Abstract
Background
Immune checkpoint inhibitors (ICIs) are an important treatment for metastatic renal cell carcinoma (mRCC). These agents may cause immune‐related adverse events (irAEs), and the relationship between irAEs and outcomes is poorly understood. We investigated the association between irAEs and clinical outcomes in patients with mRCC treated with ICIs.
Methods
We performed a retrospective study of 200 patients with mRCC treated with ICIs at Winship Cancer Institute from 2015 to 2020. Data on irAEs were collected from clinic notes and laboratory values and grades were determined using Common Terminology Criteria in Adverse Events version 5.0. The association with overall survival (OS) and progression‐free survival (PFS) was modeled by Cox proportional hazards model. Logistic regression models were used to define odds ratios (ORs) for clinical benefit (CB). Landmark analysis and extended Cox models were used to mitigate lead‐time bias by treating irAEs as a time‐varying covariate.
Results
Most patients (71.0%) were male, and one‐third of patients (33.0%) experienced at least one irAE, most commonly involving the endocrine glands (13.0%), gastrointestinal tract (10.5%), or skin (10.0%). Patients who experienced irAEs had significantly longer OS (hazard ratio [HR], 0.52; p = .013), higher chance of CB (OR, 2.10; p = .023) and showed a trend toward longer PFS (HR, 0.71; p = .065) in multivariate analysis. Patients who had endocrine irAEs, particularly thyroid irAEs, had significantly longer OS and PFS and higher chance of CB. In a 14‐week landmark analysis, irAEs were significantly associated with prolonged OS (p = .045). Patients who experienced irAEs had significantly longer median OS (44.5 vs. 18.2 months, p = .005) and PFS (7.5 vs. 3.6 months, p = .003) without landmark compared with patients who did not.
Conclusion
We found that patients with mRCC treated with ICIs who experienced irAEs, particularly thyroid irAEs, had significantly improved clinical outcomes compared with patients who did not have irAEs. This suggests that irAEs may be effective clinical biomarkers in patients with mRCC treated with ICIs. Future prospective studies are warranted to validate these findings.
Implications for Practice
This study found that early onset immune‐related adverse events (irAEs) are associated with significantly improved clinical outcomes in patients with metastatic renal cell carcinoma (mRCC) treated with immune checkpoint inhibitors (ICIs). In this site‐specific irAE analysis, endocrine irAEs, particularly thyroid irAEs, were significantly associated with improved clinical outcomes. These results have implications for practicing medical oncologists given the increasing use of ICIs for the treatment of mRCC. Importantly, these results suggest that early irAEs and thyroid irAEs at any time on treatment with ICIs may be clinical biomarkers of clinical outcomes in patients with mRCC treated with ICIs.
Keywords: Immune checkpoint inhibitors, Renal cell carcinoma, Immune‐related adverse events, Clinical biomarkers, Clinical outcomes
Short abstract
This study investigated the association between immune‐related adverse events and clinical outcomes in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors.
Introduction
Renal cell carcinoma (RCC) is one of the most common urologic cancers, with approximately 74,000 new cases and 15,000 deaths in the U.S. every year [1]. Although the prognosis in metastatic RCC (mRCC) has traditionally been poor, several new agents have been approved over the past decade that have improved response rates and survival rates compared with older agents [2]. Over the past 5 years, the development of novel immune checkpoint inhibitors (ICIs) has revolutionized the treatment of many solid tumor malignancies including mRCC. ICI‐based treatment regimens for mRCC consist of monotherapy with the programmed death protein‐1 (PD‐1) inhibitor, nivolumab, or combination therapy with dual anti–PD‐1/cytotoxic T‐lymphocyte associated protein 4 (CTLA‐4) inhibition or anti–PD‐1/vascular endothelial growth factor inhibition. Although these regimens are particularly attractive because of their promise of durable responses [3], a subset of patients do not respond to treatment. Furthermore, there have been increased efforts to identify predictive and prognostic biomarkers of response to treatment to help identify which patients would be more likely to derive clinical benefit from treatment with ICIs. Although earlier efforts have identified polybromo 1 (PBRM1) mutation as a clinically validated biomarker of response to ICI in patients with mRCC [4, 5], there has been limited investigation into the prognostic value of clinical biomarkers. Hence, the identification of clinical biomarkers of response to ICIs is an unmet need in the field of oncology.
ICIs target cell surface proteins that enable cancer cells to evade immune surveillance [6]. Although these agents generally have a favorable toxicity profile, they may induce immune dysregulation, which causes immune‐related adverse events (irAEs). These treatment‐related toxicities can affect any organ, with the most common sites of involvement being the gastrointestinal (GI) tract, skin, joints, endocrine glands, and lungs [7]. The underlying biologic explanation of these irAEs is thought to be bystander effect by activated T cells and possible cross reactivity between healthy host end‐organ tissue and malignant cells [8]. Although the majority of irAEs are mild, some patients experience moderate to severe irAEs that may be associated with organ dysfunction, declines in quality of life, and treatment discontinuation [9].
It is hypothesized that irAEs may be associated with improved oncologic outcomes given that they may be reflective of a treatment‐responsive host immune system [8]. In melanoma and non‐small cell lung cancer (NSCLC), dermatologic irAEs have been shown to be associated with improved outcomes [10, 11]. Although some studies have found an association with irAEs and improved clinical outcomes, this relationship has not been fully elucidated in mRCC. Furthermore, there are limited data on whether there is differential prognostic and predictive value of distinct end‐organ involvement by irAEs in patients with mRCC treated with ICI‐based treatment regimens. Taken together, there is an unmet need for further characterization of the relationship between incidence and sites of irAEs and clinical outcomes in patients with mRCC treated with ICI‐based treatment regimens.
In this study, we investigated the association between incidence and sites of irAEs and clinical outcomes in patients with mRCC treated with ICIs. We had a secondary aim of using advanced statistical methods to control for lead‐time bias in assessing the relationship between irAEs and clinical outcomes. We hypothesized that patients who experience irAEs would have improved clinical outcomes, given that irAEs may be indicative of a treatment‐responsive host immune system. An additional goal of this manuscript is to provide clinical data to stimulate biological investigation of possible shared antigens between healthy organ tissue and malignant RCC cells to explain why some irAEs may be more associated with oncologic outcomes than other end‐organ sites. Although this study focuses on patients with mRCC specifically, the results from this study may have broader implications for practicing medical oncologists given that ICIs are a common treatment in several malignancies.
Materials and Methods
Patients and Data
We performed a retrospective review of 200 patients with mRCC who received ICI‐based treatment regimens at Winship Cancer Institute of Emory University from 2015 to 2020. This study was approved by the Emory University Institutional Review Board. A drug administration pharmacy database was used to identify all patients who received at least one dose of an ICI at Winship Cancer Institute from 2015 to 2020. Inclusion criteria for this study were the following: (a) confirmed histologic diagnosis of RCC, (b) receipt of at least one dose of an ICI‐based treatment regimen, and (c) availability of follow‐up data for at least 1 month after initiation of ICIs.
Clinical data collected from the electronic medical record included age, race, gender, date of RCC diagnosis, RCC histologic subtype, number of systemic treatments prior to first dose of ICI, and Eastern Cooperative Oncology Group performance status (ECOG PS). The following laboratory values were also collected at the time of ICI initiation: platelet count, absolute neutrophil count (ANC), hemoglobin, calcium, and albumin. International mRCC database consortium (IMDC) risk groups were determined at the time of ICI initiation and based on the following variables: time from diagnosis to systemic treatment <1 year, platelet count > upper limit of normal (ULN), ANC > ULN, ECOG PS ≥2, corrected calcium (serum calcium, mg/dL) + 0.8 (4.0 − serum albumin level, g/dL) > ULN, and hemoglobin < lower limit of normal. Patients were characterized in IMDC risk groups based on the number of risk factors present at the time of first dose of ICI (favorable = 0 risk factors, intermediate = 1–2 risk factors, poor ≥3 risk factors). Clinical data on irAEs were collected from clinic notes, hospitalization records, laboratory values, and radiology reports. All irAEs were graded by the senior author and the study team per Common Terminology Criteria in Adverse Events version 5.0 (CTCAE v5.0). Severe irAEs were defined as grade 3 or grade 4 per CTCAE v5.0. Sites of irAEs were categorized as pulmonary, dermatologic, musculoskeletal (MSK), GI, endocrine, renal, or other. GI irAEs were further categorized as hepatic, pancreatic, or colonic irAEs. Endocrinopathies were further characterized as thyroid, adrenal, or pituitary irAEs. Data on treatments received for irAEs were also collected, including topical, oral, or intravenous immunosuppressive agents.
Overall survival (OS), progression‐free survival (PFS), and clinical benefit (CB) were used to measure clinical outcomes. OS and PFS were calculated as the number of months elapsed from first dose of ICI to date of death and radiographic or clinical progression, respectively. Patients who were lost to follow‐up were censored at date of last follow‐up. Radiographic responses to ICI were determined by reviewing clinic notes and radiology reports using RECIST v1.1. The senior author reviewed cases and determined response in situations in which the response was not clearly documented. CB was defined as a best radiographic response of complete response (CR), partial response (PR), or stable disease (SD) for ≥ 6 months per RECIST v1.1.
Statistical Analysis
Statistical analysis was conducted using SAS Version 9.4 (SAS Institute, Cary, NC), and SAS macros developed by Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute [12]. Univariate associations between each variable and the two study cohorts (irAEs vs. no‐irAEs) were calculated using the χ2 test or Fisher's exact test for categorical covariates and ANOVA for numerical covariates. The association of irAEs with OS and PFS was modeled by Cox proportional hazards model, and the multivariable models were built by a backward variable selection procedure with an α = 0.2 removal criteria. Univariate and multivariable analyses (UVA and MVA, respectively) were performed using logistic regression models to estimate odds ratios (ORs) for CB. UVA was performed to investigate the association between sites of irAEs and clinical outcomes and MVA was performed for sites of irAEs that were significantly associated with OS, PFS, and CB in UVA. MVA for all outcomes controlled for the possible confounding effect of age, race, gender, IMDC risk group, number of prior lines of systemic therapy, PD‐1 monotherapy, and clear cell RCC (ccRCC) histology. Kaplan‐Meier curves were created for survival estimates.
Lead‐Time Analysis
Landmark analysis was performed for OS or PFS to alleviate possible lead‐time bias introduced by the natural time‐dependency by irAEs. We set the landmark at 14 weeks after first dose of ICI, and patients who were lost to follow‐up or died prior to 14 weeks were excluded from the analysis. This time point was chosen because it approximately correlates clinically to the first radiographic assessment of patients with mRCC on ICI‐based treatment regimens. Patients who experienced an irAE were categorized as responders, and patients who had not responded by the landmark time were categorized as nonresponders. Kaplan‐Meier curves were generated to compare the survival estimates of the two cohorts by using log‐rank tests at landmark time [13, 14]. A supplemental analysis using an extended Cox model was also performed to assess the dynamic risk associated with irAEs, regardless of the time of irAE relative to the start of treatment. In this model, irAE status is not treated as fixed but rather allows irAE status to change when it occurs during the follow‐up period. The extended model aims to quantify how much the risk changes once patients develop irAEs [15, 16].
Results
Demographic and Baseline Disease Characteristics
Baseline demographics, disease characteristics, and descriptive statistics by irAEs are presented in Table 1. Most patients were male (71.0%) and 78.1% had ccRCC. More than three‐quarters of patients (n = 159, 79.5%) were White, whereas 19.0% (n = 38) were Black. The majority were IMDC intermediate (n = 113, 57.1%) or poor risk (n = 51, 25.8%). Anti–PD‐1 monotherapy was the most common (n = 115, 57.5%) treatment regimen, and most patients received either zero (n = 77, 38.5%) or one (n = 84, 42.0%) prior line of systemic therapy treatment. Among combination ICI regimens, anti–PD‐1/anti–CTLA‐4 was the most common (n = 56, 28.0%). Patients who received anti–PD‐1 monotherapy were significantly less likely to experience irAEs compared with those who received combination therapy (34.9% vs. 65.2%; p < .001). Most patients (n = 162, 83.1%) had an ECOG PS of 0 or 1. Half of all patients (n = 100, 50.0%) had at least three metastatic sites at the time of their first dose of ICI.
Table 1.
Descriptive statistics by immune‐related adverse events
| Covariate | n (%) | Immune‐related adverse events | Parametric p valuea | |
|---|---|---|---|---|
| No, n = 134 | Yes, n = 66 | |||
| Race | .092 | |||
| Black/Asian | 41 (20.5) | 32 (23.9) | 9 (13.6) | |
| White | 159 (79.5) | 102 (76.1) | 57 (86.4) | |
| Gender | .201 | |||
| Female | 58 (29.0) | 35 (26.1) | 23 (34.9) | |
| Male | 142 (71.0) | 99 (73.9) | 43 (65.2) | |
| Clear cell RCC | .267 | |||
| No | 42 (21.9) | 25 (19.5) | 17 (26.6) | |
| Yes | 150 (78.1) | 103 (80.5) | 47 (73.4) | |
| PD‐1 monotherapyb | <.001 | |||
| No | 85 (42.5) | 42 (31.3) | 43 (65.2) | |
| Yes | 115 (57.5) | 92 (68.7) | 23 (34.9) | |
| IMDC risk group | .274 | |||
| Favorable | 34 (17.1) | 19 (14.4) | 15 (22.7) | |
| Intermediate | 113 (57.1) | 76 (57.6) | 37 (56.1) | |
| Poor | 51 (25.8) | 37 (28.0) | 14 (21.2) | |
| Prior lines | .003 | |||
| 0 | 77 (38.5) | 43 (32.1) | 34 (51.5) | |
| 1 | 84 (42.0) | 56 (41.8) | 28 (42.4) | |
| 2 | 20 (10.0) | 18 (13.4) | 2 (3.0) | |
| ≥3 | 19 (9.5) | 17 (12.7) | 2 (3.0) | |
| Age | .833 | |||
| Mean | 62.96 | 62.61 | ||
| Median | 64 | 63 | ||
| Min | 33 | 23 | ||
| Max | 90 | 85 | ||
| SD | 11.25 | 10.55 | ||
The parametric p value is calculated by ANOVA for numerical covariates and χ2 test or Fisher's exact test for categorical covariates.
Treatment regimen breakdown: 115 PD‐1 monotherapy (57.5%), 56 immune checkpoint inhibitor (ICI)/ICI combination (28.0%), 19 ICI/vascular endothelial growth factor receptor combination (9.5%), 10 ICI/investigational agent (5%).
Abbreviations: IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; PD‐1, programmed death protein‐1; RCC, clear cell renal cell carcinoma.
Toxicity Data
The incidence and distribution of sites of irAEs are presented in Table 2. Approximately one‐third of patients (n = 66, 33.0%) experienced at least one irAE, with 24 patients (12.0%) experiencing multiple irAEs. The most common irAEs involved endocrine glands (13.0%), the GI tract (10.5%), or the skin (10.0%). Among endocrine glands involved, thyroid was the most common (n = 17, 8.5%), followed by the adrenal glands (n = 8, 4.0%) and the pituitary (n = 4, 2.0%). Among the 24 patients with multiple irAEs, 18 had endocrine involvement. MSK irAEs (n = 8, 4.0%) and renal irAEs (n = 7, 3.5%) were less common irAE sites. Gastrointestinal irAEs most commonly involved the liver (n = 14, 7.0%), followed by the pancreas (n = 5, 2.5%) and the colon (n = 2, 1.0%). Lung involvement with pneumonitis was seen in five patients (2.5%). Incidence of irAEs was significantly lower in patients receiving anti–PD‐1 monotherapy compared with patients receiving combination therapy (34.9% vs. 65.2%, p < .001). More than half of patients who experienced irAEs were treated with systemic corticosteroids (n = 41, 63.1%), and eight patients (12.3% of patients experience irAEs) received intravenous corticosteroids. There was no significant difference in clinical outcomes between patients who received systemic immunosuppression for the treatment of their irAEs and those who did not (supplemental online Table 1). A total of eight patients had baseline autoimmune disease; three of these patients experienced irAEs while on ICI.
Table 2.
Incidence and end‐organ involvement of irAEs
| Variable | n = 200, n (%) |
|---|---|
| ≥1 irAE | |
| Y | 66 (33.0) |
| N | 134 (67.0) |
| Multiple irAE | |
| Y | 24 (12.0) |
| N | 176 (88.0) |
| Pulmonary irAE | |
| Y | 5 (2.5) |
| N | 194 (97.5) |
| Dermatologic irAE | |
| Y | 20 (10.0) |
| N | 180 (90.0) |
| Endocrine irAE | |
| Y | 26 (13.0) |
| N | 173 (87.0) |
| Thyroid irAE | |
| Y | 17 (8.5) |
| N | 183 (91.5) |
| Adrenal irAE | |
| Y | 8 (4.0) |
| N | 192 (96.0) |
| Pituitary irAE | |
| Y | 4 (2.0) |
| N | 196 (98.0) |
| Renal irAE | |
| Y | 7 (3.5) |
| N | 193 (96.5) |
| MSK irAE | |
| Y | 8 (4.0) |
| N | 192 (96.0) |
| GI irAE | |
| Y | 21 (10.5) |
| N | 178 (89.0) |
| Hepatic irAE | |
| Y | 14 (7.0) |
| N | 196 (93.0) |
| Pancreatic irAE | |
| Y | 5 (2.5) |
| N | 195 (97.5) |
| Colon irAE | |
| Y | 2 (1.0) |
| N | 198 (99.0) |
| Other irAE | |
| Y | 4 (2.0) |
| N | 196 (98.0) |
Abbreviations: GI, gastrointestinal; irAE, immune‐related adverse event, MSK, musculoskeletal; N, no; Y, yes.
Data regarding the grading of irAEs per CTCAE v5.0 are provided in Table 3. A total of 100 irAEs were observed. The majority of irAEs were either grade 1 (n = 25, 25.3% of all irAEs) or grade 2 (n = 56, 56.6% of all irAEs). There were 18 severe irAEs (grade 3 n = 15; grade 4 n = 3), with more than half affecting the GI tract (n = 11). The most common severe irAEs were transaminitis (n = 6), nephritis (n = 4), and elevated lipase (n = 3). Of the 24 dermatologic irAEs, only 8.3% (n = 2) were severe. Nearly all (28 of 29, 96.6%) of the endocrine irAEs were grade 2, including all 17 thyroid irAEs. Although only seven patients experienced renal irAEs, four of these patients experienced severe (grade 3) events.
Table 3.
Grading and distribution of irAEs
| Site | Total number of events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Unknown |
|---|---|---|---|---|---|---|
| Any irAEa | 100 | 25 | 56 | 15 | 3 | 1 |
| Pulmonary | 5 | 0 | 5 | 0 | 0 | 0 |
| Dermatologic | 24 | 13 | 9 | 2 | 0 | 0 |
| Endocrineb | 30 | 0 | 28 | 1 | 0 | 1 |
| Thyroid | 17 | 0 | 17 | 0 | 0 | 0 |
| Adrenal | 9 | 0 | 9 | 0 | 0 | 0 |
| Pituitary | 4 | 0 | 2 | 1 | 0 | 1 |
| Renal | 7 | 0 | 3 | 4 | 0 | 0 |
| MSK | 8 | 5 | 3 | 0 | 0 | 0 |
| GI tractc | 21 | 3 | 7 | 8 | 3 | 0 |
| Hepatic | 14 | 2 | 6 | 6 | 0 | 0 |
| Pancreatic | 5 | 0 | 1 | 2 | 2 | 0 |
| Colon | 2 | 1 | 0 | 0 | 1 | 0 |
| Other | 5 | 4 | 1 | 0 | 0 | 0 |
All grades determined using Common Terminology Criteria for Adverse Events version 5.0.
Sum of all irAEs includes pulmonary, dermatologic, endocrine, renal, MSK, GI tract, and other categories.
Endocrine irAEs further characterized as affecting thyroid, adrenal, and pituitary glands. Total number of endocrine irAEs includes sum of thyroid irAEs, adrenal irAEs, and pituitary irAEs.
GI irAEs further characterized as having hepatic, pancreatic, or colonic involvement. Total number of GI irAE includes sum of hepatic irAEs, pancreatic irAEs, and colon irAEs.
Abbreviations: GI, gastrointestinal; irAE, immune‐related adverse events; MSK, musculoskeletal.
Association of irAEs and Clinical Outcomes
The UVA of the association between sites of irAEs and clinical outcomes is presented in supplemental online Table 2. Endocrine irAEs and thyroid irAEs were significantly associated with improved OS, PFS, and CB in UVA (all p ≤ .017), whereas GI irAEs were significantly associated with improved OS in UVA (hazard ratio [HR], 0.40; p = .040). Given that endocrine irAEs and thyroid irAEs were significantly associated with all three clinical outcomes in UVA, this relationship was investigated in MVA. The MVAs of the association between irAEs and clinical outcomes are presented in Table 4. Patients who experienced at least one irAE had significantly longer OS (HR, 0.52; 95% CI, 0.32–0.87; p = .013) and higher chance of CB (OR, 2.10; CI, 1.11–4.00; p = .023) and showed a trend toward longer PFS (HR, 0.71; CI, 0.49–1.02; p = .065) in MVA. Endocrine irAEs, specifically thyroid irAEs, were associated with significantly longer OS (endocrine HR, 0.34; CI, 0.12–0.94; p = .038; thyroid HR, 0.12; CI, 0.02–0.91; p = .04), longer PFS (endocrine HR, 0.54; CI, 0.31–0.95; p = .032; thyroid HR, 0.33; CI, 0.15–0.72; p = .005), and higher chance of CB (endocrine OR, 2.70; CI, 1.07–6.80; p = .035; thyroid OR, 9.62; CI, 2.09–44.32; p = .004) in MVA. Patients who experienced irAEs had significantly longer median OS (44.5 vs. 18.2 months; p = .005) and PFS (7.5 vs. 3.6 months; p = .0028) per Kaplan‐Meier estimation compared with patients who did not experience any irAEs (Table 4; Fig. 1). There was no significant difference in OS, PFS, or CB between patients who experienced severe irAEs and those who experienced grade 1 or 2 irAEs (supplemental online Table 1). Among the eight patients who had autoimmune disease at baseline, only one experienced CB with a best response of PR. The remaining patients either had progressive disease (n = 6) or a nonevaluable (n = 1) best response on treatment with ICI.
Table 4.
Multivariable analysis of association between irAEs and clinical outcomes
| Variable | OS | PFS | CB | |||
|---|---|---|---|---|---|---|
| HR (CI) | p value | HR (CI) | p value | OR (CI) | p value | |
| irAE, n = 66 | 0.52 (0.32–0.87) | .013a | 0.71 (0.49–1.02) | .065 | 2.10 (1.11–4.00) | .023a |
| Median, mo | 44.5 | 7.5 | ||||
| CB rate | 59% | |||||
| No irAE, n = 134 | 1 | 1 | 1 | |||
| Median, mo | 18.2 | 3.6 | ||||
| CB rate | 38% | |||||
| Thyroid analysis | ||||||
| Thyroid irAE, n = 17 | 0.12 (0.02–0.91) | .04a | 0.33 (0.15–0.72) | .005a | 9.62 (2.09–44.32) | .004a |
| No thyroid irAE, n = 183 | 1 | 1 | 1 | |||
| Endocrine analysis | ||||||
| Endocrine irAE, n = 26 | 0.34 (0.12–0.94) | .038a | 0.54 (0.31–0.95) | .032a | 2.70 (1.07–6.80) | .035a |
| No endocrine irAE, n = 174 | 1 | 1 | 1 | |||
Multivariable analysis controlled for age, race, gender, International Metastatic Rectal Cell Carcinoma Database Consortium risk group, number of prior lines of therapy, programmed death protein‐1 monotherapy, and clear cell renal cell carcinoma.
Statistical significance at α < .05.
Abbreviations: CB, clinical benefit; CI, confidence interval; HR, hazard ratio; irAE, immune‐related adverse event; OS, overall survival; PFS, progression‐free survival.
Figure 1.
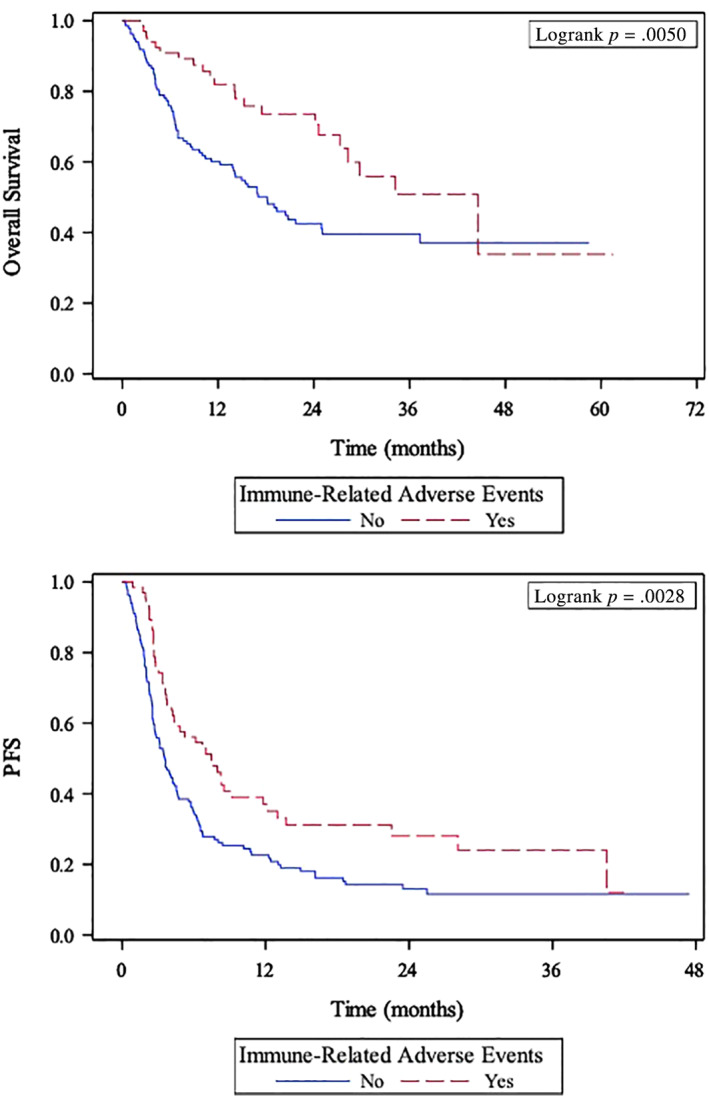
Kaplan‐Meier curves of association between immune‐related adverse events and overall survival (top panel) and progression‐free survival (bottom panel). Abbreviation: PFS, progression‐free survival.
Lead‐Time Analysis
In landmark analysis at 14 weeks after first dose of ICI, patients who did not experience irAEs has significantly shorter OS (HR, 2.10; CI, 1.00–4.03; p = .045; Fig. 2). Patients who did not experience endocrine or thyroid irAEs showed a trend toward worse OS (endocrine HR, 3.33; CI, 0.82–13.58; p = .074; thyroid HR, 4.43; CI, 0.62–31.90; p = .105) at 14 weeks. The UVA using the extended Cox model is presented in supplemental online Table 3. Once a patient experienced thyroid irAEs, the risk of dying or experiencing progression was reduced dramatically (HR, 0.133; CI, 0.019–0.958; p = .0453) in the extended Cox model. Patients who experienced any irAE or endocrine‐specific irAEs both trended toward improved OS (any irAE HR, 0.79; CI, 0.49–1.28; p = .338; endocrine irAE HR, 0.372; CI, 0.14–1.02; p = .0537) in the extended Cox model.
Figure 2.
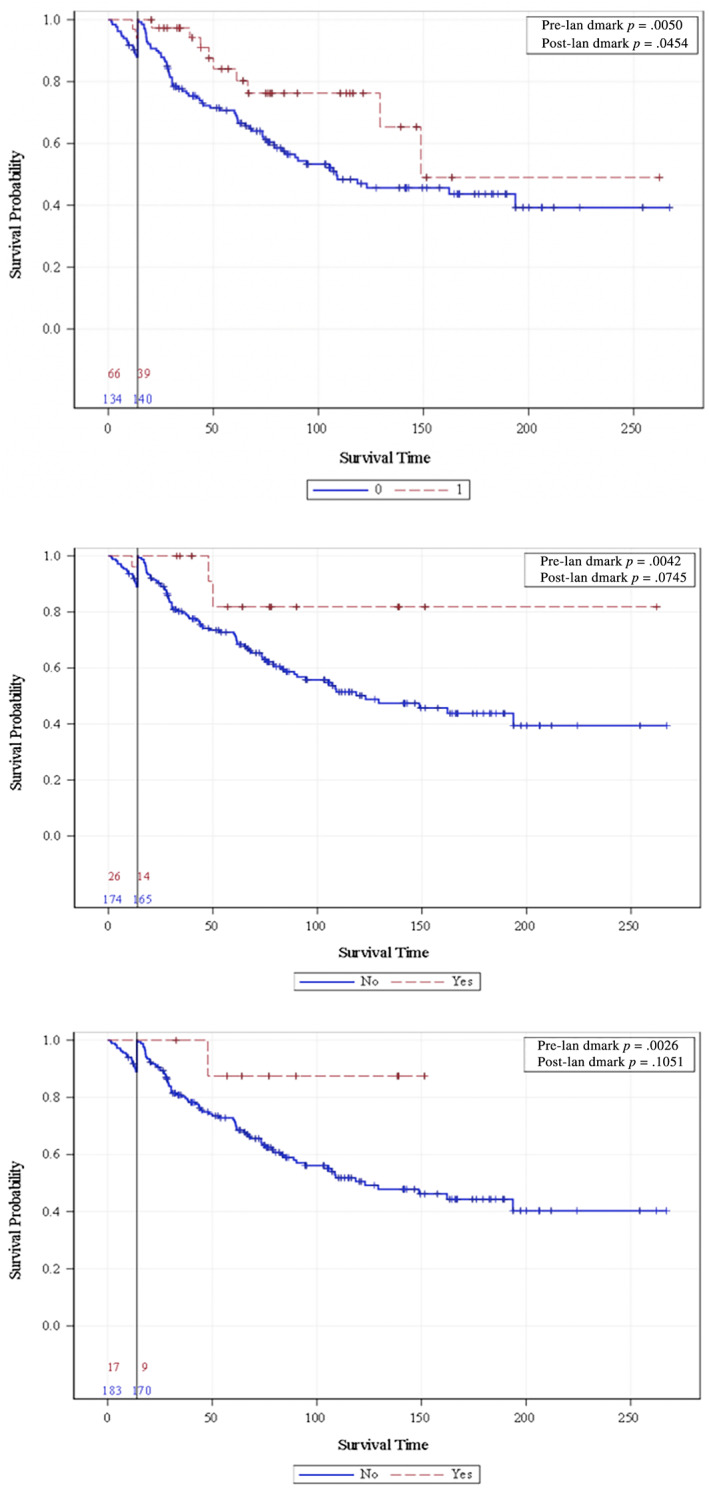
A 14‐week landmark analysis of the association between overall survival and any immune‐related adverse event (irAE; top panel), endocrine irAE (middle panel), and thyroid irAE (bottom panel).
Discussion
In this study, we reported on the incidence of irAEs and the association between irAEs and clinical outcomes in patients with mRCC treated with ICI‐based treatment regimens. Approximately one‐third of patients in this cohort experienced at least one irAE, most commonly affecting the endocrine glands, GI tract, or the skin. Notably, only 8.5% of patients experienced a severe irAE. We found that irAEs were significantly associated with prolonged OS, PFS, and increased chance of CB after controlling for several possible confounding variables. Interestingly, we found endocrine irAEs, specifically thyroid irAEs, were significantly associated with improved clinical outcomes. These findings add significantly to the current literature in mRCC regarding the relationship between irAEs and clinical outcomes, although it should be noted that our sample size of patients with endocrine (n = 26) and thyroid irAEs (n = 17) was small. Given the widespread usage of ICIs in several malignancies, these findings may have implications in many tumor types. Importantly, these clinical data suggest a possible undiscovered biological relationship between thyroid tissue and RCC that may explain why thyroid irAEs may be associated with improved oncologic outcomes in ICI‐treated patients.
Treatment‐related adverse events are thought to be reflective of a bystander process that indicates a treatment‐responsive host immune system [8]. When effective, ICIs activate T cells, which subsequently attack malignant cells. However, when T cells are activated in normal tissue, irAEs occur. It is hypothesized that patients who experience irAEs would be more likely to have a survival benefit and higher response rates compared with those who do not experience irAEs. One study of patients without melanoma treated with PD‐1 inhibitors found that irAEs were significantly associated with overall response rate [17]. A recent retrospective study of 167 patients with mRCC treated with nivolumab found that irAEs were associated with improved OS and PFS and higher response rates [18]. It should be noted, however, that these authors did not investigate the association of different sites of irAEs on clinical outcomes. Although two recent studies found an association between endocrine and thyroid irAEs and clinical outcomes in patients with NSCLC and melanoma treated with ICIs [19, 20], our study focuses specifically on patients with mRCC. One small study (n = 43) found thyroid irAEs to be significantly associated with PFS in patients with mRCC treated with ICIs [21]. However, this study did not investigate the association with OS or response rate and there were no lead‐time bias analyses performed. The data presented in our study are a significant contribution to the literature in that they are a comprehensive analysis of the relationship between incidence and sites of irAEs and clinical outcomes in patients with mRCC treated with ICI‐based treatment regimens.
Another plausible explanation for the possible association between irAEs and clinical outcomes is that there may be shared antigens between healthy tissue in specific organs and cancer cells. Furthermore, this suggests that the relationship between irAEs and outcomes may be indicative of crosstalk between the host immune system and the target tissue. This theory is supported by the relationship between vitiligo and outcomes in patients with melanoma treated with ICIs [11], given that circulating antibodies against antigens shared by both melanoma cells and normal melanocytes have been identified [22]. Interestingly, we did not find any significant association between dermatologic irAEs and outcomes in this study of patients with mRCC. An analogous relationship between acute interstitial nephritis and outcomes in mRCC has been suggested in a recent case series [23]. We were limited in our statistical assessment of the relationship between renal irAEs and oncologic outcomes given that only 3.5% of patients (n = 7) experienced renal irAEs. It should be noted, however, that one patient who experienced grade 2 nephritis had a radiographic CR to treatment and has remained progression‐free for more than 19 months despite discontinuing therapy. Similar durable responses have been observed in patients with mRCC who respond to ICIs and subsequently discontinue treatment for irAEs [24]. Larger, prospective studies with larger sample sizes of patients with mRCC with renal irAEs are needed to further elucidate the possible relationship between this specific irAE and oncologic outcomes in patients with mRCC treated with ICI‐based treatment regimens.
Endocrinopathies related to treatment ICIs have been described and are more commonly associated with CTLA‐4 inhibitors, particularly when they are used in combination with PD‐1 inhibitors such as nivolumab [25]. The relationship between thyroid irAEs in particular and clinical outcomes may be relevant for patients with mRCC treated with ICI‐based treatment regimens. Although we had a small sample size of 17 patients who experienced thyroid irAEs in this study, patients who experienced thyroid irAEs had significantly better oncologic outcomes compared with patients who did not experience thyroid irAEs. Importantly, this association was still statistically significant when controlling for lead‐time bias in the extended Cox model, which is more statistically powerful than landmark analysis. This clinical observation may have an underlying biologic explanation, as RCC has a particularly high rate of thyroid hormone receptor mutations [26], and thyroid hormone has been described as a regulator of RCC [27]. Furthermore, thyroid hormone receptor beta‐1 has been shown to act as a metastasis suppressor gene in human cancer [28]. There is no literature on shared antigens between thyroid tissue and RCC cells, but it is plausible that thyroid irAEs may be reflective of cross reactivity from a host immune system that is responding to ICI treatment in patients with mRCC. Taken together, we have provided hypothesis‐generating clinical data regarding the association between thyroid irAEs and clinical outcomes in patients with mRCC treated with ICIs, which may stimulate laboratory research investigating the biologic explanation of this relationship.
In this study, we used three statistical approaches to perform a comprehensive analysis of the relationship between irAEs and clinical outcomes: the regular Cox model, landmark analysis, and the extended Cox model. The regular Cox regression model is similar to a case‐control study, in which patients are followed from date of ICI initiation and classified into groups depending on whether or not they experienced irAEs. We performed landmark analysis at 14‐weeks after treatment initiation and the extended Cox model to control for lead‐time bias. Landmark analysis helps reduce lead‐time bias because patients who pass away within 14 weeks of treatment initiation are excluded from the analysis. We found that irAEs experienced within 14 weeks of starting ICIs were associated with significantly improved OS. This has significant clinical relevance for medical oncologists, as this suggests that onset of irAEs before the first restaging scan on ICI‐based treatment regimens may be an effective clinical biomarker of response to treatment. The drawbacks of landmark analysis are sample size reduction and arbitrary selection of landmark time, although it should be noted that we chose the 14‐week time point because it approximately correlates to the first restaging scan on ICI treatment. We also used the extended Cox model because this model assesses irAEs status dynamically and focuses on estimating the change in risk once the patient experiences an irAE, regardless of when the irAE occurred. The nonsignificance in the extended Cox model is mainly due to the short follow‐up time and the relatively small number of cases. As previously discussed, the extended Cox model UVA showed that thyroid irAEs were significantly associated with OS. This may be a stronger endorsement of thyroiditis as a clinical biomarker of outcomes in patients with mRCC treated with ICI. Regardless of the statistical method we used, we found a similar trend that irAEs are prognostic biomarkers of clinical outcomes. This was particularly true for early onset irAEs and thyroid irAEs at any point on treatment.
Although this study provides clinically relevant and hypothesis‐generating data that may be useful for practicing medical oncologists, there are several limitations that should be mentioned. First, this is a retrospective study that is inherently subject to selection bias. We attempted to mitigate the effect of this on the results by including all patients with RCC who received at least one dose of ICI and had adequate follow‐up data, regardless of RCC subtype, number of prior lines of therapy, or type of ICI‐based regimen. Second, irAEs could have been underestimated given the heterogeneity of how they were documented in the electronic medical record. Additionally, we had a small sample size of patients with thyroid irAEs (n = 17), so our results with regard to this irAE site should be evaluated with caution. Finally, because irAEs develop after initiation of treatment, their utility as a clinical biomarker should be approached with caution because of the possible effect of lead‐time bias. To alleviate the impact of this on our analysis, we used two methods to control for lead‐time bias: landmark analysis at 14‐weeks after ICI initiation and extended Cox model, which is a more statistically powerful model. Future studies should further assess the underlying biologic explanation for the association between irAEs and clinical outcomes. Furthermore, identification of possible shared antigens between thyroid tissue and malignant RCC cells may help provide a new biomarker of response to treatment with ICI.
Conclusion
We showed that patients with mRCC who experienced irAEs, particularly thyroid irAEs, had significantly improved clinical outcomes on treatment with ICI‐based regimens. In landmark analysis, patients who experienced any irAE within 14 weeks of ICI initiation had significantly longer OS. The association between thyroid irAEs and OS was also significant in the novel extended Cox analysis, further supporting the strength of this relationship. Taken together, this suggests that early irAEs and thyroid irAEs at any time on treatment may be effective clinical biomarkers of favorable outcomes in patients with mRCC treated with ICI‐based treatment regimens. These results should be considered hypothesis generating and larger, prospective studies are needed to validate these findings. Additionally, further investigation into possible shared antigens between healthy thyroid tissue and RCC cells may lead to new biomarkers of response to treatment with ICIs.
Author Contributions
Conception/design: Dylan J. Martini, Subir Goyal, Yuan Liu, Mehmet Asim Bilen
Provision of study material or patients: Jacqueline T. Brown, Lauren Yantorni, Greta Anne Russler, Sarah Caulfield, Jamie M. Goldman, Bassel Nazha, Wayne B. Harris, Haydn T. Kissick, Viraj A. Master, Omer Kucuk, Bradley C. Carthon, Mehmet Asim Bilen
Collection and/or assembly of data: Dylan J. Martini, Sean T. Evans, T. Anders Olsen, Katherine Case, Benjamin L. Magod
Data analysis and interpretation: Subir Goyal, Yuan Liu
Manuscript writing: Dylan J Martini, Subir Goyal, Yuan Liu, Mehmet Asim Bilen
Final approval of manuscript: Dylan J. Martini, Subir Goyal, Yuan Liu, Sean T. Evans, T. Anders Olsen, Katherine Case, Benjamin L. Magod, Jacqueline T. Brown, Lauren Yantorni, Greta Anne Russler, Sarah Caulfield, Jamie M. Goldman, Bassel Nazha, Wayne B. Harris, Haydn T. Kissick, Viraj A. Master, Omer Kucuk, Bradley C. Carthon, Mehmet Asim Bilen
Disclosures
Bassel Nazha: Exelixis (SAB); Bradley C. Carthon: Astellas Medivation, Pfizer, Blue Earth Diagnostics (C/A), Bristol‐Myers Squibb (Other—travel); Mehmet Asim Bilen: Exelixis, Bayer, Bristol‐Myers Squibb, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, Sanofi (C/A), Xencor, Bayer, Bristol‐Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, Pfizer (RF—inst). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 UVA of association between severe irAEs* and clinical outcomes
Supplemental Table 2: Univariate association (UVA) of sites of irAEs and clinical outcomes
Supplemental Table 3: Univariate analysis (UVA) using extended Cox model for association of irAEs and overall survival (OS)
Acknowledgments
Research reported in this publication was supported in part by the Breen Foundation and the Biostatistics Shared Resource of Winship Cancer Institute of Emory University under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Preliminary data presented in this manuscript were presented by Dylan Martini as a virtual poster presentation at the 2020 Society for ImmunoTherapy of Cancer Annual Meeting.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.SEER cancer stat facts: Kidney and renal pelvis cancer . National Cancer Institute Web site. Available at https://seer.cancer.gov/statfacts/html/kidrp.html. Accessed February 6, 2021.
- 2.FDA approved drug products . U.S. Food and Drug Administration Web site. Available at http://www.accessdata.fda.gov/scripts/cder/daf. Accessed February 6, 2021.
- 3.McDermott DF, Drake CG, Sznol M et al. Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015;33:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao D, Margolis CA, Gao W et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun DA, Ishii Y, Walsh AM et al. Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma. JAMA Oncol 2019;5:1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JM, Chen DS. Immune escape to PD‐L1/PD‐1 blockade: Seven steps to success (or failure). Ann Oncol 2016;27:1492–1504. [DOI] [PubMed] [Google Scholar]
- 7.Michot JM, Bigenwald C, Champiat S et al. Immune‐related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016;54:139–148. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Johnson DB. Immune‐related adverse events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosoya K, Fujimoto D, Morimoto T et al. Association between early immune‐related adverse events and clinical outcomes in patients with non‐small cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer 2020;21:e315–e328. [DOI] [PubMed] [Google Scholar]
- 11.Hua C, Boussemart L, Mateus C et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016;152:45–51. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Nickleach DC, Zhang C et al. Carrying out streamlined routine data analyses with reports for observational studies: Introduction to a series of generic SAS ((R)) macros. F1000Res 2018;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giobbie‐Hurder A, Gelber RD, Regan MM. Challenges of guarantee‐time bias. J Clin Oncol 2013;31:2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dafni U.Landmark analysis at the 25‐year landmark point. Circ Cardiovasc Qual Outcomes 2011;4:363–371. [DOI] [PubMed] [Google Scholar]
- 15.Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox model. New York, NY: Springer‐Verlag, 2000. [Google Scholar]
- 16.Fisher LD, Lin DY. Time‐dependent covariates in the Cox proportional‐hazards regression model. Annu Rev Public Health 1999;20:145–157. [DOI] [PubMed] [Google Scholar]
- 17.Judd J, Zibelman M, Handorf E et al. Immune‐related adverse events as a biomarker in non‐melanoma patients treated with programmed cell death 1 inhibitors. The Oncologist 2017;22:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitale MG, Pipitone S, Venturelli M et al. Correlation between immune‐related adverse event (IRAE) occurrence and clinical outcome in patients with metastatic renal cell carcinoma (mRCC) treated with nivolumab: IRAENE Trial, an Italian multi‐institutional retrospective study. Clin Genitourin Cancer 2020;18:477–488. [DOI] [PubMed] [Google Scholar]
- 19.Grangeon M, Tomasini P, Chaleat S et al. Association between immune‐related adverse events and efficacy of immune checkpoint inhibitors in non‐small‐cell lung cancer. Clin Lung Cancer 2019;20:201–207. [DOI] [PubMed] [Google Scholar]
- 20.Tarhini AA, Kang N, Lee SJ et al. Immune adverse events (irAEs) with adjuvant ipilimumab in melanoma, use of immunosuppressants and association with outcome: ECOG‐ACRIN E1609 study analysis. J Immunother Cancer 2021;9:e002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paderi A, Giorgione R, Giommoni E et al. Association between immune related adverse events and outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Cancers (Basel) 2021;13:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314–318. [PubMed] [Google Scholar]
- 23.Patel V, Elias R, Formella J et al. Acute interstitial nephritis, a potential predictor of response to immune checkpoint inhibitors in renal cell carcinoma. J Immunother Cancer 2020;8:e001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martini DJ, Hamieh L, McKay RR et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue PD‐1/PD‐L1 therapy for immune‐related adverse events. Cancer Immunol Res 2018;6:402–408. [DOI] [PubMed] [Google Scholar]
- 25.Sznol M, Postow MA, Davies MJ et al. Endocrine‐related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70–76. [DOI] [PubMed] [Google Scholar]
- 26.Rosen MD, Privalsky ML. Thyroid hormone receptor mutations found in renal clear cell carcinomas alter corepressor release and reveal helix 12 as key determinant of corepressor specificity. Mol Endocrinol 2009;23:1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szymanski L, Matak D, Bartnik E et al. Thyroid hormones as renal cell cancer regulators. J Signal Transduct 2016;2016:1362407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez‐Iglesias O, Garcia‐Silva S, Tenbaum SP et al. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res 2009;69:501–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 UVA of association between severe irAEs* and clinical outcomes
Supplemental Table 2: Univariate association (UVA) of sites of irAEs and clinical outcomes
Supplemental Table 3: Univariate analysis (UVA) using extended Cox model for association of irAEs and overall survival (OS)


