Abstract
Aims:
This post hoc analysis evaluated albumin/bilirubin (ALBI) score, an objective measure of liver function, in patients receiving pembrolizumab plus best supportive care (BSC) compared with placebo plus BSC in the KEYNOTE-240 study.
Methods:
Patients with confirmed hepatocellular carcinoma (HCC) and progression after/intolerance to sorafenib, Child–Pugh class A liver function, and Eastern Cooperative Oncology Group performance status of 0–1 were randomly assigned 2:1 to pembrolizumab 200 mg or placebo intravenously every 3 weeks plus BSC for ⩽35 cycles or until confirmed progression/unacceptable toxicity. Outcomes were assessed by ALBI grade.
Results:
Of 413 patients, at baseline 116 had an ALBI grade 1 score (pembrolizumab, n = 74; placebo, n = 42) and 279 had an ALBI grade 2 score (n = 193; n = 86). Change from baseline in ALBI score to the end of treatment was similar in both arms [difference in least squares mean, −0.039; 95% confidence interval (CI): −0.169 to 0.091]. Time to ALBI grade increase was similar in both arms [median for pembrolizumab versus placebo: 7.8 versus 6.9 months; hazard ratio (HR) = 0.863 (95% CI: 0.625–1.192)]. Regardless of baseline ALBI grade, a trend toward improved overall survival was observed with pembrolizumab [grade 1: HR = 0.725 (95% CI: 0.454–1.158); grade 2: HR = 0.827 (95% CI: 0.612–1.119)].
Conclusion:
Pembrolizumab did not adversely impact liver function compared with placebo in patients with HCC, as measured by changes in ALBI scores. A trend toward improved overall survival was observed with pembrolizumab in both ALBI grade groups.
ClinicalTrials.gov identifier: NCT02702401.
Keywords: albumin-bilirubin score, hepatocellular carcinoma, liver function, PD-1 inhibitor, pembrolizumab
Introduction
Liver cancer is a leading cause of cancer-related mortality.1 Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for 75–85% of all cases.1 Although surveillance and detection of HCC have improved over time, >50% of patients are diagnosed with advanced-stage disease [Barcelona Clinic Liver Cancer (BCLC) stage C or D] in most regions of the world (except for Taiwan and Japan).2
Systemic treatment options in the first-line setting for patients with advanced-stage HCC include sorafenib,3 lenvatinib,4 and most recently bevacizumab plus atezolizumab.5 For patients whose disease progresses on or who are unable to tolerate first-line treatment, second-line treatment options approved by US Food and Drug Administration include regorafenib,6 cabozantinib,7 ramucirumab (in patients with an alpha-fetoprotein level of ⩾400 ng/mL),8 nivolumab plus ipilimumab,9 and pembrolizumab.10,11
Pembrolizumab, a programmed death receptor 1 (PD-1)–blocking antibody, demonstrated antitumor activity and a favorable safety profile in patients with advanced HCC who were previously treated with sorafenib in the KEYNOTE-224 study [ClinicalTrials.gov identifier NCT02702414].10 Based on the results of KEYNOTE-224, pembrolizumab received accelerated approval for the treatment of patients with HCC who have previously received sorafenib.12 In a subsequent study that examined pembrolizumab plus best supportive care (BSC) compared with placebo plus BSC in patients with advanced HCC who were previously treated with sorafenib—KEYNOTE-240 [ClinicalTrials.gov identifier NCT02702401]—similar clinically significant but not statistically significant findings were observed.11 Although the findings of KEYNOTE-240 did not meet prespecified statistical criteria, the study demonstrated a favorable benefit-to-risk profile for pembrolizumab.
The prognosis of HCC is affected by underlying liver function.13 Markers of liver function include Child–Pugh score and albumin/bilirubin (ALBI) grade.14 Baseline ALBI grade has been identified as a prognostic indicator in HCC for patients receiving sorafenib15,16 and lenvatinib17 in the first-line treatment setting and cabozantinib18 and ramucirumab19 in the second-line treatment setting. The impact of baseline ALBI grade on outcomes in patients with HCC receiving pembrolizumab has not been established. Here, we present the results of a post hoc analysis from the KEYNOTE-240 study that examined outcomes by ALBI grade and liver function deterioration by increase in ALBI grade in patients with advanced HCC receiving pembrolizumab plus BSC compared with placebo plus BSC in the second-line treatment setting.
Materials and methods
Study design and patients
This was a retrospective analysis of the KEYNOTE-240 study, a double-blind, placebo-controlled, randomized, phase III study.11 Details of the study design, inclusion/exclusion criteria, primary and secondary outcome measures, and primary results have been published.11 In brief, adults with radiographically or pathologically confirmed HCC, radiographic progression after/intolerance to sorafenib, Child–Pugh A disease, BCLC stage C disease or stage B disease not amenable to/refractory to locoregional therapy, and Eastern Cooperative Oncology Group performance status of 0–1 were eligible for participation in the study. Prior therapy with an anti–PD-1, anti–programmed death ligand 1, or anti–programmed death ligand 2 agent or prior systemic therapy for advanced HCC other than sorafenib was an exclusion criterion. Patients were randomly assigned 2:1 to receive pembrolizumab 200 mg or saline placebo intravenously once every 3 weeks plus BSC for ⩽35 cycles or until confirmed progression/unacceptable toxicity, patient withdrawal of consent, or investigator decision.
The study protocol and all amendments were approved by the relevant ethics committee or institutional review board at each participating center, and the study was conducted in accordance with standards of Good Clinical Practice and the Declaration of Helsinki. The study protocol and the name of each ethics committee/institutional review board at each participating center including approval numbers are shown in the Supplemental material Table 1 online. All participants provided written informed consent. All authors had access to the study data and reviewed and approved the final manuscript for publication.
Assessments and endpoints
Response was assessed by blinded independent central review (BICR) according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). Endpoints included in this post hoc analysis were change from baseline in ALBI score, time to ALBI grade increase, overall survival (OS), progression-free survival (PFS) based on BICR as per RECIST v1.1, and objective response rate (ORR) based on BICR as per RECIST v1.1.
Statistical analysis
In this post hoc analysis, efficacy was assessed for the intention-to-treat population (all randomized patients). Time to ALBI grade increase was assessed in the as-treated population (all randomized patients who received ⩾1 dose of study treatment).
The baseline ALBI score was determined when both albumin and bilirubin results were available prior to treatment start, and the on-treatment ALBI score was determined when either albumin or bilirubin results were available at the specified day (if one of the parameters was missing, it was carried forward from the nearest prior on-treatment measurement), by log10 (bilirubin × 0.66) + (albumin × −0.085), where bilirubin is in μmol/L and albumin is in g/L. ALBI score ⩽–2.60 was equivalent to ALBI grade 1; ALBI score >−2.60 to ⩽−1.39 was equivalent to ALBI grade 2; and ALBI score >−1.39 was equivalent to ALBI grade 3.14 The association of change from baseline in ALBI score with treatment was assessed using analysis of covariance (ANCOVA), which evaluates whether the means of change from baseline in ALBI score are equal between two treatment arms while statistically controlling for the effect of the baseline ALBI score. The least squares mean that is adjusted for the baseline effect is obtained from the ANCOVA model for each treatment arm. Difference in least squares means between two treatment arms and 95% confidence intervals (CIs) is presented.
Time to ALBI grade increase was defined as the time from baseline ALBI measurement to the first postbaseline ALBI measurement that is ⩾1 grade higher than baseline ALBI grade. An exploratory analysis was conducted to evaluate time to ALBI grade increase in responders and non-responders receiving pembrolizumab. Responders were defined as patients with a best objective response of confirmed complete response or partial response based on BICR assessment per RECIST v1.1; patients who had stable disease, progressive disease, no assessment, or were not evaluable were defined as non-responders. In this analysis, ALBI grade was calculated when either albumin and bilirubin results were available at the given day. The missing value of the test was carried forward from the previous non-missing result. Hazard ratio (HR) from an unstratified Cox regression model with Efron’s method of tie handling with responder/non-responder status as a covariate is reported. OS and PFS curves were estimated using the Kaplan–Meier method for censored data. HRs from a stratified Cox regression model with Efron’s method of tie handling with treatment as a covariate are reported. Treatment difference in ORR was determined based on the stratified Miettinen and Nurminen method. In all stratified analyses, stratification was performed by factors used for randomization with small strata collapsed as prespecified in the statistical analysis plan. The data cutoff for this analysis was 2 January 2019.
Results
Patients
In KEYNOTE-240, a total of 413 patients were randomly assigned to pembrolizumab plus BSC (n = 278) or placebo plus BSC (n = 135) (Supplemental Figure 1).11 Of the 413 patients included in the intention-to-treat population, 407 patients had baseline ALBI grade available. Of the 407 patients, 116 had baseline ALBI grade 1 (pembrolizumab plus BSC, n = 74; placebo plus BSC, n = 42), 279 had baseline ALBI grade 2 (pembrolizumab plus BSC, n = 193; placebo plus BSC, n = 86), and 12 had baseline ALBI grade 3 (pembrolizumab plus BSC, n = 7; placebo plus BSC, n = 5). Data for patients with ALBI grade 3 were not analyzed because of the small sample size. A total of three of the 407 patients were excluded from the change from baseline analysis because postbaseline ALBI scores were not available.
Baseline demographics and disease characteristics were generally similar in patients with ALBI grade 1 and 2, except that a greater proportion of patients from Asia (excluding Japan) had ALBI grade 1 than ALBI grade 2 at baseline. Additionally, greater proportions of patients with ALBI grade 1 had BCLC stage C and extrahepatic disease, whereas greater proportions of patients with ALBI grade 2 had BCLC stage B and macrovascular invasion (Table 1). Among patients with ALBI grade 1, median duration of follow-up (where duration of follow-up is defined as time from randomization to database cut-off) was 21.3 months (range, 13.4–30.4) for pembrolizumab plus BSC and 22.9 months (13.3–29.0) for placebo plus BSC. Among patients with ALBI grade 2, median duration of follow-up was 21.3 months (range, 13.4–29.5) for pembrolizumab plus BSC and 21.2 months (13.6–29.5) for placebo plus BSC.
Table 1.
Patient demographics according to baseline ALBI grade.
| ALBI grade 1 | ALBI grade 2 | |||
|---|---|---|---|---|
| Characteristic | Pembrolizumab + BSC (n = 74) | Placebo + BSC (n = 42) | Pembrolizumab + BSC (n = 193) | Placebo + BSC (n = 86) |
| Age, median (range), years | 66.0 (21–82) | 64.5 (23–81) | 67.0 (18–91) | 65.5 (26–89) |
| Sex | ||||
| Male | 54 (73.0) | 34 (81.0) | 163 (84.5) | 72 (83.7) |
| Female | 20 (27.0) | 8 (19.0) | 30 (15.5) | 14 (16.3) |
| Region | ||||
| Asia (excluding Japan) | 24 (32.4) | 13 (31.0) | 43 (22.3) | 18 (20.9) |
| Europe | 27 (36.5) | 12 (28.6) | 65 (33.7) | 28 (32.6) |
| Japan | 5 (6.8) | 5 (11.9) | 34 (17.6) | 14 (16.3) |
| USA | 1 (1.4) | 2 (4.8) | 19 (9.8) | 12 (14.0) |
| Rest of worlda | 17 (23.0) | 10 (23.8) | 32 (16.6) | 14 (16.3) |
| ECOG PS | ||||
| 0 | 43 (58.1) | 29 (69.0) | 115 (59.6) | 37 (43.0) |
| 1 | 31 (41.9) | 13 (31.0) | 78 (40.4) | 49 (57.0) |
| Child–Pugh class | ||||
| A | 74 (100.0) | 42 (100.0) | 192 (99.5) | 85 (98.8) |
| A5 | 71 (95.9) | 42 (100.0) | 104 (53.9) | 43 (50.0) |
| A6 | 3 (4.1) | 0 | 88 (45.6) | 42 (48.8) |
| B | 0 | 0 | 1 (0.5) | 1 (1.2) |
| Overall BCLC stage | ||||
| B | 11 (14.9) | 5 (11.9) | 44 (22.8) | 23 (26.7) |
| C | 63 (85.1) | 37 (88.1) | 149 (77.2) | 63 (73.3) |
| HBV infectionb | 26 (35.1) | 11 (26.2) | 46 (23.8) | 18 (20.9) |
| HCV infectionc | 8 (10.8) | 1 (2.4) | 34 (17.6) | 20 (23.3) |
| Discontinuation of previous sorafenib | ||||
| Intolerance | 8 (10.8) | 5 (11.9) | 26 (13.5) | 11 (12.8) |
| PD | 66 (89.2) | 37 (88.1) | 167 (86.5) | 75 (87.2) |
| Extrahepatic disease | 59 (79.7) | 34 (81.0) | 127 (65.8) | 54 (62.8) |
| Macrovascular invasion | 4 (5.4) | 3 (7.1) | 28 (14.5) | 12 (14.0) |
| AFP ⩾200 ng/mL | 33 (44.6) | 17 (40.5) | 90 (46.6) | 37 (43.0) |
Includes Argentina, Australia, Canada, Chile, Colombia, Israel, Mexico, Norway, Russian Federation, and Turkey.
HBV infection defined as hepatitis B surface antigen positive and/or detectable HBV DNA.
HCV infection defined as anti-hepatitis C antibody positive and detectable HCV RNA.
AFP, alpha-fetoprotein; ALBI, albumin/bilirubin; BCLC, Barcelona Clinic Liver Cancer scale; BSC, best supportive care; ECOG PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; PD, progressive disease.
Change from baseline in ALBI score
Overall, change from baseline in ALBI score to the last available ALBI score measurement was similar between the pembrolizumab plus BSC and placebo plus BSC arms (difference in least squares mean, −0.039; 95% CI: −0.169 to 0.091). Similarly, change in baseline ALBI score in both arms was comparable when analyzed by ALBI grade (between-group difference in least squares mean, −0.023; 95% CI: −0.266 to 0.220 for ALBI grade 1 and −0.035; 95% CI: −0.196 to 0.126 for ALBI grade 2).
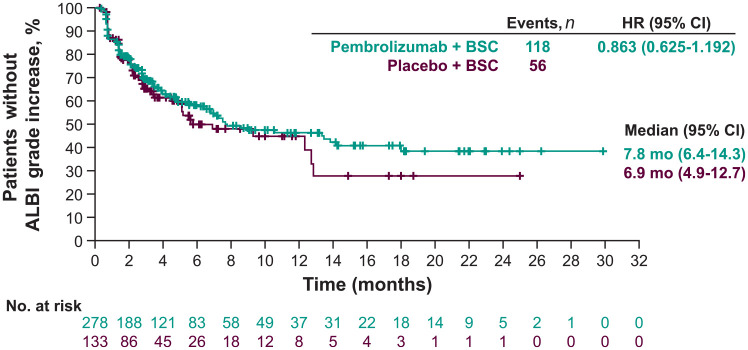
Time to ALBI grade increase
Overall, median time to ALBI grade increase (i.e. time to deterioration of liver function) was not different between the pembrolizumab plus BSC and placebo plus BSC arms (7.8 versus 6.9 months; HR = 0.863; 95% CI: 0.625–1.192 based on the as-treated population of 411 patients) (Supplemental Table 2; Figure 1). Similar results were observed when comparing the pembrolizumab plus BSC and placebo plus BSC arms for patients with ALBI grade 1 (3.4 versus 2.6 months; HR = 0.861; 95% CI: 0.534–1.388) and grade 2 (18.0 versus 12.7 months; HR = 0.928; 95% CI: 0.589–1.460), with the median time numerically longer in the pembrolizumab plus BSC arm for patients in each ALBI grade 1 and grade 2 subgroup, which suggests that pembrolizumab plus BSC does not worsen liver function compared with placebo plus BSC. Median time to ALBI grade increase was not reached in responders and was 6.8 months in non-responders receiving pembrolizumab plus BSC (HR = 0.411; 95% CI: 0.241–0.698), indicating that liver function was better preserved in responders to pembrolizumab plus BSC compared with non-responders.
Figure 1.
Kaplan–Meier estimates of time to ALBI grade increase.a,b
aTime to ALBI grade increase was defined as the time from baseline ALBI measurement to the first postbaseline ALBI measurement that is ⩾1 grade higher than baseline ALBI grade.
bData are reported for the as-treated population (n = 411).
ALBI, albumin/bilirubin; BSC, best supportive care; CI, confidence interval; HR, hazard ratio; mo, months.
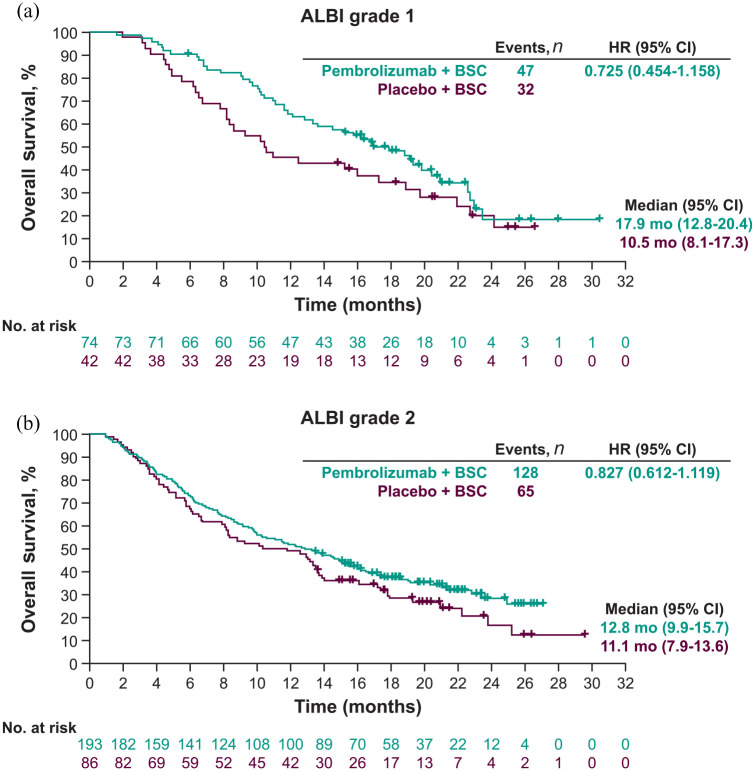
OS and PFS by ALBI grade
There was a trend toward improved OS with pembrolizumab plus BSC versus placebo regardless of baseline ALBI grade. In patients with ALBI grade 1, the median OS was 17.9 months (95% CI: 12.8–20.4) with pembrolizumab plus BSC compared with 10.5 months (95% CI: 8.1–17.3) with placebo plus BSC (HR = 0.725; 95% CI: 0.454–1.158) [Figure 2(a)]. In patients with ALBI grade 2, the median OS was 12.8 months (95% CI: 9.9–15.7) with pembrolizumab plus BSC versus 11.1 months (95% CI: 7.9–13.6) with placebo plus BSC (HR = 0.827; 95% CI: 0.612–1.119) [Figure 2(b)].
Figure 2.
Kaplan–Meier estimates of overall survival by (a) ALBI grade 1 and (b) ALBI grade 2.a
aBased on BICR as per RECIST v1.1.
ALBI, albumin/bilirubin; BICR, blinded independent central review; BSC, best supportive care; CI, confidence interval; HR, hazard ratio; mo, months; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
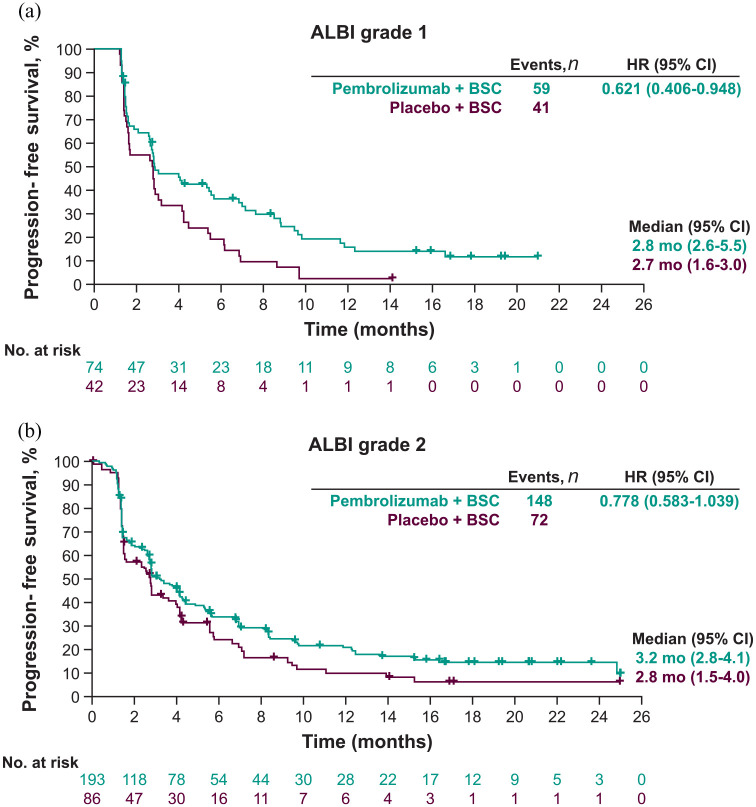
Similar to the results observed for OS, pembrolizumab plus BSC also showed a trend toward improved PFS compared with placebo plus BSC regardless of ALBI grade. In patients with ALBI grade 1, the median PFS was 2.8 months (95% CI: 2.6–5.5) with pembrolizumab plus BSC versus 2.7 months (95% CI: 1.6–3.0) with placebo plus BSC (HR = 0.621; 95% CI: 0.406–0.948) [Figure 3(a)]. In patients with ALBI grade 2, the median PFS was 3.2 months (95% CI: 2.8–4.1) with pembrolizumab plus BSC versus 2.8 months (95% CI: 1.5–4.0) with placebo plus BSC (HR = 0.778; 95% CI: 0.583–1.039) [Figure 3(b)].
Figure 3.
Kaplan–Meier estimates of progression-free survival by (a) ALBI grade 1 and (b) ALBI grade 2.a
aBased on BICR as per RECIST v1.1.
ALBI, albumin/bilirubin; BICR, blinded independent central review; BSC, best supportive care; CI, confidence interval; HR, hazard ratio; mo, months; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Response by ALBI grade
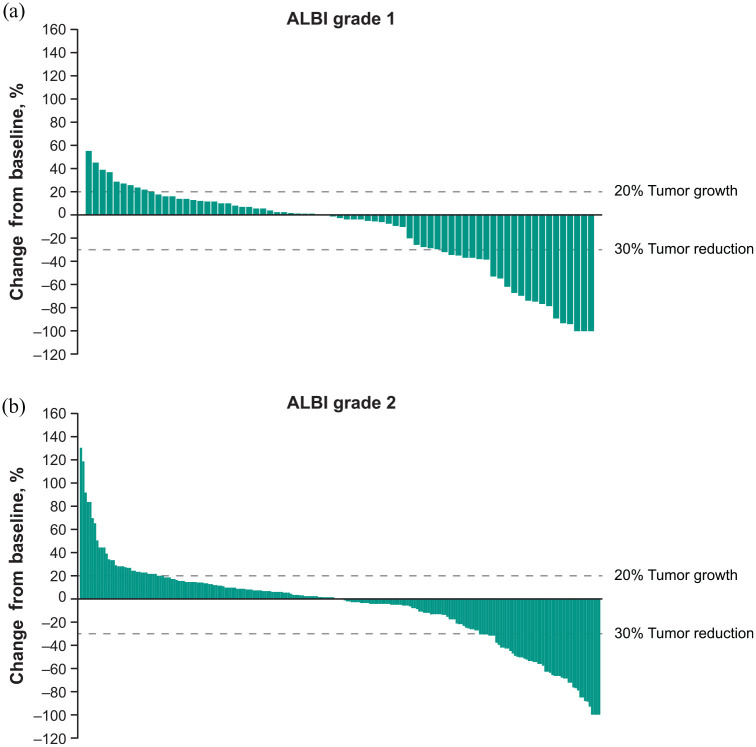
Although the difference in ORR assessed as per RECIST v1.1 between pembrolizumab plus BSC and placebo plus BSC was numerically greater for patients with ALBI grade 1 compared with ALBI grade 2 (21.7% versus 9.7%), a clinically meaningful ORR was observed in both subgroups. In patients with ALBI grade 1, the ORR was 24.3% (95% CI: 15.1–35.7) with pembrolizumab plus BSC compared with 2.4% (95% CI: 0.1–12.6) with placebo plus BSC (estimated difference 21.7%; 95% CI: 9.1–33.2). In patients with ALBI grade 2, the ORR was 15.5% (95% CI: 10.7–21.4) with pembrolizumab plus BSC versus 5.8% (95% CI: 1.9–13.0) with placebo plus BSC (estimated difference 9.7%; 95% CI: 1.5–16.4). Reductions from baseline in tumor target lesion size in patients with ALBI grade 1 and ALBI grade 2 who received pembrolizumab plus BSC are shown in Figure 4.
Figure 4.
Best percentage changes from baseline in size of target lesions by (a) ALBI grade 1 and (b) ALBI grade 2.a
aBased on BICR per RECIST v1.1.
ALBI, albumin/bilirubin; BICR, blinded independent central review; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Following disease progression, subsequent anticancer therapies were used by similar proportions of patients with ALBI grade 1 [pembrolizumab plus BSC, 47.3% (n = 35); placebo plus BSC, 52.4% (n = 22)] and grade 2 [pembrolizumab plus BSC, 41.5% (n = 80); placebo plus BSC, 43.0% (n = 37)]. Therefore, differences in hepatic reserve at baseline as measured by ALBI grade did not influence poststudy anticancer therapy use in either treatment group.
Discussion
In this post hoc analysis of the KEYNOTE-240 study, change in ALBI grade from baseline was not adversely affected by pembrolizumab plus BSC. Time to ALBI grade increase was similar in both the pembrolizumab plus BSC and placebo plus BSC arms, regardless of baseline ALBI grade. The median time to ALBI grade increase was numerically longer in the pembrolizumab plus BSC arm for patients in each ALBI grade 1 and grade 2 subgroup, suggesting that pembrolizumab plus BSC does not worsen liver function compared with placebo plus BSC. Furthermore, median time to ALBI grade increase among patients who achieved a best overall response of complete or partial response with pembrolizumab plus BSC was not reached compared with 6.8 months in patients who had stable disease, progressive disease, no assessment, or were not evaluable, indicating that liver function was better preserved in those who responded to pembrolizumab plus BSC compared with all other patients. The trend toward improvement in OS and PFS observed with pembrolizumab plus BSC compared with placebo plus BSC was maintained in each ALBI grade 1 and 2 subgroup. In addition, clinically meaningful improvement in ORR was observed with pembrolizumab plus BSC compared with placebo plus BSC in each ALBI grade 1 and 2 subgroup.
Previous studies examining baseline ALBI grade on outcomes in patients with advanced HCC report findings generally consistent with those observed in the current study. In the first-line treatment setting, a post hoc analysis of the phase III REFLECT study showed that patients receiving lenvatinib had superior response rates and improved PFS and OS outcomes compared with sorafenib regardless of ALBI grade.17 In the second-line treatment setting, an analysis of the phase III CELESTIAL study, which examined cabozantinib versus placebo in sorafenib-treated patients who received ⩽2 lines of therapy, superior survival outcomes (PFS and OS) were observed with cabozantinib versus placebo regardless of baseline ALBI grade.18 In the current study, there was a trend toward improved OS and PFS with pembrolizumab plus BSC versus placebo regardless of baseline ALBI grade. Median OS was numerically longer in patients receiving pembrolizumab plus BSC who had lower baseline ALBI grade. In the placebo group, while the early on-treatment OS was better among the patients with ALBI grade 1, median OS was not appreciably different in patients who had ALBI grade 1 and those who had ALBI grade 2. This observation likely is due to large variability of the estimate of the median in the small subgroups of patients receiving placebo plus BSC who had baseline ALBI grade 1 and 2.
Limitations
Limitations of this analysis include its post hoc exploratory design and the fact that outcomes for patients with baseline ALBI grade 3 could not be analyzed due to the small sample size. Furthermore, at the time KEYNOTE-240 was initiated, limited safety data were available for pembrolizumab in HCC. As such, a cautious approach was taken and the efficacy and safety of pembrolizumab was first evaluated in patients with well-preserved liver function before examining the effects in patients with compromised liver function. Therefore, the results reported herein may not be generalizable to patients with more advanced liver disease; however, increased use of pembrolizumab will allow the collection of additional information on this patient population. Moreover, in patients with advanced HCC, it is often difficult to determine what is causing deterioration in liver function because the tumor may be responsible for the decline or there may be deterioration in baseline liver function.
Conclusions
Pembrolizumab did not adversely impact liver function compared with placebo in patients with HCC, as measured by changes in ALBI scores. Furthermore, a trend toward improvement in OS with pembrolizumab plus BSC was observed in patients in each ALBI grade 1 and 2 subgroup.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211039928 for ALBI score and outcomes in patients with hepatocellular carcinoma: post hoc analysis of the randomized controlled trial KEYNOTE-240 by Arndt Vogel, Philippe Merle, Chris Verslype, Richard S. Finn, Andrew X. Zhu, Ann-Lii Cheng, Stephen Lam Chan, Thomas Yau, Baek-Yeol Ryoo, Jennifer Knox, Bruno Daniele, Shukui Qin, Ziwen Wei, Yanna Miteva, Usha Malhotra, Abby B. Siegel and Masatoshi Kudo in Therapeutic Advances in Medical Oncology
Acknowledgments
Medical writing and editorial assistance were provided by Lauren D’Angelo, PhD, of ApotheCom (Yardley, PA, USA), and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Prior presentation: presented in part as an e-poster presentation at the European Society for Medical Oncology (ESMO) Virtual Congress 2020, Madrid, Spain, 18–22 September 2020.
Footnotes
Conflict of interest statement: A. Vogel has received fees from Roche, Eisai, Eli Lilly, Bristol Myers Squibb, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Bayer; P. Merle has participated in advisory boards for Bayer, Eisai, Eli Lilly, Roche, AstraZeneca, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and has received a grant from and participated in an advisory board for Ipsen; C. Verslype has received a grant for research, fees for speaking, and fees for serving as a consultant for Bayer, has received a grant for research and fees for serving as a consultant for Ipsen, and has received fees for serving as a consultant for Roche and Eli Lilly; R.S. Finn has received fees and a grant to his institution from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Bayer, Eli Lilly, Bristol Myers Squibb, Eisai, Pfizer, and Roche/Genentech, and fees from AstraZeneca and CStone; A.X. Zhu reports financial activities from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Eli Lilly, Bayer, Sanofi-Aventis, Eisai, Exelixis, and Roche as potential conflicts of interest; A-L Cheng has received fees for consulting from AstraZeneca, Bristol Myers Squibb, Eisai, Merck Serono, Novartis, Ono Pharmaceutical, Exelixis, Nucleix, Ipsen Innovation, Bayer Healthcare, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Roche/Genentech, BeiGene, CSR Pharma Group, F. Hoffmann-La Roche, and IQVIA, has received travel support from Roche/Genentech, IQVIA, and Bayer Yakuhin, and has received speaker fees from Eisai, Novartis, Ono Pharmaceutical, Bayer Yakuhin, and Amgen Taiwan; S.L. Chan, B-Y Ryoo, S. Qin have nothing to disclose; T. Yau reports a consulting or advisory role for Bristol Myers Squibb, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Exelixis, Ipsen, Eisai, AstraZeneca, Bayer, Novartis, EMD Serono, AbbVie, Pfizer, Eli Lilly, Sirtex, SillaJen, Taiho, OrigiMed, New B Innovation, and H3 Biomedicine, and has received honoraria from Bristol Myers Squibb, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Exelixis, Ipsen, Eisai, AstraZeneca, Bayer, Novartis, EMD Serono, AbbVie, Pfizer, Eli Lilly, Sirtex, SillaJen, Taiho, OrigiMed, New B Innovation, and H3 Biomedicine; J. Knox has received a grant for an investigator-initiated study from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and has received fees for consulting from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Ibsen, and Roche; B. Daniele has received fees from Ipsen, Eisai, Eli Lilly, AstraZeneca, Sanofi, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Bayer, Roche, and Amgen, and non-financial support from Ipsen, Sanofi, and Bayer; Z. Wei, Y. Miteva, U. Malhotra, and A.B. Siegel are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA; M. Kudo has received a grant and fees from Eisai and EA Pharma, has received fees from Bayer, Bristol Myers Squibb, Eli Lilly, Ono Pharmaceutical, and Roche, and has received a grant from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, and AbbVie.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Data availability and materials: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Arndt Vogel, Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Carl-Neubergstrasse 1, Hannover, 30625, Germany.
Philippe Merle, Hôpital de la Croix-Rousse, Hospices Civils de Lyon, Lyon, France.
Chris Verslype, Katholieke Universiteit Leuven and University Hospitals Leuven and Leuven Cancer Institute, Leuven, Belgium.
Richard S. Finn, David Geffen School of Medicine at the University of California, Los Angeles, Los Angeles, CA, USA
Andrew X. Zhu, Massachusetts General Hospital Cancer Center and Harvard Medical School, Boston, MA, USA Jiahui International Cancer Center, Jiahui Health, Shanghai, China.
Ann-Lii Cheng, National Taiwan University Hospital and National Taiwan University Cancer Center, Zhongzheng District, Taipei.
Stephen Lam Chan, State Key Laboratory of Translational Oncology, Department of Clinical Oncology, Sir YK Pao Centre for Cancer, The Chinese University of Hong Kong, Hong Kong.
Thomas Yau, The University at Hong Kong, People’s Republic of China, Hong Kong.
Baek-Yeol Ryoo, Asan Medical Center, University of Ulsan College of Medicine, Songpa-gu, Seoul, South Korea.
Jennifer Knox, McCain Center for Pancreatic Cancer, University of Toronto, Toronto, ON, Canada.
Bruno Daniele, Ospedale del Mare, Naples, Italy.
Shukui Qin, Cancer Center of People’s Liberation Army, Jinling Hospital, Nanjing, China.
Ziwen Wei, Merck & Co., Inc., Kenilworth, NJ, USA.
Yanna Miteva, Merck & Co., Inc., Kenilworth, NJ, USA.
Usha Malhotra, Merck & Co., Inc., Kenilworth, NJ, USA.
Abby B. Siegel, Merck & Co., Inc., Kenilworth, NJ, USA
Masatoshi Kudo, Faculty of Medicine, Kindai University, Osaka-Sayama, Osaka, Japan.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Park J, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int 2015; 35: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, et al. Regorafe. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl J Med 2018; 379: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 282–296. [DOI] [PubMed] [Google Scholar]
- 9.Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol 2020; 6: e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020; 38: 193–202. [DOI] [PubMed] [Google Scholar]
- 12.KEYTRUDA® (pembrolizumab) injection, for intravenous use. Whitehouse Station, NJ: Merck Sharp & Dohme Corp, 2021. [Google Scholar]
- 13.Raoul JL, Bruix J, Greten TF, et al. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J Hepatol 2012; 56: 1080–1088. [DOI] [PubMed] [Google Scholar]
- 14.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015; 33: 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Rahman O.Impact of baseline characteristics on outcomes of advanced HCC patients treated with sorafenib: a secondary analysis of a phase III study. J Cancer Res Clin Oncol 2018; 144: 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo YH, Wang JH, Hung CH, et al. Albumin-bilirubin grade predicts prognosis of HCC patients with sorafenib use. J Gastroenterol Hepatol 2017; 32: 1975–1981. [DOI] [PubMed] [Google Scholar]
- 17.Vogel A, Frenette C, Sung MW, et al. Baseline liver function and outcomes in the phase III REFLECT study in patients with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 2020; 38: Abstract 524. [Google Scholar]
- 18.Chan SL, Miksad R, Cicin I, et al. Outcomes based on albumin-bilirubin (ALBI) grade in the phase III CELESTIAL trial of cabozantinib versus placebo in patients with advanced hepatocellular carcinoma (HCC). Ann Oncol 2019; 30: ix45–ix46. Abstract 127P. [Google Scholar]
- 19.Brandi G, Kudo M, Kang YK, et al. Ramucirumab for patients with hepatocellular carcinoma and elevated alpha-fetoprotein following sorafenib treatment: exploratory analysis of REACH-2 trial results by albumin-bilirubin grade and Child-Pugh score. Presented at EASL HCC Summit 2019, 14–16 February 2019. Lisbon, Portugal. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211039928 for ALBI score and outcomes in patients with hepatocellular carcinoma: post hoc analysis of the randomized controlled trial KEYNOTE-240 by Arndt Vogel, Philippe Merle, Chris Verslype, Richard S. Finn, Andrew X. Zhu, Ann-Lii Cheng, Stephen Lam Chan, Thomas Yau, Baek-Yeol Ryoo, Jennifer Knox, Bruno Daniele, Shukui Qin, Ziwen Wei, Yanna Miteva, Usha Malhotra, Abby B. Siegel and Masatoshi Kudo in Therapeutic Advances in Medical Oncology