Abstract
A 78-year-old female patient presented to the emergency department with syncope and dyspnoea. The left arm appeared to be cold and radial pulse was not palpable. A CT scan of the chest and left arm with intravenous contrast displayed bilateral central pulmonary embolisms in combination with a left subclavian artery embolism and an atrial septal aneurysm. Transthoracic echocardiography identified a patent foramen ovale with right-to-left shunting confirming the diagnosis of paradoxical embolism. The patient was treated with anticoagulants. In a patient presenting with a combination of a pulmonary embolism and a peripheral arterial embolism, the clinician should consider a right-to-left shunt with paradoxical embolism. In line with this, when diagnosing a peripheral arterial embolism, a central venous origin should be considered. Furthermore, when diagnosing a pulmonary embolism or other forms of venous thromboembolism, the clinician should be aware of signs of a peripheral arterial embolism.
Keywords: cardiovascular medicine, venous thromboembolism, emergency medicine, primary care, radiology
Background
Paradoxical embolism refers to the clinical phenomenon of an arterial thromboembolism originating from the venous circulation through an intracardiac or pulmonary right-to-left shunt.1 The most common intracardiac shunt is a patent foramen ovale (PFO), which is an open connection between the right and left atrium.1 After birth, this conduit between the right and left atrium closes spontaneously in most people, but remains open in 25%–30% of adults.2–5 In a patient with an intracardiac or pulmonary shunt, paradoxical embolism can occur when right-sided pressures are increased (eg, due to pulmonary embolism).6 Therefore, to determine whether paradoxical embolism has occurred through a PFO, a triad combining venous source of thrombosis, raised right atrial pressure and the presence of PFO is required.
The clinical manifestations of paradoxical embolisation are variable due to a great diversity in the site of embolisation.1 7 The most common site of embolisation is the cerebral circulation, manifesting as an ischaemic stroke. Other relatively frequent sites of embolisation are myocardial infarction, gastrointestinal ischaemia and renal infarction.7–10 Paradoxical embolism to upper extremities is relatively rare.11
The importance of recognition of a paradoxical embolism, and possible underlying heart defect such as PFO, is to prevent further arterial ischaemic events with all related morbidities and possible mortality. When a patient presents with concomitant venous and arterial embolism, the clinician should consider paradoxical embolism as a possible diagnosis.12
Case presentation
A 78-year-old female patient with Alzheimer’s dementia presented to the emergency department with syncope and dyspnoea. Initially measured saturation was 67%, and increased to 95% after using a non-rebreathing mask with 15 liters/minute of oxygen. The respiratory rate initially was 40 breaths/min, declining to 30 breaths/min with increasing saturation. Furthermore, the left arm was cold and peripheral pulsations were not palpable. There was a significant blood pressure difference between the right and the left arm, to the disadvantage of the left arm (right arm 170/80 mmHg, left arm 74/50 mmHg). Doppler assessment of the left radial and ulnar artery showed a monophasic signal, whereas the ipsilateral brachial artery showed a biphasic signal. Unlike biphasic Doppler signals, monophasic signals exhibit only one audible component, which is likely caused by an arterial obstruction.13
The ECG showed a sinus tachycardia of 110 beats/min with a right bundle branch block which was a new finding compared with an ECG of two years earlier. Arterial blood gases showed respiratory failure (pH 7.32, partial pressure of oxygen 48 mmHg, partial pressure of carbon dioxide (pCO2) 37.5 mmHg and arterial oxygen saturation of 82.3%). The relatively high pCO2 in a patient with a tachypnoea of 40 breaths/min was explained by suspected undiagnosed pulmonary disease, the quantity of smoking was unknown.
The CT scan with intravenous contrast of the chest and left arm showed bilateral central pulmonary embolisms with signs of right ventricular pressure overload and an atrial septal aneurysm (ASA). In addition, in the left subclavian artery, a blood clot was seen, which extended into the aorta. A defect was suspected between the left and right atrium due to the findings on the CT scan. The source of thrombosis was not assessed because it had no influence on the treatment. Anticoagulation therapy was started and the patient was admitted to the intensive care unit for close monitoring of her respiratory, haemodynamic status and limb ischaemia.
Investigations
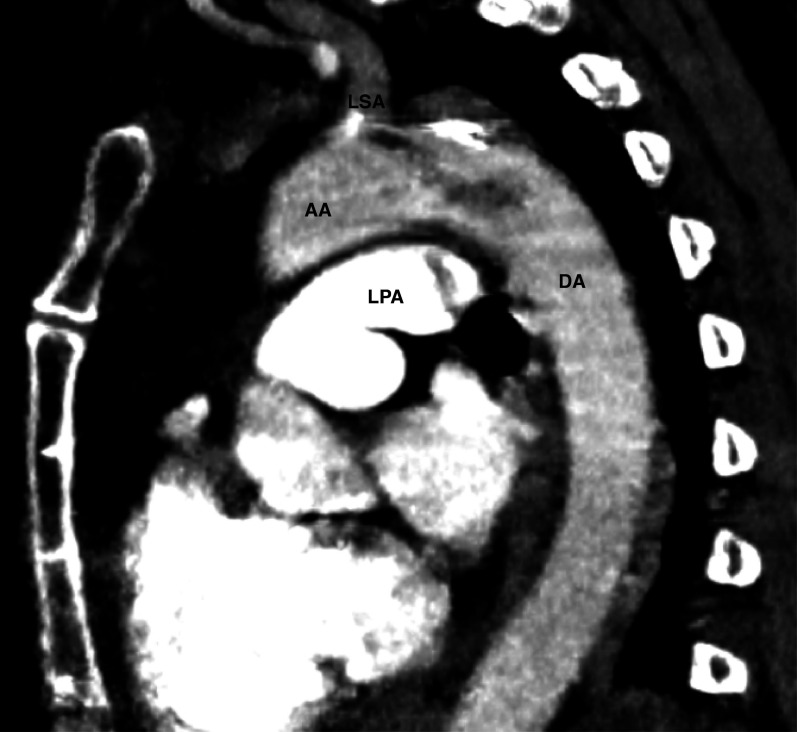
The CT scan performed at the emergency department showed bilateral central pulmonary embolisms with signs of right ventricular dilatation and an ASA (figures 1 and 2). In addition, in the left subclavian artery an embolism was seen, which extended into the aorta (figure 3).
Figure 1.
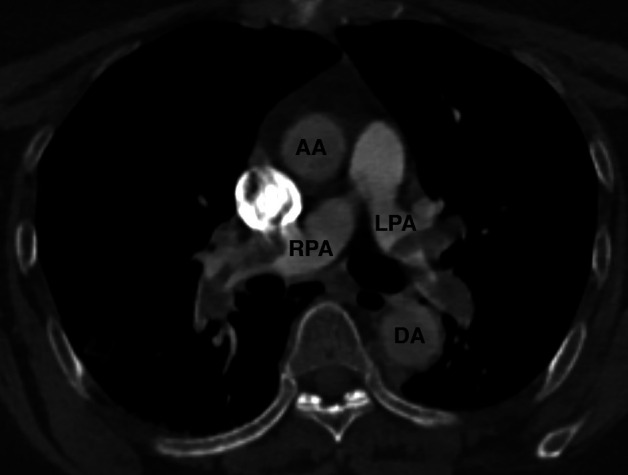
The CT scan in axial view showing bilateral pulmonary embolisms. AA, ascending aorta; DA, descending aorta; LPA, left pulmonary artery; RPA, right pulmonary artery.
Figure 2.
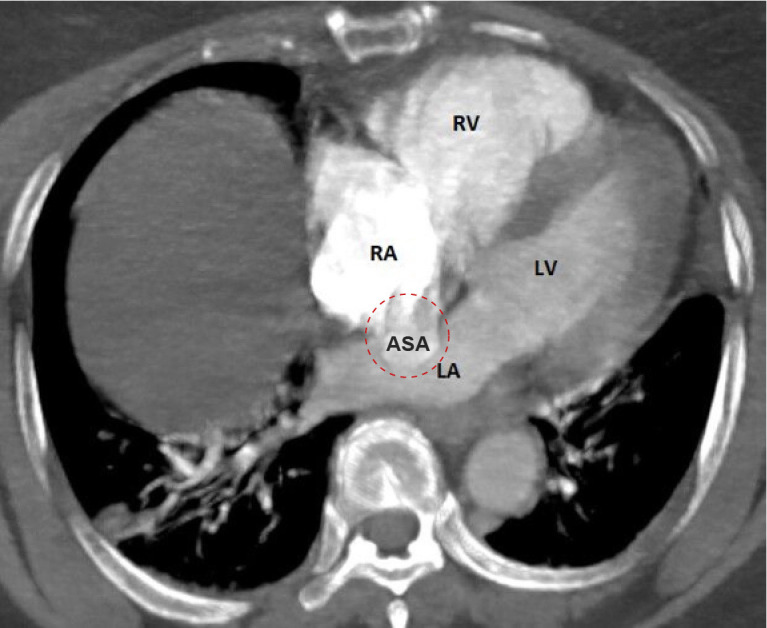
The CT scan in axial view showing an atrial septal aneurysm which is depicted in the red dashed circle with bulging of the right atrium into the left atrium. ASA, atrial septal aneurysm; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 3.
The CT scan in sagittal view showing a blood clot in the left subclavian artery extending into the aorta and a central pulmonary embolism in the left pulmonary artery. AA, ascending aorta; DA, descending aorta; LPA, left pulmonary artery; LSA, left subclavian artery.
During admission, a transthoracic echocardiogram was performed which showed a dilated right ventricle secondary to the elevated pulmonary pressure. Subsequent injection of agitated saline contrast medium into a peripheral vein during the strain phase of the Valsalva manoeuvre showed massive appearance of contrast micro-bubbles in the left atrium, which is diagnostic of a right-to-left shunt (figure 4, video 1).
Figure 4.
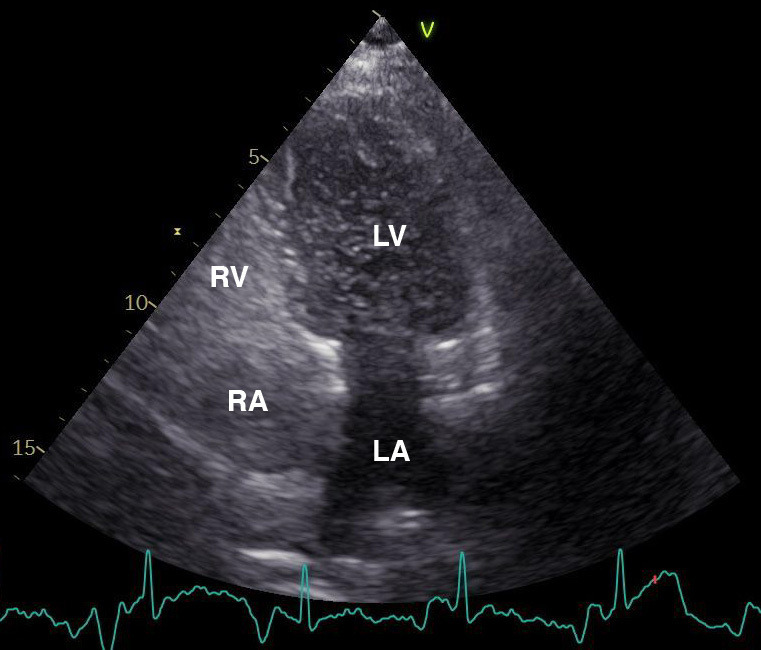
Transthoracic echocardiogram in four-chamber view showing passage of micro-bubbles from the right to the left atrium and subsequent left ventricle through the patent foramen ovale during Valsalva manoeuvre. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Video 1.
Differential diagnosis
The combination of syncope and dyspnoea leads to a differential diagnosis including acute conditions such as pulmonary embolism, myocardial infarction and aortic dissection. In the latter, pulmonary complications like dyspnoea are rare, but possible.14 The cold left arm indicated ischaemia which could be caused by an aortic dissection or, in combination with a venous thromboembolism such as pulmonary embolism, could be a result of paradoxical embolism.
Immediate CT scan of the thorax was performed to either indicate or exclude aortic dissection or pulmonary embolism and included a CT angiography of the left arm to be able to identify an arterial embolism. The CT scan showed bilateral central pulmonary embolisms and an arterial embolism in the left subclavian artery. There were no signs of aortic dissection. In addition, the ECG showed no signs of acute myocardial ischaemia.
Although the CT scan showed no obvious cardiac structural defects, it did show an ASA. ASA is strongly associated with PFO15 which is also the most common intracardiac shunt. Other right-to-left shunts which could cause paradoxical embolisms include atrial septal defect, ventricular septal defect, patent ductus arteriosus and pulmonary arteriovenous malformations.1 12 A transthoracic echocardiogram confirmed the presence of a PFO.
Treatment
The patient was initially treated with a single dose of intravenous unfractionated heparin in order to rapidly achieve a stable haemodynamic status. Subsequently, she was treated with subcutaneous low molecular weight heparin. She was briefly admitted to the intensive care unit for optimal respiratory support and monitoring of haemodynamic status and limb ischaemia. On the first day of admission, the left arm was warm and peripheral pulses were well palpable, indicating the improved blood flow. Therefore, the surgeon advised to continue the anticoagulant therapy. The patient’s respiratory and haemodynamic status was stable without further support, hence she could be transferred to the nursing ward. The physiotherapist was involved to support the patient in mobilising her left arm. After 3 days, the patient was discharged from the hospital and returned to her nursing home. Anticoagulant treatment was continued by a direct oral anticoagulant.
Outcome and follow-up
The patient has been in follow-up at the outpatient department for two months. Her family reported a stable condition. A transoesophageal echocardiography was considered to further define the characteristics of the PFO and determine if the patient would benefit from percutaneous transcatheter closure of the intracardiac shunt. Due to the comorbidities of the patient, Alzheimer’s dementia with psychotic features, the transoesophageal echocardiography and closure of the heart defect were not pursued. In consultation with the patient’s family, it was decided to refrain from invasive procedures. The patient will continue anticoagulant treatment indefinitely.
Discussion
Depending on the site of embolism, paradoxical embolism can cause severe morbidity and mortality, therefore early recognition is important. However, the diagnosis of paradoxical embolism can be challenging, due to a great diversity in clinical manifestations.1
Several cases are published describing the most common presentations such as stroke and myocardial infarction.7–9 Embolisation of the upper extremities is far less frequent.11 However, one case report described paradoxical embolisation in both upper extremities due to a PFO.16
PFO is a common heart defect which facilitates paradoxical embolism. This heart defect coexists with ASA in up to 28% of cases, adding to the pathophysiology of right-to-left shunting.17 A recent study states that in patients with PFO-associated stroke, ASA is a more important predictor of recurrent stroke than shunt size.17 How the presence of ASA contributes to paradoxical embolism in the setting of PFO is not fully understood. One hypothesis is that the presence of ASA may lead to a more frequent and wider opening of the PFO or by haemodynamically directing blood flow from the inferior vena cava towards the PFO.17
The diagnostic imaging techniques most commonly used for heart defect detection are transthoracic echocardiography and transoesophageal echocardiography, both of which can be carried out with the use of intravenous contrast agent injection to diagnose right-to-left shunting.18 Furthermore, as illustrated in this case, a CT scan can reveal an ASA.
Therapeutic options in patients with PFO include medical therapy with anticoagulants or percutaneous transcatheter closure of the PFO. Recent randomised controlled trials have shown that percutaneous PFO closure is superior to medical treatment alone to decrease stroke recurrence in adults up to 60 years with no identified alternative cause of stroke, especially in patients with high risk PFO features (eg, ASA and large shunt size).17 19 Additionally, the Risk of Paradoxical Embolism score can be used as a valuable tool in predicting recurrent ischaemic cerebrovascular events.20 Due to comorbidities of the patient presented in this case, it was decided to refrain from invasive therapy in consultation with her family. She will continue the anticoagulant treatment for the rest of her life.
Learning points.
When diagnosing a pulmonary embolism or other forms of venous thromboembolism, be aware of signs of a peripheral embolism, and vice versa when diagnosing a peripheral arterial embolism, a central venous origin should be considered.
In the diagnostic process of a patient with a suspected pulmonary embolism and peripheral (paradoxical) embolism, CT angiography can be used to detect a pulmonary embolism, atrial septal aneurysm and peripheral arterial embolism, and exclude aortic dissection.
To prevent further systemic embolisation, anticoagulant treatment is the initial step in patients with thromboembolism.
Patient factors determine if anticoagulant treatment or percutaneous transcatheter closure of the patent foramen ovale is preferable in the prevention of recurrent paradoxical embolism.
Acknowledgments
We appreciate the medical specialists mentioned below for their clinical guidance in the care of this patient: Dr HMA Hofstee (Specialist Acute Internal Medicine), C Naves (Fellow Vascular Surgery), Dr SA Mollema (Cardiologist), Dr DJ Versluis (Specialist Internal Medicine and Intensivist).
Footnotes
Contributors: All authors made substantial contributions to the conception of the article and approved the final version to be published. The article was written by TH and revised by IB, RG and TU for important intellectual content. In the emergency department, IB, RG and TU were involved in the initial care of the patient.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Next of kin consent obtained.
References
- 1.Windecker S, Stortecky S, Meier B. Paradoxical embolism. J Am Coll Cardiol 2014;64:403–15. 10.1016/j.jacc.2014.04.063 [DOI] [PubMed] [Google Scholar]
- 2.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20. 10.1016/S0025-6196(12)60336-X [DOI] [PubMed] [Google Scholar]
- 3.Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. stroke prevention: assessment of risk in a community. Mayo Clin Proc 1999;74:862–9. 10.4065/74.9.862 [DOI] [PubMed] [Google Scholar]
- 4.Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006;47:440–5. 10.1016/j.jacc.2005.10.044 [DOI] [PubMed] [Google Scholar]
- 5.Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005;112:1063–72. 10.1161/CIRCULATIONAHA.104.524371 [DOI] [PubMed] [Google Scholar]
- 6.Najib K, Heckle M, Goubran S, et al. Paradoxical emboli following a pulmonary embolus in the presence of a patent foramen ovale. Ann Transl Med 2018;6:21. 10.21037/atm.2018.01.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lak HM, Ahmed T, Nair R, et al. Simultaneous multifocal paradoxical embolism in an elderly patient with patent foramen ovale: a case report. Cureus 2020;12:e6992. 10.7759/cureus.6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budavari AI, Glenn TJ, Will KK, et al. A case of simultaneous pulmonary embolism and acute myocardial infarction secondary to a previously undiagnosed patent foramen ovale. J Hosp Med 2009;4:E5–9. 10.1002/jhm.464 [DOI] [PubMed] [Google Scholar]
- 9.Alkhalil M, Cahill TJ, Boardman H, et al. Concomitant pulmonary embolism and myocardial infarction due to paradoxical embolism across a patent foramen ovale: a case report. Eur Heart J Case Rep 2017;1:ytx010. 10.1093/ehjcr/ytx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caretta G, Robba D, Bonadei I, et al. Multiorgan paradoxical embolism consequent to acute pulmonary thromboembolism with patent foramen ovale: a case report. Cases J 2009;2:8358. 10.4076/1757-1626-2-8358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam MA, Khalighi K, Goldstein JE, et al. Paradoxical embolism-report of a case involving four organ systems. J Emerg Med 2000;19:31–4. 10.1016/S0736-4679(00)00178-5 [DOI] [PubMed] [Google Scholar]
- 12.Cachia M, Pace Bardon M, Fsadni P, et al. Systemic and venous thromboembolism: think about paradoxical embolism. BMJ Case Rep 2015;2015:bcr2015211174. 10.1136/bcr-2015-211174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim ES, Sharma AM, Scissons R, et al. Interpretation of peripheral arterial and venous Doppler waveforms: a consensus statement from the Society for vascular medicine and Society for vascular ultrasound. Vasc Med 2020;25:484–506. 10.1177/1358863X20937665 [DOI] [PubMed] [Google Scholar]
- 14.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of cardiology (ESC). Eur Heart J 2014;35:2873–926. 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 15.Mattioli AV, Aquilina M, Oldani A, et al. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J 2001;22:261–8. 10.1053/euhj.2001.2293 [DOI] [PubMed] [Google Scholar]
- 16.Kouskov OS, Nichols DJ, O'Hearn DJ. Paradoxical arterial embolism involving both upper extremities in a patient with pulmonary embolism and a patent foramen ovale. Clin Appl Thromb Hemost 2011;17:E98–101. 10.1177/1076029610387123 [DOI] [PubMed] [Google Scholar]
- 17.Turc G, Lee J-Y, Brochet E, et al. Atrial septal aneurysm, shunt size, and recurrent stroke risk in patients with patent foramen ovale. J Am Coll Cardiol 2020;75:2312–20. 10.1016/j.jacc.2020.02.068 [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi K, Yoshiyama M, Homma S. Patent foramen ovale and cryptogenic stroke. Trends Cardiovasc Med 2017;27:575–81. 10.1016/j.tcm.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 19.Abdelghani M, El-Shedoudy SAO, Nassif M, et al. Management of patients with patent foramen ovale and cryptogenic stroke: an update. Cardiology 2019;143:62–72. 10.1159/000501028 [DOI] [PubMed] [Google Scholar]
- 20.Morais LA, Sousa Lde, Fiarresga A, et al. Rope score as a predictor of recurrent ischemic events after percutaneous patent foramen ovale closure. Int Heart J 2018;59:1327–32. 10.1536/ihj.17-489 [DOI] [PubMed] [Google Scholar]