Abstract
Objective
This study aims to evaluate the effects of a symptom management intervention (SMI) based on symptom management group sessions combined with a mobile health (mHealth) application (app) on the knowledge of symptom management, the certainty of symptom self-management, symptom severity, symptom distress, medication adherence, social support, and quality of life among persons living with HIV (PLWH) in China.
Methods
A parallel randomized controlled trial with 61 PLWH was conducted in Shanghai, China. The participants in the control group (n = 30) downloaded the Symptom Management (SM) app according to their needs and preferences, and received routine follow-ups. The participants in the intervention group (n = 31) were guided to download and use the SM app, and received four tailored weekly group sessions at routine follow-ups. Each group session lasted for approximately 2 h and targeted one of the major modules of the SM app. All the outcomes were assessed at baseline and post-intervention. The study was registered with the Chinese Clinical Trial Registry (ChiCTR1900024821).
Results
The symptom management knowledge and certainty of symptom self-management were significantly improved after the intervention (all P < 0.01). Compared with the control group, the scores of symptoms reasons knowledge score improved 11.47 points (95% CI: 3.41, 19.53) and scores of symptoms self-management knowledge score improved 12.80 points (95% CI: 4.55, 21.05) in the intervention group after controlling for covariates. However, other outcomes did not show statistically significant differences between the intervention group and the control group (P > 0.05).
Conclusion
The SMI could improve PLWH’s symptom management knowledge and certainty of symptom self-management. Multi-center studies with larger sample sizes and longer follow-ups are needed to further understand the effects of SM app on ameliorating symptom severity and symptom distress. More innovative strategies are also needed to promote and maintain the sustainability of the SM app.
Keywords: China, HIV Infections, Mobile applications, Medication adherence, Quality of life, Self-management, Social support
What is known?
-
•
Persons living with HIV (PLWH) experience serious symptom burden due to many factors, such as HIV infection and virus reservoirs, opportunistic infections and comorbidities, side effects of ART medication, social discrimination and internalized stigma.
-
•
PLWH’s symptom issue may influence their antiretroviral therapy (ART) medication adherence, clinical prognosis, and quality of life. It is necessary to explore more symptom management interventions (SMIs) for PLWH.
-
•
Currently, the widespread mobile technology users provide great opportunities to implement SMIs for PLWH.
What is new?
-
•
Our SMI based on symptom management group sessions combined with a mobile health (mHealth) application (app) showed significant intervention effects on symptom management knowledge and certainty of symptom self-management among Chinese PLWH.
-
•
However, the effects of the SMI on the symptom severity, symptom distress, ART medication adherence, perceived social support, and quality of life were uncertain.
-
•
The Symptom Management (SM) app is a promising tool of symptom self-management for Chinese PLWH. However, more innovative strategies are also needed to promote and maintain the sustainability of the SM app.
1. Introduction
Human immunodeficiency virus (HIV) infection has evolved from a fatal disease to a chronic disease owing to the progress of antiretroviral therapy (ART) [1]. However, persons living with HIV (PLWH) still are confronted with significant challenges in symptom management. Many factors, such as HIV infection and virus reservoirs, opportunistic infections and comorbidities, side effects of ART medication, social discrimination and internalized stigma, may pose substantial risks to various HIV-related symptoms [[2], [3], [4]]. PLWH usually experiences many symptoms simultaneously, and the average number of symptoms experienced by PLWH can range from 8 to 17 [[5], [6], [7]]. The prevalence of common symptoms among PLWH, such as fatigue, depression, pain, rash, and insomnia, was reported ranging from 43% to 78% [[5], [6], [7]].
The HIV epidemic in China shows an increasing trend [8,9]. Chinese PLWH are at particular risk of inducing and intensifying symptoms. The National Strategies of “Four Frees and One Care for HIV/AIDS” in China provides PLWH with free ART [10]. However, only publicly-funded domestic antiretroviral medications are free of charge. The integrase strand transfer inhibitors (INSTIs), which are the internationally recommended first-line treatment medication for PLWH [11], by contrast, are imported and needed to be paid out-of-pocket. Therefore, due to the issue of affordability, instead of taking these imported INSTIs, most Chinese PLWH choose to take free ART medications, e.g., efavirenz (EFV) or lopinavir/ritonavir (LPV/r). These medications may increase their risks for medication toxicity and symptoms distress (EFV may lead to rash and sleep disorders, LPV/r may lead to diarrhea) [12]. Moreover, influenced by Chinese conservative cultural values, PLWH are confronted with more challenges of social discrimination and internalized stigma, which aggravate the prevalence and severity of their mental symptoms [13,14].
Given that symptom burden seriously influences PLWH’s ATR medication adherence, clinical prognosis, and quality of life (QoL) [3,6,15], it is particularly important to explore more symptom management interventions (SMIs) for PLWH. Zhao and colleagues’ study demonstrated the process of symptom management among PLWH as assessing PLWH’s symptoms, understanding PLWH’s symptom experience and coping ability, and treating symptoms through professional management and self-care strategies [16]. Symptom management is a lifelong task for PLWH that requires continuous efforts. Moreover, SMIs are expected to target symptom clusters as a whole instead of on a single or several symptoms as many symptoms interweave with each other and share common mechanisms [17,18]. It is also necessary to initiate interventions even PLWH are just at risk of developing symptoms. The complexity of symptom management determines the importance of improving PLWH’s knowledge to cope with symptom management issues. Improvement in health knowledge will influence motivation, improve behavior skills, and promote health behavior changes. Those factors together mediate the improvement in health outcomes [19].
Several symptom management strategies have been developed among PLWH, such as art therapy [20], community navigation intervention [21], physical activity [22], peer-based intervention [23], manual or guidebook [2,24], health education [25,26], and mobile health (mHealth) application (app) [27]. Currently, the widespread mobile technology users provide great opportunities to implement SMIs for PLWH. Given the potential to improve access to care by reducing geographical and financial barriers, mHealth apps provide feasible, accessible, and effective platforms for symptom management. Our research team developed the first culturally tailored symptom management (SM) app to provide PLWH a sustainable way in acquiring symptom management knowledge and strategies in China [28]. The main modules of this app were delivered to participants through four group sessions to help them learn about the SM app and promote their usage compliance. The primary aim of this study was to evaluate the effects of a SMI based on symptom management group sessions combined with a mHealth app on the symptom management knowledge. This study also tested the effects of SMI on the symptom severity, symptom distress, certainty of symptom self-management, ART medication adherence, perceived social support, and QoL.
2. Framework
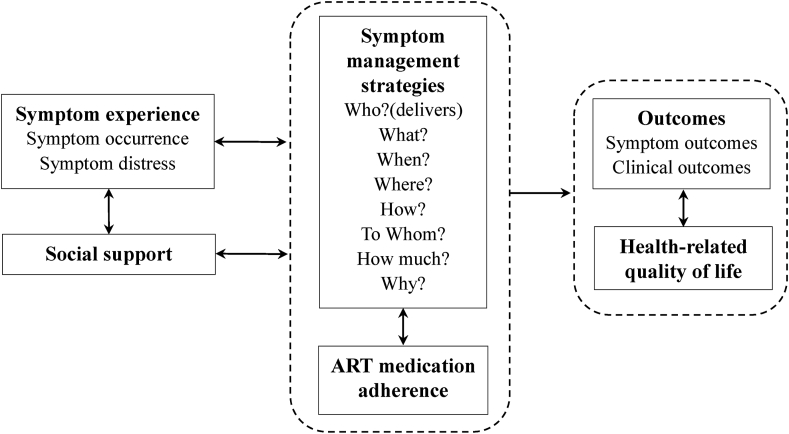
Our study is guided by a framework (Fig. 1) informed by the University of California, San Francisco (UCSF) Symptom Management Model [29] and the Self-regulatory HIV/AIDS Symptom Management Model (SSMM-HIV) [15]. Three key components of symptom management are symptom experience, management strategies, and outcomes. Symptom experience focuses on symptom occurrence (the cognitive pathway) and symptom distress (the emotional pathway). Symptom management strategies consider who, what (the nature of the strategy), when, where, how (delivered), to whom (the recipient of intervention), how much (the intervention dose) and why. Outcomes involve symptom outcomes and clinical outcomes. Social support, ART medication adherence, and QoL are equally essential components of HIV/AIDS symptom management. Guided by this framework, we designed the main functions and content of the SM app, and determined outcomes for this randomized controlled trial (RCT).
Fig. 1.
Symptom management framework. ART = antiretroviral therapy.
3. Methods
This study was approved by the research ethics committee of the School of Nursing at Fudan University (IRB#TYSA2016-3-1) and the Shanghai Public Health Center (SPHC) Affiliated with Fudan University (2019-S036-02). It was registered with the Chinese Clinical Trial Registry (ChiCTR1900024821). This study is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement of guidelines for reporting parallel group randomized trials [30].
3.1. Study design
A parallel RCT (1∶1) was conducted to examine the effects of the intervention on symptom management knowledge (symptom reasons and symptom coping), symptom certainty, QoL, perceived social support, and ART medication adherence. All these outcomes were measured at the baseline and post-intervention (four weeks after the baseline).
3.2. Setting and participants
Participants were recruited from the outpatient center at the SPHC, which is an infectious disease treatment hospital and is the only designated place distributing free ART for PLWH in Shanghai, China.
Inclusion criteria for participants were: 1) diagnosed with HIV-1 infection, 2) aged 18 or older, 3) had a smartphone installed with an Android system or iOS system, 4) receiving ART and routine follow-ups in the SPHC, and 5) provided voluntary written consent to participate in the study. Participants were excluded if they 1) were or about to be in hospital because of any of the comorbidities, such as opportunistic infections, 2) could not participate in our study because of severe mental health diseases or cognitive impairment, 3) were taking part in other HIV-related research projects at the same time.
3.3. Intervention
Our researchers introduced the SM app to all participants during the recruitment. The participants in the control group were asked to download the SM app according to their needs and preferences, and they were routinely followed up at the SPHC outpatient center, which included taking routine blood and urine tests, and receiving free ART medication. The participants in the intervention group were advised to download and use the SM app, and took part in tailored group sessions by routinely follow-ups.
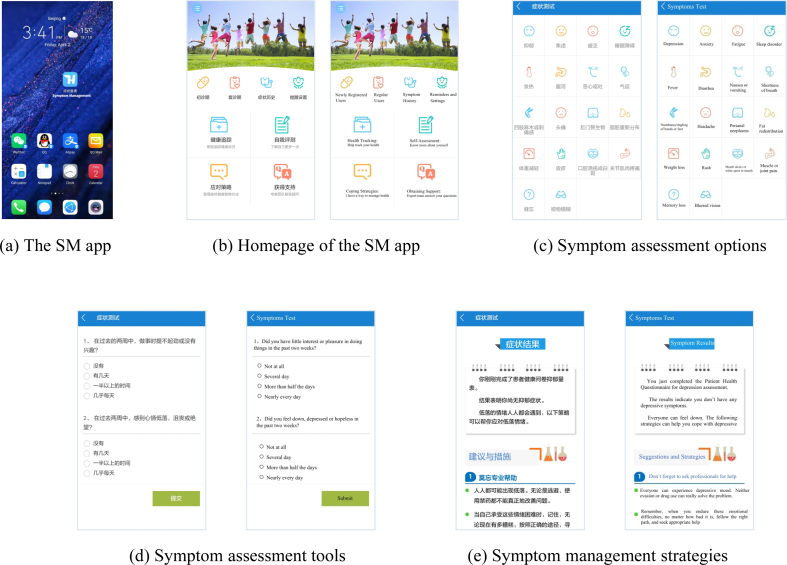
The SM app was developed by a multidisciplinary research team that includes physicians, clinical nurses, software engineers, nursing scientists, and nursing graduate students. This software was released in 2017, and was the first app that targeted symptom management for PLWH in China (Fig. 2 [a]). This app was applicable for both the iOS and Android systems, and has applied for a patent (National Copyright Administration of the People’s Republic of China: No.02700189) [28].
Fig. 2.
Screenshots of the SM app.
The design of the SM app was guided by the symptom management framework. As shown in the homepage (Fig. 2 [b]), the mobile terminal includes eight modules. Four major modules are Health Tracking, Self-Assessment, Coping Strategies, and Obtaining Support. Other modules with smaller logos are Newly Registered User, Regular User, Symptom History, and Reminders and Settings. The self-assessment module provides assessment tools for 18 common symptoms of PLWH (Fig. 2 [c]). Clicking one of the symptom buttons (such as depression), users can see the assessment tools as Fig. 1 (d). Users will receive personalized result interpretations and strategies (Fig. 1 [e]) according to their assessment submission and cutoffs. Appendix A presents all the brief self-reported tools and cutoffs for each symptom.
The symptom management framework also emphasizes on the clinical outcomes, social support, ART medication adherence, and QoL. Therefore, the SM app provides functions of tracking health indicators (such as viral load, CD4+ T-cell count, body temperature, body weight, pain, fatigue, and ART medication adherence) in the health tracking module; coping strategies that aim to improve medication adherence and QoL in the coping strategies module; and social support resources in the obtaining support module.
To increase the participants’ familiarity with the SM app and to promote their usage compliance, the participants in the intervention group were expected to attend all four tailored weekly group sessions. Each group session lasted for approximately 2 h and targeted one of the major modules in the SM app. Supported by Fudan University School of Nursing and the SPHC, a psychologist, a physician, a peer volunteer, and a head nurse were invited to implement the interventions. Each interventionist was responsible for one session. The designs of the topic and content for each session were based on the main target module of the SM app. Participants in the intervention group were invited to join a WeChat group before the first session (everyone joined voluntarily). A researcher edited notes after each group session and sent them out via the WeChat group or personally. Table 1 summarizes the content and details of the intervention sessions.
Table 1.
Content and details of the group sessions.
| Session 1 | Session 2 | Session 3 | Session 4 | |
|---|---|---|---|---|
| Topic | Mental distress and coping strategies | Interpretation for HIV disease knowledge and laboratory test report | Self-management experience sharing | How to use the medical resources provided by the hospital |
| Reporter | A psychologist with a PhD degree | A physician | A peer volunteer | A head nurse |
| Procedure | Lecture, Q&A, discussion | Lecture, Q&A, discussion, knowledge test | Lecture, Q&A, discussion | Lecture, Q&A, discussion |
| Target module | Self-assessment | Health tracking | Coping strategies | Obtaining support |
| Content | 1. Introduce the current state of mental health problems. 2. Take the mental symptom tests as an example to introduce the function of the self-assessment module. Explain the physical, mental, and social challenges that PLWH may face in their daily life. 3. Watch the videos on how to cope with depressive symptoms in the app. Participants share their experiences and coping strategies. 4. Explain and demonstrate the coping strategies for mental symptoms introduced in the app, such as cognitive behavioral therapy and relaxation training. |
1. Briefly introduce HIV and AIDS. Explain why PLWH should take laboratory tests regularly and what laboratory tests are necessary for PLWH. Explain the clinical implications and outliers for common indicators (blood routine test, urine routine test, liver function, kidney function, blood lipids, CD4+ T-cell count, viral load, medication resistance test). 2. Introduce and explain the function of the health tracking module. 3. A short quiz about the knowledge in the lecture is provided for participants on site. |
1. Share personal HIV diagnosis and disease treatment experience. 2. Introduce the function of the coping strategies module. 3. Discuss how to apply the coping strategies introduced in the app to daily self-management. |
1. Introduce the available medical resources and functions of each department in Shanghai Public Health Center affiliated with Fudan University for PLWH. Introduce the procedures of clinical visits and emergency visits, contact information, and how to deal with emergencies. Explain referral and consultation between different departments and different hospitals. 2. Introduce the function of the coping strategies module. 3. Discuss participants' needs for clinical visits and other ways to get support. |
Note: PLWH = persons living with HIV.
3.4. Measure
3.4.1. Demographic variables
We collected participants’ demographic information using a structured questionnaire. Descriptive information included age, sex, household register, race, etc. All the demographic information is presented in Table 2.
Table 2.
Baseline demographics.
| Characteristic | Full sample (n = 61) | Intervention group (n = 31) | Control group (n = 30) | χ2/Z/t | P |
|---|---|---|---|---|---|
| Age | 36.18 ± 8.51 (22–62) | 36.94 ± 8.45 (22–62) | 35.40 ± 8.64 (23–54) | 0.120 | 0.702a |
| Gender | – | 1.000b | |||
| Male | 60 (98.4) | 30 (96.8) | 30 (100.0) | ||
| Female | 1 (1.6) | 1 (3.2) | 0 (0.0) | ||
| Ethnic groups | – | 0.492b | |||
| Han | 59 (96.7) | 29 (93.5) | 30 (100.0) | ||
| Minority | 2 (3.3) | 2 (6.5) | 0 (0.0) | ||
| Educational attainment | 5.003 | 0.025c | |||
| College or less | 25 (41.0) | 17 (54.8) | 8 (26.7) | ||
| University or above | 36 (59.0) | 14 (45.2) | 22 (73.3) | ||
| Religion | 0.604 | 0.570c | |||
| No | 44 (72.1) | 21 (67.7) | 23 (76.7) | ||
| Yes | 17 (27.9) | 10 (32.3) | 7 (23.3) | ||
| Employment status | – | 1.000b | |||
| Employed | 54 (88.5) | 27 (87.1) | 27 (90.0) | ||
| Unemployed | 2 (3.3) | 1 (3.2) | 1 (3.3) | ||
| Sick leave at home | 2 (3.3) | 1 (3.2) | 1 (3.3) | ||
| Retired | 3 (4.9) | 2 (6.5) | 1 (3.3) | ||
| Household registry | 0.011 | 1.000c | |||
| Shanghai | 37 (60.7) | 19 (61.3) | 18 (60.0) | ||
| Non-shanghai | 24 (39.3) | 12 (38.7) | 12 (40.0) | ||
| Marital status | – | 0.266b | |||
| Single | 50 (82) | 24 (77.4) | 26 (86.7) | ||
| Unmarried with a stable partner | 5 (8.2) | 2 (6.5) | 3 (10.0) | ||
| Widowed | 6 (9.8) | 5 (16.1) | 1 (3.3) | ||
| Monthly income (CNY) | −1.500 | 0.134d | |||
| Less than 5,000 | 12 (19.7) | 9 (29.0) | 3 (10.0) | ||
| 5,000–10,000 | 26 (42.6) | 12 (38.7) | 14 (46.7) | ||
| More than 10,000 | 23 (37.7) | 10 (32.3) | 13 (43.3) | ||
| Months of HIV diagnosis | 25.00 (48.00) | 24.00 (56.00) | 28.50 (39.25) | −0.570 | 0.569d |
| Months of taking ART | 21.00 (33.50) | 16.00 (41.00) | 21.00 (27.75) | −0.368 | 0.713d |
| Latest CD4+ T-cell count (/μl) | −0.168 | 0.866d | |||
| Less than 300 | 19 (31.1) | 9 (29.0) | 10 (33.3) | ||
| 300–500 | 22 (36.1) | 13 (41.9) | 9 (30.0) | ||
| More than 500 | 20 (32.8) | 9 (29.0) | 11 (36.7) | ||
| Latest viral load | 0.137 | 0.772c | |||
| Undetectable | 46 (75.4) | 24 (77.4) | 22 (73.3) | ||
| Detectable | 15 (24.6) | 7 (22.6) | 8 (26.7) | ||
| ART prescription | – | 0.195b | |||
| Free ART | 56 (91.8) | 30 (96.8) | 26 (86.7) | ||
| TDF+3 TC + EFV | 33 (54.1) | 19 (61.3) | 14 (46.7) | ||
| AZT+3 TC + EFV | 7 (11.5) | 3 (9.7) | 4 (13.3) | ||
| ABC+3 TC + EFV | 5 (8.2) | 3 (9.7) | 2 (6.7) | ||
| TDF+3 TC + LPV/r | 3 (4.9) | 1 (3.2) | 2 (6.7) | ||
| AZT+3 TC + LPV/r | 4 (6.6) | 2 (6.5) | 2 (6.7) | ||
| ABC+3 TC + LPV/r | 2 (3.3) | 1 (3.2) | 1 (3.3) | ||
| AZT+3 TC + NVP | 1 (1.6) | 1 (3.2) | 0 (0) | ||
| ABC+3 TC + NVP | 1 (1.6) | 0 (0) | 1 (3.3) | ||
| Self-paying ART | 5 (8.2) | 1 (3.2) | 4 (13.3) | ||
| TDF+3 TC + DTG | 2 (3.3) | 0 (0) | 2 (6.7) | ||
| TDF+3 TC + RAL | 1 (1.6) | 0 (0) | 1 (3.3) | ||
| ABC+3 TC + DTG | 1 (1.6) | 0 (0) | 1 (3.3) | ||
| FTC/TDF + DTG | 1 (1.6) | 1 (3.2) | 0 (0) |
Note: Data are n (%), Median (IQR), or Mean ± SD (range). a independent sample t-test; b Fisher’s exact test; c chi-square test; d Wilcoxon rank-sum test. Free ART in China: TDF, 3TC, EFV, AZT, ABC, LPV/r,NVP. Self-paying ART in China: DTG, RAL, FTC/TDF. ART = antiretroviral therapy. TDF = Tenofovir disoproxil. 3TC = Lamivudine. EFV = Efavurebz. AZT = Zidovudine. ABC = Abacavir. LPV/r = Lopinavir/ritonavir. NVP = Nevirapine. DTG = Dolutegravir. RAL = Raltegravir. FTC/TDF = Emtricitabine/Tenofovir disoproxil.
3.4.2. Symptoms
Our symptom checklist was consistent with the symptom pool in the SM app. All the symptoms in the app were adopted from a modified sign and symptom checklist for HIV (SSC–HIV rev) [31] and self-completed HIV symptom index [32]. These two tools are widely used, reflecting common symptoms of PLWH, and have been applied to Chinese PLWH [17,33]. Each symptom was assessed in terms of symptom severity (range from 0 to 3), symptom distress (range from 0 to 3), knowledge of symptom reasons (range from 1 to 5), knowledge of symptom self-management (range from 1 to 5), and certainty of symptom self-management (range from 1 to 5) during the past 4 weeks.
3.4.3. Quality of life (QoL)
QoL was assessed using the Cantril Ladder instrument [34]. This assessment tool provided a picture of a ladder numbered from 0 to 10. The top of the ladder indicated the best possible QoL and the bottom of the ladder represented the worst possible QoL that participants could imagine. Participants scored their QoL on the ladder based on their conditions in the past four weeks.
3.4.4. Medication adherence
The visual analog scale (VAS) [35] was used to assess ART medication adherence. Participants were asked to mark a linear scale to indicate their adherence (ranging from 0 % to 100 %) in the past four weeks.
3.4.5. Perceived social support
One item was applied to measure perceived social support. “I feel I can get help and support in disease management.” The participants’ responses were recorded from 1 (strongly disagree) to 7 (strongly agree).
3.5. Sample size
Previous studies showed mHealth apps could greatly improve participants’ health knowledge [36,37]. Although our study is the first attempt to test the effects of SMI based on a mHealth app on the symptom management knowledge among PLWH, we anticipated a large effect size (Cohen’s d = 0.8) in the sample size calculation. We defined the power as 80 % and the statistical significance as 0.05. Considering an attrition rate of 15 %, we assigned the final sample size as 60, with at least 30 participants in each group.
3.6. Randomization
The SM app was released before the recruitment. We performed randomization after the recruitment. Participants registered their contact information (telephone number and WeChat ID) after consented to participate in our study. Researchers gave a brief introduction of the SM app to all participants. Participants could download the SM app according to their needs and preferences in site. After we completed the recruitment, one research staff who was blind in the recruitment used WPS Office software to generate the random sequence and sort the random numbers by size. The participants were randomly assigned to either the control group or the intervention group.
3.7. Allocation concealment
Participants in the intervention group were informed to participate in the group sessions, but not the assignment. Participants in the control group did not receive any message and did not know that group sessions were part of the intervention.
3.8. Blinding
Interventionists were not blinded because they were disclosed the research purpose, and they provided in-person intervention for participants. A clinical nurse who did not participate in the intervention collected data after the intervention. Participants completed online questionnaires via WeChat or the SM app, which took them 10–15 min to complete. Clinical information was collected from participants’ medical records, including length of time since the first diagnosis of HIV, length of ART, CD4+ T-cell count, viral load, and ART prescriptions.
3.9. Data analysis
Our original data were input into Stata 13.0 (Stata Crop, College Station, Texas, USA). Data restoration was double-checked by two researchers. The intent-to-treat (ITT) strategy was used to analyze the data. We applied the baseline observation carried forward (BOCF) strategy [38] to fill out the missing data after the intervention. All data from the 61 participants were included in the statistical analysis. For descriptive analyses, the continuous variables were summarized using the mean and standard deviation (SD), or median and Inter Quartile Range (IQR). The categorical variables were summarized using frequency (n) and percentage (%). All continuous variables were checked for normality (skewness < 3.0, kurtosis <8.0) [39]. Both parametric and non-parametric statistical methods, including independent sample t-test, Chi-square test, Fisher’s exact test and Wilcoxon rank-sum test were applied to compare the baseline characteristics between groups. Linear mixed models and generalized linear mixed models (negative binomial model) were conducted to test the intervention effects for continuous outcomes with and without normality, respectively. Covariates for mixed models included age, educational attainment, monthly income, and CD4+ T-cell count.
4. Results
4.1. Participant flow
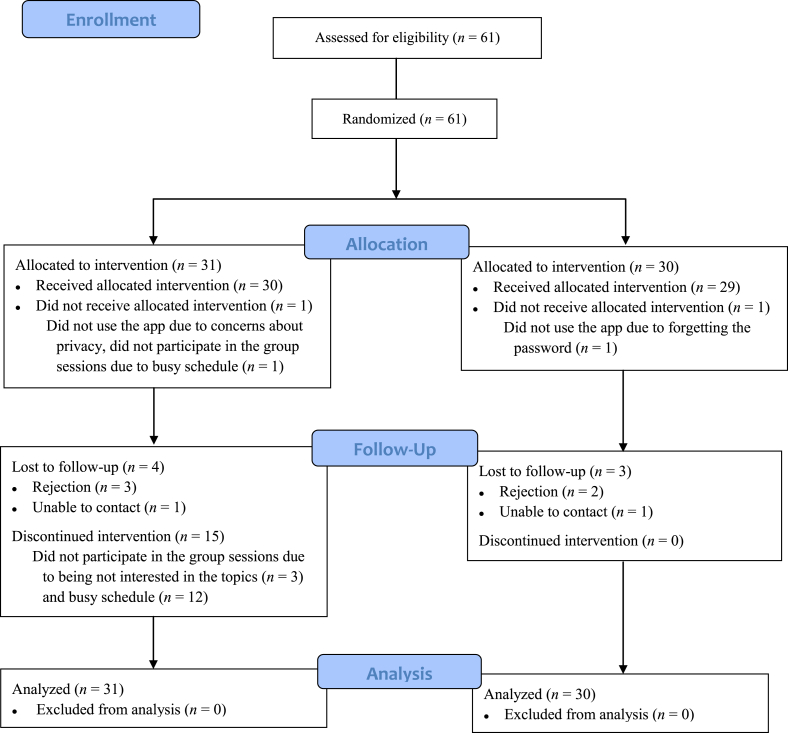
The recruitment was conducted between December 2017 and January 2018 in the SPHC outpatient center. A total of 61 participants were successfully recruited and randomly assigned to either the intervention or the control group. The intervention was implemented from January 2018 to February 2018. Seven participants dropped out after the intervention with reasons of rejection and loss of contact. After filling out the missing data based on the BOCF strategies, all the participants were included in the data analysis. The enrollment process is depicted in Fig. 3.
Fig. 3.
Enrollment summary diagram.
4.2. Participant characteristics
Table 2 describes the demographic characteristics of the participants. All the demographic variables were comparable between the two groups, except the educational attainment: participants in the intervention group had higher levels of education (P = 0.025). Of the 31 participants in the intervention group, 16 participated in symptom management group sessions and 15 did not. Participants who participated in group sessions did not show a difference in demographic characteristics with participants who did not participate in group sessions (Appendix B Table 1).
Table 3 depicts the prevalence of symptom severity and symptom distress at baseline. Fatigue, sleep problems, and difficulty remembering were the top three most common symptoms. Aside from several prevalent physical and cognitive symptoms, mental symptoms also exhibited a high prevalence. More than half of the participants reported that they had anxious symptoms or depressive symptoms. Although the prevalence of symptom distress was generally lower than symptom severity, these common symptoms also had a high prevalence of symptom distress. Approximately half of the participants considered fatigue, sleep problems, difficulty remembering, anxious symptoms and depressive symptoms as one of their symptom distresses. The majority of symptom severity and symptom distress were comparable between the two groups.
Table 3.
Frequency of symptoms at baseline.
| Symptom | Total sample (n = 61) | Intervention group (n = 31) | Control group (n = 30) | χ2 | P |
|---|---|---|---|---|---|
| Symptom severity | |||||
| Fatigue | 38 (62.3) | 18 (58.1) | 20 (66.7) | 0.480 | 0.488 |
| Dizziness | 24 (39.3) | 8 (25.8) | 16 (53.3) | 4.841 | 0.028 |
| Headache | 14 (23.0) | 8 (25.8) | 6 (20.0) | 0.291 | 0.590 |
| Fever | 10 (16.4) | 4 (12.9) | 6 (20.0) | – | 0.508a |
| Difficulty remembering | 37 (60.7) | 17 (54.8) | 20 (66.7) | 0.894 | 0.344 |
| Difficulty falling or staying asleep | 38 (62.3) | 18 (58.1) | 20 (66.7) | 0.480 | 0.488 |
| Blurred vision | 23 (37.7) | 10 (32.3) | 13 (43.3) | 0.796 | 0.372 |
| Rash, Itchy skin | 20 (32.8) | 8 (25.8) | 12 (40.0) | 1.394 | 0.238 |
| Ulcers or white spots in mouth | 20 (32.8) | 10 (32.3) | 10 (33.3) | 0.008 | 0.929 |
| Muscle and joint pain | 28 (45.9) | 13 (41.9) | 15 (50.0) | 0.399 | 0.527 |
| Numbness/tingling of hands or feet | 18 (29.5) | 9 (29.0) | 9 (30.0) | 0.007 | 0.934 |
| Decreased appetite | 14 (23.0) | 6 (19.4) | 8 (26.7) | 0.461 | 0.497 |
| Diarrhea | 31 (50.8) | 14 (45.2) | 17 (56.7) | 0.807 | 0.369 |
| Nausea or vomiting | 20 (32.8) | 7 (22.6) | 13 (43.3) | 2.980 | 0.084 |
| Fat redistribution | 18 (29.5) | 7 (22.6) | 11 (36.7) | 1.454 | 0.228 |
| Shortness of breath | 15 (24.6) | 8 (25.8) | 7 (23.3) | 0.050 | 0.823 |
| Perianal neoplasms | 12 (19.7) | 7 (22.6) | 5 (16.7) | 0.337 | 0.561 |
| Anxiety | 33 (54.1) | 12 (38.7) | 21 (70.0) | 6.011 | 0.014 |
| Depression | 36 (59.0) | 14 (45.2) | 22 (73.3) | 5.003 | 0.025 |
| Symptom distress | |||||
| Fatigue | 34 (55.7) | 15 (48.4) | 19 (63.3) | 1.380 | 0.240 |
| Dizziness | 21 (34.4) | 7 (22.6) | 14 (46.7) | 3.918 | 0.048 |
| Headache | 13 (21.3) | 7 (22.6) | 6 (20.0) | 0.061 | 0.806 |
| Fever | 9 (14.8) | 4 (12.9) | 5 (16.7) | – | 0.731a |
| Difficulty remembering | 33 (54.1) | 15 (48.4) | 18 (60.0) | 0.828 | 0.363 |
| Difficulty falling or staying asleep | 32 (52.5) | 15 (48.4) | 17 (56.7) | 0.419 | 0.517 |
| Blurred vision | 22 (36.1) | 9 (29.0) | 13 (43.3) | 1.352 | 0.245 |
| Rash, Itchy skin | 19 (31.1) | 8 (25.8) | 11 (36.7) | 0.838 | 0.360 |
| Ulcers or white spots in mouth | 15 (24.6) | 7 (22.6) | 8 (26.7) | 0.137 | 0.711 |
| Muscle and joint pain | 23 (37.7) | 11 (35.5) | 12 (40.0) | 0.132 | 0.716 |
| Numbness/tingling of hands or feet | 16 (26.2) | 8 (25.8) | 8 (26.7) | 0.006 | 0.939 |
| Decreased appetite | 13 (21.3) | 6 (19.4) | 7 (23.3) | 0.144 | 0.704 |
| Diarrhea | 28 (45.9) | 12 (38.7) | 16 (53.3) | 1.313 | 0.252 |
| Nausea or vomiting | 18 (29.5) | 6 (19.4) | 12 (40.0) | 3.124 | 0.077 |
| Fat redistribution | 18 (29.5) | 7 (22.6) | 11 (36.7) | 1.454 | 0.228 |
| Shortness of breath | 13 (21.3) | 7 (22.6) | 6 (20.0) | 0.061 | 0.806 |
| Perianal neoplasms | 10 (16.4) | 5 (16.1) | 5 (16.7) | – | 1.000a |
| Anxiety | 30 (49.2) | 11 (35.5) | 19 (63.3) | 4.731 | 0.030 |
| Depression | 35 (57.4) | 13 (41.9) | 22 (73.3) | 6.146 | 0.013 |
Note: Data are n (%). a Fisher’s exact test
4.3. Intervention effects
As demonstrated in Table 4, participants in the intervention group significantly improved their knowledge of symptom reasons and symptom self-management than the participants in the control group after the intervention (P < 0.01). Compared with the control group, the intervention group showed more significant improvement, 11.47 points (95% CI: 3.41, 19.53) in the score of symptom reasons knowledge and 12.80 points (95% CI: 4.55, 21.05) in the score of symptom self-management knowledge after controlling for covariates. Intervention effects were also significant on the certainty of symptom self-management (P < 0.01), which indicated that the SMI could also help PLWH improve their self-efficacy of symptom management. Moreover, participants who participated in group sessions in the intervention group (n = 16) showed more significant improvement after intervention compared with those who did not attend (n = 15), knowledge of symptom reasons (Z = −2.109, P = 0.035), knowledge of symptom measures (Z = −2.445, P = 0.014), and symptom certainty (Z = −2.674, P = 0.007) (Appendix B Table 2). Results of the mixed models showed that there were no statistically significant differences between the intervention and control groups in other outcomes, including symptom severity, symptom distress, ART medication adherence, and perceived social support.
Table 4.
Intervention effects on outcomes.
| Outcomes | Baseline |
After intervention |
Difference between groups |
||||
|---|---|---|---|---|---|---|---|
| Intervention group (n = 31) | Control group (n = 30) | Intervention group (n = 31) | Control group (n = 30) | Estimate | Standard error | P | |
| Knowledge of symptom reasons | 56.00 (28.00) | 45.50 (27.00) | 52.00 (30.00) | 44.50 (24.50) | 11.472 | 4.113 | 0.005 a |
| Knowledge of symptom measures | 54.00 (29.00) | 41.00 (24.00) | 54.00 (29.00) | 44.00 (25.75) | 12.803 | 4.210 | 0.002 a |
| Symptom certainty | 55.00 (30.00) | 41.50 (22.25) | 54.00 (30.00) | 44.00 (21.00) | 11.946 | 4.278 | 0.005 a |
| Number of symptoms | 4.00 (9.00) | 8.00 (7.50) | 5.00 (7.00) | 8.00 (7.00) | −1.811 | 1.160 | 0.119 a |
| Overall symptom severity | 5.00 (15.00) | 11.50 (12.25) | 6.00 (11.00) | 12.00 (12.25) | −2.898 | 2.065 | 0.161 a |
| Number of distress symptoms | 3.00 (8.00) | 7.50 (7.25) | 3.00 (8.00) | 6.00 (9.00) | −1.521 | 1.189 | 0.201 a |
| Overall symptom distress | 4.00 (14.00) | 10.00 (12.25) | 4.00 (12.00) | 10.00 (15.25) | −1.952 | 2.342 | 0.405 a |
| ART medication adherence | 100.00 (1.00) | 100.00 (0.00) | 100.00 (2.00) | 100.00 (1.00) | −6.999 | 4.281 | 0.102 a |
| Perceived social support | 4.48 ± 1.46 (2–7) | 5.13 ± 1.50 (3–7) | 5.15 ± 1.35 (1–7) | 5.04 ± 1.32 (1–7) | −0.360 | 0.280 | 0.199 b |
| Quality of life | 7.32 ± 2.17 (0–10) | 6.87 ± 2.13 (0–10) | 7.96 ± 1.74 (2–10) | 6.93 ± 1.80 (1–10) | 0.670 | 0.369 | 0.069 b |
Note: Data are Median (IQR), or Mean ± SD (range). a generalized linear mixed model; b linear mixed model.
5. Discussion
To our knowledge, this is the first known attempt in China to provide SMIs based on symptom management group sessions combined with a mHealth app for PWLH. Results of this study showed that a four-week-long SMI could significantly improve PLWH’s symptom management knowledge and self-efficacy of symptom self-management. However, the effects on the symptom severity, symptom distress, ART medication adherence, perceived social support, and QoL were uncertain.
Previous studies among other populations have confirmed the effects of mHealth apps on improving participants’ self-management knowledge [36,37,40]. In our study, we assessed symptom management knowledge in terms of symptom reasons and symptom self-management. Both dimensions showed significant differences. The SM app provided a reliable, evidence-based symptom management resource platform to help PLWH improve health knowledge. Besides, the group sessions also encouraged them to learn more about the functions of the app, discuss their questions and confusions.
The improvement in symptom certainty in the intervention group indicates that the SMI will help PLWH deal with current symptoms and cope with other symptoms that may occur in the future. We consider this result mainly due to the improvement of symptom management knowledge. PLWH may only pay attention to the current or distressing symptoms. They might not be aware of other functions and knowledge in the app before encountering problems. Group sessions could help participants become more aware that symptom management is a long-term and dynamic task. They can look for relevant information on the SM app in the future if needed. It is also necessary to learn about other relevant knowledge to deal with other potential risks and problems. Therefore, participating in group sessions is helpful for improving the use of the SM app function in full capacity.
Multiple intervention strategies have been proposed for HIV symptom management. However, their intervention effects on symptom severity and/or symptom distress were also inconsistent [21,23,27]. Particularly, Schnall et al. developed the mobile Video Information Provider (mVIP) app in the United States of America to provide PLWH with symptom management strategies for 13 symptoms, which is similar to our intervention to some extend [27]. Their 12-week intervention indicated of the 13 symptoms, five symptoms showed significant improvement in the intervention group [27]. Webel reported that a seven-week peer-based symptom management intervention for PLWH did not show significant effects on symptom intensity [23]. The direct effects of our SMI on symptom management among our participants were not significant. We speculate that several reasons may explain the results. First, the SM app is a tool to provide information and support for PLWH, but not a direct service. Although the app provides evidence-based knowledge, it requires PLWH to put it into practice. Second, our intervention only lasted four weeks, which may not be long enough to help PLWH ameliorate their symptoms. Third, the relatively small sample may not have sufficient power to detect a statistical significance for these secondary outcomes.
The effects of our SMI on ART medication adherence, perceived social support, and QoL are also uncertain in our study, which are consistent with previous HIV symptom management studies [21,23]. Given we used brief self-report tools to assess ART medication adherence, perceived social support, and QoL, these brief reporting measures may not be sensitive enough to show changes. Schnall et al. [27] also did not verify the effects of the mVIP app on ART medication adherence among PLWH via VAS. However, they showed significant improvement in ART medication adherence via the CASE Adherence Index, which had more items than VAS. Therefore, future studies should employ other more sensitive tools in assessing the effects of ART medication adherence. Besides, similar to many mHealth apps, our app only provides informational support [41], which is also an important reason that tested insignificant effects on perceived social support.
It is worth noting that more than half of the participants in the intervention group did not participate in any group sessions. We think it may be associated with the following factors. First, the intervention was conducted between January and February, and the cold weather might have discouraged participants from participating in the in-person sessions. Second, the group sessions were only conducted in the SPHC. As the only data collection site in Shanghai, it may be inconvenient for some of the participants to travel to attend group sessions. Third, reasons reported by participants for not being able to participate in group sessions included not being interested in the topics and busy schedules. We believe that participants also lack sufficient willingness to participate in group sessions. Although apps for PLWH only account for a minimal percentage of all health-related apps, existing HIV apps have generally failed to attract the attention of target audiences [42]. The design of the topics and contents at group sessions are based on the main modules from SM app. That may also be one of the important reasons for discouraging participants from attending group sessions. This phenomenon highlights the advantages and necessity of online intervention. Additionally, it also reminds researchers and practitioners of the challenges of generalizing and promoting the usage of the SM app. More innovative strategies are needed in the future to help maintain sustainability and promote the usage of the SM app. Moreover, future research is needed to learn about PLWH's experience with SMI. The SM app will continue to revise and update according to the user’s feedback.
This study has several limitations. First, only PLWH residing in Shanghai were recruited. As a single-center study, participants in our study are unable to represent PLWH as a whole in China. Second, we did not conduct follow-up after the intervention, which could not test the intervention effects for a longer-term. Third, the SM app does not have the function of recording users’ operation information (frequency, module, time length, etc.). Therefore, we did not have usability outcomes in the study.
6. Conclusion
Our study demonstrates that an SMI based on symptom management group sessions combined with a mHealth app can improve PLWH’s symptom management knowledge and certainty of symptom self-management within four weeks. Multi-center studies with larger sample sizes and longer follow-ups are needed to further understand the effects of the SM app on ameliorating symptom severity and symptom distress. More innovative strategies are warranted to promote and maintain the sustainability of the SM app.
Funding
This paper is part of a project funded by the National Natural Science Foundation of China (Grant Number 71673057) and the China Scholarship Council (No. 201906100135).
CRediT authorship contribution statement
Shuyu Han: Writing – original draft, Software, Validation, Visualization. Yaolin Pei: Writing – review & editing, Formal analysis. Rui Zhao: Writing – original draft, Project administration, Data curation, Formal analysis, Investigation. Yan Hu: Funding acquisition, Conceptualization, Methodology, Writing – review & editing. Lin Zhang: Resources, Writing – review & editing. Xiang Qi: Writing – review & editing, Software. Zheng Zhu: Supervision, Funding acquisition, Writing – review & editing. Wenxiu Sun: Resources, Project administration, Writing – review & editing. Bei Wu: Writing – review & editing, Conceptualization.
Declaration of competing interest
None to declare.
Acknowledgments
The authors would like to appreciate all the staff who helped us complete this project: Hongzhou Lu, Yinzhong Shen, Renfang Zhang, Jiangrong Wang, physicians at the Shanghai Public Health Center Affiliated with Fudan University; Meijuan Bao, Lijun Zha, Yanjuan Gan, nurses at the Shanghai Public Health Center Affiliated with Fudan University; Mingcheng Zhu, software engineer at the Shanghai Public Health Center Affiliated with Fudan University.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2021.07.002.
Contributor Information
Shuyu Han, Email: 18111170001@fuan.edu.cn.
Yaolin Pei, Email: yp22@nyu.edu.
Rui Zhao, Email: zhruicc@foxmail.com.
Yan Hu, Email: huyan@fudan.edu.cn.
Lin Zhang, Email: zhanglin@shphc.org.cn.
Xiang Qi, Email: xq450@nyu.edu.
Zheng Zhu, Email: 273183612@qq.com.
Wenxiu Sun, Email: sunwenxiu@shphc.org.cn.
Bei Wu, Email: bei.wu@nyu.edu.
Appendices. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Yoshimura K. Current status of HIV/AIDS in the ART era. J Infect Chemother. 2017;23(1):12–16. doi: 10.1016/j.jiac.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Román E., Chou F.Y. Development of a Spanish HIV/AIDS symptom management guidebook. J Transcult Nurs. 2011;22(3):235–239. doi: 10.1177/1043659611404425. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z., Hu Y., Li H.W., Bao M.J., Zhang L., Zha L.J. The implementation and evaluation of HIV symptom management guidelines:A preliminary study in China. Int J Nurs Sci. 2018;5(4):315–321. doi: 10.1016/j.ijnss.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasi M., De Biasi S., Gibellini L., Bianchini E., Pecorini S., Bacca V. Ageing and inflammation in patients with HIV infection. Clin Exp Immunol. 2017;187(1):44–52. doi: 10.1111/cei.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W.T., Shiu C., Yang J.P., Tun M.M.M., Zhang L., Wang K. Tobacco use and HIV symptom severity in Chinese people living with HIV. AIDS Care. 2020;32(2):217–222. doi: 10.1080/09540121.2019.1620169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson B., Vincent W., Meyer J.P., Kershaw T., Sikkema K.J., Heckman T.G. Depressive symptoms,physical symptoms,and health-related quality of life among older adults with HIV. Qual Life Res. 2019;28(12):3313–3322. doi: 10.1007/s11136-019-02271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K.A., Gay C., Portillo C.J., Coggins T., Davis H., Pullinger C.R. Symptom experience in HIV-infected adults:a function of demographic and clinical characteristics. J Pain Symptom Manag. 2009;38(6):882–893. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao Y.C., Xu Y., Jiang D.X., Wang X., Wang F., Yang J. Epidemiological analyses of regional and age differences of HIV/AIDS prevalence in China,2004-2016. Int J Infect Dis. 2019;81:215–220. doi: 10.1016/j.ijid.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Huang M.B., Ye L., Liang B.Y., Ning C.Y., Roth W.W., Jiang J.J. Characterizing the hiv/aids epidemic in the United States and China. Int J Environ Res Publ Health. 2015;13(1) doi: 10.3390/ijerph13010030. ijerph13010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L., Ji G.P., Lin C.Q., Liang L.J., Lan C.W. Antiretroviral therapy initiation following policy changes:Observations from China. Asia Pac J Publ Health. 2016;28(5):416–422. doi: 10.1177/1010539516650721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Günthard H.F., Saag M.S., Benson C.A., del Rio C., Eron J.J., Gallant J.E. Antiretroviral drugs for treatment and prevention of HIV infection in adults:2016 recommendations of the international antiviral society-USA panel. J Am Med Assoc. 2016;316(2):191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Professional Group - Society of Infectious Disease - Chinese Medical Association, Chinese Center for Disease Control and Prevention Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018) Chin J Intern Med. 2018;57(12):1–18. doi: 10.3760/cma.j.issn.0578-1426.2018.12. [In Chinese] [DOI] [Google Scholar]
- 13.Zang C., Guida J., Sun Y., Liu H. Collectivism culture,HIV stigma and social network support in Anhui,China:a path analytic model. AIDS Patient Care STDS. 2014;28(8):452–458. doi: 10.1089/apc.2014.0015. [DOI] [PubMed] [Google Scholar]
- 14.Xie T., Yang J.P., Simoni J.M., Shiu C.S., Chen W.T., Zhao H. Unable to be a human being in front of other people: a qualitative study of self-isolation among people living with HIV/AIDS in China. J Clin Psychol Med Settings. 2017;24(3–4):211–222. doi: 10.1007/s10880-017-9513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spirig R., Moody K., Battegay M., de Geest S. Symptom management in HIV/AIDS. Adv Nurs Sci. 2005;28(4):333–344. doi: 10.1097/00012272-200510000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Zhao R., Hu Y., Zhu Z., Han S. Research progress on symptom management in HIV positive patients. J Nurs Sci. 2018;33(7):93–96. doi: 10.3870/j.issn.1001-4152.2018.07.093. [In Chinese] [DOI] [Google Scholar]
- 17.Zhu Z., Zhao R., Hu Y. Symptom clusters in people living with HIV:A systematic review. J Pain Symptom Manag. 2019;58(1):115–133. doi: 10.1016/j.jpainsymman.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Voss J., Portillo C.J., Holzemer W.L., Dodd M.J. Symptom cluster of fatigue and depression in HIV/AIDS. J Prev Interv Community. 2007;33(1–2):19–34. doi: 10.1300/j005v33n01_03. [DOI] [PubMed] [Google Scholar]
- 19.Fisher J.D., Fisher W.A., Amico K.R., Harman J.J. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 20.Rao D., Nainis N., Williams L., Langner D., Eisin A., Paice J. Art therapy for relief of symptoms associated with HIV/AIDS. AIDS Care. 2009;21(1):64–69. doi: 10.1080/09540120802068795. [DOI] [PubMed] [Google Scholar]
- 21.Webel A., Prince-Paul M., Ganocy S., DiFranco E., Wellman C., Avery A. Randomized clinical trial of a community navigation intervention to improve well-being in persons living with HIV and other comorbidities. AIDS Care. 2019;31(5):529–535. doi: 10.1080/09540121.2018.1546819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webel A.R., Willig A.L., Liu W., Sattar A., Boswell S., Crane H.M. Physical activity intensity is associated with symptom distress in the CNICS cohort. AIDS Behav. 2019;23(3):627–635. doi: 10.1007/s10461-018-2319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webel A.R. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22(9):1029–1040. doi: 10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wantland D.J., Holzemer W.L., Moezzi S., Willard S.S., Arudo J., Kirksey K.M. A randomized controlled trial testing the efficacy of an HIV/AIDS symptom management manual. J Pain Symptom Manag. 2008;36(3):235–246. doi: 10.1016/j.jpainsymman.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Chiou P.Y., Kuo B.I.T., Lee M.B., Chen Y.M., Chuang P., Lin L.C. A programme of symptom management for improving quality of life and drug adherence in AIDS/HIV patients. J Adv Nurs. 2006;55(2):169–179. doi: 10.1111/j.1365-2648.2006.03902.x. [DOI] [PubMed] [Google Scholar]
- 26.Miles M.S., Holditch-Davis D., Eron J., Black B.P., Pedersen C., Harris D.A. An HIV self-care symptom management intervention for African American mothers. Nurs Res. 2003;52(6):350–360. doi: 10.1097/00006199-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Schnall R., Cho H., Mangone A., Pichon A., Jia H. Mobile health technology for improving symptom management in low income persons living with HIV. AIDS Behav. 2018;22(10):3373–3383. doi: 10.1007/s10461-017-2014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S.Y., Pei Y.L., Wang L.N., Hu Y., Qi X., Zhao R. The development of a personalized symptom management mobile health application for persons living with HIV in China. J Personalized Med. 2021;11(5):346. doi: 10.3390/jpm11050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodd M., Janson S., Facione N., Faucett J., Froelicher E.S., Humphreys J. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 30.Schulz K.F., Altman D.G., Moher D., Group F.T.C. CONSORT 2010 Statement:updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1) doi: 10.1136/bmj.c332. c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzemer W.L., Hudson A., Kirksey K.M., Jane Hamilton M., Bakken S. The revised sign and symptom checklist for HIV (SSC-HIVrev) J Assoc Nurses AIDS Care. 2001;12(5):60–70. doi: 10.1016/S1055-3290(06)60263-X. [DOI] [PubMed] [Google Scholar]
- 32.Justice A.C., Holmes W., Gifford A.L., Rabeneck L., Zackin R., Sinclair G. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–S90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z., Hu Y., Guo M., Williams A.B. Urban and rural differences:unmet needs for symptom management in people living with HIV in China. J Assoc Nurses AIDS Care. 2019;30(2):206–217. doi: 10.1097/jnc.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 34.Cantril H. Rutgers University Press; New Brunswick: 1965. The pattern of human concerns. [Google Scholar]
- 35.Giordano T.P., Guzman D., Clark R., Charlebois E.D., Bangsberg D.R. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5(2):74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- 36.Anderson L.M., Leonard S., Jonassaint J., Lunyera J., Bonner M., Shah N. Mobile health intervention for youth with sickle cell disease:impact on adherence,disease knowledge,and quality of life. Pediatr Blood Canc. 2018;65(8) doi: 10.1002/pbc.27081. e27081. [DOI] [PubMed] [Google Scholar]
- 37.Kang Y.N., Shen H.N., Lin C.Y., Elwyn G., Huang S.C., Wu T.F. Does a Mobile app improve patients' knowledge of stroke risk factors and health-related quality of life in patients with stroke? A randomized controlled trial. BMC Med Inf Decis Making. 2019;19(1):282. doi: 10.1186/s12911-019-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao J., Jordan D.C., Pritchett Y.L. Baseline observation carry forward:reasoning,properties,and practical issues. J Biopharm Stat. 2009;19(4):672–684. doi: 10.1080/10543400902964118. [DOI] [PubMed] [Google Scholar]
- 39.Hildreth L.A. Principles and practice of structural equation modeling. third ed. 2012. [Google Scholar]
- 40.Heikkilä, Lehtovirta, Autio Fogelholm, Valve The impact of nutrition education intervention with and without a mobile phone application on nutrition knowledge among young endurance athletes. Nutrients. 2019;11(9):2249. doi: 10.3390/nu11092249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler-Ruwisch J.M., Roess A., Robert R.C., Napolitano M.A., Chiang S. Social support for breastfeeding in the era of mHealth:A content analysis. J Hum Lactation. 2018;34(3):543–555. doi: 10.1177/0890334418773302. [DOI] [PubMed] [Google Scholar]
- 42.Muessig K.E., Pike E.C., Legrand S., Hightow-Weidman L.B. Mobile phone applications for the care and prevention of HIV and other sexually transmitted diseases:A review. J Med Internet Res. 2013;15(1):e1. doi: 10.2196/jmir.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.