Abstract
Background
Treatment options for refractory metastatic colorectal cancer (mCRC) were limited. Anlotinib is a novel multitarget tyrosine kinase inhibitor. ALTER0703 study was conducted to assess efficacy and safety of anlotinib for patients with refractory mCRC.
Materials and Methods
This was a multicenter, double‐blinded, placebo‐controlled, randomized phase III trial involving 33 hospitals in China. Patients had taken at least two lines of therapies were 2:1 randomized to receive oral anlotinib (12 mg/day; days 1–14; 21 days per cycle) or placebo, plus best supportive care. Randomization was stratified by previous VEGF‐targeting treatments and time from diagnosis to metastases. The primary endpoint was overall survival (OS). The secondary endpoints were progression‐free survival (PFS), objective response rate (ORR), disease control rate (DCR), quality of life (QoL), and safety.
Results
A total of 419 patients (anlotinib: 282; placebo: 137) were treated from December 2014 to August 2016. The median PFS was improved in anlotinib group (4.1 months; 95% confidence interval [CI], 3.4–4.5) over placebo group (1.5 months; 95% CI, 1.4–1.5), with a hazard ratio (HR) of 0.34 (95% CI, 0.27–0.43; p < .0001). However, median OS was similar between two groups (8.6 months; 95% CI, 7.8–9.7 vs. 7.2 months; 95% CI, 6.2–8.8; HR, 1.02; p = .870). Improvements of ORR and DCR were observed in anlotinib over placebo. The most common grade ≥ 3 anlotinib related adverse events were hypertension (20.92%), increased γ‐GT (7.09%), and hand‐foot skin reaction (6.38%).
Conclusion
Anlotinib was tolerated in Chinese patients with refractory mCRC. Although OS did not reach significant difference, anlotinib still provided clinical benefits by substantially prolonged PFS in these patients.
Implications for Practice
In this randomized clinical trial that included 419 patients with refractory metastatic colorectal cancer, substantial prolonged in progression‐free survival was noted in patients who received anlotinib compared with those given placebo. Improvements on objective response rate and disease control rate was also observed in anlotinib group. However, overall survival was similar between the two groups. In a word, in third‐line or above treatment of Chinese patients with refractory metastatic colorectal cancer, anlotinib provided clinical benefit by significantly prolonged progression‐free survival.
Keywords: Colorectal cancer, Metastatic, Anlotinib, Survival, Progression‐free survival, Adverse events
Short abstract
The ALTER0703 study was conducted to assess the efficacy and safety of anlotinib for patients with refractory metastatic colorectal cancer. Results are reported here.
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors, which ranked third in incidence (10.2% of the total cancer cases) and second in mortality (9.2% of the total cancer deaths) worldwide [1]. In 2015, the annual new cancer cases and deaths of CRC is 376.3 and 191.0 thousands, ranked fifth among cancers in China. [2]. Approximately a quarter of the patients with CRC present with metastases at initial diagnosis and almost half of these patients will develop metastases [3]. The standard therapies were chemotherapy based on fluorouracil with leucovorin plus either irinotecan or oxaliplatin, bevacizumab targeting vascular endothelial growth factor (VEGF), and cetuximab or panitumumab targeting epithelial growth factor receptor (EGFR) for RAS wild‐type patients. For the late‐line setting therapy in Chinese patients, regorafenib was approved for the treatment of colorectal cancer in 2017. Fruquintinib and TAS‐102 were both approved in 2019. The results of the key clinical studies that made regorafenib and fruquintinib approved in China showed that the median progression‐free survival (PFS) was 3.2 and 3.7 months, and the overall survival (OS) was 8.8 and 9.0 months, respectively. For TAS‐102, the median PFS was 2.0 months and OS was 7.8 months in TERRA study, which was conducted in Asian patients with previously treated metastatic CRC (mCRC) [4, 5, 6, 7]. Thus, there were few therapeutic options for patients who had disease progressed after standard therapy until 2014, when this trial was designed and conducted.
Standard treatment using bevacizumab revealed antiangiogenesis plays a key role in biological therapy of CRC. Furthermore, expression of VEGF, VEGF receptor 1/2, and molecules involved in proangiogenic pathways, such as fibroblast growth factor and platelet‐derived growth factor, were detected in patients with CRC [8, 9]. Anlotinib (AL3818) hydrochloride is a novel multitarget tyrosine kinase inhibitor (TKI), which targeted VEGFR 1–3, FGFR 1–4, PDGFR α/β, and stem cell factor receptor [10, 11, 12, 13]. A previous study showed that anlotinib significantly prolonged overall survival (OS) and progression‐free survival (PFS) in patients with advanced non‐small cell lung carcinoma [14]. It has been approved as a standard treatment for non‐small cell lung carcinoma, soft tissue sarcoma, and small cell lung carcinoma in China. An exploratory phase II study in 31 patients with mCRC showed encouraging results with 6.5% objective response rate (ORR), 90.3% disease control rate (DCR), and good tolerance. The median PFS and OS were 5.6 (3.8–7.6) months and 9.3 (8.5–10.2) months, respectively (unpublished data). It was revealed that anlotinib could be an evaluating option for patients with refractory mCRC.
Therefore, we conducted this ALTER0703 study, a double‐blinded, placebo‐controlled, randomized phase III trial to assess the efficacy and safety of anlotinib in patients with refractory mCRC who had disease progression after at least two lines of previous standard therapy.
Materials and Methods
Study Design
This was a multicenter, double‐blind, placebo‐controlled, randomized phase III trial involving 33 grade‐A tertiary hospitals in China. The registration number on ClinicalTrials was NCT02332499. Patients were eligible to participate when they had histological or cytological documentation of adenocarcinoma of the colon or rectum. They had to have received at least two lines of chemotherapy‐based treatments and have disease progression during or within 3 months after the last standard therapy or have stopped because of unacceptable toxic effects. Prior standard treatment must include fluoropyrimidine, oxaliplatin, or irinotecan. Previous EGFR, VEGF, and VEGFR targeting therapies were allowed to be included.
Patients
Patients had to be aged 18 years or older and have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; life expectancy of at least 3 months; and adequate bone‐marrow, liver, and renal function at the start of the trial. Patients could not participate if they had previously received anlotinib or had uncontrolled medical disorders. The supplemental online Appendix (study protocol) shows full inclusion and exclusion criteria.
This trial was approved by each center's institutional review board or independent ethics committee. All patients provided written informed consent before entering. The trial was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice requirements.
Randomization and Blinding
Patients were randomly assigned in a 2‐to‐1 ratio to receive anlotinib or placebo with a block randomization scheme (block size of 6) using a double‐blind, computerized, centric randomized list generator. Enrolled patients were stratified by previous treatment with VEGF‐targeting drugs (yes or no) and time from diagnosis to metastases (<18 months or ≥ 18 months). Patients and investigators responsible for treatment were blinded to the administrated reagents. Packaging of the anlotinib and placebo pills (supplied by Chia Tai Tianqing Pharmaceutical Group Co, Ltd) was identical and coded according to a random code list. The allocation of the unique code list was kept confidentially by the personnel who were not involved in any study assessment and will not be disclosed until the end of the trial.
Procedures
Oral anlotinib (12 mg/day) or matched placebo plus best supportive care was administered. Each cycle was defined as 2 weeks on followed by 1 week off treatment. The treatment continued until disease progression or treatment intolerance. Dose modifications (10 mg/day or 8 mg/day) of anlotinib were allowed according to the protocol‐defined dose modification criteria. Briefly, if the patient could not tolerate 12mg/day, then the dose could be reduced to 10 mg/day or 8 mg/day. If the dose of 8 mg/day was not tolerated, then treatment was terminated in accordance with the RECIST, version 1.1 [15]. Tumor assessment was performed using computed tomography or magnetic resonance imaging within 2 weeks before treatment started. Positron emission computer tomography was not be allowed for tumor assessment during screening period. After the treatment initiation, tumors were evaluated once every 2 cycles. Patient follow‐up after the last administration of the study medication was done every 8 weeks to assess clinical outcomes, including toxicity, efficacy, and survival, until the death of the patient or until the data cutoff date (August 31, 2018), whichever came first.
Outcomes
The primary endpoint was OS, defined as the time from randomization until death from any cause. The secondary endpoints were PFS, ORR, DCR, safety, and QoL. The PFS was the duration from date of randomization to disease progression or disease‐related death. The ORR was the percentage of patients who had best tumor response of complete response and partial response according to RECIST version 1.1 on the basis of investigators reviewed. The DCR was ORR plus the percentage of stable disease patients assessed by investigators according to RECIST version 1.1. Tumor response and progression were assessed radiologically every 2 cycles of medication.
The safety of the treatment was evaluated in patients who received treatment at least one cycle by the occurrence of treatment‐related adverse events (TRAEs), and the severity of the TRAEs was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. The records of TRAEs were taken during the treatment period and first 30 days after last administration.
Patient‐reported QoL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire‐Core 30 (QLQ‐C30) [16] and EuroQol‐5 dimension (EQ‐5D) at every visit before any study‐related procedures were conducted [17]. The QLQ‐C30 questionnaires involved five items of functional status (physical, role, emotional, cognitive, and social function), eight items of symptom status (fatigue, pain, nausea, dyspnea, isomnia, appetite loss, constipation, and diarrhea), health status, and financial difficult. The QLQ‐C30 ranges from 0 to 100, when 100 represents highest level of functioning and best health‐related QoL. At least 10 points of change on the QLQ‐C30 scale is considered to be clinically meaningful [16, 18]. EQ‐5D questionnaires were to evaluate performance status on mobility, self‐care, usual activities, pain/discomfort, anxiety/depression, and visual analog scale. Higher scores in EQ‐5D represent better health‐related QoL. A change of 0.06–0.12 on the EQ‐5D index and a change of 7–12 on the visual analog scale are deemed to be clinically meaningful [19].
Statistical Analysis
The sample size was calculated on the basis of the following: a hazard ratio (HR) of 0.70 for the OS with 2‐to‐1 randomization; a median OS of 9.0 months for the anlotinib group versus 6.3 months for the placebo group according to results from the phase 2 trial; a significance level of p = .025 (one‐sided); and a statistical power of 85%, with both scheduled accrual and follow‐up for 12 months. A total of 390 patients (260 in anlotinib group and 130 in placebo group) were needed to be enrolled within the scheduled accrual and follow‐up to achieve 291 death events (194 in anlotinib group and 97 in placebo group).
We assessed the efficacy in the full analysis set, which was defined as all cases treated with study drugs for at least one cycle. Baseline characteristics between the two groups were compared using t tests for continuous variables, Wilcoxon rank sum tests for ordinal categorical variables, and χ2 tests for categorical variables. The survival curves for OS and PFS were estimated with the Kaplan‐Meier method and were compared between treatment and control groups using the log‐rank test. Subgroup analysis was undertaken using univariate Cox proportional hazards regression models that, along with an interaction term (treatment and subgroup variable), tested the heterogeneity of the anlotinib and placebo subgroups. ORR, DCR for each group, and safety measurements were compared using Pearson χ2 or Fisher exact test when appropriate. Changes in QoL scores from baseline scores were assessed by Wilcoxon rank sum test. All statistical tests were carried out on the basis of a 2‐sided α = 0.05 and 95% CI. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Between December 9, 2014, and August 18, 2016, 485 patients screened for eligibility, and 421 patients were enrolled and randomly assigned to either the anlotinib group (n = 283) or the placebo group (n = 138). One patient in each group did not received treatment for at least one cycle and were excluded from final analysis. Finally, 282 patients in the anlotinib group (105 [37.2%] were female and 177 [62.8%] were male, with a mean ± SD age of 56.2 ± 10.5 years) and 137 patients in the placebo group (48 [33.6%] were female and 91 [66.4%] were male, with a mean ± SD age of 55.2 ± 10.8 years) were included in the efficacy and safety analysis (Fig. 1).
Figure 1.
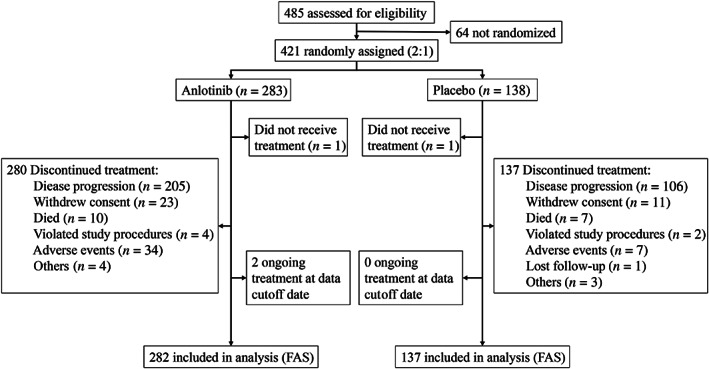
CONSORT diagram. The data cutoff date was August 31, 2018. Abbreviation: FAS, full analysis set.
The baseline information of patients is shown in Table 1. Baseline characteristics were generally balanced across the two groups, such as ECOG performance status 0 (30.1% in anlotinib vs. 23.4% in placebo) or 1 (69.9% vs. 76.6%; p = .147), time from diagnosis to metastases <18 months (83.7% vs. 82.5%) or ≥ 18 months (16.3% vs. 17.5%; p = .781), prior VEGF‐targeting therapy (30.5% vs. 31.4%), or no prior anti‐VEGF treatment (69.5% vs. 68.6%; p = .9103).
Table 1.
Baseline characteristics (intention to treat population) of anlotinib vs. placebo
| Characteristics | Anlotinib (n = 282), n (%) | Placebo (n = 137), n (%) | p value |
|---|---|---|---|
| Age n (%) | |||
| <65 | 213 (75.53) | 111 (81.02) | .2167 |
| ≥65 | 69 (24.47) | 26 (18.98) | |
| Gender n (%) | |||
| Male | 177 (62.77) | 91 (66.42) | .5156 |
| Female | 105 (37.23) | 46 (33.58) | |
| ECOG n (%) | |||
| 0 | 85 (30.14) | 32 (23.36) | .1471 |
| 1 | 197 (69.86) | 105 (76.64) | |
| Primary site of disease n (%) | |||
| Colon | 142 (50.35) | 55 (40.15) | .1073 |
| Rectum | 129 (45.74) | 78 (56.93) | |
| Both | 11 (3.90) | 4 (2.92) | |
| Left or right colon origin n (%) | |||
| Right | 39 (13.83) | 20 (14.60) | .8343 |
| Left | 230 (81.56) | 109 (79.56) | |
| Unknown | 13 (4.61) | 8 (5.84) | |
| Liver metastasis n (%) | |||
| No | 66 (23.40) | 41 (29.93) | .1540 |
| Yes | 216 (76.60) | 96 (70.07) | |
| Colon cancer surgery n (%) | |||
| No | 31 (10.99) | 16 (11.68) | .8694 |
| Yes | 251 (89.01) | 121 (88.32) | |
| Time from diagnosis of metastases n (%) | |||
| < 18 mo | 236 (83.69) | 113 (82.48) | .7809 |
| ≥ 18 mo | 46 (16.31) | 24 (17.52) | |
| KRAS mutation n (%) | |||
| Yes (+) | 112 (39.72) | 54 (39.42) | .1820 |
| No (‐) | 122 (43.26) | 50 (36.50) | |
| Unknown | 48 (17.02) | 33 (24.09) | |
| RAS/BRAF mutation n (%) | |||
| Yes (+) | 128 (45.39) | 57 (41.60) | .2750 |
| No (‐) | 103 (36.52) | 46 (33.58) | |
| Unknown | 51 (18.09) | 34 (24.82) | |
| Previous anti‐EGFR treatment n (%)a | |||
| No | 258 (91.49) | 119 (86.90) | .1914 |
| Yes | 24 (8.51) | 18 (13.10) | |
| Previous anti‐VEGF treatment n (%)a, c | |||
| No | 196 (69.50) | 94 (68.61) | .9103 |
| Yes | 86 (30.50) | 43 (31.39) | |
| Previous anti‐VEGFR treatment n (%)b | |||
| No | 254 (90.1) | 125 (91.20) | .8558 |
| Yes | 16 (5.70) | 6 (4.40) | |
| Unknown | 12 (4.20) | 6 (4.40) | |
| Previous chemotherapy n (%) | |||
| <3rd line | 142 (50.35) | 69 (50.36) | .999 |
| ≥3rd line | 140 (49.65) | 68 (49.64) | |
| Previous radiotherapy n (%) | |||
| No | 182 (64.54) | 83 (60.58) | .4508 |
| Yes | 100 (35.46) | 54 (39.42) |
Previous treatment included targeted agents in clinical trials’ setting.
Cetuximab, panitumumab, or nimotuzumab.
Regorafenib, fruquintinib, or apatinib.
Bevacizumab.
The percentage of patients received subsequent systemic antitumor therapy was 33.7% in the anlotinib and 50.4% in the placebo group (p = .001). The proportion of patients with subsequent chemotherapy in anlotinib and placebo group was 27.3% and 35.8% (p = .089), respectively (supplemental online Table 1).
By the cutoff date (August 31, 2018), the median treatment duration for anlotinib and placebo was 12 and 6 weeks, respectively. The median follow‐up duration for anlotinib was 31.0 months (95% CI, 0.6–42.2) and for placebo was 31.8 months (0.5–39.6). The median OS was 8.6 months (7.8–9.7) in the anlotinib group and 7.2 months (6.2–8.8) in the placebo group, with a HR of 1.02 (95% CI, 0.82–1.27; p = .871; Fig. 2A). Median PFS was 4.1 months (3.4–4.5) in the anlotinib group and 1.5 months (1.4–1.5) in the placebo group. Anlotinib significantly decreased the risk of disease progression by 66% with a HR of 0.34 (95% CI, 0.27–0.43; p < .0001; Fig. 3A).
Figure 2.
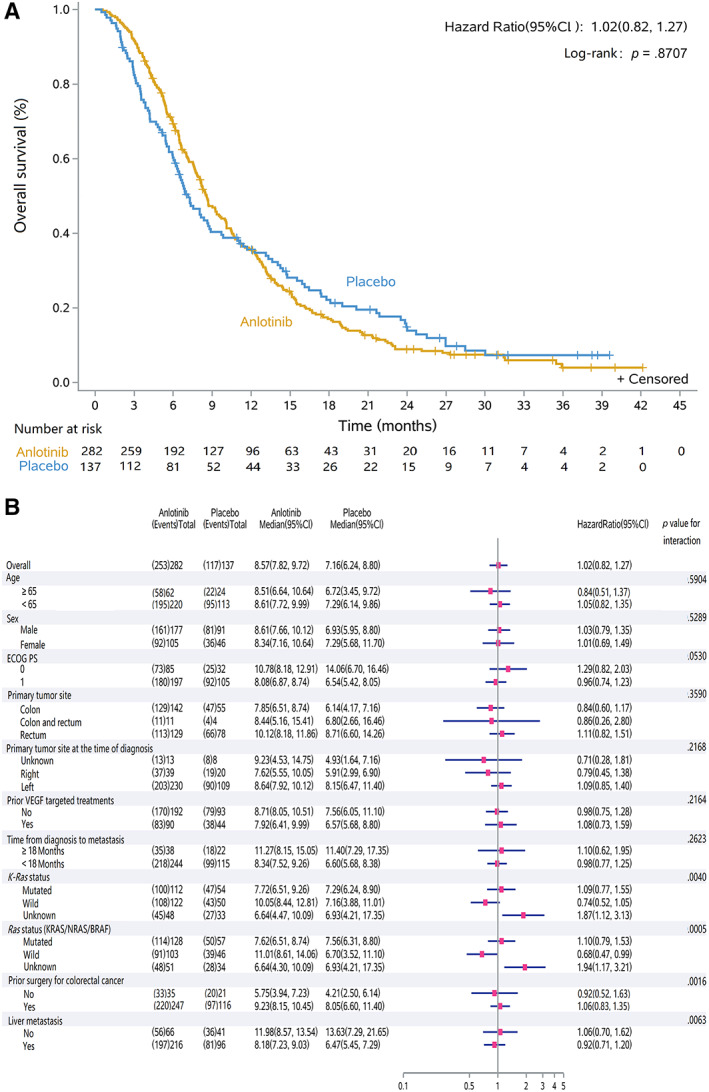
Kaplan‐Meier curve of overall survival in the intent‐to‐treat population. (A): Overall survival was 8.57 months (95% CI, 7.82–9.72) in the anlotinib group and 7.16 months (6.24–8.80) in the placebo group, with a hazard ratio of 1.02 (95% CI, 0.82–1.27, p = 0.8707). (B): Subgroup analysis for overall survival Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; VEGF, vascular endothelial growth factor.
Figure 3.
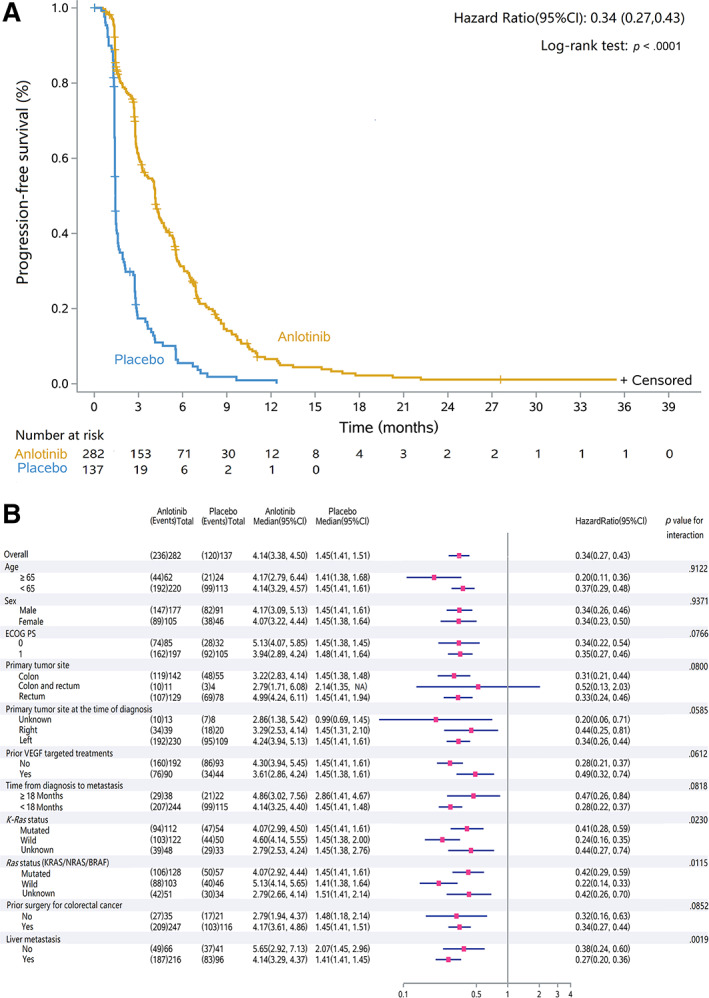
Kaplan‐Meier curve of progression‐free survival in the intent‐to‐treat population. (A): Progression‐free survival was 4.14 months (95% CI, 3.38–4.50) in the anlotinib group and 1.45 months (1.41–1.51) in the placebo group, with a hazard ratio of 0.34 (95% CI, 0.27–0.43, p < 0.0001). (B): subgroup analysis for progression‐free survival. Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; VEGF, vascular endothelial growth factor.
The post hoc OS analysis is shown in Figure 2B. In the subgroup of patients with Ras (KRAS/NRAS/BRAF) wild, median OS was 11.0 months (8.6–14.1) in the anlotinib group (n = 103) and 6.7 months (3.5–11.1) in the placebo group (n = 46), respectively, with an HR of 0.68 (0.47–0.99). However, in patients with Ras mutation, median OS was not improved by anlotinib treatment (7.6 months [6.5–8.7] in anlotinib [n = 128] and 7.6 months [6.3–8.8] in placebo [n = 57], with an HR of 1.10 [0.79–1.53]). In the subgroups of patients with K‐Ras status (wild or mutated), prior surgery (yes or no), and liver metastases (yes or no), the median OS was similar between anlotnib and placebo, nonetheless, with an interaction p < .05.
In a post hoc OS analysis of poststudy treatment, it was shown that median OS was significantly improved by anlotinib medication in patients who did not receive subsequent systemic antitumor therapy or chemotherapy. In these subgroups, the baseline characteristics were balanced in anlotinib and placebo group (supplemental online Tables 2, 3). In patients who did not receive systemic antitumor therapy, the median OS was improved in anlotinib (6.9 months [6.2–7.8]; n = 187) over placebo (4.5 months; [3.5–6.3]; n = 68), with a p of .005 (supplemental online Fig. 1A). In patients who did not had subsequent chemotherapy, the median OS in anlotinib (n = 205) and placebo (n = 88) group was 7.2 months (6.4–8.2) and 5.7 months (4.2–6.8), respectively, with a p of .017 (supplemental online Fig. 2A). In patients with systemic antitumor therapy, median OS were similar between anlotinib (13.1 months [10.7–15.3]; n = 95) and placebo (13.63 months [8.7–17.8]; n = 69), with a p of .6066 and HR of 1.09 (0.78–1.54) (supplemental online Fig. 1B). Meanwhile, the baseline characters of those patients were balanced except ECOG status (p = .027) and primary tumor site (p = .048; supplemental online Table 2). After adjusted the unbalanced baseline characters, the HR of OS was 1.064 (0.748–1.515; supplemental online Table 3). In a subgroup of patients with subsequent chemotherapy, the median OS in the anlotinib group (n = 77) and placebo group (n = 49) was 13.6 months (10.7–16.5) and 17.4 months (13.1–23.9), respectively, without significant difference (p = .182; HR = 1.31 [0.88–1.97]; supplemental online Fig. 2B). After adjusted the unbalanced baseline characters (liver metastases [p = .028], Ras status [p = .024] and KRAS status [p = .014]; supplemental online Table 4), the HR was 1.064 (0.748–1.515; supplemental online Table 5). Consistent with these, patients who did not have subsequent therapy could get better survival benefit from anlotinib treatment than placebo.
In the post hoc subgroup analysis of PFS, it favored anlotinib in almost all subgroups except primary tumor site of colon and rectum. In patients with primary tumor site in both colon and rectum, the HR of PFS was 0.52 (0.13–2.03) between the anlotinib group (n = 11) and placebo group (n = 4). Although the interaction between medication treatment and prognostic factors, such as K‐Ras, Ras status, and liver metastases was statistically significant with an interaction p < .05 (Fig. 3B). The subgroup analysis demonstrated preference of anlotinib in these subgroups.
There was no complete response. Twelve patients in the anlotinib group and one in the placebo group had a partial response, resulting in an ORR of 4.26% and 0.73%, respectively (p = .0690). DCR was significantly higher in the anlotinib group (214 [75.9%] vs. 42 [30.7%], p < .0001; supplemental online Table 6). Water‐fall plots are shown in supplemental online Figure 3.
A total of 275 (97.5%) patients in the anlotinib group and 118 (86.1%) patients in the placebo group had TRAEs. A total of 149 (52.5%) patients in the anlotinib and 27 (19.7%) patients in the placebo group experienced grade 3 or above TRAEs. Table 2 shows TRAEs that occurred in at least 5% of patients in either group. The most frequent TRAEs (>5%) of grade 3 or above in the anlotinib group were hypertension (20.9% vs. 2.9% in placebo group), increased γ‐GT (7.1% vs. 1.5%), and hand‐foot skin reaction (6.4% vs. 0.0%). There were three patients with grade 5 TRAEs overall, in which one patient with intracranial hemorrhage and one with urethral hemorrhage were recorded in the anlotinib group and one patient with asphyxia was recorded in the placebo group. Treatment‐related serious adverse events (TRSAEs) were recorded in 35 (12.4%) patients in the anlotinib and 10 (7.3%) patients in the placebo group, respectively. Sixteen out of 35 patients in the anlotinib and 2 out of 10 in the placebo group recovered by dose reducing or discontinuing. The most common TRSAE was pneumothorax, which occurred in four patients of the anlotinib group and none of the placebo group.
Table 2.
Treatment‐related adverse events (≥5%) in either group from start of treatment to 30 days after end of treatment (safety population)
| Treatment‐related adverse events | Anlotinib n = 282 | Placebo n = 137 | ||
|---|---|---|---|---|
| All, n (%) | Grade ≥ 3, n (%) | All, n (%) | Grade ≥ 3, n (%) | |
| Anorexia | 92 (32.62) | 4 (1.42) | 17 (12.41) | 0 (0) |
| Fatigue | 125 (44.3) | 7 (2.48) | 32 (23.36) | 1 (0.73) |
| Diarrhea | 95 (33.69) | 11 (3.90) | 13 (9.49) | 1 (0.73) |
| Abdominal pain | 52 (18.44) | 3 (1.06) | 10 (7.30) | 0 (0) |
| Hoarseness | 56 (19.86) | 0 (0) | 0 (0) | 0 (0) |
| Oropharynx pain | 45 (15.96) | 1 (0.35) | 3 (2.19) | 0 (0) |
| Hypertension | 190 (67.38) | 59 (20.92) | 33 (24.09) | 4 (2.92) |
| Prolonged QT | 43 (15.25) | 4 (1.42) | 12 (8.76) | 3 (2.19) |
| Hand‐foot skin reaction | 125 (44.33) | 18 (6.38) | 6 (4.38) | 0 (0) |
| Proteinuria | 115 (40.78) | 12 (4.26) | 26 (18.98) | 2 (1.46) |
| Hematuresis | 30 (10.64) | 0 (0) | 5 (3.65) | 0 (0) |
| Hypothyroidism | 50 (17.73) | 0 (0) | 4 (2.92) | 0 (0) |
| Hypertriglyceridemia | 76 (26.95) | 11 (3.90) | 14 (10.22) | 0 (0) |
| Hypercholesterolemia | 62 (21.99) | 2 (0.71) | 24 (17.52) | 1 (0.73) |
| Hypoalbuminemia | 24 (8.51) | 1 (0.35) | 5 (3.65) | 0 (0) |
| Hyponatremia | 21 (7.45) | 7 (2.48) | 6 (4.38) | 2 (1.46) |
| Anemia | 19 (6.74) | 1 (0.35) | 15 (10.95) | 1 (0.73) |
| Weight loss | 26 (9.22) | 3 (1.06) | 4 (2.92) | 1 (0.73) |
| Nausea | 25 (8.87) | 0 (0) | 9 (6.57) | 0 (0) |
| Sinus tachycardia | 21 (7.45) | 0 (0) | 8 (5.84) | 0 (0) |
| Rash | 18 (6.38) | 0 (0) | 1 (0.73) | 0 (0) |
| Emesis | 18 (6.38) | 1 (0.35) | 5 (3.65) | 0 (0) |
| Cough | 17 (6.03) | 0 (0) | 3 (2.19) | 0 (0) |
| headache | 15 (5.32) | 0 (0) | 2 (1.46) | 0 (0) |
| Constipation | 15 (5.32) | 0 (0) | 7 (5.11) | 0 (0) |
| Lipase increased | 16 (5.67) | 6 (2.13) | 2 (1.46) | 0 (0) |
| Back pain | 16 (5.67) | 0 (0) | 3 (2.19) | 0 (0) |
| Lab testing | ||||
| TSH increased | 88 (31.21) | 1 (0.35) | 3 (2.19) | 0 (0) |
| AST increased | 64 (22.70) | 3 (1.06) | 20 (14.6) | 2 (1.46) |
| ALT increased | 54 (19.15) | 2 (0.71) | 11 (8.03) | 2 (1.46) |
| Thrombocytopenia | 57 (20.21) | 5 (1.77) | 13 (9.49) | 1 (0.73) |
| γ‐GT increased | 43 (15.25) | 20 (7.09) | 13 (9.49) | 2 (1.46) |
| ALP increased | 38 (13.48) | 4 (1.42) | 9 (6.57) | 2 (1.46) |
| Positive fecal occult blood test | 33 (11.70) | 0 (0) | 5 (3.65) | 0 (0) |
| White cell decreased | 35 (12.41) | 1 (0.35) | 6 (4.38) | 0 (0) |
| Hypocalcemia | 15 (5.32) | 0 (0) | 4 (2.92) | 0 (0) |
| LDH increased | 15 (5.32) | 0 (0) | 4 (2.92) | 0 (0) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; TSH, thyroid stimulating hormone.
The proportion of patients required treatment interruption or dose reduction was 31.9% (n = 90) in the anlotinib and 12.4% (n = 17) in the placebo group. A total of 52 (18.4%) patients assigned to anlotinib and 1 (0.7%) patient assigned to placebo required dose reduction (supplemental online Table 7). The median time for dose adjustment was 7.5 cycles. Two (0.7%) patients in the anlotinib group had their dose adjusted to 8 mg/day. One was due to extremely low body surface area of 1.2m2, and the other was due to grade 2 vomiting. Grade 2–3 adverse events in hand‐foot skin reaction, hypertension and proteinuria were the most common reason for dose modification (supplemental online Table 7).
As to the QoL, it was evaluated by mean score changes in the second, fourth, and sixth treatment cycle from baseline according to EORTC QLQ‐C30 and EQ‐5D. Results of QLQ‐C30 are shown in supplemental online Figure 4. At the end of the sixth cycle, clinical meaningful functional changes in anlotinib and placebo group were physical function (−11.7 [SD, 17.6] in anlotinib vs. −11.3 [18.9] in placebo) and role function (−14.8 [25.3] vs. −11.5 [20.8]), and meaningful symptom changes were fatigue (11.4 [26.0] vs. 10.3 [25.5]), pain (14.2 [28.5] vs. 5.1 [25.8]), pyspnea (5.5 [24.4] vs. 20.5 [37.4]), appetite loss (10.2 [28.9] vs. −15.4 [37.6]), constipation (−0.8 [27.9] vs. −10.3 [25.0]), and financial difficult (−4.7 [26.0] vs. −25.6 [27.7]). Consistent with these, in the placebo group, patients suffered more pyspnea. Patients in the anlotinib group underwent more pain, appetite loss, and constipation, which were accordant with TRAEs profile. The EQ‐5D results showed clinical meaningful increase investigated in anlotinib were self‐care throughout the treatment, pain/uncomfort at the second cycle, and anxiety/depression at the fourth treatment cycle. In the placebo group, clinical meaningful increase were self‐care and anxiety/depression at the second cycle, self‐care and usual activities at the fourth cycle, and mobility and pain/uncomfort at the sixth cycle (supplemental online Fig. 5). The changes of visual analog scale in the anlotinib and placebo group were − 2.55 (17.21) and − 4.47 (22.12) at second cycle, −6.03 (17.34) and − 3.53 (18.31) at fourth cycle, and − 7.32 (16.32) and − 11.69 (24.64) at the sixth cycle, respectively, in which clinical meaningful changes were observed at sixth cycle. In the QLQ‐C30 and EQ‐5D, the deterioration of patients’ QoL in the anlotnib group was almost as same as that in the placebo group.
Discussion
This double‐blinded, placebo‐controlled, randomized trial evaluated the efficacy and safety of anlotinib in patients with advanced metastatic colorectal cancer, who had been treated with at least two lines of standard therapy. The median OS in anlotinib and placebo group were 8.6 months and 7.2 months, respectively, without significant improvement. Median PFS was significantly longer in anlotinib group (4.1 months) than placebo group (1.5 months), with 66% reducing of disease progression risk. Improvements of ORR and DCR were observed in anlotinib over placebo. In the safety profile, most common grade ≥ 3 TRAEs were hypertension, increased γ‐GT, and hand‐foot skin reaction. The QoL results indicated patients with anlotinib sustained similar deterioration of QoL and healthy status as placebo. The encouraging improvements of PFS and DCR over placebo suggested potential clinical benefit with manageable tolerance of anlotinib in refractory metastatic CRC patients. However, the significant improvement of PFS did not translate into a substantial benefit of OS in this study.
It is acceptable that subsequent treatment plays crucial role in affecting survival benefit [20, 21]. It has been reported that subsequent therapy influenced significant OS benefit in several clinical trials emerging non‐small cell lung cancer, prostate carcinoma, hepatocellular carcinoma, and breast cancer [22, 23, 24, 25]. In the Keynote‐240 trial of advanced hepatocellular carcinoma, the HR of primary endpoint OS was 0.781, which did not reach the predefined endpoint of HR = 0.65. After we re‐evaluated HR of OS by adjusting subsequent treatments, it was 0.67–0.68, much closer to the predefined one [25]. In our study, the proportion of patients who received subsequent systemic antitumor therapy in anlotinib group was significant lower than that in placebo (p = .001). Furthermore, anlotinib had brought encouraging OS benefit for patients who did not receive subsequent treatments. In patients who did not take subsequent systemic antitumor therapy, the median OS was significantly improved in anlotinib (6.9 months) than placebo (4.5 months), with a p of .0053. Meanwhile, in patients treated by subsequent systemic antitumor therapy, the median OS was similar between the two groups after adjusting unbalanced baseline factors (p = .729). It was illustrated that subsequent therapy apparently influence OS in our study. In patients who did not undergo chemotherapy after progression, the median OS prolonged by 1.48 months in anlotinib over placebo group (p = .017). However, in the subsequent chemotherapy subgroup, the median OS in placebo group was numbering longer than anlotinib without statistical difference after adjusting unbalanced baseline factors (p = .207). Overall, subsequent therapy, a bias could not be adjusted at the beginning of this trial, might be an important factor affected primary endpoint in this study.
There might be several reasons for the significant influence of subsequent therapy on OS in our study. First of all, the median follow‐up for OS analysis was 31.0 months in anlotinib and 31.8 months in placebo group, which were much longer than that in other similar phase III trials (7.4 months in CONCUR of regorafenib and 13.2 months in FRESCO of fruquintinib) [5, 6]. The longer follow‐up duration, the better data maturity, but it would increase the influence probability of subsequent therapy on OS. Second, placebo‐controlled design may bring bias to OS analysis that was the unbalanced proportion of patients received subsequent therapy. Last, patients enrolled in this study must be treated with at least two lines of prior standard treatments, but it had not been defined concretely the duration of previous treatment. Moreover, the post hoc analysis showed that the patients could get survival benefit from poststudy chemotherapy. These indicated that the some patients enrolled in this trial to receive the third‐line or subsequent therapy because they had been intolerance to toxicities of the prior chemotherapy. So some of the eligible patients might not be refractory so the poststudy chemotherapy of them could confound the OS analysis.
Comparing results with FRESCO, a phase III trial evaluated efficacy and safety of a multitarget TKI fruquintinib in China, which was conducted during the same period of our study, the median PFS was similar (3.7 months in fruquintinib group and 1.8 months in placebo group of FRESCO trial) with our study. However, the median OS was longer in fruquintinib and placebo (9.3 and 6.8 months, respectively) than that in our study [6]. When we analyzed the OS rate at different time point of these two trials, it was observed that OS rate was similar at the sixth and ninth month but was different at the 12th and 15th month. At the 12th and 15th month, the OS rate in anlotinib and placebo in this current study were both higher than that in FRESCO trial. It illustrated that the survival probability of placebo in our study was better than that of FRESCO, whereas anlotinib could bring survival benefit as the same as fruquintinib [6]. The better survival in placebo might be related to the subsequent treatments, which was a crucial bias on primary outcome in our study.
In the post hoc subgroup analysis of OS, patients with Ras wild type could get survival benefit (HR, 0.68; 0.47–0.99) from anlotinib treatment, whereas survival benefit were negative in other subgroups. Ras wild type could be a potential direction for subsequent exploratory clinical trials.
As to safety analysis, the TRAEs profile was consistent with the anlotinib ALTER 0303 study in lung cancer [14, 26]. In this study, incidence of TRSAEs in alonotinib and placebo was 35 and 10, respectively, in which 45.7% (16/35) of patients in anlotinib and 20% (2/10) in placebo recovered by dose reducing or discontinuing. The TRAEs of anlotinib were manageable. It was indicated that the deterioration of QoL in anlotnib was as same as placebo for patients in our study.
Limitations
There are several limitations of this study. First, the trial was conducted by comparing placebo. This design might lead to the more effectiveness of subsequent chemotherapy on OS outcome, and some patients enrolled might not be truly refractory to the prior therapy which could consolidate the effect. Second, screening and analysis for biomarkers were not involved in this study, which led to insufficient analysis for precision treatment of anlotinib for patients with mCRC. Third, the post hoc analysis showed that patients with Ras wild type could get survival benefit which need to be validated in further a prospective trial. Last but not least, the trial was conducted in China only, so results may differ from different ethnic groups.
Conclusion
Anlotinib is manageable and tolerant in patients with refractory metastatic CRC. Although OS did not reach significant difference, anlotinib could still provide clinical benefit by substantially prolonged PFS in patients with refractory metastatic colorectal cancer.
Author Contributions
Conception/design: Yihebali Chi, Jiangqiang Cai
Provision of study material or patients: Yihebali Chi, Yongqian Shu, Yi Ba, Yuxian Bai, Baoli Qin, Xiuwen Wang, Jianping Xiong, Nong Xu, Helong Zhang, Jianfeng Zhou, Jianming Xu, Ying Cheng, Jifeng Feng, Chunhong Hu, Yigui Chen, Zhendong Chen, Jufeng Wang, Chengxue Dang, Jianhong Wang, Yiye Wan, Yong Tang, Donglin Wang, Jiang liu, Minhui Wu, Yanhong Deng, Xingwen Li, Yongqiang Li, Jian Dong, Da Jiang, Guisheng Li, Qiong Wu, Jin Li,Yujuan Qi, Yongkun Sun, Jianqiang Cai
Collection and/or assembly of data: Yihebali Chi, Yongqian Shu, Yi Ba, Yuxian Bai, Baoli Qin, Xiuwen Wang, Jianping Xiong, Nong Xu, Helong Zhang, Jianfeng Zhou, Jianming Xu, Ying Cheng, Jifeng Feng, Chunhong Hu, Yigui Chen, Zhendong Chen, Jufeng Wang, Chengxue Dang, Jianhong Wang, Yiye Wan, Yong Tang, Donglin Wang, Jiang liu, Minhui Wu, Yanhong Deng, Xingwen Li, Yongqiang Li, Jian Dong, Da Jiang, Guisheng Li, Qiong Wu, Jin Li,Yujuan Qi, Yongkun Sun, Jianqiang Cai
Data analysis and interpretation: Yihebali Chi, Jiangqiang Cai
Manuscript writing: Yihebali Chi, Jiangqiang Cai
Final approval of manuscript: Yihebali Chi, Yongqian Shu, Yi Ba, Yuxian Bai, Baoli Qin, Xiuwen Wang, Jianping Xiong, Nong Xu, Helong Zhang, Jianfeng Zhou, Jianming Xu, Ying Cheng, Jifeng Feng, Chunhong Hu, Yigui Chen, Zhendong Chen, Jufeng Wang, Chengxue Dang, Jianhong Wang, Yiye Wan, Yong Tang, Donglin Wang, Jiang liu, Minhui Wu, Yanhong Deng, Xingwen Li, Yongqiang Li, Jian Dong, Da Jiang, Guisheng Li, Qiong Wu, Jin Li,Yujuan Qi, Yongkun Sun, Jianqiang Cai
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Supplementary Figures
Supplementary Tables
Acknowledgments
We thank all the patients and their families participated in this study. We would like to give our grateful thanks to all the investigators, site staff, and statisticians for their valuable work.
The study was sponsored by Chia Tai Tianqing Pharmaceutical Group Co., Ltd. (Lian yun gang, Jiangsu Province, China). The sponsor provided the study drugs. The sponsor had no role in the study design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Nordlinger B et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25 Suppl 3:iii1–9. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology (NCCN guidelines): Colon cancer & rectal cancer. Version 2. 2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed June 21, 2019.
- 5.Li J, Qin S, Xu R et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2015;16:619–629. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Qin S, Xu RH et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO randomized clinical trial. JAMA 2018;319:2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Kim TW, Shen L et al. Results of a randomized, double‐blind, placebo‐controlled, phase III trial of trifluridine/tipiracil (TAS‐102) Monotherapy in Asian patients with previously treated metastatic colorectal cancer: The TERRA study. J Clin Oncol 2018;36:350–358. [DOI] [PubMed] [Google Scholar]
- 8.Guler I, Askan G, Klostergaard J et al. Precision medicine for metastatic colorectal cancer: An evolving era. Expert Rev Gastroenterol Hepatol 2019;13:919–931. [DOI] [PubMed] [Google Scholar]
- 9.Manzat‐Saplacan RM, Balacescu L, Gherman C et al. The role of PDGFs and PDGFRs in colorectal cancer. Mediat Inflamm 2017; 2017:4708076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin B, Song X, Yang D et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene 2018;654:77–86. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Niu W, Du F et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi‐target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Sun M, Jiang Y et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastases via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer 2019;145:979–993. [DOI] [PubMed] [Google Scholar]
- 13.Taurin S, Yang CH, Reyes M et al. Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer 2018;28:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B, Li K, Wang Q et al. Effect of anlotinib as a third‐line or further treatment on overall survival of patients with advanced non‐small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. Jama Oncol 2018;4:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 16.Hamaguchi R, Tsuchiya T, Miyata G et al. Efficacy of oral administration of cystine and theanine in patients with colorectal cancer undergoing capecitabine‐based adjuvant chemotherapy after surgery: Study protocol for a multi‐institutional, randomised, double‐blinded, placebo‐controlled, phase II trial. BMJ Open 2018;8:e021442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchlin CT, Kavanaugh A, Merola JF et al. Bimekizumab in patients with active psoriatic arthritis: Results from a 48‐week, randomised, double‐blind, placebo‐controlled, dose‐ranging phase 2b trial. Lancet 2020;395:427–440. [DOI] [PubMed] [Google Scholar]
- 18.Osoba D, Rodrigues G, Myles J et al. Interpreting the significance of changes in health‐related quality‐of‐life scores. J Clin Oncol 1998;16:139–144. [DOI] [PubMed] [Google Scholar]
- 19.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ‐5D utility and VAS scores in cancer. Health Qual Life Out 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michiels S, Saad ED, Buyse M et al. Progression‐free survival as a surrogate for overall survival in clinical trials of targeted therapy in advanced solid tumors. Drugs 2017;77:713–719. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services Food and Drug Administration Oncology Center of Excellence, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Clinical trial endpoints for the approval of cancer drugs and biologics: Guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics. 2018.
- 22.Herbst RS, Sun Y, Eberhardt WE et al. Vandetanib plus docetaxel versus docetaxel as second‐line treatment for patients with advanced non‐small‐cell lung cancer (ZODIAC): A double‐blind, randomised, phase 3 trial. Lancet Oncol 2010;11:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccart M, Hortobagyi GN, Campone M et al. Everolimus plus exemestane for hormone‐receptor‐positive, human epidermal growth factor receptor‐2‐negative advanced breast cancer: Overall survival results from BOLERO‐2dagger. Ann Oncol 2014;25:2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gravis G, Fizazi K, Joly F et al. Androgen‐deprivation therapy alone or with docetaxel in non‐castrate metastatic prostate cancer (GETUG‐AFU 15): A randomised, open‐label, phase 3 trial. Lancet Oncol 2013;14:149–158. [DOI] [PubMed] [Google Scholar]
- 25.Finn RS, Ryoo BY, Merle P et al. Pembrolizumab as second‐line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE‐240: A randomized, double‐blind, phase III trial. J Clin Oncol 2019;38:193–202. [DOI] [PubMed] [Google Scholar]
- 26.Si X, Zhang L, Wang H et al. Management of anlotinib‐related adverse events in patients with advanced non‐small cell lung cancer: Experiences in ALTER‐0303. Thoracic Cancer 2019;10:551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Supplementary Figures
Supplementary Tables


