Significance
Distinct cell contacts like adherens junctions or desmosomes are well studied. Understanding the seemingly amorphous structures of cell–cell adhesion in many tissues still poses a challenge. Here, we explore the landscape of adhesive contacts in Xenopus gastrula tissues. Prompted by the continuous, nondiscrete variation of contact morphology, we use contact width spectra to analyze the roles of adhesion molecules. Currently, it is understood that cadherins are the main mediators of adhesion in these tissues. However, we find abundant contacts with intercellular distances far too wide for cadherin–cadherin binding. We show that alongside cadherins, glycocalyx-like structures and associated factors like heparan sulfate proteoglycans, fibronectin, and hyaluronic acid control contacts, and glycocalyx-mediated adhesion is essential for gastrula tissue architecture.
Keywords: cell adhesion, cadherin, syndecan, glycocalyx, Xenopus
Abstract
Molecular and structural facets of cell–cell adhesion have been extensively studied in monolayered epithelia. Here, we perform a comprehensive analysis of cell–cell contacts in a series of multilayered tissues in the Xenopus gastrula model. We show that intercellular contact distances range from 10 to 1,000 nm. The contact width frequencies define tissue-specific contact spectra, and knockdown of adhesion factors modifies these spectra. This allows us to reconstruct the emergence of contact types from complex interactions of the factors. We find that the membrane proteoglycan Syndecan-4 plays a dominant role in all contacts, including narrow C-cadherin–mediated junctions. Glypican-4, hyaluronic acid, paraxial protocadherin, and fibronectin also control contact widths, and unexpectedly, C-cadherin functions in wide contacts. Using lanthanum staining, we identified three morphologically distinct forms of glycocalyx in contacts of the Xenopus gastrula, which are linked to the adhesion factors examined and mediate cell–cell attachment. Our study delineates a systematic approach to examine the varied contributions of adhesion factors individually or in combinations to nondiscrete and seemingly amorphous intercellular contacts.
In various animal tissues, especially in embryos, cohesion is based on flexible cell–cell adhesion that allows for rapid morphogenetic movements (1, 2). Respective cell contacts show great variety. Tightly packed tissues with intercellular distances of a few nanometers include for example embryonic liver cell aggregates (3) or breast and prostate carcinoma (4, 5). In chick or mouse gastrula mesoderm, small contacts are interspersed with regions of nonadherent cell surface (6, 7); in other tissues, cells are in contact across large, micrometer-sized distances. While narrow contacts can be generated by the direct interaction of membrane adhesion receptors, pericellular matrix–mediated adhesion has been proposed for such wide contacts (2).
Compared with apical junctional complexes of epithelia, the structure and molecular basis of these cell contact variants have been less extensively examined. Here, we perform a comprehensive analysis of the cell contact landscape in the multilayered tissues of the Xenopus gastrula where narrow and wide contacts and interstitial gaps co-occur. In this model system, the control, strength, and role of cell adhesion in morphogenesis have been examined with an emphasis on cadherins (1, 8–10). Cadherins form 15- to 40-nm-wide contacts through interactions between their extracellular domains (11–13). Interaction releases binding energy to generate a small adhesion tension; triggers the down-regulation of the actomyosin cell cortex, which reduces cortical tension and allows the cells to mutually flatten against each other; and physically links the cells as the cadherins bind via α- and β-catenin to the actin cytoskeleton (1, 14–16).
Cadherins are thought to be the main adhesion molecules in the Xenopus gastrula, where their graded expression and interactions with modulating factors could explain cell sorting, tissue positioning, and boundary formation (8, 9, 16, 17). However, blocking cadherin function with antisense oligonucleotides does not dissociate the embryo into single cells, but into loose, flaky aggregates, and experimentally generated cadherin differentials do not induce cell sorting (18–20). Moreover, at the mesoderm–ectoderm boundary, cell contacts occur with membrane separation distances of hundreds of nanometers, too wide to be sustained by cadherins (21). These observations challenge the notion of a singular role of cadherins in the Xenopus gastrula.
Here, our systematic analysis of cell contact structure shows that gastrula regions are characterized by specific contact width spectra, with membrane separation distances ranging from 10 to 1,000 nm. To explore the control of these contact patterns and the nature of wide contacts, we interfered with the expression of C-cadherin (C-cad), the main cadherin in the gastrula, and a few other adhesion factors. Fibronectin (FN) is a glycoprotein of the extracellular matrix that occurs also as puncta on the cell surface (csFN) to mediate cell–cell adhesion (22, 23). In the Xenopus gastrula, csFN puncta support migration in endoderm and mesoderm (24–26). Of heparan sulfate (HS) membrane proteoglycans, Syndecan-4 (Syn-4) and Glypican-4 (Gpc-4) are expressed in the gastrula (27, 28), as is paraxial protocadherin (PAPC), which regulates tissue separation in the mesoderm (21, 29). Hyaluronic acid (HA) is synthesized in the gastrula by integral membrane enzymes Has-1 and Has-2 (30). It can be released into the intercellular space, remain membrane-attached through the synthases, or become reattached by HA receptors. HA cross-linkers and other matrix components integrate large HA chains into meshworks that can promote cell–cell adhesion (30, 31).
Perturbation of any of these cell surface components specifically alters the contact spectra of Xenopus gastrula tissues. It reveals unexpected roles of C-cad and Syn-4 in cell contacts and shows that adhesion across wide membrane separation distances is in large part mediated by an elaborate glycocalyx. Contact types and cell adhesion mechanisms co-occur in different mixtures in the different gastrula tissues and are regulated by adhesion factors interacting in often counterintuitive fashion. Apparently, cell–cell adhesion in simple multilayered tissues is more complex than often anticipated.
Results
Cell–Cell Contacts in the Xenopus Gastrula.
The ultrastructure of contacts between deep cells was analyzed for midgastrula dorsal tissues, from ectoderm on the outside to chordamesoderm, prechordal mesoderm, and leading-edge mesendoderm inside, and to endoderm in the center of the embryo (Fig. 1A). Within each tissue, a contact between two cells—a pair contact—stretches between two interstitial gaps at three-cell junctions (Fig. 1 B and C). Abrupt transitions from parallel to diverging cell surfaces are taken as transitions from adhesive contacts to the nonadhesive interstitium (Fig. 1C) (21, 32). Pair contacts consist of narrow and wide sections (Fig. 1C). At high magnification, narrow segments resolve into subsections from 10 to 40 nm wide. Some are filled with dense material, and membrane pits and elongate vesicles near contacts are apparent (Fig. 1D). Parallel, matching cell contours are also seen for much wider separation distances, and similar abrupt transitions at gaps suggest that wide contacts are adhesive (Fig. 1E) (21, 32). They can be visualized in live embryos by X-ray tomography (33) and in explants by fluorescent tracers (25, 34).
Fig. 1.
Cell–cell contacts in the X. laevis gastrula. (A) Midsagittal semithin section of stage 11 Xenopus gastrula outlining dorsal tissues: Ectoderm (Ecto; green), chordamesoderm (CMe; yellow), prechordal mesoderm (PCMe; orange), leading-edge mesendoderm (LEMe; red), and endoderm (Endo; blue). Arrows: D, dorsal; V, vegetal. Epithelial layer, bottle cells (purple), and ventral mesoderm (VeMe; magenta) were not analyzed. (B–F) TEM images. (B) Ectoderm with cell highlighted in green. (C) Pair contact between interstitial gaps (i) with narrow (yellow arrowheads) and wide regions (purple arrowheads). (D) Narrow <50-nm contact with sections filled with dense material (white arrowheads) and membrane pits and membrane-adjacent elongate vesicles (red arrowheads). (E) Wide contact, width determined by measuring intercellular distance in 100-nm intervals (red dotted lines). (F) Contact angle 2θ between cells at the ends of contact (i). (G) Mean relative abundance of narrow (<50 nm) and wide (>50 nm) contacts and gaps. (H) Contact angles between cells as in F. (I and J) Total length of adhesive contacts and gaps as a function of cell circumference. Error bars represent SD of the mean. For P values refer to SI Appendix, Tables S2 and S3. n = 24 embryos for all tissues. P > 0.05 n.s., P < 0.05*, P < 0.01**, P < 0.001***, P < 0.0001****. (Scale bars: black, 100 μm in A; 10 μm in B; and white, 1 μm.)
Most cell surface proteins, including cadherins, protrude less than 25 nm above the membrane (35) and can thus be accommodated in <50-nm contacts defined here as “narrow” (Fig. 1G). In ectoderm and chordamesoderm they amount to half of the cell surface, in the other tissues to a smaller fraction. Wider contacts account for 13 to 23% in any tissue (Fig. 1G). The remaining cell surface encloses interstitial gaps. Its percentage is low in ectoderm and chordamesoderm, and high in the other tissues (Fig. 1G). Most of the interstitial surface is concentrated at three-cell junctions, and only a minor fraction is dispersed in small, bubble-shaped membrane detachments within pair contacts (SI Appendix, Fig. S1 A–C).
Narrow and wide contacts are similarly adhesive, as seen from contact angles 2θ between cells (Fig. 1F). Relative adhesion strength is captured by cosθ = β*/βi with βi the tension at a cell’s free surface at a gap and β* the reduced tension of the same cell at the contact to an adjacent cell (Fig. 1F) (32). Obviously, tension reduction is in the same range at narrow and wide contacts (Fig. 1H). With an average 2θ of 50°, cosθ = 0.91, i.e., tension is reduced on average by 9%. The total length of adhesive contacts varies between tissues as a function of cell size (Fig. 1I and SI Appendix, Fig. S2 A–G′). In small chordamesoderm cells, most of the circumference is taken up by 70 µm of adhesive contacts, but counterintuitively the amount decreases with cell size, rapidly first and then gradually, to approach 40 µm for large endoderm cells. In consequence, interstitial gap size increases disproportionally with cell size (Fig. 1J). Changes in narrow and wide contact lengths compensate each other to some extent to allow for this overall trend (Fig. 1I).
Tissue-Specific Contact Spectra.
Membrane separation distances were measured at regular intervals and binned at 50-nm steps to obtain contact width spectra for gastrula tissues (Fig. 2). In all tissues, narrow <50-nm contacts are most abundant and part of a large, ubiquitous frequency peak between <50 nm and 250 nm. In the ectoderm, all contacts belong to this peak 1 (Fig. 2A and SI Appendix, Fig. S3). Chordamesoderm shows an additional, minor peak 2 at 400 to 500 nm (Fig. 2B). In prechordal mesoderm, peak 2 becomes more pronounced, and a peak 3 appears at 800 to 900 nm (Fig. 2C). Although minute, these peaks are reproducibly seen in the spectra of individual embryos (SI Appendix, Fig. S4). In leading-edge mesendoderm, peak 1 is split. Here and in endoderm, widths beyond 50 nm are more evenly distributed (Fig. 2 D and E). Overall, width frequencies are continuously distributed in contact spectra but exhibit peaks in tissue-specific patterns. In all tissues, cells are often linked across distances much larger than those bridged by cadherins.
Fig. 2.
Tissue-specific contact spectra in the X. laevis gastrula. Contact widths were binned at 50-nm intervals, and their respective abundance was determined for (A) ectoderm, (B) chordamesoderm, (C) prechordal mesoderm, (D) leading-edge mesendoderm, and (E) endoderm. Recurrent peaks are highlighted by shaded regions. n = 24 embryos for all tissues.
Adhesion Factors Differentially Control Cell Contact Patterns.
To analyze how contacts are controlled, we first examined semithin sections of C-cad, Syn-4, csFN, or Has-1/Has-2 morphants (Fig. 3). Normally, cells are tightly packed in ectoderm and chordamesoderm, less dense in other mesoderm, and separated by even spaces in endoderm (Fig. 3A). In C-cad morphants, cell packing is decreased in all tissues. Wide uniform spaces surround polygonal cells in ectoderm and endoderm, as if potentially wide contacts were normally kept narrow by C-cad. In the mesoderm the gaps between cells are more irregular (Fig. 3B). In Syn-4 morphants, cell packing is severely reduced in endoderm, to a lesser degree in mesoderm, and least in ectoderm (Fig. 3C). In FN morphants, the mesoderm is mostly affected, with gaps of irregular size and shape between cells (Fig. 3D) and Has-1/Has-2 knockdown also reduces packing density in mesoderm (Fig. 3 E and F). Coinhibition of C-cad with Syn-4, Has-1, or FN yields the C-cad–morpholino (MO) phenotype, with even wider spaces around all cells (Fig. 3 G–I). This suggests a dominant role of C-cad in cell packing control, although codepletion of Syn-4 and FN results in the same phenotype (Fig. 3J). Overall, the different adhesion factors show different patterns of tissue specificity. Interfering with any of the adhesion factors reduces cell packing at least in some tissue: Narrower contacts are replaced by interstitial gaps or by wider contacts.
Fig. 3.
Knockdown of adhesion factors disrupts cell contact patterns. (A–J) Semithin sections of single and double morphant Xenopus gastrulae. Ecto, ectoderm, Endo, endoderm. Dotted outlines demarcate leading-edge mesendoderm (red), prechordal mesoderm (orange), and chordamesoderm (yellow). White arrowheads indicate tip of archenteron invagination. Invagination is almost completely inhibited in treated embryos except in C, where it is increased. Dorsal is to the Left, animal to the Top. (Scale bars, 100 μm.)
The impact of treatments on gastrulation movements is surprisingly specific. Only Has-1 knockdown interferes noticeably with the advance of the leading-edge mesendoderm (Fig. 3 E and H). On the other hand, all treatments affect convergent extension of the chordamesoderm and the associated processes of mesoderm involution and archenteron formation (36–38) (Fig. 3). Convergent extension is generally inhibited, but Syn-4 knockdown accelerates it (Fig. 3C), as if some type of contact normally present in chordamesoderm attenuated the movement.
Adhesion Factors Modulate Contact Spectra.
The role of adhesion factors was examined in more detail in ectoderm and prechordal mesoderm. To visualize how the factors affected contact patterns, contact spectra of untreated tissues were subtracted from those of morphants to generate difference spectra, which were interpreted in conjunction with transmission electron microscopy (TEM) images of contacts.
C-cadherin and Syndecan-4.
Knockdown of C-cad reduces <50-nm contacts in ectoderm (Fig. 4 A, B, B′, and E) and prechordal mesoderm (Fig. 4 F, G, G′, and J). However, 50- to 100-nm contacts in ectoderm (Fig. 4B′) and all peak 1 contacts in prechordal mesoderm (Fig. 4G′) are also diminished, revealing a role of C-cadherin in wide contacts where direct cadherin–cadherin binding could not be involved. Loss of contacts increases interstitial gaps (Fig. 4 B′, E, G′, and J). The Syn-4–MO difference spectrum is similar, with contact reduction being even more pronounced (Fig. 4 C′, E, H′, and J). C-cad/Syn-4 double morphants show Syn-4–MO-like spectra (Fig. 4 D, D′, I, and I′). Notably, the prevalence of <50-nm contacts is not further reduced when C-cad is inhibited in Syn-4 morphants (Fig. 4 E and J). Since half of these narrow contacts rely on C-cad, but almost all on Syn-4, essentially none of the <50-nm contacts depends on C-cad alone. In wider contacts, the same dependence of C-cad on Syn-4 is seen in ectoderm (Fig. 4E), but less in prechordal mesoderm (Fig. 4J). In the double morphants, peaks 2 and 3 are reduced (Fig. 4I′).
Fig. 4.
Knockdown of C-cadherin and Syndecan-4. (A–D and F–I) TEM images of wild-type, C-cad, and Syn-4 morphant ectoderm and prechordal mesoderm. Single cells are highlighted in green or orange. (B′–D′ and G′–I′) Difference spectra for ectoderm and prechordal mesoderm. The Left-most, white column in each graph denotes interstitial gaps. Colored columns indicate changes in abundance, which are statistically significant (P < 0.05) and represent ≥50% changes in morphants compared with wild type (blue, decrease; red, increase). Gray shades highlight positions of recurrent peaks. (E and J) Abundance of narrow and wide contacts and of gaps. Error bars represent SD of the mean. For P values refer to Datasets S2 and S3. (Scale bars: black, 10 μm; white, 15 μm.) Wild type: n = 24 embryos; C-cad-MO: n = 14; Syn-4-MO: n = 10; and C-cad-MO+Syn-4-MO: n = 8.
Despite their similar spectra, cell shapes differ in C-cad and Syn-4 morphants. Upon C-cad knockdown, enlarged interstitial gaps at three-cell junctions extend far laterally between cells (Fig. 4 B and G). By contrast, cell outlines are serrate in Syn-4 morphants. Interstitial spaces form within pair contacts as shallow surface indentations, and contact is reduced to small spots where thin processes connect cells (Fig. 4 C and H). In consequence, contacts are strongly reduced (Fig. 4 C, E, H, and J) while packing density is less affected (Fig. 3C). This cell shape is also seen in Syn-4–MO chordamesoderm engaged in convergent extension (SI Appendix, Fig. S5 B and B′) and in leading-edge mesendoderm translocating across the ectoderm (39) (SI Appendix, Fig. S5 A and A′). In C-cad/Syn-4 double morphants, fewer of the stitch-like contacts are present (Fig. 4I).
Fibronectin.
The ectoderm is barely impacted by FN-MO (Figs. 3D and 5 A, B, and E), but in prechordal mesoderm, wide contacts are reduced (Fig. 5 F, G, G′, and J). All three peaks are affected, peaks 2 and 3 most strongly when FN is codepleted with C-cad or Syn-4 (Fig. 5 H′ and I′) or in C-cad/Syn-4 double morphants, suggesting a requirement for C-cad, Syn-4, and csFN. In the 100- to 250-nm range, reduction in FN/Syn-4 double morphants exceeds that of Syn-4 morphants, arguing for some Syn-4–independent csFN contacts. In FN or FN/C-cad double morphants, cells are rounder and less attached (Fig. 5 F–H), upon FN/Syn-4 double knockdown cell edges are slightly serrate (Fig. 5 D and I). Although only 3% adhesive contact is left, contacts are evenly distributed along the cell perimeter, and cells remain polygonal and regularly spaced (Fig. 5I).
Fig. 5.
Knockdown of FN. (A–D and F–I) TEM images of wild-type and FN, C-cad/FN, and FN/Syn-4 morphant ectoderm and prechordal mesoderm, with single cells highlighted. (B′–D′ and G′–I′) Difference spectra for ectoderm and prechordal mesoderm. Colored columns show changes in abundance as in Fig. 4. Positions of recurrent peaks are shaded in gray. (E and J) Summary of the abundance of narrow and wide contacts and of gaps. Error bars represent SD of the mean. For P values refer to Datasets S2 and S3. (Scale bars: black, 10 μm; white, 15 μm.) Wild type: n = 24 embryos; FN-MO: n = 12; C-cad-MO+FN-MO: n = 15; and FN-MO+Syn-4-MO: n = 9.
HA synthases, Gpc-4, and PAPC.
In Has-1 or -2 morphant ectoderm the abundance of interstitial gaps is decreased and <50-nm contacts are increased (Fig. 6 A–C, B′, C′, and E), consistent with an antiadhesive role of HA that normally prevents expansion of narrow contacts. Coinhibition of C-cad with Has-1 abolishes narrow contacts almost completely (Fig. 6 D, D′, and E), showing that their expansion at the expense of gaps requires C-cadherin. Further, it reveals that Syn-4, which supports all narrow contacts, is not sufficient to maintain these but corequires either C-cad or HA, uncovering a parallel adhesion-promoting role of HA in ectoderm.
Fig. 6.
Knockdown of HA synthases. (A–D and F–H) TEM images of wild-type and Has-1, Has-2, and C-cad/Has-1 morphant ectoderm and prechordal mesoderm; single cells are highlighted. (B′–D′ and G′–H′) Difference spectra for ectoderm and prechordal mesoderm. Colored columns show changes in abundance as in Fig. 4. (E and I) Abundance of narrow and wide contacts and of gaps. Error bars represent SD of the mean. For specific P values refer to Datasets S2 and S3. (Scale bars: black, 10 μm; white, 15 μm.) Wild type: n = 24 embryos; Has-1-MO: n = 10; Has-2-MO: n = 11; and C-cad-MO+Has-1-MO: n = 9.
A second feature of Has-1/Has-2 morphants is the selective loss of 50- to 100-nm contacts in ectoderm (Fig. 6 B′ and C′) and prechordal mesoderm (Fig. 6 G′ and H′). These contacts seem to be replaced by wider ones beyond peak 1. Apparently, factors can normally restrict the widths of some moderately wide contacts, such that if these factors are removed, the contacts are not dismantled but assume the next possible width configuration. In prechordal mesoderm, Has-1 or -2 knockdown in addition partially reduces contacts >100 nm (Fig. 6 B′, C′, G′, and H′, red arrowheads). In contrast to ectoderm, interstitial surface is increased, narrow contacts are not promoted (Fig. 5I), and thus cell packing is reduced (Fig. 6 F–H).
Two factors, the proteoglycan Gpc-4 and the protocadherin PAPC, affect contact patterning similarly to HA synthases. The Gpc-4 difference spectra resemble those of Has-1/Has-2. The 50- to 100-nm contacts are down-regulated, and in ectoderm but not mesoderm, the <50-nm contacts are increased at the expense of interstitial gaps (SI Appendix, Fig. S6 C–E). Knockdown of either Syn-4 or Gpc-4 reduces heparan sulfate staining strongly and to the same degree (SI Appendix, Fig. S7), suggesting that the factors do not contribute additively, but as essential parts of a single structure. Apparently Gpc-4 cooperates with Syn-4 and HA in a specific contact type that contains much of the cell surface heparan sulfate. The cell shape of Gpc-4 morphants resembles Has-1/Has-2 morphants in the ectoderm, whereas the serrate shape of Syn-4 morphants is reproduced in the prechordal mesoderm (SI Appendix, Fig. S6 A–B′). PAPC is only expressed in mesoderm. Here, it is the only factor whose depletion reduces interstitial gaps and promotes narrow <50-nm contacts (SI Appendix, Fig. S8 A–D), apparently assuming the roles that HA and Gpc-4 play in the ectoderm. At the same time, PAPC-MO reduces contacts in the 50- to 200-nm-width range, similarly to Has1/2 or Gpc-4 knockdown, consistent with a role in the Syn-4/HA/Gpc-4–dependent structure.
La3+ Stains a Gastrula Cell Glycocalyx at Free Surfaces and at Contacts.
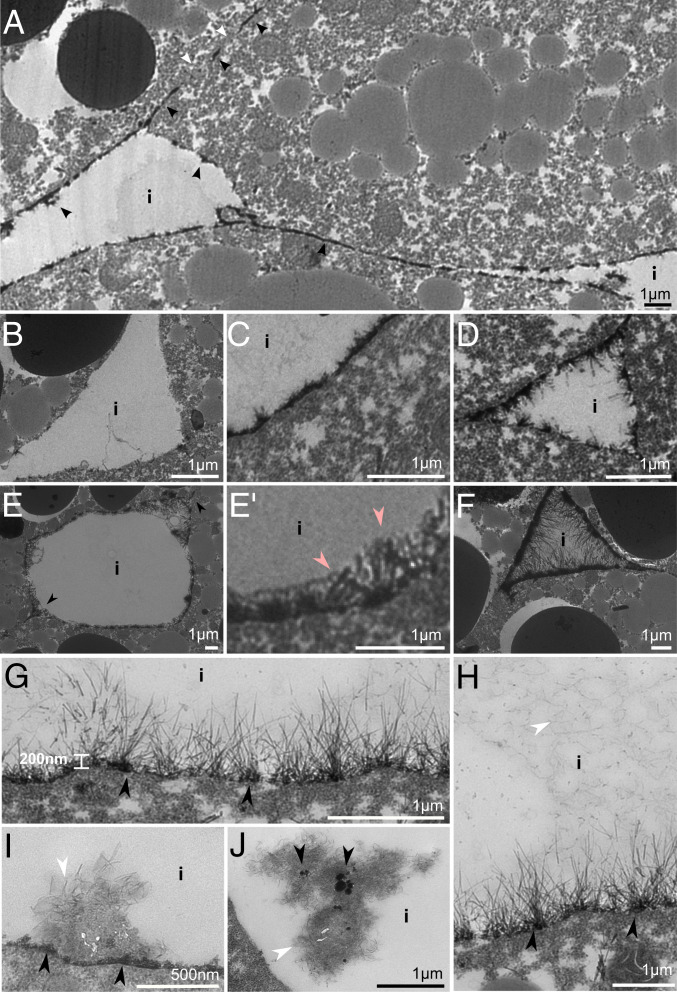
A cell coat that contains syndecans, glypicans, and HA is the endothelial glycocalyx (40). Lanthanum (La3+) can be used to stain glycocalyces (41), and in the gastrula, stretches of cell contacts and gap surface are labeled (Fig. 7A). Some gaps display little to no staining (Fig. 7B), and others, dense plaques with or without protruding filbrils (Fig. 7C). Often, plaques extend a few thick bush-like fibrils (Fig. 7 D and E). Bushes can be embedded in a faintly stained material to form a layer, which is continuous across cell–cell borders (Fig. 7 E and E′). In a few gaps, straight delicate filaments extend outward for up to a micrometer from plaques, which appear as 200-nm-wide zones of aggregated dense lumps at higher magnifications (Fig. 7 F–H). Such fine-fibril structures are not seen in the ectoderm. Inside gaps, fuzzy fibrils (Fig. 7H) or aggregates of straight and fuzzy fibrils are seen. The aggregates are attached to the dense layer at the cell surface or are unattached in gaps and harbor dense lumps (Fig. 7 I and J).
Fig. 7.
La3+ staining of interstitial gaps. (A) Low magnification view of La3+-stained stretches in contacts and interstitium. Interstitial gap (i) surfaces (B) displaying little to no lanthanum staining, (C) contiguous, dense plaques; (D–E′) a bush-like glycocalyx; or (F–G) a fine fibrillar glycocalyx based on dense plaques or a zone of dense lumps (black arrowheads). (E and E′) A bush-like glycocalyx is embedded in a faintly stained material (pink arrowheads). (H) Gaps are often filled with fine, wavy fibrils (white arrowhead). (I) Aggregates of straight and wavy fibrils attached to dense plaques on the cell surface or (J) unattached in the interstitium, together with dense lumps.
The La3+ staining patterns of gaps recur in contacts. Homogeneous dense plaques that can alternate with unlabeled stretches occur in contacts changing gradually in width from 15 to 120 nm (Fig. 8 A–D). Staining at contact-gap transitions (Fig. 8B), or in contacts that connect cells by taut processes (SI Appendix, Fig. S1 A and B), indicates an adhesive role of this glycocalyx. In contacts wider than 120 nm, La3+ stained fibrils appear. Some plaques extend thick-filament bushes (Fig. 8 E and F). Narrow, homogeneous plaque sections can widen into bush-filled ones and become narrow and homogeneous again (Fig. 8E) or widen into gaps (Fig. 8F). Plaques on opposite membranes are often out of register, and fibrils run from a plaque toward a plaque-free surface opposite. Fewer and shorter fibrils originate at plaques where membranes are closer together (Fig. 8F and SI Appendix, Fig. S1C). In a different configuration, fine fibrils extend more than 350 nm from plaques into the space between cells with little overlap between opposite sides (Fig. 8G). In all contacts, La3+ label often extends into cells, and isolated patches are seen near contacts (Fig. 8). In rarer instances, fibril aggregates and plaque material together seem to be shed from opposite cell surfaces, folding into a structure that keeps membranes widely separated (Fig. 8H).
Fig. 8.
La3+ staining of cell–cell contacts. (A) Narrow contact 15 to 30 nm wide. (B) La3+-stained contact increasing from 30 to 100 nm from Left to Right, ending at interstitial gap. (C) A 30- to 70-nm-wide contact with alternating La3+-stained and unstained stretches. (D) Homogeneously stained contact, width increasing from 40 to 120 nm, ending in unstained wide contact (Right). Patches of cytoplasmic staining are visible near the contact. (E) La3+-stained bush-like glycocalyx with thick fibrils in 125- to 250-nm-wide contact ending on the Right in unstained 125-nm contact. (F) Wide contact gradually opening into an interstitial gap. Bush-like glycocalyx with thick fibrils extending from dense plaques, often in alternating positions on opposite membranes (Inset). (G) A 350-nm-wide contact with fine fibrils emanating from dense plaques. (H) Micrometer-wide contact with fibril aggregates and dense plaques extending between opposing cell surfaces. Interstitial gaps, i. Black arrowheads indicate La3+-stained stretches; white arrowheads, unstained stretches; and orange arrowheads, cytoplasmic La3+ staining near contacts.
Discontinuous La3+ staining suggests that glycocalyx occurs in puncta like other adhesion factors. Thus, C-cad forms rows of puncta (SI Appendix, Fig. S9 A, C, and I) that continue from contacts into gaps (SI Appendix, Fig. S9A) with reduced puncta size and density (Fig. 7I). csFN shows a similar pattern (SI Appendix, Fig. S9 B and J). Strings of C-cad puncta run between csFN puncta or form parallel rows, but individual puncta can overlap (SI Appendix, Fig. S9 C and D). Syn-4 puncta can align with C-cad puncta (SI Appendix, Fig. S9 E and E′) or form two parallel rows, with C-cad between rows or merged with one (SI Appendix, Fig. S9 F and F′). This pattern resembles internalized Syn-4 at contacts between cultured cells (42) and the intracellular La3+ staining near contacts (Fig. 8). Heparan sulfate antibodies stain a Syn-4–like pattern (SI Appendix, Fig. S9 G, H, K, and L). The length distribution of La3+ patches resembles that of heparan sulfate or Syn-4 puncta but differs from C-cad or csFN puncta (SI Appendix, Fig. S9 I–M), consistent with La3+ mainly labeling heparan sulfate–containing glycocalyx.
In C-cad and FN morphants, the dense plaque layer is present in gaps and contacts, but neither thick nor thin straight fibrils extend from it (Fig. 9 A and B). Aggregates of curved and entangled fine fibrils are loosely attached to the plaque layer or, together with included dense lumps, floating in gaps (Fig. 9 A–B′) like bona fide shedding products. This suggests roles for C-cad and csFN in stabilizing fibrils, or fibril-containing forms of glycocalyx. Unlike specific C-cad knockdown, removing Ca2+ from the medium interferes effectively with the function of all classic cadherins. However, cells still remain mutually attached in loose, flaky aggregates. La3+ stained glycocalyx is still retained between cells and enriched at points of contact (SI Appendix, Fig. S10 A–C), in agreement with incomplete tissue dissociation and cadherin-independent adhesion.
Fig. 9.
Modulation of glycocalyx structure by adhesion factors. TEM images of La3+-stained structures in morphants. (A and A′) C-cad and (B and B′) FN morphant prechordal mesoderm with plaques (black arrowheads), thin fibrils (white arrowheads), and (A′ and B′) fibril aggregates containing dense lumps in interstitium (i). (C and C′) Has-1 morphant prechordal mesoderm with plaque layer in 120-nm contact (white arrow) and in gap (i), and (C′) with 40-nm plaque layers on either side of 200-nm wide contact. (D) Has-2 morphant ectoderm, glycocalyx-filled contact from 15 to 130 nm wide. (D′) Gap with plaque layer in Has-1-MO mesoderm. (E and E′) Syn-4 morphant mesoderm. (E) A 200- to 400-nm contact with small, sparse lumps (black arrowheads) and a fibril (white arrowheads). (E′) Fibril aggregates without lumps.
In Has-1/2 morphants, no thick or thin fibrils protrude from the plaque layer or form aggregates in gaps (Fig. 9 C–D′). The plaque layer is unusually thin between cells, often filling contacts only 10 nm wide and indicating a loss of the space-filling role of HA (Fig. 9 D and D′), or leaving a central zone in wide contacts unstained (Fig. 9C′). Thus, HA controls the width of glycocalyx and is essential for its fibril components. Knockdown of Syn-4 eliminates La3+ staining except for small dots, suggesting that Syn-4 is essential for all types of glycocalyx. The few remaining contacts are typically the width of peak 2 contacts and not filled with La3+ stained material (Fig. 9 E and E′ and SI Appendix, Fig. S1D). An increased number of lump-free fibril aggregates in gaps may indicate shedding of HA that lacks anchoring to plaques (Fig. 9E′). Gpc-4 knockdown similarly compromises the glycocalyx, but to a smaller degree (SI Appendix, Fig. S6F). Altogether, Syn-4 is essential for all forms of glycocalyx, probably by organizing the plaque layer, HA for adding fibril elements, and C-cad and csFN for stabilizing fibrils and the whole glycocalyx at the cell surface.
Discussion
Cell–Cell Adhesion in the Xenopus Gastrula.
In Xenopus gastrula tissues, contact widths covering a 100-fold range show continuous frequency distributions when binned at 50-nm steps. Single bins can be affected experimentally, however, arguing against a blurring of distinct peaks due to a lack of resolution. Also, individual contacts often change their width gradually along their length while transitioning between width-specific La3+ staining patterns. In the Rana gastrula, where contact widths were binned at 5-nm instead of 50-nm steps, the frequency distribution is also continuous (43). In general, membrane separation distances may be flexible when long, chain-like molecules form amorphous meshworks whose fuzzy surfaces interpenetrate to mediate adhesion.
The adhesion factors examined act mostly on broad width ranges. This is unexpected for the C-cadherin molecule. Its length restricts membrane distances compatible with homophilic binding to below 40 nm (12), yet its single knockdown affects contacts up to 250 nm wide. C-cadherin could promote adhesion at wide contacts by binding to yet unidentified extracellular components. E-cadherin was found to bind numerous factors, e.g., integrinα2β1 or ephrinB1 (44), consistent with unconventional heterophilic yet narrow contacts. Similar binding to matrix molecules would not have been detected in this study that was restricted to transmembrane proteins, but nonjunctional roles of C-cadherin (44) could reflect such interactions. Interestingly, the shed extracellular domain of C-cadherin is also present in cell–cell contacts, and its overexpression does not reduce adhesion, but affects gastrulation, consistent with a role in contact modulation (45). However, the main contribution of cadherins to adhesion derives from the down-regulation of the actomyosin cortex upon cell contact (14, 16). Such overall cortical tension reduction supports all contacts. The tendency of cells to round up is diminished, and the stress on any interacting adhesion molecules and the off rates in their binding kinetics are consequently lowered (46), thus increasing adhesion.
We identified Syn-4 instead of C-cadherin as the main regulator of cell adhesion in the embryo. By extending 30 to 45 nm from the membrane (47), Syn-4 could directly form narrow contacts. At wider contacts, binding to ligands via its HS side chains, which can extend up to 500 nm (40) could mediate adhesion (48), or Syn-4 could become an extracellular factor by being shed (49). Like cadherins, Syn-4 can regulate cortical F-actin, through PKCα and Rho GTPases (48, 50) or indirectly via binding to cadherin-11 or integrins (48, 51). csFN and HA can mediate adhesion via cellular receptors. csFN was detected by immuno-TEM in 30- to 50-nm contacts as well as in >100-nm globules that connected adjacent cells (52, 53). The FN example, like the essential role of HA in narrow contacts, demonstrate that the sheer size of a molecule is not restricting its contact occurrence; conformation and orientation relative to the membrane of the often chain-like molecules seem more relevant.
From a comprehensive Xenopus transcript expression database (54), we extracted a list of putative adhesion-related transmembrane or extracellular factors expressed in the Xenopus midgastrula, hinting at the complexity of the system (Dataset S1). The seven factors examined here are among the 90 most highly expressed ones. Not all categories of adhesion-promoting factors are represented in our exploratory survey. For example, lectins are evolutionarily ancient mediators of metazoan cell–cell adhesion (3), and although several galectins are expressed in the Xenopus gastrula (55), these are expected to perform a host of intracellular and extracellular roles not well characterized in this system. From the few factors examined, some principles of their functioning can nevertheless be derived.
Direct and indirect roles of the same factors in different contact types explains their pleiotropic effects in contact spectra, and all contacts require at least one or two of the factors examined. Thus, contact types emerge from tight-knit interaction networks. The roles of factors can be characterized as 1) general modifiers of contact abundance, like C-cadherin or csFN; 2) being coessential with other factors for specific multimolecular adhesion structures like glycocalyx types; 3) regional factors that perform functions executed by other factors in other regions, like PAPC; or 4) being redundant. Contact types behave as units in that they can dissolve when a component is removed or change into narrower or wider contacts. The regular decrease of total adhesive contact length with cell size and the decrease of contact abundance with contact width hint at superimposed global control mechanisms that organize tissues.
Contact Types and Glycocalyx Adhesion.
From our difference spectra and La3+ staining patterns, some gastrula contact types can be tentatively reconstructed. We infer an elaborate glycocalyx in the 50- to 125-nm-width range in ectoderm and mesoderm, which depends on Syn-4, Gpc-4, and HA, and exhibits homogeneous La3+ staining with heparan sulfate being contributed by Syn-4 and Gpc-4 (Fig. 10). Syndecans, glypicans, and hyaluronic acid are signature components of the endothelial apical glycocalyx (40, 56), and pulmonary and cardiac capillary surfaces are covered with dense homogeneous plaques (41, 57). We refer to this structure as glycocalyx I (Fig. 10C). The 50- to 100-nm contacts depend also on csFN in mesoderm, and on C-cad in mesoderm and ectoderm (Fig. 10 A and B), factors that stabilize the glycocalyx. In the mesoderm, its difference spectrum identifies PAPC as a putative component of glycocalyx I.
Fig. 10.
Model of glycocalyx-mediated contacts in the Xenopus gastrula. Reconstruction of contact types in ectoderm (A) and prechordal mesoderm (B), based on difference spectra and La3+ staining. Solid and dashed lines indicate regions of width spectrum that are strongly or weakly reduced by single adhesion factor knockdown. Gray inhibitory arrows indicate supposed indirect inhibition of contacts. Gray box, reconstructed contact types; asterisks, results from double knockdown were used in deductions. (C) La3+ staining reveals different glycocalyx structures in the Xenopus gastrula. Only La3+-binding components of surface coats are visualized (dark red); others like FN or yet unidentified factors (pink) must be assumed to contribute.
La3+ homogeneously stains narrow 10- to 50-nm contacts, which depend on Syn-4, and on C-cad or HA (Fig. 10 A and B). In the remainder of peak 1, from 125 to 250 nm, contacts depend on Syn-4 and C-cad in the ectoderm, and additionally on Gpc-4, HA, and PAPC in the mesoderm. La3+ staining identifies an elaborate glycocalyx composed of bush-like units and resembling the endothelial glycocalyx of cerebral capillaries (57), termed here glycocalyx II (Fig. 10C). In mesoderm, it or a modification of it also occurs at low frequency in wider contacts beyond peak 1 (Fig. 10B). A wide contact glycocalyx III, characterized by fine straight fibrils in a brush-like structure, occurs in mesoderm but not ectoderm (Fig. 10 B and C). It resembles the brush-like coatings on some cardiac endothelial cells (58). The fibrils in glycocalyx II and III are absent in Has morphants, indicating that they depend on or consist of HA chains.
In the Xenopus gastrula, an elaborate glycocalyx is present at cell contacts, particularly at abrupt transitions into gaps and at stitch-like contacts. Moreover, depletion of Syn-4, Gpc-4, Has-1/2, or PAPC diminishes contacts and simultaneously affects La3+ staining. This suggests that the glycocalyx mediates adhesion, by a mechanism that remains yet to be examined. The protruding fibrils of glycocalyx II bushes of two cells are not entangled to a large extent, but instead bushes contact the opposite membranes in antiparallel fashion and each other laterally. This allows for asymmetric contacts, e.g., between negatively charged fibrils on one side and positive ligands on the opposite membrane. It suggests a zippering mechanism where the height of a free glycocalyx layer is the same as the width of two interpenetrating glycocalyces in contacts. Such a width relationship has previously been described for the type I glycocalyx of mutually adhering blood cells (59).
Apart from adhesion, antiadhesive roles of the glycocalyx could contribute to contact spacing in the gastrula. Glycocalyx III fibrils show little interdigitation at contacts, and occasional large fibril aggregates, possibly formed during glycocalyx shedding, could maintain large membrane separations by resisting cells being squeezed together. However, in view of the general prevalence of high contact angles at contact-gap transitions that indicate adhesion, we presume that repellent functions of the glycocalyx are rare.
A substantial fraction of contacts is not La3+ stained. For contacts <40 nm, cadherin adhesion could be responsible, supported indirectly by Syn-4. At wider contacts, HA and csFN could participate in the formation of heparan sulfate–free adhesive pericellular matrices (2). Intriguingly, residual stitch-like contacts in Syn-4 morphants are La3+ negative and in the width range of peak 2. Codepletion of csFN with Syn-4 causes the strongest reduction of adhesive contact frequency, diminishes peak 2, and reduces stitch contacts. This is consistent with peak 2 contacts being based on csFN and persisting after Syn-4 knockdown.
Glycocalyx and other pericellular matrix adhesion must differ mechanistically from membrane receptor–based adhesion (2). When cells attach to each other through such a cell coat, a transient supracellular structure is formed whose elasticity is linked to adhesion strength. Multiple weak interactions, including purely physical effects like chain entanglement, add up to a rather nonspecific overall adhesion, and the behavior of this bulky, fuzzy adhesion complex in cell-on-cell migration and cell rearrangement has yet to be analyzed. Interestingly, knockdown of Syn-4, which removes most of the La3+ positive cell coat and strongly reduces cell attachment, accelerates convergent extension. Removal of adhesive glycocalyx by internalization could be rate limiting in normal cell-on-cell movement. Endosomal recycling of Syn-4 is indeed essential for cell spreading in vitro (42) and for epiboly in the zebrafish embryo (60).
Cells in Syn-4 or Gpc-4 morphants bear a striking resemblance to the mesoderm in chick or mouse gastrulae (6, 7, 61) or the limb bud mesenchyme (62), where cell surfaces are concave, and cells are connected via thin processes. Thus, elimination in the frog gastrula of a single gene product generates a fundamental tissue type that forms naturally in other animals during an epithelial–mesenchymal transition and may possibly be characterized by a lack of widespread glycocalyx adhesion. Residual cell–cell contact areas are not coalescing into few patches but are spaced out over the whole cell surface, which seems to be sufficient for tissue cohesion and the execution of sophisticated morphogenetic movements like convergent extension of chordamesodem (63) or vertical telescoping of leading-edge mesendoderm (39).
Major points for further investigation are raised by our results. Novel roles of cadherins in cell adhesion, related to cadherin shedding, nonjunctional activities, and putative extracellular binding partners seem to deserve attention. Likewise, the adhesive role of syndecans and their interactions with cadherin functions are underexplored. Examining the mechanisms, mechanics, and functional roles of glycocalyx-mediated cell–cell adhesion will yield novel insights into modes of tissue cohesion, cell-on-cell migration, and the control of extracellular space.
Materials and Methods
For details, see SI Appendix, SI Materials and Methods.
TEM.
To prepare samples for TEM (21, 32), Xenopus laevis stage 11 gastrulae were fixed in 4% paraformaldehyde and 2.5% glutaraldehyde, bisected, and postfixed in 1% osmium tetroxide (OsO4). For visualization of the glycocalyx, 1% lanthanum nitrate (Sigma-Aldrich Canada) was added (64). To prepare dissociated samples, gastrulae were punctured and placed in Ca2+/Mg2+-free Modified Barths Solution for 30 min before fixation. Samples were dehydrated in ethanol, embedded in Spurr’s resin, and sectioned on a Leica EM UC6 microtome. Ultrathin sections were stained with 3% uranyl acetate and Reynold’s lead citrate and viewed under a Hitachi HT7700 microscope.
Analysis of TEM Images.
Differences in cell and yolk platelet size were used to identify tissues in TEM images of normal gastrulae where tissue boundaries had previously been determined using marker genes (65), and in treated embryos where gastrulation movements are affected (SI Appendix, Fig. S2) (66). Cell surfaces were considered in contact when contours of adjacent cells paralleled each other. Abrupt divergence of the membranes marked the end of a contact at an interstitial gap. Separation distances between membranes were measured every 100 nm along continuous lines between cells in a tissue (SI Appendix, Fig. S11A), and distances were binned in 50-nm steps to obtain contact spectra (SI Appendix, Figs. S3 and S4). The error due to random tilting of sectioning plane relative to the plane of a contact was estimated from the width variation of tight junctions at the gastrula surface (67, 68) (SI Appendix, Fig. S11 B and C), which suggested an up to 1.5-fold apparent increase in contact width possible. Abundances of narrow 0- to 50-nm contacts, wide contacts (50 to 1,000 nm), and gaps were collated in summary profiles (Fig. 1G). Difference spectra for each treatment were derived by subtracting wild-type contact spectra bin by bin from morphant spectra. Variance was analyzed using one-way ANOVA (see SI Appendix, SI Materials and Methods for significance values).
Morpholino Antisense Oligonucleotides.
Two-cell stage X. laevis embryos were microinjected with previously characterized translation-blocking morpholino antisense oligonucleotides (GeneTools). For a list of morpholinos, their sequences, injection doses, and published efficiencies, please refer to SI Appendix, Table S4. For a general evaluation of the morpholino knockdown approach see ref. 69.
Immunostaining.
Stage 11 gastrulae were fixed in 4% paraformaldehyde, dissected, incubated in mouse monoclonal antibodies against C-cadherin, rabbit antiserum against X. laevis fibronectin, polyclonal rabbit antibody against Syn-4 (26), or mouse monoclonal IgM against heparan sulfate, and in secondary antibodies AffiniPure Cy3-goat-anti-rabbit IgG or AffiniPure Cy2-goat-anti-mouse IgG. Images were taken on a Leica TCS SP8 confocal microscope.
Supplementary Material
Acknowledgments
We thank A. Chong of the Cell and Systems Biology imaging facility for help with TEM imaging, and S. Parent, Y. Huang, O. Luu, and A. Miles for suggestions to improve the manuscript. Funding was provided to R.W. by the Canadian Institutes of Health Research (project grant PJT-15614).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107953118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Gumbiner B. M., Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622–634 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Winklbauer R., Dynamic cell-cell adhesion mediated by pericellular matrix interaction – A hypothesis. J. Cell Sci. 132, jcs231597 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Timpe L., Martz E., Steinberg M. S., Cell movements in a confluent monolayer are not caused by gaps: Evidence for direct contact inhibition of overlapping. J. Cell Sci. 30, 293–304 (1978). [DOI] [PubMed] [Google Scholar]
- 4.Kastendieck H., Altenähr E., Burchardt P., Proceedings: Light and electron microscopy findings on the infiltrating growth of prostatic carcinoma [in German]. Verh. Dtsch. Ges. Pathol. 57, 463–474 (1973). [PubMed] [Google Scholar]
- 5.Lloreta J., Mariñoso M. L., Corominas J. M., Cañas M. A., Serrano S., Medullary carcinoma of the breast: An ultrastructural morphometric study of nine cases. Ultrastruct. Pathol. 21, 499–507 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Batten B. E., Haar J. L., Fine structural differentiation of germ layers in the mouse at the time of mesoderm formation. Anat. Rec. 194, 125–141 (1979). [DOI] [PubMed] [Google Scholar]
- 7.Granholm N. H., Baker J. R., Cytoplasmic microtubules and the mechanism of avian gastrulation. Dev. Biol. 23, 563–584 (1970). [DOI] [PubMed] [Google Scholar]
- 8.Kühl M., Wedlich D., Xenopus cadherins: Sorting out types and functions in embryogenesis. Dev. Dyn. 207, 121–134 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Winklbauer R., Cell adhesion in amphibian gastrulation. Int. Rev. Cell Mol. Biol. 278, 215–275 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Winklbauer R., Parent S. E., Forces driving cell sorting in the amphibian embryo. Mech. Dev. 144 (Pt A), 81–91 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Miyaguchi K., Ultrastructure of the zonula adherens revealed by rapid-freeze deep-etching. J. Struct. Biol. 132, 169–178 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Sivasankar S., Brieher W., Lavrik N., Gumbiner B., Leckband D., Direct molecular force measurements of multiple adhesive interactions between cadherin ectodomains. Proc. Natl. Acad. Sci. U.S.A. 96, 11820–11824 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boggon T. J., et al., C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296, 1308–1313 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Amack J. D., Manning M. L., Knowing the boundaries: Extending the differential adhesion hypothesis in embryonic cell sorting. Science 338, 212–215 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Maître J.-L., Heisenberg C.-P., Three functions of cadherins in cell adhesion. Curr. Biol. 23, R626–R633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winklbauer R., Cell adhesion strength from cortical tension – An integration of concepts. J. Cell Sci. 128, 3687–3693 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Fagotto F., Gumbiner B. M., Beta-catenin localization during Xenopus embryogenesis: Accumulation at tissue and somite boundaries. Development 120, 3667–3679 (1994). [DOI] [PubMed] [Google Scholar]
- 18.Heasman J., et al., A functional test for maternally inherited cadherin in Xenopus shows its importance in cell adhesion at the blastula stage. Development 120, 49–57 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya H., et al., Cadherin-dependent differential cell adhesion in Xenopus causes cell sorting in vitro but not in the embryo. J. Cell Sci. 125, 1877–1883 (2012). [DOI] [PubMed] [Google Scholar]
- 20.David R., et al., Tissue cohesion and the mechanics of cell rearrangement. Development 141, 3672–3682 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Luu O., et al., PAPC mediates self/non-self-distinction during Snail1-dependent tissue separation. J. Cell Biol. 208, 839–856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng H.-C., Abdel-Ghany M., Elble R. C., Pauli B. U., Lung endothelial dipeptidyl peptidase IV promotes adhesion and metastasis of rat breast cancer cells via tumor cell surface-associated fibronectin. J. Biol. Chem. 273, 24207–24215 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Huang L., et al., Protein kinase Cepsilon mediates polymeric fibronectin assembly on the surface of blood-borne rat breast cancer cells to promote pulmonary metastasis. J. Biol. Chem. 283, 7616–7627 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Winklbauer R., Conditions for fibronectin fibril formation in the early Xenopus embryo. Dev. Dyn. 212, 335–345 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Wen J. W., Winklbauer R., Ingression-type cell migration drives vegetal endoderm internalisation in the Xenopus gastrula. eLife 6, e27190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagel M., Winklbauer R., PDGF-A suppresses contact inhibition during directional collective cell migration. Development 145, dev162651 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Muñoz R., Moreno M., Oliva C., Orbenes C., Larraín J., Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat. Cell Biol. 8, 492–500 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Galli A., Roure A., Zeller R., Dono R., Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Development 130, 4919–4929 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Medina A., Swain R. K., Kuerner K. M., Steinbeisser H., Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. EMBO J. 23, 3249–3258 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeGrendele H. C., Estess P., Picker L. J., Siegelman M. H., CD44 and its ligand hyaluronate mediate rolling under physiologic flow: A novel lymphocyte-endothelial cell primary adhesion pathway. J. Exp. Med. 183, 1119–1130 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nandi A., Estess P., Siegelman M. H., Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J. Biol. Chem. 275, 14939–14948 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Barua D., Parent S. E., Winklbauer R., Mechanics of fluid-filled interstitial gaps. II. Gap Characteristics in Xenopus embryonic ectoderm. Biophys. J. 113, 923–936 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moosmann J., et al., X-ray phase-contrast in vivo microtomography probes new aspects of Xenopus gastrulation. Nature 497, 374–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damm E. W., “PDGF-A signalling regulates radially oriented movements of mesoderm cells during gastrulation in Xenopus,” PhD thesis, University of Toronto, Toronto, ON (2014).
- 35.Son S., et al., Molecular height measurement by cell surface optical profilometry (CSOP). Proc. Natl. Acad. Sci. U.S.A. 117, 14209–14219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ewald A. J., Peyrot S. M., Tyszka J. M., Fraser S. E., Wallingford J. B., Regional requirements for Dishevelled signaling during Xenopus gastrulation: Separable effects on blastopore closure, mesendoderm internalization and archenteron formation. Development 131, 6195–6209 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Evren S., et al., EphA4-dependent Brachyury expression is required for dorsal mesoderm involution in the Xenopus gastrula. Development 141, 3649–3661 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Winklbauer R., “Chapter Nine – Mesoderm and endoderm internalization in the Xenopus gastrula” in Current Topics in Developmental Biology, Solnica-Krezel L., Ed. (Academic Press, 2020), pp. 243–270. [DOI] [PubMed] [Google Scholar]
- 39.Nagel M., et al., Capillarity and active cell movement at mesendoderm translocation in the Xenopus gastrula. Development 148, dev198960 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Weinbaum S., Tarbell J. M., Damiano E. R., The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9, 121–167 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Okada H., et al., Three-dimensional ultrastructure of capillary endothelial glycocalyx under normal and experimental endotoxemic conditions. Crit. Care 21, 261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann P., et al., Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell 9, 377–388 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Johnson K. E., The extent of cell contact and the relative frequency of small and large gaps between presumptive mesodermal cells in normal gastrulae of Rana pipiens and the arrested gastrulae of the Rana pipiens female x Rana catesbeiana male hybrid. J. Exp. Zool. 179, 227–237 (1972). [DOI] [PubMed] [Google Scholar]
- 44.Shafraz O., Xie B., Yamada S., Sivasankar S., Mapping transmembrane binding partners for E-cadherin ectodomains. Proc. Natl. Acad. Sci. U.S.A. 117, 31157–31165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seifert K., Ibrahim H., Stodtmeister T., Winklbauer R., Niessen C. M., An adhesion-independent, aPKC-dependent function for cadherins in morphogenetic movements. J. Cell Sci. 122, 2514–2523 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Bell G. I., Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978). [DOI] [PubMed] [Google Scholar]
- 47.Cruz-Chu E. R., Malafeev A., Pajarskas T., Pivkin I. V., Koumoutsakos P., Structure and response to flow of the glycocalyx layer. Biophys. J. 106, 232–243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roper J. A., Williamson R. C., Bass M. D., Syndecan and integrin interactomes: Large complexes in small spaces. Curr. Opin. Struct. Biol. 22, 583–590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elfenbein A., Simons M., Syndecan-4 signaling at a glance. J. Cell Sci. 126, 3799–3804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afratis N. A., Karamanou K., Piperigkou Z., Vynios D. H., Theocharis A. D., The role of heparins and nano-heparins as therapeutic tool in breast cancer. Glycoconj. J. 34, 299–307 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Langhe R. P., et al., Cadherin-11 localizes to focal adhesions and promotes cell-substrate adhesion. Nat. Commun. 7, 10909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W. T., Singer S. J., Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J. Cell Biol. 95, 205–222 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hedman K., Vaheri A., Wartiovaara J., External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J. Cell Biol. 76, 748–760 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanai I., Peshkin L., Jorgensen P., Kirschner M. W., Mapping gene expression in two Xenopus species: Evolutionary constraints and developmental flexibility. Dev. Cell 20, 483–496 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoji H., Nishi N., Hirashima M., Nakamura T., Characterization of the Xenopus galectin family. Three structurally different types as in mammals and regulated expression during embryogenesis. J. Biol. Chem. 278, 12285–12293 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Reitsma S., Slaaf D. W., Vink H., van Zandvoort M. A. M. J., oude Egbrink M. G. A., The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 454, 345–359 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ando Y., et al., Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci. Rep. 8, 17523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia Y., Fu B. M., Investigation of endothelial surface glycocalyx components and ultrastructure by single molecule localization microscopy: Stochastic Optical Reconstruction Microscopy (STORM). Yale J. Biol. Med. 91, 257–266 (2018). [PMC free article] [PubMed] [Google Scholar]
- 59.Soler M., et al., Adhesion-related glycocalyx study: Quantitative approach with imaging-spectrum in the energy filtering transmission electron microscope (EFTEM). FEBS Lett. 429, 89–94 (1998). [DOI] [PubMed] [Google Scholar]
- 60.Lambaerts K., et al., Syntenin, a syndecan adaptor and an Arf6 phosphatidylinositol 4,5-bisphosphate effector, is essential for epiboly and gastrulation cell movements in zebrafish. J. Cell Sci. 125, 1129–1140 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trelstad R. L., Hay E. D., Revel J. D., Cell contact during early morphogenesis in the chick embryo. Dev. Biol. 16, 78–106 (1967). [DOI] [PubMed] [Google Scholar]
- 62.Singley C. T., Solursh M., The use of tannic acid for the ultrastructural visualization of hyaluronic acid. Histochemistry 65, 93–102 (1980). [DOI] [PubMed] [Google Scholar]
- 63.Shindo A., Wallingford J. B., PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 343, 649–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson K. E., Changes in the cell coat at the onset of gastrulation in Xenopus laevis embryos. J. Exp. Zool. 199, 137–142 (1977). [DOI] [PubMed] [Google Scholar]
- 65.Damm E. W., Winklbauer R., PDGF-A controls mesoderm cell orientation and radial intercalation during Xenopus gastrulation. Development 138, 565–575 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Nakatsuj N., Studies on the gastrulation of amphibian embryos: Light and electron microscopic observation of a urodele Cynops pyrrhogaster. J. Embryol. Exp. Morphol. 34, 669–685 (1975). [PubMed] [Google Scholar]
- 67.Farquhar M. G., Palade G. E., Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furuse M., Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2, a002907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blum M., De Robertis E. M., Wallingford J. B., Niehrs C., Morpholinos: Antisense and sensibility. Dev. Cell 35, 145–149 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.