Abstract
Background
In Ontario, Canada, patient‐reported outcome (PRO) evaluation through the Edmonton Symptom Assessment System (ESAS) has been integrated into clinical workflow since 2007. As stage IV non‐small cell lung cancer (NSCLC) is associated with substantial disease and treatment‐related morbidity, this province‐wide study investigated moderate to severe symptom burden in this population.
Materials and Methods
ESAS collected from patients with stage IV NSCLC diagnosed between 2007 and 2018 linked to the Ontario provincial health care system database were studied. ESAS acquired within 12 months following diagnosis were analyzed and the proportion reporting moderate to severe scores (ESAS ≥4) in each domain was calculated. Predictors of moderate to severe scores were identified using multivariable Poisson regression models with robust error variance.
Results
Of 22,799 patients, 13,289 (58.3%) completed ESAS (84,373 assessments) in the year following diagnosis. Patients with older age, with high comorbidity, and not receiving active cancer therapy had lower ESAS completion. The majority (94.4%) reported at least one moderate to severe symptom. The most prevalent were tiredness (84.1%), low well‐being (80.7%), low appetite (71.7%), and shortness of breath (67.8%). Most symptoms peaked at diagnosis and, while declining, remained high in the following year. On multivariable analyses, comorbidity, low income, nonimmigrants, and urban residency were associated with moderate to severe symptoms. Moderate to severe scores in all ESAS domains aside from anxiety were associated with radiotherapy within 2 weeks prior, whereas drowsiness, low appetite and well‐being, nausea, and tiredness were associated with systemic therapy within 2 weeks prior.
Conclusion
This province‐wide PRO analysis showed moderate to severe symptoms were prevalent and persistent among patients with metastatic NSCLC, underscoring the need to address supportive measures in this population especially around treatments.
Implications for Practice
In this largest study of lung cancer patient‐reported outcomes (PROs), stage IV non‐small cell lung cancer patients had worse moderate‐to‐severe symptoms than other metastatic malignancies such as breast or gastrointestinal cancers when assessed with similar methodology. Prevalence of moderate‐to‐severe symptoms peaked early and remained high during the first year of follow‐up. Symptom burden was associated with recent radiation and systemic treatments. Early and sustained PRO collection is important to detect actionable symptom progression, especially around treatments. Vulnerable patients (e.g., older, high comorbidity) who face barriers in attending in‐person clinic visits had lower PRO completion. Virtual PRO collection may improve completion.
Keywords: Non‐small cell lung cancer, Stage IV, Edmonton Symptom Assessment System, Symptom burden, Patient‐reported outcomes
Short abstract
Reporting a study in Canada, this article analyzes utilization of patient‐reported outcomes and symptom burden among patients with stage IV non‐small cell lung cancer, providing a basis for the development of strategies to address gaps in symptom management in this patient population.
Introduction
Lung cancer is the most common cause of cancer‐related death worldwide, including in Canada, where it accounts for 26% of cancer‐related deaths [1, 2]. Non‐small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer [3]. Most patients with NSCLC present with incurable stage IV disease at diagnosis, historically with dismal 5‐year survival of less than 10% according to a recent Surveillance, Epidemiology, and End Results study between 2013 and 2017 [3]. Even with newer therapies, such as immunotherapy and targeted therapy, by nature of the patient's incurable disease, the importance of symptom management remains crucial [4, 5]. Previous studies have demonstrated that lung cancer is associated with high symptom burden, which has strong implications on overall quality of life (QoL), caregiver burden, and health system resource utilization [6, 7]. It has been reported that depression is common among patients with stage IV NSCLC owing to its inherent high symptom burden and low survival [8]. In addition, patients with lung cancer may present with a unique constellation of symptoms as a consequence of local and regional tumor burden of structures within the thorax, such as dyspnea, hemoptysis, and chest pain [9].
Historically, the quantification of cancer‐related symptoms and treatment toxicities were through health care providers recording adverse events in the medical record, as ascertained and graded from the provider perspective [10]. Recently, patient‐reported outcome (PRO) measures have been increasingly used in the clinical setting to elicit patients’ own response of symptoms resulting from disease or treatment. PROs present a unique opportunity to understand the patient‐centered perspectives and areas where intervention might improve symptoms. Studies suggest that measuring and responding to PROs is associated with improved QoL, symptom management, and survival, while reducing emergency department visits among patients with advanced cancers [11, 12].
Our current understanding of PROs utility in patients with stage IV lung cancer is limited to smaller cohort studies [9, 13]. Quantification of patient‐reported symptom burden and identification of factors associated with high symptom burden in a large population receiving routine cancer care are important to assess the unmet needs of patients with stage IV NSCLC and may aid decision‐making on population‐level health care resource allocation. To investigate these issues, the aim of this study was to analyze PRO utilization, as well as patient symptom burden and trajectory among patients with stage IV NSCLC in the 12 months following diagnosis from the entire province of Ontario, Canada. This study will then provide the basis for the development of strategies to address gaps in symptom management in this patient population.
Materials and Methods
Study Overview
In 2007, Cancer Care Ontario (CCO) implemented a province‐wide program whereby all regional cancer centers systematically collected PROs via the Edmonton Symptom Assessment System (ESAS) questionnaire at outpatient cancer clinic visits. This study used these prospectively collected ESAS PROs linked to routinely collected administrative data acquired through patient interactions within the universal, single‐payer health care system in Ontario (2018 population of 14.3 million), Canada. ESAS is a validated and reliable patient‐reported outcome measure assessing the severity of nine common cancer‐associated symptoms: anxiety, depression, drowsiness, lack of appetite, nausea, pain, shortness of breath, tiredness, and impaired well‐being [14, 15]. Patients rate each symptom from 0 (no symptoms) to 10 (worst possible symptom) on the ESAS form during clinical encounters, which are collected as part of their records. The administrative data included all patients with a valid Ontario Health Insurance Plan (OHIP) number. The provincial ESAS data are consolidated and made available through the ICES (formally known as Institute for Clinical Evaluative Sciences) database. The study was approved by the Sunnybrook Health Sciences Centre research ethics board and adhered to data confidentiality and privacy policies of ICES. The study was conducted and reported following the Reporting of Studies Conducted Using Observational Routinely Collected Data statement [16].
Study Cohort
Patients diagnosed with stage IV lung cancer between January 2007 and September 2018 were identified in the Ontario Cancer Registry (OCR) using the International Classification of Disease for Oncology (ICD‐O) topography codes (ICD‐O‐3 codes: C34.0–34.3, C34.8, and C34.9). ICD‐O‐3 histology codes were used to identify non‐small cell histologies and to exclude carcinoid, mesothelioma, and small cell cancer histologies (supplemental online Appendix 1). OCR captures 95% of Ontario incident cancer diagnoses including the staging data since 1964, excluding nonmelanoma skin cancers [17]. Patients were excluded if they were aged <18 or > 99 years, if they had histology inconsistent with NSCLC, or if the follow‐up period was less than 6 months with no confirmed death. Patients with additional cancer diagnosis between 5 years before and 1 year after NSCLC diagnosis were also excluded to eliminate noise due to symptoms related to additional cancer diagnosis and treatments. Follow‐up was current to September 30, 2019, allowing a minimum follow‐up of 1 year for all patients.
Data Sources
The following linked administrative data sets were used to capture baseline clinical characteristics, ESAS scores, and covariates: (a) OCR; (b) Cancer Activity Level Reporting (ALR); (c) OHIP database containing billing claims from clinicians, including physicians, laboratories, groups, and out‐of‐province health care providers; (d) CCO Symptoms Management Reporting Database; (e) Ambulatory Care Reporting System; (f) Canadian Institute of Health Information Discharge Abstract Database and Same Day Surgery; (g) Registered Persons Database (RPDB); (h) the 2006 Canadian Census; and (i) Permanent Resident Database of Immigration, Refugees, and Citizenship Canada (IRCC). Details on the use of each data source are contained in supplemental online Appendix 1.
Outcomes
The primary outcome of interest was the prevalence of moderate to severe symptoms, defined as an ESAS score ≥4 [18], reported each month within 1 year after diagnosis. Date of diagnosis was defined as the earlier between the recorded date of lung cancer diagnosis or the first day of delivery of cancer treatments. If more than one ESAS score was reported by a patient in a month, the highest score was used.
Covariates
All baseline characteristics were measured at the time of diagnosis. Age and sex were acquired from the RPDB. Rural residence was defined according to Rurality Index of Ontario [19] scored 0–100 based on the postal code of patients’ primary home, which considers population size, population density, and health care resources of where patients primarily reside: major urban (0–9), non‐major urban (10–44), or rural (≥45). Neighborhood income quintiles were categorized based on the median income of a patient's residential postal code using Canadian census data. Comorbidity was assessed using the Elixhauser comorbidity index, based on health service use in the 24 months prior to lung cancer diagnosis [20]. Elixhauser comorbidity indices were summed in a total score that was categorized as a dichotomous variable: low (0–3) and high (≥4) comorbidity burden, as per prior studies [20, 21]. From IRCC data, patients who immigrated to Canada (including refugees) were defined as immigrants; otherwise, they were defined as nonimmigrants.
Radiation (to any site) and systemic therapies received by a patient were identified from OHIP physician billing claims and ALR activity. To assess the associations between treatment delivery to peak symptom severities, the administrations of radiation and systemic therapies were included as time‐dependent covariates, whereby ESAS scores within 2 weeks following the (onset of) therapy were examined. The year of diagnosis was a covariate as a continuous variable. To define number of months from diagnosis until the time of ESAS recording, timing of ESAS were categorized in 30‐day intervals from the day of diagnosis.
Statistical Analysis
Of the patients with an ESAS score, proportions of patients reporting at least one moderate to severe (≥4) score in each ESAS domain within the 12‐month follow‐up period were then tabulated. Patients who received surgery were censored at the date of surgery, as they may represent patients with oligometastatic disease with better outcomes compared with the typically incurable patients with stage IV disease [22]. Symptom trajectories were plotted with line graphs depicting the proportion of patients with moderate to severe symptoms out of all patients with recorded ESAS in each month from diagnosis; median number of moderate to severe scores per patient was also reported each month. For each symptom, the highest monthly prevalence within 1 year was defined as “peak” and the lowest prevalence as “nadir.” Sensitivity analyses of symptom trajectories were also performed for patients who survived 12 months after diagnosis.
Potential predictors of moderate to severe scores for each symptom were analyzed using multivariable modified Poisson regression models with robust error variance. As analyses were performed for nine ESAS domains, Bonferroni‐correction α of 0.006 was used (familywise α of 0.05). The relevant variables were included a priori based on clinical relevance and existing literature [17, 23]; all variables were kept in the final model. Using a Bonferroni‐adjusted α of 0.006, results are reported as relative risk (RR) with 99.4% confidence interval (99.4% CI). Results were considered statistically significant if p < .006.
Baseline characteristics were reported and stratified based on whether a patient completed at least one ESAS or none within 12 months after diagnosis. Categorical and ordinal variables were reported as frequencies and proportions. Continuous variables were reported as medians and interquartile ranges (IQRs). Characteristics of patients who completed and did not complete at least one ESAS score were compared using χ2 tests for independence. Incomplete ESAS questionnaires were excluded from final analyses. All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Study Cohort and ESAS Completion
A total of 22,799 patients with stage IV lung cancer diagnosed between January 2007 and September 2018 were identified as meeting the study inclusion criteria. Among those, 13,289 (58.2%) had at least one completed ESAS (reported all nine symptom scores), with a total of 84,373 unique ESAS completed. Among patients with completed ESAS, 78.2% and 40.1% completed at least two and six ESAS, respectively.
The median follow‐up period among patients with completed ESAS was 7.9 months (IQR: 3.7–12.2), and 63.3% died within 1 year of follow‐up after diagnosis. Among patients with completed ESAS, median age was 68 (IQR: 60–75) years, and 48.2% were females. Of these, 127 (1.0%) underwent surgery. Details of patient characteristics based on ESAS completion are summarized in Table 1. Patients who did not complete any ESAS were more likely to be older, with higher comorbidity index, and from neighborhood with lower income quintile. ESAS completion was lower in patients not receiving active cancer treatments and diagnosed before 2013. Immigration status and residency rurality were similar between patients with and without completed ESAS.
Table 1.
Characteristics of patients with stage IV non‐small cell lung cancer diagnosed in Ontario between January 2007 to September 2018 based on ESAS completion
| Characteristics | No reported ESAS or incomplete ESAS surveys (n = 9,510) | Reported ESAS (n = 13,289) | Standardized difference |
|---|---|---|---|
| Sex | |||
| Female | 4,289 (45.1) | 6,406 (48.2) | .06 |
| Male | 5,221 (54.9) | 6,883 (51.8) | .06 |
| Age, median (IQR), yr | 71 (63‐79) | 68 (60‐75) | .28 |
| Elixhauser comorbidity index | |||
| 4 or more | 1,306 (13.7) | 1,288 (9.7) | .13 |
| Less than 4 | 8,204 (86.3) | 12,001 (90.3) | .13 |
| Immigration status | |||
| Immigrant | 729 (7.7) | 964 (7.3) | .02 |
| Nonimmigrant | 8,781 (92.3) | 12,325 (92.7) | .02 |
| Lung cancer treatments | |||
| Systemic therapy and radiation | 663 (7.0) | 4,221 (31.8) | .66 |
| Systemic therapy only | 825 (8.7) | 2,075 (15.6) | .21 |
| Radiation only | 2,674 (28.1) | 4,815 (36.2) | .17 |
| No active treatment | 5,348 (56.2) | 2,178 (16.4) | .91 |
| Neighborhood income quintile | |||
| Q1 | 2,411 (25.4) | 2,868 (21.6) | .09 |
| Q2 | 2,138 (22.5) | 2,910 (21.9) | .01 |
| Q3 | 1,876 (19.7) | 2,610 (19.6) | .00 |
| Q4 | 1,623 (17.1) | 2,499 (18.8) | .05 |
| Q5 (highest income) | 1,417 (14.9) | 2,370 (17.8) | .08 |
| Unknown | 45 (0.5) | 32 (0.2) | .04 |
| Residence | |||
| Major urban | 6,548 (68.9) | 8,604 (64.7) | .09 |
| Non‐major urban | 2,272 (23.9) | 3,654 (27.5) | .08 |
| Rural | 690 (7.3) | 1,031 (7.8) | .02 |
| Diagnosis year | |||
| 2007–2012 | 5,109 (53.7) | 5,221 (39.3) | .29 |
| 2013–2018 | 4,401 (46.3) | 8,068 (60.7) | .29 |
Data are shown as n (%).
Abbreviations: ESAS, Edmonton Symptom Assessment Score; IQR, interquartile range.
Prevalence and Trajectories of Moderate to Severe Symptoms Reported on ESAS
Among patients who completed at least one ESAS, nearly all (94.4%) reported at least one moderate to severe score through ESAS within 12 months after diagnosis. The most prevalent moderate to severe ESAS symptoms within 12 months after diagnosis were tiredness (84.1%), lack of well‐being (80.7%), low appetite (71.7%), and shortness of breath (67.8%; Fig. 1). Monthly peaks and nadirs of these symptoms were as follows: 67.6% (month 1) and 47.0% (month 12) for tiredness; 64.6% (month 1) and 43.3% (month 10) for lack of well‐being; 50.5% (month 1) and 33.1% (month 10) for low appetite; and 51.1% (month 1) and 35.8% (month 10) for shortness of breath (Fig. 2). Nausea was the least common moderate to severe symptom reported (12‐month prevalence: 34.6%, monthly peak and nadir: 18.7% [month 2] and 11.0% [month 10]). In all nine ESAS domains, symptom severity peaked at 1 or 2 months after diagnosis and demonstrated downward trajectories in the subsequent months (Fig. 2). Symptoms with the largest prevalence change (from peak to nadir) during follow‐up were anxiety (23.8%), lack of well‐being (21.2%), tiredness (20.6%), and lack of appetite (17.3%). The smallest change was observed in nausea (7.7%). The median moderate to severe score was 5 for 1 to 3 months after diagnosis, and 4 in subsequent follow‐up periods. Median time until first radiotherapy was 36 days (IQR: 21–64) from diagnosis, whereas median time until first systemic therapy was 57 days (IQR: 36–90) after diagnosis.
Figure 1.
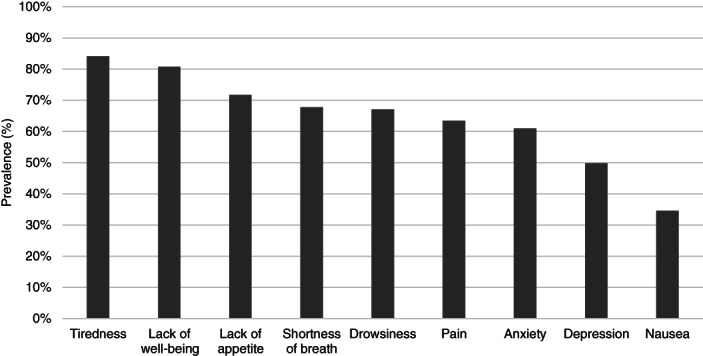
Bar graphs depicting prevalence of patients with stage IV NSCLC (n = 13,289) who reported at least one moderate to severe (≥4) score in each domain of the Edmonton Symptom Assessment Score within 12 months of the follow‐up period. The symptom domains were ordered from the highest to lowest 12‐month prevalence.
Figure 2.
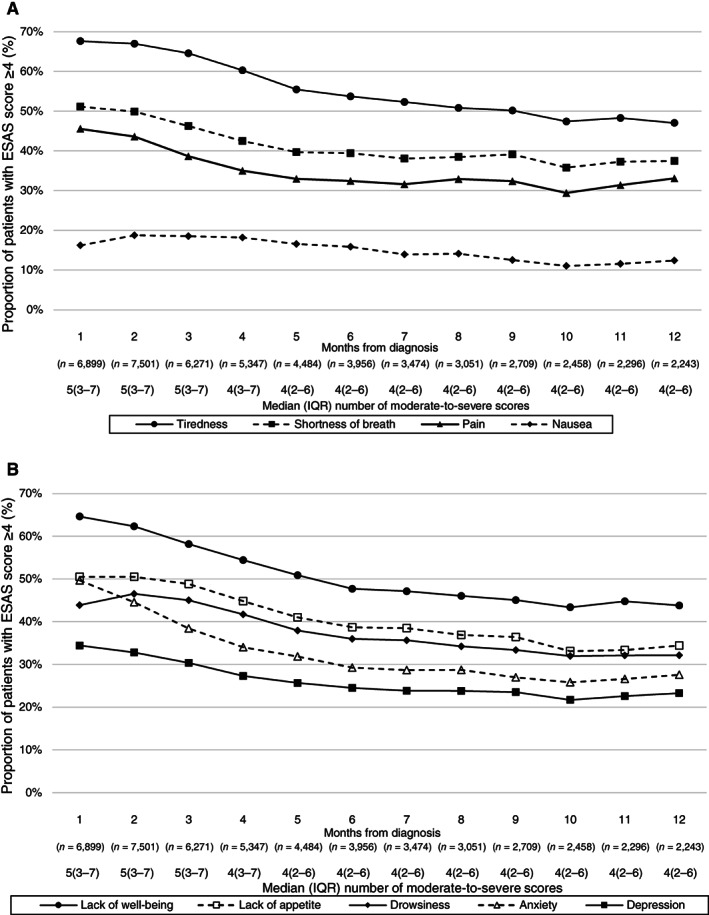
(A and B): Line graphs depicting trajectories of patient proportions reporting a moderate to severe (≥4) score in each domain of ESAS at each month up to 12 months after diagnosis of stage IV non‐small cell lung cancer (n = 13,289). The median number of moderate to severe symptoms per patient each month was also reported. Abbreviations: ESAS, Edmonton Symptom Assessment Score; IQR, interquartile range.
The symptom trajectory of the subset of patient surviving past 12 months (n = 4,791) after diagnosis is shown in Figure 3. Tiredness (84.1%), lack of well‐being (75.7%), low appetite (65.0%), and shortness of breath (62.0%) were also the most prevalent symptoms in this subset of patients, whereas nausea (15.3%) was the least prevalent. Monthly peaks and nadirs of these symptoms were as follows: 54.6% (month 1) and 42.7% (month 10) for tiredness; 53.9% (month 1) and 38.3% (month 7) for lack of well‐being; 37.2% (month 3) and 28.7% (month 10) for low appetite; 39.9% (month 1) and 31.1% (month 7) for shortness of breath; and 13.9% (month 2) and 9.0% (month 10) for nausea (Fig. 3). Among these, the median moderate to severe score was 4 in the months following diagnosis up to a year, aside from month 5 and 10, where the median was 3.
Figure 3.
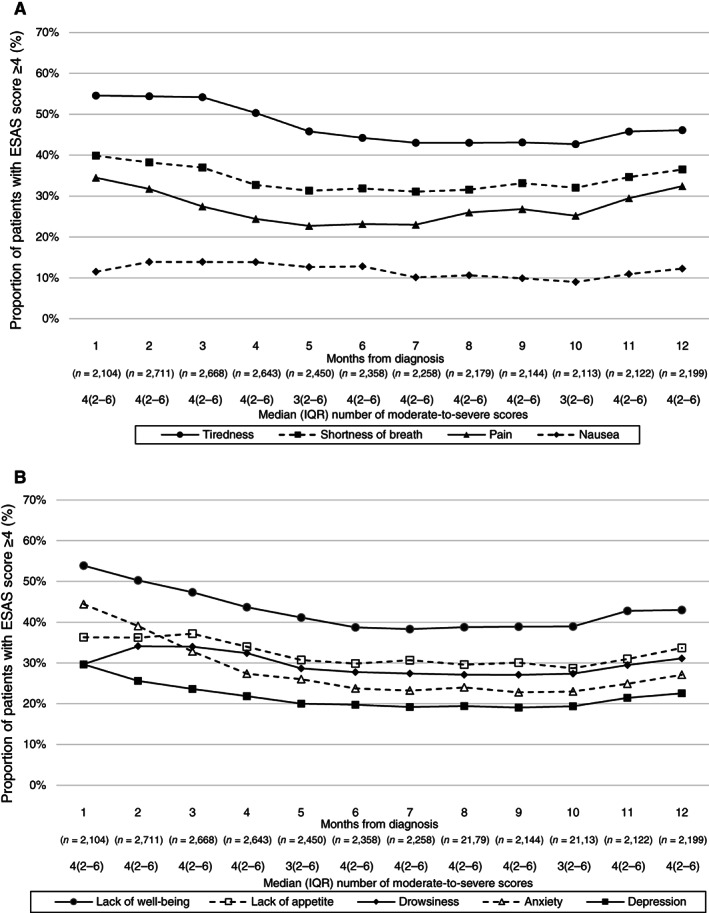
(A and B): Line graphs depicting trajectories of patient proportions reporting a moderate to severe (≥4) score in each domain of ESAS at each month among patients surviving at least 12 months after stage IV non‐small cell lung cancer diagnosis (n = 4,791). The median number of moderate to severe symptoms per patient each month was also reported. Abbreviations: ESAS, Edmonton Symptom Assessment Score; IQR, interquartile range.
Factors Associated with Moderate to Severe Symptoms
There were significant associations between moderate to severe ESAS symptom scores and patient and treatment characteristics (Table 2). Older patients were less likely to report moderate to severe nausea (RR: 0.67–0.78 among patients ≥70 years) and pain (RR: 0.78–0.89 among patients ≥60 years); patients from age groups ≥80 years reported higher moderate to severe lack of appetite (RR: 1.13), whereas age groups ≥70 years reported higher shortness of breath (RR: 1.08–1.11) and tiredness (RR: 1.08–1.11).
Table 2.
Multivariable modified Poisson regression analysis of the association between patient and treatment characteristics and moderate to severe ESAS scores in the 12 months after stage IV non‐small cell lung cancer diagnosis for all symptoms
| Characteristic | Relative Risk (Bonferroni‐corrected [99.4%] confidence interval) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anxiety | Depression | Drowsiness | Lack of appetite | Nausea | Pain | Shortness of Breath | Tiredness | Lack of well‐being | |
| Age group, yr | |||||||||
| 18–49 | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| 50–59 |
1.11 (0.98–1.25) |
1.11 (0.95–1.29) |
1.06 (0.95–1.18) |
1.03 (0.94–1.14) |
1.03 (0.87–1.23) |
1 (0.9–1.11) |
1.09 (0.97–1.23) |
1.04 (0.96–1.12) |
1.02 (0.94–1.1) |
| 60–69 |
1.03 (0.91–1.15) |
0.99 (0.85–1.14) |
1.01 (0.9–1.12) |
1.03 (0.94–1.13) |
0.85 (0.72–1.01) |
0.89 (0.8–0.98)a |
1.10 (0.99–1.23) |
1.03 (0.96–1.11) |
0.98 (0.91–1.06) |
| 70–79 |
1.01 (0.89–1.13) |
0.99 (0.86–1.15) |
1.04 (0.94–1.16) |
1.07 (0.98–1.18) |
0.78 (0.66–0.93)a |
0.85 (0.77–0.95)a |
1.18 (1.06–1.32)a |
1.08 (1.01–1.16)a |
1.01 (0.94–1.09) |
| 80 and older |
0.97 (0.85–1.1) |
1 (0.86–1.18) |
1.06 (0.95–1.19) |
1.13 (1.02–1.25)a |
0.67 (0.54–0.82)a |
0.78 (0.7–0.88)a |
1.19 (1.05–1.34)a |
1.11 (1.03–1.2)a |
1.05 (0.96–1.14) |
| Sex | |||||||||
| Male | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| Female |
1.14 (1.09–1.19)a |
1.01 (0.95–1.07) |
0.97 (0.93–1.01) |
1.02 (0.98–1.05) |
1.14 (1.06–1.23)a |
0.93 (0.89–0.97)a |
0.88 (0.84–0.92)a |
1 (0.97–1.02) |
1 (0.97–1.03) |
| Elixhauser Comorbidity Index | |||||||||
| Low (<4) | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| High (≥4) |
1.03 (0.95–1.11) |
1.08 (0.98–1.19) |
1.05 (0.98–1.13) |
1.04 (0.97–1.11) |
1.08 (0.95–1.24) |
1.07 (0.99–1.15) |
1.12 (1.05–1.19)a |
1.06 (1.01–1.1)a |
1.05 (1–1.11)a |
| Immigration status | |||||||||
| Nonimmigrant | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| Immigrant |
0.91 (0.82–1) |
1.03 (0.92–1.15) |
0.85 (0.78–0.94)a |
0.93 (0.86–1.01) |
0.92 (0.79–1.07) |
0.98 (0.9–1.07) |
0.87 (0.79–0.95)a |
0.94 (0.89–0.99)a |
0.93 (0.88–0.99)a |
| Income quintile | |||||||||
| Q5 (highest income) | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| Q1 |
1.08 (0.99–1.16) |
1.13 (1.03–1.24)a |
1.1 (1.03–1.18) |
1.04 (0.98–1.11) |
1.17 (1.04–1.32)a |
1.15 (1.07–1.23)a |
1.12 (1.05–1.19)a |
1.07 (1.02–1.12)a |
1.04 (0.99–1.09) |
| Q2 |
1.03 (0.96–1.11) |
1.06 (0.96–1.16) |
1.05 (0.98–1.13) |
1.05 (0.99–1.12) |
1.09 (0.97–1.23) |
1.12 (1.04–1.2)a |
1.06 (0.99–1.13) |
1.03 (0.98–1.08) |
1.02 (0.97–1.07) |
| Q3 |
1.04 (0.96–1.12) |
1.07 (0.97–1.18) |
1.05 (0.98–1.12) |
1.05 (0.99–1.12) |
1.12 (0.99–1.27) |
1.09 (1.01–1.18)a |
1.02 (0.96–1.1) |
1.04 (0.99–1.08) |
1.03 (0.98–1.08) |
| Q4 |
1.04 (0.97–1.13) |
1.05 (0.95–1.16) |
1.02 (0.95–1.1) |
1.01 (0.94–1.07) |
1.04 (0.92–1.18) |
1.04 (0.96–1.12) |
1.03 (0.96–1.1) |
1.01 (0.97–1.06) |
1.01 (0.96–1.07) |
| Rurality | |||||||||
| Major urban | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| Non‐major urban |
0.97 (0.92–1.02) |
0.9 (0.84–0.96)a |
0.98 (0.93–1.03) |
0.98 (0.94–1.02) |
0.97 (0.89–1.05) |
0.95 (0.9–1) |
1.01 (0.96–1.06) |
0.97 (0.94–1) |
0.94 (0.91–0.97)a |
| Rural |
0.94 (0.86–1.03) |
0.83 (0.74–0.94)a |
0.98 (0.9–1.06) |
0.97 (0.91–1.05) |
0.83 (0.71–0.97)a |
0.96 (0.88–1.05) |
1.01 (0.94–1.09) |
0.96 (0.9–1.01) |
0.97 (0.91–1.03) |
| Diagnosis year | |||||||||
| 2007–2018 |
0.98 (0.98–0.99)a |
0.99 (0.98–0.99)a |
0.99 (0.99–1) |
0.96 (0.96–0.97)a |
0.97 (0.96–0.98)a |
0.99 (0.98–0.99)a |
0.99 (0.98–0.99)a |
0.99 (0.99–0.99)a |
0.99 (0.98–0.99)a |
| Systemic therapy within 2 weeks prior to highest monthly ESAS score | |||||||||
| Yes |
0.97 (0.93–1.01) |
1.01 (0.97–1.06) |
1.08 (1.05–1.12)a |
1.06 (1.02–1.09)a |
1.25 (1.17–1.34)a |
1.02 (0.98–1.06) |
1.01 (0.98–1.05) |
1.05 (1.02–1.07)a |
1.03 (1–1.06)a |
| No | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| Radiation within 2 weeks prior to highest monthly ESAS score | |||||||||
| Yes |
1.03 (0.99–1.08) |
1.11 (1.06–1.17)a |
1.28 (1.23–1.33)a |
1.17 (1.12–1.21)a |
1.48 (1.38–1.6)a |
1.2 (1.16–1.25)a |
1.06 (1.02–1.1)a |
1.15 (1.12–1.18)a |
1.1 (1.07–1.14)a |
| No | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| Months from diagnosis | |||||||||
| 1 | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] | 1 [Ref.] |
| 2 |
0.9 (0.86–0.93)a |
0.94 (0.89–0.99)a |
1.04 (0.99–1.09) |
0.99 (0.95–1.03) |
1.09 (0.99–1.19) |
0.94 (0.9–0.99)a |
0.97 (0.94–1.01) |
0.98 (0.95–1) |
0.96 (0.93–0.99)a |
| 3 |
0.78 (0.74–0.82)a |
0.9 (0.84–0.95)a |
1.04 (0.99–1.09) |
0.97 (0.93–1.02) |
1.08 (0.98–1.19) |
0.86 (0.82–0.91)a |
0.92 (0.88–0.96)a |
0.97 (0.94–0.99)a |
0.91 (0.88–0.94)a |
| 4 |
0.71 (0.67–0.75)a |
0.85 (0.79–0.9)a |
1.02 (0.97–1.07) |
0.93 (0.88–0.98)a |
1.11 (0.99–1.23) |
0.82 (0.77–0.86)a |
0.86 (0.82–0.91)a |
0.93 (0.9–0.96)a |
0.88 (0.84–0.91)a |
| 5 |
0.68 (0.64–0.72)a |
0.82 (0.76–0.87)a |
0.96 (0.91–1.02) |
0.88 (0.83–0.92)a |
1.04 (0.93–1.16) |
0.8 (0.75–0.84)a |
0.84 (0.8–0.88)a |
0.89 (0.85–0.92)a |
0.84 (0.81–0.88)a |
| 6 |
0.63 (0.6–0.68)a |
0.79 (0.74–0.85)a |
0.94 (0.89–0.99)a |
0.85 (0.8–0.9)a |
1.01 (0.9–1.13) |
0.8 (0.75–0.85)a |
0.85 (0.81–0.89)a |
0.87 (0.84–0.91)a |
0.81 (0.77–0.84)a |
| 7 |
0.64 (0.6–0.69)a |
0.8 (0.75–0.87)a |
0.97 (0.91–1.03) |
0.87 (0.82–0.92)a |
0.92 (0.81–1.04) |
0.8 (0.75–0.85)a |
0.85 (0.81–0.9)a |
0.87 (0.83–0.9)a |
0.81 (0.78–0.85)a |
| 8 |
0.64 (0.6–0.68)a |
0.81 (0.75–0.87)a |
0.94 (0.89–1) |
0.85 (0.8–0.91)a |
0.94 (0.83–1.07) |
0.85 (0.79–0.9)a |
0.87 (0.82–0.92)a |
0.86 (0.82–0.9)a |
0.81 (0.77–0.85)a |
| 9 |
0.62 (0.58–0.66)a |
0.81 (0.75–0.88)a |
0.95 (0.89–1.01) |
0.86 (0.81–0.91)a |
0.86 (0.75–0.99)a |
0.86 (0.8–0.92)a |
0.89 (0.84–0.95)a |
0.86 (0.82–0.9)a |
0.8 (0.76–0.85)a |
| 10 |
0.62 (0.58–0.67)a |
0.8 (0.74–0.87)a |
0.95 (0.89–1.02) |
0.82 (0.77–0.88)a |
0.8 (0.7–0.93)a |
0.82 (0.77–0.88)a |
0.87 (0.82–0.92)a |
0.85 (0.81–0.89)a |
0.8 (0.76–0.85)a |
| 11 |
0.64 (0.59–0.69)a |
0.83 (0.76–0.9)a |
0.97 (0.9–1.04) |
0.84 (0.79–0.9)a |
0.85 (0.74–0.99)a |
0.88 (0.82–0.94)a |
0.9 (0.85–0.96)a |
0.86 (0.82–0.9)a |
0.83 (0.79–0.88)a |
| 12 |
0.66 (0.62–0.71)a |
0.85 (0.79–0.93)a |
1 (0.94–1.07) |
0.89 (0.83–0.95)a |
0.93 (0.8–1.07) |
0.93 (0.87–0.99)a |
0.92 (0.87–0.98)a |
0.87 (0.82–0.91)a |
0.84 (0.79–0.88)a |
Statistically significant using Bonferroni correction (p < .006).
Abbreviations: ESAS, Edmonton Symptom Assessment System; Ref., reference.
Females were more likely than males to report moderate to severe anxiety (RR: 1.14; 99.4% CI: 1.09–1.19) and nausea (RR: 1.14; 99.4% CI: 1.06–1.23) and less likely to report moderate to severe pain (RR: 0.93; 99.4% CI: 0.89–0.97) and shortness of breath (RR: 0.88; 99.4% CI: 0.84–0.92). Moderate to severe shortness of breath, tiredness, and lack of well‐being were more common among patients with high baseline Elixhauser comorbidity index (RR: 1.05–1.12). Immigrants were less likely to report moderate to severe drowsiness, shortness of breath, tiredness, and lack of well‐being (RR: 0.85–0.94).
Compared with patients from neighborhoods with the highest income quintile, patients from neighborhoods with the lowest income quintile reported higher moderate to severe scores in depression, nausea, pain, shortness of breath, and tiredness (RR: 1.07–1.17); higher moderate to severe pain was observed among patients from three lower quintiles (RR: 1.09–1.15). Compared with major urban residents, rural residents reported lower depression and nausea (RR: 0.83 for both), whereas non‐major urban residents reported lower depression and lack of well‐being (RR: 0.90–0.94).
In terms of symptom prevalence over time, a lower prevalence of moderate to severe scores was observed within 2 months after diagnosis for anxiety, depression, pain, and lack of well‐being; within 3 months for shortness of breath and tiredness; within 4 months for lack of appetite; and within 9 months for nausea. Aside from less drowsiness 6 months after diagnosis, this domain's prevalence seemed unchanged during follow‐up. A later year of diagnosis was associated with a lower risk of moderate to severe scores in all ESAS domains but drowsiness (RR: 0.96–0.99 per 1‐year increment).
There were significant associations between delivery of systemic or radiation treatments within 2 weeks prior to symptom peak and moderate to severe symptom scores. Higher drowsiness, lack of appetite, nausea, tiredness, and lack of well‐being were associated with systemic therapy delivery within 2 weeks prior (RR: 1.03–1.25). Higher moderate to severe scores in all ESAS domains aside from anxiety were associated with radiotherapy delivered within 2 weeks prior (RR: 1.06–1.48).
Discussion
This province‐wide analysis of ESAS from 22,799 patients with stage IV NSCLC reporting a total of 84,373 unique ESAS assessments represents, to our knowledge, the largest lung cancer PRO cohort published worldwide. Our cohort demonstrated high, persistent symptom burden among patients with stage IV NSCLC up to a year after diagnosis (Figs. 1, 2, 3). With nearly all patients who completed ESAS (94.4%) reporting at least one moderate to severe symptom within the 12 months of diagnosis, this population exhibited higher moderate to severe symptoms compared with other malignancies including breast, head and neck, central nervous system, and pancreatic cancers (Fig. 1) [17, 24]. In our population, nausea and drowsiness were the only symptoms in which prevalence increased after diagnosis (Fig. 2). Although difficult to distinguish with current data, these findings may relate to the side effects of cancer‐directed treatments, such as radiation and systemic therapy, as well as some supportive therapies such as opioids [25, 26].
Analysis of symptom trajectories among patients surviving at least 12 months after diagnosis demonstrated lower baseline moderate to severe symptom prevalence but less decrease of the prevalence over the 12‐month period compared with the whole cohort (Figs. 2, 3). The decreasing moderate to severe symptom prevalence in the whole cohort over time may reflect the better symptom burden among longer surviving patients, who may have favorable cancer biology, less extensive metastatic disease, and better baseline performance status. This reflected the symptom persistence among patients with stage IV NSCLC.
Our analyses demonstrated notable associations between patient baseline characteristics and the occurrence of moderate to severe symptoms (Table 2). Similar to patients with stage I–III NSCLC, the most prevalent symptoms were tiredness, lack of well‐being, low appetite, and shortness of breath [23]. Older patients were at higher risk of low appetite, shortness of breath, and tiredness but at lower risk of nausea and pain. Females reported higher anxiety and nausea but lower shortness of breath and pain. High comorbidity index, major urban residence, and nonimmigrants are associated with higher moderate to severe symptoms in some ESAS domains such as shortness of breath or pain, which could be alleviated with supportive therapies. Ensuring PRO completion for symptom identification will be especially important for these high‐risk patients. Understanding patient characteristics that may be associated with increased risk of certain symptom constellations may aid in devising strategic supportive care initiatives. As an example, higher depression, nausea, pain, shortness of breath, and tiredness is increased in patients with lower income. To address this barrier, upstream referrals to the relevant care providers and creation of frameworks to systematically improve access are examples of initiatives that could be piloted to address patient‐centered needs [27].
Our results suggest that patients with metastatic NSCLC exhibited worse symptom burden when compared with other advanced, incurable malignancies such as metastatic breast, gastric, and esophageal cancers [28, 29, 30]. The degree of early symptom burden from our results suggests that almost all patients with stage IV NSCLC would benefit from supportive interventions such as psychosocial or palliative care referral soon after diagnosis. Indeed, a randomized trial showed QoL improvement and survival prolongation with early palliative care for patients with stage IV NSCLC [4]. Nonetheless, although referral to palliative care after a diagnosis of metastatic NSCLC may be helpful in the management of symptom and side effects, this strategy is not feasible in broader practice owing to the limited availability of specialized palliative care services (especially in lower resource settings), as well as patient/provider hesitancy for early involvement [31, 32]. Instead, clinician‐initiated palliative referrals guided by institutional criteria and/or identification of patients who may best benefit on the basis of symptom burden thresholds warrants additional evaluation [31].
A recent study investigating ESAS among patients with cancer in Ontario indicated that a high symptom burden was a predictor of adverse events such as unplanned hospitalizations and emergency room visits [33]. We would suggest that early PRO completion (e.g., ESAS) may help with timely symptom detection and thus alert clinicians about the need for specific supportive measures in this population. For instance, a worsening dyspnea score serves as an early indicator for actionable diagnoses such as pleural effusion or pneumonitis needing further investigations with chest x‐ray and routine blood work.
The rate of ESAS completion has increased since 2013 compared with prior, indicating its increasing use since the program implementation in 2007. A study in 2019 reported increasingly uniform rates of ESAS completion between Ontario regions since its deployment [34]. Encouragingly, there are also significant associations between year of diagnosis and lower reported moderate to severe symptoms in all ESAS domains, which may reflect progress in the awareness and effectiveness of supportive managements and treatments for patients with stage IV NSCLC [35]. Importantly, an Ontario study showed that more documentations and clinical actions such as addition of symptom‐directed medications or referrals are triggered by high ESAS symptom scores [36].
Nonetheless, patients who are older, with higher comorbidity, or not receiving active cancer treatment were less likely to complete ESAS (Table 1); these patients may have fewer visits to cancer centers where ESAS are systematically collected. The surge in virtual health care adoption due to the COVID‐19 pandemic may present an opportunity to implement PRO collection without in‐person clinic visits [37]. Systematic verbal collection of the ESAS scores during these virtual encounters may be a method to address a particularly vulnerable patient population within the current evolving clinical workflow.
There were significant associations between patient‐reported moderate to severe scores across multiple ESAS domains and the administration of radiotherapy 2 weeks prior to peak symptoms (Table 2). Several factors may contribute to these associations. These patients commonly received radiotherapy early after diagnosis, corresponding to the period of highest symptom burden in this population (Fig. 2). Radiotherapy in this setting is often used to palliate symptoms such as hemoptysis, pain, and shortness of breath [38]. The effect of radiotherapy can take weeks to manifest [39], which, in addition to its associated side effects, highlights the need for heightened supportive care during this period. Regarding systemic therapy, its delivery 2 weeks prior to peak symptoms was associated with higher drowsiness, lack of appetite, tiredness, and lack of well‐being but lower anxiety (Table 2). Nausea is a common side effect of platinum‐based chemotherapies used in stage IV NSCLC, which can be effectively managed with modern antiemetics [40]. A key caveat of these associations is that it does not necessarily infer causality between symptoms and treatment, a topic our group plans to investigate in more detail in future work.
Additional limitations of our study warrant mention. First, more than 40% of the population did not complete any ESAS, representing a substantial data loss. These nonrespondents were more likely to be older, to be of higher comorbidity, and to not receive any active cancer treatment, all features that may be particularly associated with vulnerability. Patients who do not complete PROs may have their symptoms unaddressed and experienced delay in accessing treatments, which may lead to cessation of therapies and worse clinical outcomes [13]. Second, administrative documentation processes involved in the large databases used for the analysis may introduce random error to the results. Third, the rates of ESAS collection may not be uniform between centers within the study period, reflecting differential rates of ESAS uptake in Ontario since its province‐wide implementation in 2007. Lastly, our current data set lacks information on tumor biomarkers such as epidermal growth factor receptor mutations or ALK rearrangements, which guide the choice of systemic therapy in the modern era [41, 42]. Despite these limitations, a major strength of our study lies in its large size in the context of a province‐wide PRO implementation within routine clinical care, improving its generalizability. Future studies are planned to investigate the frequency of focused symptom‐specific interventions, health care resource utilization, and the patient stakeholder perspective of our PRO research.
Conclusion
This province‐wide study of PRO cohort data of patients with stage IV NSCLC demonstrated that moderate to severe symptoms were high and persistent and peaked early after diagnosis. This supports the importance of early and sustained PRO collection for identification of symptoms that may be amenable to intervention in these patients. Characteristics of patients less likely to complete any ESAS were identified; considerations to address this can include remote ESAS collection through telemedicine or virtual clinics, which are increasingly being used within our health system.
Author Contributions
Conception/design: Michael C. Tjong, Mark Doherty, Hendrick Tan, Wing C. Chan, Haoyu Zhao, Victoria Delibasic, Natalie G. Coburn, Alexander V. Louie
Provision of study material or patients: Wing C. Chan, Haoyu Zhao, Natalie G. Coburn, Alexander V. Louie
Collection and/or assembly of data: Michael C. Tjong, Wing C. Chan, Haoyu Zhao, Natalie G. Coburn, Alexander V. Louie
Data analysis and interpretation: Michael C. Tjong, Mark Doherty, Hendrick Tan, Wing C. Chan, Haoyu Zhao, Julie Hallet, Gail Darling, Biniam Kidane, Frances C. Wright, Alyson Mahar, Laura E. Davis, Victoria Delibasic, Ambika Parmar, Nicole Mittmann, Natalie G. Coburn, Alexander V. Louie
Manuscript writing: Michael C. Tjong, Mark Doherty, Hendrick Tan, Wing C. Chan, Haoyu Zhao, Julie Hallet, Gail Darling, Biniam Kidane, Frances C. Wright, Alyson Mahar, Laura E. Davis, Victoria Delibasic, Ambika Parmar, Nicole Mittmann, Natalie G. Coburn, Alexander V. Louie
Final approval of manuscript: Michael C. Tjong, Mark Doherty, Hendrick Tan, Wing C. Chan, Haoyu Zhao, Julie Hallet, Gail Darling, Biniam Kidane, Frances C. Wright, Alyson Mahar, Laura E. Davis, Victoria Delibasic, Ambika Parmar, Nicole Mittmann, Natalie G. Coburn, Alexander V. Louie
Disclosures
Mark Doherty: Merck, AstraZeneca, Eisai, Boehringer Ingelheim, Roche, Takeda (C/A), Merck, AstraZeneca (RF); Julie Hallet: Ipsen, Advanced Accelerator Applications (H); Biniam Kidane: AstraZeneca (SAB); Natalie G. Coburn: AstraZeneca (H); Alexander V. Louie: RefleXion, Varian Medical Systems Inc., AstraZeneca (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Acknowledgments
This research was funded by an Ontario Institute of Cancer Research Grant. N.G.C. receives salary support from the Sherif and MaryLou Hanna Chair in Surgical Oncology Research and is the Clinical Lead for Patient‐Reported Outcomes and Symptom Management for Ontario Health‐Cancer Care Ontario.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DR, Weir HK, Demers AA et al. Projected estimates of cancer in Canada in 2020. Cmaj 2020;192:E199–E205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone A, Krapcho M et al. SEER Cancer Statistics Review (CSR), 1975‐2017. National Cancer Institute 2017. Available at https://seer.cancer.gov/csr/1975_2017/. Accessed August 18, 2020.
- 4.Temel J, Greer J, Muzikansky A et al. Early palliative care for patients with metastatic non–small‐cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 5.Ferrell BR, Temel JS, Temin S et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:96–112. [DOI] [PubMed] [Google Scholar]
- 6.Bremner KE, Krahn MD, Warren JL et al. An international comparison of costs of end‐of‐life care for advanced lung cancer patients using health administrative data. Palliat Med 2015;29:918–928. [DOI] [PubMed] [Google Scholar]
- 7.Warren JL, Barbera L, Bremner KE et al. End‐of‐life care for lung cancer patients in the United States and Ontario. J Natl Cancer Inst 2011;103:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvo N, Zeng L, Zhang L et al. Frequency of reporting and predictive factors for anxiety and depression in patients with advanced cancer. Clin Oncol 2012;24:139–148. [DOI] [PubMed] [Google Scholar]
- 9.Iyer S, Roughley A, Rider A et al. The symptom burden of non‐small cell lung cancer in the USA: A real‐world cross‐sectional study. Support Care Cancer 2014;22:181–187. [DOI] [PubMed] [Google Scholar]
- 10.Atherton PJ, Watkins‐Bruner DW, Gotay C et al. The complementary nature of patient‐reported outcomes and adverse event reporting in cooperative group oncology clinical trials: A pooled analysis (NCCTG N0591). J Pain Symptom Manage 2015;50:470–479.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbera L, Sutradhar R, Howell D et al. Does routine symptom screening with ESAS decrease ED visits in breast cancer patients undergoing adjuvant chemotherapy? Support Care Cancer 2015;23:3025–3032. [DOI] [PubMed] [Google Scholar]
- 13.Ben Bouazza Y, Chiairi I, El Kharbouchi O et al. Patient‐reported outcome measures (PROMs) in the management of lung cancer: A systematic review. Lung Cancer 2017;113:140–151. [DOI] [PubMed] [Google Scholar]
- 14.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;88:2164–2171. [DOI] [PubMed] [Google Scholar]
- 15.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: Past, present and future developments. J Pain Symptom Manag 2017;53:630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benchimol EI, Smeeth L, Guttmann A et al. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) Statement. PLoS Med 2015;12:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tung S, Davis LE, Hallet J et al. Population‐level symptom assessment following pancreaticoduodenectomy for adenocarcinoma. JAMA Surg 2019;154:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selby D, Cascella A, Gardiner K et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage 2010;39:241–249. [DOI] [PubMed] [Google Scholar]
- 19.Kralj B.Measuring ‘rurality’ for purposes of health‐care planning: An empirical measure for Ontario. Ont Med Rev 2000;67:33–52. [Google Scholar]
- 20.Azzalini L, Chabot‐Blanchet M, Southern DA et al. A disease‐specific comorbidity index for predicting mortality in patients admitted to hospital with a cardiac condition. CMAJ 2019;191:E299–E307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jawitz OK, Wang Z, Boffa DJ et al. The differential impact of preoperative comorbidity on perioperative outcomes following thoracoscopic and open lobectomies. Eur J Cardiothorac Surg 2017;51:169–174. [DOI] [PubMed] [Google Scholar]
- 22.Palma DA, Olson R, Harrow S et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long‐term results of the SABR‐COMET phase II randomized trial. J Clin Oncol 2020;38:2830–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirpara DH, Gupta V, Davis LE et al. Severe symptoms persist for up to one year after diagnosis of stage I‐III lung cancer: An analysis of province‐wide patient reported outcomes. Lung Cancer 2020;142:80–89. [DOI] [PubMed] [Google Scholar]
- 24.Bubis LD, Davis L, Mahar A et al. Symptom burden in the first year after cancer diagnosis: An analysis of patient‐reported outcomes. J Clin Oncol 2018;36:1103–1111. [DOI] [PubMed] [Google Scholar]
- 25.Singh P, Yoon SS, Kuo B. Nausea: A review of pathophysiology and therapeutics. Therap Adv Gastroenterol 2016;9:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nersesyan H, Slavin KV. Current aproach to cancer pain management: Availability and implications of different treatment options. Ther Clin Risk Manag 2007;3:381–400. [PMC free article] [PubMed] [Google Scholar]
- 27.Yorio JT, Yan J, Xie Y et al. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clin Lung Cancer 2012;13:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ecclestone C, Chow R, Pulenzas N et al. Quality of life and symptom burden in patients with metastatic breast cancer. Support Care Cancer 2016;24:4035–4043. [DOI] [PubMed] [Google Scholar]
- 29.Bubis LD, Delibasic V, Davis LE et al. Patient‐reported symptoms in metastatic gastric cancer patients in the last 6 months of life. Support Care Cancer 2021;29:515–524. [DOI] [PubMed] [Google Scholar]
- 30.Merchant SJ, Kong W, Brundage M et al. Symptom evolution in patients with esophageal and gastric cancer receiving palliative chemotherapy: A population‐based study. Ann Surg Oncol 2020;28:79–87. [DOI] [PubMed] [Google Scholar]
- 31.Mathews J, Hannon B, Zimmermann C. Models of integration of specialized palliative care with oncology. Curr Treat Options Oncol 2021;22:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conlon MSC, Caswell JM, Santi SA et al. Access to palliative care for cancer patients living in a northern and rural environment in Ontario, Canada: The effects of geographic region and rurality on end‐of‐life care in a population‐based decedent cancer cohort. Clin Med Insights Oncol 2019;13:1179554919829500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noel C, Sutradhar R, Hallet J et al. Symptom burden as a predictor of emergency room use and unplanned hospitalization in patients with head and neck cancer: A population‐based study. J Clin Oncol 2020;38:12084. [DOI] [PubMed] [Google Scholar]
- 34.Barbera L, Lee F, Sutradhar R. Use of patient‐reported outcomes in regional cancer centres over time: A retrospective study. CMAJ Open 2019;7:E101–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simoff MJ, Lally B, Slade MG et al. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013;143(suppl 5):e455S–e497S. [DOI] [PubMed] [Google Scholar]
- 36.Seow H, Sussman J, Martelli‐Reid L et al. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. J Oncol Pract 2012;8:e142–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster P.Virtual health care in the era of COVID‐19. Lancet 2020;395:1180–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langendijk JA, Ten Velde GPM, Aaronson NK et al. Quality of life after palliative radiotherapy in non‐small cell lung cancer: A prospective study. Int J Radiat Oncol Biol Phys 2000;47:149–155. [DOI] [PubMed] [Google Scholar]
- 39.Macbeth FR, Bolger JJ, Hopwood P et al. Randomized trial of palliative two‐fraction versus more intensive 13‐fraction radiotherapy for patients with inoperable non‐small cell lung cancer and good performance status. Clin Oncol 1996;8:167–175. [DOI] [PubMed] [Google Scholar]
- 40.Ranganath P, Einhorn L, Albany C. Management of chemotherapy induced nausea and vomiting in patients on multiday cisplatin based combination chemotherapy. Biomed Res Int 2015;2015:943618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mok TS, Wu YL, Ahn MJ et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw AT, Kim D‐W, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information


