Abstract
Titanium particles as a product of degradation have been detected in periimplant oral tissues and it has been assumed that implants were the source of these particles. Periimplantitis sites had higher concentrations of particles in comparison to healthy implant sites. Several factors have been identified in the degradation of dental implant surface, such as mechanical wear, contact with chemical agents, and the effects of biofilm adhesion. Titanium particles silently prompt the immune-system activation and generate a pro-inflammatory response in macrophages, T lymphocytes and monocytes. During the activation, inflammatory cytokines are released including, granulocyte–macrophage colony-stimulating factor (GM-CSF), prostaglandin, and TNF-α, IL-1β, IL-6. The nanoparticles depict unique features such as high level of biological reactivity and potentially harmful compared to microparticles since they have a relatively greater surface area to volume ratio. Allergic response to titanium as a cause of implant failure has not been well documented. Evidence demonstrating biological complication due to titanium particles release includes peri-implant tissue inflammation that lead terminally to implant loss. There is a biological probability for a relation between the presence of titanium particles and ions, biological complication, and corrosion, but there is no justifiable evidence for unidirectional series of causative actions.
Keywords: Titanium implant, Titanium particles, Implant degradation, Periimplantitis, Implant wear, Implant corrosion
1. Introduction
There is a steady rise in the use of dental implants to restore function and improve aesthetics to enhance quality of life for partially or fully edentulous patients. Clinical research conducted during the past five decades has led to improvements in the design of dental implants and their surface topography generating a better understanding of the relation between implant surface and the bone and soft tissue biology. Titanium is the predominant material used for dental implant fixtures due to their biocompatibility, high resistance to corrosion, and high tensile strength, and this was reflected clinically in the high survival rates and long-term stability. Nevertheless, there have been concerns regarding implant material degradation, allergic reactions, and chronic peri-implant inflammation leading to failure and loss of implant [[1], [2], [3]]. Alterations to the physico-chemical composition and/or modifications of the surface layer of implant materials are continuously being investigated to avoid failure of implants and performing reimplantation operations [4].
It has been suggested that the surface topography of implants could influence changes in peri-implant bone levels and in-turn influence the occurrence of biological complications such as periimplantitis. Previous studies have found higher concentrations of titanium particles in desquamated cells from the peri-implant mucosa having peri-implantitis compared to soft tissues around clinically health implants [5,6]. Besides, there were higher quantities of dissolved titanium found in the submucosal biofilm of implants with peri-implantitis compared to those with healthy peri-implant tissues [7]. From these findings, we can infer that when titanium particles are released into the tissues, they may contribute to the pathogenesis of peri-implant diseases [8]. This concern contributes to the current interest in the value of other implant materials like zirconia [9]. Javed et al. [10] and Siddiqi et al. [11] have conducted reviews on hypersensitivity and allergic reactions associated with titanium implants; both studies could not rule titanium as a cause of hypersensitivity, and there is no proof of titanium causing allergic reactions. Independent of allergic pathways, the leached debris from implants may have toxic or pro-inflammatory potential to cause harm to peri-implant tissues [12]. Following these controversies, the aim of this review is to provide a summary of evidence of the association, if any, between tribocorrosion and the release of titanium particles towards the complications of dental implant therapy.
2. Materials and methods
The following electronic databases were primarily searched to identify relevant studies: Medline, Google Scholar, and PubMed, using the terms” dental” OR “implant” OR “hypersensitivity” OR “particles” OR “tribocorrosion” OR “allergy” OR “complications” OR “survival” together with (AND) “titanium” OR “Ti.” OR “TiO2”. Furthermore, the bibliographies of selected suitable articles and previously published reviews dealing with the biological complications of implants were searched to identify other potentially relevant material. We included clinical studies and pertinent in vivo and in vitro experiments. Two authors independently evaluated the titles and abstracts of the selected articles followed by a thorough joint discussion of the authors to establish and resolve any disagreement between them concerning the articles.
3. Results and discussion
The use of different keyword combinations was applied to each of the selected databases with no time period limits. A total of 122 papers were identified and only ninety relevant articles were selected and retrieved for final analysis. The review included, 13 clinical studies, 8 in-vivo animal studies, 36 in vitro studies and the rest are systematic review and literature review articles.
3.1. Dental implants as the source of titanium particles in human tissues
Titanium particles as a product of degradation have been detected in both intra-oral and extra-oral tissues. In cases of patients who had dental implants, it has been assumed that the implants were the source of these particles. In these patients, titanium particles could be found in the submucosal plaque, peri-implant bone and/or soft tissues, and in distant lymph nodes [13,14]. Schliephake et al. [15] confirmed through in-vivo studies that traces of titanium were present in tissue specimens of the kidneys, lungs and liver five months from the time of implant placement. Further studies [16,17] have suggested that titanium particles were transported in the bloodstream by phagocytic cells or plasma proteins to organs such as the liver, lungs, spleen, or abdominal lymph nodes.
A systematic review by Suárez-López del Amo et al. [18] showed that it is common for titanium particles to be found surrounding peri-implant tissues. Peri-implantitis sites had higher concentrations of the particles in comparison to healthy implant sites. Titanium particles were mostly detected inside epithelial cells, macrophages, connective tissue and bone. The most common methods through which titanium is released into peri-implant tissues are from friction during implant placement procedures, implant surface corrosion, and fretting phenomena at the implant-abutment interphase. In addition to these, Olmedo et al. [6] reported that implant debridement procedures during maintenance visits was another feasible method of particle release. Implant surface cleaning with mechanical and chemical means that occurs during prophylaxis and therapy of peri-implantitis and peri-implant mucositis could also lead to the release of titanium particles.
3.2. Factors contributing to degradation of dental implants
Metallic biomaterials such as dental implants and its frameworks, abutments, orthodontic devices, and maxillofacial surgical structures [19] are susceptible to tribocorrosion damage, in the oral environment, which is a process where material degradation is caused by the combined effect of corrosion and wear. Corrosion refers to the chemical or electrochemical reactions of the material, while wear is the mechanical material degradation caused by the friction that occurs between two surfaces such as between a dental implant and its supra-structure or abutment [20]. Tribocorrosion can hence be defined as the scientific field that focuses on the inter-relationship of wear and chemical and/or electrochemical processes [21]. Notably, there are specific oral conditions, that include food intake, oral microbiota, fluoridated dentifrices, salivary pH and biomechanical loading that when in-combined, may significantly increase corrosion and speed up the degradation process, and subsequently having a direct influence on the survival of dental implant [22].
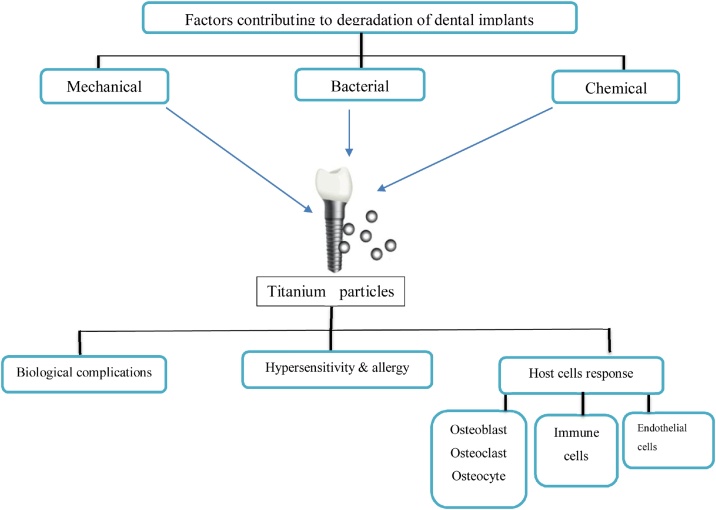
In its elementary form, titanium has a quick and strong reaction with oxygen forming a stable protective film of titanium dioxide around the exposed surface that prevent further oxidization of the deeper parts. However, under other common environmental conditions, such as exposure to sea water, titanium does not show any visible corrosion, even over periods of several years. Nonetheless, inside an oral environment, titanium may at times be exposed to local conditions that may foster corrosion [23]. Designing in-vivo studies on wear measurements are highly complex and time-consuming; hence, dental wear analysis is usually simulated and evaluated in an artificial saliva environment. Several factors have been identified to be involved in the degradation of dental implant surface and the release of titanium particles, such as mechanical wear, contact with chemical agents, and the effects of biofilm adhesion (Fig. 1).
Fig. 1.
Diagram showing release of titanium particles from dental implant and the consequent complications, based on this narrative review.
3.2.1. Mechanical wear-related degradation
Dental implant surface can undergo mechanical wear during the implant placement surgery, fitting of implant-supported prosthesis procedure, preventive mechanical cleaning and therapy of peri-implant infections, and as a result of friction caused by micro-movements between the implant and its supra-structure during function. The protective oxide layer that forms on surface of titanium structures keeps the release of corrosion products at a very low level; however, no metallic material is completely resistant to corrosion or ionization when present in a living tissue environment [24]. The disruption of the oxide layer can occur mechanically, with such damages resulting in titanium particles being released. In accordance with the theory of passivity, the protective oxide film curtails the dissolution of the metal ions and varies in stability inside the human body [25]. The possible degradation mechanisms of surface oxide layer are wearing, corrosion or a combination of both. This could cause deterioration of the metal surface and release of particles into the peri-implant region [24,26]. Fretting corrosion occurs when there are small oscillating movements between two materials that are interacting with each other (e.g. bone-implant and implant-abutment interaction) in a corrosive oral environment [27]. Fretting causes the protective oxide layer to rupture, and initiates cracks and forms reactive metal atoms on the surface layer that are vulnerable to corrosion [28]. So, fretting-wear refers to chipping, while adhesive-wear refers to plastic deformation.
Deppe et al. [20] conducted an in vitro study that demonstrated oral implants could trigger the release of particles that are stripped off their surface during insertion into bone. Meyer et al. [29] in an in-vivo study confirmed presence of titanium in peri-implant bone after the placement of implants in the jawbone of pigs. The scanning electron microscopy showed an abundance of titanium particles around implants with rough surface, as well as higher particle deposition in the crestal part of the bone. These findings are confirmed by Suárez‐López del Amo et al. [18] who conducted tests on varied implant surfaces (sandblasted large-grit, acid-etched/hydrophilic, dual-acid-etched, sandblasted large grit acid-etched, fluoride-modified, and large grit, phosphate-enriched titanium oxide) and found round elongated or small angular titanium debris present in the crestal area of the osteotomy site. Wear of the abutment and the implant could be promoted by micromotion, and this would widen the micro-gap allowing microleakage and invasion of bacteria. Prophylaxis and therapy of peri-implantitis and peri-implant mucositis are procedures concerned with the cleaning of the surfaces of implants within an oral environment with chemical and mechanical means. There are clinicians who even advocate implant surface modification macroscopically with the use of rotary instrument and such interventions in the treatment of advance peri-implant lesion will undoubtedly lead to direct mechanical surface damage and particle leach [30,31]. While several modifications of rotary instruments were tested to result in a much smoother implant surface outcome [30,32], such procedures still cause contamination of the surrounding tissues with significant quantities of abrasion debris of polishing instruments and titanium particles.
3.2.2. Chemical agents-related degradation
There are chemical agents that would decrease the protection provided by the oxide layer forming on the surface of dental implants, causing a corrosion process. This may occur as a result of interaction with products that contain fluoride or acid used for dental treatment or prophylaxis. An in vitro study explored the different effects of chemical agents on titanium alloy (Ti-6Al-4 V) and on commercially pure titanium after surface rubbing and immersion [33]. Adverse pitting and discoloration were seen in the specimens that were treated with hydrogen peroxide (15%), citric acid, peroxyacetic acid (35%), and chlorhexidine (0.12%, 1%). Titanium particles was detected on all rubbing swabs treatment using energy-dispersive spectroscopy analysis. The chemotherapeutic agents altered the wettability of the surface, while chlorhexidine 0.12% created cytotoxic effects on the implant surface with a potential for distorting its biocompatibility [34]. Barao et al. [35] conducted an immersion tests in fluoride at low pH, demonstrating titanium ion release, which is usually slow, but increased noticeably. There was various corrosion sequence observed when both titanium grade II and IV implants contacted with saliva containing fluoride ions. It has been suggested that there was a decrease in the protective properties of the oxide layer when the fluoride ions were incorporated [36].
Antimicrobial mouthwashes prescription is a common practice after implant placement procedures and during peri-implantitis treatment [37]. There is reliable documentation that oral mouthwashes [[38], [39], [40]] and fluoride concentration cause damage on the surface of metallic biomaterials. Analyses of corrosion were done after immersion of Ti6Al4V discs into different mouthwashes solutions (0.05% cetylpyridinium chloride, 0.12% chlorhexidine digluconate, artificial saliva, and 3% hydrogen peroxide) for 14 days. Electrochemical impedance spectroscopy analysis showed decreased corrosion resistance only for those discs that were exposed to 3% hydrogen peroxide; this was also true in discs’ surface topography. Discs exposure to 0.05% cetylpyridinium chloride and 0.12% chlorhexidine digluconate did not modify any of their parameters [41]. However, it was seen that 0.2% chlorhexidine digluconate might induce pitting corrosion [42]. The most noticeable clinical sign of titanium release is “metallosis,” a dark tissue discoloration seen in tissues that are adjacent to implants [43].
3.2.3. Bacterial biofilm-related degradation
When pH is low, there is a potential for titanium degradation due to reduce corrosion resistance An acidic environment generally favors the exchange of ions between saliva and implant surface. that is triggering higher rates of corrosion [35]. The oral microbiota ferment dietary sugars to organic acids and increases the chances of dental implants corrosion and degradation [44]. Unlike orthopedic devices, dental implants are typically exposed to an environment containing abundance of microorganisms. Adherent bacterial biofilm is usually formed on all exposed implant surfaces within the oral cavity [45]. During the development of these biofilms, presence of bacteria alter the environmental conditions, oxygen concentration levels and pH value [46], hence promoting inflammatory reactions in the adjacent tissues [47]. Normally, the bacteria are located on the external implant surface, but they may also be found between secondary parts and implant body, such as prosthesis, abutment, and screws interfaces [48]. Inner space contamination occurs during secondary parts insertion or later via micro-gaps in between components [49].
Sridhar et al. [50] investigated the influence of early planktonic bacteria colonization on the implant surface; and found that after 22 days of immersing implants in suspensions of Streptococcus mutans, corrosion characteristics were seen in microscopic deformation form and mild rusting on implants’ edge, pitting deformation, and discoloration. A study carried by Mathew et al. [44] established that lipopolysaccharides (LPS) from bacteria activated exchange of ions between saliva and titanium; hence, decreasing the corrosion resistance and surging the roughness of titanium surface. Generally, high LPS concentration and lower pH tend to increase titanium corrosion [51]. An in vitro study demonstrated a significant increase in bacteria adhesion to immersed implants in a fluoridated medium of (1500 ppm, pH 5.5), compared to a control solution [52].
It is believed that the roughness of the surface prompts accumulation of bacteria where they sustain corrosion process further and cause peri-implant mucositis; therefore, suggesting corrosion as a factor that contributes to peri-implantitis. Besides, the surface roughness effect on in vivo biofilm make up was showed by Rimondini et al. [53]. A historical analysis was done on 272 implants obtained due to biological failures over a duration of 16 years showed loss of titanium particles from the implant surface and internal threads along with the internal portions of implants and presence of bacteria in the micro-gap [54]. A study by Safioti et al. [7] compared samples from 30 patients containing dissolved titanium in the submucosal plaque, showed that diseased sample sites had significantly more bacteria and manifested high degrees of titanium ions than in healthy sites specimens. As dental implants are exposed to microbiota from the oral environment, it is feasible to expect that oral microorganisms would affect the tribocorrosion of implants surfaces [55].
When implants were placed in the oral cavity, a salivary pellicle instantly formed on dental implant surface. Finally, the implant surface was covered by the oral bacteria, thus making a biofilm [56]. The interfacial discrepancies among abutments and implants produce a prosthetic gap that encourages microbial colonization [36]. The acidic metabolites made by bacteria are associated with increasing tendency of titanium corrosion [55]. The carbohydrates metabolism and biofilm development change the pH and oxygen level when the acidic substances are released. Bacteria colonization lead to pH alteration, acidic substances released and prompts localization of titanium corrosion [57]. Therefore, reducing the pH level through production of lactic acidic encourages diffusion and accumulation of fluoride ions by the extracellular biofilm matrix that triggers to the surge of titanium corrosion [36]. In a study carried out by Mathew et al. [44] on the association between LPS and titanium surface degradation, they found that LPS which is a membrane consisting of gram-negative bacteria, influences the corrosion behavior of pure titanium and TiAIV alloy negatively. Similarly, the production of hydrogen peroxide (H202) during inflammatory response affects titanium corrosion resistance negatively [57]. Moreover, the particle release affects the biofilm development, bacteria endotoxins accumulation and peri-implants tissue health [58]. Generally, the studies showed that acidic environment encourages the process that lead to release of titanium particles and have a high tendency to induce bacterial biofilms inflammatory processes.
3.3. Influence of titanium particles on immune, bone, and tissue cells
3.3.1. Immune cells-implant interaction
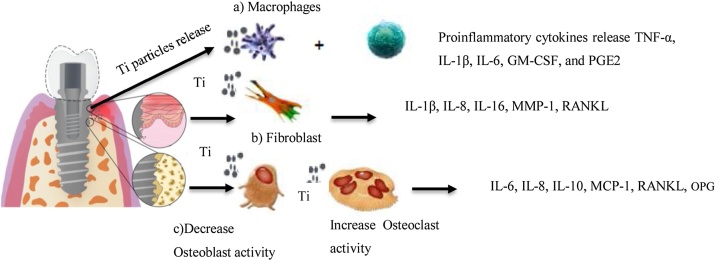
The remarkable aspect of titanium particles is that they prompt the immune-system activation and generate a pro-inflammatory response in macrophages, T lymphocytes and monocytes. During the activation, inflammatory cytokines are released including, granulocyte–macrophage colony-stimulating factor (GM-CSF), prostaglandin, and TNF-α, IL-1β, IL-6 as shown in (Fig. 2) [12]. In case of cell cultures that are primed with titanium particles and Escherichia coli LPS (100 ng/mL), it enhances the release and activation of IL-1β in a dose-dependent manner. The primary stimulus is the LPS that increase pro-IL-1β expression in the macrophages. The secondary stimulus is the titanium particles that activates cascade NLRP3 inflammasome caspase releasing a mature IL-1β. However, titanium nanoparticles and microparticles show distinct aspects of action in respective cells. The nanoparticles depict unique features such as high level of biological reactivity and potentially harmful compared to microparticles since they have a relatively greater surface area to volume ratio [59]. The aggregation and surface area of nanoparticulate materials may change once they are introduced into the biological milieu. The nanoparticles may also aggregate in a similar microparticles size and alter their own identity by the host; thus, significantly reducing the inflammatory response [59]. The levels of particles aggregation should be taken into account when considering dose- and size-dependent toxicity, since the particle size may differently affect the severity of toxicity [60].
Fig. 2.
Schematic representative diagram of interaction at (a) immune cells-implant, (b) soft tissue-implant, (c) bone-implant, based on this narrative review.
Understanding the biological effects of different sized particles on periimplant tissues and on body systems is a new challenge to nanotoxicology and biocompatibility studies [61]. The surface of dental implant may be a potential source of release of both microparticles and nanoparticles into the biological milieu. Little is known about the biological effects, biodistribution, and final destination in the body, of titanium nanoparticles. Although, microparticles and nanoparticles can be chemically similar, their specific physico–chemical properties may cause different biological responses. However, nanoparticles have a larger surface to volume ratio, they are biologically more reactive and potentially more toxic and harmful to human health than microparticles [62].
3.3.2. Bone-implant interaction
The aseptic implant loosening in orthopedic reconstructive surgery is a serious complication related to wear particles, and it is characterized by osteolysis at the bone-implant interface [45]. Studies have pointed out that titanium particles compromise the balance between bone resorption and bone formation in two ways; directly through differently activate osteoclasts and osteoblasts [63], and indirectly by inducing the secretion of inflammatory cytokines which is produced by lymphocytes and macrophages [64,65]. According to Pioletti et al. [66] at low concentration titanium particles (0.05), the osteoblast was not affected. Nonetheless, at higher concentrations of 0.15%–1% in contact with osteoblasts, the viability was significantly reduced. Titanium ions may affect the osteoclasts differentiation through influencing the expression of receptor activator of nuclear factor ĸB ligand (RANKL) and osteoprotegerin (OPG) in the osteoblast, in vitro [67] (Fig. 2). Besides, it depicts that titanium contact to human osteoclast precursors could cause their differentiation into mature osteoclasts, which may later corrode substrate from the metal and take up the particles [68].
A study conducted by Wachi et al. [65] showed that the exposure of Porphyromonas gingivalis-lipopolysaccharide (PG-LPS) to a titanium concentration of 9 ppm increased the surged RANKL/OPG ratio in the bone. In this case, the titanium ions could play a part in the inflammatory reaction that could lead to bone resorption that is observed in peri-implantitis. However, when the concentration of titanium ions is less than 9 ppm, it does not influence osteoblasts viability [69]. But, when the concentration is at 9 ppm, it increases the RANKL mRNA expression in osteoblasts which are known by their ability to internalize wear particles; modifying the primary cell mechanism and may end up in cell necrosis [12]. So, maturation and differentiation of osteoclasts that are induced by metal ions and particles may exacerbate peri-implantits. In the same manner, titanium particle cytotoxicity may influence bone marrow stem cells as reported in previous work by [70]. A study carried out by Ribeiro et al. [71] showed that in the intricate biological environment, anatase nanoparticles form bio-complexes (mixture of proteins and ions) which act as a kind of ‘Trojan-horse’ internalization by osteoblasts. Furthermore, anatase nanoparticles-induced modifications on osteoblasts viability and internalization.
3.3.3. Gingival tissue-implant interaction
When a metal come into contacts with gingival tissues, it experiences an electrochemical process that has a high tendency to liberate ions. These ions form complexes together with host protein that activates the immune system; the response of lymphocyte to serum protein complex with metal particles from implants was examined in vitro [72]. The detection of an increase in the expression of Toll-like receptor-4, and bacterial LPS was achieved by injecting 9 ppm titanium ions in gingival epithelium [65]. Titanium ions could compete with bacterial LPS to modulate gingival epithelial cells response by changing the sensitivity to oral bacteria. Nevertheless, the viability of gingival epithelial cells is significantly decrease when the concentration of titanium ions is higher than 13 ppm, and this may induce cell necrosis [73].
The epithelium was considered a physical barrier to titanium particles, with the ability to establish innate immune response. An in vitro study investigated on how the homeostasis of oral epithelial cells is affected by titanium particles [18]. The damage of oral epithelium cells may be activated by titanium particles with surface containing phosphate-enriched titanium oxide or that had been fluoride-modified [74]. Fibroblasts have shown some of the reactions to titanium particles. The titanium wear particles in the peri-implant soft-tissue are toxic to epithelial cells such as fibroblast, through cellular DNA damage [12]. Therefore, this process influences the integrity of epithelial barrier, enhancing bacterial colonization where the infection reaction and inflammatory response are increased [75].
The in vitro combined exposure of fibroblasts from peri-implant granulation tissue to titanium dioxide particles and P. gingivalis had the strongest effect on gene expression of tumor necrosis factor TNF-α, IL-6, and IL-8 (Fig. 2) [76]. In such cases, the titanium particles play a role in inflammation exacerbation mainly caused by biofilm accompanied by peri-implant tissue bacteria. Berryman et al. [77] showed a mixture of acute and chronic inflammatory cells within peri-implantitis tissues and a remarkable cytokine RANKL overexpression, with an overexpression of TGF-B1 and IL-33 as well in areas infiltrated with titanium particles. Wilson et al. [78] reported similar findings, whereby chronic inflammatory cells infiltration was seen in peri-implantitis suggesting the presence of titanium particles initiated chronic inflammation in those tissues.
3.4. Titanium particles-related hypersensitivity and allergic reaction
Allergy is an acute immunological response to antigen contact. It could be either an immediate humoral response as a result of antibody/antigen complexes reactions such as in type I, II, and III; or delayed cell-mediated response, type IV [79]. Implant related allergic reaction is more commonly associated with type IV delayed hypersensitivity. Although titanium is preferred as an inert material in dental implants, it has a high probability to encourage type I and IV reactions. An allergic response caused by titanium is responsible for implants failure in some instances; the study has documented that titanium allergy risk is prevalent to patients who are sensitive to metals [80]. Titanium does not exist in its free form since it is highly reactive when exposed to oxygen forming titanium dioxide. It has been hypothesized that titanium dioxide tends to bind with proteins, and the compounds may evoke a hypersensitivity reaction.
A critical review by Fage et al. [81] has recorded cases believed to be titanium allergy and accounted several clinical signs and symptoms such as swelling, redness, and vesicular lesions of the skin overlaying titanium devices such as implants and screws. A facial eczema emerged after placement of two titanium implants for a mandibular overdenture; and complete remission was achieved upon removal of the titanium material [82]. Furthermore, dermatitis, rash, pruritus, redness, urticaria, and facial eczema allergic reactions were detected in a retrospective study on titanium implant allergy conducted by Sicilia et al. [83]. When memory lymphocyte immune-stimulation assay (MELISA) was used against ten metals including titanium among 56 patients following dental implants placement, the results showed that 21 of the patients were tested positive for titanium. However, when the implants were removed, all the 56 patients showed a relief of clinical symptoms. The same test was repeated for 15 sensitized patients, and they manifested a normalization in MELISA reactivity [84]. To compare the potential hypersensitivity reactions to dental implants, it is crucial to consider that all dental implants will inevitably be contaminated by bacteria during surgery, although this is not the case in indwelling devices.
According to Bonsignore, et al. [85], microbial residue and adherent LPS manifest a negative interaction with peri-implant tissues; this render the evidence for existence of allergy or hypersensitivity against titanium. Furthermore, there was often a temporal relationship between tissue exposure to titanium and the incidence of tissue reactions. Although hypersensitivity and allergic reaction associated with titanium release into oral tissues have been justified; it is not clear whether such phenomena play any significant part in epidemiology of the peri-implant diseases [86]. Based on the present evidence, and the availability of a few reported cases, it is doubtful whether hypersensitivity or allergy to titanium has any relation to epidemiology of peri-implant condition or if it play any role in the disease process. In contrary, Siddiqi et al. [11] showed that titanium prompts hypersensitivity in some patients and could have played a part in implant failure.
3.5. Titanium particles-related biological complications
The biological complication due to titanium particles includes peri-implant tissue inflammation that leading terminally to implant loss [77,87]. Peri-implant diseases could be peri-implant mucositis or peri-implantitis. They are characterized by the presence or absence of peri-implant bone loss, respectively. In the sixth European Workshop consensus report on the Periodontology (6 EWOP), “peri-implant mucositis” was described as inflammatory lesions involving mucosa only, whereas the lesions in “peri-implantitis” sites extend to supporting bone [88]. Thus, dental implants biological problems comprise earlier failure, prompted by poor or absence of osseointegration, bone resorption that is typically associated with an infection, and inflammatory lesions limited to the mucosa that end up in implant loosening. In addition, other factors such as tobacco smoking, premature loading, and other systemic condition including diabetes mellitus predisposes the risk of biological problems and modify the healing process accompanied by implant loss that usually happens early [[89], [90], [91]].
Souza et al. [92] showed in a review about corrosive pathways of titanium dental implants and their adverse biological effects following exposure to several therapeutic products like mouthwash. Oral dentifrices have a negative impact on corrosion resistance characteristics of the titanium dioxide film and promote release of titanium particles. In addition, fluoride toothpaste and bleaching agents, can amplify the chemical reactivity of titanium since fluoride ions interact with the titanium dioxide film. Moreover, bacteria and oral biofilm accumulation lower the oral pH level, thus inducing titanium corrosion at a much lower fluoride concentration. According to Suarez-Lopez Del Amo et al. [18], periimplantitis sites showed higher number of titanium particles when compared to healthy implants sites. This could be linked to the role of titanium wear debris in the mechanism of peri-implant disease development.
4. Conclusion
Today it is evident that titanium is the most successful material used for dental implant fixtures due their biocompatibility, high resistance to corrosion, and high tensile strength, which is reflected clinically in the high survival rates and long-term stability. However, the degradation products and wear particles have been identified in oral and extra-oral tissues consequently to the release of titanium particles from dental implants due to mechanical wear, contact to chemical agents, and interaction with substances produced by adherent biofilm and inflammatory cells. There has been concern on it demerits on patient safety and conformability; hence, numerous studies have been carried to establish evidence on its effectiveness and related complication. In that case, it is imperative to further evaluate the titanium dental implants on different perspectives such as response to oral cells, allergic complication, mechanical wear, and general degrading factors.
The two types, namely: (a) toxic/pro-inflammatory and (b) hypersensitivity/allergic effects, which are host reaction, ought to be well-thought-out in relation to a reaction to the presence of titanium dental implants. The fact raised by the existence of allergy or hypersensitivity against titanium is rare, where a temporal association is shown between occurrence of tissue reaction and titanium implantation. Nonetheless, the specificity of the reaction is poor and could be related to other aspects of implant placement. Many in vitro and in vivo experiment have demonstrated remarkable titanium particles leach that have pro-inflammatory and toxic effects in the peri-implant environment. Furthermore, it has shown modulating factor effects for instance, the size of released particles and relation with LPS molecules. Typically, titanium particles are detected in peri-implant mucosa and a much higher amount is seen from diseased site mucosal specimens. However, a high concentration of titanium ions in the diseased sites could be the consequence of corrosion caused by the activity of inflammatory cells and bacteria present in peri-implantitis lesions, rendering a lower pH in the peri-implant environment.
Thus, there is a biological probability for a relation between the presence of titanium particles and ions, biological complication, and corrosion, but there is no justifiable evidence for unidirectional series of causative actions. Different factors may take part in response to external foreign bodies such as titanium particles, inflammation and immune response, corrosion, and microorganism with various feedback loops: wear and corrosion together with environmental factors lead to material degradation in a process called tribocorrosion which leads to titanium particles release. Therefore, titanium particles and ions compromise the normal function of the cell; hence, increasing the possibility of inflammation under various circumstances. The findings show that inflammation interferes with functions and composition of biofilm and causes corrosion. Titanium remains predominant even though there have been concerns about negative complication. These findings have influenced the need to carry out future research to further improve and make titanium dental implant experience better for patients who need tooth replacement.
Funding
This project was supported by a research grant from University of Sharjah.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Waad Kheder, Email: wkheder@sharjah.ac.ae.
Sausan Al Kawas, Email: sausan@sharjah.ac.ae.
Khaled Khalaf, Email: kkhalaf@sharjah.ac.ae.
A.R. Samsudin, Email: drabrani@sharjah.ac.ae.
References
- 1.Albrektsson T., Branemark P.I., Hansson H.A., Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52(155):70. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 2.Buser D., Sennerby L., De Bruyn H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000. 2017;73(1):7–21. doi: 10.1111/prd.12185. [DOI] [PubMed] [Google Scholar]
- 3.Hoornaert A., Vidal L., Besnier R., Morlock J., Louarn G., Layrolle P. Biocompatibility and osseointegration of nanostructured titanium dental implants in minipigs. Clin Oral Implants Res. 2020 doi: 10.1111/clr.13589. [DOI] [PubMed] [Google Scholar]
- 4.Ossowska A., Zieliński A. The mechanisms of degradation of titanium dental implants. Coatings. 2020;10(9):836. [Google Scholar]
- 5.He X.L., Reichl F.X., Wang Y., Michalke B., Milz S., Yang Y. Analysis of titanium and other metals in human jawbones with dental implants — a case series study. Dent Mater. 2016;32:1042–1051. doi: 10.1016/j.dental.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Olmedo D.G., Nalli G., Verdú S., Paparella M.L., Cabrini R.L. Exfoliative cytology and titanium dental implants: a pilot study. J Periodontol. 2013;84(1):78–83. doi: 10.1902/jop.2012.110757. [DOI] [PubMed] [Google Scholar]
- 7.Safioti L.M., Kotsakis G.A., Pozhitkov A.E., Chung W.O., Daubert D.M. Increased levels of dissolved titanium are associated with peri‐implantitis–a cross‐sectional study. J Periodontol. 2017;88(5):436–442. doi: 10.1902/jop.2016.160524. [DOI] [PubMed] [Google Scholar]
- 8.Halperin‐Sternfeld M., Zigdon‐Giladi H., Machtei E.E. The association between shallow vestibular depth and peri‐implant parameters: a retrospective 6 years longitudinal study. J Clin Periodontol. 2016;43(3):305–310. doi: 10.1111/jcpe.12504. [DOI] [PubMed] [Google Scholar]
- 9.Cionca N., Hashim D., Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000. 2017;73(1):241–258. doi: 10.1111/prd.12180. [DOI] [PubMed] [Google Scholar]
- 10.(a) Javed F., Ahmed H.B., Crespi R., Romanos G.E. Role of primary stability for successful osseointegration of dental implants: factors of influence and evaluation. Interv Med Appl Sci. 2013;5(4):162–167. doi: 10.1556/IMAS.5.2013.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Siddiqi A., Payne A.G., De Silva R.K., Duncan W.J. Titanium allergy: could it affect dental implant integration? Clin Oral Implants Res. 2011;22(7):673–680. doi: 10.1111/j.1600-0501.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqi A., Payne A.G., De Silva R.K., Duncan W.J. Titanium allergy: could it affect dental implant integration? Clin Oral Implants Res. 2011;22(7):673–680. doi: 10.1111/j.1600-0501.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 12.Noronha Oliveira M., Schunemann W., Mathew M.T., Henriques B., Magini R.S., Teughels W. Can degradation products released from dental implants affect peri‐implant tissues? J Periodont Res. 2018;53(1):1–11. doi: 10.1111/jre.12479. [DOI] [PubMed] [Google Scholar]
- 13.Deppe H., Greim H., Brill T., Wagenpfeil S. Titanium deposition after peri-implant care with the carbon dioxide laser. Int J Oral Maxillofac Implants. 2002;17(5) [PubMed] [Google Scholar]
- 14.Frisken K.W., Dandie G.W., Lugowski S., Jordan G. A study of titanium release into body organs following the insertion of single threaded screw implants into the mandibles of sheep. Aust Dent J. 2002;47(3):214–217. doi: 10.1111/j.1834-7819.2002.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 15.Schliephake H., Reiss G., Urban R., Neukam F.W., Guckel S. Metal release from titanium fixtures during placement in the mandible: an experimental study. Int J Oral Maxillofac Implants. 1993;8(5) [PubMed] [Google Scholar]
- 16.Olmedo D., Guglielmotti M.B., Cabrini R.L. An experimental study of the dissemination of titanium and zirconium in the body. J Mater Sci Mater Med. 2002;13(8):793–796. doi: 10.1023/a:1016131310025. [DOI] [PubMed] [Google Scholar]
- 17.Urban R.M., Jacobs J.J., Tomlinson M.J., Gavrilovic J., Black J., Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. JBJS. 2000;82(4):457. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Suárez‐López del Amo F., Garaicoa‐Pazmiño C., Fretwurst T., Castilho R.M., Squarize C.H. Dental implants‐associated release of titanium particles: a systematic review. Clin Oral Implants Res. 2018;29(11):1085–1100. doi: 10.1111/clr.13372. [DOI] [PubMed] [Google Scholar]
- 19.Bryant M., Neville A. Fretting corrosion of CoCr alloy: effect of load and displacement on the degradation mechanisms. Proc Inst Mech Eng H J Eng Med. 2017;231(2):114–126. doi: 10.1177/0954411916680237. [DOI] [PubMed] [Google Scholar]
- 20.Deppe H., Wolff C., Bauer F., Ruthenberg R., Sculean A., Mücke T. Dental implant surfaces after insertion in bone: an in vitro study in four commercial implant systems. Clin Oral Investig. 2018;22(3):1593–1600. doi: 10.1007/s00784-017-2262-4. [DOI] [PubMed] [Google Scholar]
- 21.Trino L.D., Bronze-Uhle E.S., Ramachandran A., Lisboa-Filho P.N., Mathew M.T., George A. Titanium surface bio-functionalization using osteogenic peptides: surface chemistry, biocompatibility, corrosion and tribocorrosion aspects. J Mech Behav Biomed Mater. 2018;81:26–38. doi: 10.1016/j.jmbbm.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa B.C., Alves A.C., Toptan F., Pinto A.M., Grenho L., Fernandes M.H. Exposure effects of endotoxin-free titanium-based wear particles to human osteoblasts. J Mech Behav Biomed Mater. 2019;95:143–152. doi: 10.1016/j.jmbbm.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Kim K.T., Eo M.Y., Nguyen T.T.H., Kim S.M. General review of titanium toxicity. Int J Implant Dent. 2019;5(1):10. doi: 10.1186/s40729-019-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revathi A., Borrás A.D., Muñoz A.I., Richard C., Manivasagam G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater Sci Eng C. 2017;76:1354–1368. doi: 10.1016/j.msec.2017.02.159. [DOI] [PubMed] [Google Scholar]
- 25.Noumbissi S., Scarano A., Gupta S. A literature review study on atomic ions dissolution of titanium and its alloys in implant dentistry. Materials. 2019;12(3):368. doi: 10.3390/ma12030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zavanelli R.A., Henriques G.E.P., Ferreira I., de Almeida Rollo João M.D. Corrosion-fatigue life of commercially pure titanium and Ti-6Al-4V alloys in different storage environments. J Prosthet Dent. 2000;84(3):274–279. doi: 10.1067/mpr.2000.108758. [DOI] [PubMed] [Google Scholar]
- 27.Manivasagam G., Dhinasekaran D., Rajamanickam A. Recent patents on corrosion science. 2010. Biomedical implants: corrosion and its prevention-a review. [Google Scholar]
- 28.Gittens R.A., Olivares-Navarrete R., Tannenbaum R., Boyan B.D., Schwartz Z. Electrical implications of corrosion for osseointegration of titanium implants. J Dent Res. 2011;90(12):1389–1397. doi: 10.1177/0022034511408428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer U., Bühner M., Büchter A., Kruse‐Lösler B., Stamm T., Wiesmann H.P. Fast element mapping of titanium wear around implants of different surface structures. Clin Oral Implants Res. 2006;17(2):206–211. doi: 10.1111/j.1600-0501.2005.01184.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramel C.F., Lüssi A., Özcan M., Jung R.E., Hämmerle C.H., Thoma D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin Oral Implants Res. 2016;27(7):776–781. doi: 10.1111/clr.12682. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz F., John G., Becker J. The influence of implantoplasty on the diameter, chemical surface composition, and biocompatibility of titanium implants. Clin Oral Investig. 2017;21(7):2355–2361. doi: 10.1007/s00784-016-2030-x. [DOI] [PubMed] [Google Scholar]
- 32.Bollen C.M., Papaioanno W., Van Eldere J., Schepers E., Quirynen M., Van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri‐implant mucositis. Clin Oral Implants Res. 1996;7(3):201–211. doi: 10.1034/j.1600-0501.1996.070302.x. [DOI] [PubMed] [Google Scholar]
- 33.Wheelis S.E., Gindri I.M., Valderrama P., Wilson T.G., Jr, Huang J., Rodrigues D.C. Effects of decontamination solutions on the surface of titanium: investigation of surface morphology, composition, and roughness. Clin Oral Implants Res. 2016;27(3):329–340. doi: 10.1111/clr.12545. [DOI] [PubMed] [Google Scholar]
- 34.Kotsakis G.A., Lan C., Barbosa J., Lill K., Chen R., Rudney J. Antimicrobial agents used in the treatment of peri‐implantitis alter the physicochemistry and cytocompatibility of titanium surfaces. J Periodontol. 2016;87(7):809–819. doi: 10.1902/jop.2016.150684. [DOI] [PubMed] [Google Scholar]
- 35.Barão V.A., Mathew M.T., Assunção W.G., Yuan J.C., Wimmer M.A., Sukotjo C. Stability of cp‐Ti and Ti‐6 Al‐4 V alloy for dental implants as a function of saliva pH–an electrochemical study. Clin Oral Implants Res. 2012;23(9):1055–1062. doi: 10.1111/j.1600-0501.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- 36.Souza J.C., Barbosa S.L., Ariza E.A., Henriques M., Teughels W., Ponthiaux P. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater Sci Eng C. 2015;47:384–393. doi: 10.1016/j.msec.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 37.Heitz-Mayfield L.J., Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29 doi: 10.11607/jomi.2014suppl.g5.3. [DOI] [PubMed] [Google Scholar]
- 38.Golvano I., García I., Conde A., Tato W., Aginagalde A. Influence of fluoride content and pH on corrosion and tribocorrosion behaviour of Ti13Nb13Zr alloy in oral environment. J Mech Behav Biomed Mater. 2015;49:186–196. doi: 10.1016/j.jmbbm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Licausi M., Muñoz A.I., Borrás V.A. Influence of the fabrication process and fluoride content on the tribocorrosion behaviour of Ti6Al4V biomedical alloy in artificial saliva. J Mech Behav Biomed Mater. 2013;20:137–148. doi: 10.1016/j.jmbbm.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Sivakumar B., Kumar S., Narayanan T.S. Fretting corrosion behaviour of Ti–6Al–4V alloy in artificial saliva containing varying concentrations of fluoride ions. Wear. 2011;270(3–4):317–324. [Google Scholar]
- 41.Faverani L.P., Barao V.A.R., Pires M.F.A., Yuan J.C., Sukotjo C., Mathew M.T. Corrosion kinetics and topography analysis of Ti–6Al–4V alloy subjected to different mouthwash solutions. Mater Sci Eng C. 2014;43:1–10. doi: 10.1016/j.msec.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 42.Quaranta A., Ronconi L.F., Di Carlo F., Vozza I., Quaranta M. Electrochemical behaviour of titanium in ammine and stannous fluoride and chlorexidine 0.2% mouthwashes. Int J Immunopathol Pharmacol. 2010;23(1):335–343. doi: 10.1177/039463201002300132. [DOI] [PubMed] [Google Scholar]
- 43.Romesburg J.W., Wasserman P.L., Schoppe C.H. Metallosis and metal-induced synovitis following total knee arthroplasty: review of radiographic and CT findings. J Radiol Case Rep. 2010;4(9):7. doi: 10.3941/jrcr.v4i9.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathew M.T., Barão V.A., Yuan J.C., Assunção W.G., Sukotjo C., Wimmer M.A. What is the role of lipopolysaccharide on the tribocorrosive behavior of titanium? J Mech Behav Biomed Mater. 2012;8:71–85. doi: 10.1016/j.jmbbm.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Fürst M.M., Salvi G.E., Lang N.P., Persson G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res. 2007;18(4):501–508. doi: 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 46.Marshall A., Ries M.D., Paprosky W. How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J Am Acad Orthop Surg. 2008;16:S1–S6. doi: 10.5435/00124635-200800001-00003. [DOI] [PubMed] [Google Scholar]
- 47.Meyer S., Giannopoulou C., Courvoisier D., Schimmel M., Müller F., Mombelli A. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res. 2017;28(8):1005–1012. doi: 10.1111/clr.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cosyn J., Van Aelst L., Collaert B., Persson G.R., De Bruyn H. The peri‐implant sulcus compared with internal implant and suprastructure components: a microbiological analysis. Clin Implant Dent Relat Res. 2011;13(4):286–295. doi: 10.1111/j.1708-8208.2009.00220.x. [DOI] [PubMed] [Google Scholar]
- 49.Persson L.G., Lekholm U., Leonhardt Å, Dahlen G., Lindhe J. Bacterial colonization on internal surfaces of Brånemark system® implant components. Clin Oral Implants Res. 1996;7(2):90–95. doi: 10.1034/j.1600-0501.1996.070201.x. [DOI] [PubMed] [Google Scholar]
- 50.Sridhar S., Wilson T.G., Jr, Palmer K.L., Valderrama P., Mathew M.T., Prasad S. In vitro investigation of the effect of oral bacteria in the surface oxidation of dental implants. Clin Implant Dent Relat Res. 2015;17:e562–e575. doi: 10.1111/cid.12285. [DOI] [PubMed] [Google Scholar]
- 51.Barao V.A., Mathew M.T., Assunção W.G., Yuan J.C., Wimmer M.A., Sukotjo C. The role of lipopolysaccharide on the electrochemical behavior of titanium. J Dent Res. 2011;90(5):613–618. doi: 10.1177/0022034510396880. [DOI] [PubMed] [Google Scholar]
- 52.Correa C.B., Pires J.R., Fernandes-Filho R.B., Sartori R., Vaz L.G. Fatigue and fluoride corrosion on Streptococcus mutans adherence to titanium‐based implant/component surfaces. J Prosthodont Implant Esthetic Reconstr Dent. 2009;18(5):382–387. doi: 10.1111/j.1532-849X.2009.00463.x. [DOI] [PubMed] [Google Scholar]
- 53.Rimondini L., Farè S., Brambilla E., Felloni A., Consonni C., Brossa F. The effect of surface roughness on early in vivo plaque colonization on titanium. J Periodontol. 1997;68(6):556–562. doi: 10.1902/jop.1997.68.6.556. [DOI] [PubMed] [Google Scholar]
- 54.Scarano A., Assenza B., Piattelli M., Iezzi G., Leghissa G.C., Quaranta A. A 16–year study of the microgap between 272 human titanium implants and their abutments. J Oral Implantol. 2005;31(6):269–275. doi: 10.1563/753.1. [DOI] [PubMed] [Google Scholar]
- 55.Figueiredo‐Pina C.G., Guedes M., Sequeira J., Pinto D., Bernardo N., Carneiro C. On the influence of Streptococcus salivarius on the wear response of dental implants: an in vitro study. J Biomed Mater Res B Appl Biomater. 2019;107(5):1393–1399. doi: 10.1002/jbm.b.34231. [DOI] [PubMed] [Google Scholar]
- 56.Busscher H.J., Rinastiti M., Siswomihardjo W., Mei H.C. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89:657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 57.Mabilleau G., Bourdon S., Joly-Guillou M.L., Filmon R., Baslé M.F., Chappard D. Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium. Acta Biomater. 2006;2(1):121–129. doi: 10.1016/j.actbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Suárez-López del Amo F., Rudek I., Wagner V.P., Martins M.D., O’Valle F., Galindo-Moreno P. Titanium activates the DNA damage response pathway in oral epithelial cells: a pilot study. Int J Oral Maxillofac Implants. 2017;32(6) doi: 10.11607/jomi.6077. [DOI] [PubMed] [Google Scholar]
- 59.Guglielmotti M.B., Domingo M.G., Steimetz T., Ramos E., Paparella M.L., Olmedo D.G. Migration of titanium dioxide microparticles and nanoparticles through the body and deposition in the gingiva: an experimental study in rats. Eur J Oral Sci. 2015;123(4):242–248. doi: 10.1111/eos.12190. [DOI] [PubMed] [Google Scholar]
- 60.L’Azou B., Jorly J., On D., Sellier E., Moisan F., Fleury-Feith J. In vitro effects of nanoparticles on renal cells. Part Fibre Toxicol. 2008;5:22. doi: 10.1186/1743-8977-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marcos E.B., Deborah R.T., Emilio R., María L.P., Pablo E., Raúl J.R. Impact through time of different sized titanium dioxide particles on biochemical and histopathological parameters. J Biomed Mater Res A. 2014;102(5):1439–1448. doi: 10.1002/jbm.a.34822. [DOI] [PubMed] [Google Scholar]
- 62.Revell P.A. The biological effects of nanoparticles. Nanotechnol Percept. 2006;2:283–298. [Google Scholar]
- 63.Wang W., Ferguson D.J., Quinn J.M., Simpson A.H.R., Athanasou N.A. Biomaterial particle phagocytosis by bone-resorbing osteoclasts. J Bone Joint Surg Br. 1997 doi: 10.1302/0301-620x.79b5.7780. [DOI] [PubMed] [Google Scholar]
- 64.Wang W., Ferguson D.J., Quinn J.M., Simpson A.H.R., Athanasou N.A. Biomaterial particle phagocytosis by bone-resorbing osteoclasts. J Bone Joint Surg Br. 1997;79(5):849–856. doi: 10.1302/0301-620x.79b5.7780. [DOI] [PubMed] [Google Scholar]
- 65.Wachi T., Shuto T., Shinohara Y., Matono Y., Makihira S. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology. 2015;327:1–9. doi: 10.1016/j.tox.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 66.Pioletti D.P., Takei H., Kwon S.Y., Wood D., Sung K.P. The cytotoxic effect of titanium particles phagocytosed by osteoblasts. J Biomed Mater Res. 1999;46(3):399–407. doi: 10.1002/(sici)1097-4636(19990905)46:3<399::aid-jbm13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 67.Koide M., Maeda H., Roccisana J.L., Kawanabe N., Reddy S.V. Cytokine regulation and the signaling mechanism of osteoclast inhibitory peptide‐1 (OIP‐1/hSca) to inhibit osteoclast formation. J Bone Miner Res. 2003;18(3):458–465. doi: 10.1359/jbmr.2003.18.3.458. [DOI] [PubMed] [Google Scholar]
- 68.Cadosch D., Al‐Mushaiqri M.S., Gautschi O.P., Meagher J., Simmen H., Filgueira L. Biocorrosion and uptake of titanium by human osteoclasts. J Biomed Mater Res A. 2010;95(4):1004–1010. doi: 10.1002/jbm.a.32914. [DOI] [PubMed] [Google Scholar]
- 69.Mine Y., Makihira S., Nikawa H., Murata H., Hosokawa R., Hiyama A. Impact of titanium ions on osteoblast-, osteoclast-and gingival epithelial-like cells. J Prosthodont Res. 2010;54(1):1–6. doi: 10.1016/j.jpor.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Meng B., Chen J., Guo D., Ye Q., Liang X. The effect of titanium particles on rat bone marrow stem cells in vitro. Toxicol Mech Methods. 2009;19(9):552–558. doi: 10.3109/15376510903401716. [DOI] [PubMed] [Google Scholar]
- 71.Ribeiro A.R., Gemini-Piperni S., Travassos R., Lemgruber L., Silva R.C., Rossi A.L. Trojan-like internalization of anatase titanium dioxide nanoparticles by human osteoblast cells. Sci Rep. 2016;6(1):1–11. doi: 10.1038/srep23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hallab N.J., Mikecz K., Vermes C., Skipor A., Jacobs J.J. Differential lymphocyte reactivity to serum‐derived metal–protein complexes produced from cobalt‐based and titanium‐based implant alloy degradation. J Biomed Mater Res. 2001;56(3):427–436. doi: 10.1002/1097-4636(20010905)56:3<427::aid-jbm1112>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 73.Makihira S., Mine Y., Nikawa H., Shuto T., Iwata S., Hosokawa R. Titanium ion induces necrosis and sensitivity to lipopolysaccharide in gingival epithelial-like cells. Toxicol Vitr. 2010;24(7):1905–1910. doi: 10.1016/j.tiv.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 74.Wei X., Zhang X., Zuscik M.J., Drissi M.H., Schwarz E.M., O’Keefe R.J. Fibroblasts express RANKL and support osteoclastogenesis in a COX‐2‐dependent manner after stimulation with titanium particles. J Bone Miner Res. 2005;20(7):1136–1148. doi: 10.1359/JBMR.050206. [DOI] [PubMed] [Google Scholar]
- 75.Dini C., Costa R.C., Sukotjo C., Takoudis C.G., Mathew M.T., Barão V.A. Progression of bio-tribocorrosion in implant dentistry. Front Mech Eng. 2020;6(1) [Google Scholar]
- 76.Irshad M., Scheres N., Crielaard W., Loos B.G., Wismeijer D., Laine M.L. Influence of titanium on in vitro fibroblast–Porphyromonas gingivalis interaction in peri‐implantitis. J Clin Periodontol. 2013;40(9):841–849. doi: 10.1111/jcpe.12136. [DOI] [PubMed] [Google Scholar]
- 77.Berryman Z., Bridger L., Hussaini H.M., Rich A.M., Atieh M., Tawse-Smith A. Titanium particles: an emerging risk factor for peri-implant bone loss. Saudi Dent J. 2020;32(6):283–292. doi: 10.1016/j.sdentj.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson T.G., Jr, Valderrama P., Burbano M., Blansett J., Levine R., Kessler H., Rodrigues D.C. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015 doi: 10.1902/jop.2014.140363. [DOI] [PubMed] [Google Scholar]
- 79.Chaturvedi T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys) Indian J Dent Res. 2009;20(1):91. doi: 10.4103/0970-9290.49068. [DOI] [PubMed] [Google Scholar]
- 80.Chuang S.K., Cai T., Douglass C.W., Wei L.J., Dodson T.B. Frailty approach for the analysis of clustered failure time observations in dental research. J Dent Res. 2005;84(1):54–58. doi: 10.1177/154405910508400109. [DOI] [PubMed] [Google Scholar]
- 81.Fage S.W., Muris J., Jakobsen S.S., Thyssen J.P. Titanium: a review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Derm. 2016;74(6):323–345. doi: 10.1111/cod.12565. [DOI] [PubMed] [Google Scholar]
- 82.Egusa H., Ko N., Shimazu T., Yatani H. Suspected association of an allergic reaction with titanium dental implants: a clinical report. J Prosthet Dent. 2008;100(5):344–347. doi: 10.1016/S0022-3913(08)60233-4. [DOI] [PubMed] [Google Scholar]
- 83.Sicilia A., Cuesta S., Coma G., Arregui I., Guisasola C., Ruiz E. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin Oral Implants Res. 2008;19(8):823–835. doi: 10.1111/j.1600-0501.2008.01544.x. [DOI] [PubMed] [Google Scholar]
- 84.Muller K., Valentine-Thon E. Hypersensitivity to titanium: clinical and laboratory evidence. Neuroendocrinol Lett. 2006;27(1):31–35. [PubMed] [Google Scholar]
- 85.Bonsignore L.A., Anderson J.R., Lee Z., Goldberg V.M., Greenfield E.M. Adherent lipopolysaccharide inhibits the osseointegration of orthopedic implants by impairing osteoblast differentiation. Bone. 2013;52(1):93–101. doi: 10.1016/j.bone.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mombelli A., Hashim D., Cionca N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin Oral Implants Res. 2018;29:37–53. doi: 10.1111/clr.13305. [DOI] [PubMed] [Google Scholar]
- 87.Berglundh T., Persson L., Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29:197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. [DOI] [PubMed] [Google Scholar]
- 88.Lindhe J., Meyle J., Group D of the European Workshop on Periodontology Peri‐implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol. 2008;35:282–285. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 89.Atieh M.A., Alsabeeha N.H., Faggion Jr C.M., Duncan W.J. The frequency of peri‐implant diseases: a systematic review and meta‐analysis. J Periodontol. 2013;84(11):1586–1598. doi: 10.1902/jop.2012.120592. [DOI] [PubMed] [Google Scholar]
- 90.Derks J., Tomasi C. Peri‐implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42:S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 91.Stacchi C., Berton F., Perinetti G., Frassetto A., Lombardi T., Khoury A. Risk factors for peri-implantitis: Effect of history of periodontal disease and smoking habits. A systematic review and meta-analysis. J Oral Maxillofac Res. 2016;7(3) doi: 10.5037/jomr.2016.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Souza J., Apaza-Bedoya K., Benfatti C.A., Silva F.S., Henriques B. A comprehensive review on the corrosion pathways of titanium dental implants and their biological adverse effects. Metals. 2020;10(9):1272. [Google Scholar]