Abstract
Lessons Learned
Preclinical studies have demonstrated that Src inhibition through dasatinib synergistically enhances the antitumor effects of oxaliplatin.
In this phase II, single‐arm study, FOLFOX with dasatinib in previously untreated patients with mPC only showed only modest clinical activity, with a progressive‐free survival of 4 months and overall survival of 10.6 months.
Continued investigation is ongoing to better understand the role of Src inhibition with concurrent 5‐fluorouracil and oxaliplatin in a subset of exceptional responders.
Background
Src tyrosine kinase activity is overexpressed in many human cancers, including metastatic pancreatic cancer (mPC). Dasatinib is a potent inhibitor of Src family of tyrosine kinases. This study was designed to investigate whether dasatinib can synergistically enhance antitumor effects of FOLFOX regimen (FOLFOX‐D).
Methods
In this single‐arm, phase II study, previously untreated patients received dasatinib 150 mg oral daily on days 1–14, oxaliplatin 85 mg/m2 intravenous (IV) on day 1 every 14 days, leucovorin (LV) 400 mg/m2 IV on day 1 every 14 days, 5‐fluorouracil (5‐FU) bolus 400 mg/m2 on day 1 every 14 days, and 5‐FU continuous infusion 2,400 mg/m2 on day 1 every 14 days. Primary endpoint was progression‐free survival (PFS) with preplanned comparison to historical controls.
Results
Forty‐four patients enrolled with an estimated median PFS of 4.0 (95% confidence interval [CI], 2.3–8.5) months and overall survival (OS) of 10.6 (95% CI, 6.9–12.7) months. Overall response rate (ORR) was 22.7% (n = 10): one patient (2.3%) with complete response (CR) and nine patients (20.5%) with partial response (PR). Fifteen patients (34.1%) had stable disease (SD). Nausea was the most common adverse event (AE) seen in 35 patients (79.5%).
Conclusion
The addition of dasatinib did not appear to add incremental clinical benefit to FOLFOX in untreated patients with mPC.
Keywords: FOLFOX, Dasatinib, Metastatic pancreatic cancer, Src
Discussion
mPC treatment remains an area of active investigation because of its aggressive natural course and a lack of durable response seen with available cytotoxic therapies. This single‐arm, phase II, open label study of FOLFOX‐D showed that addition of dasatinib did not demonstrate an additional benefit to FOLFOX, with an observed median PFS of 4 months, OS of 10.6 months, and ORR of 23% (Figure 1; Table 1). The primary endpoint of the study was PFS (targeting a 50% improvement from 4 to 6 months). PFS was chosen as the primary endpoint as we did not expect a robust response rate (RR) given the natural history of mPC.
Figure 1.
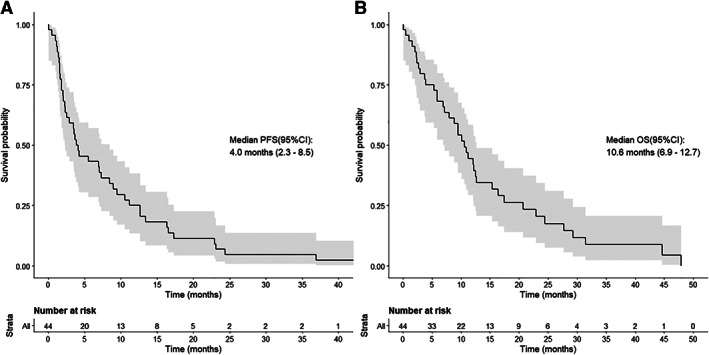
Kaplan‐Meier curves of median PFS shown on the left (A) and median OS shown on the right (B). Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression‐free survival.
Table 1.
Best response
| Response | Investigator assessment (n = 44) | Patients, %a |
|---|---|---|
| Overall response rate (95% CI)b | 10 | 22.7 (11.5–37.8) |
| Clinical benefit rate (95% CI) | 25 | 56.8 (41.0–71.7) |
| Best responseb | ||
| Complete response | 1 | 2.3 |
| Partial response | 9 | 20.5 |
| Stable disease | 15 | 34.1 |
| Progressive disease | 8 | 18.2 |
| Could not be evaluatedc | 11 | 25.0 |
Percentages may not total 100 because of rounding.
Overall response rate and best response were derived from the responses as assessed at specific time points according to the RECIST version 1.1.
Presumed to be most likely related to progressive disease as patients discontinued therapy for clinical progression prior to obtaining image confirmation.
Abbreviation: CI, confidence interval.
FOLFOX‐D regimen was tolerable, as all AEs were within the safety profile of the individual agents and the majority of AEs being Common Terminology Criteria for Adverse Events (CTCAE) grades ≤3. Nausea (n = 35, 79.5%) and fatigue (n = 33, 75%) were the two most common AEs.
Dasatinib‐mediated knockdown of Src family kinases members has been shown to reduce cancer cell growth and proliferation in prostate cancer, head and neck cancer, non‐small cell lung cancer, colon cancer, and sarcoma cancer lines [1]. Oxaliplatin leads to intracellular reactive oxygen species (ROS) generation, and ROS, in turn, potently activates Src [2]. Preclinical models have also shown that combination of dasatinib and oxaliplatin result in significantly reduced tumor volume [2], and this served as the rationale of the design of the study. However, we have subsequently learned from other investigations recently reported that the effect of dasatinib and oxaliplatin on Src modulation may be more complex than initially understood. For instance, in clinical studies of patients with patients metastatic colorectal cancer, dasatinib was unable to consistently and fully suppress Src levels in peripheral blood mononuclear cells at the tested dose of 150 mg daily [3]. Additionally, it has also been recently proposed that dasatinib reduces 5‐FU‐triggered apoptosis by modulating Src‐dependent caspase‐9 phosphorylation [4]. Together, these data may help to further explain our clinical findings. Of note, we plan to measure p‐Src expression in our tissue and serum samples to confirm adequate inhibition, particularly in the cohort of responders, as a next step in this line of investigation.
Trial Information
| Disease | Pancreatic cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | None |
| Type of Study | Phase II, single‐arm |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoint | Overall response rate, overall survival, toxicity |
| Additional Details of Endpoints or Study Design | |
| Patients: Patient provided a written informed consent prior to participating in the study. Target population included patients with histologically or cytologically proven pancreatic adenocarcinoma with evidence of metastatic disease on diagnostic imaging studies. Patients had measurable disease per RECIST v1.1 and were ECOG 0–2. Patients did not have any prior chemotherapy or radiotherapy for mPC, but previous chemotherapy or radiotherapy was allowed for nonmetastatic pancreatic cancer; however, the diagnosis of metastatic disease had to be more than 6 months after completion of prior treatment. Patients had to have a patent biliary system, and surgical bypass or internal stent was allowed, if there was concern for obstructive potential during the course of the study. Patients were allowed to receive therapeutic anticoagulation as long as those on coumadin were on a stable dose for >3 weeks, and international normalized ratio was stable between 2 and 3 (documented on two sequential occasions prior to enrollment). Additional key inclusion criteria included patients ≥18, with an adequate organ and marrow function, defined as total bilirubin <1.5 times the upper limit of normal (ULN), aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase ≤ 2.5 times ULN, serum creatinine <1.5 × ULN, hemoglobin ≥9g/dL, absolute neutrophil count ≥1500 per mm3, and platelet count ≥100,000 per mm.3 Key exclusion criteria included women with child‐bearing potential who were unwilling or unable to use an acceptable method of contraception for the entire study period and for at least 4 weeks after the last dose of study drug, history of known brain metastases or carcinomatous meningitis, recent major surgery within 4 weeks, and concurrent medical conditions that would increase the risk of toxicity, including clinically significant pleural or pericardial effusion required therapeutic thoracentesis or chest tube placement, pericardiocentesis, or causing ≥ grade 2 dyspnea. A full list of inclusion and exclusion criteria are listed in the study protocol. | |
| Study Treatment: Patients received dasatinib 150 mg daily on days 1–14, oxaliplatin 85 mg/m2 on day 1 every 14 days, leucovorin 400mg/m2 on day 1 every 14 days, 5‐fluorouracil bolus 400mg/m2 on day 1 every 14 days, and 5‐fluorouracil continuous infusion 2400 mg/m2 on day 1 every 14 days. One treatment cycle was equal to 14 days. | |
| Endpoints: The primary endpoint of the study was PFS (targeting a 50% improvement from 4 to 6 months), and it was defined as the time from treatment start to the first of either (a) documented disease progression or (b) death as a result of any cause. Patients who were lost to follow‐up were censored at the day of their last objective tumor assessment. Secondary endpoints included OS, ORR, and toxicity. OS was defined as the time from the date of treatment start to the date of death from any cause. If the patient was alive at the end of the follow‐up period or was lost to follow‐up, OS was censored on the last date the patient was known to be alive. ORR was defined as the proportion of patients achieving a best overall response of complete or partial response (CR + PR), according to RECIST v1.1, from the start of treatment until disease progression or recurrence. PFS and OS durations were estimated using the Kaplan‐Meier method, together with a 95% CI. Patients were contacted for survival status every 8 weeks until death or patient withdrawal. | |
| SD was defined by RECIST version 1.1 measurements as a component of best overall response. It was calculated from the start of treatment time until the criteria for progression were met, taking as reference the smallest measurements recorded since the treatment started. Upon treatment discontinuation, subjects were contacted every 8 weeks to assess survival status. Clinical benefit rate (CBR) was defined as equal to the objective RR plus the proportion of patients attaining stable disease (CR + PR + SD). | |
| Investigator's Analysis | Level of activity did not meet planned endpoint |
Drug Information
| 5‐Fluorouracil | |
| Generic Name | 5‐Fluorouracil |
| Trade Name | Adrucil |
| Drug Type | Antimetabolite |
| Dose | 400 mg/m2 bolus followed by 2,400 mg/m2 continuous infusion over 46 hours mg/m2 |
| Route | Bolus followed by continuous infusion over 46 hours |
| Schedule of Administration | 5‐Fluorouracil was given on day 1 every 14 days |
| Oxaliplatin | |
| Generic Name | Oxaliplatin |
| Trade Name | Eloxatin |
| Drug Type | Platinum containing compound |
| Dose | 85 mg/m2 |
| Route | IV |
| Schedule of Administration | Oxaliplatin was given on day 1 every 14 days |
| Leucovorin | |
| Generic Name | Leucovorin |
| Trade Name | Folinic acid |
| Drug Type | Folic acid analog |
| Dose | 400 mg/m2 |
| Route | IV |
| Schedule of Administration | Leucovorin was given on day 1 every 14 days |
| Dasatinib | |
| Generic Name | Dasatinib |
| Trade Name | Sprycel |
| Company Name | Bristol‐Myers Squibb |
| Drug Type | Small molecule |
| Drug Class | BCR‐Abl |
| Dose | 150 mg per flat dose |
| Route | oral (po) |
| Schedule of Administration | Dasatinib was given on days 1–14 |
Patient Characteristics
| Number of Patients, Male | 29 |
| Number of Patients, Female | 15 |
| Stage | IV |
| Age | Median (range): 64 (29–80) years |
| Number of Prior Systemic Therapies | 0 |
| Performance Status: ECOG |
0 — 23 1 — 18 2 — 3 3 — 0 Unknown — 0 |
| Other | No patient received prior chemotherapy for mPC. However, seven (15.9%) received prior chemotherapy or radiotherapy in the neoadjuvant oradjuvant setting. |
Primary Assessment Method
| Title | Response assessment |
| Number of Patients Screened | 65 |
| Number of Patients Enrolled | 44 |
| Number of Patients Evaluable for Toxicity | 44 |
| Number of Patients Evaluated for Efficacy | 44 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 1 (2.3%) |
| Response Assessment PR | n = 9 (20.5%) |
| Response Assessment SD | n = 15 (34.1%) |
| Response Assessment PD | n = 8 (18.2%) |
| Response Assessment OTHER | n = 11 (25%) |
| (Median) Duration Assessments PFS | 4.0 Months, CI: 2.3–8.5 |
| (Median) Duration Assessments TTP | 9.8 Months, CI: 8.6–19.5 |
| (Median) Duration Assessments OS | 10.6 Months, CI: 6.9–12.7 |
| Outcome Notes | ORR was observed in 10 patients (22.7%). CBR was observed in 25 patients (56.8%). |
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Level of activity did not meet planned endpoint |
Pancreatic cancer (PC) is projected to soon be the second leading cause of cancer‐related mortality. For response rate (mPC), systemic cytotoxic therapy was historically limited to 5‐fluorouracil (5‐FU) and leucovorin, which produced response rates (RRs) of <10%, clinical benefit rates (CBRs) of <10%, and median overall survival (OS) of 4.5 months [5, 6]. With the introduction of gemcitabine in the 1990s showing clinical benefit as monotherapy [7, 12], multiple gemcitabine‐based combinations were evaluated, but none of these combinations improved OS [13]. Combining biologic therapies like the epidermal growth factor receptor inhibitor erlotinib with gemcitabine has only shown marginal clinical benefit (OS, 6.24 vs. 5.91 months, p = .038) [14].
However, the combination of gemcitabine and nab‐paclitaxel yielded improved efficacy over gemcitabine alone, essentially changing practice as first‐line therapy for the management of patients with mPC (median OS, 8.5 vs. 6.7 months, p > .0001) [15]. As a gemcitabine‐free alternative regimen, FOLFIRINOX has also been established as an effective first‐line therapy for mPC, with OS 11.1 versus 6.8 months observed with gemcitabine alone (p < .0001) [16]. FOLFOX regimen is typically used in the second line following gemcitabine‐based treatments, demonstrating a 36% disease control rate and 1.7 month progressive‐free survival (PFS) [17]. Given the efficacy of fluoropyrimidine‐based regimens in first and subsequent lines of therapy as well as well‐established side effect profile, FOLFOX has presented itself as an attractive backbone to be studied in combination with novel therapies.
This open label, single‐arm, prospective study was designed to investigate the efficacy of FOLFOX with dasatinib (FOLFOX‐D) as a means to inhibit Src. Patient enrollment schema and patient demographics are shown in Figure 2 and Table 2, respectively. The study showed modest antitumor activity of FOLFOX‐D with an overall response rate of 23%, CBR of 57% , PFS of 4 months, and OS of 10.6 months. Although not directly compared through randomization in this single‐arm study, the 4 month PFS (primary endpoint) does not appear to be better than existing gemcitabine‐based (PFS, 5.5 months; OS, 8.5 months) or fluoropyrimidine‐based first‐line regimens (PFS, 6.4 months; OS, 11.1 months) [15, 16]. The combination also did not suggest a radiographic benefit by adding dasatinib to FOLFOX when compared with response rates observed by FOLFOX alone in untreated locally advanced or mPC (partial response, 27.6%; stable disease, 34.5%; and CBR of 62%) [18].
Figure 2.
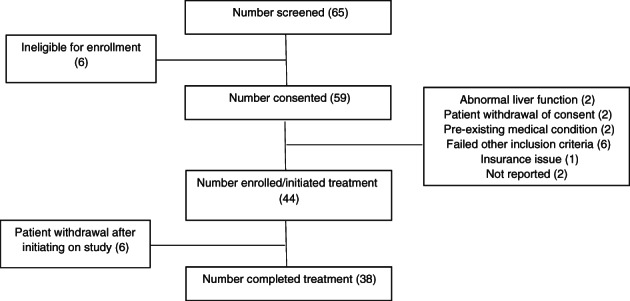
CONSORT diagram of enrollment. All patients who met enrollment eligibility criteria were included in the analysis. Patients who received at least one dose of a study drug were included in the safety analysis.
Table 2.
Demographics and baseline characteristics (n = 44)
| Characteristic | n = 44, n (%) |
|---|---|
| Age group | |
| 18 to <50 | 5 (11.4) |
| 50 to <65 | 18 (40.9) |
| ≥65 | 21 (47.7) |
| Gender | |
| Male | 29 (65.9) |
| Female | 15 (34.1) |
| Race | |
| White | 38 (86.4) |
| Black | 4 (9.1) |
| Asian | 2 (4.5) |
| ECOG performance status at enrollment | |
| 0 | 23 (52.3) |
| 1 | 18 (40.9) |
| 2 | 3 (6.8) |
| Prior surgery for pancreatic cancer | |
| Yes | 7 (15.9) |
| No | 37 (84.1) |
| Prior radiotherapy for pancreatic cancer | |
| Yes | 8 (18.2) |
| No | 36 (81.8) |
| Prior chemotherapy for pancreatic cancer | |
| Yes (Gemcitabine‐based) | 7 (15.9) |
| No | 37 (84.1) |
Abbreviation: ECOG, Eastern Cooperative Operative Group.
The FOLFOX‐D regimen was associated with adverse events (AEs) that were otherwise anticipated and easily managed with supportive care medications. Nausea (35 patients, 79.5%) and fatigue (33 patients, 75%) were the two most common AEs observed (ten most common AEs as shown in Table 3). The majority of the AEs were CTCAE grade <3 (all AEs grade 1–5 are shown in Table 4). Grade 4 events included neutropenia in four patients (9.1%), oral mucositis in one patient (2.3%), upper gastrointestinal hemorrhage in one patient (2.3%), sepsis in one patient (2.3%), hydrocephalus in one patient (2.3%), depression in one patient (2.3%), and respiratory failure in one patient (2.3%). Four patients (9.1%) died while on active treatment from disease complications not attributed to the treatment.
Table 3.
Ten most common adverse events with FOLFOX with dasatinib
| Adverse event | CTCAE toxicity grade | |
|---|---|---|
| All grade, n (%) | Grade ≥3, n (%) | |
| Nausea | 34 (77.3) | 10 (22.7) |
| Fatigue | 32 (72.7) | 21 (47.7 ) |
| Vomiting | 25 (56.8) | 8 (18.2) |
| Abnormal liver function tests | 23 (52.3) | 3 (6.8) |
| Diarrhea | 22 (50) | 2 (6.8) |
| Anorexia | 18 (40.9) | 2 (4.5) |
| Anemia | 14 (31.8) | 6 (13.6) |
| Constipation | 12 (27.3) | |
| Dysgeusia | 11 (25) | |
| Neutropenia | 9 (20.5) | |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; NA, not applicable.
Table 4.
All adverse reactions grouped by organ system
| Toxicity category, CTCAE Terms | CTCAE Grade | Grand totalb | |||||
|---|---|---|---|---|---|---|---|
| Missing, n (%) | 1, n a (%) | 2, n a (%) | 3, n a (%) | 4, n a (%) | 5, n a (%) | ||
| Blood and lymphatic system disorders | 19 (43.2) | ||||||
| Anemia | 10 (22.7) | 12 (27.3) | 8 (18.2) | 17 (38.6) | |||
| Febrile neutropenia | 2 (4.5) | 2 (4.5) | |||||
| Cardiac disorders | 6 (13.6) | ||||||
| Atrial fibrillation | 1 (2.3) | 1 (2.3) | |||||
| Atrial flutter | 1 (2.3) | 1 (2.3) | |||||
| Cardiac disorders: other, specify | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Pericardial effusion | 2 (4.5) | 2 (4.5) | |||||
| Supraventricular tachycardia | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| Endocrine disorders | 2 (4.5) | ||||||
| Hypothyroidism | 2 (4.5) | 2 (4.5) | |||||
| Eye disorders | 6 (13.6) | ||||||
| Dry eye | 1 (2.3) | 1 (2.3) | |||||
| Eye disorders: other, specify | 1 (2.3) | 1 (2.3) | 1 (2.3) | 3 (6.8) | |||
| Watering eyes | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Gastrointestinal disorders | 43 (97.7) | ||||||
| Abdominal distension | 2 (4.5) | 1 (2.3) | 1 (2.3) | 4 (9.1) | |||
| Abdominal pain | 2 (4.5) | 9 (20.5) | 8 (18.2) | 5 (11.4) | 17 (38.6) | ||
| Ascites | 2 (4.5) | 5 (11.4) | 6 (13.6) | ||||
| Bloating | 1 (2.3) | 4 (9.1) | 1 (2.3) | 4 (9.1) | |||
| Cheilitis | 1 (2.3) | 1 (2.3) | |||||
| Colitis | 2 (4.5) | 2 (4.5) | |||||
| Constipation | 1 (2.3) | 16 (36.4) | 6 (13.6) | 19 (43.2) | |||
| Dental caries | 1 (2.3) | 1 (2.3) | |||||
| Diarrhea | 3 (6.8) | 23 (52.3) | 4 (9.1) | 3 (6.8) | 24 (54.5) | ||
| Dry mouth | 2 (4.5) | 2 (4.5) | |||||
| Dysphagia | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Esophagitis | 1 (2.3) | 1 (2.3) | |||||
| Fecal incontinence | 2 (4.5) | 2 (4.5) | |||||
| Flatulence | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Gastric ulcer | 1 (2.3) | 1 (2.3) | |||||
| Gastroesophageal reflux disease | 2 (4.5) | 4 (9.1) | 4 (9.1) | ||||
| Gastrointestinal disorders: other, specify | 3 (6.8) | 5 (11.4) | 3 (6.8) | 1 (2.3) | 8 (18.2) | ||
| Hemorrhoids | 1 (2.3) | 3 (6.8) | 3 (6.8) | ||||
| Ileus | 1 (2.3) | 1 (2.3) | |||||
| Lower gastrointestinal hemorrhage | 1 (2.3) | 1 (2.3) | |||||
| Mucositis oral | 2 (4.5) | 8 (18.2) | 8 (18.2) | 2 (4.5) | 1 (2.3) | 16 (36.4) | |
| Nausea | 2 (4.5) | 26 (59.1) | 14 (31.8) | 11 (25) | 35 (79.5) | ||
| Oral dysesthesia | 1 (2.3) | 1 (2.3) | |||||
| Oral pain | 4 (9.1) | 1 (2.3) | 5 (11.4) | ||||
| Small intestinal obstruction | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| Toothache | 1 (2.3) | 1 (2.3) | |||||
| Upper gastrointestinal hemorrhage | 1 (2.3) | 1 (2.3) | |||||
| Vomiting | 2 (4.5) | 12 (27.3) | 10 (22.7) | 9 (20.5) | 26 (59.1) | ||
| General disorders and administration site conditions | 39 (88.6) | ||||||
| Chills | 1 (2.3) | 4 (9.1) | 1 (2.3) | 5 (11.4) | |||
| Death NOSc | 4 (9.1) | 4 (9.1) | |||||
| Edema face | 1 (2.3) | 2 (4.5) | 2 (4.5) | ||||
| Edema limbs | 2 (4.5) | 3 (6.8) | 2 (4.5) | 1 (2.3) | 7 (15.9) | ||
| Edema trunk | 1 (2.3) | 1 (2.3) | |||||
| Fatigue | 2 (4.5) | 27 (61.4) | 13 (29.5) | 8 (18.2) | 33 (75) | ||
| Fever | 1 (2.3) | 8 (18.2) | 4 (9.1) | 11 (25) | |||
| Flu‐like symptoms | 3 (6.8) | 1 (2.3) | 4 (9.1) | ||||
| Gait disturbance | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| General disorders and administration site conditions: other, specify | 3 (6.8) | 1 (2.3) | 4 (9.1) | ||||
| Infusion related reaction | 1 (2.3) | 1 (2.3) | |||||
| Localized edema | 1 (2.3) | 2 (4.5) | 1 (2.3) | 3 (6.8) | |||
| Malaise | 1 (2.3) | 1 (2.3) | |||||
| Noncardiac chest pain | 1 (2.3) | 1 (2.3) | |||||
| Pain | 1 (2.3) | 1 (2.3) | 2 (4.5) | 3 (6.8) | |||
| Immune system disorders | 2 (4.5) | ||||||
| Allergic reaction | 2 (4.5) | 2 (4.5) | |||||
| Infections and infestations | 17 (38.6) | ||||||
| Anorectal infection | 1 (2.3) | 1 (2.3) | |||||
| Biliary tract infection | 1 (2.3) | 1 (2.3) | |||||
| Bronchial infection | 1 (2.3) | 1 (2.3) | |||||
| Esophageal infection | 1 (2.3) | 1 (2.3) | |||||
| Infections and infestations: other, specify | 3 (6.8) | 2 (4.5) | 3 (6.8) | 6 (13.6) | |||
| Sepsis | 2 (4.5) | 2 (4.5) | |||||
| Sinusitis | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Skin infection | 1 (2.3) | 1 (2.3) | |||||
| Upper respiratory infection | 1 (2.3) | 7 (15.9) | 7 (15.9) | ||||
| Urinary tract infection | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Injury, poisoning, and procedural complications | 9 (20.5) | ||||||
| Bruising | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Fall | 3 (6.8) | 1 (2.3) | 4 (9.1) | ||||
| Intraoperative venous injury | 1 (2.3) | 1 (2.3) | |||||
| Vascular access complication | 1 (2.3) | 1 (2.3) | |||||
| Wound dehiscence | 1 (2.3) | 1 (2.3) | |||||
| Investigations | 26 (59.1) | ||||||
| Alanine aminotransferase increased | 5 (11.4) | 1 (2.3) | 2 (4.5) | 6 (13.6) | |||
| Alkaline phosphatase increased | 10 (22.7) | 3 (6.8) | 10 (22.7) | ||||
| Aspartate aminotransferase increased | 8 (18.2) | 1 (2.3) | 1 (2.3) | 8 (18.2) | |||
| Blood bilirubin increased | 3 (6.8) | 3 (6.8) | 1 (2.3) | 4 (9.1) | |||
| Creatinine increased | 5 (11.4) | 1 (2.3) | 1 (2.3) | 5 (11.4) | |||
| INR increased | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| Investigations: other, specify | 1 (2.3) | 1 (2.3) | |||||
| Neutrophil count decreased | 3 (6.8) | 8 (18.2) | 8 (18.2) | 4 (9.1) | 15 (34.1) | ||
| Platelet count decreased | 7 (15.9) | 8 (18.2) | 2 (4.5) | 12 (27.3) | |||
| Weight loss | 9 (20.5) | 8 (18.2) | 1 (2.3) | 12 (27.3) | |||
| Metabolism and nutrition disorders | 31 (70.5) | ||||||
| Anorexia | 2 (4.5) | 8 (18.2) | 15 (34.1) | 2 (4.5) | 22 (50) | ||
| Dehydration | 4 (9.1) | 2 (4.5) | 6 (13.6) | ||||
| Glucose intolerance | 3 (6.8) | 1 (2.3) | 4 (9.1) | ||||
| Hypercalcemia | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| Hypoalbuminemia | 7 (15.9) | 2 (4.5) | 1 (2.3) | 8 (18.2) | |||
| Hypocalcemia | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Hypoglycemia | 1 (2.3) | 1 (2.3) | |||||
| Hypokalemia | 2 (4.5) | 2 (4.5) | 2 (4.5) | 5 (11.4) | |||
| Hyponatremia | 12 (27.3) | 4 (9.1) | 13 (29.5) | ||||
| Hypophosphatemia | 1 (2.3) | 1 (2.3) | |||||
| Metabolism and nutrition disorders: other, specify | 1 (2.3) | 1 (2.3) | |||||
| Musculoskeletal and connective tissue disorders | 12 (27.3) | ||||||
| Arthralgia | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Arthritis | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Back pain | 1 (2.3) | 3 (6.8) | 1 (2.3) | 1 (2.3) | 5 (11.4) | ||
| Bone pain | 1 (2.3) | 1 (2.3) | |||||
| Chest wall pain | 1 (2.3) | 1 (2.3) | |||||
| Flank pain | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Generalized muscle weakness | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Musculoskeletal and connective tissue disorder: other, specify | 1 (2.3) | 3 (6.8) | 3 (6.8) | ||||
| Musculoskeletal deformity | 1 (2.3) | 1 (2.3) | |||||
| Myalgia | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Pain in extremity | 1 (2.3) | 1 (2.3) | 1 (2.3) | 3 (6.8) | |||
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 (2.3) | ||||||
| Tumor pain | 1 (2.3) | 1 (2.3) | |||||
| Nervous system disorders | 29 (65.9) | ||||||
| Concentration impairment | 1 (2.3) | 1 (2.3) | |||||
| Dizziness | 1 (2.3) | 6 (13.6) | 1 (2.3) | 1 (2.3) | 7 (15.9) | ||
| Dysesthesia | 2 (4.5) | 1 (2.3) | 3 (6.8) | ||||
| Dysgeusia | 6 (13.6) | 7 (15.9) | 12 (27.3) | ||||
| Facial nerve disorder | 1 (2.3) | 1 (2.3) | |||||
| Headache | 2 (4.5) | 4 (9.1) | 1 (2.3) | 1 (2.3) | 6 (13.6) | ||
| Hydrocephalus | 1 (2.3) | 1 (2.3) | |||||
| Nervous system disorders: other, specify | 1 (2.3) | 1 (2.3) | 1 (2.3) | 3 (6.8) | |||
| Paresthesia | 2 (4.5) | 1 (2.3) | 3 (6.8) | ||||
| Peripheral sensory neuropathy | 26 (59.1) | 7 (15.9) | 8 (18.2) | 27 (61.4) | |||
| Sinus pain | 1 (2.3) | 1 (2.3) | |||||
| Tremor | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| Psychiatric disorders | 20 (45.5) | ||||||
| Anxiety | 1 (2.3) | 6 (13.6) | 2 (4.5) | 9 (20.5) | |||
| Confusion | 1 (2.3) | 1 (2.3) | |||||
| Depression | 1 (2.3) | 1 (2.3) | 1 (2.3) | 1 (2.3) | 4 (9.1) | ||
| Hallucinations | 1 (2.3) | 1 (2.3) | |||||
| Insomnia | 2 (4.5) | 4 (9.1) | 2 (4.5) | 1 (2.3) | 8 (18.2) | ||
| Psychiatric disorders: other, specify | 1 (2.3) | 1 (2.3) | |||||
| Restlessness | 1 (2.3) | 1 (2.3) | |||||
| Renal and urinary disorders | 11 (25) | ||||||
| Hematuria | 1 (2.3) | 1 (2.3) | |||||
| Renal and urinary disorders: other, specify | 4 (9.1) | 1 (2.3) | 1 (2.3) | 6 (13.6) | |||
| Urinary frequency | 3 (6.8) | 1 (2.3) | 4 (9.1) | ||||
| Urinary incontinence | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| Urinary urgency | 1 (2.3) | 1 (2.3) | |||||
| Reproductive system and breast disorders | 4 (9.1) | ||||||
| Genital edema | 1 (2.3) | 1 (2.3) | |||||
| Gynecomastia | 1 (2.3) | 1 (2.3) | |||||
| Pelvic pain | 1 (2.3) | 1 (2.3) | |||||
| Reproductive system and breast disorders: other, specify | 1 (2.3) | 2 (4.5) | 3 (6.8) | ||||
| Respiratory, thoracic, and mediastinal disorders | 18 (40.9) | ||||||
| Aspiration | 1 (2.3) | 1 (2.3) | |||||
| Cough | 1 (2.3) | 2 (4.5) | 3 (6.8) | ||||
| Dyspnea | 4 (9.1) | 5 (11.4) | 1 (2.3) | 8 (18.2) | |||
| Epistaxis | 4 (9.1) | 4 (9.1) | |||||
| Hiccups | 1 (2.3) | 1 (2.3) | 2 (4.5) | 4 (9.1) | |||
| Laryngopharyngeal dysesthesia | 1 (2.3) | 1 (2.3) | |||||
| Nasal congestion | 1 (2.3) | 1 (2.3) | |||||
| Pleural effusion | 9 (20.5) | 5 (11.4) | 2 (4.5) | 9 (20.5) | |||
| Pneumonitis | 1 (2.3) | 1 (2.3) | |||||
| Postnasal drip | 1 (2.3) | 1 (2.3) | |||||
| Productive cough | 1 (2.3) | 1 (2.3) | |||||
| Respiratory failure | 1 (2.3) | 1 (2.3) | |||||
| Respiratory, thoracic, and mediastinal disorders: other, specify | 1 (2.3) | 1 (2.3) | 2 (4.5) | ||||
| Sore throat | 2 (4.5) | 2 (4.5) | 3 (6.8) | ||||
| Skin and subcutaneous tissue disorders | 16 (36.4) | ||||||
| Alopecia | 4 (9.1) | 4 (9.1) | |||||
| Dry skin | 1 (2.3) | 1 (2.3) | |||||
| Palmar‐plantar erythrodysesthesia syndrome | 3 (6.8) | 2 (4.5) | 1 (2.3) | 3 (6.8) | |||
| Periorbital edema | 6 (13.6) | 6 (13.6) | |||||
| Pruritus | 1 (2.3) | 1 (2.3) | 1 (2.3) | ||||
| Rash maculo‐papular | 1 (2.3) | 6 (13.6) | 1 (2.3) | 1 (2.3) | 7 (15.9) | ||
| Skin and subcutaneous tissue disorders: other, specify | 1 (2.3) | 3 (6.8) | 3 (6.8) | 5 (11.4) | |||
| Urticaria | 1 (2.3) | 1 (2.3) | |||||
| Vascular disorders | 10 (22.7) | ||||||
| Flushing | 1 (2.3) | 1 (2.3) | |||||
| Hot flashes | 1 (2.3) | 1 (2.3) | |||||
| Hypertension | 1 (2.3) | 3 (6.8) | 4 (9.1) | ||||
| Hypotension | 1 (2.3) | 1 (2.3) | |||||
| Thromboembolic event | 4 (9.1) | 1 (2.3) | 4 (9.1) |
In each column, n represents a unique adverse event (AE), not necessarily an individual patient
In this column, n represents the total number of patients with the listed AE.
Not attributed to study treatment.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; INR, international normalized ratio; NOS, not otherwise specified.
Preclinical data had shown dasatinib has an antitumor effect on PC cell lines [19], although dasatinib had not shown clinical benefit in addition to gemcitabine in PC [20, 21]. C‐Src protein is a member of Src family kinases (SFsK) that are encoded by the Src gene. Knockdown of SFKs in human PC cell lines has shown to reduce cancer cell proliferation, migration, and invasion [22], a mechanism in part explained by restoration of E‐cadherin expression [23]. Oxaliplatin has been shown to activate intracellular reactive oxygen species (ROS), and ROS consequently activates Src. Src blockade with dasatinib was shown to increase oxaliplatin activity synergistically in human cell lines in vitro, with the effect on cell line growth measured by synergy analysis and combination index calculations showing a supra‐additive effect, and in vivo, with a 92% reduction in tumor volume relative to untreated controls (p < .01) as compared with no statistically significant reduction in tumor size with dasatinib or oxaliplatin as monotherapies [2]. In addition, Src inhibition has been postulated to reverse 5‐FU chemoresistance [24]. This preclinical data served as the rationale of the design of our study.
We have subsequently learned from other recently reported investigations that the effect of dasatinib and oxaliplatin on Src modulation may be more complex than initially understood. The combination of FOLFOX plus dasatinib was recently reported to not demonstrate a meaningful clinical response in refractory colorectal cancer, thought to be in part because of failure to consistently and fully inhibit Src at clinically achievable doses of dasatinib (150 mg daily dose). Additionally, posttranslational work demonstrated an increase in Src levels following oxaliplatin therapy as another potential mechanism of resistance [2, 3].
Fu et al. also recently showed that Src knockout mouse embryonic fibroblasts and human colon cancer cells demonstrate 5‐FU treatment resistance [25]. Specifically, 5‐FU can cause DNA damage and induce apoptosis through recruitment of caspase‐9, which is a main member of caspase family of proteins that are involved in endogenous apoptosis [2, 4]. Fu and colleagues showed that dasatinib reduced the 5‐FU apoptotic effect on colon carcinoma cell lines through reduced cleavage of caspase‐3, caspase‐7, caspase‐9, and poly ADP‐ribose polymerase [2, 4]. Thus, there are several potential mechanistic reasons why dasatinib did not appear to add clinical benefit to FOLFOX in our trial and this remains an area of active exploratory and translational investigation.
Although the study results are informative, we do note some study limitations. This was a single‐arm study with relatively modest sample size. Given the natural history of mPC, a robust objective RR was not anticipated, hence PFS was picked as the primary endpoint and historical controls were used as comparators. For this reason, we also used historical controls as a comparison, a design that lends itself to inherent patient selection bias and challenges in cross‐study comparisons. Additional correlative studies are also ongoing to profile these patients, assess for other biomarkers associated with exceptional response, and measure Src levels in tissue and plasma samples to ensure that adequate Src inhibition took place. These studies are continuing as part of our translational investigations.
Disclosures
Thomas J. George: Tempus Laboratories (C/A), Bristol‐Myers Squibb, Merck, AstraZeneca, Lilly, Bayer, Incyte, Tesaro, GSK, Ipsen, Seattle Genetics, Genentech, Astellas (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.govIdentifier: NCT01652976
- Sponsor: University of Florida
- Principal Investigator: Thomas J. George
- IRB Approved: Yes
References
- 1.Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. The Oncologist 2011;16:566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopetz S, Lesslie DP, Dallas NA et al. Synergistic activity of the src family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res 2009;69:3842–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parseghian CM, Parikh NU, Wu JY et al. Dual inhibition of EGFR and c‐Src by cetuximab and dasatinib combined with folfox chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res 2017;23:4146–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Y, Yang G, Xue P et al. Dasatinib reduces 5‐Fu‐triggered apoptosis in colon carcinoma by directly modulating Src‐dependent caspase‐9 phosphorylation. Cell Death Discov 2018;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuigan A, Kelly P, Turkington RC et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCaprio JA, Mayer RJ, Gonin R et al. Fluorouracil and high‐dose leucovorin in previously untreated patients with advanced adenocarcinoma of the pancreas: Results of a phase II trial. J Clin Oncol 1991;9:2128–2133. [DOI] [PubMed] [Google Scholar]
- 8.Glimelius B, Hoffman K, Sjödén PO et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593–600. [DOI] [PubMed] [Google Scholar]
- 9.Burris HA, Moore MJ, Andersen J et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 10.Mohammad AA. Advanced pancreatic cancer: The standard of care and new opportunities. Oncol Rev 2018;12:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storniolo AM, Enas NH, Brown CA et al. An investigational new drug treatment program for patients with gemcitabine: Results for over 3000 patients with pancreatic carcinoma. Cancer 1999;85:1261–1268. [PubMed] [Google Scholar]
- 12.Berlin JD, Catalano P, Thomas JP et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group trial E2297. J Clin Oncol 2002;20:3270–3275. [DOI] [PubMed] [Google Scholar]
- 13.Stathis A and Moore MJ. Advanced pancreatic carcinoma: Current treatment and future challenges. Nat Rev Clin Oncol 2010;7:163–172. [DOI] [PubMed] [Google Scholar]
- 14.Moore MJ, Goldstein D, Hamm J et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol 2007;25:1960‐1966. [DOI] [PubMed] [Google Scholar]
- 15.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 17.Zaanan A, Trouilloud I, Markoutsaki T et al. FOLFOX as second‐line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer 2014;14:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosn M, Farhat F, Kattan J et al. FOLFOX‐6 combination as the first‐line treatment of locally advanced and/or metastatic pancreatic cancer. Am J Clin Oncol 2007;30:15–20. [DOI] [PubMed] [Google Scholar]
- 19.Morton JP, Karim SA, Graham K et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 2010;139:292–303. [DOI] [PubMed] [Google Scholar]
- 20.Evans TRJ, Van Cutsem E, Moore MJ et al. Phase 2 placebo‐controlled, double‐blind trial of dasatinib added to gemcitabine for patients with locally‐advanced pancreatic cancer. Ann Oncol 2017;28:354–361. [DOI] [PubMed] [Google Scholar]
- 21.Renouf DJ, Moore MJ, Hedley D et al. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs 2012;30:779–786. [DOI] [PubMed] [Google Scholar]
- 22.Je DW, YM O, Ji YG et al. The inhibition of SRC family kinase suppresses pancreatic cancer cell proliferation, migration, and invasion. Pancreas 2014;43:768–776. [DOI] [PubMed] [Google Scholar]
- 23.Dosch AR, Dai X, Gaidarski AA III et al. Src kinase inhibition restores E‐cadherin expression in dasatinib‐sensitive pancreatic cancer cells. Oncotarget 2019;10:1056–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ischenko I, Camaj P, Seeliger H et al. Inhibition of src tyrosine kinase reverts chemoresistance toward 5‐fluorouracil in human pancreatic carcinoma cells: An involvement of epidermal growth factor receptor signaling. Oncogene 2008;27:7212–7222. [DOI] [PubMed] [Google Scholar]
- 25.Fu Y, Yang G, Zhu F et al. Antioxidants decrease the apoptotic effect of 5‐Fu in colon cancer by regulating Src‐dependent caspase‐7 phosphorylation. Cell Death Dis 2014;5:e983. [DOI] [PMC free article] [PubMed] [Google Scholar]


