Abstract
Background
To reduce the overall exposure to dexamethasone (DEX) in patients receiving cisplatin‐based chemotherapy, we evaluated the noninferiority of DEX on day 1, with or without low‐dose DEX on days 2 and 3, combined with an oral fixed‐dose combination of netupitant and palonosetron (NEPA), compared with the guideline‐consistent use of 4‐day DEX.
Patients and Methods
In this open‐label, multicenter study, chemotherapy‐naïve patients undergoing high‐dose cisplatin (≥70 mg/m2), were given NEPA and DEX (12 mg) on day 1 and randomized (1:1:1 ratio) to receive either (a) no further DEX (DEX1), (b) oral DEX (4 mg daily) on days 2–3 (DEX3), or (c) DEX (4 mg twice daily) on days 2–4 (DEX4). The primary efficacy endpoint was complete response (CR: no emesis and no rescue medication) during the 5‐day overall phase. The noninferiority margin was set at −15% difference (DEX1 or DEX3 minus DEX4). Secondary efficacy endpoints included complete protection (CP: CR and none or mild nausea).
Results
Two‐hundred twenty‐eight patients, 76 in each arm, were assessable. Noninferiority was met for both DEX‐sparing regimens and the reference arm, with overall phase CR rates of 76.3% in each of the DEX1 and DEX3 arms and 75.0% in the DEX4 arm (95% confidence interval, −12.3% to 15% for each comparison). During the overall phase, CP rates were similar between groups.
Conclusion
A simplified regimen of NEPA plus single‐dose DEX offers comparable chemotherapy‐induced nausea and vomiting prevention throughout 5 days post‐chemotherapy with the advantage of sparing patients additional doses of DEX in the high–emetic‐risk setting of cisplatin‐based chemotherapy.
Implications for Practice
Dexamethasone (DEX) has traditionally played an integral role in the management of chemotherapy‐induced emesis. Although generally considered safe, even short‐term DEX use is associated with various side effects, and some evidence suggests that concurrent steroids may reduce the efficacy of immunotherapies. This study demonstrates comparable antiemetic control during the 5 days post‐chemotherapy with a simplified regimen of netupitant/palonosetron plus single‐dose DEX versus the standard 4‐day DEX reference treatment in high‐dose cisplatin. This represents a clinically relevant achievement as it not only simplifies antiemetic prophylaxis but also offers an opportunity to appropriately use in patients where caution with corticosteroid use is advised.
Keywords: Cisplatin, Dexamethasone, Netupitant, Palonosetron, Chemotherapy‐induced nausea and vomiting
Short abstract
This study was designed to test whether two different dexamethasone‐sparing regimens, when administered with NEPA, might provide the opportunity to reduce the total corticosteroid dose while maintaining the same degree of chemotherapy‐induced nausea and vomiting control in patients undergoing cisplatin‐based chemotherapy.
Introduction
Corticosteroids such as dexamethasone (DEX) continue to play a key role for the prevention of chemotherapy‐induced nausea and vomiting (CINV) and are recommended by antiemetic guidelines to be used in conjunction with other agents such as 5‐hydroxytryptamine‐3 (5‐HT3) receptor antagonists (RAs) and neurokinin‐1 (NK‐1) RAs [1, 2]. Although DEX is generally considered safe when used in combination with other antiemetic agents [3], corticosteroids can cause a range of side effects. In a survey investigating self‐reported severity of DEX‐related side effects in 60 patients receiving moderately emetogenic chemotherapy (MEC) with DEX given for acute and delayed prophylaxis of CINV, patients reported moderate‐to‐severe side effects with insomnia, indigestion/epigastric discomfort, agitation, increased appetite, weight gain, and acne the week after chemotherapy [4]. Because patients undergo multiple consecutive cycles of the same chemotherapy in clinical practice, there has been growing interest in minimizing DEX dose/frequency in each cycle of therapy [5]. It has been hypothesized that the pharmacologically distinct 5‐HT3RA palonosetron could achieve protection against delayed CINV, without the need for multiple‐day DEX in MEC [6]. In 2010, a noninferiority phase III study demonstrated that in patients treated with palonosetron on day 1, the administration of DEX only once before MEC was not associated with significant loss in antiemetic control during the 5‐day observation period when compared with palonosetron on day 1 administered with DEX on days 1–3 [7]. In this proof‐of‐concept trial the DEX‐sparing strategy was explored in patients with breast cancer undergoing the combination of an anthracycline and cyclophosphamide (AC), who represent a population at particularly high risk for CINV [1, 2]. Updated guidelines have now included AC in the category of highly emetogenic chemotherapy (HEC) and recommend the addition of an NK‐1RA to a 5‐HT3RA and DEX as antiemetic prophylaxis [1, 2]. A simplified but guideline‐consistent DEX‐sparing strategy was evaluated in a pivotal trial that evaluated the efficacy of single‐dose oral fixed‐dose combination of the NK‐1RA, netupitant, and palonosetron (NEPA), versus palonosetron, both with single‐dose DEX administered before AC on day 1 [8]. The proportion of patients with breast cancer achieving complete response (CR) during the delayed phase (primary efficacy end point) was significantly higher in the NEPA arm compared with the palonosetron arm, as well as during the overall and acute phases. These findings combined with the efficacy of NEPA in preventing CINV in patients undergoing cisplatin‐based HEC [9], led us to evaluate the DEX‐sparing strategy administered with NEPA in the cisplatin setting where multiple doses of prophylactic DEX each cycle are currently recommended [1, 2].
The current, investigator‐initiated, phase III study was designed to test whether two different DEX‐sparing regimens, when administered with NEPA, might provide the opportunity to reduce the total corticosteroid dose while maintaining the same degree of CINV control in patients undergoing cisplatin‐based chemotherapy.
Materials and Methods
Study Design
This was a phase III, open‐label, multicenter, randomized, three‐arm study that aimed to evaluate the noninferiority of two DEX‐sparing regimens when combined with oral NEPA versus the guideline‐consistent DEX regimen in patients receiving cisplatin‐containing chemotherapy. The study was conducted in 24 Italian centers from November 2016 to November 2019. The study was done in compliance with the Declaration of Helsinki and the study protocol was approved by the institutional review boards and the Ethics Committees of each participating institution. All patients included in this study provided written, informed consent. This study was registered on the European Union Clinical Trials Register (EudraCT number 2015‐005704‐29) and on ClinicalTrials.gov (NCT04201769).
Study Population
Eligible patients were ≥ 18 years of age with a confirmed diagnosis of non‐small cell lung cancer (NSCLC), were chemotherapy‐naïve, and were scheduled to receive the first course of cisplatin (≥70 mg/m2)‐based chemotherapy. Patients could receive cisplatin either alone or in combination with antineoplastic agents with low or minimal emetogenic potential [1, 2]. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate hematologic, hepatic, and renal functions. Main exclusion criteria included patients who were scheduled to receive either concurrent chemo‐radiation therapy for NSCLC or radiation therapy to the abdomen or pelvis within 1 week before chemotherapy initiation, and patients with symptomatic brain metastases, contraindications for corticosteroid use, routine use of corticosteroids or any other agent with antiemetic potential, and nausea or vomiting within 24 hours before chemotherapy initiation.
Antiemetic Treatments and Random Assignment
Patients in each arm received oral NEPA (netupitant 300 mg/palonosetron 0.50 mg) 1 hour before the administration of cisplatin on the first day of chemotherapy (day 1) (Table 1). Prophylactic DEX was administered as follows: patients in each arm received DEX 12 mg intravenously a maximum of 30 minutes before the administration of cisplatin on day 1; in the DEX1 arm, patients received no additional doses of corticosteroid; in the DEX3 arm, patients received oral DEX 4 mg on days 2 and 3; and in the DEX4 arm, patients received oral DEX 4 mg twice daily on days 2 to 4 (reference arm) (Table 1). Random assignment was centrally done using a computer‐generated, allocation list with a block size of 6 to ensure a balance in sample size across groups over time. Patients were allowed to take rescue medication throughout the study period for nausea or vomiting, if necessary. The choice of recommended rescue medicine was either DEX or metoclopramide and was at the discretion of each investigator.
Table 1.
Treatment regimens and dosing schedule
| Treatment regimen | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| Treatment arm DEX1 | NEPA + IV DEX 12 mg | |||
| Treatment arm DEX3 | NEPA + IV DEX 12 mg | Oral DEX 4 mg QD | Oral DEX 4 mg QD | |
| Treatment arm DEX4 | NEPA + IV DEX 12 mg | Oral DEX 4 mg b.i.d. | Oral DEX 4 mg b.i.d. | Oral DEX 4 mg b.i.d. |
Abbreviations: DEX, dexamethasone; IV, intravenous; NEPA, fixed‐dose combination of netupitant and palonosetron; QD, once daily.
Procedures and Outcomes
During days 1–5, patients recorded the following items into their symptom diary every 24 hours: the number of emetic episodes and the time of first vomiting; the severity of nausea using a Likert scale (0, no nausea; 1, mild nausea; 2, moderate nausea; 3, severe nausea); the number of rescue medications and the time of the first administration. On day 6 from the initiation of cisplatin, the patients assessed the severity of DEX‐related side effects, such as indigestion/heartburn or reflux, insomnia, agitation, hiccups, and facial rash/acne, using a four‐point categorical scale (0, not at all; 1, a little bit; 2, quite a bit; 3, very much), and entered the results of these assessments into their symptom diary. Adverse events (AEs) were evaluated by the investigators according to the Common Terminology Criteria for Adverse Events version 4.3 during the overall study period.
The primary endpoint was the proportion of patients achieving CR (no emetic episode and no use of rescue medication) in the overall phase (0–120 hours from the initiation of cisplatin). Secondary endpoints included the proportion of patients who achieved the following during the overall, acute (0–24 hours) and delayed (>24–120 hours) phases: CR (not including overall phase), complete protection (CP; no emetic episode, no use of rescue medication, and no more than mild nausea), no emesis, no nausea, no significant nausea (NSN; defined as no more than mild nausea), and no use of rescue medication.
Statistical Analysis
The proportion of patients achieving CR during the overall phase was compared between treatment arms sequentially: first for the DEX1 arm with DEX4 arm comparison and then for the DEX3 arm with DEX4 arm comparison. The “Fixed Sequence Procedure” was used to preserve the overall type I family‐wise error rate for testing the two noninferiority hypotheses at the one‐sided 0.025 significance level. The associated hierarchical order was chosen because we considered the noninferiority hypothesis for the first comparison (DEX1 with DEX4) to be most clinically important. The testing procedure was stopped, and all the remaining null hypotheses were accepted if an acceptance occurred. Confidence intervals (CIs) of the risk difference (RD) in the proportion of patients achieving CR were calculated by resorting to a generalized linear model with identity link function, binomial distribution and using treatment group as dummy covariate. To accept the noninferiority hypothesis, the lower boundary of the two‐sided 95% CI on the RD between the CRs was to be greater than −15%, with 15% used as the prefixed noninferiority margin. A sample size of 210 eligible and assessable patients when randomized in a 1:1:1 ratio (that is 70 patients in each arm) achieved 80% power to detect a noninferiority margin equal to 15% in the prioritized comparison “DEX1 arm vs. DEX4 arm” assuming that the proportion of CR during the overall phase would be 90% in the reference arm [9] and a one‐sided type I error rate equal to 0.025. Assuming an attrition rate of 4%, at least 73 eligible and assessable patients per arm needed to be enrolled.
Analysis of the primary endpoint was performed for both the intention‐to‐treat (ITT) cohort (all randomized patients who received study treatment) and the per‐protocol (PP) cohort (all patients who completed study and who were compliant with the study protocol), with the PP cohort being the primary population for the efficacy analyses. All secondary efficacy analyses used the same methods as the primary endpoint, without testing for noninferiority. All statistical analyses were performed by using the SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Of the 252 patients randomized, 22 did not fill out the symptom diary for unknown reasons (seven patients in the DEX1 arm, nine in the DEX3 arm, and six in the DEX4 arm). One patient randomized to DEX1 had a protocol violation due to the taking of rescue medication before the occurrence of CINV, while one patient randomized to DEX4 received cisplatin dose <70 mg/m2. Consequently, 228 (76 patients in each study arm) and 252 represented the PP and safety populations, respectively (Fig. 1). Demographic data and baseline characteristics for patients in the ITT cohort are shown in Table 2. The majority of patients evaluated (67%) were male, with a numerically higher proportion of women randomized to the DEX3 arm. All three treatment groups were comparable regarding the dose of cisplatin administered. Only one patient receiving concurrent immunotherapy for NSCLC was included in this study.
Figure 1.
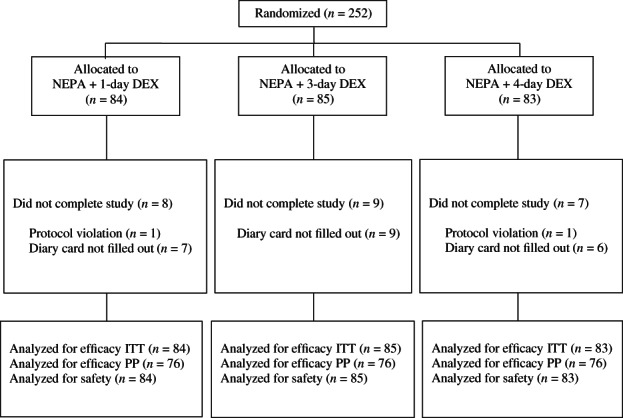
CONSORT diagram. Abbreviations: DEX, dexamethasone; ITT, intention‐to‐treat cohort; NEPA, fixed‐dose combination of netupitant and palonosetron; PP per‐protocol cohort.
Table 2.
Baseline demographic and clinical characteristics (intention‐to‐treat cohort, n = 252)
| Characteristic | NEPA + DEX1 (n = 84) | NEPA + DEX3 (n = 85) | NEPA + DEX4 (n = 83) |
|---|---|---|---|
| Age, yr | |||
| Mean ± SD | 63.9 ± 7.2 | 62.7 ± 7.9 | 63.3 ± 8.2 |
| Median (range) | 66 (44–79) | 63 (34–77) | 64 (40–76) |
| Gender, n (%) | |||
| Male | 60 (71.4) | 51 (60) | 58 (69.9) |
| Female | 24 (28.6) | 34 (40) | 25 (30.1) |
| BMI (kg/m2), mean ± SD | 24.7 ± 4.2 | 24.6 ± 4.2 | 25 ± 4.0 |
| ECOG score, n (%) | |||
| 0 | 66 (78.6) | 70 (82.4) | 62 (74.7) |
| 1 | 18 (21.4) | 15 (17.6) | 21 (25.3) |
| Cisplatin | |||
| Mean ± SD | 75 ± 4.2 | 75.3 ± 4.4 | 74.3 ± 5.9 |
| Dose 70 mg/m2, n (%) | 20 (23.8) | 21 (24.7) | 21a (25.6) |
| Dose >70 mg/m2, n (%) | 64 (76.2) | 64 (75.3) | 61 (74.4) |
| Concomitant chemotherapy, n (%) | |||
| Pemetrexed | 37 (44.0) | 42 (49.4) | 44 (53.0) |
| Gemcitabine | 22 (26.2) | 21 (24.7) | 22 (26.5) |
| Vinorelbine | 21 (25.0) | 18 (21.2) | 14 (16.9) |
| Other | 4 (4.8) | 4 (4.7) | 3 (3.6) |
One patient received cisplatin dose <70 mg/m2 in the DEX4 arm.
Abbreviations: BMI, body mass index; DEX1, dexamethasone day 1; DEX3, dexamethasone day 1 to 3; DEX4, dexamethasone day 1 to 4; ECOG, Eastern Cooperative Oncology Group; NEPA, fixed‐dose combination of netupitant and palonosetron.
Primary Efficacy Analyses
The proportion of patients achieving CR during the overall phase in the PP cohort (primary endpoint) is presented in Figure 2. Noninferiority of each DEX‐sparing regimen compared with the reference treatment was demonstrated, as the lower boundaries of the 95% CI of the difference with the reference arm were greater than the preset threshold of −15% difference. Similar result of both DEX‐sparing regimens was also observed during the delayed phase. In the ITT cohort, CR rates during both the overall and delayed phases were 69.1%, 68.2%, and 68.7% for the DEX1, DEX3, and DEX4 arms, respectively; noninferiority of DEX1 and DEX4, and DEX3 and DEX4 was also demonstrated in this cohort.
Figure 2.
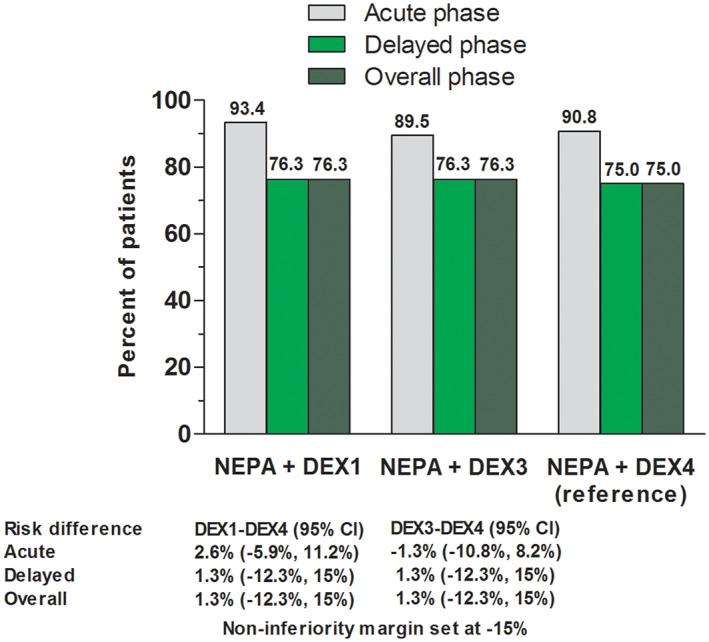
Proportion of patients with complete response (no emesis and no use of rescue medication) in per‐protocol cohort. Noninferiority of each dexamethasone‐sparing regimen compared with the reference treatment was demonstrated, as the lower boundaries of the 95% confidence interval of the difference with the reference arm were greater than the preset threshold of −15%. Abbreviations: CI, confidence interval; DEX1, dexamethasone day 1; DEX3, dexamethasone day 1–3; DEX4, dexamethasone day 1–4; NEPA, fixed‐dose combination of netupitant and palonesetron.
Secondary Efficacy Analyses
Secondary efficacy analyses are presented in Table 3. Response rates for all efficacy endpoints at all time intervals were similar for the DEX‐sparing regimens and the reference treatment, except on acute phase in which fewer patients in the DEX3 arm had no nausea and NSN compared with patients receiving the reference treatment. Although the proportion of patients with no nausea was numerically higher for the DEX4 arm than DEX1 and DEX3 arms during the delayed and overall phases, the proportion of patients with NSN was similar for all treatment groups during the delayed and overall phases.
Table 3.
Rates of secondary efficacy endpoints in the study (per‐protocol cohort)
| Endpoint, time period (hours) | NEPA + DEX1 (n = 76) | NEPA + DEX3 (n = 76) | NEPA + DEX4 (n = 76) | ||
|---|---|---|---|---|---|
| % of patients | RD, % (95% CIa) | % of patients | RD, % (95% CIa) | % of patients | |
| No emetic episodes | |||||
| Acute (0–24) | 96.1 | −2.6 (−7.7–2.4) | 96.1 | −2.6 (−7.7–2.4) | 98.7 |
| Delayed (>24–120) | 92.1 | −5.3 (−12.3–1.8) | 90.8 | −6.6 (−14.0–0.9) | 97.4 |
| Overall (0–120) | 92.1 | −5.3 (−12.3–1.8) | 90.8 | −6.6 (−14.0–0.9) | 97.4 |
| Complete protectionb | |||||
| Acute (0–24) | 90.8 | 1.3 (−8.2–10.8) | 80.3 | −9.2 (−20.5–2.1) | 89.5 |
| Delayed (>24–120) | 73.7 | 6.6 (−7.9–21.1) | 67.1 | 0 (−14.9–14.9) | 67.1 |
| Overall (0–120) | 73.7 | 6.6 (−7.9–21.1) | 67.1 | 0 (−14.9–14.9) | 67.1 |
| No nausea | |||||
| Acute (0–24) | 73.7 | −10.5 (−23.4–2.3) | 68.4 | −15.8 (−29.1 to −2.5c) | 84.2 |
| Delayed (>24–120) | 50.0 | −10.5 (−26.2–5.2) | 48.7 | −11.8 (−27.6–3.9) | 60.5 |
| Overall (0–120) | 48.7 | −11.8 (−27.6–3.9) | 46.1 | −14.5 (−30.2–1.2) | 60.5 |
| No significant nausea | |||||
| Acute (0–24) | 93.4 | −3.9 (−10.6–2.7) | 86.8 | −10.5 (−18.9 to −2.1c) | 97.3 |
| Delayed (>24–120) | 77.6 | 1.3 (−12.1–14.7) | 73.7 | −2.6 (−16.4–11.1) | 76.3 |
| Overall (0–120) | 77.6 | 1.3 (−12.1–14.7) | 73.7 | −2.6 (−16.4–11.1) | 76.3 |
| No rescue use | |||||
| Acute (0–24) | 97.4 | 6.6 (−0.9–14.0) | 92.1 | 1.3 (−7.6–10.2) | 90.8 |
| Delayed (>24–120) | 78.9 | 3.9 (−9.4–17.3) | 80.3 | 5.3 (−8.0–18.5) | 75.0 |
| Overall (0–120) | 78.9 | 3.9 (−9.4–17.3) | 80.3 | 5.3 (−8.0–18.5) | 75.0 |
The 95% confidence interval for the risk difference between the DEX1 or DEX3 groups and the DEX4 group.
Complete protection defined as no emetic episodes, no use of rescue medication, and no more than mild nausea.
Two‐sided p value < .05 in a post hoc superiority contrast.
Abbreviations: CI, confidence interval; DEX1, dexamethasone day 1; DEX3, dexamethasone day 1 to 3; DEX4, dexamethasone day 1 to 4; NEPA, fixed‐dose combination of netupitant and palonosetron; RD, risk difference.
Safety
Table 4 shows patient‐reported severity of prespecified DEX‐related side effects. Although the proportion of patients who graded their severity of any DEX‐related side effect on day 6 as either “quite a bit” or “very much” was higher in the DEX3 and/or DEX4 arms than in the DEX1 arm, the differences were small and not clinically significant. The most common treatment‐related AEs are listed in supplemental online Table 1. Grade 3 gastritis (n = 1) and fatigue (n = 1) related to antiemetic treatment were reported in two patients in the DEX3 arm.
Table 4.
Patient‐reported severity of dexamethasone‐related side effects
| Side effect | NEPA + DEX1 n (%) | NEPA + DEX3 n (%) | NEPA + DEX4 n (%) |
|---|---|---|---|
| Indigestion/heartburn or reflux | (n = 74) | (n = 74) | (n = 74) |
| Not at all | 36 (48.6) | 33 (44.6) | 38 (51.4) |
| A little bit | 17 (23.0) | 16 (21.6) | 17 (23.0) |
| Quite a bit | 10 (13.5) | 16 (21.6) | 14 (18.9) |
| Very much | 11 (14.9) | 9 (12.2) | 5 (6.8) |
| Insomnia | (n = 75) | (n = 74) | (n = 75) |
| Not at all | 43 (57.3) | 38 (51.4) | 35 (46.7) |
| A little bit | 19 (25.3) | 17 (23.0) | 21 (28.0) |
| Quite a bit | 8 (10.7) | 13 (17.6) | 16 (21.3) |
| Very much | 5 (6.7) | 6 (8.1) | 3 (4.0) |
| Hiccups | (n = 75) | (n = 74) | (n = 75) |
| Not at all | 52 (69.3) | 46 (62.2) | 43 (57.3) |
| A little bit | 12 (16.0) | 22 (29.7) | 16 (21.3) |
| Quite a bit | 9 (12.0) | 4 (5.4) | 10 (13.3) |
| Very much | 2 (2.7) | 2 (2.7) | 6 (8.1) |
| Agitation | (n = 75) | (n = 74) | (n = 75) |
| Not at all | 40 (53.3) | 37 (50.0) | 34 (45.3) |
| A little bit | 21 (28.0) | 14 (18.9) | 26 (34.7) |
| Quite a bit | 11 (14.7) | 19 (25.7) | 14 (18.7) |
| Very much | 3 (4.0) | 4 (5.4) | 1 (1.3) |
| Facial rash/ acne | (n = 75) | (n = 74) | (n = 75) |
| Not at all | 70 (93.3) | 61 (82.4) | 69 (92.1) |
| A little bit | 5 (6.7) | 8 (10.8) | 4 (5.3) |
| Quite a bit | 0 | 4 (5.4) | 1 (1.3) |
| Very much | 0 | 1 (1.4) | 1 (1.3) |
Abbreviations: DEX1, dexamethasone day 1; DEX3, dexamethasone day 1 to 3; DEX4, dexamethasone day 1 to 4; NEPA, fixed‐dose combination of netupitant and palonosetron.
Discussion
The recent European Society for Medical Oncology guidance for supportive care during the COVID‐19 pandemic recommends that a reduced dose of DEX on day 1 without additional use in the following days should be considered even in HEC [10]. Compelling clinical research demonstrated that the DEX‐sparing strategy evaluating palonosetron in combination with single‐dose DEX, with or without an NK‐1RA, was not associated with significant loss in antiemetic control during the 5‐day period following the administration of AC‐based HEC [7, 8, 11]. Current guidelines recommend prophylaxis consisting of a 5‐HT3RA, an NK‐1RA and multiple‐day DEX, with or without olanzapine, for the prevention of CINV induced by cisplatin [1, 2]. For this challenging setting of CINV, we designed a randomized study that included two investigational arms in which patients received oral NEPA in combination with DEX administered either on day 1 only (the optimal DEX‐sparing strategy) [7, 8] or also on days 2 and 3 but administered at a lower dose compared with the DEX 8 mg recommended by guidelines [1, 2]. Administration of the multidrug prophylaxis of NEPA plus DEX only once per cycle before cisplatin would represent a clinically relevant achievement. This approach would simplify antiemetic prophylaxis as well as improve adherence to guidelines in daily clinical practice [12].
The present study is a proof of concept. For the first time, this study demonstrates that comparable control of vomiting and nausea can be achieved with DEX given on day 1 only compared with additional DEX doses on days 2–4, when combined with NEPA in patients receiving cisplatin‐based HEC. The CR rate during the overall phase in the DEX3 arm was also noninferior to that observed in the reference DEX4 arm. Because the overall efficacy findings did not change substantially between the two investigational regimens, the results observed in the DEX3 arm provide additional support of the efficacy of the optimal DEX‐sparing strategy in the challenging setting of cisplatin. Although a recent phase III study by Ito et al. [13] demonstrated that administration of DEX on days 2 and 3 can be spared when combined with palonosetron and 3‐day aprepitant in patients treated with HEC, the majority of patients (77%) were women undergoing AC, a setting where DEX is not recommended on days 2–3. Therefore, post hoc subgroup analyses were performed in the patients who received cisplatin; however, noninferiority of the DEX‐sparing regimen was not demonstrated in this cisplatin subset [13]. Therefore, the study was inconclusive about the value of the DEX‐sparing strategy in the management of cisplatin‐induced CINV [14].
Overall, secondary efficacy endpoints analyzed during the delayed and overall phases are supportive of the noninferiority outcome of the primary endpoint between the DEX1 and DEX3 arms and the reference arm. As nausea remains a key issue in CINV control, it is encouraging that the results of both investigational arms were very close to the reference ones also for the composite endpoint of CP and comparable rates of NSN were seen for all treatment arms.
To put the efficacy findings into clinical perspective, clinicians should kept in mind the following key points: (a) all patients received cisplatin at a dose of 70 mg/m2 or greater, (b) within the reference arm, prophylactic DEX was administered at the guideline‐recommended dose for 4 days [1, 2], and (c) the proportion of patients achieving overall CR in the reference arm is quite consistent with the CR rate observed in a recent phase III study of NEPA plus 4‐day DEX in cisplatin [15]. Recently, the authors of a superiority phase III study claimed that olanzapine 5 mg combined with palonosetron, standard 3‐day aprepitant and 4‐day DEX could be the new standard antiemetic prophylactic regimen to improve control of CINV, especially nausea, in patients undergoing cisplatin‐based HEC [16]. Our findings call into question the clinical relevance of multiple‐day DEX to control CINV caused by cisplatin, in light of the DEX‐sparing strategy with NEPA, which also offers clinicians an opportunity to considerably simplify the four‐drug prophylaxis containing olanzapine. Therefore, clinicians might wish to add olanzapine to the DEX‐sparing regimen to improve nausea control in the challenging setting of cisplatin [1, 2].
In the study by Ito et al. [13] only hot flushes and tremors were observed more frequently among the patients receiving multiple‐day DEX during the delayed phase in cycle 1. We did not observe any noteworthy differences across groups with respect to the self‐reported incidence and severity of prespecified DEX‐related side effects during the 5‐day observation period. These findings might be explained by the fact that patient reporting of treatment‐related side effects is highly subjective and, because collective side effects were reported only on day 6, these results may be underreported. However, recent prospective evidence also highlights the importance of evaluating side effects associated with cumulative DEX doses over multiple consecutive cycles of chemotherapy [17, 18, 19].
The study has some limitations. First, this study was unblind and this could have potentially influenced the results. However, the consistency of the overall findings observed between the two DEX‐sparing regimens supports the validity of the study results. Second, females represented only 33% of the study population; however, this figure is consistent with recent evidence regarding patients undergoing cisplatin‐containing chemotherapy [15, 16]. In addition, gender, which was well balanced for the DEX1 and DEX4 arms (the primary comparison within the study), should not have influenced the results.
Conclusion
DEX‐sparing on days 2–4 can be an equally effective option for preventing CINV in the high–emetic‐risk setting of cisplatin‐based chemotherapy, when combined with NEPA. The simplified regimen of NEPA plus single‐dose DEX also permits administration of three‐drug prophylaxis only once per cycle prior to cisplatin.
Author Contributions
Conception/design: Luigi Celio, Emilio Bria, Sara Pilotto
Provision of study material or patients: Diego Cortinovis, Alessio Aligi Cogoni, Luigi Cavanna, Olga Martelli, Simona Carnio, Elena Collovà, Federica Bertolini, Fausto Petrelli, Alessandra Cassano, Rita Chiari, Francesca Zanelli, Salvatore Pisconti, Isabella Vittimberga, Antonietta Letizia, Andrea Misino, Angela Gernone, Sara Pilotto
Collection and/or assembly of data: Diego Cortinovis, Alessio Aligi Cogoni, Luigi Cavanna, Olga Martelli, Simona Carnio, Elena Collovà, Federica Bertolini, Fausto Petrelli, Alessandra Cassano, Rita Chiari, Francesca Zanelli, Salvatore Pisconti, Isabella Vittimberga, Antonietta Letizia, Andrea Misino, Angela Gernone, Sara Pilotto
Data analysis and interpretation: Erminio Bonizzoni, Luigi Celio, Emilio Bria, Sara Pilotto
Manuscript writing: Luigi Celio, Emilio Bria
Final approval of manuscript: Luigi Celio, Diego Cortinovis, Alessio Aligi Cogoni, Luigi Cavanna, Olga Martelli, Simona Carnio, Elena Collovà, Federica Bertolini, Fausto Petrelli, Alessandra Cassano, Rita Chiari, Francesca Zanelli, Salvatore Pisconti, Isabella Vittimberga, Antonietta Letizia, Andrea Misino, Angela Gernone, Erminio Bonizzoni, Sara Pilotto, Sabino De Placido, Emilio Bria
Disclosures
Luigi Celio: Italfarmaco SpA, Kyowa Kirin (C/A); Sara Pilotto: AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Merck, Roche (C/A), Astrazeneca, Bristol‐Myers Squibb (RF—outside the submitted manuscript); Emilio Bria: Roche, Pfizer (SAB), AstraZeneca, Roche (RF), Merck Sharpe & Dohme, AstraZeneca, Celgene, Pfizer, Helsinn, Eli Lilly & Co., Bristol‐Myers Squibb, Novartis, Roche (Other—speakers’ and travels’ fee). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table S1 Most common treatment‐related adverse events
Acknowledgments
We thank the patients, nurses, and data managers who participated in this study. We also thank Italfarmaco S.p.A., Milan, Italy, for the generous gift of NEPA. Emilio Bria is supported by Institutional funds of Università Cattolica del Sacro Cuore (UCSC‐project D1‐2019‐2020). Emilio Bria is currently supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) under Investigator Grant (IG) no. IG20583. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1.Roila F, Molassiotis A, Herrstedt J et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27(suppl 5):v119–133. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Kris MG, Basch E et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:3240–3261. [DOI] [PubMed] [Google Scholar]
- 3.Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: Dosing, efficacy, and tolerability analysis. Ann Oncol 2007;18:233–240. [DOI] [PubMed] [Google Scholar]
- 4.Vardy J, Chiew KS, Galica J et al. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 2006;94:1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy‐induced nausea and vomiting (CINV): A comprehensive review. Ann Oncol 2015;26:1081–1090. [DOI] [PubMed] [Google Scholar]
- 6.Celio L, Denaro A, Canova S et al. Clinical update on palonosetron in the management of chemotherapy‐induced nausea and vomiting. Tumori 2008;94:447–452. [DOI] [PubMed] [Google Scholar]
- 7.Aapro M, Fabi A, Nolè F et al. Double‐blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 2010;21:1083–1088. [DOI] [PubMed] [Google Scholar]
- 8.Aapro M, Rugo H, Rossi G et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed‐dose combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 2014;25:1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesketh PJ, Rossi G, Rizzi G et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following highly emetogenic chemotherapy: A randomized dose ranging pivotal study. Ann Oncol 2014;25:1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ESMO guidelines: supportive care strategies during the COVID‐19 pandemic . Available at https://www.esmo.org/guidelines/cancer‐patient‐management‐during‐thecovid‐19‐pandemic/supportive‐care‐in‐the‐covid‐19‐era. Accessed on May 21, 2021.
- 11.Celio L, Bonizzoni E, Zattarin E et al. Impact of dexamethasone‐sparing regimens on delayed nausea caused by moderately or highly emetogenic chemotherapy: A meta‐analysis of randomised evidence. BMC Cancer 2019;19:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aapro M, Molassiotis A, Dicato M et al. The effect of guideline‐consistent antiemetic therapy on chemotherapy‐induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER). Ann Oncol 2012;23:1986–1992. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Tsuda T, Minatogawa H et al. Placebo‐controlled, double‐blind phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin‐1 receptor antagonist and palonosetron in high‐emetogenic chemotherapy. J Clin Oncol 2018;36:1000–1006. [DOI] [PubMed] [Google Scholar]
- 14.Celio L, Bonizzoni E, Aapro M. Is the dexamethasone‐sparing strategy ready for cisplatin? Too early for an answer. J Clin Oncol 2018;36:2741–2742. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Lu S, Feng J et al. A randomized phase III study evaluating the efficacy of single‐dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy‐induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol 2018;29:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto H, Abe M, Tokuyama O et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy‐induced nausea and vomiting (J‐FORCE): A multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2020;21:242–249. [DOI] [PubMed] [Google Scholar]
- 17.Han HS, Park JC, Park SY et al. A prospective multicenter study evaluating secondary adrenal suppression after antiemetic dexamethasone therapy in cancer patients receiving chemotherapy: A Korean South West Oncology Group study. The Oncologist 2015;20:1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura M, Ishiguro A, Muranaka T et al. A prospective observational study on effect of short‐term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO‐01). The Oncologist 2017;22:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong Y, Han HS, Lee HD et al. A pilot study evaluating steroid‐induced diabetes after antiemetic dexamethasone therapy in chemotherapy‐treated cancer patients. Cancer Res Treat 2016;48:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table S1 Most common treatment‐related adverse events


