Abstract
In June 2020, the U.S. Food and Drug Administration granted accelerated approval to selinexor for the treatment of adult patients with relapsed or refractory diffuse large B‐cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy. Approval was based on SADAL, a multicenter trial of selinexor monotherapy in patients with DLBCL after two to five systemic regimens. Efficacy was based on independent review committee–assessed objective response rate (ORR) and duration of response using Lugano criteria. In 134 patients treated with the approved dosage (60 mg orally on days 1 and 3 of each week), the ORR was 29% (95% confidence interval, 22–38), with complete response in 13% and with 38% of responses lasting at least 6 months. Gastrointestinal toxicity developed in 80% of patients, hyponatremia in 61%, central neurological toxicity (such as dizziness and mental status changes) in 25%, and ocular toxicity in 18%. New or worsening grade 3 or 4 thrombocytopenia, lymphopenia, neutropenia, anemia, or hyponatremia developed in ≥15%. Adverse reactions led to selinexor dose interruption in 61% of patients, dose reduction in 49%, and permanent discontinuation in 17%, with thrombocytopenia being the leading cause of dose modifications. Postmarketing studies will evaluate reduced dosages of selinexor and further evaluate clinical benefit in patients with relapsed or refractory DLBCL.
Implications for Practice
Selinexor is a new potential option for adults with relapsed or refractory diffuse large B‐cell lymphoma, not otherwise specified, in the third‐line setting or beyond. Toxicities are typically manageable but can be difficult to tolerate and necessitate close monitoring and supportive care.
Keywords: Diffuse large B‐cell lymphoma, Non‐Hodgkin lymphoma, Transformed lymphoma, Selinexor
Short abstract
This review provides a regulatory overview of the U.S. FDA approval of selinexor for the treatment of adult patients with relapsed or refractory diffuse large B‐cell lymphoma after at least two lines of systemic therapy.
Introduction
Although hematopoietic stem cell transplantation (HSCT) is potentially curative for patients with relapsed or refractory (R/R) diffuse large B‐cell lymphoma (DLBCL), management is especially challenging for patients who have chemoresistant lymphoma, comorbidities, or other issues that preclude HSCT or relapse despite HSCT [1, 2, 3, 4]. Treatment goals for such patients are highly individualized and include palliation of symptoms, possibly prolongation of survival, and in some cases, bridging to allogeneic HSCT or other cellular therapies. Chimeric antigen receptor (CAR) T‐cell therapies can produce durable complete remissions (CRs) in patients with multiply pretreated, resistant DLBCL, and three such products are now approved by the U.S. Food and Drug Administration (FDA) for large B‐cell lymphoma in the third or later line [5, 6, 7, 8]. However, their application is limited by restricted availability, stringent selection criteria [9], and the waiting period associated with product manufacture.
The treatment landscape has recently expanded for patients with R/R DLBCL to include drugs with diverse mechanisms of actions. Since 2017, the FDA has granted multiple approvals for this setting, ranging from the three CD19‐directed CAR T‐cell therapies [5, 6, 7] to polatuzumab vedotin (a CD79b‐directed antibody‐drug conjugate) in combination with bendamustine and rituximab [10], tafasitamab (a CD19‐directed antibody) in combination with lenalidomide [11], loncastuximab tesirine (a CD19‐directed antibody‐drug conjugate) [12], and selinexor [13].
Selinexor is an orally administered nuclear export inhibitor that was first approved, in combination with dexamethasone, for selected patients with relapsed or refractory multiple myeloma [14] (Table 1). Inhibition of exportin 1 is hypothesized to restore normal tumor suppressor pathways and lead to selective apoptosis of neoplastic cells [15]. In June 2020, after a priority review, the FDA extended the approval of single‐agent selinexor to patients with relapsed or refractory DLBCL, not otherwise specified (NOS), including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy. The recommended dosage in DLBCL is 60 mg orally on days 1 and 3 of each week. Herein, we summarize the FDA review and regulatory considerations with this marketing application.
Table 1.
Selinexor background information
| Structure and pharmacologic class | Small‐molecule nuclear export inhibitor. |
| Mechanism of action | Selinexor reversibly inhibits nuclear export of tumor suppressor proteins, growth regulators, and mRNAs of oncogenic proteins by blocking XPO1. XPO1 inhibition leads to accumulation of tumor suppressor proteins in the nucleus, reductions in several oncoproteins, cell cycle arrest, and apoptosis of cancer cells. |
| Pharmacokinetics |
Metabolized by CYP3A, UGTs, and GSTs. Appropriate dosing with concomitant strong CYP3A inhibitors is not determined. Dose reduction is not required for renal impairment or mild hepatic impairment. |
| Approval in DLBCLa | For the treatment of adult patients with relapsed or refractory DLBCL, NOS, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy (2020). |
| Other approvals |
In combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy (2020). In combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti‐CD38 monoclonal antibody (2019). |
Under accelerated approval.
Abbreviations: DLBCL, diffuse large B‐cell lymphoma; UGT, UDP‐glucuronosyltransferase; GST, glutathione S‐transferase; NOS, not otherwise specified; XPO1, exportin 1.
Trial Design
The application was based on SADAL (study KCP‐330‐009; NCT02227251), a multicenter, open‐label phase II trial of selinexor as a single agent in adults with relapsed or refractory DLBCL NOS after two to five systemic regimens [13]. The original design randomized patients to receive either 60 mg or 100 mg of selinexor orally twice weekly, with a 28‐day cycle length. Earlier protocol versions dosed selinexor twice weekly on weeks 1, 2, and 3 of each 4‐week cycle, whereas later versions (from version 5.0 onward) dosed selinexor twice weekly each week. Treatment continued until disease progression or unacceptable toxicity. After interim analysis, the 100 mg arm was discontinued because of similar efficacy with excess toxicity compared with the 60 mg arm. The study proceeded with the 60 mg dose, administered on days 1 and 3 of each week with antiemetic prophylaxis including a 5‐HT3 antagonist. Recipients of this 60 mg dosage comprise the FDA's efficacy and primary safety population (n = 134). Safety from all patients treated on SADAL was also reviewed.
Eligible patients were not candidates for autologous HSCT. Earlier protocol versions (prior to version 6.0) required at least 2 weeks since last lymphoma therapy. During the study, eligibility criteria were changed to require at least 60 days, rather than 2 weeks, since last lymphoma therapy, with a minimum of 98 days required if the disease was refractory to the last systemic therapy. Other requirements included a life expectancy >3 months, Eastern Cooperative Oncology Group performance status ≤2, absolute neutrophil count ≥1,000/μL, platelet count ≥75,000/μL, adequate hepatic function, and no known central nervous system lymphoma.
The primary endpoint was objective response rate (ORR), as assessed by an independent review committee (IRC) using Lugano criteria [16]. The primary objective was to evaluate the ORR with selinexor compared with a minimally effective threshold of 15%. The FDA adjudicated the efficacy results.
Results
Characteristics of the analysis population are summarized in Table 2. Of the 134 patients evaluated, the median age was 67 years with one quarter being aged ≥75. Most had de novo DLBCL NOS (75%) or DLBCL arising from a low‐grade lymphoma (23%). Patients received a median of two prior systemic therapies for DLBCL (63% of patients), with 34% having three to five prior therapies. Refractory disease to the most recent therapy was documented in 28% of patients. The median time from the most recent therapy to selinexor initiation was 3.6 months in this refractory subgroup and 5.4 months overall.
Table 2.
Characteristics of the efficacy and safety population (n = 134)
| Parameter | Result |
|---|---|
| Baseline characteristics | |
| Age | |
| Median (range) | 67 (35–91) |
| ≥75 years, n | 33 (25%) |
| Diagnosis, n | |
| DLBCL NOS, de novo | 101 (75%) |
| DLBCL NOS, transformed | 31 (23%) |
| HGBLa | 2 (1.5%) |
| ECOG performance status: 0 or 1, n | 118 (88%) |
| Outcome of last systemic therapy, n | |
| Refractory (<PR) | 37 (28%) |
| ≥PR | 92 (69%) |
| Unknown | 5 (4.7%) |
| Months since last systemic therapy, median (Q1, Q3) | |
| All patients | 5.4 (3.5, 11.1) |
| Refractory to last therapy | 3.6 (3.3, 5.0) |
| Prior systemic therapies, nb | |
| Median (range) | 2 (1–5) |
| 1 | 4 (3.0%) |
| 2 | 84 (63%) |
| 3 | 32 (24%) |
| 4 or 5 | 14 (10%) |
| Prior regimens, n | |
| Autologous HSCT | 40 (30%) |
| CAR T cells | 0 (0%) |
| Stage: III or IV, n | 101 (75%) |
| Bulky disease (≥7.5 cm): Present, n | 27 (20%) |
| Treatment characteristics | |
| Selinexor exposure (60 mg twice weekly dosage) | |
| Months on selinexor, median (Q1, Q3) | 2.1 (1.2, 5.5) |
| Action because of adverse reaction, n | |
| Dose reduction | 65 (49%) |
| Interruption | 82 (61%) |
| Discontinuation | 23 (17%) |
| Days to first modification of any cause, median (Q1, Q3) | |
| Dose reduction | 42 (29, 76) |
| Interruption | 36 (23, 60) |
| Any modification | 29 (22, 43) |
| Number of dose reductions, n | |
| 1 | 42 (31%) |
| 2 | 12 (11%) |
| 3 or more | 11 (8%) |
High‐grade B‐cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement.
For patients with transformed lymphoma, indicates the number of systemic therapies for the diffuse large B‐cell lymphoma component.
Abbreviations: CAR, chimeric antigen receptor; DLBCL NOS, diffuse large B‐cell lymphoma, not otherwise specified; ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation; PR, partial response; Q1, 25th percentile; Q3, 75th percentile.
Efficacy
The determination of efficacy was based on IRC‐assessed ORR and duration of response (DOR). Efficacy is summarized in Table 3, and a waterfall plot of best overall response is shown in Figure 1A. The ORR was 29% (95% confidence interval, 22%–38%) and the CR rate was 13% by Lugano criteria, with a median time to first response of 8.1 weeks (Table 1). The FDA designated cases of clinical progressive disease (PD) without radiographic confirmation as PD, in contrast to the IRC designation of not evaluable.
Table 3.
Independent review committee–assessed efficacy in relapsed or refractory diffuse large B‐cell lymphoma
| Parameter | Selinexor 60 mg twice weekly (n = 134) |
|---|---|
| Best overall responsea | |
| Objective response, n (%) [95% CI] | 39 (29%) [22%–38%] |
| Complete response, n (%) [95% CI] | 18 (13%) [5%–16%] |
| Partial response | 21 (16%) |
| Stable disease | 11 (8%) |
| Progressive disease | 68 (51%) |
| Not evaluable | 16 (12%) |
| Time to first response, median (range), weeks | 8.1 (6.7–16.4) |
| Duration of responseb | |
| Patients maintaining response at 3 months, n/N | 22/39 (56%) |
| Patients maintaining response at 6 months, n/N | 15/39 (38%) |
| Patients maintaining response at 12 months, n/N | 6/39 (15%) |
Assessed by an independent review committee using Lugano criteria, with U.S. Food and Drug Administration adjudication.
Calculated from the date of first objective response to date of last adequate disease assessment, relapse or progression, or death from any cause.
Abbreviation: CI, confidence interval.
Figure 1.
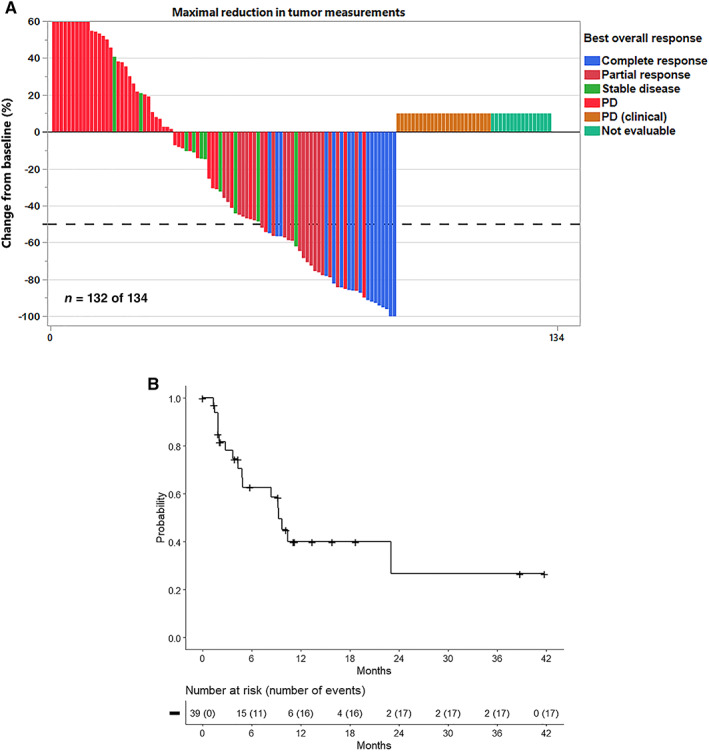
Efficacy outcomes. (A): Waterfall plot of best overall response per Lugano criteria in recipients of the 60 mg twice weekly dosage of selinexor. Patients with radiographically unevaluable disease are arbitrarily assigned a change from baseline of +10% to allow representation on the graph. This includes patients with clinical evidence of progressive disease that was not radiographically confirmed. The maximum change from baseline is arbitrarily capped at +60%. Of two patients not shown, one had partial response, and one had stable disease. (B): Kaplan‐Meier estimate of duration of response (n = 39). Abbreviation: PD, progressive disease.
Of the 39 of 134 patients who achieved an objective response, 38% maintained response for at least 6 months, and 15% maintained response for at least 12 months (Table 3 and Fig. 1B). The median DOR was reached, but the estimate may be unstable in part because of the small number of responses (Fig. 1B).
On exploratory subgroup analysis, ORR was numerically lower in patients with bulky disease per IRC (response in 1/27 vs. 38/107 in patients with nonbulky disease) and in patients with refractory disease to last systemic therapy (response in 6/37 vs. 33/92 in patients with sensitive disease to last therapy). ORRs were similar by type of lymphoma (de novo vs. transformed) and number of prior lines (two vs. three to five), although comparisons are limited by sample size.
Safety and Tolerability
An overview of safety is next provided, with a focus on the tolerability of the recommended dosage for patients with DLBCL.
Safety Overview
With the 60 mg twice weekly dosage (n = 134), serious adverse reactions (ARs) occurred in 46% of patients with DLBCL, most often from infection (21% of patients). Grade ≥ 4 ARs occurred in 35%. The most common ARs (incidence ≥20%), excluding laboratory abnormalities, were fatigue, nausea, diarrhea, appetite decrease, weight decrease, constipation, vomiting, and pyrexia (Table 4). Central neurological ARs, such as dizziness and mental status changes, occurred in 25% of patients, and grade 3 or higher infection occurred in 25%. New or worsening grade 3–4 laboratory abnormalities in ≥15% of patients included thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia (Table 4). Grade ≥ 3 cytopenias first occurred after a median of 4 to 5 weeks on selinexor. Fatal ARs occurred in 3.7% of patients within 30 days and 5.2% of patients within 60 days of last treatment, primarily from infection.
Table 4.
Selected adverse reactions in patients with diffuse large B‐cell lymphoma (n = 134)
| Adverse reaction by categorya | Selinexor 60 mg twice weekly | |
|---|---|---|
| Any grade, % | Grade 3 or 4, % | |
| Adverse reactions (>10%), excluding laboratory termsa | ||
| Gastrointestinal | ||
| Nausea | 57 | 6 |
| Diarrhea | 37 | 3.0 |
| Constipation | 29 | 0 |
| Vomiting | 28 | 1.5 |
| Metabolic or nutritional | ||
| Appetite decreased | 37 | 3.7 |
| Weight decreased | 30 | 0 |
| Nervous system | ||
| Dizziness | 16 | 0.7 |
| Taste disorder | 13 | 0 |
| Mental status changes | 11 | 3.7 |
| Ocular: Vision blurred | 11 | 0.7 |
| Other disorders | ||
| Fatigue | 63 | 15 |
| Pyrexia | 22 | 4.5 |
| Cough | 18 | 0 |
| Upper respiratory tract infection | 17 | 1.5 |
| Edema | 17 | 2.2 |
| Musculoskeletal pain | 15 | 2.2 |
| Hypotension | 13 | 3.0 |
| New or worsening laboratory abnormalities (>5% grade 3 or 4)b | ||
| Hematologic | ||
| Platelet count decrease | 86 | 49c |
| Hemoglobin decrease | 82 | 25c |
| Lymphocyte count decrease | 63 | 37c |
| Neutrophil count decrease | 58 | 31c |
| Chemistry | ||
| Sodium decrease | 62c | 16d |
| Phosphate decrease | 34 | 11 |
| Potassium decrease | 23 | 7 |
Includes events occurring up to 30 days after therapy completion. Toxicities were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Denominator varies, based on the number of patients with at least one post‐treatment value.
Grade 4 incidences: thrombocytopenia 18%, anemia 0%, lymphopenia 5%, neutropenia 9%.
Grade 1 is sodium level between 130 mmol/L and the lower limit of normal, grade 3 is 120–130 mmol/L, and grade 4 is <120 mmol/L, with no grade 2 hyponatremia in this scale.
The U.S. prescribing information (USPI) for selinexor includes warnings and precautions for thrombocytopenia, neutropenia, gastrointestinal toxicity, hyponatremia, serious infection, neurological toxicity, embryo‐fetal toxicity, and cataracts [17]. Selected toxicities are next briefly described.
Gastrointestinal Toxicity
Gastrointestinal toxicity is a leading toxicity of selinexor and requires close monitoring and supportive care. In the primary safety population, 80% of patients developed gastrointestinal ARs (grade 3–4, 13%), including nausea (57% after antiemetic prophylaxis), vomiting, and diarrhea (Table 4). Anorexia, weight loss, and disordered taste, such as dysgeusia, were common (Table 4), as were grade 1–2 electrolyte imbalances [17]. 5‐HT3 receptor antagonists and other antiemetics are indicated before and throughout treatment with selinexor. To what extent dexamethasone prophylaxis mitigates selinexor‐induced nausea and vomiting is not established. Interruption, dose reduction, or discontinuation of selinexor may be warranted based on the severity of the gastrointestinal toxicity. Antidiarrheal agents, intravenous fluids, electrolyte repletion, and nutritional support may be indicated.
Hyponatremia
In the primary safety population, analysis of laboratory data revealed that 62% of patients developed hyponatremia, with 16% developing grade 3 hyponatremia (sodium 120–130 mmol/L per Common Terminology Criteria for Adverse Events, version 4.0). The incidence of hyponatremia was underestimated in the adverse event data, with hyponatremia being reported as an adverse event in 11% of patients (grade 3; 8%). Hyponatremia tended to develop in the first cycle, with a median time to onset of 2.1 weeks (25th and 75th percentiles, 1.1 and 4.1 weeks). The mechanism of selinexor‐associated hyponatremia is not well understood. In approximately 63% of cases, hyponatremia developed in the context of gastrointestinal toxicity such as nausea, vomiting, diarrhea, anorexia, and dehydration. Interruption and dose reduction of selinexor may be warranted, in addition to the standard evaluation and management of hyponatremia.
Neurological Toxicity
The selinexor USPI has a warning and precaution for neurologic events, including dizziness, mental status changes (including delirium and confusion), depressed level of consciousness, and syncope. In the main safety population (n = 134), such neurologic events were reported in 25% of patients, with a 6% incidence of grade 3–4 events. The most common manifestation was dizziness (16% of all patients), followed by mental status changes (11%) such as confusion, cognitive disorders, and hallucination. The median time to onset was 4 weeks, and 68% of affected patients had resolution, with or without sequelae, after a median of 2 weeks. The degree to which selinexor directly causes these neurological symptoms is unclear because volume depletion (from gastrointestinal toxicity), orthostatic hypotension, concomitant medications, and/or concurrent illness may also cause or contribute to such symptoms. However, the incidence and types of neurological toxicity are similar across clinical trials of selinexor in DLBCL and multiple myeloma [17]. Risk mitigation measures include optimizing concomitant medications and volume status to avoid worsening of dizziness and mental status changes, refraining from driving until the neurological toxicity fully resolves, and instituting fall precautions [17].
Tolerability, Dose Modifications, and Dose Discontinuations
Table 2 summarizes the exposure to selinexor in the primary analysis population. In SADAL, the recommended dosage of selinexor for DLBCL (60 mg twice weekly) tended to be difficult to tolerate, and the majority of patients required dose modification. ARs led to permanent treatment discontinuation in 17% of patients, dose interruption in 61%, and dose reduction in 49%, with 17% of all patients having two or more dose reductions (Table 2). The median time to first dose modification (reduction or interruption) was 4 weeks, with the leading causes being thrombocytopenia (40% of all patients), neutropenia (16%), fatigue (16%), nausea (10%), and anemia (10%). The median time on the starting dose was 5.7 weeks (range, 1 day to 19.6 months; Fig. 2), with 83% of first dose reductions occurring within the first 3 months.
Figure 2.
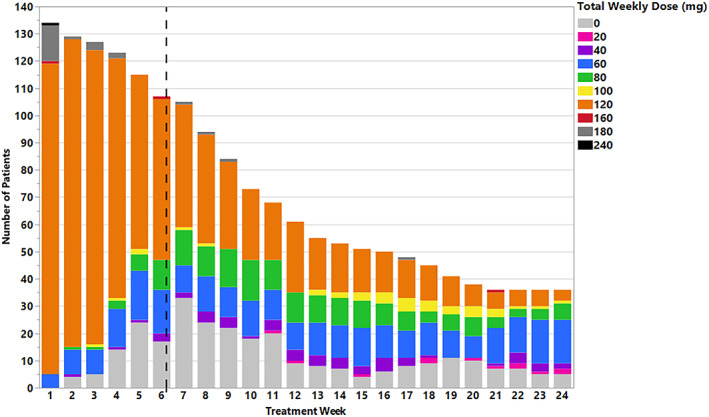
Exposure to selinexor in the primary analysis population (n = 134). Selinexor treatment duration and number of patients at each selinexor dose level are shown through 24 weeks (six cycles). The median duration on the starting dose was 5.7 weeks.
For patients treated with the approved dosage regimen in SADAL, the median duration on selinexor was 2.1 months, with 22% of patients treated for at ≥6 months and 12% treated for ≥12 months. The relative dose intensity (RDI) tended to be low, with 48% of patients having an RDI of <70%. Overall, the median selinexor exposure was 100 mg per week. However, only 7 of 39 responders (18%) had an average weekly dose intensity of at least 100 mg per week after achieving response, consistent with the frequent dose modifications for patients who remained on treatment with selinexor.
On exploratory analysis, higher selinexor exposure was statistically significantly associated with increased incidence or earlier onset of safety events such as grade ≥ 3 decreased appetite, grade ≥ 3 thrombocytopenia, grade ≥ 2 fatigue, grade ≥ 2 vomiting, and grade ≥ 1 blurred vision, as well as selinexor dose modifications and discontinuations because of treatment‐emergent ARs. Conversely, there was no evident relationship between selinexor exposure and efficacy in patients with DLBCL.
Clinical Pharmacology
Selinexor exhibits dose‐proportional pharmacokinetics at the dosages approved for DLBCL and multiple myeloma, with no significant accumulation when given once or twice weekly. Selinexor is metabolized by CYP3A, multiple UDP‐glucuronosyltransferases, and glutathione S‐transferases, with a mean half‐life of 6 to 8 hours for a single dose.
The exposure of selinexor is not significantly affected by age (18–94 years), sex, body weight (36–168 kg), ethnicity, mild to severe renal impairment (estimated creatinine clearance 15–89 mL/min), mild hepatic impairment, or administration with food. No dose modification is necessary when selinexor is coadministered with up to a 1,000 mg daily dose of acetaminophen. The effects of concomitant strong CYP3A inhibitors and moderate to severe hepatic impairment on selinexor pharmacokinetics have not been fully characterized. Postmarketing requirements to determine appropriate selinexor dosing for these patients are currently being conducted.
Regulatory Considerations
Based on the SADAL study, selinexor received accelerated approval for patients with relapsed or refractory DLBCL NOS after two or more lines of systemic therapy. Selinexor showed modest, but clinically meaningful, efficacy in patients with relapsed or refractory DLBCL at the approved dosage, based on ORR and durability of response, and carried an acceptable safety profile.
There were multiple regulatory considerations, which are briefly summarized below.
Benefit/Risk Determination
The benefit/risk determination for selinexor involved careful consideration (Table 5). The toxicities of selinexor necessitated frequent monitoring and dose interruption and/or reduction in the majority of patients in the context of modest clinical activity. On descriptive analysis of quality of life (QoL) outcomes, which was an exploratory endpoint in SADAL, declines in QoL scores during the first two cycles were observed in both responding and nonresponding patients based on the Functional Assessment of Cancer Therapy–Lymphoma questionnaire. Selinexor toxicity may have contributed to such outcomes. However, multiple limitations of the analysis include the single‐arm, open‐label study design; measurement challenges (such as potential overlap of disease and treatment‐related symptoms); and missing data. The FDA review team deemed the risks and overall benefit/risk profile of selinexor to be acceptable for the DLBCL indication (Table 5). Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial or trials.
Table 5.
U.S. Food and Drug Administration benefit/risk analysis: Selinexor for relapsed or refractory DLBCL
| Parameter | Summary |
|---|---|
| Unmet medical need | Patients with relapsed or refractory DLBCL after two or more regimens tend to have few effective treatment options. |
| Clinical benefit | In a trial for R/R DLBCL after two to five systemic therapies (SADAL), of 134 patients treated with selinexor 60 mg twice weekly, IRC‐assessed ORR was 29% (95% CI, 22%–38%) and CR rate was 13%. Of patients who achieved response, 38% maintained response for ≥6 months, and 15% maintained a response for ≥12 months. |
| Risks | The USPI for selinexor has warnings and precautions for thrombocytopenia, neutropenia, gastrointestinal toxicity, hyponatremia, serious infection, neurological toxicity, embryo‐fetal toxicity, and cataracts. |
| Of the 134 patients in SADAL treated with 60 mg twice weekly, serious ARs occurred in 46% of patients. ARs led to selinexor dose interruption in 61%, dose reduction in 49%, and permanent discontinuation in 17%. Thrombocytopenia was a leading cause of dose modification and serious ARs. Gastrointestinal toxicity occurred in 80% (grade 3–4, 13%), including nausea in 58% and vomiting in 29% with use of antiemetic prophylaxis. Central neurologic toxicities occurred in 25% (grade 3–4, 6%), most frequently dizziness and mental status changes. Nonlaboratory ARs in ≥20% of patients were fatigue, nausea, diarrhea, appetite decrease, weight decrease, constipation, vomiting, and pyrexia. Grade 3–4 laboratory abnormalities in ≥15% were thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia, with all‐grade hyponatremia developing in 62%. | |
| Uncertainties |
Verification of clinical benefit in a confirmatory trial(s) in DLBCL is pending. The optimal dosage of selinexor for DLBCL is not confirmed. Safety data in patients with moderate or severe hepatic impairment are limited. Safety and dosing of selinexor with concomitant strong CYP3A inhibitors are under study. |
| Conclusions |
The overall benefit/risk of selinexor is acceptable for the approved DLBCL indication. Selinexor dosed at 60 mg twice weekly can be difficult to tolerate and often requires dose modifications. More frequent monitoring for actionable toxicities is warranted during the first 3 months. Prophylactic antiemetics, including 5‐HT3 receptor antagonists, are indicated throughout treatment. Given the risk of neurologic toxicities, concomitant medications and supportive care should be optimized, with fall precautions and avoidance of driving as appropriate. |
Abbreviations: AR, adverse reaction; CI, confidence interval; CR, complete remission; DLBCL, diffuse large B‐cell lymphoma; IRC, independent review committee; ORR, objective response rate; R/R, relapsed or refractory; USPI, U.S. prescribing information.
Dosage in DLBCL
Notably, it is not clear that the optimal dosage of selinexor has been identified for the DLBCL indication. In patients with R/R DLBCL, the approved dosage (60 mg twice weekly on days 1 and 3 of each week) is based on dose escalation studies in patients with advanced hematologic malignancies [18, 19] or solid tumors [15], coupled with the SADAL study. Single‐agent activity in lymphoid malignancies was noted at doses as low as 6 mg/m2 [19]. Starting dosages less than 60 mg twice weekly were not thoroughly evaluated in patients with DLBCL. Data from early cohorts in SADAL revealed excessive toxicity with 100 mg twice weekly dosing and difficulty tolerating 60 mg twice weekly dosing with frequent dose modifications. Increase in selinexor exposure was associated with increase in the probability of dose modification and some ARs. Therefore, the FDA has required a postmarketing study to evaluate dosages lower than 60 mg twice weekly in patients with DLBCL.
With a median time to first dose modification of 4 weeks and median time to first dose reduction of 7 weeks, close monitoring is especially warranted during the first three cycles of selinexor. Based on the FDA's review of this marketing application, the selinexor USPI was revised to extend the period of more frequent safety monitoring for actionable toxicities (first 3 months), add guidelines for ocular toxicity, advise fall precautions as appropriate because of potential neurological toxicity, and advise patients against driving and hazardous activities until the neurological toxicity resolves.
Indication Statement
In contrast to SADAL, which allowed no more than five prior systemic therapies for DLBCL, the approved indication statement for DLBCL does not cap the number of prior therapies. There are few efficacy and safety data with selinexor in patients with more than three prior systemic therapies, and there are no data in patients with more than five such therapies. Both efficacy and tolerability could decrease in patients with more heavily pretreated disease. The FDA considered limiting the indication statement to two to five prior lines of systemic therapy, mirroring the protocol eligibility criteria. A broader indication was granted based on the observed clinical activity of selinexor in patients with four to five prior systemic therapies, the detailed safety information and toxicity management guidelines in the selinexor USPI, and the high unmet medical need of patients with multiply relapsed or progressive DLBCL.
Generalizability of the Results
The FDA has concern about the generalizability of the study results to the overall DLBCL population because of selection bias. The protocol's stringent eligibility criteria, including the unusually long washout period since the last therapy, end‐organ function requirements, and ≥ 3 month life expectancy, limit the generalizability of the findings. Notably, the washout period was extended mid‐study from 2 weeks to at least 60 days based on the number of on‐study deaths from progressive disease. A longer washout period became required for patients with refractory DLBCL to last systemic therapy (≥14 weeks) than for patients with responsive disease. Such waiting times may be prohibitive for many patients with active, aggressive lymphoma. A protocol amendment may have introduced additional study bias by liberalizing the statistical criterion for success (reducing the minimally effective ORR threshold from 20% to 15%) after preliminary efficacy results were available.
Conclusion
Despite the limitations of the study data, the results of SADAL support the determination that selinexor has clinically meaningful activity, and is reasonably likely to confer clinical benefit, in the intended population. Close monitoring for treatment‐related toxicities is, however, of utmost importance. Postmarketing study data would be needed to confirm the clinical benefit of selinexor, such as an improvement in progression‐free survival, overall survival, or patient‐reported outcomes. As a postmarketing requirement, a randomized, placebo‐controlled phase III trial of chemoimmunotherapy with or without selinexor is planned in patients with relapsed or refractory DLBCL (NCT04442022). Given the importance of dose optimization, the phase III trial is preceded by a dose‐finding study to inform the dosage of selinexor when combined with chemoimmunotherapy.
Author Contributions
Conception/design: Yvette L. Kasamon
Collection and/or assembly of data: Yvette L. Kasamon, Lauren S.L. Price, Ruo‐Jing Li, Yu‐Te Wu
Data analysis and interpretation: Yvette L. Kasamon, Lauren S.L. Price, Olanrewaju O. Okusanya, Nicholas C. Richardson, Ruo‐Jing Li, Lian Ma, Yu‐Te Wu, Marc Theoret, Richard Pazdur, Nicole J. Gormley
Manuscript writing: Yvette L. Kasamon, Lauren S.L. Price, Olanrewaju O. Okusanya, Nicholas C. Richardson, Ruo‐Jing Li, Lian Ma, Yu‐Te Wu, Marc Theoret, Richard Pazdur, Nicole J. Gormley
Final approval of manuscript: Yvette L. Kasamon, Lauren S.L. Price, Olanrewaju O. Okusanya, Nicholas C. Richardson, Ruo‐Jing Li, Lian Ma, Yu‐Te Wu, Marc Theoret, Richard Pazdur, Nicole J. Gormley
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
Note: This is a U.S. Government work. There are no restrictions on its use.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.Zelenetz AD, Gordon GI, Abramson JS et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): B‐Cell Lymphomas. Version 1.2021. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed February 2, 2021.
- 2.Crump M, Neelapu SS, Farooq U et al. Outcomes in refractory diffuse large B‐cell lymphoma: Results from the international SCHOLAR‐1 study. Blood 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Den Neste E, Schmitz N, Mounier N et al. Outcomes of diffuse large B‐cell lymphoma patients relapsing after autologous stem cell transplantation: An analysis of patients included in the CORAL study. Bone Marrow Transplant 2017;52:216–221. [DOI] [PubMed] [Google Scholar]
- 4.Van Den Neste E, Schmitz N, Mounier N et al. Outcome of patients with relapsed diffuse large B‐cell lymphoma who fail second‐line salvage regimens in the international CORAL study. Bone Marrow Transplant 2016;51:51–57. [DOI] [PubMed] [Google Scholar]
- 5.Bouchkouj N, Kasamon YL, de Claro RA et al. FDA approval summary: Axicabtagene ciloleucel for relapsed or refractory large B‐cell lymphoma. Clin Cancer Res 2019;25:1702–1708. [DOI] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 7.Neelapu SS, Locke FL, Bartlett NL et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramson JS, Palomba ML, Gordon LI et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B‐cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020;396:839–852. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PC, Abramson JS. Patient selection for chimeric antigen receptor (CAR) T‐cell therapy for aggressive B‐cell non‐Hodgkin lymphomas. Leuk Lymphoma 2020;61:2561–2567. [DOI] [PubMed] [Google Scholar]
- 10.Sehn LH, Herrera AF, Flowers CR et al. Polatuzumab vedotin in relapsed or refractory diffuse large B‐cell lymphoma. J Clin Oncol 2020;38:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salles G, Duell J, Gonzalez Barca E et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B‐cell lymphoma (L‐MIND): A multicentre, prospective, single‐arm, phase 2 study. Lancet Oncol 2020;21:978–988. [DOI] [PubMed] [Google Scholar]
- 12.Caimi PF, Ai W, Alderuccio JP et al. Loncastuximab tesirine in relapsed or refractory diffuse large B‐cell lymphoma (LOTIS‐2): A multicentre, open‐label, single‐arm, phase 2 trial. Lancet Oncol 2021;22:790–800. [DOI] [PubMed] [Google Scholar]
- 13.Kalakonda N, Maerevoet M, Cavallo F et al. Selinexor in patients with relapsed or refractory diffuse large B‐cell lymphoma (SADAL): A single‐arm, multinational, multicentre, open‐label, phase 2 trial. Lancet Haematol 2020;7:e511–e522. [DOI] [PubMed] [Google Scholar]
- 14.Chari A, Vogl DT, Gavriatopoulou M et al. Oral selinexor‐dexamethasone for triple‐class refractory multiple myeloma. N Engl J Med 2019;381:727–738. [DOI] [PubMed] [Google Scholar]
- 15.Abdul Razak AR, Mau‐Soerensen M, Gabrail NY et al. First‐in‐class, first‐in‐human phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol 2016;34:4142–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: The Lugano classification. J Clin Oncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.XPOVIO (selinexor) tablets, for oral use. Prescribing information. Karyopharm Therapeutics Inc. Available at https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed April 15, 2021.
- 18.Chen C, Siegel D, Gutierrez M et al. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood 2018;131:855–863. [DOI] [PubMed] [Google Scholar]
- 19.Kuruvilla J, Savona M, Baz R et al. Selective inhibition of nuclear export with selinexor in patients with non‐Hodgkin lymphoma. Blood 2017;129:3175–3183. [DOI] [PubMed] [Google Scholar]


