Significance
Restoration of postinjury brain function is a signal neuroscience challenge. Animal models of stroke recovery demonstrate time-limited windows of heightened motor recovery, similar to developmental neuroplasticity. However, no equivalent windows have been demonstrated in humans. We report a randomized controlled trial applying essential elements of animal motor training paradigms to humans, to determine the existence of an analogous sensitive period in adults. We found a similar sensitive or optimal period 60 to 90 d after stroke, with lesser effects ≤30 d and no effect 6 mo or later after stroke. These findings prospectively demonstrated the existence of a sensitive period in adult humans. We urge the provision of more intensive motor rehabilitation within 60 to 90 d after stroke onset.
Keywords: stroke rehabilitation, stroke, critical period, neuronal plasticity, time factors
Abstract
Restoration of human brain function after injury is a signal challenge for translational neuroscience. Rodent stroke recovery studies identify an optimal or sensitive period for intensive motor training after stroke: near-full recovery is attained if task-specific motor training occurs during this sensitive window. We extended these findings to adult humans with stroke in a randomized controlled trial applying the essential elements of rodent motor training paradigms to humans. Stroke patients were adaptively randomized to begin 20 extra hours of self-selected, task-specific motor therapy at ≤30 d (acute), 2 to 3 mo (subacute), or ≥6 mo (chronic) after stroke, compared with controls receiving standard motor rehabilitation. Upper extremity (UE) impairment assessed by the Action Research Arm Test (ARAT) was measured at up to five time points. The primary outcome measure was ARAT recovery over 1 y after stroke. By 1 y we found significantly increased UE motor function in the subacute group compared with controls (ARAT difference = +6.87 ± 2.63, P = 0.009). The acute group compared with controls showed smaller but significant improvement (ARAT difference = +5.25 ± 2.59 points, P = 0.043). The chronic group showed no significant improvement compared with controls (ARAT = +2.41 ± 2.25, P = 0.29). Thus task-specific motor intervention was most effective within the first 2 to 3 mo after stroke. The similarity to rodent model treatment outcomes suggests that other rodent findings may be translatable to human brain recovery. These results provide empirical evidence of a sensitive period for motor recovery in humans.
Restoration of human brain function after anatomic injury is a challenge to current neuroscience. Importantly, findings in animal models suggest that there are short-lived phenomena after stroke in mature animals that are similar to early developmental periods of heightened neural plasticity (1–6). Within days after a neuronal injury, rats show time-sensitive increases in dendritic branching peaking 2 to 3 wk postinjury (7), followed by a behavior-dependent pruning of newly formed dendritic arbors (8, 9). Intensive motor training provided to rodents within such periods has been shown to restore full motor function (1, 5, 10). However, there has previously been no direct evidence of similar time-limited responsiveness to intensive motor training in human adults. Here we present such evidence: a randomized controlled trial supporting the existence of such a sensitive period for motor recovery after stroke in human patients.
Restoration of human brain function after a neuroanatomical injury like stroke is a pressing challenge not only for scientific reasons but also for its profound clinical significance. Americans suffer a stroke once every 40 s, with ∼750,000 new strokes each year (11). Two-thirds of the individuals with stroke do not recover the necessary upper extremity (UE) function for usual activities by 6 mo, when motor recovery typically plateaus (12–14). To date the majority of randomized stroke rehabilitation trials have demonstrated limited efficacy in motor recovery (15–26).
Importantly, findings from rodent models of stroke suggest that there may exist brief, time-sensitive phenomena when the poststroke circuitry is especially responsive to training. Forelimb motor training during these preferred time windows is associated with nearly full recovery of forelimb function compared with animals given the same motor training outside the window (1, 5, 10). Biernaskie et al. (1) randomized lesioned rodents to receive focused forelimb motor training at 5, 14, or 30 d after injury. They found the best response to training started at 5 d postlesion, an intermediate response occurred at 14 d, and training started at 30 d showed no difference compared with controls. Purposeful forelimb activity is an important component of this time-sensitive recovery: Animals housed in enriched environments that encouraged exploratory forelimb movements demonstrated superior functional recovery compared with animals housed in standard cages after stroke (5). Zeiler et al. (10) recently demonstrated that mice given a second ischemic lesion to the medial premotor cortex show nearly full recovery after previously incomplete forelimb motor recovery from a primary ischemic lesion to the caudal forelimb area. The second lesion was hypothesized to reopen a neuroplastic window closed after the first lesion. Analogous phenomena have not yet been demonstrated in human adults after stroke. In this paper we will present findings that demonstrate the existence of a poststroke sensitive period in adult humans.
These time-sensitive improvements in motor function after stroke have also been linked to a period of heightened physiological neuroplasticity, similar to the critical or sensitive periods observed in juvenile circuitry at various times in the developing nervous system (27–30). A critical or sensitive period, which typically manifests in the early developing nervous system, is a time-limited window of heightened neuroplasticity when neural circuits are highly malleable and especially responsive to external stimuli, which can shape the resulting connectivity patterns of the circuitry (28, 31–33). After stroke to the motor system in rodents a similar sensitive period is thought to be triggered in perilesional tissue by the cascade of events that occur from the neural injury, beginning with the responses of surrounding cells and then the loss of input to the connected neurons beyond the infarct (29, 30, 34–37). Changes in the perilesional region are hypothesized to create the cellular and molecular context (37–40) in which new or enhanced neural connections and therefore new pathways for motor control can be produced. However, this occurs only if training and practice, reinforcing the appropriate signals for effective motor behavior, takes place simultaneously. Importantly, training before or after a receptive neural context is in place may have no functional effect (1). Indeed, intensive training too soon may result in lesion expansion (41, 42) and reduce benefits of subsequent recovery in rodents (43). Similar negative effects of training too early have been reproduced in human stroke trials (16, 17). In the absence of training, no connections or dysfunctional connections may develop (8, 44, 45).
It may be surprising to suggest sensitive periods that are manifest in adult circuitry, since most well-known critical periods occur during early development (33, 46–50). However, there are examples of the adult neural circuits showing opening or reopening of critical periods as a result of environmental and epigenetic triggers. For example, bonding in sheep occurs in mother ewes within 2 to 4 h (up to 24 h) of giving birth; during this brief period (but not before or after) the ewe will permit a lamb to nuzzle and she will become imprinted on its smell (51, 52). Chronic administration of fluoxetine in adult rats reopens the ocular dominance plasticity and leads to recovery of adult amblyopia (53). Valproate has been demonstrated to reopen critical period-like learning in human adults for absolute pitch discrimination (54), which ordinarily occurs only during early development (55, 56). Several recent studies have demonstrated that histone acetylation in the adult brain, through epigenetic remodeling of the chromatin structure, reopens critical period-like plasticity in the adult visual cortex (57–59). Of note, exposure of adult animals to enriched environments (60) reopens critical period plasticity in adult rats through epigenetic modifications triggered by the histone acetylation mechanism.
The Present Study
The Critical Periods After Stroke Study (CPASS) is a phase II, randomized, controlled trial designed to test if a critical or sensitive period exists after stroke during which patients are particularly responsive to intensive motor training. If a critical or sensitive period is present, then intensive motor therapy during this period should lead to superior and sustained UE motor outcomes, as compared with the same amount of intensive motor therapy provided at other times. In the United States, nearly all patients receive standard rehabilitation therapies; these constitute a barrier to experimental manipulation because of resistance from institutional review boards and attitudes and beliefs of clinicians and patients. Our experimental study-related treatment, provided to participants beyond their standard rehabilitation therapy, was devised to standardize treatment procedure and intensity across groups regardless of treatment location (hospital or home). This bolus of additional motor training will hereafter be referred to as intensive motor therapy. As described in detail in Materials and Methods, it consisted of 20 h of UE motor training based on a standardized yet individualized shaping protocol. We hypothesized that, compared with individuals randomized to the control condition or to the subacute (2 to 3 mo after onset) or chronic (6 to 9 mo after onset) time points, persons randomized to the early (acute) time point would show greater UE motor improvement as measured by the Action Research Arm Test (ARAT) (61) assessed during 1 y poststroke. However, calculating this outcome effect in humans is somewhat more complex than assessing an effect at a single time point in rodents. While time of intensive training and measuring outcomes can be precise when administered in the laboratory, in humans the assessment at time of stroke, provision of training, and assessment at 6 and 12 mo may occur only approximately at the desired time points, due to the availability of the patients and their other life circumstances. In addition, with the smaller effects expected in humans (who receive training in addition to standard care) as compared with rodents (from whom we can withhold treatment in the controls), we need to carefully consider the differences between groups in how much improvement remains possible in the types of movements assessed by the ARAT before hitting the ARAT ceiling. Our analysis is therefore not of the raw ARAT score at the 1-y time point after stroke—which would be confounded by all of these other variables—but instead is of each individual patient’s raw ARAT score improvement over the year, taking into account the starting ARAT score at randomization, the precise days at which the starting assessment, treatment, 6-mo, and 12-mo assessments occurred, and the proportion of remaining improvement that was possible on the ARAT scale. These variables are taken into account through a longitudinal unbalanced repeated measures analysis that is explained in more detail in Materials and Methods and in SI Appendix.
The overall goal for the CPASS trial was primarily to elicit a timing signal for critical period plasticity in adult humans recovering from stroke. If a critical period was identified, then future studies can optimize the content and dose of intensive motor therapy needed for maximum recovery, thus best improving clinical outcomes in persons with stroke.
Results
Study Participants and Baseline Characteristics.
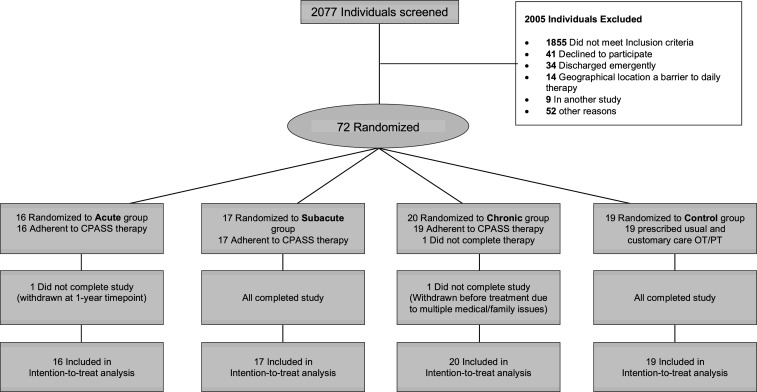
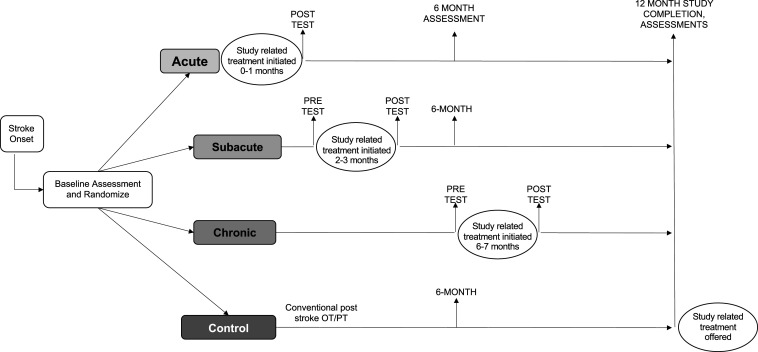
Table 1 presents the study inclusion and exclusion criteria. We screened 2,077 patients admitted for stroke rehabilitation at MedStar National Rehabilitation Hospital between November 2014 and October 2018 in order to enroll 72 participants. Participants were adaptively randomized to the acute (n = 16), subacute (n = 17), chronic (n = 20), or control (n = 19) groups. Fig. 1 presents the CONSORT diagram. Fig. 2 illustrates the flow of patients through the study. Seventy of the 72 enrolled participants completed the primary outcome assessment at 12 mo. Table 2 shows the key participant demographics and study group baseline characteristics.
Table 1.
CPASS inclusion and exclusion criteria
| Inclusion criteria |
| 1. Ischemic or hemorrhagic stroke (with confirmatory neuroimaging) within 28 d of admission to inpatient rehabilitation |
| 2. Age ≥21 y |
| 3. Able to participate in first study-related treatment session within 30 d of stroke onset |
| 4. Able to participate in all study-related activities, including 1-y follow up and blood draws |
| 5. Persistent hemiparesis leading to impaired UE function as indicated by a score ≥1 on the NIHSS motor arm score, and motor impairment judged clinically appropriate as defined by one or more of the following: |
| a. Proximal UE voluntary activity indicated by a score of ≥3 on the upper arm item of the motor assessment scale; wrist and finger movements are not required |
| b. Manual muscle test (MMT) score ≥2 on shoulder flexion and either elbow flexion or extension or |
| c. Active range of motion (AROM) to at least 50% of range in gravity eliminated position for shoulder flexion or abduction, and for any of the following motions: elbow flexion, elbow extension, wrist flexion, wrist extension, finger flexion or finger extension |
| 6. Score of ≤8 on the Short Blessed Memory Orientation and Concentration scale |
| 7. Follows two-step commands |
| 8. No UE injury or conditions that limited use prior to the stroke |
| 9. Prestroke independence: Modified Rankin Scale score of 0 or 1 |
| Exclusion criteria |
| 1. Inability to give informed consent |
| 2. Prior stroke with persistent motor impairment or other disabling neurologic conditions such as multiple sclerosis, Parkinsonism, ALS, dementia requiring medication |
| 3. Rapidly evolving motor function |
| 4. Clinically significant fluctuations in mental status in the 72 h prior to randomization |
| 5. Hemispatial neglect as determined by an asymmetry >3 errors on the Mesulam symbol cancellation test |
| 6. Not independent prior to stroke (determined by scores of <95 on Barthel Index or >1 on modified Rankin scale |
| 7. Dense sensory loss indicated by a score of 2 on NIHSS sensory item |
| 8. Ataxia out of proportion to weakness in the affected arm as defined by a score ≥1 on the NIHSS limb ataxia item |
| 9. Active or prior (within 2 y) psychosis |
| 10. Active or prior (within 2 y) substance abuse |
| 11. Not expected to survive 1 y due to other illnesses (cardiac disease, malignancy, etc.) |
| 12. Received UE botulinum toxin within 6 mo (other medications do not exclude) |
Fig. 1.
CONSORT diagram for the CPASS trial. Seventy-two individuals were adaptively randomized to receive CPASS therapy acute (≤30 d poststroke), subacute (2 to 3 mo), chronic (≥6 mo poststroke), or the control group.
Fig. 2.
Study design. Baseline assessment occurred <30 d from stroke onset, and participants were randomized to one of four groups: acute, received additional 20 h of therapy initiated within 30 d from stroke onset; subacute, received additional 20 h initiated within 2 to 3 mo from stroke onset; chronic, received additional 20 h 6 to 7 mo after onset; controls, received standard rehabilitation. Adapted from ref. 100, which is licensed under CC BY 4.0.
Table 2.
Demographic characteristics and scores on baseline study measures (n = 72) shown by group
| Total sample (n = 72) | Acute (n = 16) | Subacute (n = 17) | Chronic (n = 20) | Control (n = 19) | |
| Age, y | 62.8 ± 11.5 | 61.8 ± 11.3 | 63.9 ± 10.8 | 67.3 ± 9.8 | 58 ± 12.6 |
| Sex (female) | 36 (50) | 11 (68.8) | 6 (35.2) | 11 (55) | 8 (42.1) |
| Race | |||||
| Caucasian | 10 (13.9) | 1 (6.3) | 2 (11.8) | 5 (25) | 2 (10.5) |
| African American | 60 (83.3) | 13 (81.3) | 15 (88.2) | 15 (75) | 17 (89.5) |
| American Indian, Alaskan | 0 | 0 | 0 | 0 | 0 |
| Asian | 1 (1.4) | 1 (6.3) | 0 | 0 | 0 |
| Native Hawaiian, Pacific Islander | 1 (1.4) | 1 (6.3) | 0 | 0 | 0 |
| Dominant UE affected | 33 (45.8) | 9 (56.2) | 8 (47) | 9 (45) | 7 (36.8) |
| Stroke type | |||||
| Ischemic | 69 (95.8) | 16 (100) | 17 (100) | 17 (85) | 19 (100) |
| Hemorrhagic | 3 (4.2) | 0 | 0 | 3 (15) | 0 |
| Total NIHSS | 4.9 ± 1.7 | 4.9 ± 1.9 | 4.9 ± 2.1 | 4.6 ± 1.5 | 5.3 ± 1.6 |
| Total ARAT | 15.8 ± 13.8 | 16.8 ± 16.2 | 13.4 ± 11.4 | 20.3 ± 15.7 | 12.3 ± 10.5 |
| Days from stroke onset to randomization | 15.4 ± 4.5 | 15.6 ± 4 | 14.8 ± 4.6 | 15.3 ± 4.4 | 16.1 ± 5 |
| Hours of study-specific therapy received | 19.7 ± 1.7 | 18.8 ± 2.9 | 20 ± 0.3 | 20.2 ± 0.7 | — |
Baseline demographics of study participants per group. Numbers in parentheses indicate percentages. Categorical variables are shown as counts and percentages; continuous variables are described using means and SDs. Controls received standard rehabilitation; therefore controls’ “hours of study-specific therapy received” is empty.
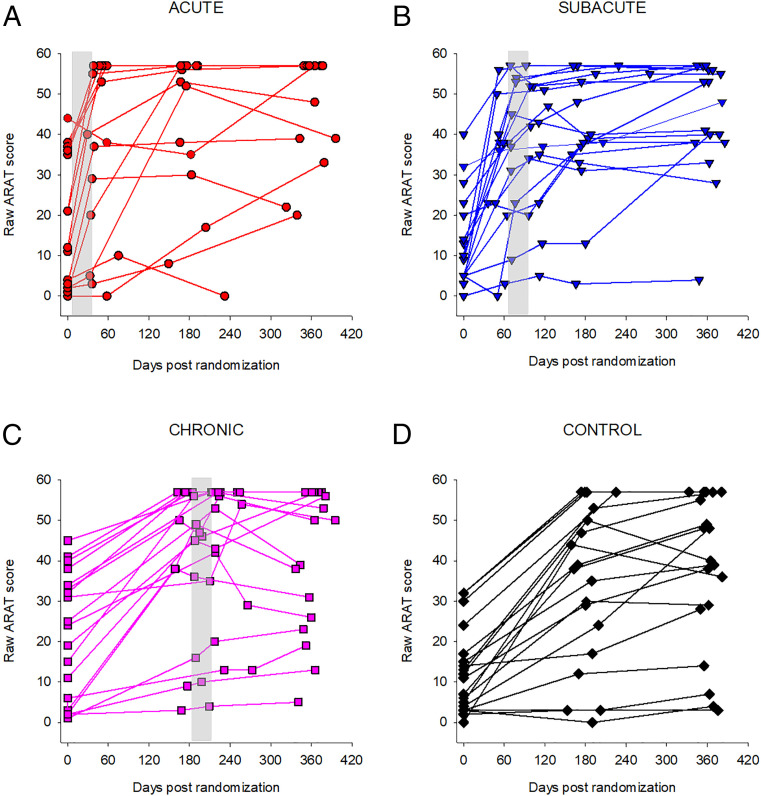
There were no significant differences among the treatment groups for any of the demographic variables. Consistent with the general population of patients admitted to MedStar National Rehabilitation Hospital and the population of Washington, DC, the CPASS sample was predominantly African American (83.3%) and 50% female. The mean age of the participants was 62.8 ± 11.5 y. Most of the participants had ischemic strokes with a mean National Institutes of Health Stroke Scale (NIHSS) score of 4.9 ± 1.7. The mean time from stroke onset to randomization was 15.4 ± 4.5 d. The dominant side of the body was affected in 45.8% of the participants. Individual motor recovery trajectories throughout the 12-mo study duration, measured using ARAT on the affected UE, are shown in Fig. 3. SI Appendix, Table S1 shows the study measures applied at different time points throughout the study.
Fig. 3.
(A–D) Individual trajectories of raw ARAT scores posttroke, by treatment group. Vertical gray bars show average timing of the intervention in each group.
Significant Effect of Timing the Therapy Manipulation Early after Stroke: Subacute and Acute Groups Improve Significantly More than Controls.
A longitudinal (unbalanced repeated measures) model computed by the GEE (generalized estimating equations) was utilized for data analysis to test differences in overall UE recovery of the four groups between baseline and the 12-mo time point. An unbalanced repeated measures model is necessary to accommodate missing data and the actual times of measurement around the original preset assessment schedule (62). Unbalanced designs have become the rule rather than the exception in human clinical trials, as patient follow-ups rarely occur at exactly planned, equally spaced time points (63–65). This analytic approach was selected because it uses all available data for each subject and allows specification of both time-varying and individual difference variables within the same analysis (66, 67). The longitudinal model, which focuses on average changes in response over time and the impact of covariates on these changes, was used to determine whether additional motor training introduced at different time points after stroke affected UE motor recovery. Longitudinal ARAT scores from the four groups were used to estimate the overall UE motor recovery between baseline and the 12-mo time point. These scores were adjusted for baseline ARAT scores and days since stroke onset to the baseline evaluation.
Fig. 3 shows the individual participants’ trajectories in raw total ARAT scores poststroke, organized by treatment group. ARAT scores in the chronic and the control groups at 6 mo and 12 mo are spread throughout the ARAT score range, suggesting relatively poor recovery for many individuals. In contrast, subacute group scores at 6 mo tend to cluster in the top half of the ARAT score range and do so even more at 12 mo.
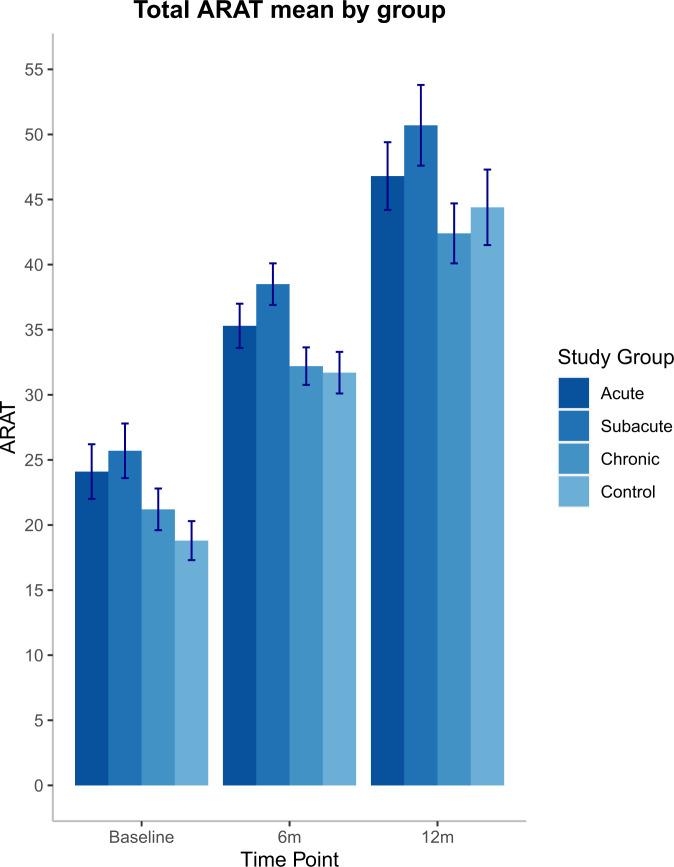
Fig. 4 presents the mean total ARAT scores and SEs for each group over time from the longitudinal model, computed using the GEE method, as is required for appropriate analysis due to the unequal spacing of assessments and training (these data along with ARAT scores from pre- and posttreatment are shown in SI Appendix, Fig. S1). These group means bear out what can be seen in the individual trajectories in Fig. 3. The subacute group (given 20 extra therapy hours initiated 2 to 3 mo poststroke) improved significantly more than the control group (difference between subacute and control ARAT = +6.87 ± 2.63 points, P = 0.009). Similarly, the acute group (20 extra therapy hours initiated within the 30-d poststroke window) showed significantly more improvement than the controls (difference in ARAT between acute and control groups = +5.25 ± 2.59 points, P = 0.043). In contrast, the chronic group (20 extra therapy hours initiated at ≥6 mo poststroke) showed no significant difference compared with controls (difference in ARAT = +2.41 ± 2.25, P = 0.29). Baseline ARAT (P < 0.001) and number of days from stroke onset to randomization (P = 0.003) were other significant predictors of the mean ARAT score.
Fig. 4.
Mean total ARAT scores (with SEs) for each group at each time point, from the longitudinal model. (The 6-mo assessment score for the chronic group is their pretreatment assessment.)
Notably, the improvement in the subacute group was greater than the minimal clinically important difference (MCID) for mean ARAT scores compared with the control group (ARAT MCID = 5.7 points) (68, 69). Thus, the increase in arm function recovery was not only statistically significant but the magnitude of improvement was large enough to be perceived as functionally meaningful by the subacute group participants. The acute group improvement was statistically significant but did not achieve the magnitude needed to meet MCID criteria. The magnitude of additional recovery in the chronic group was not statistically significant and did not exceed the ARAT MCID.
Discussion
CPASS is the first early stroke rehabilitation trial in humans to demonstrate superior recovery of UE motor function in an experimental group (at 2 to 3 mo after and at 30 d) compared with a control group receiving traditional occupational and physical therapy at 12 mo. The same effect was not observed when additional therapy was initiated 6 mo poststroke. The methodology of this study was carefully designed to determine whether human responsiveness is analogous to rodent responses when the study elements were matched as closely as possible without substantially disrupting the standard of care. Our study goal was met: Under rigorously controlled conditions for a human trial in a realistic clinical setting we are able to present strong evidence for what appears to be, indeed, a critical or sensitive period in human stroke recovery. We showed that study-specific UE motor rehabilitation and time poststroke combine synergistically during a time-limited window to produce enhanced UE motor recovery after stroke. Similar gains in recovery were absent when the same motor rehabilitation was provided at a later time. Moreover, we have shown that such intensive motor training is not only effective but is also feasible within current US acute and subacute rehabilitation treatment environments.
There are several important findings from this trial. First, study-specific intervention in the subacute phase (2 to 3 mo poststroke) led to significantly greater UE motor recovery compared with controls. Second, the acute group (≤30 d poststroke) showed smaller but statistically significant improvement over controls. Third, the same intervention provided later, at ≥6 mo to the chronic group, showed no significant differences compared with controls. Fourth, the early gains in motor recovery observed in the subacute and acute groups were sustained long-term for at least up to 12 mo after stroke. Finally, subacute group gains in UE motor recovery were large enough in magnitude to meet the published criteria for meaningful clinical improvement on the measure.
What is the clinical meaning of these improved motor skills? Clinical trialists use the MCID as a means of determining the effectiveness of the intervention on domains of interest to the person. The MCID has been defined as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient’s management” (68). In this study, the MCID (68, 70–72) refers to improved ability of the participants to execute functional movements associated with performance of activities of daily living requiring hand and arm use. The subacute group achieved improvement in UE function that is greater than the ARAT MCID. Although the acute group showed statistically significant increase in ARAT compared with controls, the acute group’s scores narrowly missed the MCID threshold. Although most of our participants did not achieve a full resolution of their motor impairment, our results are consistent with recent rodent motor recovery findings, which also demonstrate residual forelimb motor impairments. Rodent studies create precise reproducible brain lesions; this differs from the clinical situation in human trials, where the lesions are more heterogeneous (73), perhaps also accounting for some of the differences in degree of outcome. CPASS data, by demonstrating the presence of a critical period poststroke in the clinical trial context, opens several avenues to expand the science of poststroke motor recovery in adult humans poststroke.
It is also important to note that, while the effects of this study may appear to be small and do not restore our patients to full motor function, they are extremely important in our field. Few UE rehabilitation trials have demonstrated an intervention effect that is bigger than the CPASS primary outcome’s MCID (15–26). This makes the effect size of our critical-period-dependent improvements in motor function especially significant for translating animal data into meaningful UE recovery in humans poststroke. Comparable UE stroke rehabilitation trials have demonstrated limited efficacy. For instance, the VECTORS phase II trial (16) enrolled participants at 9.6 ± 4.5 d (compared with 15.4 ± 4.5 d in CPASS); VECTORS reported no significant difference between its two groups at 90 d. Thus, the VECTORS “low-dose group” receiving 2 h/d of rehabilitation with restraint of the unaffected hand in a mitt for 60% of the waking hours (to encourage use of the affected hand) did no better than their control group receiving traditional rehabilitation of the same intensity as their low-dose group. Both the VECTORS and AVERT (17) trials showed poorer outcomes with very early higher-intensity motor training. The ICARE phase III RCT (15) compared two different treatment strategies, structured task-oriented UE training and an intensive high-repetition training, compared with a control group receiving conventional occupational therapy. ICARE reported no significant differences between groups at 12 mo poststroke. The primary exception is the EXCITE phase III trial (74–76) showed superior UE motor recovery at 12 mo in an “early” group receiving constraint-induced movement therapy (CIMT) 3 to 9 mo poststroke compared with controls receiving conventional rehabilitation. The “early” group’s treatment timing (3 to 9 mo) in EXCITE was slightly later than CPASS’ subacute intervention time (2 to 3 mo) and overlaps some with our chronic group’s intervention timing (>6 mo poststroke), which showed no difference compared with controls in CPASS. EXCITE trial’s early group improvements may, however, be dose-related, not linked to a critical period, because in a follow-up study the EXCITE investigators demonstrated that the same intensity of CIMT provided to their controls after 12 mo led to the same motor improvement in controls as in their experimental early group (3 to 9 mo poststroke) in the first study. It is likely that in the EXCITE study the intensity of CIMT dose compared with traditional outpatient rehabilitation led to the superior motor recovery in the early group. The EXCITE trial used a cross-over design, wherein the control group was provided CIMT similarly intense as that of the early group, but at a much later time point (at 12 mo) they showed similar improvement in function. In contrast, CPASS improvements are time-sensitive; the CPASS chronic group (>6 mo) receiving the same intervention but outside the preferred window of opportunity showed no significant improvement compared with CPASS controls. It is likely the poststroke critical period stretches beyond 3 mo but ends before 6 mo considering the EXCITE and CPASS findings. Follow-up studies are needed for finer determination of the boundaries of the poststroke critical period. The CPASS results, by demonstrating presence of critical period poststroke in a clinical context, open several avenues for the exploration of the science of poststroke motor recovery.
Clinical Evidence for a Poststroke Critical Period.
The heightened gains in UE motor recovery found here appear to result from a synergistic coupling between a specific window of time poststroke during the lesioned brain’s recovery and the type of motor rehabilitation focused on training functional UE movements that were of the highest priority to each individual person.
Typical inpatient rehabilitation sessions in the United States last ∼39 min/d for ∼12 d poststroke (77); on average, persons with stroke move their impaired UE 3.7 ± 3.1 h/d (78) at this poststroke time point. Outpatient rehabilitation sessions in the United States last ∼36 min/d with patients engaging in an average of 12 purposeful movements in an otherwise unstructured treatment session, continuing for a few weeks (79). CPASS added to this an innovative study-specific 20-h intervention that emphasized participant autonomy and intrinsic motivation coupled with task shaping and massed practice. This approach demonstrates that patients can tolerate much more intensive motor training than is traditionally provided. In addition, the finding of greatest benefit from additional therapy at the subacute phase (2 to 3 mo after stroke) warrants consideration of extending outpatient rehabilitation to this time period.
Given that we have shown that human recovery appears to have a critical period like that observed in the rodent model, we can also speculate that similar physiological processes may underpin the enhanced recovery observed in our subacute and acute participant groups. The enhancement effect of the task-specific training in rodents is constrained around optimal times poststroke (1, 10) that physiologically are associated with strong increases in synaptic activity in the perinfarct regions, greater dendritic branching and complexity (5), and stability of the newly formed dendritic spines in line with behavioral recovery of forelimb function over time (80). These processes are similar to the effects of purposeful activity working synergistically to achieve optimal plasticity in other domains, for example in sensorimotor integration during locomotion in rodents (81), auditory map plasticity in adult barn owls (82), and in language acquisition (83, 84). In adult humans with amblyopia, 40 to 80 h of video-game training leads to substantial improvement of visual function in amblyopia (85) compared with the conventional eye patching alone.
CPASS findings suggest optimal periods for intensive motor training when UE motor rehabilitation interventions are most effective. Our results point to the subacute period. However, it is possible that there is a point in the acute period that is equally effective. Unfortunately, our design did not allow such resolution. Our findings diverge somewhat from the rodent model where animals treated earlier (but not too early) tend to have the best recovery. This discordance might be attributed to the differences between rodent and human brains as well as to the social and emotional forces at play in humans who must accommodate the sudden and unanticipated changes associated with stroke. It should also be noted that CPASS was a phase II trial; a larger multicenter trial is needed to extend the findings and confirm the superiority of the subacute treatment window.
Conclusions and Future Directions.
CPASS provides strong evidence for a sensitive or critical period in human brain recovery and confirms the relevance of rodent models to the study of stroke recovery in humans. Our results also have important implications for the redesign of optimal stroke rehabilitation programs, which we believe can be done within the constraints of existing clinical care systems. There remains a series of questions that are crucial to be addressed in subsequent studies. First, a more precise definition of the timing and duration of the sensitive or critical period is needed. Evidence in both rodents and humans suggests that intensive training too soon after injury can reduce recovery (16, 17) and possibly even increase the size of the lesion (41, 42, 86), and training too late after injury provides no additional benefit (1, 87). Second, more information is needed about the interaction between the timing of treatment and treatment intensity. The goal of the CPASS study was to elicit a signal for the existence of a sensitive period and not to determine the optimal dose of motor training within these treatment periods. It is quite possible that the most effective treatment dose varies within the sensitive period and that higher or lower doses of intensive motor treatment may further optimize UE outcomes. Now that the optimal time period for intensive motor treatment is known, a focused study of treatment dose can be conducted.
Moreover, it is possible that time and treatment dose may interact in more complex ways within the two early time periods. In the present results we found a signal for plasticity in the acute group that was not as robust as in the subacute period. A lower training dose might have enhanced outcomes in the acute group, consistent with the literature on deleterious effects of intensive therapy provided “too early” in animal models (16, 41, 42, 86) and human stroke (16, 17). In contrast, a higher dose of therapy might be useful in the chronic phase to determine if residual plasticity can be harnessed when recovery seems to have plateaued (14). A subsequent dose-defining trial can thus accelerate our understanding of critical period plasticity and also provide definitive neurorehabilitation guidelines in the restoration of UE motor function across the recovery timeline of stroke. Future stroke rehabilitation trials should also include neurophysiologic and imaging measures in acute, subacute, and chronic periods to elucidate the mechanisms at the cellular and molecular level involved in critical period like neuroplasticity in adult humans poststroke. This information is essential to the development of future treatment modalities, which might include neurostimulation, growth factors, or cellular replacement therapies that could amplify the effects of intensive motor training. In the future, a further understanding of the mechanisms underlying these timing effects can allow us to extend and enhance the treatment timeline, with the ultimate aim of producing full motor recovery in human patients.
Materials and Methods
The trial was approved by the Institutional Review Board at MedStar National Rehabilitation Hospital/MedStar Health Research Institute (protocol no. 2014-065). All study participants provided informed consent prior to engaging in study procedures. Fig. 2 shows the study design.
Participants.
Screening and recruitment.
Persons with stroke were primarily recruited from the inpatient stroke service at the MedStar National Rehabilitation Hospital, the Washington, DC site for the National Institute of Neurological Disorders and Stroke StrokeNet, and DC area hospitals (MedStar Washington Hospital, MedStar Georgetown University Hospital, and peripheral MedStar hospitals). Participants were screened via daily inpatient hospital admission logs and approached for recruitment to the study within the first few days of admission. Participants provided informed consent prior to randomization. To preclude severe cognitive or sensory impairments participants were excluded for hemispatial neglect as determined by an asymmetry of more than three errors on the Mesulam Symbol Cancellation Test (88) and a score of ≥8 on the Short-Blessed Memory Orientation and Concentration scale (89). Full trial inclusion and exclusion criteria are listed in Table 1. Fig. 2 shows the participant flow through the study.
Randomization.
Participants were adaptively randomized (90) into one of four study groups (acute, subacute, chronic, or control) by the study statisticians after informed consent and baseline assessment data were entered into a database. Study groups were balanced with respect to participant age, number of days from stroke onset to baseline evaluation, stroke type (ischemic versus hemorrhagic), baseline ARAT score, concordance (whether the dominant or nondominant UE is affected), and overall stroke severity measured using the NIHSS (91) at baseline. The adaptive randomization we developed was based on methods described by Meinert (92), Signorini et al. (93), and Atkinson (94). This scheme was created to prevent imbalances while avoiding the problems of small numbers within blocks that stratification can produce.
Study Measures.
The primary outcome measure was the ARAT (61, 95). The ARAT is a standardized assessment that evaluates functional limitations of the UE. It uses a 4-point ordinal scale of 19 items, where 0 indicates no movement and 3 indicates normal movement. Item scores are summed to create four subscale scores: grasp (18 point maximum), grip (12 point maximum), pinch (18 point maximum), and gross motor (9 point maximum). The total scale has a maximum score of 57, indicating normal UE performance (96). The ARAT has been shown to be reliable, valid, and responsive to change across a variety of time points poststroke (69, 96–99).
The ARAT and other secondary study measures were administered at baseline, pretreatment, posttreatment, 6-mo, and 1-y time points. The baseline, 6-mo, and 12-mo evaluations were standard for all treatment groups. However, the pre- and posttreatment assessments were linked to the start and completion of study treatments, which varied across the groups (see Fig. 2 for participant flow through the trial and SI Appendix, Fig. S1 for all assessments for each group). All study measures were administered by evaluators blinded to the treatment condition. SI Appendix, Table S1 outlines the various study measures at each evaluation time point from the study.
Intervention.
All participants received standard of care (both inpatient and outpatient) interdisciplinary rehabilitation treatments as prescribed by their physician and clinical therapy teams. The CPASS intervention was modeled as closely as clinically feasible in the US system and ethically appropriate (100) to the Biernaskie et al. (1) rodent study. In addition to standard rehabilitation treatment that all participants received from their clinicians, we provided an additional 20 h of intensive UE motor training based on a standardized, yet highly individualized, shaping protocol adapted from VECTORS, our previous UE stroke recovery trial and recent stroke rehabilitation trials (15, 16, 75, 87, 101, 102). Unlike the VECTORS or EXCITE trials, no constraint was applied to the less-affected hand or arm. Since the goal of CPASS was to identify the timing of a potential critical period of increased responsiveness to activity-based therapy and elicit an enhanced and persistent motor response during that therapy, we did not explore optimal dosing for the training provided in the present trial (100). Based on our prior work, an additional 10 h of UE therapy was sufficient to alter motor outcomes (16). As a result, to ensure a signal (if one was present) we chose to deliver 20 h of additional UE therapy in a brief period of time to each participant to enhance poststroke motor recovery. The treatment was based on four fundamental principles of learning that guide rodent motor training trials as applied to humans: 1) massed practice (30), 2) intrinsic motivation (103–105), 3) detailed task-specific analysis and grading (106–108), and 4) positive reinforcement (109–112). Typically, therapy sessions ranged from 30 min (inpatient/acute group) to 3 h per session (out-patient/subacute and chronic groups). This training focused on the use of the more-affected UE in ADL and leisure tasks agreed upon by the patient and study therapist. The treatment was delivered in the inpatient rehabilitation setting (for those randomized to the early group) or outpatient clinic settings (for subacute and chronic groups) whenever possible. Adherence to CPASS therapy protocol was defined as at least 15 h of therapy within 42 d in the allocated group’s timeline.
Power Analysis, Sample Size Estimation, and Final Sample.
Before study initiation a power analysis was conducted to estimate the sample size needed to test the study hypotheses. The estimated study sample of 64 subjects (16 per study arm) was based on demonstrating a moderate effect size (0.425 as observed in preliminary data) in the primary endpoint, the ARAT at 1 y in an ANOVA model, with 80% power at a significance level of 5%. Persons with stroke with moderate UE motor impairment who demonstrated motor arm score ≥1 on the NIHSS were recruited. Individuals with terminal illness were excluded. An interim analysis with blinded data were conducted by the study’s principal investigator (A.W.D.) and statistician (M.T.T.). This analysis indicated that the treatment groups were imbalanced and an additional eight participants were recruited, randomized, and treated.
Statistical Analysis.
Demographic and baseline characteristics were summarized using mean ± SD, median (minimum, maximum) for continuous variables, and counts and percentages for categorical variables. A longitudinal model for unbalanced repeated measures computed by GEE with robust SE estimates (113, 114) was utilized to determine if timing therapy at different time points after stroke affects overall motor recovery during the course of 1-y follow-up, adjusting for baseline ARAT and days since stroke onset. Group means and SEs for total ARAT scores were estimated at baseline, preintervention, postintervention, and 6 mo and 12-mo poststroke. All analyses were performed with R software (115).
This repeated-measures model was chosen to allow unequally spaced measurements (differing numbers of days for individual patients between the baseline assessment and other assessment time points). Because this unequal spacing of measurements is a common feature of clinical trials in human subjects, it has been widely used in studies across multiple diseases from chronic to acute since the late 1980s (62–67). For example, it has been used in several large NIH-funded studies including the Framingham heart study, AIDS and HIV studies (64, 66), renal disease study (65), and T cell follow-up studies in myeloma (116) This model requires fitting using new computation tools, such as the GEE, that are not required in the more classic equally spaced repeated-measures data more common in preclinical studies. This repeated measures model is generally referred to as a longitudinal model in human subjects studies that involve measurements at unequally spaced follow-up times.
Supplementary Material
Acknowledgments
This work was supported by funds from the Center for Brain Plasticity and Recovery at Georgetown University and MedStar National Rehabilitation Hospital, NIH/National Institute of Neurological Disorders and Stroke StrokeNet SCANR Grant 5U24NS107222-03, NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development National Center for Medical Rehabilitation Research Grant K12HD093427, NIH/National Institute on Deafness and Other Communication Disorders Grant R01 DC016902, and by MedStar National Rehabilitation Hospital clinicians and study participants.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026676118/-/DCSupplemental.
Data Availability
Anonymized data have been deposited in Open Science Framework (https://osf.io/wrk3x/). To observe HIPAA protections for human subject information, click to request access to the dataset. Access will be granted by the corresponding author.
References
- 1.Biernaskie J., Chernenko G., Corbett D., Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J. Neurosci. 24, 1245–1254 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hensch T. K., Bilimoria P. M., Re-opening windows: Manipulating critical periods for brain development. Cerebrum 2012, 11 (2012). [PMC free article] [PubMed] [Google Scholar]
- 3.Reh R. K., et al., Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. U.S.A. 117, 23242–23251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takesian A. E., Hensch T. K., Balancing plasticity/stability across brain development. Prog. Brain Res. 207, 3–34 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Biernaskie J., Corbett D., Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J. Neurosci. 21, 5272–5280 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allred R. P., Cappellini C. H., Jones T. A., The “good” limb makes the “bad” limb worse: Experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav. Neurosci. 124, 124–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones T. A., Schallert T., Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 581, 156–160 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Schallert T., Jones T. A., “Exuberant” neuronal growth after brain damage in adult rats: The essential role of behavioral experience. J. Neural Transplant. Plast. 4, 193–198 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones T. A., Schallert T., Use-dependent growth of pyramidal neurons after neocortical damage. J. Neurosci. 14, 2140–2152 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeiler S. R., et al., Paradoxical motor recovery from a first stroke after induction of a second stroke: Reopening a postischemic sensitive period. Neurorehabil. Neural Repair 30, 794–800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virani S. S.et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee , Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 141, e139–e596 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Kwakkel G., Kollen B. J., van der Grond J., Prevo A. J., Probability of regaining dexterity in the flaccid upper limb: Impact of severity of paresis and time since onset in acute stroke. Stroke 34, 2181–2186 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen H. S., et al., Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 76, 406–412 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen H. S., Nakayama H., Raaschou H. O., Olsen T. S., Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys. Med. Rehabil. Clin. N. Am. 10, 887–906 (1999). [PubMed] [Google Scholar]
- 15.Winstein C. J.et al.; Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE) Investigative Team , Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: The ICARE randomized clinical trial. JAMA 315, 571–581 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dromerick A. W., et al., Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS): A single-center RCT. Neurology 73, 195–201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernhardt J.et al.; AVERT Trial Collaboration group , Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet 386, 46–55 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Lo A. C., et al., Robot-assisted therapy for long-term upper-limb impairment after stroke. N. Engl. J. Med. 362, 1772–1783 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yelnik A. P.et al.; AMOBES Group , AMOBES (Active Mobility Very Early After Stroke): A randomized controlled trial. Stroke 48, 400–405 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Kwakkel G.et al.; EXPLICIT-Stroke Consortium , Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: The EXPLICIT-Stroke randomized clinical trial. Neurorehabil. Neural Repair 30, 804–816 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Cramer S. C.et al.; National Institutes of Health StrokeNet Telerehab Investigators , Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: A randomized clinical trial. JAMA Neurol. 76, 1079–1087 10.1001/jamaneurol.2019.1604. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodgers H., et al., Robot Assisted Training for the Upper Limb after Stroke (RATULS): A multicentre randomised controlled trial. Lancet 394, 51–62 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saposnik G.et al.; Stroke Outcomes Research Canada , Efficacy and Safety of Non-Immersive Virtual Reality Exercising in Stroke Rehabilitation (EVREST): A randomised, multicentre, single-blind, controlled trial. Lancet Neurol. 15, 1019–1027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomeroy V. M., et al., Functional strength training versus movement performance therapy for upper limb motor recovery early after stroke: A RCT. Efficacy and Mechanism Evaluation 5, 1–112 (2018). [PubMed] [Google Scholar]
- 25.Brunner I., et al., Virtual Reality Training for Upper Extremity in Subacute Stroke (VIRTUES): A multicenter RCT. Neurology 89, 2413–2421 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Adie K., et al., Does the use of Nintendo Wii SportsTM improve arm function? Trial of WiiTM in stroke: A randomized controlled trial and economics analysis. Clin. Rehabil. 31, 173–185 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Hensch T. K., Critical period mechanisms in developing visual cortex. Curr. Top. Dev. Biol. 69, 215–237 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Hensch T. K., Critical period regulation. Annu. Rev. Neurosci. 27, 549–579 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Murphy T. H., Corbett D., Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 10, 861–872 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Kleim J. A., Jones T. A., Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 51, S225–S239 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Hensch T. K., Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Newport E. L., Bavelier D., Neville H. J., “Critical thinking about critical periods: Perspectives on a critical period for language acquisition” in Language, Brain and Cognitive Development: Essays in Honor of Jacques Mehler, Dupoux E., Ed. (MIT Press, 2001), pp. 481–502. [Google Scholar]
- 33.Knudsen E. I., Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 16, 1412–1425 (2004). [DOI] [PubMed] [Google Scholar]
- 34.MacLellan C. L., Langdon K. D., Botsford A., Butt S., Corbett D., A model of persistent learned nonuse following focal ischemia in rats. Neurorehabil. Neural Repair 27, 900–907 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Corbett D., Jeffers M., Nguemeni C., Gomez-Smith M., Livingston-Thomas J., Lost in translation: Rethinking approaches to stroke recovery. Prog. Brain Res. 218, 413–434 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Carmichael S. T., Wei L., Rovainen C. M., Woolsey T. A., New patterns of intracortical projections after focal cortical stroke. Neurobiol. Dis. 8, 910–922 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Carmichael S. T., Kathirvelu B., Schweppe C. A., Nie E. H., Molecular, cellular and functional events in axonal sprouting after stroke. Exp. Neurol. 287, 384–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krakauer J. W., Carmichael S. T., Corbett D., Wittenberg G. F., Getting neurorehabilitation right: What can be learned from animal models? Neurorehabil. Neural Repair 26, 923–931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeiler S. R., Should we care about early post-stroke rehabilitation? Not yet, but soon. Curr. Neurol. Neurosci. Rep. 19, 13 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Zeiler S. R., Krakauer J. W., The interaction between training and plasticity in the poststroke brain. Curr. Opin. Neurol. 26, 609–616 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humm J. L., Kozlowski D. A., James D. C., Gotts J. E., Schallert T., Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 783, 286–292 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Kozlowski D. A., James D. C., Schallert T., Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J. Neurosci. 16, 4776–4786 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okabe N., et al., Very early initiation reduces benefits of poststroke rehabilitation despite increased corticospinal projections. Neurorehabil. Neural Repair 33, 538–552 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Nudo R. J., Mechanisms for recovery of motor function following cortical damage. Curr. Opin. Neurobiol. 16, 638–644 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Nudo R. J., Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci. 7, 887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barkat T. R., Polley D. B., Hensch T. K., A critical period for auditory thalamocortical connectivity. Nat. Neurosci. 14, 1189–1194 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiesel T. N., Hubel D. H., Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017 (1963). [DOI] [PubMed] [Google Scholar]
- 48.Belford G. R., Killackey H. P., The sensitive period in the development of the trigeminal system of the neonatal rat. J. Comp. Neurol. 193, 335–350 (1980). [DOI] [PubMed] [Google Scholar]
- 49.Knudsen E. I., Experience alters the spatial tuning of auditory units in the optic tectum during a sensitive period in the barn owl. J. Neurosci. 5, 3094–3109 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Immelmann K., “Song development in the Zebra Finch and other estrildid finches” in Bird Vocalizations, Hinde R. A., Ed. (Cambridge University Press, 1969), pp. 64–74. [Google Scholar]
- 51.Poindron P., et al., Amniotic fluid is important for the maintenance of maternal responsiveness and the establishment of maternal selectivity in sheep. Animal 4, 2057–2064 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Vince M. A., Newborn lambs and their dams: The interaction that leads to sucking. Adv. Stud. Behav. 22, 239–268 (1993). [Google Scholar]
- 53.Maya Vetencourt J. F., et al., The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320, 385–388 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Gervain J., et al., Valproate reopens critical-period learning of absolute pitch. Front. Syst. Neurosci. 7, 102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levitin D. J., Zatorre R. J., On the nature of early music training and absolute pitch: A reply to Brown, Sachs, Cammuso, and Folstein. Music Percept. 21, 105–110 (2003). [Google Scholar]
- 56.Russo F. A., Windell D. L., Cuddy L. L., Learning the “special note”: Evidence for a critical period for absolute pitch acquisition. Music Percept. 21, 119–127 (2003). [Google Scholar]
- 57.Putignano E., et al., Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron 53, 747–759 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Borrelli E., Nestler E. J., Allis C. D., Sassone-Corsi P., Decoding the epigenetic language of neuronal plasticity. Neuron 60, 961–974 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maya Vetencourt J. F., Tiraboschi E., Spolidoro M., Castrén E., Maffei L., Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur. J. Neurosci. 33, 49–57 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Baroncelli L., et al., Experience affects critical period plasticity in the visual cortex through an epigenetic regulation of histone post-translational modifications. J. Neurosci. 36, 3430–3440 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yozbatiran N., Der-Yeghiaian L., Cramer S. C., A standardized approach to performing the action research arm test. Neurorehabil. Neural Repair 22, 78–90 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Fitzmaurice G. M., Ravichandran C., A primer in longitudinal data analysis. Circulation 118, 2005–2010 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Colosimo E. A., Fausto M. A., Freitas M. A., Pinto J. A., Practical modeling strategies for unbalanced longitudinal data analysis. J. Appl. Stat. 39, 2005–2013 (2012). [Google Scholar]
- 64.Jenson H., et al., Natural history of primary Epstein-Barr virus infection in children of mothers infected with human immunodeficiency virus type 1. J. Infect. Dis. 179, 1395–1404 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelson R. G.et al.; Diabetic Renal Disease Study Group , Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 335, 1636–1642 (1996). [DOI] [PubMed] [Google Scholar]
- 66.Ma Y., Mazumdar M., Memtsoudis S. G., Beyond repeated-measures analysis of variance: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg. Anesth. Pain Med. 37, 99–105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cnaan A., Laird N. M., Slasor P., Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 16, 2349–2380 (1997). [DOI] [PubMed] [Google Scholar]
- 68.Lang C. E., Edwards D. F., Birkenmeier R. L., Dromerick A. W., Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch. Phys. Med. Rehabil. 89, 1693–1700 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van der Lee J. H., et al., The intra- and interrater reliability of the action research arm test: A practical test of upper extremity function in patients with stroke. Arch. Phys. Med. Rehabil. 82, 14–19 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Beaton D. E., Boers M., Wells G. A., Many faces of the minimal clinically important difference (MCID): A literature review and directions for future research. Curr. Opin. Rheumatol. 14, 109–114 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Jaeschke R., Singer J., Guyatt G. H., Measurement of health status. Ascertaining the minimal clinically important difference. Control. Clin. Trials 10, 407–415 (1989). [DOI] [PubMed] [Google Scholar]
- 72.Jayadevappa R., Cook R., Chhatre S., Minimal important difference to infer changes in health-related quality of life-a systematic review. J. Clin. Epidemiol. 89, 188–198 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Edwardson M. A., et al., Reduced upper limb recovery in subcortical stroke patients with small prior radiographic stroke. Front. Neurol. 10, 454 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolf S. L., et al., The EXCITE stroke trial: Comparing early and delayed constraint-induced movement therapy. Stroke 41, 2309–2315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf S. L.et al.; EXCITE Investigators , Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA 296, 2095–2104 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Wolf S. L., et al., Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: The EXCITE randomised trial. Lancet Neurol. 7, 33–40 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Latham N. K., et al., Occupational therapy activities and intervention techniques for clients with stroke in six rehabilitation hospitals. Am. J. Occup. Ther. 60, 369–378 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Barth J., et al., Characterizing upper extremity motor behavior in the first week after stroke. PLoS One 15, e0221668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lang C. E., MacDonald J. R., Gnip C., Counting repetitions: An observational study of outpatient therapy for people with hemiparesis post-stroke. J. Neurol. Phys. Ther. 31, 3–10 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Clark T. A., et al., Rehabilitative training interacts with ischemia-instigated spine dynamics to promote a lasting population of new synapses in peri-infarct motor cortex. J. Neurosci. 39, 8471–8483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaneko M., Stryker M. P., Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife 3, e02798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bergan J. F., Ro P., Ro D., Knudsen E. I., Hunting increases adaptive auditory map plasticity in adult barn owls. J. Neurosci. 25, 9816–9820 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi D., Bruderer A. G., Werker J. F., Sensorimotor influences on speech perception in pre-babbling infants: Replication and extension of Bruderer et al. (2015). Psychon. Bull. Rev. 26, 1388–1399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bruderer A. G., Danielson D. K., Kandhadai P., Werker J. F., Sensorimotor influences on speech perception in infancy. Proc. Natl. Acad. Sci. U.S.A. 112, 13531–13536 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li R. W., Ngo C., Nguyen J., Levi D. M., Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol. 9, e1001135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schallert T., Kozlowski D. A., Humm J. L., Cocke R. R., Use-dependent structural events in recovery of function. Adv. Neurol. 73, 229–238 (1997). [PubMed] [Google Scholar]
- 87.Lang C. E., et al., Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase II, single-blind, randomized, controlled trial. Ann. Neurol. 80, 342–354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mesulam M.-M., Dementia: Its definition, differential diagnosis, and subtypes. JAMA 253, 2559–2561 (1985). [PubMed] [Google Scholar]
- 89.Katzman R., et al., Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am. J. Psychiatry 140, 734–739 (1983). [DOI] [PubMed] [Google Scholar]
- 90.Yuan A., Dromerick A. W., Tan M. T., Phase II multi-arm clinical trial designs and randomization with covariates via empirical weighting. J. Clin. Trials 4, 2–7 (2014). [Google Scholar]
- 91.Brott T., et al., Measurements of acute cerebral infarction: A clinical examination scale. Stroke 20, 864–870 (1989). [DOI] [PubMed] [Google Scholar]
- 92.Meinert C. L., Clinical Trials: Design, Conduct and Analysis (Oxford University Press, 2012), vol. 39. [Google Scholar]
- 93.Signorini D. F., et al., Dynamic balanced randomization for clinical trials. Stat. Med. 12, 2343–2350 (1993). [DOI] [PubMed] [Google Scholar]
- 94.Atkinson A. C., Optimum biased coin designs for sequential clinical trials with prognostic factors. Biometrika 69, 61–67 (1982). [Google Scholar]
- 95.Lyle R. C., A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 4, 483–492 (1981). [DOI] [PubMed] [Google Scholar]
- 96.Lang C. E., Wagner J. M., Dromerick A. W., Edwards D. F., Measurement of upper-extremity function early after stroke: Properties of the action research arm test. Arch. Phys. Med. Rehabil. 87, 1605–1610 (2006). [DOI] [PubMed] [Google Scholar]
- 97.van der Lee J. H., Beckerman H., Lankhorst G. J., Bouter L. M., The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J. Rehabil. Med. 33, 110–113 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Hsieh C.-L., Hsueh I.-P., Chiang F.-M., Lin P.-H., Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing 27, 107–113 (1998). [DOI] [PubMed] [Google Scholar]
- 99.De Weerdt W., Harrison M., Measuring recovery of arm-hand function in stroke patients: A comparison of the Brunnstrom-Fugl-Meyer test and the Action Research Arm test. Physiother. Can. 37, 65–70 (1985). [Google Scholar]
- 100.Dromerick A. W., et al., Critical periods after stroke study: Translating animal stroke recovery experiments into a clinical trial. Front. Hum. Neurosci. 9, 231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bernhardt J., Dewey H., Thrift A., Collier J., Donnan G., A Very Early Rehabilitation Trial For Stroke (AVERT): Phase II safety and feasibility. Stroke 39, 390–396 (2008). [DOI] [PubMed] [Google Scholar]
- 102.Tilson J. K., et al., Characterizing and identifying risk for falls in the LEAPS study: A randomized clinical trial of interventions to improve walking poststroke. Stroke 43, 446–452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Winstein C. J., Kay D. B., Translating the science into practice: Shaping rehabilitation practice to enhance recovery after brain damage. Prog. Brain Res. 218, 331–360 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Savage T., Shaping: The link between rats and robots. Connect. Sci. 10, 321–340 (1998). [Google Scholar]
- 105.Sunderland A., Tuke A., Neuroplasticity, learning and recovery after stroke: A critical evaluation of constraint-induced therapy. Neuropsychol. Rehabil. 15, 81–96 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Winstein C. J.et al.; ICARE Investigative Team , Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE): A randomized controlled trial protocol. BMC Neurol. 13, 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ingvaldsen R., Whiting H. J., Modern views on motor skill learning are not ‘representative’! Hum. Mov. Sci. 16, 705–732 (1997). [Google Scholar]
- 108.Keetch K. M., Schmidt R. A., Lee T. D., Young D. E., Especial skills: Their emergence with massive amounts of practice. J. Exp. Psychol. Hum. Percept. Perform. 31, 970–978 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Dobkin B. H.et al.; SIRROWS Group , International randomized clinical trial, Stroke Inpatient Rehabilitation With Reinforcement of Walking Speed (SIRROWS), improves outcomes. Neurorehabil. Neural Repair 24, 235–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taub E., et al., An operant approach to rehabilitation medicine: Overcoming learned nonuse by shaping. J. Exp. Anal. Behav. 61, 281–293 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dragoi V., Staddon J. E., The dynamics of operant conditioning. Psychol. Rev. 106, 20–61 (1999). [DOI] [PubMed] [Google Scholar]
- 112.Skinner B. F., About Behaviorism (Vintage, 2011). [Google Scholar]
- 113.Zeger S. L., Liang K.-Y., Albert P. S., Models for longitudinal data: A generalized estimating equation approach. Biometrics 44, 1049–1060 (1988). [PubMed] [Google Scholar]
- 114.Fitzmaurice G., Davidian M., Verbeke G., Molenberghs G., “Generalized estimating equations for longitudinal data analysis” in Longitudinal Data Analysis (Handbooks of Modern Statistical Methods, CRC Press, Boca Raton, FL, 2009), chap. 3. [Google Scholar]
- 115.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2020). [Google Scholar]
- 116.Rapoport A. P., et al., Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood 117, 788–797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data have been deposited in Open Science Framework (https://osf.io/wrk3x/). To observe HIPAA protections for human subject information, click to request access to the dataset. Access will be granted by the corresponding author.