Significance
Type I fusion machines rearrange from prefusion to postfusion conformations to induce viral-cell entry, with structure-based stabilization of the metastable prefusion forms of these machines yielding promising vaccine candidates. However, with human metapneumovirus (HMPV), both prefusion and postfusion forms of the fusion (F) glycoprotein yield equivalent titers. We therefore tried an alternative strategy, the creation of interprotomer disulfides (IP-DSs) linking together each of the three protomers of the F trimer. In mice, IP-DS stabilization induced significantly higher titers than non–IP-DS–stabilized trimers. In macaques, improvement was more modest; nonetheless, IP-DS–stabilized trimers, in either prefusion or postfusion forms, could induce neutralizing titers many times the average titer observed in a healthy human cohort, suggesting their utility as vaccine immunogens.
Keywords: disulfide-based stabilization, nonhuman primates, paramyxoviruses, structure-based vaccine design, type I fusion machines
Abstract
Human metapneumovirus (HMPV) is a major cause of respiratory disease worldwide, particularly among children and the elderly. Although there is no licensed HMPV vaccine, promising candidates have been identified for related pneumoviruses based on the structure-based stabilization of the fusion (F) glycoprotein trimer, with prefusion-stabilized F glycoprotein trimers eliciting significantly higher neutralizing responses than their postfusion F counterparts. However, immunization with HMPV F trimers in either prefusion or postfusion conformations has been reported to elicit equivalent neutralization responses. Here we investigate the impact of stabilizing disulfides, especially interprotomer disulfides (IP-DSs) linking protomers of the F trimer, on the elicitation of HMPV-neutralizing responses. We designed F trimer disulfides, screened for their expression, and used electron microscopy (EM) to confirm their formation, including that of an unexpected postfusion variant. In mice, IP-DS–stabilized prefusion and postfusion HMPV F elicited significantly higher neutralizing responses than non–IP-DS–stabilized HMPV Fs. In macaques, the impact of IP-DS stabilization was more measured, although IP-DS–stabilized variants of either prefusion or postfusion HMPV F induced neutralizing responses many times the average titers observed in a healthy human cohort. Serological and absorption-based analyses of macaque responses revealed elicited HMPV-neutralizing responses to be absorbed differently by IP-DS–containing and by non–IP-DS–containing postfusion Fs, suggesting IP-DS stabilization to alter not only the immunogenicity of select epitopes but their antigenicity as well. We speculate the observed increase in immunogenicity by IP-DS trimers to be related to reduced interprotomer flexibility within the HMPV F trimer.
Human metapneumovirus (HMPV) is a globally widespread respiratory pathogen (1–13) primarily affecting infants, the elderly, and those that are immune compromised (reviewed in ref. 14). Disease symptoms are similar to those of the closely related respiratory syncytial virus (RSV) (15), with rates of hospitalization in older adults approaching those of influenza (6). Phylogenetic analysis (SI Appendix, Fig. S1) shows HMPV to comprise two related subtypes, A and B, which are closely related to avian metapneumoviruses, from which HMPV might have evolved (1, 16, 17).
There is currently no licensed vaccine or treatment for HMPV (2–4, 18). Recently, promising vaccine candidates for RSV as well as for parainfluenza viruses types 1 through 4 have been developed using structure-based vaccine design, in which prefusion-stabilized versions of the fusion (F) glycoprotein have been used to induce high titer-neutralizing responses; this approach has succeeded in animal models (19–23) and for RSV in recent human clinical trials (24). Unfortunately, prefusion F-based stabilization with the HMPV F glycoprotein does not induce improved neutralization titers compared to postfusion HMPV F (18, 25, 26).
In the absence of a difference in titers induced by prefusion versus postfusion forms of HMPV F, we explored whether there was a structure-based vaccine approach that might increase neutralizing titers elicited by HMPV F. With both human and bovine RSV F, stabilization in the prefusion conformation with multiple disulfide bonds significantly increased neutralizing titers (27, 28). The increase was especially evident with interprotomer disulfides (IP-DSs), covalently cross-linking together protomers within the trimer (27). We therefore sought to test the impact of disulfides—and especially IP-DSs—on the elicitation of HMPV-neutralizing responses. We evaluated the prefusion HMPV F structure (PDB ID 5WB0) (26) for sites suitable for the introduction of either intraprotomer- or IP-DS–bonding mutations, which we then synthesized, expressed, and tested antigenically. We determined cryoEM structures to delineate F conformation and atomic-level details of stabilization and assessed immunogenicity in mice and rhesus macaques. Overall, IP-DSs—of both prefusion and postfusion F—induced significantly higher HMPV-neutralizing responses than non–IP-DS–stabilized variants, suggesting that in addition to fixing a particular conformation, IP-DSs appear to be capable of enhancing the immunogenicity of neutralizing responses.
Results
Design and Characterization of Intraprotomer and IP-DSs That Stabilize the Prefusion HMPV F Trimer.
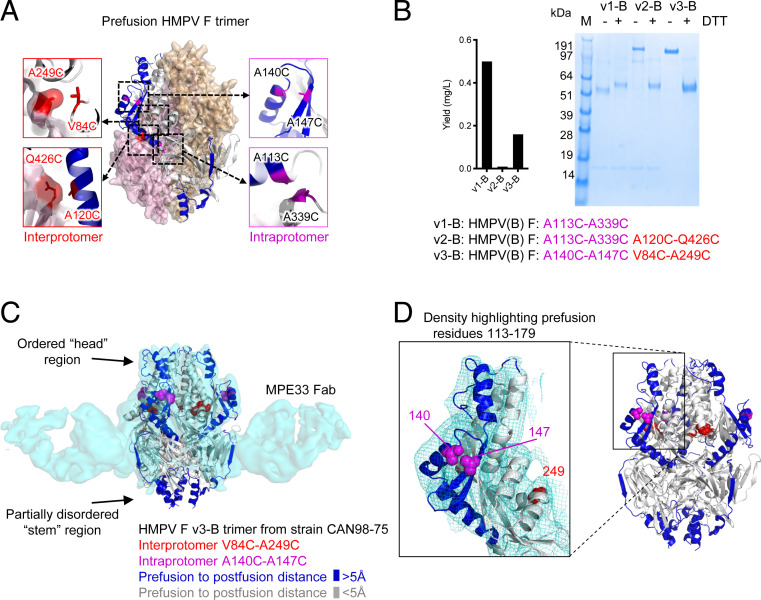
Based on the prefusion structure of trimeric HMPV F (26), we identified residues with appropriate distances for disulfide bond formation either within a protomer (intraprotomer disulfides) or between protomers (IP-DSs). We also analyzed the HMPV F structure for regions that moved more than 5 Å between prefusion and postfusion conformations. Six potential disulfides, three intraprotomer (A113C to A339C; A140C to A147C; and K450C to S470C) and three IP-DS (A63C to K188C; V84C to A249C; and A120C to Q426C) were within disulfide bonding distance and proximal to residues that moved substantially between prefusion and postfusion conformations (Fig. 1A), suggesting that their formation would stabilize the prefusion conformation.
Fig. 1.
Interprotomer disulfide-based stabilization of HMPV F trimer in a prefusion state. (A) Structure-based design of interprotomer disulfides based on the prefusion structure of HMPV F (PDB ID 5WB0) (26); additional designs are shown in SI Appendix, Fig. S1. (B) Properties of disulfide-stabilized prefusion HMPV Fs. Left, expression level. Right, SDS-PAGE analysis of interprotomer disulfide-stabilized HMPV F prefusion variants without (−) and with (+) reducing agent (DTT, dithiothreitol). (C) Cryo-EM 3D reconstruction at 4.8-Å resolution of the MPE33 Fab in complex with prefusion HMPV F trimer stabilized with interprotomer 84C-249C and intraprotomer 140C-147C. Density is shown in transparent cyan, with HMPV F in ribbons, colored by distance between prefusion and postfusion conformation (>5 Å, blue; <5 Å, gray, based on PDB ID 5WB0 and 5L1X); atoms for interprotomer disulfides are highlighted in red, and those for intraprotomer disulfides in magenta. (D) Close-up of prefusion residues 113 to 179 in the head region, where there is clear density showing residues 113 to 179 to be in the prefusion conformation.
HMPV F variants incorporating engineered disulfides often did not show favorable antigenicity (Dataset S1), especially in combination, likely reflecting poor expression, but from the B2 strain CAN98-75 of HMPV, we succeeded in making three HMPV F variants: v1-B, with a single intraprotomer disulfide 113C-339C as well as cavity-filling mutations T160F and I177L; v2-B, with additional IP-DS 120C-426C; and v3-B, with two disufides, the intraprotomer 140C-147C and the IP-DS 84C-249C (Figs. 1B and SI Appendix, Fig. S2 A and B). Expression yields were particularly low for variant v2-B where we obtained only about 0.05 mg/L by transient transfection. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) indicated variants with interprotomer disulfide to run as higher molecular weight species in the absence of reducing reagent (Fig. 1 B, Right). Variants v1-B and v2-B were recognized well by the prefusion-specific antibody MPE8 (29), but variant v3-B was not (SI Appendix, Fig. S2C), despite negative stain electron micrographs showing trimeric forms that appeared to be primarily in a prefusion conformation (SI Appendix, Fig. S3 A–C).
To confirm the overall architecture of the disulfide-stabilized prefusion HMPV F, we determined the cryo-electron microscopy (cryo-EM) structure of HMPV F v3-B in complex with antibody MPE33 to 4.8 Å from 33,058 particles (Fig. 1C and SI Appendix, Table S1). The MPE33 antibody bound to a “stem” epitope about a third of the way toward the viral membrane. Reconstruction density in the “head” region fit well with the HMPV prefusion 5WB0 coordinates, but less well in the stem region toward the viral membrane, consistent with a flexible or partially disorded stem region. The fit in the head was especially good in the 113 to 179 region (Fig. 1D), which changes substantially between prefusion and postfusion conformations (SI Appendix, Fig. S4A), clearly indicating the v3-B structure to be in a prefusion conformation.
Characterization of an HMPV F Variant with Triple Potential Disulfides.
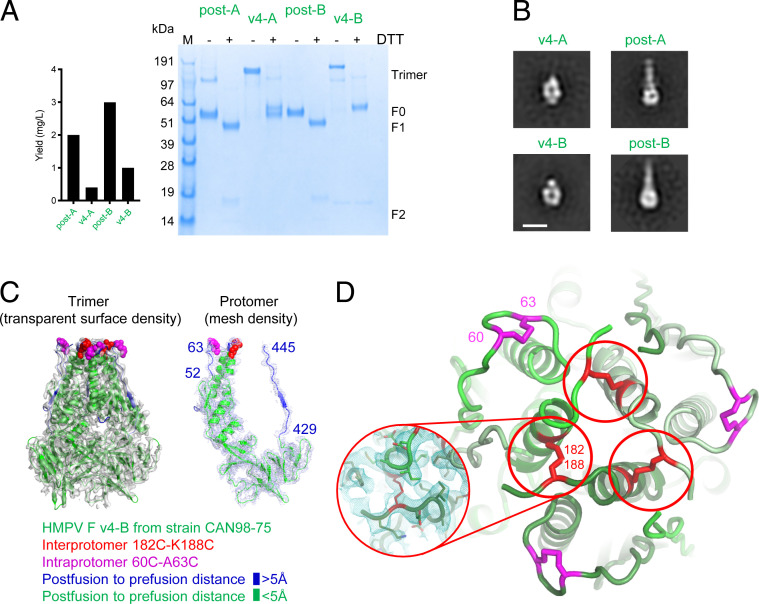
We observed reasonable expression from an HMPV F variant in both A1 strain NL/1/00 (v4-A) and B2 strain CAN98-75 (v4-B), designed with three potential disulfides between 63C-188C, 140C-147C, and 450C-470C (Fig. 2 A, Left). By SDS-PAGE analysis, this triple-disulfide variant in both subtypes formed IP-DSs, as judged by the presence of a higher molecular weight band, which appeared to correspond to a covalent-linked trimer in the absence of reducing agent (Fig. 2 A, Right). Antigenic analysis indicated recognition of both postfusion and triple disulfide variants by antibodies MPE33 and MPF5, though not by the prefusion-specific MPE8 (SI Appendix, Fig. S2C). Negative stain electron micrographs of variants v4-A and v4-B showed this variant to not fit into either the classical pre- or postfusion forms of HMPV F (Fig. 2B), lacking a classic postfusion tail with a head region that was smaller than observed with prefusion F variants, v1 to v3.
Fig. 2.
Interprotomer disulfide-based stabilization of HMPV F trimer in a postfusion state. (A) Properties of postfusion F proteins of HMPV subtypes A1 (NL/1/00) and B2 (CAN98-75) and corresponding HMPV F variants v4-A and v4-B. Left, expression levels. Right, SDS-PAGE analysis of postfusion and IP-DS–stabilized variants v4. (SI Appendix, Fig. S2 shows construct details.) (B) Negative-stain electron micrographs of postfusion and IP-DS–stabilized variants v4 in subtype A and B. (Scale bar: 10 nm.) (C) Cryo-EM 3D reconstruction at 3.3-Å resolution of HMPV F v4-B. Left, trimeric F with 3D reconstruction density gray, HMPV F trimer is displayed in ribbon and colored by distance between postfusion and prefusion conformation (>5 Å, blue; <5 Å, green); atoms for interprotomer disulfides are highlighted in red, and those for intraprotomer disulfides in magenta. Right, 3D reconstruction density for an HMPV F protomer displayed with trimer. (D) Enlarged view of the HMPV F v4-B apex, with engineered disulfide bonds shown in red (interprotomer) and magenta (intraprotomer) at an orientation of 90° from C (Inset shows 182 to 188 disulfide with 3D reconstruction density).
To provide atomic-level definition, we determined the cryo-EM structure for HMPV F v4 from subtype B at 3.2-Å resolution from 59,432 particles (Fig. 2B and SI Appendix, Table S1). Residues 19 to 90 and 181 to 445 were well defined in the density. This includes residues 52 to 63 and 429 to 445, which are substantially different between post- and prefusion conformations (these residues are colored blue in Fig. 2C and SI Appendix, Fig. S6). Residues 182 and 188, which formed an IP-DS, with Cys188 introduced by mutation, and Cys182 naturally occurring, and residues 60 to 63, which formed an intraprotomer disulfide bond, are colored in red and magenta, respectively (Fig. 2D and SI Appendix, Fig. S4B). Notably, residues 91 to 179 and 446 to 485, which typically form an extended helical region in the postfusion conformation, were disordered in this molecule. Unmasked density suggests the 182 to 188 IP-DS interrupted the native helix and redirected the region proceeding, from residues 91 to 179, outward radially. Overall, the cryo-EM–defined disulfide pattern for HMPV F v4 comprised an intraprotomer disulfide between 60C and 63C and an IP-DS between 182C and 188C, with mutations A140C, A147C, K450C, and S470C, which were not defined in the cryo-EM reconstruction and likely comprise free cysteines.
Based on the cryo-EM–confirmed disulfides (SI Appendix, Fig. S4B), we describe the HMPV F v4 with intraprotomer disulfide 60C to A63C and IP-DS 182C to K188C hereafter and in Fig. 2. Thus the cryo-EM structure of the designed triple disulfide variant revealed it to fold into a postfusion-like conformation,with two unexpected disulfides, one intraprotomeric, and the other interprotomeric in character.
IP-DS–Stabilized HMPV F Trimers Elicit Significantly Higher Neutralizing Responses.
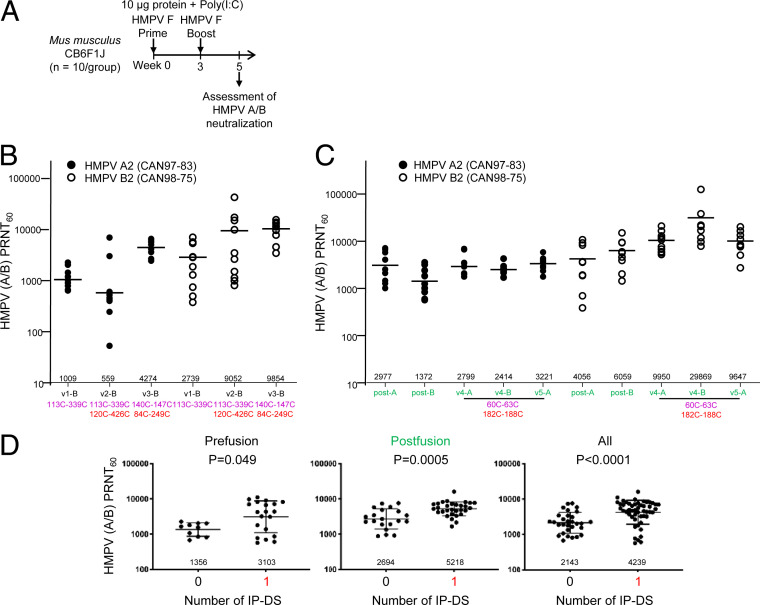
To evaluate the ability of the IP-DS–containing variants of HMPV F to elicit HMPV-neutralizing responses, we immunized CB6F1/J mice with 10 µg doses of each of the designed HMPV F glycoprotein variants (Fig. 3A). We combined the immunogens with 10 μg polyinosinic–polycytidylic acid (poly-I:C) adjuvant at weeks 0 and 3 and measured the ability of week 5 sera in plaque reduction neutralization tests (PRNTs). Two viruses were assessed, the subgroup A virus CAN97-83 and the subgroup B virus CAN98-75 (Fig. 3A).
Fig. 3.
Interprotomer disulfide-stabilized HMPV F variants induce high titer-neutralizing responses in mice. (A) Immunization regimen for CB6F1J mice (n = 10/group) with two HMPV F immunizations followed by serum analysis at week 5. (B) Elicited HMPV neutralization by prefusion F immunogens; experiment was carried out in two sets of mice, one set with v1-B and v3-B and a second set with v2-B. (C) Elicited HMPV neutralization by postfusion F immunogens; immunization experiment was carried out in a single set of mice. v4-B is significantly more immunogenic than postfusion F (P value = 0.0009 based on Mann–Whitney test; even if the highest response is removed, it would still be statistically better, with a P value = 0.002 based on a Mann–Whitney test). (D) Comparison of titers elicited by IP-DS–stabilized prefusion and postfusion forms of HMPV F.
In a first experiment, we immunized with the three prefusion immunogens described in Fig. 1 and assessed neutralization against both the more distantly related HMPV A2 virus, as well as the more closely related HMPV B2 virus. In all cases, the highest titers were observed with variant v3-B, with both an intraprotomer disulfide (140C-147C) and an IP-DS (84C-249C). Neutralizing titers against the more distantly related A2 HMPV, however, were in some cases substantially lower (up to 10-fold) than against the more closely related B strain of HMPV (Fig. 3B), complicating analyses of the impact of disulfides on neutralizing titers.
In a second experiment with postfusion immunogens, we immunized with both HMPV F variants, v4-A and v4-B (Fig. 3C). We also tested another version of variant v4 (named variant 5), in which the engineered mutants for one of the intraprotomer disulfides, K450C to S470C, were reverted (Fig. 3C and SI Appendix, Fig. S2 A and B). The highest neutralizing responses were observed for HMPV F v4-B—with average geometric mean titers of almost 30,000—as assessed against the homologous B virus CAN98-75. Neutralizing responses, however, were signficantly decreased when assessed against the more distantly related A2 virus, with average geometric mean titers of ∼3,000. By comparison, the neutralization titers elicited by HMPV F v4-A—the A1 version of HMPV F variant v4—showed higher neutralizing titers when assessed against the more distantly related B2 strain, with titers against the more closely related A2 strain about threefold lower.
The lack of a consistent ratio of neutralization for strains more closely or more distantly related to the viral sequence of the immunogen may relate to the multiple factors influencing immunogenicity, including differential immunogenicity of neutralizing epitopes as observed with RSV F subtypes (30). Differences may also relate to differing sensitivity of the viruses themselves, as titers against the B2 viruses were consistently higher than against A2 virus. Despite these confounding factors, when we combined neutralization results from both closely and more distantly related viruses to delineate the impact of IP-DSs, in both postfusion (P = 0.049) and in prefusion (P ≤ 0.005) forms, IP-DS–containing immunogens induced significantly higher HMPV-neutralizing titers (Fig. 3D).
IP-DS–Stabilized HMPV Fs Induce High Titer-Neutralizing Responses in Macaques.
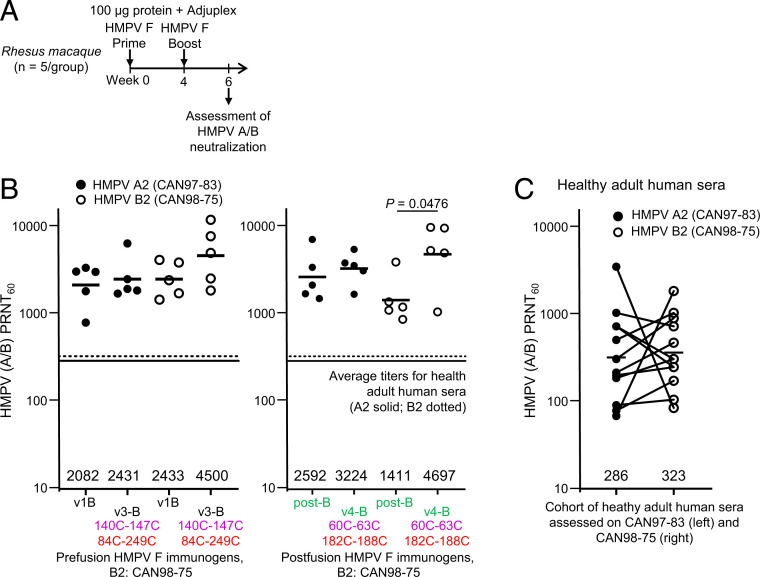
Our observation that the IP-DS–stabilized postfusion conformation of HMPV F (v4-B) elicited the highest neutralizing responses was unexpected in light of the prefusion forms of the closely related orthopneumo- and respiroviruses inducing higher titers (19, 27, 31). The IP-DS–stabilized prefusion conformation of HMPV F (v3-B), however, did elicit the second highest neutralizing responses. To test the generality of these responses, we sought to replicate them with rhesus macaques (nonhuman primates [NHPs]). We used an immunization regimen comprising immunization with 100 µg F protein at weeks 0 and 4, and Adjuplex as an adjuvant, and assessed serum neutralizing titers at week 6 (Fig. 4A). We tested four groups of five NHPs. In the first group, we used v1-B as the standard prefusion F, and in the second group we used the subtype B HMPV F variant V3-B, which contained the IP-DS 84C-249C. In the third group, we used the standard postfusion F, and in the fourth group we used the subtype B HMPV F variant v4-B, which contained the IP-DS 182C-188C, which we confirmed by cryo-EM.
Fig. 4.
Immunization of rhesus macaques shows interprotomer disulfide-stabilized variants of either prefusion or postfusion HMPV F induce neutralizing responses many times the average titer in healthy adult humans. (A) Immunization regimen for rhesus (n = 5/group) with two HMPV F immunizations followed by serum analysis at week 6. (B) Neutralizing responses graphed with geometric mean titers provided (analyses versus number of IP-DSs are provided in SI Appendix, Fig. S5B). (C) Neutralization titers from 12 human subjects. Adult human sera were assessed for HMPV plaque reduction neutralization titers using subtype A and subtype B HMPV viruses. Lines connect titers from the same donor. Geometric mean titers against A1 and B2 subtype viruses are provided.
When assessed against the subgroup A2 virus CAN97-83 and the subgroup B2 virus CAN98-75, the IP-DS–containing subgroup B prefusion F induced higher average titers than the non–IP-DS prefusion control v1-B (Fig. 4B), averaging 2,431 against the heterologous subgroup A2 virus and 4,500 against the homologous B2 virus (Fig. 4B). The IP-DS–containing subgroup B postfusion F also induced higher average titers than the non–IP-DS postfusion control, and these were statistically significant with the B virus, where titers averaged just under 5,000 (Fig. 4B). Overall, IP-DS responses were statistically significantly higher only for the autologous B2 virus (SI Appendix, Fig. S5B).
To provide context for these titers, we assessed human serum from 12 healthy adults on the same two viruses. The average titers were 286 against the subgroup A2 virus CAN97-83 and 323 against the subgroup B2 virus CAN98-75 (Fig. 4C). Notably, the average level of neutralizing responses induced in NHPs by immunization with the IP-DS–containing HMPV F variant v4 were 10-fold or more higher than the average level of serum neutralization in this healthy adult cohort.
Serological Assessment of Serum Responses from Macaques.
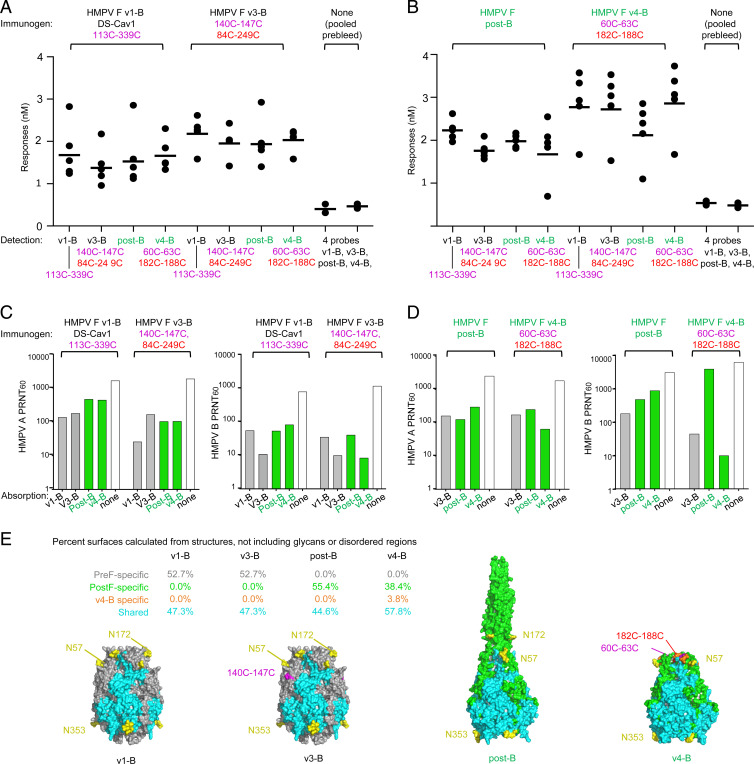
To provide insight into the high titer HMPV neutralization induced by HMPV F variants v3-B and v4-B immunization of macaques, we assessed both prebleed and week 6 serological responses (Fig. 5 A and B). Various HMPV F trimers, including variant v1, v3, v4, as well as post-F, were used to probe elicited responses, as quantified by biolayer interferometer (BLI). Low responses (less than 1 nm) were observed with prebleed serum against a mixture of HMPV F variants. For week 6 prefusion v1 and v3 sera, all four probes yielded similar responses, indicating elicited responses to both prefusion and postfusion antigenic surfaces. Overall, the response level in v1 sera were lower, however, than the v3 sera, indicating v3 to be more immunogenic. For week 6 postfusion (post-F) and v4 sera, all four probes generally yielded similar responses, the sole exception being post-F response in v4 sera, which was substantially lower. These suggested post-F to induce immunogenic responses to both prefusion and postfusion antigenic surfaces, while v4 predominantly elicited a prefusion response. The overall level of response elicited by v4, however, was much higher than post-F, indicating v4 to be substantially more immunogenic. Collectively, these results indicate the IP-DS–stablized variant v3-B to elicit moderate titer responses against both prefusion and postfusion, whereas v4-B elicited higher titer responses that appeared to be more “prefusion F” than “postfusion F” in nature. In both cases, the IP-DS–stabilized versions showed higher responses to probes, suggesting IP-DS to have enhanced overall immunogenicity.
Fig. 5.
Serum analysis indicates neutralizing antibodies to mainly target epitopes shared between prefusion and interprotomer disulfide-stabilized postfusion HMPV F. Serum antibody binding analysis for rhesus macaques immunized with HMPV prefusion (A) and postfusion (B) F (post-B) or stabilized postfusion F v4-B (n = 5/group) using two prefusion HMPV F (black identifiers) and two postfusion HMPV F versions (green identifiers) as probes. (C and D) Neutralizing antibody responses for pooled rhesus serum (n = 5/group) of each group was absorbed with prefusion (v3-B), postfusion (post-B), and interprotomer disulfide-stabilized postfusion HMPV F (v4-B), respectively. (E) Immunogen shown with surfaces colored as being pre-F, post-F, or immunogen specific or shared between pre-F and post-F, with Inset providing % surfaces. Glycans N57 and N172 at the apex of the prefusion spike are likely shifting immune recognition away from this site, which is a prevalent site of neutralization in RSV.
We also used absorption coupled to neutralization assessment to determine which of the elicited responses were capable of neutralizing HMPV. For prefusion v1 and v3 immunogens (Fig. 5C), absorption with v1, v3, post-F, and v4 all showed similar responses, though with less adsorption against the divergent A2 subtype versus the homologous B2 virus. For immunogens post-F and v4 (Fig. 5D), when assessed against the heterologous A2 virus, both prefusion F (v3) and postfusion F (v4-B or post-B) absorption reduced neutralizing titers similarly, suggesting elicited neutralizing titers against HMPV A2 virus to be focused on neutralizing epitopes shared between prefusion and postfusion forms of HMPV F (Fig. 5 D, Left). However, when these immunogens were used to adsorb and serum then tested against the more closely related B virus, the post-B standard absorbed little of the variant v4-B–induced neutralization (Fig. 5 D, Right); this suggested the very high autologous neutralizing titers elicited by HMPV F variant v4-B to be related to neutralizing epitopes shared with prefusion HMPV F (such as displayed by v3-B).
We analyzed surfaces of v1, v3, post-F, and v4 immunogens for regions structurally shared or specific to pre-F and post-F conformations (Fig. 5E). As the difference between v1-B and v3-B primarily related to disulfide bonds that were not accessible to the surface—the surface properties of these immunogens were similar—with about equal shares of pre-F–specific surface areas and areas shared with the post-F conformation. Importantly, however, two glycans were present at residues N57 and N72, shielding the pre-F–specific apex of these HMPV F trimers. The difference between post-B and v4-B was more evident, as the large post–F-specific coiled-coil helix was in an alternative highly flexible conformation in the v4-B structure and was not observed in the cryo-EM data; of the remaining visualized surface, almost 60% was shared with the prefusion conformation.
Discussion
Globally, humans have acquired substantial immunity to HMPV, with HMPV disease impacting those that have not been exposed to HMPV (e.g., infants and young children) or with weakened immunity (e.g., the elderly). We observed that in healthy adult donors, neutralization titers to HMPV subgroups A and B were moderate, with geometric means of around 300 (Fig. 4C); since adults are generally resistant to HMPV infection, this level of neutralizing titer would thus appear to be protective, though more extensive epidemiological data or human challenge experiments are needed to quantify the relationship between neutralizing titer and protection.
In the context of the increased neutralizing titers elicited by F glycoproteins stabilized with disulfides, especially with IP-DSs added to RSV F (27), we hypothesized IP-DS–stabilized HMPV F trimers would induce high titer protective responses. With IP-DS–stabilized HMPV F v3-B stabilized in a prefusion form (Figs. 1 and 3), we observed strong neutralizing responses, averaging 4,000 to 10,000 in mice and in NHPs of ∼2,000 to 4,500, and with IP-DS–stabilized HMPV v4-B stabilized in a postfusion form (Figs. 2–4), we observed geometric mean neutralizing titers in mice of ∼2,000 to 30,000 and in NHPs of ∼3,000 to 5,000. This observed level of HMPV F-induced neutralization was many times the average titer observed in our heathy adult cohort. While we do not know the protective titer in humans for HMPV, we note that both the overall titers in immunized NHPs and in the healthy human cohort are similar to the titers observed in our prior work with the DS-Cav1–stabilized RSV F, which recently yielded promising clinical trial results (20, 24).
In general, the ability of postfusion HMPV F to induce high titer-neutralizing responses was unexpected in light of repeated failure of postfusion RSV F to induce similarly high responses (19, 27, 30). One possible explanation may relate to the additional N-linked glycosylation on prefusion HMPV F, proximal to the potent neutralizing epitopes at the F trimer apex, which has been shown to dominate the neutralization of RSV F (32). Many potently neutralizing RSV F antibodies target prefusion-specific epitopes (including antigenic site 0, at the apex of the trimer) (19); however in prefusion HMPV F, the antigenic site 0 equivalent is shielded by the presence of two N-linked glycans at residues N57 and N172. Glycan shielding from these two site 0 equivalent glycans appears to dampen the immune response to prefusion-specific epitopes, thereby increasing the relative immunogenicy of neutralizing responses to epitopes that are common to both prefusion and postfusion conformations of HMPV F.
The increase in neutralizing titer related specifically to IP-DS stabilization appeared moderate (Fig. 3D). This relatively modest observed increase in the context of very high overall levels of elicited neutralization likely stems from confounding effects of subtype specificity as well as the substantial increase in titer related to immunization with F in either prefusion or postfusion forms (Figs. 3 and 4). One possible explanation for the increase relates to the high flexibility we observed with HMPV F, as evidenced by “blurring” of negative-stain electron micrographs of non–IP-DS–stabilized prefusion Fs (SI Appendix, Fig. S2); such flexibility may substantially decrease the immunogenicity of quaternary response, with IP-DS–reducing protomer-to-protomer flexibility within trimers leading to increased immunogenicity.
Indeed, reduced flexibility was observed with IP-DS versions of RSV F, which also induced higher neutralizing titers (27). Overall, the IP-DS–related increase in neutralizing titer likely relates to an increase in immunogenicity (Fig. 5), and this likely derives from alteration of intrinsic properties of the HMPV F variants, such as flexibility, as it was observed in both mice and macaques. In this context, we note that the cryo-EM structure indicated regions both of reduced flexibility (e.g., IP-DS–proximal regions) and of increased flexibility (e.g., the disordered “tail”). By and large, it seems likely that the observed level of neutralizing titer derives from complex interactions between each F variant and the immune system, such as related to the differential immunogenicity observed for different subtypes of RSV F (30).
Overall, IP-DS–stabilized versions of trimeric HMPV F appear to be promising vaccine immunogens able to elicit high titer-neutralizing responses. We were surprised to observe variant v4 of HMPV F to be in a postfusion conformation, and further characterization will be needed to understand the folding pathway leading to the altered disulfide partnering of residue 182, which normally partners with residue 60, but in variant v4 partners with residue 188. Current results, nonetheless, demonstrate the utility of IP-DSs in increasing the elicited neutralizing titer of HMPV F immunogens in both prefusion and postfusion forms, a finding of importance to the development of an effective HMPV vaccine, and structure-based vaccines in general.
Materials and Methods
Design of prefusion-stabilizing disulfide bonds in HMPV F were performed based on the prefusion HMPV F structure (5WB0). Proteins were expressed in 293F cells transfected with Turbo293 transfection reagent (SPEED BioSystem) and purified by a serial His6-strepTagII purification approach. Purified recombinant proteins were analyzed for antigenicity by biolayer interferometry (BLI/Octet) with HMPV F-specific antibodies. Proteins were also analyzed by negative-stain EM. Cryo-EM data for the HMPV F-Fab complexes were collected using a Titan Krios microscope with Leginon and map reconstruction carried out by cryoSPARC. CB6F1/J mice were immunized to evaluate elicitation of neutralizing sera with 10 μg of HMPV F immunogen adjuvanted with Poly-IC. NHPs were immunized with 100 μg of HMPV F immunogen adjuvanted with Adjuplex. Sera were assessed for neutralization against HMPV A2 subtype (strain CAN97-83) and B2 subtype (CAN98-75). Serum reactivity to immunogens was probed by BLI/Octet and by adsorption coupled to neutralization. Details are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank members of the Structural Biology Section and the Structural Bioinformatics Core Section of the Vaccine Research Center for helpful comments, and members of the Electron Microscopy Group at the New York Structural Biology Center for assistance with data collection. Support for this work was provided by the Intramural Research Programs of the Vaccine Research Center and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. This work was also supported in part by federal funds from the Frederick National Laboratory for Cancer Research, NIH, under contract HHSN261200800001E. Some of this work was performed at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center, supported by grants from the Simons Foundation (SF349247), NYSTAR, and the NIH National Institute of General Medical Sciences (GM103310). The work at the Institute for Research in Biomedicine was partially supported by the Swiss Vaccine Research Institute and by the European Research Council (grant 670955 BROADimmune).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2106196118/-/DCSupplemental.
Data Availability
Structure data have been deposited in the Electron Microscopy Data Bank (EMDB) (EMD-23605 and EMD-23625) and Protein Data Bank (PDB) (7LZE).
References
- 1.van den Hoogen B. G., et al., A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7, 719–724 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell C. J., Simões E. A. F., Hurwitz J. L., Vaccines for the paramyxoviruses and pneumoviruses: Successes, candidates, and hurdles. Viral Immunol. 31, 133–141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S., et al., Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 373, 415–427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain S., et al., Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis C. R., et al., Incidence, morbidity, and costs of human metapneumovirus infection in hospitalized children. J. Pediatric Infect. Dis. Soc. 5, 303–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widmer K., et al., Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J. Infect. Dis. 206, 56–62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams J. V., et al., Human metapneumovirus infection in children hospitalized for wheezing. J. Allergy Clin. Immunol. 115, 1311–1312 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams J. V., et al., Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350, 443–450 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esper F., et al., A 1-year experience with human metapneumovirus in children aged <5 years. J. Infect. Dis. 189, 1388–1396 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiris J. S., et al., Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg. Infect. Dis. 9, 628–633 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falsey A. R., Erdman D., Anderson L. J., Walsh E. E., Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187, 785–790 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Peret T. C., et al., Characterization of human metapneumoviruses isolated from patients in North America. J. Infect. Dis. 185, 1660–1663 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin G., et al., Virological features and clinical manifestations associated with human metapneumovirus: A new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 186, 1330–1334 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Panda S., Mohakud N. K., Pena L., Kumar S., Human metapneumovirus: Review of an important respiratory pathogen. Int. J. Infect. Dis. 25, 45–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Hoogen B. G., et al., Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J. Infect. Dis. 188, 1571–1577 (2003). [DOI] [PubMed] [Google Scholar]
- 16.van den Hoogen B. G., Bestebroer T. M., Osterhaus A. D., Fouchier R. A., Analysis of the genomic sequence of a human metapneumovirus. Virology 295, 119–132 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Njenga M. K., Lwamba H. M., Seal B. S., Metapneumoviruses in birds and humans. Virus Res. 91, 163–169 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Pilaev M., et al., Evaluation of pre- and post-fusion human metapneumovirus F proteins as subunit vaccine candidates in mice. Vaccine 38, 2122–2127 (2020). [DOI] [PubMed] [Google Scholar]
- 19.McLellan J. S., et al., Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342, 592–598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLellan J. S., et al., Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340, 1113–1117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krarup A., et al., A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 6, 8143 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang B., et al., Enhanced neutralizing antibody response induced by respiratory syncytial virus prefusion F protein expressed by a vaccine candidate. J. Virol. 89, 9499–9510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart-Jones G. B., et al., A cysteine zipper stabilizes a pre-fusion F glycoprotein vaccine for respiratory syncytial virus. PLoS One 10, e0128779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crank M. C., et al., A proof of concept for structure-based vaccine design targeting RSV in humans. Science 365, 505–509 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Más V., et al., Engineering, structure and immunogenicity of the human metapneumovirus F protein in the postfusion conformation. PLoS Pathog. 12, e1005859 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battles M. B., et al., Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein. Nat. Commun. 8, 1528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyce M. G., et al., Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat. Struct. Mol. Biol. 23, 811–820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B., et al., Protection of calves by a prefusion-stabilized bovine RSV F vaccine. NPJ Vaccines 2, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corti D., et al., Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature 501, 439–443 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Joyce M. G., et al., Crystal structure and immunogenicity of the DS-Cav1-stabilized fusion glycoprotein from respiratory syncytial virus subtype B. Pathog. Immun. 4, 294–323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart-Jones G. B. E., et al., Structure-based design of a quadrivalent fusion glycoprotein vaccine for human parainfluenza virus types 1-4. Proc. Natl. Acad. Sci. U.S.A. 115, 12265–12270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngwuta J. O., et al., Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 7, 309ra162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structure data have been deposited in the Electron Microscopy Data Bank (EMDB) (EMD-23605 and EMD-23625) and Protein Data Bank (PDB) (7LZE).