Abstract
Background and Aims:
While the United States is in the midst of an overdose epidemic, effective treatments are underutilized and commonly discontinued. Innovations in medication delivery, including an extended-release formulations, have the potential to improve treatment access and reduce discontinuation. We sought to assess extended-release buprenorphine discontinuation among individuals with opioid use disorder (OUD) in a real-world, nationally representative cohort.
Setting:
United States
Participants:
Commercially insured individuals initiating one of four FDA-approved medications for opioid use disorder (MOUD) in 2018: extended-release buprenorphine, extended-release naltrexone, mucosal buprenorphine (mono- or co-formulated with naloxone), or methadone.
Measurements:
Our primary outcome was medication discontinuation, defined as a gap of more than 14 days between the end of one prescription or administration and the subsequent dose.
Findings:
We identified 14,358 individuals initiating MOUD in 2018, including 204 (1%) extended-release buprenorphine, 1,173 (8%) extended-release naltrexone, 12,171 (85%) mucosal buprenorphine, and 810 (6%) methadone initiations. Three months after initiation, 50% (95% confidence interval [CI] 40%−60%) of extended-release buprenorphine, 64% (95% CI 61%−69%) of extended-release naltrexone, 34% (95% CI 33%−35%) of mucosal buprenorphine, and 58% (95% CI 54%−62%) of methadone initiators had discontinued treatment.
Conclusions:
Across all treatment groups, medication discontinuation was high, and in this sample of early adopters with limited follow-up time, we found no evidence that extended-release buprenorphine offered a retention advantage compared to other MOUD in real-world settings. Retention continues to represent a major obstacle to treatment effectiveness, and interventions are needed to address this challenge even as new MOUD formulations become available.
Keywords: opioid use disorder, extended-release buprenorphine, medication for opioid use disorder, retention
1. Introduction
Since the 1980s, drug overdose rates in the United States (US) have been rising at alarming rates, driven in the 2010s by the presence of illicitly produced fentanyl—a potent synthetic opioid—in the illicit drug supply.1–3 A synthesis of multiple data sets estimated at least 1.5 million insured people in the U.S. have an opioid use disorder (OUD) balanced between commercial (41%) and public (16% Medicare and 43% Medicaid) beneficiaries,4 and this number is increasing.5,6 Medications for opioid use disorder (MOUD) substantially reduce the risk of opioid overdose,7 but less than 25% of individuals with OUD initiate MOUD.8,9 Additionally, among those who do initiate treatment, discontinuation is common – 50% or greater by 12 months in many clinical cohorts.8–12 There are three MOUD approved for use by the Food and Drug Administration (FDA), and multiple formulations are available: mucosal buprenorphine (M-BUP, mono- or co-formulated with naloxone), dosed daily; methadone, dosed daily often in a supervised clinical setting; extended-release injectable naltrexone, dosed monthly (XR-NTX); and an extended-release buprenorphine implant, dosed every 6 months. A formulation of buprenorphine approved in the United States in 2017, extended-release depot buprenorphine (XR-BUP), dosed monthly, may offer an attractive alternative that could increase retention.13–15 Randomized controlled trials have demonstrated the efficacy of XR-BUP over placebo,16 and non-inferiority compared to M-BUP combinations,17–19 for abstinence from opioid use. Qualitative research suggests that XR-BUP may be appealing to individuals because of its convenient dosing schedule.20 While additional clinical trials are ongoing in several populations who might benefit from a depot injection, including individuals with prior overdose or exposed to fentanyl, individuals released from correctional settings,21,22 veterans,23 and pregnant women,24 there is a lack of evidence comparing XR-BUP to other MOUD or characterizing its initiation, use, and discontinuation in real-world settings. This is important given potential challenges of XR-BUP including increased cost and rigorous storage requirements as a controlled substance.19,24,25
In this study, we characterize initiation, use, and discontinuation of XR-BUP in a real-world cohort to understand the lessons from early adoption and compare discontinuation rates to established alternatives of M-BUP, XR-NTX, and methadone. These emerging real-world data can begin to build the evidence base to inform the MOUD treatment decisions faced by patients, clinicians, payers, and policymakers.
2. Methods
2.1. Population and cohort design
We utilized the IBM Watson MarketScan Commercial Claims and Encounters Database (MarketScan) to identify MOUD use. MarketScan is a nationally representative data set of US individuals covered by employer-sponsored health insurance, and contains detailed data on inpatient and outpatient medical claims, and outpatient pharmacy administrative claims. Given that XR-BUP was not approved until late 2017 (on November 30, 2017), we use 2018 data covering over 27 million unique individuals to establish our cohort. We compared XR-BUP to three other FDA-approved medication treatments for OUD: methadone, XR-NTX, and M-BUP. We identified MOUD treatment using national drug code numbers in outpatient pharmacy claims that included details of the date the prescription was filled and the number of days supply. For extended-release products and methadone, Health Common Procedure Coding System (HCPCS) codes for in-office administration (supplemental appendix). To identify the initiating prescribing event, we isolated the first prescribing or administration event preceded by a three-month washout period for all MOUDs,8 so each individual was enrolled in the dataset from three months prior to their index prescribing event (as early as October 1st, 2017 for initiation in January 1, 2018) through December 31st, 2018. For methadone, XR-NTX, and M-BUP we defined the washout period as free of any other MOUD prescriptions or initiations, while for XR-BUP we allowed for induction with M-BUP as recommended in the prescribing information package insert.26 As XR-NTX is also used to treat alcohol use disorder, we required XR-NTX to be preceded by a diagnosis of OUD in the 30 days prior to initiation as evidence that the medication was being used to treat this condition. We excluded implantable buprenorphine as there were only claims for three documented administrations in two individuals in 2018.
2.2. Outcome measures and analysis
2.2.1. Characterizing the XR-BUP cohort.
We first characterized the uptake and utilization of XR-BUP and compared the cohort of individuals prescribed XR-BUP with those prescribed other MOUD, using a chi-square or Fisher’s exact test. We compared the composition of each medication cohort based on demographic and clinical characteristics available in claims data including sex, age (under 30 vs. 30 and older as in previous literature8,27), region of residence (Northeast, Midwest, South, West), as well as whether the beneficiary was the primary holder of the plan, or a spouse or dependent. We also included a modified Elixhauser comorbidity score27,28 from the 30 days prior to initiation, which captures general comorbidity burden, but excludes illicit drug and alcohol use (which are captured separately). This is important to capture as individuals with competing health priorities may be more likely to discontinue treatment, and to understand MOUD prescribing patterns as the potential convenience of a monthly dosed medication may be helpful for these individuals. We also assessed differences in individual components of the comorbidity index as a supplemental analysis. We included the presence of non-opioid concurrent illicit drug (including amphetamines, cocaine, non-medical marijuana, hallucinogens, and sedatives) use and alcohol use in the 30 days prior to initiation as a dichotomous variable.
2.2.2. Discontinuation: XR-BUP and other MOUD.
Next, we compared the uptake of XR-BUP with the utilization of M-BUP, XR-NTX, and methadone over the same period by calculating the time on treatment of each medication. We also characterized the length of gaps between prescriptions. We calculated the proportion who discontinued by three months, defined as consistent medication coverage, via in-office administration or filled prescription, with a gap of no more than 14 days in coverage, and calculated the 95% confidence interval for each proportion. We assessed the effect of censorship in two ways. First, we included all medication initiations from January 1st through December 31st, 2018 and looked for discontinuation by three months – those who initiated the medication with fewer than 3 months left in the calendar year, but had not discontinued by December 31st, 2018, were included among those who did not discontinue. Second, we included only those initiating before October 1st, 2018 so that we only measured 3-month discontinuation among those we observed for three months. We report both of these measures to transparently describe the emerging use of XR-BUP. The first discontinuation measure provides a snapshot of discontinuation as of December 31st, 2018, recognizing that uptake of XR-BUP was increasing through the calendar year. The second discontinuation measure fully accounts for person-time in the three-month discontinuation definition. Finally, we summarized the number of individuals who initiated a different MOUD within 14 days of discontinuing their initial medication to measure the frequency of medication switching.
2.2.3. Characterizing XR-BUP prescribing guidance concordance.
We then examined concordance with XR-BUP prescribing information instructions as of December 2018: (1) recommended dosing schedule of two 300 mg injections followed by 100 mg injections; (2) initiation with mucosal buprenorphine for at least 7 days prior to XR-BUP initiation, and; (3) a gap of at least 26 days and not more than 45 days between administrations.29 The XR-BUP package insert was later updated to allow for a 300mg dose to cover 60 days as needed for travel, etc.26 We assessed how often this occurred in our data, although these updated guidelines were not published until February 2020, outside of our observation period.
2.2.4. Ethical approval.
Because these data are de-identified, the Boston University Institutional Review Board ruled this study Not Human Subjects Research.
3. Results
3.1. Overall MOUD initiation
We identified 14,358 individuals initiating medications for opioid use disorder in 2018, including 204 (1%) XR-BUP, 1,173 (8%) XR-NTX, 12,171 (85%) M-BUP, and 810 (6%) methadone initiations (Table 1). The days of medication supplied at initiation was 4-weeks for injectable formulations (defined by package insert), one day for dispensed methadone (defined by the HCPCS code), and varied for M-BUP, with 13% initiating with a prescription covering 7 or fewer days, 15% 8–14 days, and the remainder initiated with a 30 day prescription. There were several notable differences among the initiating cohorts.
Table 1:
Characteristics of individuals initiating medication for opioid use disorder in 2018
| XR-BUP | XR-NTX | M-BUP | Methadone | p-value of difference* | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Total | 204 | 100% | 1,173 | 100% | 12,171 | 100% | 810 | 100% | |
| Sex | |||||||||
| Male | 128 | 63% | 715 | 61% | 7,802 | 64% | 496 | 61% | 0.08 |
| Female | 76 | 37% | 458 | 39% | 4,369 | 36% | 314 | 39% | |
| Age | |||||||||
| <30 | 76 | 37% | 696 | 59% | 3,924 | 32% | 213 | 26% | <0.01 |
| 30 or older | 128 | 63% | 477 | 41% | 8,247 | 68% | 597 | 74% | |
| Region | |||||||||
| Northeast | 53 | 26% | 397 | 34% | 2,529 | 21% | 223 | 28% | <0.01 |
| Midwest | 30 | 15% | 233 | 20% | 2,290 | 19% | 115 | 14% | |
| South | 102 | 50% | 388 | 33% | 5,522 | 45% | 367 | 45% | |
| West | 19 | 9% | 154 | 13% | 1,808 | 15% | 105 | 13% | |
| Unknown | 0 | 0% | 1 | 0% | 13 | 0% | 0 | 0% | |
| Concurrent substance use at initiation** | |||||||||
| Alcohol | 49 | 24% | 720 | 61% | 2,005 | 16% | 76 | 9% | <0.01 |
| Amphetamines | 22 | 11% | 320 | 27% | 1,111 | 9% | 28 | 3% | <0.01 |
| Marijuana | 29 | 14% | 237 | 20% | 932 | 8% | 31 | 4% | <0.01 |
| Cocaine | 39 | 19% | 387 | 33% | 1,128 | 9% | 54 | 7% | <0.01 |
| Hallucinogens | 5 | 2% | 34 | 3% | 96 | 1% | 2 | 0% | <0.01 |
| Sedatives | 35 | 17% | 440 | 38% | 1,550 | 13% | 45 | 6% | <0.01 |
| Elixhauser comorbidity index*** | |||||||||
| 0 | 119 | 58% | 493 | 42% | 7,743 | 64% | 593 | 73% | <0.01 |
| 1 | 49 | 24% | 391 | 33% | 2,564 | 21% | 118 | 15% | |
| 2 | 20 | 10% | 173 | 15% | 1,034 | 8% | 58 | 7% | |
| 3+ | 16 | 8% | 116 | 10% | 830 | 7% | 41 | 5% | |
| Insurance Coverage | |||||||||
| Primary holder | 103 | 50% | 428 | 36% | 6,688 | 55% | 497 | 61% | <0.01 |
| Spouse | 48 | 24% | 186 | 16% | 3,031 | 25% | 174 | 21% | |
| Dependent | 53 | 26% | 559 | 48% | 2,452 | 20% | 139 | 17% | |
XR-BUP = extended-release buprenorphine; XR-NTX = extended-release naltrexone; M-BUP = sublingual buprenorphine (mono- or co-formulated with naloxone)
The p-value of difference is based on the chi-square statistic from the distribution difference in the given characteristic among the medications initiated. Taking age as an example, the p-value indicates there is a statistically significantly difference in the distribution of individuals under 30 and those 30 and over among each medication initiation type.
Diagnosis code in 30 days before first initiation, only includes use documented in the billing record
Diagnosis code in 30 days before first initiation and modified to exclude drug use as a comorbidity
3.2. Demographic differences among MOUD initiations
First, younger individuals were more often initiating injectable MOUD, particularly XR-NTX, evidenced by a higher proportion of individuals under 30 (59% XR-NTX and 37% XR-BUP vs. 32% in M-BUP and 26% for methadone, p<0.01). Next, a higher proportion of individuals who were listed as dependents on the insurance plan, rather than primary plan holder or spouses, were represented among those receiving injectable MOUD (48% XR-NTX and 26% XR-BUP vs. 20% M-BUP and 17% methadone, p<0.01, Table 1).
3.3. Clinical differences among MOUD initiations
For each non-opioid substance, individuals initiating XR-NTX had the highest prevalence of documented concurrent substance use (ranging from 3% with hallucinogen use, to 61% with alcohol use), followed by XR-BUP (ranging from 2% with hallucinogen use to 24% with alcohol use). In addition, individuals treated with injectable MOUD had higher comorbidity burden; 10% of XR-NTX initiators had 3 or more non-drug use Elixhauser comorbidities in the month prior to MOUD initiation, followed by 8% of the XR-BUP cohort, 7% in M-BUP, and 5% in methadone, (p<0.01). This difference was largely driven by increased documented prevalence of depression in those receiving XR-NTX (supplemental appendix).
3.4. Discontinuation differences among MOUD initiations
The majority of individuals initiating an MOUD discontinued (a gap of more than 14 days between medications) before the end of follow-up, including 51% (n=105, 95% confidence interval [CI] 45%−58%)) of those starting XR-BUP, 74% (868, 95% CI 71%−77%)) of XR-NTX initiations, 53% (6,493, 95% CI 52%−54%) of M-BUP starts, and 69% (557, 95% CI 66%−72%) of individuals who initiated methadone. Figure 1 presents the Kaplan-Meier curves showing time to discontinuation, while incorporating differences in follow-up time. The median time from initiation until discontinuation among those who discontinued was 47 days for those starting XR-BUP (interquartile range [IQR] 28–73 days), 48 days for XR-NTX (IQR 28–84 days), 71 days for M-BUP (IQR 36–122 days), and 32 days for methadone (IQR 29–60 days). A Wilcoxon rank test of homogeneity indicated a significant (p<0.01) difference in discontinuation time among the treatments. Among those enrolled for at least three months after initiation, 64% (95% CI 55%−73%) of those initiating XR-BUP, 67% (95% CI 64%−70%) of those initiating XR-NTX, 34% (95% CI 33%−35%) of those initiating M-BUP, and 59% (95% CI 55%−62%) of those initiating methadone discontinued. The sample size of those enrolled for at least 3 months after initiation was markedly smaller than the unrestricted cohort, but the differences among discontinuation rates at 3 months was significantly different (chi-square test p<0.01) (n=100 XR-BUP, 839 XR-NTX, 8,888 M-BUP, 628 methadone). In general, those who discontinued by 3 months were more often under 30 years of age, managing multiple comorbidities, and diagnosed with concurrent substance use (supplemental appendix).
Figure 1: Time-to-medication discontinuation among individuals treated for opioid use disorder in a 2018 United States commercially insured population.
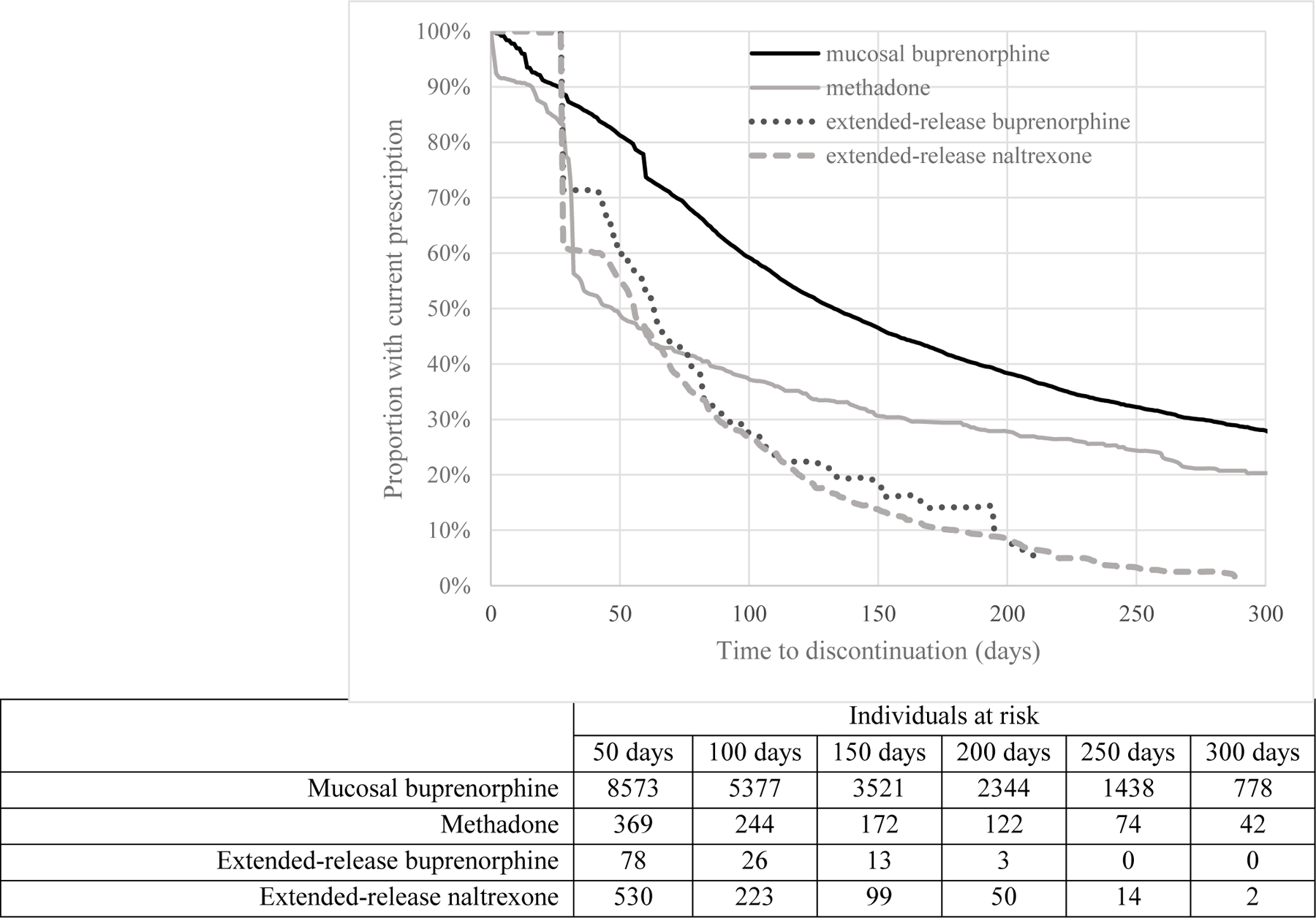
This Kaplan–Meier survival curve displays the time to discontinuation for individuals prescribed injectable buprenorphine, injectable naltrexone, mucosal buprenorphine (mono- or co-formulated with naloxone), and methadone. Injectable formulations have no discontinuation prior to 4-weeks, reflecting the fact that once the medication is injected, an individuals is adherent for the duration of the extended-release medication, compared to mucosal buprenorphine, which may be prescribed for different lengths of time, and methadone, which is dispensed daily. The horizontal axis displays the time to discontinuation in days while the vertical axis displays the proportion of the population with a current prescription.
3.5. Characterizing medication “switching” and the effect on discontinuation
Few individuals initiated a different MOUD within 14 days of discontinuing the initial MOUD (“switched”); this occurred in 3% of XR-NTX discontinuations (27 individuals switched to M-BUP), 1% of methadone discontinuations (4 to M-BUP), and 1% of M-BUP discontinuations (52 to XR-NTX and 2 to methadone). Among those discontinuing XR-BUP, 15% (16 individuals) switched to another MOUD, all to M-BUP. We then excluded switches from the discontinuation numbers to estimate discontinuation from any medication rather than discontinuation from the initiating medication as was estimated in section 3.4. This implies 44% (95% CI 37%−50%) discontinuation among those initiating with XR-BUP; 72% (95% CI 69%−74%) for XR-NTX; 53% (95% CI 52%−54%) for M-BUP, and; 68% (95% CI 65%−71%) for methadone. Among those with at least three months of follow-up, the revised discontinuation proportions are: 50% (95% CI 40%−60%) for XR-BUP; 65% (95% CI 61%−68%) for XR-NTX; 34% (95% CI 33%−35%) for M-BUP, and; 58% (95% CI 54%−62%) in methadone.
3.6. Describing concordance with prescribing guidelines for XR-BUP
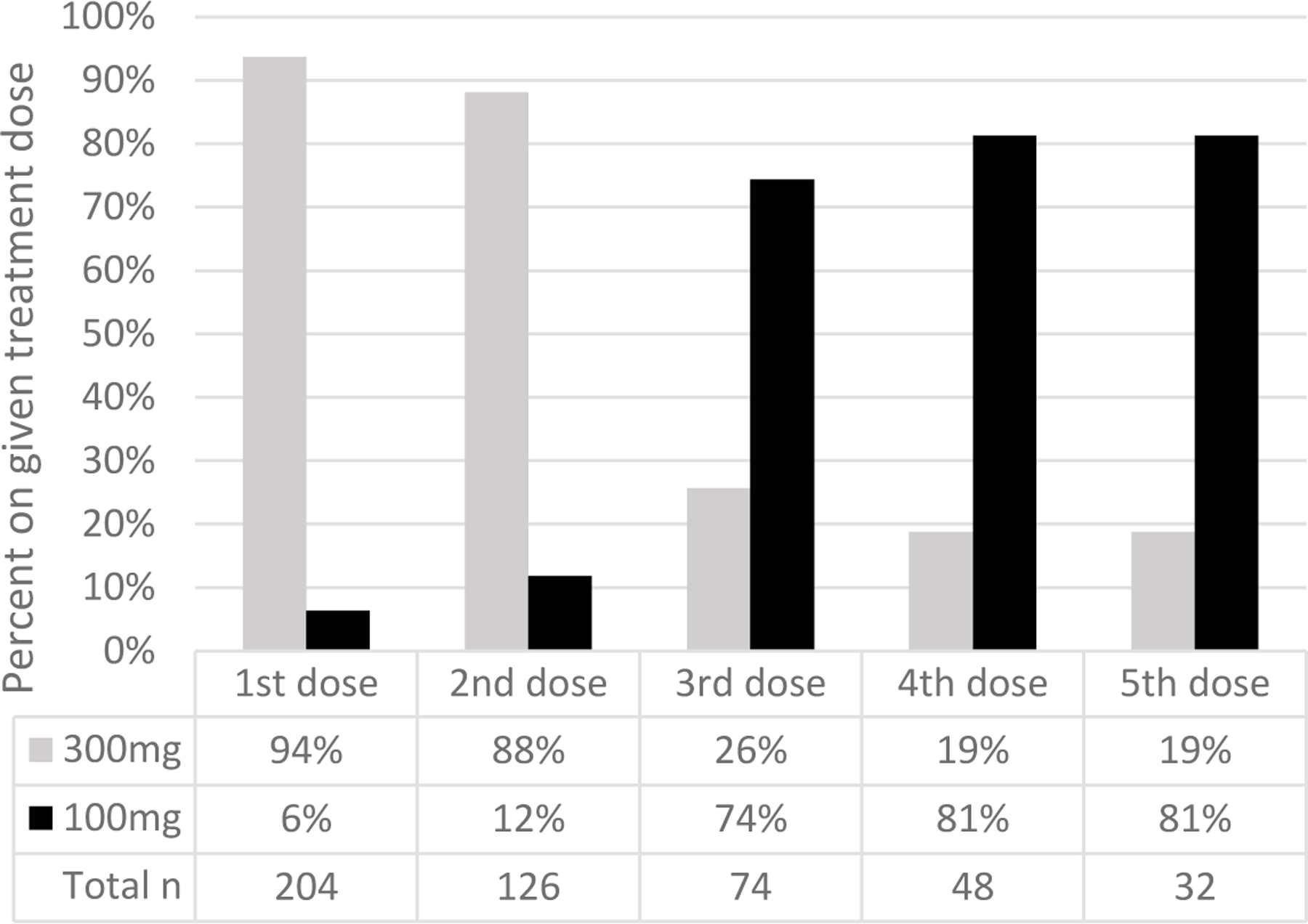
We captured several measures of utilization of XR-BUP among the 204 early adopters. Based on prescriptions filled, XR-BUP demonstrated a ramp-up of utilization over calendar-year 2018, while other treatments were stable or slightly decreasing (Figure 2). Overall, 527 total administrations were observed among the 204 individuals initiating XR-BUP. XR-BUP dosing guidelines recommend 300 mg for the first two administrations followed by 100 mg every administration after that, and our real-world data showed broad adherence to these instructions. Ninety-four percent of first administrations were 300 mg, 88% of second administrations were 300 mg, then 74% of third administrations were 100 mg, and 81% of fourth and fifth administrations were 100 mg (Figure 3). Just four individuals (2%) were given 100mg for each of the first three doses, and 17 (8%) received 300mg for all three first doses (a 300 mg maintenance dose is guideline-recommended for patients for which the benefits outweigh the harms26). To assess how often individuals were receiving a 300 mg dose every other month we examined individuals with at least three doses that were between 6 and 10 weeks apart. We only found one individual with this schedule, and those doses were 300 mg, 100 mg, and 100 mg – we did not find evidence of 300 mg used every other month as allowed in updated guidelines. Next, we examined how often initiation of XR-BUP was preceded by a prescription of M-BUP. In the 90 days prior to XR-BUP initiation, 171 (84%) individuals received at least one prescription for M-BUP, while 33 (16%) had no evidence of M-BUP. Finally, we assessed overlap and gaps between doses of XR-BUP. In the 313 cases of subsequent XR-BUP doses, 44 (14%) were administered before 26 days had passed from the prior administration (among these the median time from the prior administration was 23 days ranging from 14 to 25 days). Thirty-seven (12%) were administered after more than 45 days had elapsed from the prior administration (median of 83 days between administrations ranging from 47 to 128 days). Based on our definition of discontinuation, any dose that was administered more than 6 weeks apart (4 weeks of medication coverage plus a two week gap) met the criteria for discontinuation.
Figure 2: Histograms of medication uptake by month and medication type in a commercially insured cohort of individuals initiating medication for opioid use disorder in 2018.
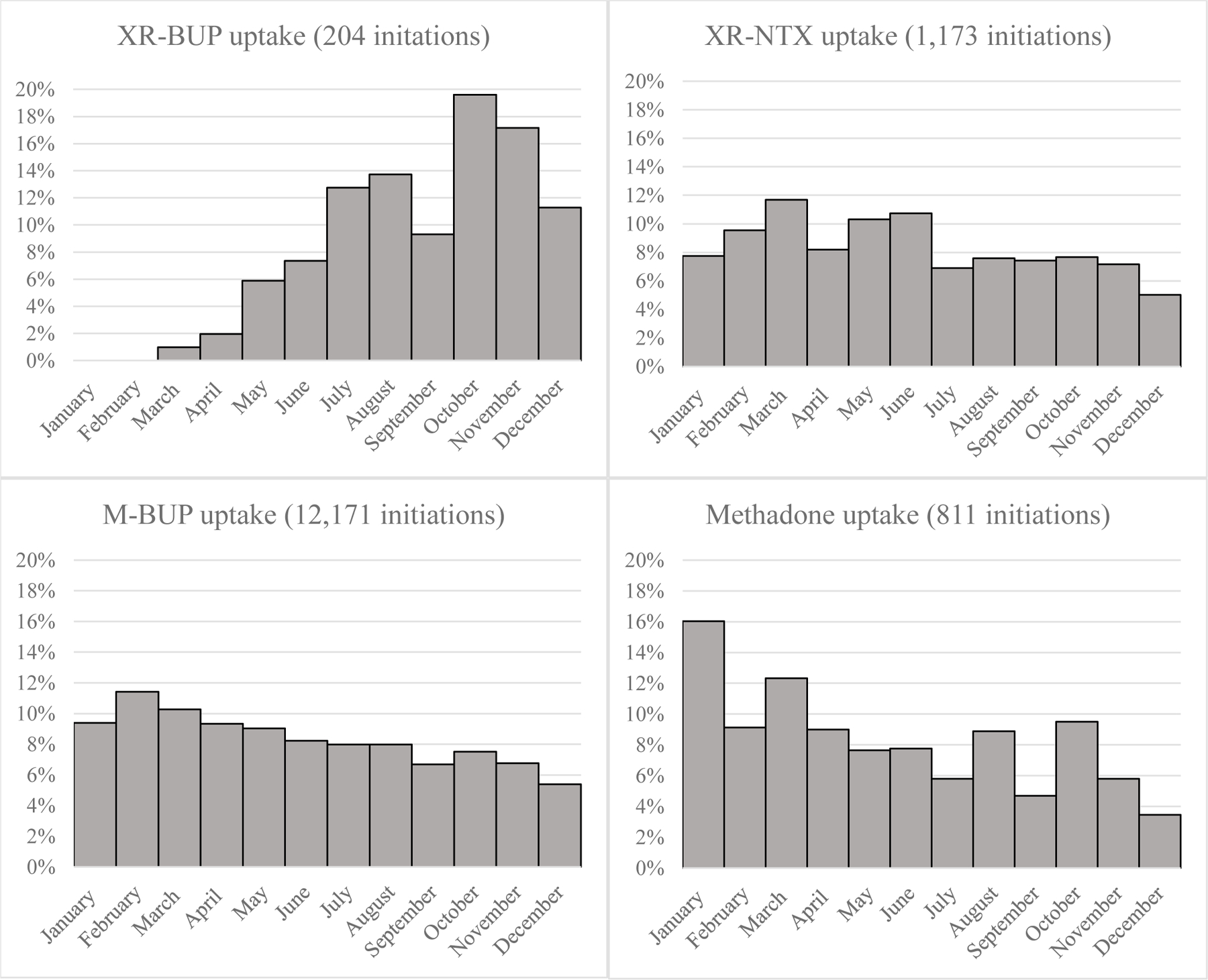
XR-BUP=Extended-release buprenorphine; XR-NTX=extended-release naltrexone; M-BUP=sublingual buprenorphine (mono- or co-formulated with naloxone). For each histogram, the vertical axis is the percent of total initiations of the given medication that occurred on a given month. For example, of all M-BUP initiations, just under 10% occurred in January.
Figure 3: Extended-release buprenorphine strength over dosing time in a commercially insured cohort of 204 individuals in 2018.
4. Discussion
While it is clear that new approaches are needed to address the opioid overdose epidemic in the US, retention on MOUD continues to be a major barrier to OUD treatment efficacy at the population level. In our assessment of emerging XR-BUP data in a nationally representative, commercially insured population, we found discontinuation, defined as a 14-or-more day gap in medication coverage, was common across all MOUD studied. There is an urgent need to address discontinuation across MOUD given recent work, highlighted by the National Institute on Drug Abuse,30 showing that current low adherence rates are one of the primary barriers to reducing opioid-related overdose on an individual and population level.31 As in previous work,27 we found that M-BUP was by far the most common MOUD, representing 85% of the sample. We did find that those initiating on XR-BUP more often switched to another MOUD upon discontinuation of XR-BUP compared to other MOUDs, and that fact made XR-BUP look attractive as of December 31, 2018 with the lowest proportion discontinued at that point in time (44% vs. 53% for M-BUP with non-overlapping confidence intervals). However, this was due in part to the significant ramp up of XR-BUP uptake at the end of the year, so on average those on XR-BUP had less follow-up time (and thus less time to discontinue). This ramp up in uptake has been seen among Medicaid-covered individuals as well32, and a positive sign for increasing MOUD access and choice. Assessing those with at least three months of follow-up, M-BUP had the lowest rate of discontinuation by a substantive margin over XR-BUP (34% vs. 50%, p<0.01).
Our research reveals patient-centered flexibility of choice of MOUD, via shared decision making, for example, is likely key to improving adherence as more than 15% of those leaving XR-BUP were later initiated on M-BUP. Some of the patient-centered aspects of shared decision making that warrant further study include attention to cost barriers, accommodating dose adjustment or co-prescribing with M-BUP, or using XR-BUP as a taper off MOUD for patients desiring that option.33 We saw the most dramatic discontinuation in the first month after initiation, so this may be the most clinically relevant time to provide that flexibility and support.
Even with important retention obstacles to overcome, development of novel medication options remains an important piece of addressing the opioid crisis at an individual level. Qualitative research exploring the medication preferences of patients has found that opinions are diverse and that treatment characteristics that may be appealing for one patient (long-acting formulations reducing treatment) are a disadvantage for another (long-acting formulations remove the morning routine and “purpose” provided by daily dosing).34 For this reason, it is important that all MOUD are available during the patient-clinician decision-making process such that treatment decisions can meet the unique needs of individual patients. Previous work has shown that more than half (59%) of patients receive behavioral health treatment for OUD, and just as many seek inpatient care as do MOUD (both approximately 15%).35 Combinations of MOUD with behavioral and residential treatment should be considered in shared decision-making treatment planning.
We are encouraged by the close alignment we found between treatment dosing guidelines for XR-BUP and real-world use of this novel medication, but findings of two specific populations need further study. First, we found that younger aged individuals more likely to initiate injectable medications. Previous literature has demonstrated younger age is associated with higher discontinuation,8,27 and while a sophisticated adjusted analysis is beyond the scope of this paper, we found that individuals under 30 had higher discontinuation through the study period (supplemental appendix). More research is needed to understand why younger individuals are disproportionately initiating injectable medications, whether by choice, family pressure, generational differences in MOUD preference, or other barriers to or stigma surrounding non-injectable MOUD. Second, individuals with current non-OUD substance use and higher comorbidity burden were more represented in injectable MOUD, and it is not immediately obvious why this would be the case. It could be that some providers interpret long-acting drugs as more suitable for individuals with multiple use disorder or “complex cases,” or it could be that these individuals tried other MOUDs in the past before our washout and we are capturing a new treatment attempt. Previous research has not shown compelling evidence that XR-NTX was beneficial for those with concurrent substance use,27 but as more data emerges this hypothesis could be tested among those receiving XR-BUP. Our finding that discontinuation is high across MOUD has been shown in previous studies. We also report factors such as age, comorbidities, utilization of inpatient care, as well as variation in provider specialty and place of treatment initiation that have been previously described.8,27 Further work should contextualize these findings to develop relevant interventions. More evidence is also needed to understand the disconnect between qualitative studies suggesting that injectable medications are acceptable and may provide a convenience benefit, and emerging real-world data showing a high rate of discontinuation in injectable MOUD. Earlier research of XR-NTX hypothesized that this difference could be driven by the lack of withdrawal symptoms when discontinuing an antagonist compared to an agonist such as M-BUP.8 While there is little data available yet for XR-BUP, reduced withdrawal symptoms have been reported in at least one case series among people treated with XR-BUP seeking to discontinue.36
A major strength of this study is that our large, nationally representative cohort allows us to track the emerging utilization of XR-BUP in the real-world and compare that utilization to existing MOUD, using data from actual medication administrations and filled prescriptions. However, there are several limitations inherent to this type of commercial claims data. First, our cohort includes those who are commercially insured, and it is possible that retention patterns we observed may be different among those with public insurance, particularly for methadone which is more difficult to receive under commercial insurance due to a burdensome pre-authorization process.37 Additionally, we identified methadone administration with a procedure code specific to OUD rather than pain.38 This approach does not allow us to see the formulation or dosage given. And while not common in the U.S., there may be differences in injectable versus oral methadone administration that should be considered, particularly in places where injectable methadone treatment is an established practice, such as the United Kingdom.39 Second, while we observe administration of long-acting medications and methadone in either outpatient or inpatient settings, for M-BUP we are limited to outpatient pharmacy records, so may be missing initiation of M-BUP in inpatient settings such as drug detoxification centers or inpatient hospital settings. Third, we did not account for inpatient addiction care or psychosocial interventions. In examining XR-BUP, other MOUD are the most relevant comparators. MOUD treatments have demonstrated better outcomes than inpatient40 or psychosocial35 care alone, specifically associated with better survival. The National Academy of Sciences Engineering and Medicine has specifically concluded that MOUD saves lives and should not be withheld due to a lack of availability of behavioral interventions.41 However, many individuals with OUD have comorbid mental health diagnoses and psychosocial concerns,42 and both the American Society of Addiction Medicine (ASAM)43 and the National Institute for Health and Care Excellence (NICE)44 recommend psychosocial support and treatment in conjunction with pharmacological treatment. For patients for whom MOUD is not enough, psychosocial components of care warrant strengthening with approaches like cognitive behavioral therapy, contingency management, and residential treatment. Fourth, we are only able to measure what is documented in administrative billing records. For example, polysubstance use is common among individuals with OUD, and the prevalence of concurrent use we find, particularly for M-BUP and methadone patients, is lower that we might expect45 – studies of methadone maintenance programs have found concurrent cocaine use of over 20%, for example.46,47 Instead, our estimates should be interpreted as concurrent use that was severe enough or particularly relevant to the course of care to be documented by the provider. Our type of data also precludes us from examining shared decision making as we might with chart review. Understanding how patients and clinicians work together to establish the approach to OUD treatment is important for developing strategies to promote access and retention.
5. Conclusion
OUD presents a major public health challenge, and treatment discontinuation is an important barrier to the effectiveness of MOUD. We assessed the uptake, use, and adherence to a new MOUD, XR-BUP, in a real-world setting. While XR-BUP was the least prescribed of the MOUD we examined, uptake increased over 2018 and use of the medication was largely concordant with induction and dosing instructions. However, we found that all MOUD, including XR-BUP, had low retention over the analysis period. Long-acting depot formulations of medications for opioid use disorder are an important advance, but they are not a panacea. The effectiveness of MOUD to address the opioid overdose epidemic will be severely limited unless the discontinuation challenge is addressed, even as new medications enter the market.
Supplementary Material
Highlights.
Studied 204 early adopters of injectable buprenorphine compared to other medications
Discontinuation is common across opioid use disorder medications
No evidence that injectable buprenorphine offered a retention advantage
Good concordance with injectable buprenorphine prescribing guidelines
Acknowledgments
Author disclosures
Role of funding source: This work was supported by the National Institutes of Health (grant numbers P30DA040500, R01046527, R01CE002999 and K23DA044085). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Colon-Berezin C, Nolan ML, Blachman-Forshay J, Paone D. Overdose Deaths Involving Fentanyl and Fentanyl Analogs - New York City, 2000–2017. MMWR Morb Mortal Wkly Rep 2019;68:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladden RM, Martinez P, Seth P. Fentanyl Law Enforcement Submissions and Increases in Synthetic Opioid-Involved Overdose Deaths - 27 States, 2013–2014. MMWR Morb Mortal Wkly Rep 2016;65:837–43. [DOI] [PubMed] [Google Scholar]
- 3.Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davenport S, Matthews K. Opioid use disorder in the United States: Diagnosed prevalence by payer, age, sex, and state Washington, DC: Milliman; 2018. [Google Scholar]
- 5.Barocas JA, White LF, Wang J, et al. Estimated Prevalence of Opioid Use Disorder in Massachusetts, 2011–2015: A Capture-Recapture Analysis. Am J Public Health 2018:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuter P, Caulkins JP, Midgette G. Heroin Use Cannot Be Measured Adequately with a General Population Survey. Addiction 2021. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 2017;377:391–4. [DOI] [PubMed] [Google Scholar]
- 8.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat 2018;85:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in Receipt of Buprenorphine and Naltrexone for Opioid Use Disorder Among Adolescents and Young Adults, 2001–2014. JAMA Pediatr 2017;171:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. J Subst Abuse Treat 2010;39:22–31. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein ZM, Kim HW, Cheng DM, et al. Long-term retention in Office Based Opioid Treatment with buprenorphine. J Subst Abuse Treat 2017;74:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis 2016;35:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling W, Nadipelli VR, Solem CT, et al. Patient-centered Outcomes in Participants of a Buprenorphine Monthly Depot (BUP-XR) Double-blind, Placebo-controlled, Multicenter, Phase 3 Study. Journal of addiction medicine 2019;13:442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larance B, Degenhardt L, Grebely J, et al. Perceptions of extended-release buprenorphine injections for opioid use disorder among people who regularly use opioids in Australia. Addiction 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariani JJ, Mahony A, Iqbal MN, Luo SX, Naqvi NH, Levin FR. Case Series: Rapid Induction Onto Long Acting Buprenorphine Injection for High Potency Synthetic Opioid Users. Am J Addict 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 2019;393:778–90. [DOI] [PubMed] [Google Scholar]
- 17.Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and Monthly Subcutaneous Buprenorphine Depot Formulations vs Daily Sublingual Buprenorphine With Naloxone for Treatment of Opioid Use Disorder: A Randomized Clinical Trial. JAMA Intern Med 2018;178:764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lintzeris N, Leung SY, Dunlop AJ, et al. A randomised controlled trial of sublingual buprenorphine-naloxone film versus tablets in the management of opioid dependence. Drug Alcohol Depend 2013;131:119–26. [DOI] [PubMed] [Google Scholar]
- 19.Opioid dependence: buprenorphine prolonged-release injection (Buvidal). 2019 (Accessed 14 Feb, 2021, at https://www.nice.org.uk/advice/es19/chapter/Key-messages.)
- 20.Parsons G, Ragbir C, D’Agnone O, Gibbs A, Littlewood R, Hard B. Patient-Reported Outcomes, Experiences and Satisfaction with Weekly and Monthly Injectable Prolonged-Release Buprenorphine. Subst Abuse Rehabil 2020;11:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy SM, Laiteerapong N, Pho MT, et al. Health economic analyses of the justice community opioid innovation network (JCOIN). J Subst Abuse Treat 2021:108262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy SM, Jeng PJ, Poole SA, et al. Health and economic outcomes of treatment with extended-release naltrexone among pre-release prisoners with opioid use disorder (HOPPER): protocol for an evaluation of two randomized effectiveness trials. Addict Sci Clin Pract 2020;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A Study Comparing Oral Buprenorphine and Injectable Buprenorphine for the Treatment of Opioid Use Disorder (VA-BRAVE) 2020. at https://clinicaltrials.gov/ct2/show/NCT04375033.)
- 24.Socias ME, Nolan S. Can Extended-release Injectable Medications Help Curb United States and Canada’s Opioid Overdose Epidemic? Journal of addiction medicine 2021;15:15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Major E, Fusco CW, White Z, Wilson CG, Fagan EB. Long-acting, Injectable Buprenorphine: Great Promise, but Significant Barriers to Use. N C Med J 2020;81:210–1. [DOI] [PubMed] [Google Scholar]
- 26.Sublocade Package Insert 2020. at https://www.sublocade.com/Content/pdf/prescribing-information.pdf.)
- 27.Morgan J, Schackman B, Weinstein Z, Walley A, Linas B. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug and Alcohol Dependence 2019;200:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 29.Sublocade Package Insert 2017. at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209819s000lbl.pdf.)
- 30.Volkow ND, Blanco C. Interventions to Address the Opioid Crisis-Modeling Predictions and Consequences of Inaction. JAMA Netw Open 2021;4:e2037385. [DOI] [PubMed] [Google Scholar]
- 31.Linas BP, Savinkina A, Madushani R, et al. Projected Estimates of Opioid Mortality After Community-Level Interventions. JAMA Netw Open 2021;4:e2037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shover CL. Availability of Extended-Release Buprenorphine to Treat Opioid Use Disorders Among Medicaid-Covered Patients. Psychiatr Serv 2020:appips202000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shover CL. Commentary on Larance et al. (2020): Priorities and concerns of people who use opioids are key to scaling up XR-buprenorphine. Addiction 2020;115:1306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunders EC, Moore SK, Walsh O, et al. Perceptions and preferences for long-acting injectable and implantable medications in comparison to short-acting medications for opioid use disorders. J Subst Abuse Treat 2020;111:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open 2020;3:e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritvo AD, Calcaterra SL, Ritvo JI. Using Extended Release Buprenorphine Injection to Discontinue Sublingual Buprenorphine: A Case Series. Journal of addiction medicine 2020. [DOI] [PubMed] [Google Scholar]
- 37.Polsky D, Arsenault S, Azocar F. Private Coverage of Methadone in Outpatient Treatment Programs. Psychiatr Serv 2020;71:303–6. [DOI] [PubMed] [Google Scholar]
- 38.Hadland SE, Bagley SM, Rodean J, et al. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder. JAMA Pediatr 2018;172:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strang J, Metrebian N, Lintzeris N, et al. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. The Lancet 2010;375:1885–95. [DOI] [PubMed] [Google Scholar]
- 40.Morgan JR, Barocas JA, Murphy SM, et al. Comparison of Rates of Overdose and Hospitalization After Initiation of Medication for Opioid Use Disorder in the Inpatient vs Outpatient Setting. JAMA Netw Open 2020;3:e2029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.In: Mancher M, Leshner AI, eds. Medications for Opioid Use Disorder Save Lives Washington (DC)2019. [PubMed] [Google Scholar]
- 42.Hooker SA, Sherman MD, Lonergan-Cullum M, et al. Mental Health and Psychosocial Needs of Patients Being Treated for Opioid Use Disorder in a Primary Care Residency Clinic. J Prim Care Community Health 2020;11:2150132720932017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Journal of addiction medicine 2015;9:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institute for Health and Care Excellence. Coexisting severe mental illness and substance misuse: community health and social care services National Institute for Health and Clinical Excellence London; 2016. [Google Scholar]
- 45.Lin LA, Bohnert ASB, Blow FC, et al. Polysubstance use and association with opioid use disorder treatment in the US Veterans Health Administration. Addiction 2021;116:96–104. [DOI] [PubMed] [Google Scholar]
- 46.Taylor OD. Poly Substance Use in Methadone Maintenance Therapy (MMT) Patients. Journal of Human Behavior in the Social Environment 2015;25:822–9. [Google Scholar]
- 47.Shahid H, Bhatt M, Sanger N, et al. Association Between Family Factors and Illicit Polysubstance Use Amongst Methadone Maintenance Patients with Opioid Use Disorder. International Journal of High Risk Behaviors and Addiction 2018;7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


