Significance
Intestinal bacteria produce extracellular ATP in the lumen during their growth, which drives host immune responses. To avoid excessive immune reactions in the intestinal mucosa, luminal ATP is finely tuned. However, how the concentration of luminal ATP is controlled in the colon remains poorly understood. Here, we discovered that ATP-hydrolyzing enzyme E-NTPD8 acts as an immunomodulator in the colon. Entpd8 deficiency led to high sensitivity to DSS-induced colitis in mice, which was due to the sustained survival of colonic neutrophils. Extracellular ATP suppressed apoptosis by inducing metabolic alteration toward glycolysis via P2X4R in neutrophils. These results reveal the mechanism preventing innate intestinal pathology through modulation of myeloid cell metabolism and may serve to identify therapeutic targets for IBD.
Keywords: adenosine triphosphate, colitis, E-NTPD8, immunometabolism, P2X4R
Abstract
Extracellular adenosine triphosphate (ATP) released by mucosal immune cells and by microbiota in the intestinal lumen elicits diverse immune responses that mediate the intestinal homeostasis via P2 purinergic receptors, while overactivation of ATP signaling leads to mucosal immune system disruption, which leads to pathogenesis of intestinal inflammation. In the small intestine, hydrolysis of luminal ATP by ectonucleoside triphosphate diphosphohydrolase (E-NTPD)7 in epithelial cells is essential for control of the number of T helper 17 (Th17) cells. However, the molecular mechanism by which microbiota-derived ATP in the colon is regulated remains poorly understood. Here, we show that E-NTPD8 is highly expressed in large-intestinal epithelial cells and hydrolyzes microbiota-derived luminal ATP. Compared with wild-type mice, Entpd8−/− mice develop more severe dextran sodium sulfate–induced colitis, which can be ameliorated by either the depletion of neutrophils and monocytes by injecting with anti–Gr-1 antibody or the introduction of P2rx4 deficiency into hematopoietic cells. An increased level of luminal ATP in the colon of Entpd8−/− mice promotes glycolysis in neutrophils through P2x4 receptor–dependent Ca2+ influx, which is linked to prolonged survival and elevated reactive oxygen species production in these cells. Thus, E-NTPD8 limits intestinal inflammation by controlling metabolic alteration toward glycolysis via the P2X4 receptor in myeloid cells.
The intestinal microbiota contribute to reinforcing epithelial integrity and shaping the immune system via metabolically derived signaling molecules (1–3). However, alterations in microbial metabolites and their translocation following intestinal dysbiosis are implicated in the pathogenesis of chronic disorders, such as inflammatory bowel diseases (IBD) including Crohn’s disease (CD) and ulcerative colitis (UC) (4, 5). Extracellular adenosine triphosphate (ATP) is released by microbes and immune cells in the intestine (6–8) and drives immune responses through the P2X1-7 and P2Y1, 2, 11 receptors (9). To avoid inappropriate immune reactions in the intestine, luminal ATP is strictly controlled by epithelial ATP-hydrolyzing ectoenzymes, such as ectonucleotide pyrophosphatase/phosphodiesterases (E-NPPs) and ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). E-NPP3 on the epithelial cells depresses the apoptosis of plasmacytoid dendritic cells (DCs) in the small intestine and Peyer’s patches (10). In addition, E-NTPD7 in small-intestinal epithelial cells hydrolyzes luminal ATP, thus inhibiting excessive T helper 17 (Th17) responses (11). However, how the concentration of luminal ATP produced by commensal bacteria is regulated in the large intestine remains undetermined.
Although intestinal phagocytes such as monocytes, macrophages (Mϕ), DCs, and neutrophils have some protective effects, these cells can also function in pathological conditions (12–14). In patients with IBD, inflamed sites of the intestinal mucosa have more inflammatory DCs and Mϕ, many of which are dysfunctional (15). In addition, an enhanced neutrophil accumulation in the intestinal mucosa of UC patients correlates with disease severity (16–18). Accordingly, experimental murine colitis can be abrogated by inhibiting the recruitment of monocytes and neutrophils to the intestinal lamina propria by using anti-CCR2 (19) or -Gr-1 (20–22) antibody or by the targeted deletion of CCR2 (23) or β7-integrin (24). Thus, the number of intestinal phagocytes and their physiological functions must be tightly tuned to prevent the intestinal inflammation. However, the mechanisms by which the activity of monocytes and neutrophils that have infiltrated into the intestinal mucosa are regulated remain poorly understood.
Different immune cell populations have distinct nutrient utilizations and cellular metabolisms (25), which are involved in their differentiation, proliferation, functions, longevity, and epigenetic modification (25–27). Microbial components and metabolites, such as lipopolysaccharide (28) and short chain fatty acids (29), can switch to glycolysis in monocytes and T cells, respectively. The small molecule dimethyl fumarate (DMF), which suppresses glycolysis in lymphocytes and myeloid cells (30), abrogates chemically induced colitis in mice (31, 32), which indicates that inadequate glycolysis activation is involved in the pathogenesis of intestinal inflammation. However, the mechanism underlying the adaptation of intestinal myeloid cells to environmental factors that fuel glycolysis is unclear.
In this study, we investigated the immunomodulatory function of ATP-hydrolyzing ectoenzyme E-NTPD8 in maintenance of the gut homeostasis. Mice with Entpd8 deficiency had an increased concentration of luminal ATP in their colons, which led to the prolonged survival of neutrophils owing to the facilitation of glycolysis by the P2X4 receptor (P2X4R), thereby exacerbating dextran sodium sulfate (DSS)-induced colitis. Therefore, the clearance of extracellular ATP by E-NTPD8 is essential for the prevention of innate intestinal pathology by inhibiting a metabolic alteration toward glycolysis in myeloid cells.
Results
Entpd8−/− Mice Have Higher Levels of Luminal ATP in the Colon.
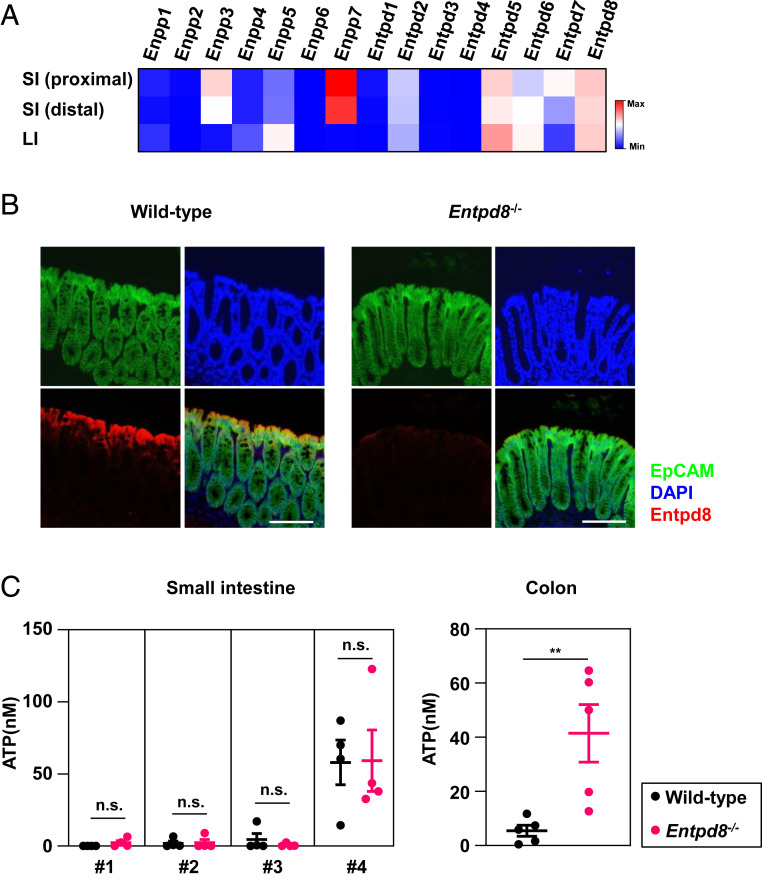
The molecular mechanism underlying regulation of the luminal ATP concentration in the large intestine, unlike the small intestine, remains poorly understood. To address this knowledge gap, we performed RNA-sequencing (RNA-seq) on small and large-intestinal epithelial cells and comprehensively analyzed their expression patterns of ectonucleotidases. In large-intestinal epithelial cells, the expression levels of Enpp5, Entpd5, Entpd6, and Entpd8 were higher than those of other molecules (Fig. 1A). Among these candidate targets, E-NTPD8 was identified as capable of hydrolyzing ATP and UTP (33, 34). To evaluate whether E-NTPD8 is involved in the hydrolysis of extracellular ATP, we added an ATP solution to cultured HEK293 cells that had transfected with an Entpd8-expression or empty vector and measured the concentration of ATP remaining in the culture supernatants at 5 min after the addition (SI Appendix, Fig. S1A). E-NTPD8–expressing HEK293 cells had a lower concentration of ATP in their supernatants compared with control cells, which indicates that E-NTPD8 regulates the extracellular ATP concentration. In addition, expression of ENTPD8 was severely reduced in epithelial cells of patients with UC (SI Appendix, Fig. S1 B and C), indicating the involvement of E-NTPD8 in the pathogenesis of UC.
Fig. 1.
E-NTPD8 in intestinal epithelial cells hydrolyzes luminal ATP in the colon. (A) A heat map of differentially expressed Enpps and Entpds in epithelial cells isolated from the small or large intestine. (B) Expression of E-NTPD8 in the large intestine (red, E-NTPD8; green, EpCAM; and blue, DAPI). (Scale Bar, 100 μm.) Data are representative of two independent experiments. (C) Concentrations of luminal ATP in the small intestine (Left) and the colon (Right). Smaller numbers of the small intestine represent more proximal sites. Mean ± SEM from four (small intestine) or five (colon) independent experiments are shown. **P < 0.01; n.s., not significant.
To assess the physiological roles of E-NTPD8, we generated Entpd8−/− mice by using gene targeting (SI Appendix, Fig. S1 D and E). The epithelial cells of the small and large intestines in Entpd8−/− mice were confirmed to lack Entpd8 expression (SI Appendix, Fig. S1F). In the colon of wild-type mice, E-NTPD8 protein expression was observed in epithelial cells but not in lamina propria cells (Fig. 1B), whereas it was completely undetectable in the colon of Entpd8−/− mice. To determine whether E-NTPD8 hydrolyzes extracellular ATP in the intestine, we measured luminal ATP levels in the small and large intestines (Fig. 1C). There were no differences in the concentrations of luminal ATP in any of the four parts of the small intestine between wild-type and Entpd8−/− mice. In contrast, a higher level of luminal ATP in the colon was observed for Entpd8−/− mice compared with wild-type mice. These findings indicate that E-NTPD8 is essential for maintaining the concentration of luminal ATP in the colon.
Entpd8 Deficiency Exacerbates Microbiota-Mediated Colitis.
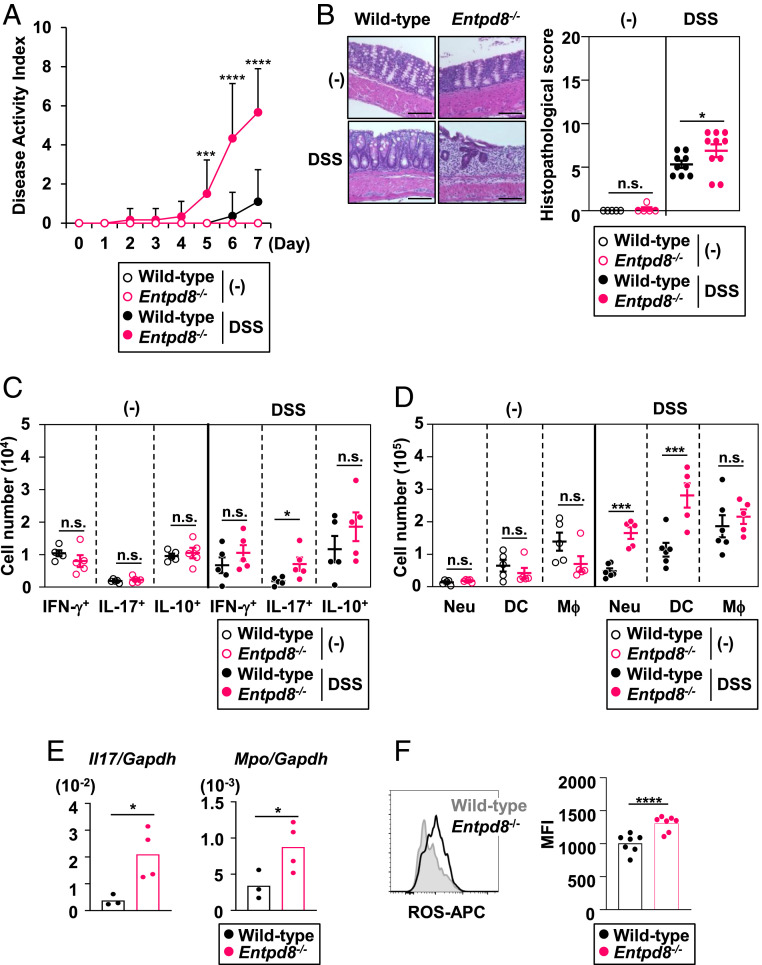
Excessive amounts of extracellular ATP are known to mediate the exacerbation of large-intestinal inflammation in mice (35). Therefore, we examined the sensitivity of Entpd8−/− mice to DSS-induced colitis (Fig. 2). Compared with wild-type mice orally administered 3% DSS in drinking water for 7 d, similarly treated Entpd8−/− mice exhibited more severe clinical symptoms, including bleeding and pasty stool, which are summarized by their higher disease activity index (DAI) scores (Fig. 2A). Additionally, Entpd8−/− mice showed worsened large-intestinal histopathology compared with wild-type mice after DSS administration (Fig. 2B).
Fig. 2.
Entpd8−/− mice suffer from severe DSS-induced colitis. Mice were orally administered 3% DSS or left untreated for 7 d. (A) The DAI scores of DSS-administered or untreated wild-type (n = 11 or 5, respectively) or Entpd8−/− (n = 12 or 5, respectively) mice (mean values ± SEM). ***P < 0.005; ****P < 0.001. (B) Hematoxylin-eosin staining (Left) and histopathological scores (Right) of colons from DSS-administered or untreated wild-type (n = 9 or 5, respectively) or Entpd8−/− (n = 10 or 5, respectively) mice (mean values ± SEM). (Scale Bars, 100 μm.) *P < 0.05; n.s., not significant. (C) Cell numbers of IFN-γ–, IL-17–, or IL-10–producing CD4+ T cells in the large-intestinal lamina propria from DSS-administered or untreated wild-type or Entpd8−/− mice (n = 5 for all groups) (mean values ± SEM). *P < 0.05; n.s., not significant. (D) Cell numbers of the indicated innate myeloid cell types in the colonic lamina propria from DSS-administered or untreated wild-type (n = 6 or 5, respectively) or Entpd8−/− (n = 6 or 5, respectively) mice (mean values ± SEM). ***P < 0.005; n.s., not significant. (E) Expression levels of Il17 and Mpo in the lamina propria cells from the large intestine of DSS-administered wild-type (n = 3) or Entpd8−/− (n = 4) mice (mean values ± SD). *P < 0.05. (F) Baseline levels of intracellular ROS in colonic Gr-1+ CD11+ cells from DSS-administered wild-type (n = 7) or Entpd8−/− (n = 7) mice (mean values ± SD). All data are from at least two independent experiments. ****P < 0.001.
In a steady state, the numbers of interferon (IFN)-γ+, interleukin (IL)-17+, and IL-10+ CD4+ T cells (Fig. 2C) and myeloid cells (Fig. 2D), such as Ly6G+ CD11b+ neutrophils, CD64− CD11b+ Ly6Cintermediate DCs, and CD64+ CD11b+ Mϕ, were normal in the colons of Entpd8−/− mice. However, after DSS administration, accumulation of IL-17+ CD4+ T cells, neutrophils, and CD64− CD11b+ Ly6Cintermediate DCs was promoted in Entpd8−/− mice compared with wild-type mice (Fig. 2 C and D). In accordance with increase in the numbers of Th17 cells and neutrophils following DSS administration in Entpd8−/− mice, the expression levels of Il17a and Mpo were higher in the large-intestinal lamina propria of these mice than in wild-type mice (Fig. 2E). Furthermore, the level of reactive oxygen species (ROS) production in neutrophils was elevated in the colon of DSS-treated Entpd8−/− mice relative to that in wild-type mice (Fig. 2F).
To examine whether E-NTPD8 in nonhematopoietic or hematopoietic cells influences sensitivity to colitis, we generated bone marrow (BM) chimeric mice. Wild-type and Entpd8−/− recipients were received either wild-type or Entpd8-deficient BM cells, after which they were administered 3% DSS (SI Appendix, Fig. S2). Similar DAI scores and levels of colon histopathology were observed in wild-type recipients transplanted with wild-type or Entpd8-deficient BM cells (SI Appendix, Fig. S2 A and B). Regardless of whether the BM cells were from a wild-type or Entpd8−/− donor, the Entpd8−/− recipients had elevated DAI scores associated with more severe large-intestinal pathology compared with those of wild-type recipients, which indicates that E-NTPD8 in nonhematopoietic cells, possibly epithelial cells, is essential for suppression of colitis.
Intestinal bacteria have been reported to release ATP in the lumen (6–8, 35). To investigate whether E-NTPD8 mediates the clearance of commensal bacteria–derived ATP, mice were administered an antibiotics mixture (ABX) in their drinking water for 12 wk, and the ATP level in their feces was measured (SI Appendix, Fig. S3A). In untreated Entpd8−/− mice, the fecal ATP concentration was significantly higher than that in wild-type mice. However, ABX treatment reduced the fecal concentration of ATP in Entpd8−/− mice to a level similar to that in wild-type mice. In accordance with the reduced level of fecal ATP in ABX-treated Entpd8−/− mice, these mice had markedly lower DAI score with less severe large-intestinal pathology following DSS administration compared with untreated Entpd8−/− mice (SI Appendix, Fig. S3 B and C). These findings suggest that E-NTPD8 in epithelial cells controls the concentration of luminal ATP released by commensal bacteria in the colon and thereby provides resistance to DSS-induced colitis.
Depletion of Monocytes and Neutrophils but Not Adaptive Immune Cells Abrogates DSS-Induced Colitis in Entpd8−/− Mice.
During DSS administration, Entpd8−/− mice suffered from severe colitis accompanied by increased numbers of IL-17+ CD4+ T cells, neutrophils, and DCs in the lamina propria of the colon. To determine whether E-NTPD8 is required for limiting Th17 cell–mediated intestinal pathology, we generated Entpd8−/− Rag2−/− mice and administered 2.5% DSS to these mice for 5 d. Compared with Rag2−/− mice, Entpd8−/− Rag2−/− mice had higher DAI scores (SI Appendix, Fig. S4A) and higher numbers of neutrophils and DCs in the large-intestinal lamina propria (SI Appendix, Fig. S4B). We also depleted CD4+ T cells in wild-type and Entpd8−/− mice using anti-CD4 antibody before and during DSS administration (SI Appendix, Fig. S4C). The numbers of CD4+ T cells in the colonic lamina propria of both wild-type and Entpd8−/− mice were severely reduced by anti-CD4 antibody treatment (SI Appendix, Fig. S4D). However, DAI scores and histopathological scores of the colon remained higher in Endpd8−/− mice with anti-CD4 antibody injection compared with wild-type mice (SI Appendix, Fig. S4 E and F), indicating that CD4+ T cells are dispensable for exaggeration of colitis in Entpd8−/− mice.
Previous studies have demonstrated that the depletion of neutrophils and monocytes by using anti–Gr-1 antibody reduces inflammatory manifestations in chemically induced colitis (20–22, 36). To define whether a promoted accumulation of neutrophils and DCs is involved in development of severe colitis in Entpd8−/− mice, anti–Gr-1 antibody or control IgG was intraperitoneally injected into mice (SI Appendix, Fig. S5). The Entpd8−/− mice injected with control IgG displayed more severe disease manifestations with more profound histopathology of the colon compared with similarly treated wild-type mice (SI Appendix, Fig. S5 A and B). However, the ablation of neutrophils and monocytes by treatment with anti–Gr-1 antibody mitigated the severity of colitis in Entpd8−/− mice, as shown by the lower DAI scores and less severe large-intestinal pathology (SI Appendix, Fig. S5 A and B). These results indicate that the hydrolysis of luminal ATP by E-NTPD8 prevents innate intestinal pathology.
The ATP–P2X4R Axis in Myeloid Cells Is Involved in the Exacerbation of Colitis in Entpd8−/− Mice.
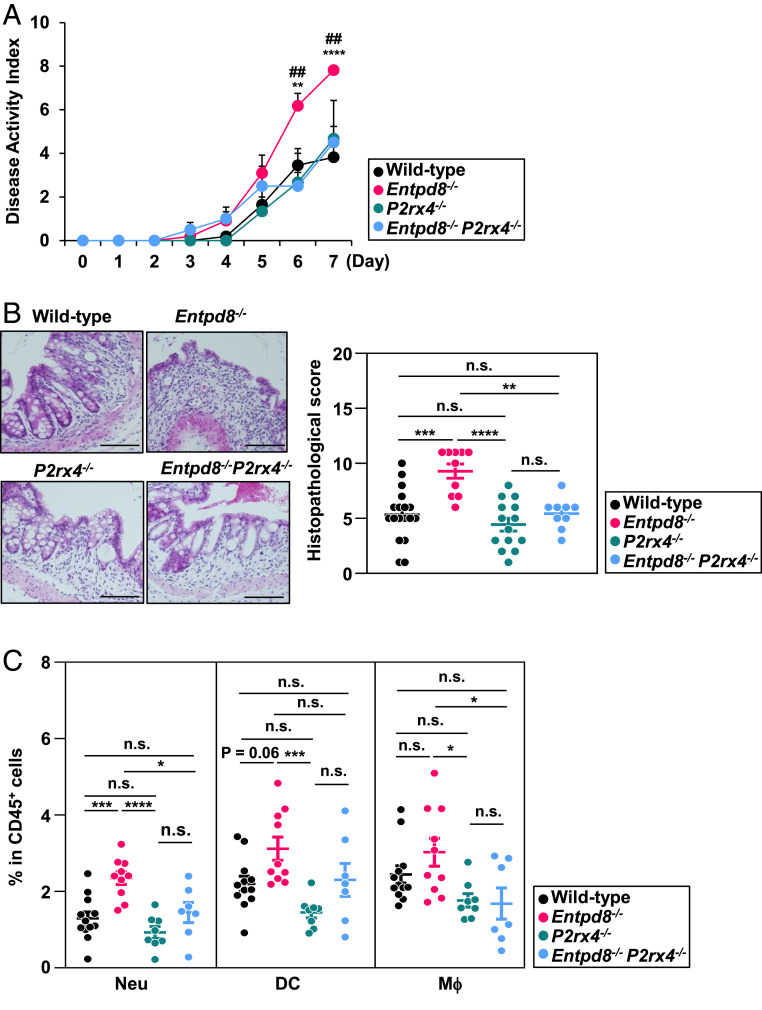
To determine the signaling pathway responsible for the induction of pathological innate immune responses in the colon of Entpd8−/− mice, we investigated the expression patterns of ATP receptors in intestinal epithelial cells (SI Appendix, Fig. S6A) and colonic myeloid cells (SI Appendix, Fig. S6B) by performing an RNA-seq analysis. Among P2 purinergic receptors, P2rx4, an ATP-gated Ca2+ channel, was expressed at a high level in both colonic epithelial cells and myeloid cells. Thus, we attempted to examine whether P2X4R signaling contributes to the development of severe colitis in Entpd8−/− mice by generating Entpd8−/− P2rx4−/− mice. Under homeostatic condition, the numbers of neutrophils, DCs, and Mϕ in the large-intestinal lamina propria of P2rx4−/− mice were similar to those of wild-type mice (SI Appendix, Fig. S6C). In addition, the permeability of intestinal epithelial cells to fluorescein isothiocyanate-conjugated dextran was normal in P2rx4−/− mice (SI Appendix, Fig. S6D). During DSS administration, either the clinical parameters or histopathology of the colon in P2rx4−/− mice were similar to those in wild-type mice (Fig. 3 A and B). However, the introduction of a P2rx4 deficiency into Entpd8−/− mice lessened the level of disease activity and ameliorated the large-intestinal histopathology (Fig. 3 A and B). In this context, Entpd8−/−P2rx4−/− mice showed lower levels of neutrophils and Mϕ accumulation in the colon compared with Entpd8−/− mice (Fig. 3C), as well as partial reduction in the accumulation of DCs.
Fig. 3.
DSS-induced colitis is mitigated in P2rx4−/− Entps8−/− mice. Wild-type, Entpd8−/−, P2rx4−/−, or P2rx4−/− Entpd8−/− mice were administered 3% DSS for 7 d. (A) The DAI scores of wild-type (n = 16), Entpd8−/− (n = 11), P2xr4−/− (n = 8), and P2xr4−/− Entpd8−/− (n = 8) mice (mean values ± SD). **P < 0.01, ****P < 0.001. ##P < 0.01. *P denotes significance between wild-type and Entpd8−/− mice. #P denotes significance between Entpd8−/− and P2xr4−/− Entpd8−/− mice. All data are from three independent experiments. (B) Representative sections (Left) and histopathological scores (Right) of the colon from wild-type (n = 18), Entpd8−/− (n = 10), P2xr4−/− (n = 14), and P2xr4−/− Entpd8−/− (n = 9) mice. All data are mean values ± SEM from three independent experiments. **P < 0.01, ***P < 0.005, ****P < 0.001; n.s., not significant. (Scale Bars, 100 μm.) (C) Frequencies of the indicated innate myeloid cell types in the large-intestinal lamina propria of wild-type (n = 12), Entpd8−/− (n = 10), P2xr4−/− (n = 8), and P2xr4−/− Entpd8−/− (n = 7) mice (mean values ± SEM). All data are from two independent experiments. *P < 0.05, ***P < 0.005, ****P < 0.001; n.s., not significant.
We further investigated whether P2X4R activation in either hematopoietic or nonhematopoietic cells contributes to the aggravation of colitis in Entpd8−/− mice, Entpd8−/− recipients were transplanted with P2rx4+/+ or P2rx4−/− BM cells and then administered 3% DSS for 7 d (SI Appendix, Fig. S7). The DSS-administered Entpd8−/− recipients reconstituted with P2rx4−/− BM cells showed less severe bleeding and better stool consistency, as presented by their lower DAI scores, relative to DSS-administered mice reconstituted with P2rx4+/+ BM cells (SI Appendix, Fig. S7A). Accordingly, the severity of colonic histopathology was alleviated in P2rx4−/− BM cell recipients (SI Appendix, Fig. S7B). Moreover, the frequencies and numbers of neutrophils, DCs, and Mϕ were drastically reduced in the colonic lamina propria of P2rx4−/− BM cell recipients (SI Appendix, Fig. S7C). These findings demonstrate that activation of P2X4R signaling pathway in hematopoietic cells is responsible for the exaggeration of colitis in Entpd8−/− mice. To assess the effect of microbial communities on P2X4R-mediated aggravation of colitis in Entpd8−/− mice, we analyzed the fecal microbiota composition (SI Appendix, Fig. S8). Results of 16S ribosomal RNA gene sequencing analysis revealed that the phylum-level fecal bacterial composition in Entpd8−/− mice was not changed compared with those in wild-type and Entpd8−/− P2rx4−/− mice, which indicates that development of severe innate intestinal pathology in Entpd8−/− mice is not due to dysbiosis.
Extracellular ATP Inhibits the Apoptosis of Colonic Gr-1+ CD11b+ Cells.
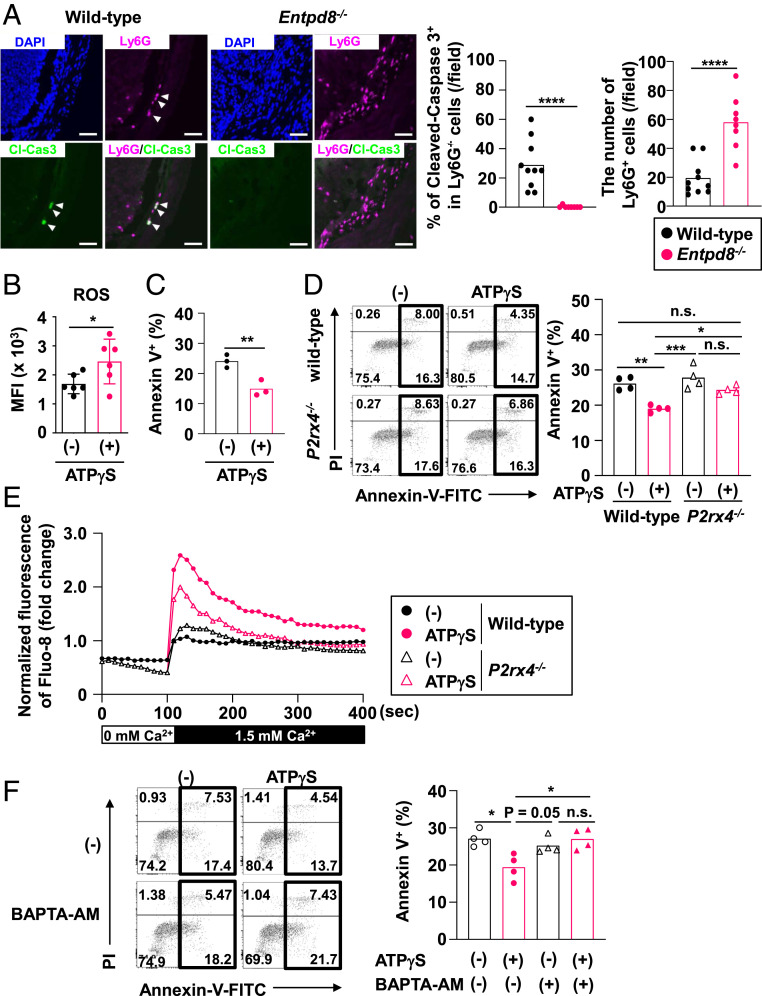
In DSS-treated wild-type mice, the number of Ly6G+ neutrophils with cleaved-caspase 3 was increased, indicating that many neutrophils undergo apoptosis during DSS treatment (Fig. 4A). However, the number of cleaved-caspase 3–positive neutrophils was barely detected in the colonic lamina propria of DSS-treated Entpd8−/− mice. In addition, the injection of anti-Gr1 antibody alleviated DSS-induced colitis in Entpd8−/− mice (SI Appendix, Fig. S5). Therefore, we analyzed the effects of extracellular ATP on the activities of Gr-1+ CD11b+ cells. To evaluate the effector functions, colonic Gr-1+ CD11b+ cells were cultured for 3 h in the presence or absence of ATP-γS, which is a nonhydrolysable ATP. ATP-γS treatment promoted ROS production in colonic Gr-1+ CD11b+ cells (Fig. 4B). We further analyzed the resistance to cell death of colonic Gr-1+ CD11b+ cells that had been stimulated with or without ATP-γS for 5 h by staining the cells with propidium iodide and annexin V. The frequency of annexin V–positive cells was lower in the ATP-γS–stimulated cells (Fig. 4C).
Fig. 4.
Sustained Ca2+ influx via P2X4 receptor mediates the inhibition of apoptosis in Gr-1+ CD11b+ cells. (A) Immunohistochemistry of Ly6G+ cleaved-Caspase 3 (Cl-Cas3)+ cells in the colon from mice administered 3% DSS for 7 d (Left), the number of Ly6G cells (Right), and the frequency of Cl-Cas3+ cells in Ly6G+ cells (Middle) in a field (wild-type: 10 fields and Entpd8−/−: 8 fields). Images are representative of three independent experiments (blue, DAPI; green, Cl-Cas3; and magenta, Ly6G (Scale bar, 50 μm). ****P < 0.001. (B) Flow cytometry–based analysis of ROS production in colonic Gr-1+ CD11b+ cells stimulated with or without ATP-γS for 3 h (mean values ± SD). *P < 0.05. Data were pooled from six independent experiments. (C) The frequencies of annexin V+ cells among Gr-1+ CD11b+ cells from the colon following stimulation with or without ATP-γS for 5 h. All data are from three independent experiments. **P < 0.01. (D) Flow cytometric dot plots (Left) and the frequencies of annexin V+ cells (Right) among Gr-1+ CD11b+ cells from the colonic lamina propria of wild-type or P2rx4−/− mice after their stimulation with or without ATP-γS for 5 h. All data are from four independent experiments. *P < 0.05, **P < 0.01, ***P < 0.005; n.s., not significant. (E) Cytosolic Ca2+ level in Gr-1+ CD11b+ cells from the large-intestinal lamina propria of wild-type or P2rx4−/− mice. All data are representative of two independent experiments. (F) Flow cytometric dot plots (Left) and the frequencies of annexin V+ cells (Right) in Gr-1+ CD11b+ cells from the colons of BALB/c mice following treatment with or without ATP-γS in the presence or absence of BAPTA-AM for 5 h. All data are from four independent experiments. *P < 0.05; n.s., not significant.
We then analyzed whether the effect of ATP-γS on colonic Gr1+ CD11b+ cells is dependent on P2X4R. An ATP-γS–dependent inhibition of apoptosis was not observed in P2rx4−/− Gr-1+ CD11b+ cells (Fig. 4D), which indicates that P2X4R signaling is responsible for prolonging the lifespan of colonic neutrophils. Previous studies have demonstrated that P2X4R is essential for sustaining the ATP-evoked Ca2+ signaling in Mϕ (37), neutrophils (38), and T cells (39). We observed that the peak level of cytosolic Ca2+ was higher in ATP-γS–stimulated Gr-1+ CD11b+ cells from the colon of wild-type mice compared with those of P2rx4−/− mice (Fig. 4E). Furthermore, the increased Ca2+ level was sustained in wild-type cells but not P2rx4−/− cells. Thus, we investigated whether activation of Ca2+ signaling is required for the acquisition of resistance to cell death by colonic Gr-1+ CD11b+ cells following extracellular ATP stimulation. Loading colonic Gr-1+ CD11b+ cells with the Ca2+ chelator 1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) prevented the ATP-γS–induced reduction of apoptotic cell death in these cells (Fig. 4F). These results suggest that ATP-induced Ca2+ mobilization via P2X4R mediates prolonged survival of colonic Gr-1+ CD11b+ cells.
Extracellular ATP–Induced Promotion of Glycolysis Results in the Inhibition of Apoptosis in Colonic Neutrophils.
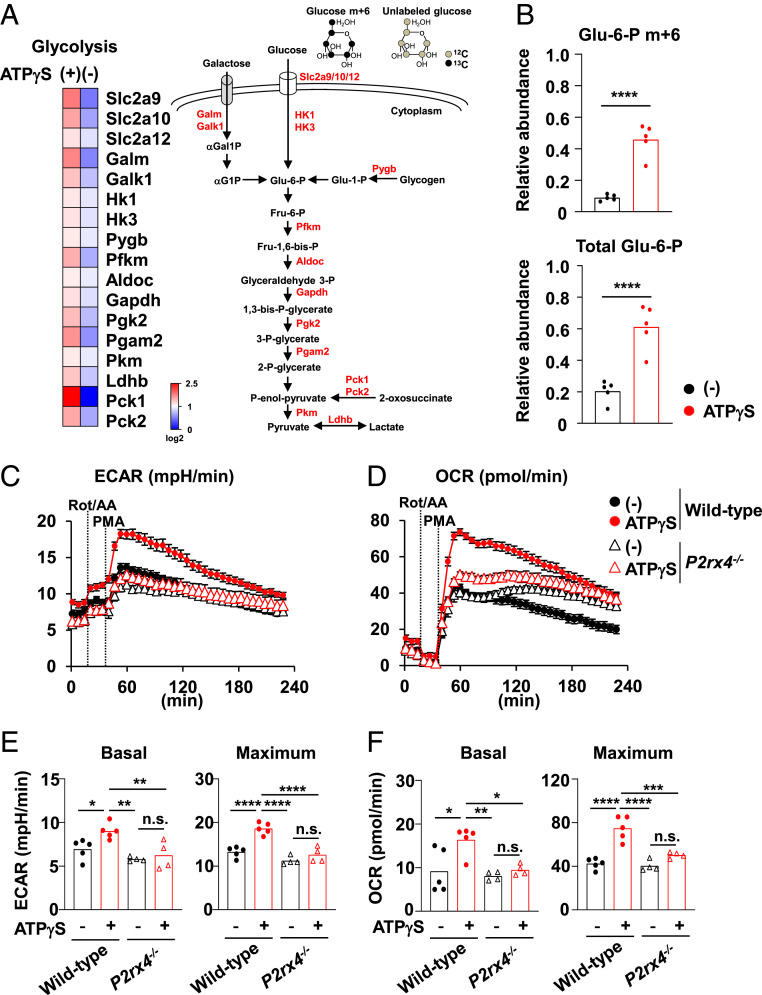
Previous studies have demonstrated that the Ca2+ signaling promotes glycolytic metabolism in neutrophils (39, 40), which is linked to the reduction of apoptosis in these cells (40). Therefore, we examined whether extracellular ATP influences cellular metabolism in Gr-1+ CD11b+ cells. An RNA-seq analysis revealed that ATP-γS stimulation in colonic Gr-1+ CD11b+ cells up-regulated their expression of molecules involved in the glycolytic pathway (Fig. 5A). In agreement with those data, the level of maximum glycolysis following phorbol myristate acetate (PMA) stimulation, as determined by measuring extracellular acidification rates (ECAR), was increased in colonic Gr-1+ CD11b+ cells pretreated with ATP-γS compared with that in untreated cells (SI Appendix, Fig. S9A). In addition, the maximum levels of the oxygen consumption rate (OCR) were augmented in ATP-γS–stimulated colonic Gr-1+ CD11b+ cells (SI Appendix, Fig. S9A). Gr-1+ CD11b+ cells are further divided into Ly6G+ CD11b+ neutrophils and Ly6C+ CD11b+ monocytes (41, 42). To define whether neutrophils or monocytes are sensitive to ATP, we isolated Ly6G+ CD11b+ neutrophils and Ly6C+ CD11b+ monocytes from the colonic lamina propria and analyzed their responses to ATP-γS (SI Appendix, Fig. S9B). Colonic Ly6G+ CD11b+ neutrophils exhibited ATP-γS–dependent elevation of the maximum ECAR and OCR (SI Appendix, Fig. S9C), but Ly6C+ CD11b+ monocytes did not (SI Appendix, Fig. S9D). ATP-γS–dependent up-regulation of the maximum ECAR and OCR in neutrophils was inhibited by BAPTA-AM treatment (SI Appendix, Fig. S9E). We next explored the abundance of glycolytic intermediate glucose-6-phosphate (G6P) in colonic neutrophils cultured in the presence of U-13C6 glucose by liquid chromatography-mass spectrometry (40) (Fig. 5 A and B). Consistent with the up-regulation of ECAR, relative abundances of Glu-6-P m+6, in which all six carbons are 13C, and total Glu-6-P, which includes both unlabeled Glu-6-P and all mass isotopomers of Glu-6-P (m+1 to m+6), were increased in colonic neutrophils stimulated with ATP-γS. These findings indicate that extracellular ATP promotes neutrophil glycolysis in the colon through activation of the Ca2+ signaling. To address whether the extracellular ATP–mediated promotion of glycolysis contributes to the suppression of apoptosis, glycolytic activity was inhibited by 2 deoxy-D-glucose (2DG) in colonic Gr-1+ CD11b+ cells cultured in the presence or absence of ATP-γS, and the apoptosis rates of these cells were analyzed (SI Appendix, Fig. S10A). Treatment with 2DG abrogated the ATP-γS–induced decrease in the frequency of annexin-positive cells among Gr-1+ CD11b+ cells. The ATP-γS–dependent elevation of glycolytic pathway was more evident in Ly6G+ CD11b+ neutrophils than Ly6C+ CD11b+ monocytes (SI Appendix, Fig. S9 C and D). Accordingly, ATP-γS–dependent suppression of apoptosis was observed in Ly6G+ CD11b+ neutrophils, but not in Ly6C+ CD11b+ monocytes (SI Appendix, Fig. S10B). These results suggest that an increased level of glycolysis is crucial for the inhibition of apoptosis by extracellular ATP in colonic neutrophils.
Fig. 5.
Promotion of glycolysis in neutrophils through P2X4 receptor. (A) A heat map (Left) of the genes related with glycolysis in the Gr-1+ CD11b+ cells stimulated with or without ATP-γS for 2.5 h. Scheme of glycolytic pathway (Right). (B) Relative abundances of Glu-6-P m+6 and total Glu-6-P in colonic Ly6G+ CD11b+ neutrophils cultured in the presence of U-13C6 glucose with or without 100 μM ATP-γS for 3 h. Glu-6-P; glucose 6-phosphate. These data are pooled from five independent experiments. ****P < 0.001. (C–F) The ECAR (C and E) and OCR (D and F) in Gr-1+ CD11b+ cells from the colons of wild-type or P2rx4−/− mice following pretreatment with or without ATP-γS for 3 h. The bar graphs in E and F show the average values of basal and maximum ECAR (E) and OCR (F) from four wells. All data are representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001; n.s., not significant.
To investigate whether P2X4R provides the signal for promoting glycolysis, Gr-1+ CD11b+ cells from the colons of wild-type or P2rx4−/− mice were stimulated with ATP-γS for 3 h, after which their ECAR and OCR were measured (Fig. 5 C–F). We observed ATP-γS–dependent elevations in the basal and maximum values of ECAR in wild-type Gr-1+ CD11b+ cells, whereas these changes were not induced in P2rx4−/− cells (Fig. 5 C and E). In addition, higher basal and maximum OCR values were observed in ATP-γS–stimulated wild-type cells but not P2rx4−/− cells compared with unstimulated cells (Fig. 5 D and F). These findings indicate that a P2X4R-induced Ca2+ influx is required for promotion of glycolysis in colonic neutrophils.
Discussion
In this study, we showed that E-NTPD8 in epithelial cells exerts an immunomodulatory function. The clearance of microbiota-derived ATP by E-NTPD8 is essential for inhibiting the prolonged survival of neutrophils and monocytes by discouraging the P2X4R-mediated promotion of glycolysis, thereby abrogating innate intestinal pathology.
Extracellular ATP released by intestinal commensals suppresses the production of IgA and down-regulates its affinity for commensal bacteria (43, 44). We found a lower frequency of IgA+ plasma cells among the CD45+ hematopoietic cells in the colon of Entpd8−/− mice, as compared with wild-type mice (7.97 ± 0.86% and 10.82 ± 4.04%; P = 0.031) under steady state conditions. This finding indicates that E-NTPD8–mediated hydrolysis of ATP might be necessary for inhibiting the reduction of IgA+ plasma cells in the colon. Although we found a higher number of IL-17+ CD4+ T cells and lower number of IgA+ plasma cells in the colon of Entpd8−/− mice compared with wild-type mice, more severe large-intestinal pathology was induced in Entpd8−/− mice, even in mice lacking adaptive lymphocytes as a result of a Rag2 gene deletion, which suggests that altered adaptive immune responses do not account for the pathological intestinal inflammation found in Entpd8−/− mice at least during the acute phase of DSS-induced colitis.
Previous studies showed that augmentation of the intracellular Ca2+ level boosts glycolysis in T lymphocytes by inducing glycolytic enzyme expression through the activation of transcription factors, such as HIF1-α and NFAT (45, 46). Similarly, enhanced HIF1-α expression in neutrophils resulting from either a Phd2 deficiency or hypoxia up-regulates glycolysis (40, 47), leading sustained neutrophil survival (40). In the present study, we demonstrated that ATP-γS induces the expression of glycolysis-related molecules in colonic Gr-1+ CD11b+ cells (Fig. 5A), suggesting that extracellular ATP-induced metabolic alteration toward glycolysis in colonic neutrophils might be initiated by transcriptional modification via the P2X4R-mediated activation of the Ca2+ signaling.
Several studies have demonstrated that a metabolic switch to glycolysis in neutrophils precipitates ROS production by up-regulating NADPH oxidase, which is required for pathogen clearance (48, 49). In accordance with the promoted glycolysis in ATP-γS–exposed neutrophils, ROS production was also elevated in these cells in the current study. Furthermore, a higher level of ROS production during DSS-induced colitis was observed in the colonic neutrophils from Entpd8−/− mice than in those from wild-type mice. Thus, in Entpd8−/− mice, the activation of glycolysis by microbiota-derived ATP may be linked to the enhancement of ROS production other than the inhibition of apoptosis in colonic neutrophils.
In this study, we showed that, in addition to P2rx4, colonic Gr-1+ CD11b+ cells also express P2rx7 and P2ry2. P2rx4−/− Gr-1+ CD11b+ cells stimulated with extracellular ATP showed transient elevation of intracellular Ca2+ level, which may depend on either P2X7R or P2Y2R. However, the introduction of a P2rx4 deficiency into Entpd8−/− mice completely mitigated the myeloid cell-mediated exaggeration of colitis, which indicates that P2X4R is the most relevant P2 purinergic receptor for the acquisition of immunopathological phenotypes in colonic neutrophils in Entpd8−/− mice. The present study found that colonic epithelial cells, likely myeloid cells, express P2rx4. In fecal microbiota of P2rx4−/− mice, the relative abundance of Bacteroidetes was higher than those in wild-type mice, while the relative abundance of Proteobacteria was lower. These results suggest that epithelial cell P2X4R contributes to the maintenance of gut microbial community.
Collectively, our results demonstrate that regulation of luminal ATP by E-NTPD8 is required for the metabolic adaptation of myeloid cells in the colon, where microbiota constitutively secrete ATP, and that disruptions to this regulation lead to severe intestinal inflammation. Growing evidence suggests that targeting the metabolic reprograming occurring within immune cells has therapeutic potential for treating autoimmune diseases and inflammatory disorders (50). The anti-inflammatory drug DMF, which is used in the treatment of psoriasis and multiple sclerosis, down-regulates glycolysis in lymphocytes and myeloid cells via inactivation of the glycolytic enzyme GAPDH (30). Interestingly, DMF suppresses dinitrobenzene sulfuric acid–induced colitis by preventing neutrophil accumulation and activation (31). As in Entpd8−/− mice, the clinical disease activity in UC patients is correlated with an elevated number of infiltrated neutrophils (16), which exhibit higher ROS production (51) and increased survival time (52–55). Although genome-wide association study did not indicate any UC-related mutations of the ENTPD8 gene, there remains the possibility that neutrophil-mediated pathology in UC patients is associated with a reduced expression of ENTPD8 resulting from the perturbation of epigenetic or posttranscriptional modification. Thus, it is important that future research investigates the expression of E-NTPD8 at the protein level in colonic epithelial cells as well as the fecal/luminal concentration of ATP in UC patients, which will provide insights for developing immunotherapeutic interventions targeting the modification of metabolic processes in colonic myeloid cells.
Materials and Methods
Detailed information on the materials, methods, and associated references can be found in SI Appendix, SI Materials and Methods.
Human Samples.
Colon samples were obtained from five patients with UC and seven patients with colorectal cancer. Patient characteristics are provided in SI Appendix, Fig. S1B. The colon was washed in phosphate-buffered saline (PBS) to remove feces and placed in Hank’s balanced salt solution (HBSS) containing 20 mM ethylenediaminetetraacetic acid (EDTA) and then incubated at room temperature. After 3 min, epithelial cells were peeled off from the colon and washed in PBS, after which they were isolated total RNA to analyzed expression of ATP-hydrolyzing enzymes. This study was approved by the Ethical Committee of Osaka University School of Medicine (10261-12). We obtained written informed consent from all patients to use their samples and data.
Generation of Entpd8−/− Mice.
To generate Entpd8−/− mice, a targeted vector was constructed by replacing exons 4 to 9 of Entpd8 with a neomycin-resistance gene cassette, and a gene encoding herpes simplex virus thymidine kinase driven by a phosphoglycerate kinase promoter was inserted into the genomic fragment for use in negative selection. After V6.5 embryonic stem cells were transfected with the targeted vector, the resulting colonies that were doubly resistant to G418 and ganciclovir were selected and screened with PCR and Southern blot analyses. Homologous recombinants were microinjected into blastocysts from female C57BL/6 mice, and the heterozygous F1 progeny were intercrossed to obtain Entpd8-deficient mice. The Entpd8-deficient mice and their wild-type and heterozygous littermates from these intercrosses were identified from the results of Southern blot, Northern blot, and real-time RT-PCR analyses and then used for subsequent experiments. The Entpd8-deficient mice were backcrossed to BALB/c mice for at least 10 generations, and the Entpd8-deficient mice and their wild-type littermates from the intercrosses of heterozygous mice were used for the experiments.
Metabolic Analysis.
The ECAR and OCR were measured with a XFe96 Extracellular Flux Analyzer (Agilent Technologies). Gr-1+ CD11b+, Ly6G+ CD11b+, or Ly6C+ CD11b+ cells were isolated from the colons of wild-type or P2rx4−/− mice that had been administered 3% DSS for 6 d. Cell-Tak–coated XF96 cell culture microplate was seeded with 1 × 105 cells that were then stimulated with or without 100 μM ATP-γS for 3 h. The culture medium was replaced with Agilent Seahorse XF Roswell Park Memorial Institute medium (Agilent Technologies) supplemented with 1 mM pyruvate (Agilent Technologies), 10 mM glucose (Agilent Technologies), and 2 mM L-glutamine (Agilent Technologies), after which the ECAR and OCR were analyzed under basal conditions and upon stimulation with 1 μg/mL PMA following treatment with 0.5 μM rotenone and antimycin A (Rot/AA).
RNA-seq Analysis.
To isolate large and small-intestinal epithelial cells from C57BL/6J mice, murine intestines were opened longitudinally and washed with PBS to remove feces. The extracted intestines were placed in HBSS containing 5 mM EDTA and incubated at 37 °C for 20 min in a shaking water bath. After removing the intestinal tissues, the suspended epithelial cells were centrifuged at 780 × g for 5 min at 4 °C and washed with PBS. To analyze the expression levels of ATP receptors, myeloid cells, such as CD64− DCs, CD64+ Mϕ, and Gr-1+ CD11b+ cells, were isolated from the colons of healthy BALB/c mice. Gr-1+ CD11b+ cells were isolated from BALB/c mice that had been administered 3% DSS for 6 d, and these cells were stimulated with 100 μM ATP-γS for 2.5 h to identify extracellular ATP-inducible genes. Total RNA was extracted from these cells with an RNeasy Mini kit (Qiagen) in accordance with the manufacture’s protocol. Full-length complementary DNA was generated by using a SMART-Seq HT Kit (Takara Bio). An Illumina library was then prepared by using a Nextera DNA Library Preparation Kit (Illumina). Sequencing was performed on an Illumina HiSEq 2500 sequencer (Illumina) in 75-base single-end mode. Sequenced reads were mapped to the mouse reference genome sequence (mm10) with TopHat version 2.0.12. Fragments per kilobase of exons per million mapped fragments (FPKMs) were calculated using Cufflinks version 2.1.1. Among calculated genes with a normalized FPKM value of >1.0 in ATP-γS–stimulated Gr-1+ CD11b+ cells, 7,126 were up-regulated >1.3-fold from ATP-γS–stimulated Gr-1+ CD11b+ cells compared with untreated cells (Fig. 5A). A heat map of Enpp1-7 and Entpd1-8 (Fig. 1A) and graphs showing the expression levels of purinergic P2 receptors (SI Appendix, Fig. S6 A and B) were generated from the FPKM values.
Statistical Analysis.
Differences between the control and experimental groups were evaluated by a two-tailed unpaired Student’s t test or two-way ANOVA followed by Tukey’s multiple comparisons test using GraphPad Prism 8.4.3 for Windows (GraphPad Software Inc.). Differences where P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Y. Magota for technical assistance, C. Hidaka and H. Matsui for secretarial assistance, and Katie Oaklry, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript. This work was supported by the Japan Agency for Medical Research and Development under grant number 19gm1010004, the National Cancer Center Research and Development Fund under grant number No. 28-A-7 to K.T., a Grant-in-Aid for Scientific Research under grant number 17K08881, and the Japan Agency for Medical Research and Development under grant number 19gm6210016 to H.K.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100594118/-/DCSupplemental.
Data Availability
Gene data have been deposited in the National Center Biotechnology Information Gene Expression Omnibus (GSE160379, GSE160380, and GSE160381). All other study data are included in the article and/or SI Appendix.
References
- 1.Zhang Z., Tang H., Chen P., Xie H., Tao Y., Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct. Target. Ther. 4, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavelle A., Sokol H., Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 223–237 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Kayama H., Okumura R., Takeda K., Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 38, 23–48 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Levy M., Kolodziejczyk A. A., Thaiss C. A., Elinav E., Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Tilg H., Zmora N., Adolph T. E., Elinav E., The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 20, 40–54 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Hironaka I., et al., Glucose triggers ATP secretion from bacteria in a growth-phase-dependent manner. Appl. Environ. Microbiol. 79, 2328–2335 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwase T., et al., Isolation and identification of ATP-secreting bacteria from mice and humans. J. Clin. Microbiol. 48, 1949–1951 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mempin R., et al., Release of extracellular ATP by bacteria during growth. BMC Microbiol. 13, 301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Virgilio F., Dal Ben D., Sarti A. C., Giuliani A. L., Falzoni S., The P2X7 receptor in infection and inflammation. Immunity 47, 15–31 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Furuta Y., et al., E-NPP3 controls plasmacytoid dendritic cell numbers in the small intestine. PLoS One 12, e0172509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusu T., et al., Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J. Immunol. 190, 774–783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Németh T., Sperandio M., Mócsai A., Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 19, 253–275 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Honda M., Kubes P., Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat. Rev. Gastroenterol. Hepatol. 15, 206–221 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Papayannopoulos V., Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Caër C., Wick M. J., Human intestinal mononuclear phagocytes in health and inflammatory bowel disease. Front. Immunol. 11, 410 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bressenot A., et al., Comparing histological activity indexes in UC. Gut 64, 1412–1418 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg L., et al., Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin. Gastroenterol. Hepatol. 11, 991–996 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Souza H. S., Fiocchi C., Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13, 13–27 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Zigmond E., et al., Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Zhou J., et al., Targeting EZH2 histone methyltransferase activity alleviates experimental intestinal inflammation. Nat. Commun. 10, 2427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y. F., et al., Ficolin-A/2, acting as a new regulator of macrophage polarization, mediates the inflammatory response in experimental mouse colitis. Immunology 151, 433–450 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X., Liu B., Sartor R. B., Jobin C., Phosphatidylinositol 3-kinase-γ signaling promotes Campylobacter jejuni-induced colitis through neutrophil recruitment in mice. J. Immunol. 190, 357–365 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bain C. C., et al., Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schippers A., et al., β7-Integrin exacerbates experimental DSS-induced colitis in mice by directing inflammatory monocytes into the colon. Mucosal Immunol. 9, 527–538 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makowski L., Chaib M., Rathmell J. C., Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 295, 5–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Egervari G., Wang Y., Berger S. L., Lu Z., Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishton R. J., Sukumar M., Restifo N. P., Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 26, 94–109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachmandas E., et al., Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat. Microbiol. 2, 16246 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Luu M., Visekruna A., Short-chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 49, 842–848 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Kornberg M. D., et al., Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360, 449–453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casili G., et al., Dimethyl fumarate reduces inflammatory responses in experimental colitis. J. Crohn’s Colitis 10, 472–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X., et al., Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem. Pharmacol. 112, 37–49 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Fausther M., et al., Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G785–G795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robson S. C., Sévigny J., Zimmermann H., The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2, 409–430 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atarashi K., et al., ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Nemoto Y., et al., Negative feedback regulation of colitogenic CD4+ T cells by increased granulopoiesis. Inflamm. Bowel Dis. 14, 1491–1503 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Layhadi J. A., Turner J., Crossman D., Fountain S. J., ATP evokes Ca2+ responses and CXCL5 secretion via P2X4 receptor activation in human monocyte-derived macrophages. J. Immunol. 200, 1159–1168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledderose C., et al., Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J. Clin. Invest. 128, 3583–3594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao Y., et al., Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J. Biol. Chem. 289, 26794–26803 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadiku P., et al., Prolyl hydroxylase 2 inactivation enhances glycogen storage and promotes excessive neutrophilic responses. J. Clin. Invest. 127, 3407–3420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaillon S., et al., Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 20, 485–503 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Gordon S., Taylor P. R., Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Proietti M., et al., ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat. Commun. 10, 250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perruzza L., et al., T follicular helper cells promote a beneficial gut ecosystem for host metabolic homeostasis by sensing microbiota-derived extracellular ATP. Cell Rep. 18, 2566–2575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahlfuss S., et al., STIM1-mediated calcium influx controls antifungal immunity and the metabolic function of non-pathogenic Th17 cells. EMBO Mol. Med. 12, e11592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaeth M., et al., Store-operated Ca2+ entry controls clonal expansion of T cells through metabolic reprogramming. Immunity 47, 664–679.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walmsley S. R., et al., Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med. 201, 105–115 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soto-Heredero G., Gómez de Las Heras M. M., Gabandé-Rodríguez E., Oller J., Mittelbrunn M., Glycolysis–A key player in the inflammatory response. FEBS J. 287, 3350–3369 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jun H. S., Weinstein D. A., Lee Y. M., Mansfield B. C., Chou J. Y., Molecular mechanisms of neutrophil dysfunction in glycogen storage disease type Ib. Blood 123, 2843–2853 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pålsson-McDermott E. M., O’Neill L. A. J., Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 30, 300–314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sedghi S., et al., Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut 34, 1191–1197 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lampinen M., Sangfelt P., Taha Y., Carlson M., Accumulation, activation, and survival of neutrophils in ulcerative colitis: Regulation by locally produced factors in the colon and impact of steroid treatment. Int. J. Colorectal Dis. 23, 939–946 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Hanai H., et al., Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: A prospective, uncontrolled, pilot study. Clin. Gastroenterol. Hepatol. 1, 28–35 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Brannigan A. E., et al., Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock 13, 361–366 (2000). [DOI] [PubMed] [Google Scholar]
- 55.McCarthy D. A., Rampton D. S., Liu Y. C., Peripheral blood neutrophils in inflammatory bowel disease: Morphological evidence of in vivo activation in active disease. Clin. Exp. Immunol. 86, 489–493 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene data have been deposited in the National Center Biotechnology Information Gene Expression Omnibus (GSE160379, GSE160380, and GSE160381). All other study data are included in the article and/or SI Appendix.