;408:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, Li X, Lu Y, Qiu S, Fan Z.ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis. Cancer Lett. 2016;381:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma H, Liu Y, Shi M, et al. Theranostic, pH-responsive, doxorubicin-loaded nanoparticles inducing active targeting and apoptosis for advanced gastric cancer. Biomacromolecules. 2015;16:4022-4031. [DOI] [PubMed] [Google Scholar]
- 19.Szász AM, Lánczky A, Nagy Á, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322-49333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH.EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738-746. [DOI] [PubMed] [Google Scholar]
- 21.Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F.Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lordick F, Kang YK, Chung H-C, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [DOI] [PubMed] [Google Scholar]
- 23.Arienti C, Pignatta S, Tesei A.Epidermal growth factor receptor family and its role in gastric cancer. Front Oncol. 2019;9:1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raimúndez E, Keller S, Zwingenberger G, et al. Model-based analysis of response and resistance factors of cetuximab treatment in gastric cancer cell lines. PLoS Comput Biol. 2020;16:e1007147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Qian G, Zhang H, et al. HER3 targeting sensitizes HNSCC to cetuximab by reducing HER3 activity and HER2/HER3 dimerization: evidence from cell line and patient-derived xenograft models. Clin Cancer Res. 2017;23:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA.Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [DOI] [PubMed] [Google Scholar]
- 27.Silva VA, Lafont F, Benhelli-Mokrani H, et al. Rapid diminution in the level and activity of DNA-dependent protein kinase in cancer cells by a reactive nitro-benzoxadiazole compound. Int J Mol Sci. 2016;17:E703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick F.Mutant onco-proteins as drug targets: successes, failures, and future prospects. Curr Opin Genet Dev. 2011;21:29-33. [DOI] [PubMed] [Google Scholar]
- 29.Moyer MP, Armstrong A, Aust JB, Levine BA, Sirinek KR.Effects of gastrin, glutamine, and somatostatin on the in vitro growth of normal and malignant human gastric mucosal cells. Arch Surg. 1986;121:285-288. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Liu R, Shuai Y, et al. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br J Cancer. 2020;122:82-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Liu Y, Zhao TL, et al. Topotecan induces apoptosis via ASCT2 mediated oxidative stress in gastric cancer. Phytomedicine. 2019;57:117-128. [DOI] [PubMed] [Google Scholar]
- 32.Jeon YJ, Khelifa S, Ratnikov B, et al. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell. 2015;27:354-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Wang Y, Warden C, Chen S.Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J Steroid Biochem Mol Biol. 2015;149:118-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354211045349 for Inhibition of Glutamine Uptake Improves the Efficacy of Cetuximab on Gastric Cancer by Huanrong Ma, Jingjing Wu, Minyu Zhou, Jianhua Wu, Zhenzhen Wu, Li Lin, Na Huang, Wangjun Liao and Li Sun in Integrative Cancer Therapies