Abstract
The chemokine ligand CCL2 and its receptor CCR2 are implicated in the initiation and progression of various cancers. CCL2 can activate tumour cell growth and proliferation through a variety of mechanisms. By interacting with CCR2, CCL2 promotes cancer cell migration and recruits immunosuppressive cells to the tumour microenvironment, favouring cancer development. Over the last several decades, a series of studies have been conducted to explore the CCL2‐CCR2 signalling axis function in malignancies. Therapeutic strategies targeting the CCL2‐ CCR2 axis have also shown promising effects, enriching our approaches for fighting against cancer. In this review, we summarize the role of the CCL2‐CCR2 signalling axis in tumorigenesis and highlight recent studies on CCL2‐CCR2 targeted therapy, focusing on preclinical studies and clinical trials.
The chemokine ligand CCL2 and its receptor CCR2 are implicated in the initiation and progression of various cancers. The CCL2‐CCR2 signalling axis plays a critical role in the promotion of pathological angiogenesis, the survival and invasion of tumour cells, and the recruitment of immune inhibitory cells. Therefore, CCL2 and CCR2 enable us to explore the sophisticated mechanisms underlying cancer development and provide potential options for treating malignant tumours.
1. INTRODUCTION
Chemokines are small‐molecule proteins that exert their functions by binding to G protein‐coupled chemokine receptors (GPCRs) expressed on the cell surface.1, 2, 3 Initially, chemokines were described as mediators that induced immune cell infiltration and migration to specific inflammatory response sites. Subsequent studies revealed that chemokines participated in different biological processes, especially in the development of malignant tumours.4, 5 Chemokines can interact with tumour cells and the tumour microenvironment, promoting tumour occurrence and progression.6 Therapeutic methods targeting chemokines and their receptors have been developed from bench to bedside and have shown promising prospects.
To date, over 50 chemokines and 19 different chemokine receptors have been identified in human beings.7 According to the number and spacing of the conserved cysteine residues in the N‐terminus, chemokines are categorized into four main subfamilies: CXC, CC, C and CX3C.3, 8 Most chemokines belong to the CC and CXC subfamilies. In the CC‐chemokine sub‐family, a total of five monocyte chemoattractant proteins (MCP) have been identified: CCL2 (MCP‐1), CCL8 (MCP‐2), CCL7 (MCP‐3), CCL13 (MCP‐4) and CCL12 (MCP‐5).9, 10 Among them, CCL2 has similar sequence homology with other family members. For example, CCL8 and CCL7 have 62% and 71% amino acid identity with CCL2, respectively.11 After binding to their corresponding ligands, chemokine receptors undergo conformational changes that make G proteins bind to intracellular loop epitopes and the carboxy‐terminal tail of the receptors. Chemokine receptor activation induces a series of intracellular signals, resulting in cell motility and exerting diverse biological effects in the related target cells.12, 13
CCL2, also known as monocyte chemoattractant protein‐1 (MCP‐1), was initially isolated and purified from the culture supernatants of peripheral blood mononuclear cells and tumour cell lines in 1989.14, 15, 16 CCL2 was the first discovered and well investigated CC chemokine, preferentially binding to its receptor CCR2.11 Previous studies indicated that the CCL2‐CCR2 signalling axis played a role in the promotion of pathological angiogenesis, the survival and invasion of tumour cells, and the recruitment of immune inhibitory cells.17, 18, 19 Consequently, CCL2 and CCR2 enable us to explore the sophisticated mechanisms underlying cancer development and provide potential options for treating malignant tumours. In the present review, we introduce the mechanisms of the CCL2‐CCR2 axis in the process of tumorigenesis. We also focus on the research progress on the CCL2‐CCR2 axis in both preclinical studies and clinical trials.
2. BIOLOGICAL CHARACTERISTICS OF THE CCL2‐CCR2 SIGNALLING AXIS
CCL2 is a 13 kDa protein composed of 76 amino acids, and its coding gene is mapped at human chromosome 17 (chr. 17, q11.2).11, 20 A wide range of cells can produce CCL2, including tumour cells, endothelial cells, fibroblasts, epithelial cells, smooth muscle cells and myeloid cells.21 Moreover, CCL2 is able to regulate the infiltration and migration of various cells such as monocytes, memory T lymphocytes and natural killer (NK) cells, playing critical roles in the immune response.22
CCL2 mainly binds to the receptor CCR2. Structurally, the N‐terminal tail at the end of CCL2 is a significant determinant of CCR2 binding affinity and efficacy.23 Functionally, the binding of CCL2 and its cognate receptor CCR2 is essential for initiating signal transduction pathways and stimulating cell migration. In addition to CCR2, CCL2 can bind to other receptors. For example, the binding of CCL2 and CCR4 is involved in mammary gland development and promotes breast cancer progression.24 Moreover, CCL2 can bind to atypical receptors (ACKR1 and ACKR2). ACKRs and typical chemokine receptors differ structurally in the substituted DRYLAIV amino acid motif in the second intracellular loop of the ACKR. These atypical receptors cannot generate signals through G proteins and lack chemotactic activity. Therefore, they are often referred to as ‘scavenger receptors’.25, 26 Despite this, multiple research results have shown that CCR2 was the primary receptor of CCL2.27
CCR2 belongs to the GPCRs and contains an N‐terminal extracellular domain and seven conserved transmembrane domains. Based on the difference in terminal carboxyl tails, CCR2 is divided into CCR2A and CCR2B (Figure 1), and they may function through different signalling pathways.28, 29 CCR2B is the predominant isoform of the CCR2 surface receptors and can be trafficked well to the cell surface, but CCR2A is detected predominantly in the cytoplasm.30 CCR2 functions by binding to different ligands, including CCL2, CCL7, CCL8, CCL12, CCL13 and CCL16, leading to the redundancy and promiscuity of the chemokine family. Nevertheless, it is this redundancy that has evolutionary significance for maintaining chemokine system activity and stability.31 Meanwhile, among the different ligands known to bind to CCR2, CCL2 has significantly higher activity than the other ligands.32
FIGURE 1.
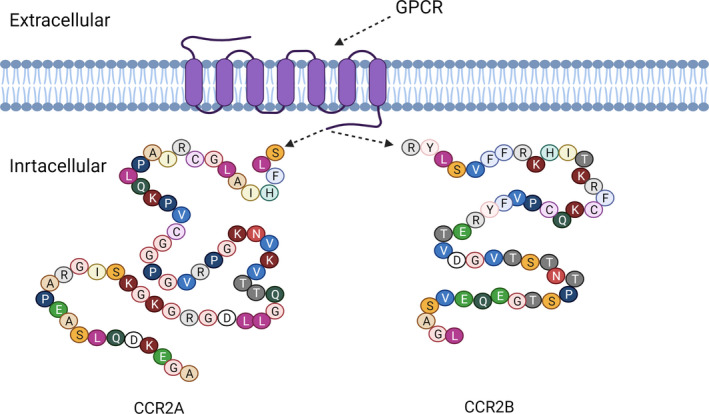
The structure of human CCR2A and CCR2B molecules. CCR2 belongs to the family of G protein‐coupled receptors (GPCRs) and exists as two splice variants that are CCR2A and CCR2B. CCR2A and CCR2B have been found to differ only in their terminal carboxyl tails
As the mainly functional receptor of CCL2, CCR2 is widely expressed by different types of cells, including dendritic cells (DCs),33 endothelial cells,34 monocytes28 and various cancer cells. Moreover, we also discover that CCR2 is expressed at low levels in neutrophils and lymphocytes.35 CCR2 upregulation is closely related to the recurrence and metastasis of advanced cancers. Once CCR2 is triggered by its ligand CCL2, various G protein‐mediated signalling cascades within the cell will be activated, such as the phosphatidylinositol 3‐kinase (PI3K)/AKT, mitogen‐activated protein kinase (MAPK)/p38 and Janus kinase (JAK)/STAT3.36, 37, 38 The activation of these signalling pathways plays a vital role in anti‐apoptosis, angiogenesis, and cell migration, leading to oncogenic advancement. The CCL2‐CCR2 axis is also implicated in the progression of numerous human diseases, such as inflammatory pain,39 atherosclerosis,40 neuroinflammatory diseases,41 rheumatoid arthritis42 and diabetic nephropathy.43 Therefore, the CCL2‐CCR2 axis is considered an appealing target for the treatment of these diseases.
3. ROLE OF THE CCL2‐CCR2 SIGNALLING AXIS IN CANCER PATHOGENESIS
It has been revealed that the CCL2‐CCR2 signalling axis participated in the occurrence and progression of a wide range of malignancies, such as breast cancer,44 prostate cancer,45 lung cancer,46 hepatocellular cancer,47 pancreatic cancer,48 nasopharyngeal carcinoma49 and renal cancer.50 Hence, numerous clinical studies have identified this axis as a predictor of early diagnosis and poor prognosis, which is shown in Table 1.
TABLE 1.
Role of CCL2‐CCR2 axis as a prognostic factor in various cancers
| Cancer types | Functions of the CCL2‐CCR2 axis |
|---|---|
| Prostate cancer | High serum CCL2 level is associated with poor overall survival and can be regarded as a diagnostic marker.178 |
| Breast cancer | CCL2 expression is an independent risk factor for disease‐free survival (DFS) and correlated with poor prognosis.105 |
| Lung adenocarcinoma | High expression of CCL2 is correlated with unfavourable overall survival (OS) and poor disease‐free survival (DFS).175 |
| Gastric cancer | CCL2 can be a predictive biomarker for patients prognosis and tumour aggressiveness.179, 180 |
| Colorectal cancer | High expression of CCL2 is associated with poor overall survival (OS) in colorectal cancer patients.181 |
| Pancreatic cancer | High level of serum CCL2 is strongly associated with poor survival and prognosis.182, 183 |
| Nasopharyngeal carcinoma | Overexpression of CCL2/CCR2 are significantly associated with poor overall survival (OS) in NPC patient.184, 185 |
| Cervical cancer | CCL2 expression is a marker of cervical cancer, and enhanced expression of CCR2 may correlate with poor overall survival.186 |
| Ovarian cancer | The overexpression of CCR2 shortens overall and progression‐free survival in patients with ovarian cancer.187 |
| Liver cancer | CCL2 is an independent prognostic factor for patients and appears to be a promising complementary biomarker for HCC diagnosis.188, 189 |
| Kidney cancer | The CCL2/CCR2 axis can be regarded as an independent prognostic factor for non‐metastatic clear‐cell renal cell carcinoma patients after surgical treatment.50, 190 Furthermore, high CCL2 expression is related to worse clinical stage and poor overall survival (OS) in patients with clear‐cell renal cell carcinoma.191 |
| Bladder cancer | CCL2 expression is associated with poor overall survival (OS).192 |
| Oesophageal cancer | The expression of CCL2 is an independent predictor of overall survival (OS) and predicts poor prognosis.102 |
| Osteosarcoma | CCL2 expression is correlated with tumour prognosis in patients with osteosarcoma.193 |
| Thyroid carcinoma | The expression of CCL2 is an independent predictive factor for recurrence of papillary thyroid carcinoma.194 |
| Salivary adenoid cystic carcinoma | The overexpression of CCL2 is associated with the clinical progression and poor prognosis of SACC.104 |
| Glioma | CCL2 overexpression is correlated with the reduced overall survival of patients.61 |
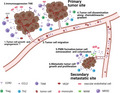
Tumorigenesis is a dynamic and complicated process that consists of three stages including initiation, progression and metastasis.51 The CCL2‐CCR2 signalling axis participates in different stages of tumorigenesis. The axis sustains tumour cell growth and proliferation at the primary tumour site. When malignant cells break away from their original locations to metastasize, the CCL2‐CCR2 axis can stimulate cancer cells to invade surrounding tissues, entry to the circulatory system and spread along a specific chemotactic gradient to metastatic sites.17 After reaching a specific secondary organ and/or tissue, surviving circulating tumour cells can colonize successfully and continue to grow by interacting with the tumour microenvironment. A diagram of the mechanism of CCL2‐CCR2‐mediated tumour pathogenesis is shown in Figure 2.
FIGURE 2.
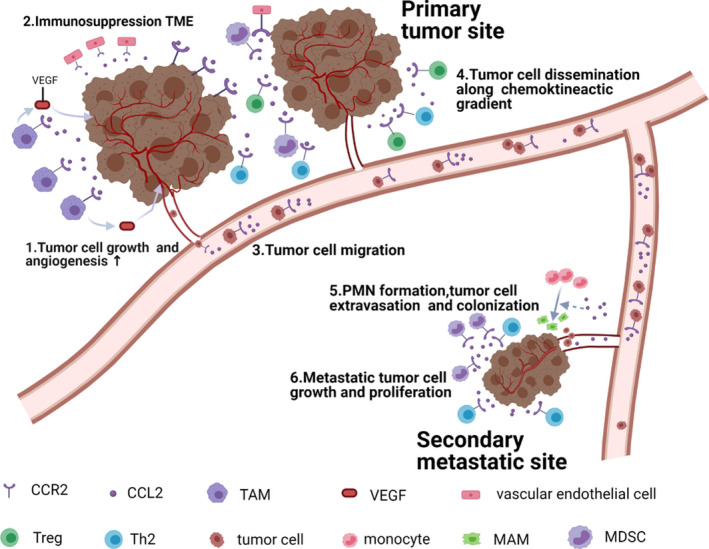
The role of the CCL2‐CCR2 axis in tumour development. CCL2 is mainly produced by tumour cells and surrounding stromal cells. By interacting with CCR2, CCL2 sustains tumour cell growth and proliferation. CCL2 is capable of recruiting CCR2+ TAMs, MDSCs and Th2 cells to create an immunosuppression microenvironment that contributes to tumour progression. Besides, TAM‐secreted vascular endothelial growth factor (VEGF) promotes tumour angiogenesis through the interaction with CCR2+ vascular endothelial cells. In the secondary sites, CCL2 facilitates the differentiation of monocytes into MAMs and the formation of pre‐metastatic niche, accelerating metastatic tumour cell colonization and growth
3.1. Tumour growth and proliferation
Primary tumour growth is a multi‐step and complicated process involving various growth factors and signalling pathways regulation. Previous studies have shown that both stromal‐ and tumour‐derived CCL2 could directly stimulate tumour cell growth and proliferation.36, 52 Lu et al53 found that CCL2 might accelerate tumour growth by acting as an autocrine or paracrine growth factor. Moreover, CCL2 promoted cancer cell to achieve self‐renew and reproduce by activating a series of signal transduction pathways downstream of GPCRs.
Different signalling pathways activated by the CCL2‐CCR2 axis are involved in tumorigenesis (Figure 3). It was reported that CCL2 could protect prostate cancer PC‐3 cells from autophagic death and prolong the survival of tumour cells in serum‐free conditions via activating PI3K/AKT signalling.54, 55 What is more, activated PI3K/AKT signalling remarkably promoted the expression of Survivin, a small protein belonged to the inhibitor of apoptosis protein family, suppressing both autophagy and apoptosis of cancer cell. Moreover, CCL2 hindered the activation of docetaxel‐induced apoptosis‐associated protein caspase‐3 to support tumour cell growth. This process was also correlated with the PI3K/AKT pathway activation, and CCL2 overexpression contributed to the activation of AKT by promoting its phosphorylation at Ser473.56 In addition, CCL2 regulated cancer cell viability, motility and survival by the activation of mitogen‐activated protein kinase (MAPK).57 CCL2 derived from cancer‐associated mesothelial cells could elevate ovarian cancer malignant potential, which was dependent on p38‐MAPK signalling.38 In vitro experiments, researchers demonstrated that recombinant hCCL2 dramatically promoted cancer cell proliferation by activating the MAPK/ERK pathway and enhanced the MEK and ERK1/2 phosphorylation levels.58
FIGURE 3.
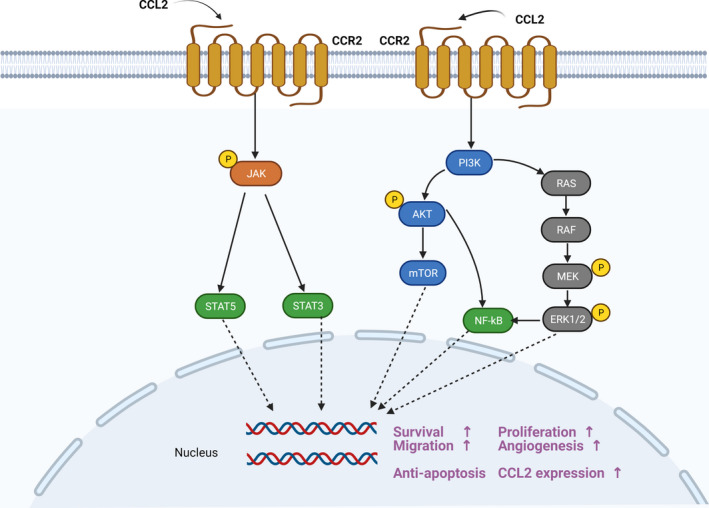
The CCL2‐CCR2 axis and transduction pathway. CCR2 is a typical G‐protein‐coupled receptor. When CCR2 binds to its ligand CCL2, a series of downstream signals are activated, such as JAK/STAT, p38MAPK and PI3K/AKT. Then, multiple transcription factors and genes are mobilized, leading to tumour cell growth, proliferation, migration and the increased CCL2 expression
One of the major issues with cancer eradication is the acquisition of resistance to both chemotherapies and radiotherapies in due course treatment. CCL2 can decrease drug‐induced cytotoxicity to support tumour cell growth by inhibiting proapoptotic autophagy and is regarded as an intervention target for chemotherapy resistance.59 Similarly, CCL2 also contributes to the chemoresistance of lung cancer cells to docetaxel, which may be attributed to cell stress responses.56 Furthermore, ablative radiotherapy increased the production of tumour‐derived CCL2 in a pancreatic ductal adenocarcinoma model, and the increased CCL2 promoted the recruitment of Ly6C+CCR2+ monocytes in tumour sites, thereby accelerating tumour development.60
CCL2 can also recruit various immune cells (such as myeloid‐derived suppressor cells, MDSCs) to form an immunosuppressive microenvironment, which allows tumour cell to evade the body's immune surveillance and supports tumour cell proliferation.61, 62 Overall, the tumorigenic processes mediated by the CCL2‐CCR2 axis involve a series of cellular or molecular signalling, the acquisition of chemoresistance and radioresistance, and the recruitment of multiple immunosuppressive cells. Therefore, more attention should be paid to comprehensively investigate the underlying action mechanisms of the CCL2‐CCR2 axis in tumour proliferation in the future.
3.2. Tumour angiogenesis
Tumour angiogenesis is a critical process in tumour progression and consists of tightly regulated events that include basement membrane degradation, endothelial cell migration and proliferation, and capillary tube formation. Angiogenesis also involves various stimulatory factors such as vascular endothelial growth factor (VEGF).63 In cancer, tumour cells acquire invasive behaviours and induce robust angiogenesis.64 Based on the more profound understanding of the CCL2‐CCR2 axis, scientists found that this axis could act as a mediator of angiogenesis and lead to extensive neovascularization. The CCL2‐CCR2 axis can mediate pathological angiogenesis regulation both directly and indirectly.
CCL2 could bind to CCR2 expressed on vascular endothelial cells and directly stimulated angiogenesis in the absence of inflammatory infiltration. Treatment with a CCL2 neutralizing antibody showed obvious inhibition of the CCL2‐modulated angiogenic effect and reduced the metastatic lesions.65 CCL2 also stimulates angiogenesis through the activation of transcription factors. Recently, Stamatovic et al66 demonstrated that the transcription factor Ets‐1 played a crucial role in CCL2‐mediated angiogenesis. CCL2 probably exerted its angiogenic activity by upregulating Est‐1 target molecules such as β3 integrin. Furthermore, CCL2‐mediated Est‐1 activation was associated with the ERK1/2 cascade, and the inhibition of ERK1/2 with PD98059 obviously decreased Ets‐1 phosphorylation and then inhibited angiogenesis.
On the other hand, CCL2 induced angiogenesis through indirect ways that were correlated with the recruitment of inflammatory cells.67, 68 Tumour‐associated macrophages (TAMs) recruited by CCL2 upregulated the expression of angiogenic factors such as VEGF. In a rat glioblastoma model, TAMs recruitment was blocked after the administration of CCL2 inhibitor, thus reducing angiogenesis and inhibiting tumour growth.69 High expression levels of matrix metalloproteinase‐9 (MMP9) also contributed to tumour vascularization. After CCL2 administration, intratumoral MMP9 expression was increased significantly and the survival time was declined dramatically in tumour‐bearing mice.70
In brief, CCL2 promotes tumour angiogenesis by recruiting CCR2+ vascular endothelial cells and inflammatory cells and by stimulating the expression of angiogenic factors. Based on the finding that CCL2 inhibition limits tumour angiogenesis,71 we presume that CCL2 may be a potential anti‐angiogenesis target in cancer therapy.
3.3. Tumour metastasis
Tumour metastasis is an integrated multi‐step process by which cancer cells disperse from the original tumour sites through the blood or lymphatic system to the surrounding tissues or distant organs, and remains the leading cause of death among cancer patients.72 Metastasis involves a series of events regarding epithelial‐mesenchymal transition (EMT), tumour cell invasion of the basement membrane and entry into the bloodstream to form circulating tumour cells (CTC), formation of pre‐metastatic niches, homing of cancer cell and tumour growth at the metastatic site.73, 74 The CCL2‐CCR2 signalling axis contributes to cancer cell abscission, invasion and migration, playing a pivotal role in tumour metastasis.
3.3.1. The CCL2‐CCR2 axis induces epithelial‐mesenchymal transition
At the initial stage of metastasis, tumour cells acquire an invasive phenotype that can break down the extracellular matrix (ECM) to facilitate metastasis.17 Then, epithelial‐mesenchymal transition (EMT) reduces the adhesion between cells and facilitates cell crossing of the basement membrane.75 Numerous studies have shown that the EMT programme was involved in modulating the metastasis and spread of diverse tumour cells. IL‐6, a cytokine, was reported to support tumour cell invasion by inducing EMT effectively. Besides, the addition of CCL2 dramatically enhanced this EMT‐like morphological change compared with IL‐6 alone, which was associated with the phosphorylation of STAT3.76 Pharmacological interruption of the CCL2‐CCR2‐STAT3 signal markedly suppressed EMT and decreased tumour cell migration.37 Furthermore, CCL2 induced EMT through the upregulation of transcription factor Snail and the activation of the Hedgehog pathway.47, 77
Matrix metalloproteinases (MMPs) are a multigene family of metal‐dependent endopeptidases that play important roles in EMT and contribute to tumour metastasis.78 The CCL2‐CCR2 signalling axis upregulates MMP2 and MMP9 expression to promote cancer cell infiltration and migration. In a human chondrosarcoma model, MMP9 expression was increased in CCL2‐treated cells. However, after the transfection of MMP9‐small interfering RNA(siRNA), MMP9 expression was inhibited and the CCL2‐mediated tumour cell migration was also reduced.79 NF‐κB was a highly conserved transcription factor and was also responsible for enhancing CCL2‐mediated MMP‐9 expression and cell motility.80 Besides, MMP2 was discovered to engage in CCR2‐mediated EMT, whereas CCR2‐siRNA transfection could reduce MMP2 activity, indicating the specific binding between MMP2 and CCR2.81
3.3.2. The CCL2‐CCR2 axis induces tumour cell extravasation
When tumour cells enter the lymphatic system or bloodstream, they become circulating tumour cells (CTCs) and disseminate along chemotactic gradients. In the new sites, tumour cells exude and continue to colonize. This step is associated with the interactions between the host factors in the target organs and the spreading tumour cells. In tumour cell extravasation, scientists found a population of cells derived from a subset of inflammatory monocyte precursors, known as metastasis‐associated macrophages (MAMs).82, 83 A study conducted by Kitamura et al84 indicated that CCL2‐CCR2 axis activation was able to promote CCL3 secretion by recruited MAMs. Meanwhile, the secretion of CCL3 led to enhanced interaction between MAMs and cancer cells and prolonged MAMs retention in tumour sites, which accelerated the extravasation of malignant cells. In addition, CCR2 deficiency prevented tumour cell extravasation. Researchers also noticed that the phosphorylation of JAK2 was increased in lung homogenates from MC‐38‐injected mice but no or minor JAK2 phosphorylation was detected in CCR2−/− mice. Similarly, STAT5 and p38MAPK could not be activated in CCR2−/− tumour tissues after tumour cell injection, indicating that CCL2‐induced tumour cell migration had a close relationship with JAK2‐STAT5 and p38MAPK pathway activation.85
3.3.3. The CCL2‐CCR2 axis induces the homing of tumour cell
After malignant cell exudation from the circulatory system, their growth in secondary sites is also critical for cancer metastasis. Dating back to the 1980s, the English surgeon Stephen Paget proposed the ‘seed and soil’ hypothesis, which suggested that the spread of tumour cells was controlled by the interaction between the cancer cells (seed) and the host organ (soil).86, 87 Subsequently, in 2005, Kaplan and colleagues discovered that bone marrow‐derived hematopoietic progenitor cells clustered to tumour‐specific pre‐metastatic sites and formed cellular clusters before tumour cells arrived, which indicated the existence of ‘pre‐metastatic niche (PMN)’.88
The PMN is considered as fertile soil for the survival and growth of metastatic tumour cell and represents an aberrant, tumour growth‐favouring microenvironment.86, 89 It has been reported that CCL2 contributed to the establishment of the PMN and the homing of tumour cells. CCL2 secreted by stromal cells or tumour cells could recruit inflammatory monocytes to stimulate PMN formation and create a suitable environment for cancer cell colonization.90 Intriguingly, in a hypoxic environment, CCL2 induced by tumour cells may inhibit the maturation of natural killer cells in PMN and reduce the ability of NK cells to eliminate incoming CTCs, accelerating the homing of cancer cells.91
In both the early and late stages of metastasis, the CCL2‐CCR2 signalling axis also recruits diverse immune cells to form an immunosuppressive TME that allows tumour cells to evade the body's immune surveillance, which will be discussed in the next section. Altogether, CCL2 and its receptor CCR2 are upregulated in tumours and engaged in tumour metastasis through the induction of EMT, the promotion of tumour cell extravasation and the establishment of a PMN.
4. ROLE OF THE CCL2‐CCR2 AXIS IN THE TUMOUR MICROENVIRONMENT
In the past few decades, cancer researches mainly focused on malignant cells and related gene regulation. More recently, the concept of the tumour microenvironment (TME) is becoming widely accepted. The dynamic interplay between the tumour and its microenvironment has a significant influence on carcinogenesis. Other than tumour cells, the TME is composed of a great variety of nonmalignant cells such as fibroblasts, mesenchymal stem cells, haemolymph endothelial cells and numerous infiltrating leukocytes.92 Some of these cells can upregulate CCL2 expression and participate in tumour progression. Herein, we will discuss the relationship between the CCL2‐CCR2 axis and these nonmalignant cells in the TME, with an emphasis on immune cells and fibroblasts (Figure 4).
FIGURE 4.
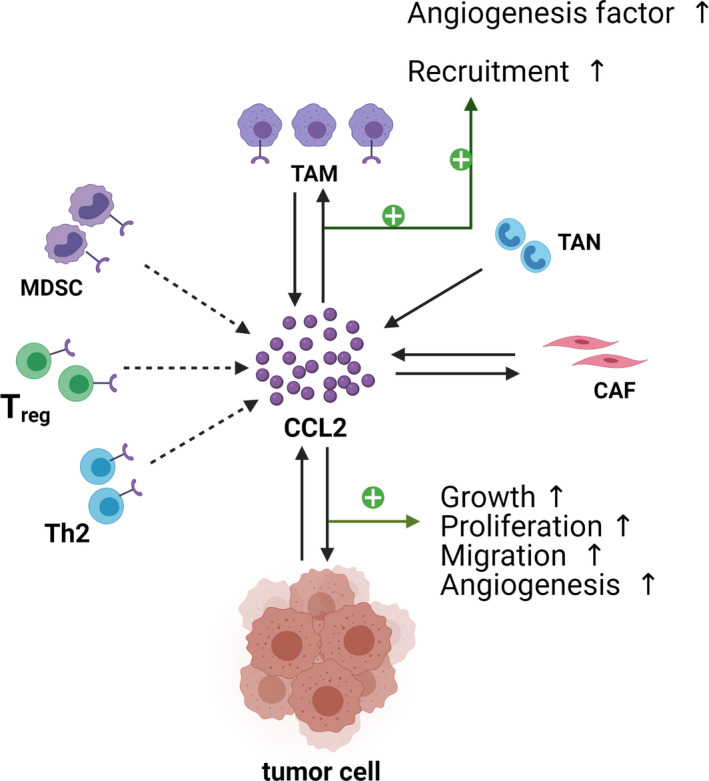
CCL2 and the tumour microenvironment. In the tumour microenvironment, cancer cells interact with various cells to promote the occurrence and development of tumours. CCL2 can recruit various immunosuppressive cells to the TME and weaken the anti‐tumor immune response. In addition to tumour cells, other cells are able to secrete CCL2, such as TAMs, TANs and CAFs
4.1. Macrophages
As one of the leucocytes in the innate immune system, macrophages exist in nearly all organs and tissues. In the events of tissue injury, infection and cancer, monocytes are recruited to the related sites by chemotactic signals and further differentiate into macrophages to participate in the body's immune response.93 Macrophages can differentiate into two different types: classically activated (or inflammatory) macrophages (M1) and alternatively activated (or wound‐healing) macrophages (M2), a process termed ‘macrophage polarization’.94 Generally, M2 macrophage is characterized in vitro by the production of IL‐12low IL‐23low IL‐10high TGF‐βhigh, whereas M1 macrophage is characterized by IL‐12high IL‐23high IL‐10low.94 Furthermore, a frequently used marker to identify M2 macrophages is Arginase‐1, whereas Arginase‐1 is also induced in M1 spectrum macrophages. Therefore, scientists usually favour an approach using combinations of markers (or a lack of marker expression) to identify macrophage subsets.95 For instance, the analysis of the transcriptional signature of murine M1 and M2 macrophages showed that CD38, G protein coupled receptor 18 (Gpr18) and Formyl peptide receptor 2 (Fpr2) are useful markers for M1, whereas Early growth response protein 2 (Egr2) and c‐Myc were M2‐exclusive.96 Functionally, M1 macrophages promote inflammation and exhibit anti‐tumour properties. In contrast to M1, M2 macrophages play an immunosuppressive role that is conducive to tissue repair and promotes tumour progression.97 Tumour‐associated macrophages (TAMs) are a type of macrophages aggregated in the microenvironment of tumour‐infiltrating tissues and are mainly differentiated from bone marrow‐derived mononuclear cells.98 TAMs are generally considered as a specialized phenotype of M2‐like macrophages as they have similar functions with M2 macrophages and facilitate tumour growth by inducing immune suppression.99, 100
The CCL2‐CCR2 axis promotes monocytes' recruitment from the peripheral blood to the tumour sites, where these monocytes further differentiate into TAMs.99, 101 TAMs accelerate tumour proliferation and metastasis by inducing immune escape. Yang et al102 found that in oesophageal cancer, tissues with high CCL2 expression showed a corresponding increase in the number of cells expressing CD68 (a common TAM marker). Besides, increased expression of PD‐L2 in TAMs supported immune escape through the PD‐1 pathway. In metastatic colorectal cancer, a large number of TAMs infiltrate the liver to facilitate metastatic cancer cell growth, which is driven by TAM‐mediated immunosuppression.103 Intriguingly, not only does the CCL2‐CCR2 axis promote tumour progression by recruiting and reprogramming TAMs,104 but TAMs also support cancer invasion and metastasis by secreting CCL2.105 Such crosstalk and mutual promotion provide a positive induction for TAMs in the TME and establish favourable conditions for tumour growth and metastasis.
4.2. Neutrophils
Neutrophils are derived from a common committed myeloid progenitor cell and are considered as effectors of the innate immune response. Neutrophils coordinate multiple immune processes including chronic inflammatory, autoimmunity and cancer.106 Meanwhile, neutrophils have been uncovered in the TME of various cancers. Tumour‐associated neutrophils (TANs), similar to macrophages, have anti‐ and pro‐tumour heterogeneities modulated by the TME.107, 108 The extensive TAN infiltration was observed to have a positive relationship with the advanced stage and poor prognosis of oral squamous cell carcinoma. Melatonin could delay cancer progression by inhibiting CCL2 release from TANs.109 Furthermore, CCL2‐positive TANs were widely distributed in the primary tumour sites and regional lymph nodes,110 suggesting that they might facilitate tumour progression through CCL2 expression.
4.3. Myeloid‐derived suppressor cells
Myeloid‐derived suppressor cells (MDSCs) were initially described in the 1980s and accumulated in the TME.111 MDSCs are a group of immature immunosuppressive cells derived from the bone marrow, and their notable function is to suppress T‐cell response.112, 113 Generally, MDSCs are divided into two types: polymorphonuclear MDSC (PMN‐MDSC), which are morphologically and phenotypically similar to neutrophils, and another is monocytic MDSC (M‐MDSC) that is similar to monocytes.114, 115 The CCL2‐CCR2 axis is required for MDSCs functional specialization. CCL2 promoted the progression of colorectal cancer and enhanced the polymorphonuclear MDSC population and function. Mechanismly, CCL2 regulated T‐cell‐suppressive activity mediated by PMN‐MDSC through the activation of STAT3 and the reactive oxygen species (ROS) production in PMN‐MDSC.116 Moreover, the CCL2‐CCR2 axis modulated the migration of M‐MDSC to tumour tissues and metastatic environment.117
Previous studies showed that the CCL2‐CCR2 axis also played a key role in the recruitment of MDSCs into the TME, which protected tumour cells from immune‐mediated killing.118 In a murine glioma model, one week after GL261 cells had been injected into mice, flow cytometric analysis of the tumour tissues showed that CCR2 was largely localized to MDSC populations. These CCR2+ MDSCs were recruited to the glioma microenvironment in response to CCL2, whereas MDSCs lacking CCR2 failed to maximally accumulate in the TME.61 Furthermore, MDSCs in the microenvironment prevented the influx of CD8+ T cell. Meanwhile, the CD8+T cell/MDSC ratio was increased within tumours derived from CCL2 deletion mice, and elevated levels of CD8+ T cells restored anti‐tumour immunity to inhibit tumour development.119
4.4. T cells
T cells are leukocytes that express T‐cell receptors (TCRs). T cells are divided into two types: CD8+ cytotoxic lymphocytes (CTL) that recognize peptides presented by MHC I and CD4+ helper (Th) cells that recognize peptides presented by MHC II.120 There are several subtypes of CD4+ T cell, among which Th1 and Th2 cells are most common and important. Th1‐derived cytokines induce pro‐inflammatory responses and exhibit anti‐tumour effects, whereas Th2‐secreted cytokines produce anti‐inflammatory effects and support tumour progression.120, 121
The secretion and expression of CCL2 is associated with Th2 polarization.122 CCR2 can be expressed in Th2 cells, and the CCL2‐CCR2 axis may facilitate Th2 effector cells' differentiation and maturation through the reduction of IL‐12 produced by antigen presenting cells and the increase of IL‐4 secreted by activated T cells.123 Moreover, CCL2 secreted from the TME could promote Th2 cell aggregation. In concert with this, researchers found that the ratio of Th1/Th2 was prominently decreased within tumour tissues of high CCL2 expression.124
In nearly all cancers, the number of regulatory T cell (Treg) is typically increased. Treg is a type of CD4+ T cell, and can foster tumour metastasis and progression by coordinating immunosuppressive effects.125 The majority of studies showed that the CCL2‐CCR2 axis was crucial for Treg recruitment and accumulation in the TME.126, 127 In a murine model of head and neck squamous cell carcinoma, the elevation of CCL2 after radiotherapy was involved in oncogenic transformation and further mediated the recruitment of CCR2+ Treg.128 Further, CCR2+ Treg represented high immunosuppressive activity and played a central role in suppressing anticancer responses. Although CCR2+ Treg accumulated in various cancers, it could be depleted by low‐dose cyclophosphamide, which allowed the definition of a novel target for improving chemotherapic efficacy.129
4.5. Fibroblasts
Cancer‐associated fibroblasts (CAFs) are one of the most abundant stromal components in the TME. Although their origin remains unclear, it is generally believed that CAFs might result from the activation of local tissue‐resident fibroblasts.130, 131 Shen et al132 revealed that miRNAs‐mediated FoxO3a/VEGF/CCL2 signalling played a prominent role in converting normal fibroblasts (NFs) into CAFs. The authors further indicated that CCL2 upregulation had a potential promoting effect on CAFs production. Intriguingly, CAFs also foster tumour metastasis by secreting CCL2. The overexpression of NF‐κB and STAT3 considerably stimulated the CCL2 secretion in cocultured CAFs.133, 134 Besides, in pancreatic cancer, tumour cells educate fibroblasts via the secretion of IL‐8 and CCL2, leading to the generation of CAFs and similar metastasis‐associated fibroblasts (MAFs).135
Collectively, the above results suggest that the CCL2‐CCR2 axis is upregulated during cancer progression and has a close relationship with cancer initiation, progression and metastasis. Blockade of the CCL2‐CCR2 axis is a potential therapeutic option for cancer treatment.
5. CCL2‐CCR2‐ASSOCIATED TARGETED THERAPY
The CCL2‐CCR2 signalling axis has a multifaceted involvement in a wide range of cancers, and accumulated experimental evidence is paving the way for future clinical research. Furthermore, blockade of the CCL2‐CCR2 axis has attracted extensive attention and represents promising anti‐tumour activity in both preclinical studies and clinical trials. Here, we mainly report the therapeutic strategies targeting the CCL2‐CCR2 axis.
5.1. Preclinical studies targeting CCL2‐CCR2 axis
5.1.1. Inhibition of CCL2
A number of preclinical studies have used various CCL2 inhibitors or antibodies to delay tumour progression. Blockade of CCL2 achieves tumour suppression through diverse ways such as blocking CCL2‐mediated signalling pathways, inhibiting the immunosuppressive cell recruitment and increasing the number of tumour‐killing cells.
C1142
C1142 is a rat/mouse chimeric monoclonal antibody that can specifically neutralize CCL2. This antibody has yielded encouraging results in different cancer models as a single agent or combination with standard chemotherapies.136 In a glioma model, C1142 treatment significantly reduced the total numbers of CD11b+CD45+ TAMs and CD11b+Gr1+ MDSCs, inhibiting tumour proliferation and prolonging the survival of glioma‐bearing mice.137 C1142 also inhibited tumour cell migration by blocking the infiltration of TAMs into tumour sites.138 In addition, C1142 also enhanced hepatic NK‐cell cytotoxicity and increased IFNγ production, resulting in the inhibition of tumour progression.139 Administration of C1142 along with anticancer vaccine augmented CD8+ T‐cell numbers and reduced tumour volume, suggesting that combining CCL2 antibody with the vaccine could be a promising tactic for treating cancer.140
Bindarit
Bindarit is an anti‐inflammatory indazole derivative that can inhibit the synthesis of CCL2, with a potential inhibitory function in tumour development. Targeting CCL2 with bindarit induces tumour regression, which has been confirmed in some preclinical studies. Bindarit is able to impair the organization of endothelial cells in the formation of blood vessels to inhibit tumour angiogenesis.141 Moreover, bindarit impairs the TME by preventing TAM and MDSC infiltration, which limits tumour growth and delays tumour progression.142, 143 Further, bindarit inhibited tumour cell proliferation and migration in vitro through negative regulation of the NF‐κB and AKT signalling pathway.142
mNOX‐36
A CCL2 inhibitor, mNOX‐36 suppressed TAM recruitment in a rat glioblastoma multiforme model. Interestingly, the combination therapy of mNOX‐36 and bevacizumab reduced both the tumour size and blood volume.69 The safety and validity of mNOX‐36 require further evaluation in clinical trials.
Curcumin
The expression and activity of CCL2 can be inhibited by curcumin, an active polyphenolic compound of the powdered turmeric (Curcuma longa) root. In a mouse model of colon cancer, plasma CCL2 was reduced after curcumin feedings.144 Protein kinase C (PKC), an important intracellular signalling mediator, is known to upregulate CCL2 secretion and is associated with tumour cell invasion. Curcumin significantly blocks CCL2‐induced cell invasion and motility through the inhibition of PKC. Furthermore, Curcumin also reduces CCL2‐mediated MMP9 expression that contributes to tumour progression.145
Carlumab
Carlumab, also known as CNTO 888, is a human anti‐CCL2 antibody. Carlumab inhibits PC‐3 cell proliferation and migration in vitro. Investigators found that the combination of carlumab and docetaxel was more efficacious than docetaxel alone by inducing tumour regression.146 To date, using carlumab to treat cancer has been investigated in clinical trials, and we will discuss its efficacy in the related section.
5.1.2. Inhibition of CCR2
CCR2 is overexpressed in immunosuppressive cells and tumour cells, but its relatively low expression in most normal tissues make this receptor a promising target in cancer.
RS 504393
RS 504393 is a selective CCR2 antagonist that delays tumour progression by inhibiting the infiltration of immunosuppressive cells into tumour sites. Researchers found that RS 504393 improved the survival time of mice with bladder cancer by blocking the recruitment of MDSCs.147 Following RS 504393 treatment, the number of TAMs and the tumour volume were significantly reduced in tumour‐bearing mice,104 suggesting that targeting CCR2 with RS504393 might be a potential therapeutic option for cancer.
RS 102895
RS 102895 is a CCR2 antagonist that blocks CCL2 signalling through the occupation of a binding site on the extracellular side of CCR2.148 RS 102895 treatment reduced prostate cancer PC‐3 cell invasion from 197 to 85 percent.149 Moreover, CCL2 regulated breast cancer cell growth and migratory ability, whereas RS 102895 could attenuate these regulatory effects partially due to the downregulation of MMP‐9 expression.150
CAS445479‐97‐0
CCL2 promotes lung cancer cells' growth, migration and invasion. CAS445479‐97‐0, a CCR2 antagonist, reversed the induction of A549 cell proliferation by CCL2 in a dose‐dependent manner. In addition, CAS445479‐97‐0 was able to inhibit A549 cell migratory capacity mediated by CCL2, which could be attributed to the downregulation of MMP‐9 expression.151 Therefore, targeting CCR2 with CAS445479‐97‐0 may be an attractive therapeutic strategy for patients with lung cancer.
GMME1
GMME1 is a novel fusion protein that functions as a CCR2‐specific tumoricidal agent. GMME1 is composed of granulocyte macrophage colony‐stimulating factor (GM‐CSF) in tandem with N‐terminal truncated monocyte chemotactic protein‐1 (MCP1 6‐76).152 Rafei et al152 developed it and confirmed its anti‐tumour activity. GMME1 specifically blocked CCR2‐associated STAT3 phosphorylation and induced tumour cell apoptosis, thereby inhibiting tumour proliferation.
Teijin Compound 1
Teijin Compound 1(TC1) is a specific CCR2 antagonist and is developed to block the binding of human CCL2 to CCR2b.153 In a trans‐endothelial tumour cell migration experiment, TC1 was observed to inhibit cancer cell migration. TC1 also reduced vascular permeability and prevented the generation of pulmonary metastases.154
BMS CCR2 22
BMS CCR2 22 is a potent and high affinity CCR2 antagonist. In a metastatic liver tumour model (MC38), BMS CCR2 22 treatment decreased the infiltration of TAMs into tumour sites effectively and increased the efficacy of FOLFOX chemotherapy, thereby improving the survival time of tumour‐bearing mice.155
747
Recently, Yao et al156 reported a natural product from Abies georgei, named 747. 747 exhibited sensitivity and selectivity as a CCR2 antagonist and could block the CCL2‐CCR2 axis. 747 showed anti‐tumour activity by inhibiting TAM recruitment and elevating CD8+ T‐cell numbers. In addition, blockade of TAMs enhanced the anti‐tumour efficacy of sorafenib. The discovery of 747 provided a novel perspective on the development of CCR2 antagonists and enriched the therapeutic options against cancer.
Others
CCX872, a small molecule antagonist of CCR2, can impede MDSC invasion into tumour sites.119 The CCR2 inhibitor, PF‐04136309 can decrease tumour‐infiltrating inflammatory monocytes and macrophages, promoting anti‐tumour immunity in mice.48 Targeting CCR2 with CCX872 and PF‐04136309 for treating cancer has been investigated in clinical trials, which is shown in Table 2.
TABLE 2.
Overview of clinical trials targeting the CCL2‐CCR2 axis
| Target | Drug name | Conditions | Phase | Status | Trial number |
|---|---|---|---|---|---|
| CCL2 | Carlumab | Patients with metastatic castrate‐resistant prostate cancer. | Phase II | Completed | NCT00992186 |
| Combination with chemotherapy in patients with solid tumours. | Phase I | Completed | NCT01204996 | ||
| Patients with solid tumours. | Phase I | Completed | NCT00537368 | ||
| CCR2 | MLN1202 | Cancer patients with bone metastases. | Phase Ⅱ | Completed | NCT01015560 |
| CCR2/CCR5 | BMS‐813160 | With or without GVAX for locally advanced pancreatic ductal adenocarcinomas. | Phase Ⅰ/Ⅱ | Recruiting | NCT03767582 |
| Combination with Nivolumab, Gemcitabine and Nab‐paclitaxel in borderline resectable and locally advanced pancreatic ductal adenocarcinoma (PDAC). | Phase Ⅰ/Ⅱ | Recruiting | NCT03496662 | ||
| Combination with chemotherapy or Nivolumab in patients with pancreatic cancer. | Phase Ⅰ/Ⅱ | Not recruiting | NCT03184870 | ||
| Combination treatments in patients with advanced renal cell carcinoma. | Phase Ⅱ | Recruiting | NCT02996110 | ||
| Combination treatments in patients with hepatocellular carcinoma. | Phase Ⅱ | Recruiting | NCT04123379 | ||
| CCR2 | CCX872‐B | Patients with pancreatic adenocarcinoma. | Phase Ⅰ | Not recruiting | NCT02345408 |
| Preoperative stereotactic body radiation therapy for pancreatic adenocarcinoma. | Phase Ⅰ/Ⅱ | Withdrawn | NCT03778879 | ||
| CCR2 | PF‐04136309 | Patients with borderline resectable and locally advanced pancreatic adenocarcinoma. | Phase Ⅰ | Completed | NCT01413022 |
| Combination with Gem/Nab‐P in first‐line metastatic pancreatic patients. | Phase Ⅱ | Terminated | NCT02732938 |
5.1.3. Combined therapy
The pathogenesis is complicated in cancer. Blockade of the CCL2‐CCR2 axis alone may cause unexpected side effects. Therefore, combining inhibition therapy of CCL2‐CCR2 axis with current treatment tactics for different cancers is necessary. In pancreatic adenocarcinoma, TANs and TAMs are recruited to the TME, mediating immune escape and favouring tumour growth. In patients with pancreatic adenocarcinoma, blockade of CCR2 prevented the accumulation of TAM in tumour sites but led to a compensatory CXCR2+ TANs influx, which possibly limited the intended treatment efficacy.157 A preclinical study reported a dual targeted strategy, where CCR2 inhibition combined with CXCR2 blockade resulted in notable tumour suppression and improved survival in tumour‐bearing mice. Further, dual targeting of CCR2+ TAMs and CXCR2+ TANs restored anti‐tumour immunity and improved the chemotherapeutic effect, providing the theoretical basis for targeting both CCR2 and CXCR2 in future clinical trials.157
The immune system is involved in almost every stage of cancer initiation and progression. Programmed cell death protein 1 (PD‐1) is an immune inhibitory receptor and is particularly expressed on cytotoxic T cells. PD‐1 plays a vital role in promoting self‐tolerance and inhibiting immune responses by binding to its ligand PD‐L1 or PD‐L2.158, 159, 160 Interestingly, recent studies have found that the CCL2‐CCR2 axis could induce immune escape through PD‐1 signalling in cancer.102 More recently, Fein and colleagues found that CCR2 secreted by tumour cells orchestrated immune evasion by depressing CD103+ DC maturation and suppressing CTL activity. Besides, CCR2−/− cancer cells had lower surface levels of PD‐L1 compared with CCR2+/+ cancer cells.161 Combination therapy of CCR2 antagonist and anti‐PD‐1 also improved anti‐tumour effects compared with monotherapy.162, 163 It would be valuable to further explore the prospects of combining CCL2‐CCR2 axis blockade with PD‐1‐directed immunotherapies in multiple tumours.
5.2. Clinical trials targeting the CCL2‐CCR2 axis
The efficacy of CCL2‐CCR2 axis inhibition has been widely investigated in numerous preclinical experiments. CCL2 and CCR2 can function as diagnostic biomarkers,164 and are correlated with the prognosis and poor overall survival in cancer patients.105, 165 Given the critical roles of the CCL2‐CCR2 signalling axis in tumorigenesis, a series of clinical trials targeting this axis have been launched continually (Table 2).
5.2.1. CCL2 neutralizing antibody
Targeting CCL2 is based on the assumption that CCL2 neutralizing antibodies can bind to CCL2 and block the recruitment and differentiation of macrophages. Carlumab (CNTO 888), a human anti‐CCL2 neutralizing antibody, can bind to human CCL2 and exhibits broad spectrum of preclinical anticancer activity.166 In a phase I study of the safety of carlumab in patients with solid tumours (NCT00537368), forty‐four patients with advanced solid tumours were enrolled. No dose‐limited toxicity (DLT) was observed in the carlumab‐treated patients, indicating that carlumab was generally well tolerated. However, in thirty‐three evaluable patients, none of them achieved an objective response, but durable stable disease (SD) was observed in four patients.167 In a phase II study of carlumab in metastatic castration‐resistant prostate cancer (NCT00992186), only 34% of patients maintained stable disease for more than three months, and none of the forty‐four patients evaluable for prostate specific antigen (PSA) response had a response. Notably, the concentrations of median free CCL2 sharply declined from baseline at 4 hours after carlumab treatment, but total CCL2 concentrations increased to 1000‐fold higher than baseline after one week.168 Similarly, in an open‐label, multicentre phase Ib study (NCT01204996), researchers found that carlumab sequestered CCL2 for only a short time and did not generate obvious anti‐tumour responses.169 The reasons for these unexpected results may be the weak affinity of carlumab with CCL2 and the inadequate clearance of the circulating CCL2‐complex.
Collectively, although no significant adverse reaction was found in these patients, the therapeutic effects of carlumab were not satisfactory. In the future, how to permanently suppress CCL2 levels may be the focus of research.
5.2.2. CCR2 antibodies and inhibitors
Antibodies and inhibitors are used to interfere with CCR2 binding to its cognate ligand CCL2 and to block the activation of latent CCR2. Both steps are critical for CCR2‐induced pro‐tumorigenic effects and immune escape. CCR2‐associated targeted therapy has made progress in recent years.
PF‐04136309
PF‐04136309 is a CCR2 inhibitor that exhibited anti‐tumour activity in an orthotopic model of murine pancreatic cancer.48 Based on its anticancer potential, PF‐04136309 has been tested in clinical trials. A non‐randomized open‐label phase IIb study (NCT01413022) assessed the effect of PF‐04136309 in combination with FOLFIRINOX chemotherapy (oxaliplatin and irinotecan plus leucovorin and fluorouracil) in patients with pancreatic cancer. In all patients treated with FOLFIRINOX plus PF‐04136309, 32 patients (97%) achieved the control of local tumours, and 16 patients (49%) achieved an objective tumour response. Nevertheless, none of all patients achieved an objective response in the FOLFIRINOX chemotherapy alone group.170 What's more, after the treatment of FOLFIRINOX plus PF‐04136309, the mean ratio of blood to bone‐marrow CCR2+ monocytes in patients was significantly decreased compared with that in the FOLFIRINOX alone group (1.06 vs. 6.46), which indicated that FOLFIRINOX plus PF‐04136309 could prevent CCR2+ monocyte egress from the bone marrow to the peripheral blood and might affect the anti‐tumour immunity.170
Another phase I study (NCT02732938) was initiated to evaluate the effect of administering PF‐04136309 combined with gemcitabine and nab‐paclitaxel in patients with metastatic pancreatic cancer. All patients (21) received PF04136309 at a starting dose of 500 mg or 750 mg twice daily (BID). The final result showed that 14 patients (66.7%) experienced treatment‐related serious adverse events (SAEs), which might attribute to at least one of the three exposure drugs (PF‐04136309, nab‐paclitaxel, or gemcitabine). By the cut‐off date, the objective response rate (ORR) was 23.8% (95% CI = 8.2%‐47.2%) in all 21 patients who received the treatment, but the overall survival (OS) was not assessed in this trial. In the 500 mg BID group (n = 17), the median progression‐free survival (mPFS) was 5.3 months and the ORR was 29.4% (95% CI = 10.3%‐56.0%).171 Overall, PF‐04136309 in combination with chemotherapy showed clinically meaningful efficacy and survival benefit in patients with pancreatic cancer,170 but the value of PF‐04136309 in treating cancer patients remains to be further explored and more evidence for larger samples is also needed.
MLN1202
MLN1202 is an anti‐CCR2 monoclonal antibody. In a phase II clinical trial, researchers assessed the efficacy of MLN1202 in forty‐four patients with bone metastases (NCT01015560). Among forty‐three eligible patients, forty‐one patients completed the study, and only 7.14% had severe adverse reactions. To some extent, MLN1202 was well tolerated in patients. After forty‐three days of MLN1202 treatment, the urinary N‐telopeptide value (a primary assessment indicator) was decreased in 14 patients, indicating some positive effects of MLN1202 for cancer treatment.
Propagermanium
Propagermanium (3‐oxygermylpropionic acid polymer) is an organogermanium compound that blocks CCR2 signalling.35 In a phase I dose‐escalation study, a total of twelve patients with breast cancer were enrolled. The patients were all at tumour‐node‐metastasis (TNM) stage I, II, or III. After treatment with propagermanium, the serum IL‐6, which participated in tumour angiogenesis, was downregulated in a dose‐dependent manner, suggesting that propagermanium may have the potential to inhibit angiogenesis and prevent cancer metastasis. In the future, a phase II clinical trial of propagermanium will be conducted to assess its antimetastatic potential.172
CCX872‐B
CCX872‐B is a specific CCR2 antagonist. A phase Ib trial was designed to investigate the safety and efficacy of CCX872‐B in patients with pancreatic adenocarcinoma (NCT02345408). The enrolled patients (50) received FOLFIRINOX (fluorouracil [5‐FU], leucovorin, irinotecan, oxaliplatin) plus 150 mg CCX872 QD or BID for 12 weeks. From the perspective of survival improvement, all‐subjects overall survival (OS) at 18 months was 29%,173 whereas the OS was only 18.6% at 18 months for FOLFIRINOX regimen alone,174 suggesting that CCX872 might be a promising option for cancer treatment.
BMS‐813160
BMS‐813160 is a dual CCR2/CCR5 antagonist. Several clinical trials are currently evaluating the combination therapy of BMS‐813160 and chemotherapies or immunotherapies for treating cancer. (NCT 03767582, NCT03496662, NCT03184870, NCT02996110, NCT04123379) The anti‐tumour effects of BMS‐813160 have not been disclosed.
6. CONCLUSION
Although CCL2 was initially identified as a mediator of inflammation, increasing evidence has linked CCL2 with human diseases, particularly cancer. Deeper insight into the mechanisms of the CCL2‐CCR2 axis would provide new directions for better understanding of malignant tumours. The CCL2‐CCR2 signalling axis sustains primary tumour cell survival and proliferation through angiogenesis and different signalling pathways. Moreover, the axis can induce immunosuppressive cell entry to the TME, accelerating tumour growth and metastasis. The expression levels of CCL2 and CCR2 can be regarded as a predictor of poor prognosis in patients with cancer. Nevertheless, researchers noticed that high CCL2 expression was associated with favourable overall survival and progression‐free survival in patients with lung squamous cell carcinoma (LUSC). They speculated that the possible reason is that most TAMs differentiated into the M1 subtype in LUSC.175 Similarly, CCL2 secreted by hepatic macrophage can stimulate the establishment of an M1‐dominant hepatic macrophage phenotype.176
CCL2 neutralizing antibodies or CCR2 antagonists have been developed to potentiate anti‐tumour effects and have produced encouraging results in some preclinical studies. However, targeting CCL2 with carlumab did not appear to have a significant effect in clinical trials, suggesting that anti‐CCR2 may be a compensatory approach for the deficiency of targeting CCL2. In pancreatic cancer, CCR2 antagonists alone or combined with chemotherapeutic agents can control local tumours and are well tolerated in patients. Additionally, in several cancer models, scientists discovered that the CCL2‐CCR2 signalling axis could induce tumour immune evasion through PD‐1 signalling. Furthermore, the combined treatment of anti‐PD‐1 and inhibiting the CCL2‐CCR2 axis is more effective than monotherapy. It might be promising to move this combination therapy regimen into a clinical trial in the near future.
In conclusion, the CCL2‐CCR2 signalling axis covers a wide range of tumour cells' activities and is involved in both the early and late steps of tumorigenesis. A series of therapeutic strategies targeting this axis have enriched our approaches for fighting cancer and have entered clinical trials. However, it is important to note that discontinuing CCL2 inhibition exacerbates tumour metastasis,177 suggesting the need to develop new therapies to achieve long‐lasting CCL2 inhibition. Moreover, combination therapy of anti‐PD‐1 and CCL2‐CCR2 axis inhibition may be a novel and promising therapeutic approach for improving anti‐tumour efficacy, which provides new directions for cancer immunotherapy and warrants further study in clinical trials.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
YQW and XWW offered main direction and significant guidance of this manuscript. MSX and YW drafted the manuscript. RLX illustrated the figures and tables for the manuscript. All authors approved the final manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation Regional Innovation and Development (U19A2003), the Excellent Youth Foundation of Sichuan Scientific Committee Grant in China (No. 2019JDJQ008).
Xu M, Wang Y, Xia R, Wei Y, Wei X. Role of the CCL2‐CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021;54:e13115. 10.1111/cpr.13115
Maosen Xu and Yang Wang contributed equally to the work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944‐2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhusal RP, Foster SR, Stone MJ. Structural basis of chemokine and receptor interactions: key regulators of leukocyte recruitment in inflammatory responses. Protein Sci. 2020;29(2):420‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harbor Perspect Biol. 2015;7(5):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukaida N, Baba T. Chemokines in tumor development and progression. Exp Cell Res. 2012;318(2):95‐102. [DOI] [PubMed] [Google Scholar]
- 5.Ono SJ, Nakamura T, Miyazaki D, Ohbayashi M, Dawson M, Toda M. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003;111(6):1185‐1199. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540‐550. [DOI] [PubMed] [Google Scholar]
- 8.Arimont M, Sun SL, Leurs R, Smit M, de Esch IJP, de Graaf C. Structural analysis of chemokine receptor‐ligand interactions. J Med Chem. 2017;60(12):4735‐4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacon K, Baggiolini M, Broxmeyer H, et al. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22(10):1067‐1068. [DOI] [PubMed] [Google Scholar]
- 10.Yadav A, Saini V, Arora S. MCP‐1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411(21–22):1570‐1579. [DOI] [PubMed] [Google Scholar]
- 11.Van Coillie E, Van Damme J, Opdenakker G. The MCP eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10(1):61‐86. [DOI] [PubMed] [Google Scholar]
- 12.Ben‐Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25(3):357‐371. [DOI] [PubMed] [Google Scholar]
- 13.Salazar N, Zabel BA. Support of tumor endothelial cells by chemokine receptors. Front Immunol. 2019;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushima K, Larsen CG, Dubois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell‐line. J Exp Med. 1989;169(4):1485‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu JI, Leonard EJ. Purification and amino‐acid analysis of 2 human glioma‐derived monocyte chemoattractants. J Exp Med. 1989;169(4):1449‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura T, Robinson EA, Tanaka S, Appella E, Leonard E. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin ‐Stimulated human blood mononuclear leukocytes. J Immunol (Baltimore, Md: 1950). 1989;142:1956‐1962. [PubMed] [Google Scholar]
- 17.Lim SY, Yuzhalin AE, Gordon‐Weeks AN, Muschel RJ. Targeting the CCL2‐CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7(19):28697‐28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulbe H, Levinson NR, Balkwill F, Wilson JL. The chemokine network in cancer ‐ much more than directing cell movement. Int J Dev Biol. 2004;48(5–6):489‐496. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura T. The chemokine MCP‐1 (CCL2) in the host interaction with cancer: a foe or ally? Cell Mol Immunol. 2018;15(4):335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121‐127. [DOI] [PubMed] [Google Scholar]
- 21.Bianconi V, Sahebkar A, Atkin SL, Pirro M. The regulation and importance of monocyte chemoattractant protein‐1. Curr Opin Hematol. 2018;25(1):44‐51. [DOI] [PubMed] [Google Scholar]
- 22.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein‐1 (MCP‐1): an overview. J Interferon Cytokine Res. 2009;29(6):313‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huma ZE, Sanchez J, Lim HD, et al. Key determinants of selective binding and activation by the monocyte chemoattractant proteins at the chemokine receptor CCR2. Sci Signal. 2017;10(480):13. [DOI] [PubMed] [Google Scholar]
- 24.Xiong W, Tan J, Guo Y, Chen S, Fan L, Li Y. Notch3 knockout suppresses mouse mammary gland development and inhibits the proliferation of 4T1 murine mammary carcinoma cells via CCL2/CCR4 axis. Front Cell Develop Biol. 2020;8:594372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachelerie F, Graham GJ, Locati M, et al. New nomenclature for atypical chemokine receptors. Nat Immunol. 2014;15(3):207‐208. [DOI] [PubMed] [Google Scholar]
- 26.Lokeshwar BL, Kallifatidis G, Hoy JJ. Atypical chemokine receptors in tumor cell growth and metastasis. Adv Cancer Res. 2020;145:1‐27. [DOI] [PubMed] [Google Scholar]
- 27.Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C‐C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100(10):2552‐2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular‐cloning and functional expression of 2 monocyte chemoattractant protein‐1 receptors reveals alternative splicing of the carboxyl‐terminal tails. Proc Natl Acad Sci USA. 1994;91(7):2752‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apel AK, Cheng RKY, Tautermann CS, et al. Crystal structure of CC chemokine receptor 2A in complex with an orthosteric antagonist provides insights for the design of selective antagonists. Structure. 2019;27(3):427. [DOI] [PubMed] [Google Scholar]
- 30.Wong LM, Myers SJ, Tsou CL, Gosling J, Arai H, Charo IF. Organization and differential expression of the human monocyte chemoattractant protein 1 receptor gene ‐ evidence for the role of the carboxyl‐terminal tail in receptor trafficking. J Biol Chem. 1997;272(2):1038‐1045. [DOI] [PubMed] [Google Scholar]
- 31.Tang P, Wang JM. Chemokines: the past, the present and the future. Cell Mol Immunol. 2018;15(4):295‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation‐therapeutic opportunities and pharmacological challenges. Pharmacol Rev. 2013;65(1):47‐89. [DOI] [PubMed] [Google Scholar]
- 33.Sozzani S, Luini W, Borsatti A, et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997;159(4):1993‐2000. [PubMed] [Google Scholar]
- 34.Weber KSC, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells ‐ implications for MCP‐1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19(9):2085‐2093. [DOI] [PubMed] [Google Scholar]
- 35.Yumimoto K, Sugiyama S, Mimori K, Nakayama KI. Potentials of C‐C motif chemokine 2‐C‐C chemokine receptor type 2 blockers including propagermanium as anticancer agents. Cancer Sci. 2019;110(7):2090‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C‐C motif) ligand 2 in promoting prostate cancer growth. JNCI‐J Natl Cancer Inst. 2010;102(8):522‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izumi K, Fang LY, Mizokami A, et al. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2‐induced STAT3 activation. EMBO Mol Med. 2013;5(9):1383‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasui H, Kajiyama H, Tamauchi S, et al. CCL2 secreted from cancer‐associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38‐MAPK pathway. Clin Exp Metas. 2020;37(1):145‐158. [DOI] [PubMed] [Google Scholar]
- 39.Ma S‐B, Xian H, Wu W‐B, et al. CCL2 facilitates spinal synaptic transmission and pain via interaction with presynaptic CCR2 in spinal nociceptor terminals. Mol Brain. 2020;13(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franca CN, Izar MCO, Hortencio MNS, et al. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin Sci. 2017;131(12):1215‐1224. [DOI] [PubMed] [Google Scholar]
- 41.Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224(1–2):93‐100. [DOI] [PubMed] [Google Scholar]
- 42.Moadab F, Khorramdelazad H, Abbasifard M. Role of CCL2/CCR2 axis in the immunopathogenesis of rheumatoid arthritis: Latest evidence and therapeutic approaches. Life Sci. 2021;269:119034. [DOI] [PubMed] [Google Scholar]
- 43.Du Q, Fu Y‐X, Shu A‐M, et al. Loganin alleviates macrophage infiltration and activation by inhibiting the MCP‐1/CCR2 axis in diabetic nephropathy. Life Sci. 2020;272:118808. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Yang Z, Lu W, et al. Chemokines and chemokine receptors: a new strategy for breast cancer therapy. Cancer Med. 2020;9(11):3786‐3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y, Chen Q, Corey E, et al. Activation of MCP‐1/CCR2 axis promotes prostate cancer growth in bone. Clin Exp Metas. 2009;26(2):161‐169. [DOI] [PubMed] [Google Scholar]
- 46.Ding M, He SJ, Yang J. MCP‐1/CCL2 mediated by autocrine loop of PDGF‐BB promotes invasion of lung cancer cell by recruitment of macrophages via CCL2‐CCR2 axis. J Interferon Cytokine Res. 2019;39(4):224‐232. [DOI] [PubMed] [Google Scholar]
- 47.Zhuang H, Cao G, Kou C, Liu T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial‐mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol Rep. 2018;39(1):21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19(13):3404‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CH, Su LJ, Tsai HT, Hwang CF. ELF‐1 expression in nasopharyngeal carcinoma facilitates proliferation and metastasis of cancer cells via modulation of CCL2/CCR2 signaling. Cancer Manage Res. 2019;11:5243‐5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang ZW, Xie HY, Zhou L, et al. CCL2/CCR2 axis is associated with postoperative survival and recurrence of patients with non‐metastatic clear‐cell renal cell carcinoma. Oncotarget. 2016;7(32):51525‐51534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang MN, Zhao JZ, Zhang LS, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein‐1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25(4):611‐619. [DOI] [PubMed] [Google Scholar]
- 53.Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein‐1 (MCP‐1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66(12):1311‐1318. [DOI] [PubMed] [Google Scholar]
- 54.Martínez‐García D, Manero‐Rupérez N, Quesada R, Korrodi‐Gregório L, Soto‐Cerrato V. Therapeutic strategies involving survivin inhibition in cancer. Med Res Rev. 2019;39(3):887‐909. [DOI] [PubMed] [Google Scholar]
- 55.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3‐kinase/AKT‐dependent survivin up‐regulation. J Biol Chem. 2008;283(36):25057‐25073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang T, Zhan QY, Peng XD, Qiu ZM, Zhao TT. CCL2 influences the sensitivity of lung cancer A549 cells to docetaxel. Oncol Lett. 2018;16(1):1267‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein‐ and p42/44 mitogen‐activated protein kinase (MAPK)‐dependent mechanisms. J Biol Chem. 2012;287(43):36593‐36608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W, Wang L, Zhang JJ, et al. Purification of recombinant human chemokine CCL2 in E. coli and its function in ovarian cancer. 3 Biotech. 2021;11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu WX, Wei Q, Han MJ, et al. CCL2‐SQSTM1 positive feedback loop suppresses autophagy to promote chemoresistance in gastric cancer. Int J Biol Sci. 2018;14(9):1054‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalbasi A, Komar C, Tooker GM, et al. Tumor‐derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23(1):137‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang AL, Miska J, Wainwright DA, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid‐derived suppressor cells. Can Res. 2016;76(19):5671‐5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartwig T, Montinaro A, von Karstedt S, et al. The TRAIL‐induced cancer secretome promotes a tumor‐supportive immune microenvironment via CCR2. Mol Cell. 2017;65(4):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines. 2017;5(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457‐474. [DOI] [PubMed] [Google Scholar]
- 65.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP‐1: direct role of MCP‐1 in angiogenesis and tumor progression. Blood. 2000;96(1):34‐40. [PubMed] [Google Scholar]
- 66.Stamatovic SM, Keep RF, Mostarica‐Stojkovic M, Andjelkovic AV. CCL2 regulates angiogenesis via activation of Ets‐1 transcription factor. J Immunol. 2006;177(4):2651‐2661. [DOI] [PubMed] [Google Scholar]
- 67.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein‐1. Int J Cancer. 1999;82(5):765‐770. [DOI] [PubMed] [Google Scholar]
- 68.Stathopoulos GT, Psallidas I, Moustaki A, et al. A central role for tumor‐derived monocyte chemoattractant protein‐1 in malignant pleural effusion. J Natl Cancer Inst. 2008;100(20):1464‐1476. [DOI] [PubMed] [Google Scholar]
- 69.Cho HR, Kumari N, Vu HT, Kim H, Park CK, Choi SH. Increased antiangiogenic effect by blocking CCL2‐dependent macrophages in a rodent glioblastoma model: correlation study with dynamic susceptibility contrast perfusion MRI. Sci Rep. 2019;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu J, Zhong H, Chu T, et al. Role of anlotinib‐induced CCL2 decrease in anti‐angiogenesis and response prediction for nonsmall cell lung cancer therapy. Eur Resp J. 2019;53(3):1801562. [DOI] [PubMed] [Google Scholar]
- 71.Bartneck M, Schrammen PL, Mockel D, et al. The CCR2(+) macrophage subset promotes pathogenic angiogenesis for tumor vascularization in fibrotic livers. Cell Mol Gastroenterol Hepatol. 2019;7(2):371‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science (New York, NY). 2011;331(6024):1559‐1564. [DOI] [PubMed] [Google Scholar]
- 73.Guan XM. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adekoya TO, Richardson RM. Cytokines and chemokines as mediators of prostate cancer metastasis. Int J Mol Sci. 2020;21(12):4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suarez‐Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11(7):805‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen W, Gao Q, Han SQ, Pan F, Fan W. The CCL2/CCR2 axis enhances IL‐6‐induced epithelial‐mesenchymal transition by cooperatively activating STAT3‐Twist signaling. Tumor Biol. 2015;36(2):973‐981. [DOI] [PubMed] [Google Scholar]
- 77.Li S, Lu J, Chen Y, et al. MCP‐1‐induced ERK/GSK‐3 beta/Snail signaling facilitates the epithelial‐mesenchymal transition and promotes the migration of MCF‐7 human breast carcinoma cells. Cell Mol Immunol. 2017;14(7):621‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roy R, Morad G, Jedinak A, Moses MA. Metalloproteinases and their roles in human cancer. Anat Rec. 2020;303(6):1557‐1572. [DOI] [PubMed] [Google Scholar]
- 79.Tang CH, Tsai CC. CCL2 increases MMP‐9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF‐κB signaling pathway. Biochem Pharmacol. 2012;83(3):335‐344. [DOI] [PubMed] [Google Scholar]
- 80.Tang CH, Tsai CC. CCL2 increases MMP‐9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF‐kappa B signaling pathway. Biochem Pharmacol. 2012;83(3):335‐344. [DOI] [PubMed] [Google Scholar]
- 81.Li HJ, Li H, Li XP, et al. C‐C chemokine receptor type 2 promotes epithelial‐to‐mesenchymal transition by upregulating matrix metalloproteinase‐2 in human liver cancer. Oncol Rep. 2018;40(5):2734‐2741. [DOI] [PubMed] [Google Scholar]
- 82.Qian BZ, Deng Y, Im JH, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4(8):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qian B‐Z, Pollard JW. New tricks for metastasis‐associated macrophages. Breast Cancer Res. 2012;14(4):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitamura T, Qian BZ, Soong D, et al. CCL2‐induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis‐associated macrophages. J Exp Med. 2015;212(7):1043‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf MJ, Hoos A, Bauer J, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2‐Stat5 and p38MAPK pathway. Cancer Cell. 2012;22(1):91‐105. [DOI] [PubMed] [Google Scholar]
- 86.Liu Q, Zhang HF, Jiang XL, Qian CY, Liu ZQ, Luo DY. Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol Cancer. 2017;16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akhtar M, Haider A, Rashid S, Al‐Nabet ADMH. Paget's “seed and soil” theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol. 2019;26(1):69‐74. [DOI] [PubMed] [Google Scholar]
- 88.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1‐positive haematopoietic bone marrow progenitors initiate the pre‐metastatic niche. Nature. 2005;438(7069):820‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peinado H, Zhang HY, Matei IR, et al. Pre‐metastatic niches: organ‐specific homes for metastases. Nat Rev Cancer. 2017;17(5):302‐317. [DOI] [PubMed] [Google Scholar]
- 90.Qian BZ, Li JF, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast‐tumour metastasis. Nature. 2011;475(7355):222‐U129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sceneay J, Chow MT, Chen A, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72(16):3906‐3911. [DOI] [PubMed] [Google Scholar]
- 92.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669‐682. [DOI] [PubMed] [Google Scholar]
- 93.Cassetta L, Pollard JW. Tumor‐associated macrophages. Curr Biol. 2020;30(6):R246‐R248. [DOI] [PubMed] [Google Scholar]
- 94.Funes SC, Rios M, Escobar‐Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines (vol 41, pg 14, 2014). Immunity. 2014;41(2):339‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jablonski KA, Amici SA, Webb LM, et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10(12):e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin YX, Xu JX, Lan HY. Tumor‐associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin YX, Xu JX, Lan HY. Tumor‐associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C, Xu X, Wei S, et al. Tumor‐associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9(1):e001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549‐555. [DOI] [PubMed] [Google Scholar]
- 101.Chen CH, He W, Huang J, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. 2018;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang H, Zhang QN, Xu M, et al. CCL2‐CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD‐1 signaling in esophageal carcinogenesis. Mol Cancer. 2020;19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grossman JG, Nywening TM, Belt BA, et al. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology. 2018;7(9):e1470729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang ZH, Li H, Wang WQ, et al. CCL2/CCR2 axis promotes the progression of salivary adenoid cystic carcinoma via recruiting and reprogramming the tumor‐associated macrophages. Front Oncol. 2019;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li D, Ji H, Niu X, et al. Tumor‐associated macrophages secrete CC‐chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020;111(1):47‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liew PX, Kubes P. The neutrophil's role during health and disease. Physiol Rev. 2019;99(2):1223‐1248. [DOI] [PubMed] [Google Scholar]
- 107.SenGupta S, Subramanian BC, Parent CA. Getting TANned: how the tumor microenvironment drives neutrophil recruitment. J Leukoc Biol. 2019;105(3):449‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8(3):125‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu HB, Wu BL, Ma G, et al. Melatonin represses oral squamous cell carcinoma metastasis by inhibiting tumor‐associated neutrophils. Am J Transl Res. 2017;9(12):5361‐5374. [PMC free article] [PubMed] [Google Scholar]
- 110.Fujita S, Ikeda T. The CCL2‐CCR2 axis in lymph node metastasis from oral squamous cell carcinoma: an immunohistochemical study. J Oral Maxillofac Surg. 2017;75(4):742‐749. [DOI] [PubMed] [Google Scholar]
- 111.Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer. 2018;18(6):359‐376. [DOI] [PubMed] [Google Scholar]
- 112.Law AMK, Valdes‐Mora F, Gallego‐Ortega D. Myeloid‐derived suppressor cells as a therapeutic target for cancer. Cells. 2020;9(3):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gabrilovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid‐derived suppressor cells in tumor‐bearing mice. J Immunol. 2008;181(8):5791‐5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid‐derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chun E, Lavoie S, Michaud M, et al. CCL2 promotes colorectal carcinogenesis by enhancing polymorphonuclear myeloid‐derived suppressor cell population and function. Cell Rep. 2015;12(2):244‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor‐induced myeloid deviation: when myeloid‐derived suppressor cells meet tumor‐associated macrophages. J Clin Invest. 2015;125(9):3365‐3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Do HTT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers (Basel). 2020;12(2):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flores‐Toro JA, Luo DF, Gopinath A, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci USA. 2020;117(2):1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro‐tumor immunity. Cytokine Growth Factor Rev. 2010;21(1):3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chraa D, Naim A, Olive D, Badou A. T lymphocyte subsets in cancer immunity: friends or foes. J Leukoc Biol. 2019;105(2):243‐255. [DOI] [PubMed] [Google Scholar]
- 122.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of T(H)2 polarization by the chemokine monocyte chemoattractant protein‐1. Nature. 2000;404(6776):407‐411. [DOI] [PubMed] [Google Scholar]
- 123.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2(2):102‐107. [DOI] [PubMed] [Google Scholar]
- 124.Mandal PK, Biswas S, Mandal G, et al. CCL2 conditionally determines CCL22‐dependent Th2‐accumulation during TGF‐β‐induced breast cancer progression. Immunobiology. 2018;223(2):151‐161. [DOI] [PubMed] [Google Scholar]
- 125.Najafi M, Farhood B, Mortezaee K. Contribution of regulatory T cells to cancer: a review. J Cell Physiol. 2019;234(6):7983‐7993. [DOI] [PubMed] [Google Scholar]
- 126.Vasanthakumar A, Chisanga D, Blume J, et al. Sex‐specific adipose tissue imprinting of regulatory T cells. Nature. 2020;579(7800):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ge YZ, Bohm HH, Rathinasamy A, et al. Tumor‐specific regulatory T cells from the bone marrow orchestrate antitumor immunity in breast cancer. Cancer Immunol Res. 2019;7(12):1998‐2012. [DOI] [PubMed] [Google Scholar]
- 128.Mondini M, Loyher PL, Hamon P, et al. CCR2‐dependent recruitment of tregs and monocytes following radiotherapy is associated with TNFα‐mediated resistance. Cancer Immunol Res. 2019;7(3):376‐387. [DOI] [PubMed] [Google Scholar]
- 129.Loyher PL, Rochefort J, de Chanville CB, et al. CCR2 influences T regulatory cell migration to tumors and serves as a biomarker of cyclophosphamide sensitivity. Can Res. 2016;76(22):6483‐6494. [DOI] [PubMed] [Google Scholar]
- 130.Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer‐associated fibroblasts. Nat Rev Cancer. 2020;20(3):174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen XM, Song EW. Turning foes to friends: targeting cancer‐associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99‐115. [DOI] [PubMed] [Google Scholar]
- 132.Shen H, Yu XB, Yang FM, et al. Reprogramming of normal fibroblasts into cancer‐associated fibroblasts by miRNAs‐mediated CCL2/VEGFA signaling. PLoS Genet. 2016;12(8):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li X, Xu Q, Wu Y, et al. A CCL2/ROS autoregulation loop is critical for cancer‐associated fibroblasts‐enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis. 2014;35(6):1362‐1370. [DOI] [PubMed] [Google Scholar]
- 134.Yang XG, Lin YL, Shi YH, et al. FAP promotes immunosuppression by cancer‐associated fibroblasts in the tumor microenvironment via STAT3‐CCL2 signaling. Can Res. 2016;76(14):4124‐4135. [DOI] [PubMed] [Google Scholar]
- 135.Pausch TM, Aue E, Wirsik NM, et al. Metastasis‐associated fibroblasts promote angiogenesis in metastasized pancreatic cancer via the CXCL8 and the CCL2 axes. Sci Rep. 2020;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moisan F, Francisco EB, Brozovic A, et al. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol Oncol. 2014;8(7):1231‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu XM, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol. 2011;104(1):83‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9(7):556‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Teng KY, Han JF, Zhang XL, et al. Blocking the CCL2‐CCR2 axis using CCL2‐neutralizing antibody is an effective therapy for hepatocellular cancer in a mouse model. Mol Cancer Ther. 2017;16(2):312‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fridlender ZG, Buchlis G, Kapoor V, et al. CCL2 blockade augments cancer immunotherapy. Can Res. 2010;70(1):109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gazzaniga S, Bravo AI, Guglielmotti A, et al. Targeting tumor‐associated macrophages and inhibition of MCP‐1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127(8):2031‐2041. [DOI] [PubMed] [Google Scholar]
- 142.Zollo M, Di Dato V, Spano D, et al. Targeting monocyte chemotactic protein‐1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metas. 2012;29(6):585‐601. [DOI] [PubMed] [Google Scholar]
- 143.Liu SH, Gao HW, Gao CZ, Liu WG, Xing DG. Bindarit attenuates pain and cancer‐related inflammation by influencing myeloid cells in a model of bone cancer. Arch Immunol Ther Exp. 2018;66(3):221‐229. [DOI] [PubMed] [Google Scholar]
- 144.Murphy EA, Davis JM, McClellan JL, Gordon BT, Carmichael MD. Curcumin's effect on intestinal inflammation and tumorigenesis in the Apc(Min/+) mouse. J Interferon Cytokine Res. 2011;31(2):219‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herman JG, Stadelman HL, Roselli CE. Curcumin blocks CCL2‐induced adhesion, motility and invasion, in part, through down‐regulation of CCL2 expression and proteolytic activity. Int J Oncol. 2009;34(5):1319‐1327. [PMC free article] [PubMed] [Google Scholar]
- 146.Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Can Res. 2007;67(19):9417‐9424. [DOI] [PubMed] [Google Scholar]
- 147.Mu XY, Wang RJ, Yao ZX, et al. RS 504393 inhibits M‐MDSCs recruiting in immune microenvironment of bladder cancer after gemcitabine treatment. Mol Immunol. 2019;109:140‐148. [DOI] [PubMed] [Google Scholar]
- 148.Mirzadegan T, Diehl F, Ebi B, et al. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists ‐ binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275(33):25562‐25571. [DOI] [PubMed] [Google Scholar]
- 149.Lindholm PF, Sivapurapu N, Jovanovic B, Kajdacsy‐Balla A. Monocyte‐induced prostate cancer cell invasion is mediated by chemokine ligand 2 and nuclear factor‐κB activity. J Clin Cell Immunol. 2015;6(2):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Han R, Gu SZ, Zhang YJ, et al. Estrogen promotes progression of hormone‐dependent breast cancer through CCL2‐CCR2 axis by upregulation of Twist via PI3K/AKT/NF‐kappa B signaling. Sci Rep. 2018;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.An J, Xue Y, Long MJ, Zhang G, Zhang JH, Su H. Targeting CCR2 with its antagonist suppresses viability, motility and invasion by downregulating MMP‐9 expression in non‐small cell lung cancer cells. Oncotarget. 2017;8(24):39230‐39240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rafei M, Deng JS, Boivin MN, et al. A MCP1 fusokine with CCR2‐specific tumoricidal activity. Mol Cancer. 2011;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Imai N, Shiota T, Kataoka K, et al. Small molecule inhibitors of the CCR2b receptor. Part 1: discovery and optimization of homopiperazine derivatives. Bioorg Med Chem Lett. 2004;14(21):5407‐5411. [DOI] [PubMed] [Google Scholar]
- 154.Roblek M, Calin M, Schlesinger M, et al. Targeted delivery of CCR2 antagonist to activated pulmonary endothelium prevents metastasis. J Controlled Release. 2015;220:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grossman JG, Nywening TM, Belt BA, et al. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology. 2018;7(9):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yao WB, Ba Q, Li XG, et al. A natural CCR2 antagonist relieves tumor‐associated macrophage‐mediated immunosuppression to produce a therapeutic effect for liver cancer. Ebiomedicine. 2017;22:58‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nywening TM, Belt BA, Cullinan DR, et al. Targeting both tumour‐associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67(6):1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Han YY, Liu DD, Li LH. PD‐1/PD‐L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727‐742. [PMC free article] [PubMed] [Google Scholar]
- 159.Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD‐1/PD‐L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol. 2019;234(2):1313‐1325. [DOI] [PubMed] [Google Scholar]
- 160.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science (New York, NY). 2020;367(6477):525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fein MR, He X‐Y, Almeida AS, et al. Cancer cell CCR2 orchestrates suppression of the adaptive immune response. J Exp Med. 2020;217(10):e20181551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tu MM, Abdel‐Hafiz HA, Jones RT, et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun Biol. 2020;3(1):720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tu MM, Abdel‐Hafiz HA, Jones RT, et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun Biol. 2020;3(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lubowicka E, Przylipiak A, Zajkowska M, et al. Plasma chemokine CCL2 and its receptor CCR2 concentrations as diagnostic biomarkers for breast cancer patients. Biomed Res Int. 2018;2018:2124390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li R, Zhang H, Liu H, et al. High expression of C‐C chemokine receptor 2 associates with poor overall survival in gastric cancer patients after surgical resection. Oncotarget. 2016;7(17):23909‐23918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Obmolova G, Teplyakov A, Malia TJ, et al. Structural basis for high selectivity of anti‐CCL2 neutralizing antibody CNTO 888. Mol Immunol. 2012;51(2):227‐233. [DOI] [PubMed] [Google Scholar]
- 167.Sandhu SK, Papadopoulos K, Fong PC, et al. A first‐in‐human, first‐in‐class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71(4):1041‐1050. [DOI] [PubMed] [Google Scholar]
- 168.Pienta KJ, Machiels JP, Schrijvers D, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 (CCL2), in metastatic castration‐resistant prostate cancer. Invest New Drugs. 2013;31(3):760‐768. [DOI] [PubMed] [Google Scholar]
- 169.Brana I, Calles A, LoRusso PM, et al. Carlumab, an anti‐C‐C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open‐label, multicenter phase 1b study. Target Oncol. 2015;10(1):111‐123. [DOI] [PubMed] [Google Scholar]
- 170.Nywening TM, Wang‐Gillam A, Sanford DE, et al. Targeting tumour‐associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single‐centre, open‐label, dose‐finding, non‐randomised, phase 1b trial. Lancet Oncol. 2016;17(5):651‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Noel M, O'Reilly EM, Wolpin BM, et al. Phase 1b study of a small molecule antagonist of human chemokine (C‐C motif) receptor 2 (PF‐04136309) in combination with nab‐paclitaxel/gemcitabine in first‐line treatment of metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2020;38(3):800‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Masuda T, Noda M, Kogawa T, et al. Phase I dose‐escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020;111(3):924‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Linehan D, Noel MS, Hezel AF, et al. Overall survival in a trial of orally administered CCR2 inhibitor CCX872 in locally advanced/metastatic pancreatic cancer: correlation with blood monocyte counts. J Clin Oncol. 2018;36(5):92. [Google Scholar]
- 174.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817‐1825. [DOI] [PubMed] [Google Scholar]
- 175.Li L, Liu YD, Zhan YT, et al. High levels of CCL2 or CCL4 in the tumor microenvironment predict unfavorable survival in lung adenocarcinoma. Thorac Cancer. 2018;9(7):775‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li L, Wei W, Li ZZ, et al. The spleen promotes the secretion of CCL2 and supports an M1 dominant phenotype in hepatic macrophages during liver fibrosis. Cell Physiol Biochem. 2018;51(2):557‐574. [DOI] [PubMed] [Google Scholar]
- 177.Bonapace L, Coissieux MM, Wyckoff J, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515(7525):17. [DOI] [PubMed] [Google Scholar]
- 178.Izumi K, Mizokami A, Lin H‐P, et al. Serum chemokine (CC motif) ligand 2 level as a diagnostic, predictive, and prognostic biomarker for prostate cancer. Oncotarget. 2016;7(7):8389‐8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang J, Yan Y, Cui XH, et al. CCL2 expression correlates with Snail expression and affects the prognosis of patients with gastric cancer. Pathol Res Pract. 2017;213(3):217‐221. [DOI] [PubMed] [Google Scholar]
- 180.Tao LL, Shi SJ, Chen LB, Huang GC. Expression of monocyte chemotactic protein‐1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World J Gastroenterol. 2014;20(15):4421‐4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu YY, Pan J, Pan X, et al. Klotho‐mediated targeting of CCL2 suppresses the induction of colorectal cancer progression by stromal cell senescent microenvironments. Mol Oncol. 2019;13(11):2460‐2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Feng L, Qi Q, Wang P, et al. Serum level of CCL2 predicts outcome of patients with pancreatic cancer. Acta Gastro‐Enterologica Belgica. 2020;83(2):295‐299. [PubMed] [Google Scholar]
- 183.Lewis HL, Chakedis JM, Talbert E, et al. Perioperative cytokine levels portend early death after pancreatectomy for ductal adenocarcinoma. J Surg Oncol. 2018;117(6):1260‐1266. [DOI] [PubMed] [Google Scholar]
- 184.Yang J, Lv X, Chen JN, et al. CCL2‐CCR2 axis promotes metastasis of nasopharyngeal carcinoma by activating ERK1/2‐MMP2/9 pathway. Oncotarget. 2016;7(13):15632‐15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lu X, Qian C‐N, Mu Y‐G, et al. Serum CCL2 and serum TNF‐alpha ‐ Two new biomarkers predict bone invasion, post‐treatment distant metastasis and poor overall survival in nasopharyngeal carcinoma. Eur J Cancer. 2011;47(3):339‐346. [DOI] [PubMed] [Google Scholar]
- 186.Huang T, Fan Q, Wang YW, et al. Schwann cell‐derived CCL2 promotes the perineural invasion of cervical cancer. Front Oncol. 2020;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fader AN, Rasool N, Vaziri SAJ, et al. CCL2 expression in primary ovarian carcinoma is correlated with chemotherapy response and survival outcomes. Anticancer Res. 2010;30(12):4791‐4798. [PubMed] [Google Scholar]
- 188.Li XG, Yao WB, Yuan Y, et al. Targeting of tumour‐infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157‐167. [DOI] [PubMed] [Google Scholar]
- 189.Wang W‐W, Ang SF, Kumar R, et al. Identification of serum monocyte chemoattractant protein‐1 and prolactin as potential tumor markers in hepatocellular carcinoma. PLoS One. 2013;8(7):e68904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang Y, Zhai C, Chang Y, et al. High expression of chemokine CCL2 is associated with recurrence after surgery in clear‐cell renal cell carcinoma. Urol Oncol‐Semin Orig Invest. 2016;34(5):238.e19–e26. [DOI] [PubMed] [Google Scholar]
- 191.Arakaki R, Yamasaki T, Kanno T, et al. CCL2 as a potential therapeutic target for clear cell renal cell carcinoma. Cancer Med. 2016;5(10):2920‐2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eckstein M, Epple E, Jung R, et al. CCL2 expression in tumor cells and tumor‐infiltrating immune cells shows divergent prognostic potential for bladder cancer patients depending on lymph node stage. Cancers. 2020;12(5):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu J‐F, Chen P‐C, Chang T‐M, Hou C‐H. Monocyte chemoattractant protein‐1 promotes cancer cell migration via c‐Raf/MAPK/AP‐1 pathway and MMP‐9 production in osteosarcoma. J Exp Clin Cancer Res. 2020;39(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tanaka K, Kurebayashi J, Sohda M, et al. The expression of monocyte chemotactic protein‐1 in papillary thyroid carcinoma is correlated with lymph node metastasis and tumor recurrence. Thyroid. 2009;19(1):21‐25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


