Abstract
This case series reports six patients (four men and two women; median age, 38 years; interquartile range, 26–48 years) who presented with vaccine-induced thrombocytopenia and thrombosis beginning 3–26 days after receiving the first dose of the ChAdOx1 nCoV-19 (AstraZeneca) vaccine for COVID-19. The patients were admitted to a general hospital between 9 and 31 days after the first dose. All patients had strongly detected antiplatelet factor 4 antibodies and severe thrombosis. Laboratory features included thrombocytopenia and elevated d-dimer levels. Thrombotic events were predominantly venous; two patients had arterial or mixed arterial and venous thrombosis. All patients recovered after receiving intravenous immunoglobulin and nonheparin-based anticoagulation.
© RSNA, 2021
An earlier incorrect version appeared online. This article was corrected on August 18, 2021.
Summary
Vaccine-induced thrombotic thrombocytopenia rarely complicates ChAdOx1 nCoV-19 (AstraZeneca) vaccination and presents with extensive thrombosis, blood clots at atypical sites, asymptomatic thrombus, thrombocytopenia, and raised d-dimer levels.
Introduction
This case series demonstrates rare thromboembolic events and thrombocytopenia after receiving the first dose of the ChAdOx1 nCoV-19 (AstraZeneca) vaccine. To our knowledge, no thromboembolic events have been found in randomized safety studies of the AstraZeneca vaccine (1,2).
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare syndrome of immune-driven thrombosis and thrombocytopenia, which typically presents 5–28 days after vaccination. At present, there is no clear indication of risk factors, although younger age has been suggested. Clinical features include thrombocytopenia, high d-dimer levels, positive antiplatelet factor 4 (PF4) antibodies, and thrombotic events (3,4).
Detected anti-PF4 antibodies on heparin-induced thrombocytopenia (HIT) enzyme-linked immunosorbent assay of the immunoglobulin G subclass can recognize PF4-platelet neoantigens. They evoke a pronounced immune response, leading to thrombosis by platelet activation, and are heparin independent in contrast to HIT. Reported sites of thromboembolism are atypical. They include venous, arterial, intracranial, and abdominal sites (5), which is more akin to patients with myeloproliferative disorders or paroxysmal nocturnal hemoglobinuria. This hospital-based case series highlights imaging and hematologic findings in VITT.
Materials and Methods
Waiving ethical approval, this is a retrospective single-center study of consecutive patients admitted to a large district general hospital (Queen Alexandra Hospital, Portsmouth, England), with VITT between March 2021 and May 2021. Enzyme-linked immunosorbent assay (PF4 IgG, Immucor GTI Diagnostics) was used to help detect anti-PF4 antibodies; an optical density greater than 0.4 was the cutoff for a positive HIT test result. Arterial and venous thromboses were depicted with CT, MRI, and abdominal US.
Results
Patient Characteristics
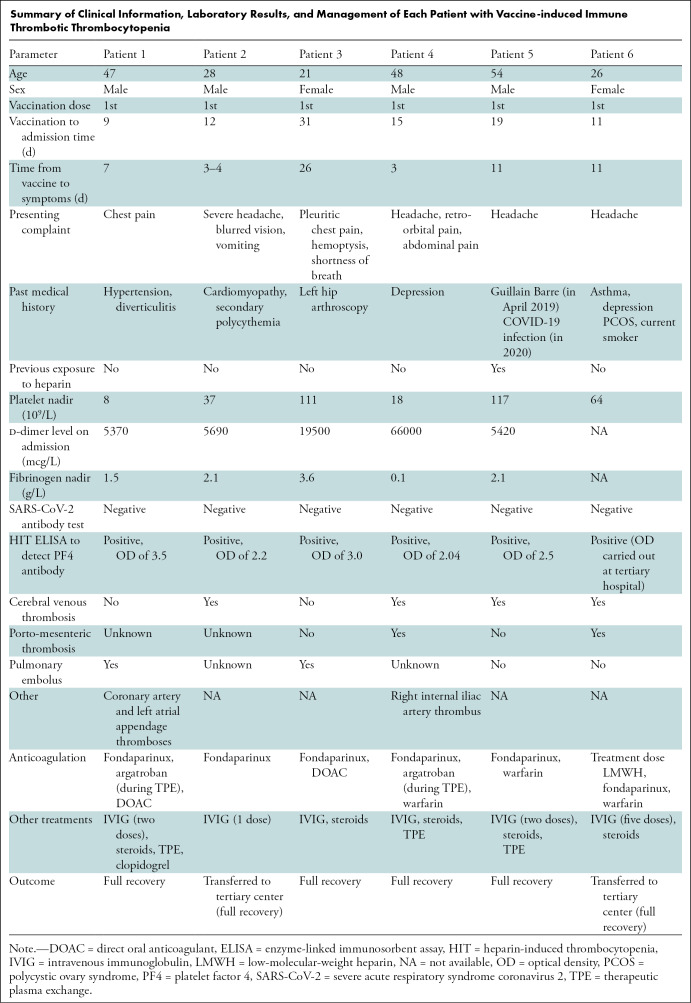
Six patients (four men; median age, 38 years; interquartile range [IQR], 26–48 years]) were admitted following vaccination with thrombocytopenia. Four patients had cerebral venous thrombosis, two had pulmonary emboli, one had portomesenteric thrombosis, one also had pelvic arterial thrombosis, and another developed coronary artery thrombosis. Two patients were transferred to a tertiary center, and one required intensive care. Clinical information, laboratory results, and treatment are summarized in the Table.
Summary of Clinical Information, Laboratory Results, and Management of Each Patient with Vaccine-induced Immune Thrombotic Thrombocytopenia
All patients were admitted between 9 and 31 days following the first vaccine dose with symptoms developing 3–26 days after inoculation. One patient was taking the oral contraceptive pill, and another had a history of secondary polycythemia. All patients continued to improve on 1-month follow-up. Treatment included nonheparin-based anticoagulation, steroids, intravenous immunoglobulin, and therapeutic plasma exchange.
Laboratory Testing
Nadir platelet count ranged from 8–117 × 109 per liter, with a median value of 50 × 109 per liter (n = 6 [IQR, 18–111 × 109]). The d-dimer level was elevated in all patients (median, 5690 mcg/liter; n = 5 [IQR, 5395–42750 mcg/liter]). Activated partial thromboplastin time and the international normalized ratio were normal in all patients. Fibrinogen level was very low (0.1 g/L) in patient 4 leading to cryoprecipitate support (median value, 2.1; n = 5 [IQR, 0.8–2.85]). High troponin level was found in patients 1 and 3 who presented with coronary artery thrombosis and pulmonary embolism, respectively. No patient had prior history of thrombosis, signs of hemolysis, or evidence of red cell fragments on blood film. All patients had high optical densities on HIT enzyme-linked immunosorbent assay (optical density median value, 2.5 [IQR, 0.8–2.85]).
CT, MRI, and US Findings
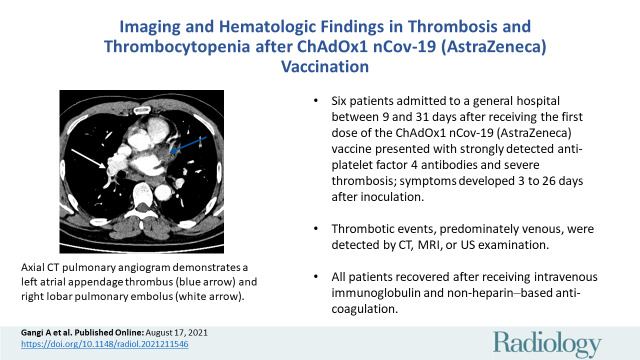
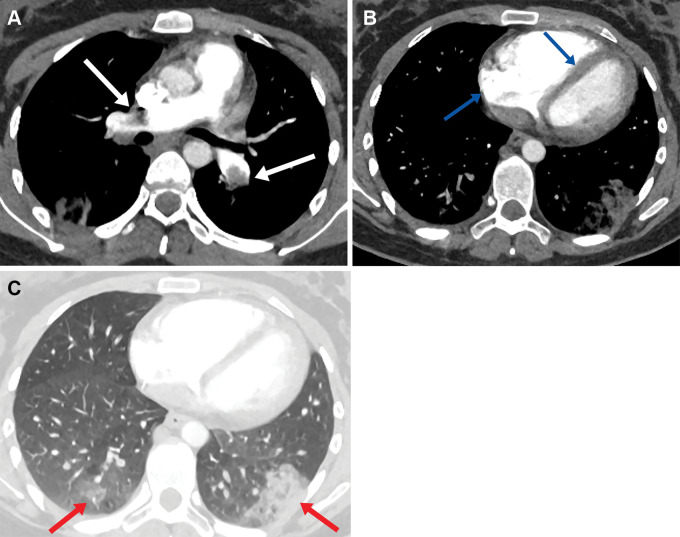
Patient 1 was admitted with a posterior-inferior ST-elevation myocardial infarction. Diagnostic angiogram demonstrated thrombosis within the proximal circumflex and the posterior descending arteries, and the patient underwent percutaneous coronary intervention. No significant atheroma was identified. The CT pulmonary angiogram obtained on day 4 due to increased oxygen requirement showed multiple pulmonary emboli and a large left atrial appendage thrombus (Fig 1A) in addition to bilateral ground-glass opacification within the lungs (Fig 1B). He developed acute kidney injury on day 6 and imaging did not confirm intra-abdominal thrombosis. Laboratory features included thrombocytopenia, high d-dimer level, and strongly positive anti-PF4 antibodies (Table).
Figure 1:
Axial CT pulmonary angiogram images in a 47-year-old man demonstrate (A) a left atrial appendage thrombus (blue arrow) right lobar pulmonary embolus (white arrow), and (B) bilateral ground-glass opacfication.
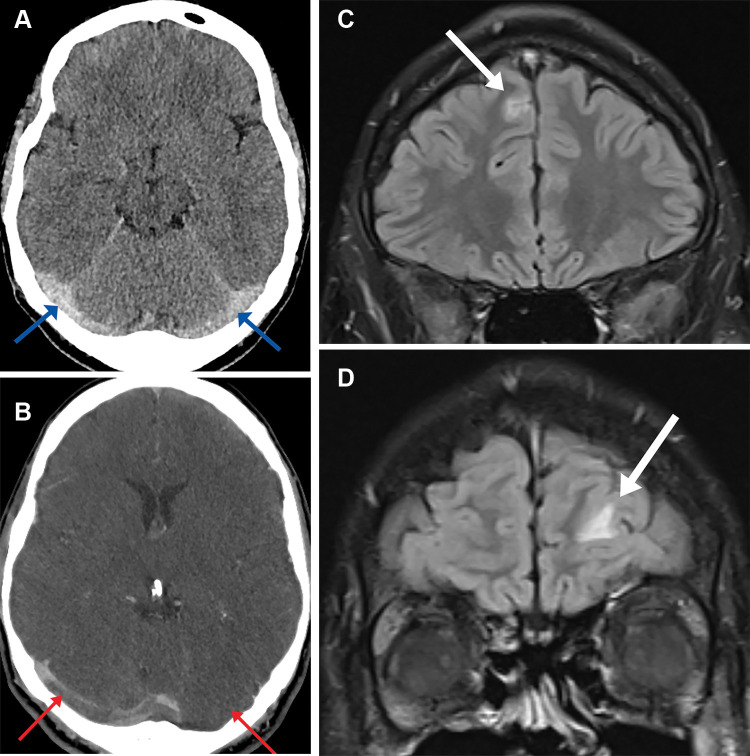
Patient 2 was admitted with a headache and blurred vision. Noncontrast head CT demonstrated hyperdensity involving the superior sagittal sinus and bilateral transverse sinuses. CT venography confirmed thrombotic disease within this distribution (Fig 2A, 2B). His condition deteriorated further as he developed seizures and his Glasgow Coma Scale score decreased. He was transferred to a tertiary intensive therapy unit for consideration of decompressive craniotomy. A week following initial admission, the patient developed new left-sided weakness, variable sensory signs, and brisk reflexes. Brain MRI images demonstrated high T2 signal within the frontal lobes bilaterally thought to represent venous infarcts (Fig 2C, 2D).
Figure 2:
(A) Axial head CT image in a 28-year-old man shows hyperdense bilateral transverse cerebral sinuses (arrows) and (B) axial CT venogram image demonstrates filling defects within the transverse cerebral sinuses bilaterally (arrows). (C-D) T2 coronal fluid-attenuated inversion recovery brain MRI images demonstrate small foci of high T2 sig- nal within the frontal lobes bilaterally thought to represent venous infarcts (arrows).
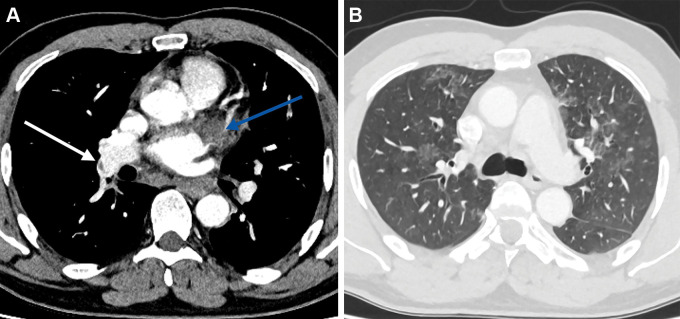
Patient 3 presented with shortness of breath, hemoptysis, and pleuritic chest pain; an admission CT pulmonary angiogram showed extensive pulmonary emboli (Fig 3A), features of right heart strain (Fig 3B), and pulmonary infarcts (Fig 3C). Abdominal US and CT venography did not demonstrate portal vein, hepatic vein, or cerebral venous thrombosis. An echocardiogram showed right ventricular impairment and tricuspid regurgitation.
Figure 3:
(A) Axial CT pulmonary angiogram image in a 21-year-old woman shows bilateral central pulmonary emboli (arrows) with (B) enlargement of the right heart and flattening of the intraventricular septum in keeping with right heart strain (arrows). (C) Lung window axial CT pulmonary angiogram demonstrates bilateral peripheral areas of opacification in keeping with pulmonary infarcts (arrows).
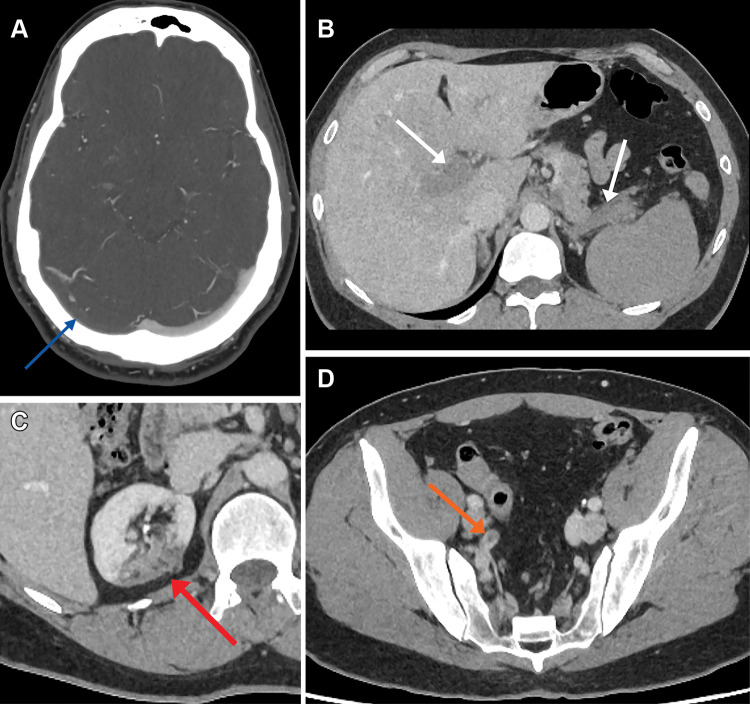
Patient 4 presented with headache, retro-orbital pain, pleuritic chest pain, and abdominal pain. He had a low platelet count and high d-dimer level. CT venography showed thrombosis within the right transverse sinus (Fig 4A) and right jugular vein. CT of the abdomen and pelvis demonstrated extensive occlusive thrombi within the main portal vein, right and left portal vein branches (Fig 4B), superior mesenteric vein, and splenic vein. In addition, CT showed acute thrombus within the right renal infarct (Fig 4C) and within the right internal iliac artery (Fig 4D).
Figure 4:
(A) Axial CT venogram image in a 48-year-old man shows a right transverse sinus filling defect in keeping with thrombosis (arrow). (B) Axial portal venous CT of the abdomen and pelvis demonstrates portal vein and splenic vein thromboses (arrows) in addition to (C) right upper pole renal infarct (arrow) and (D) acute right internal iliac artery thrombus (arrow).
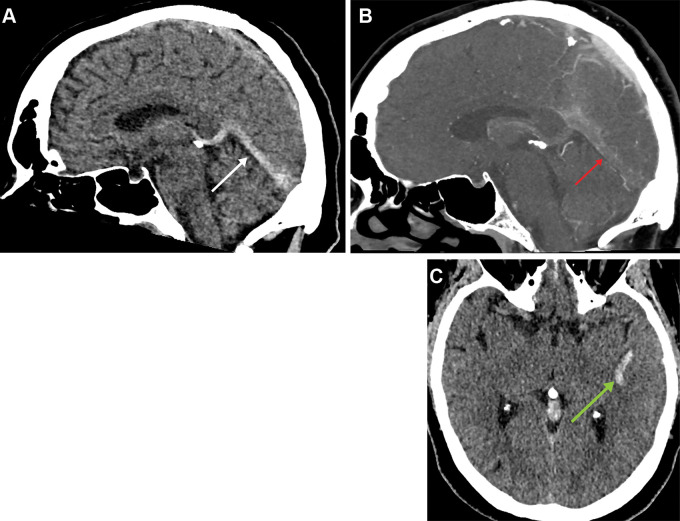
Patient 5 presented with a headache, and CT demonstrated asymmetric hyperdensity within the left transverse, sigmoid, and straight sinuses. Extensive filling defects within the left transverse, sigmoid, and straight sinuses (Fig 5A, 5B) in addition to the left jugular vein was confirmed with subsequent CT venography. No further thrombosis was identified at CT of the chest, abdomen, and pelvis. One day following admission, the patient developed new seizures. A repeat head CT showed a 2-cm left temporal cortical venous hemorrhage (Fig 5C).
Figure 5:
(A) Sagittal head CT in a 54-year-old man shows a hyperdense straight sinus (arrow) confirmed with (B) CT venogram (arrow). (C) Axial head CT shows a 2-cm left temporal lobe cortical venous hemorrhage (arrow).
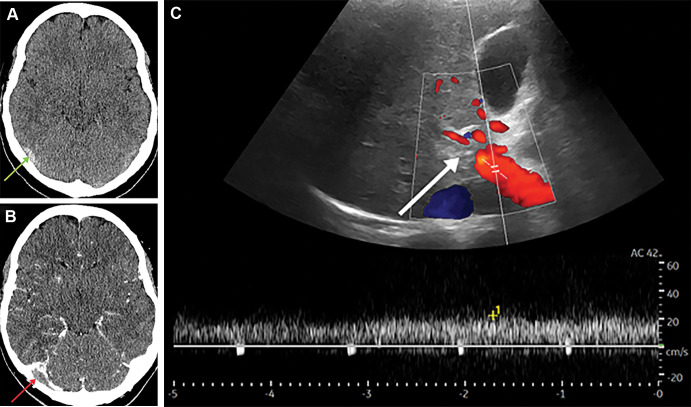
Patient 6 was admitted with headaches, photophobia, and nausea. Head CT demonstrated hyperdensity of the inferior sagittal and transverse sinuses (Fig 6A). The patient was transferred to the tertiary neurologic center. Thrombus within the straight sinus, bilateral transverse sinuses (Fig 6B), and right internal jugular vein was confirmed with subsequent CT venography. Abdominal US was performed on day 6 due to raised alanine transaminase level, which confirmed intrahepatic main and right portal vein thrombosis (Fig 6C) with suspected cavernous transformation.
Figure 6:
(A) Axial noncontrast head CT in a 27-year-old woman shows a hyperdense right transverse sinus (arrow) confirmed with (B) CT venogram with a filling defect (arrow). (C) Doppler US image shows no flow within the intrahepatic main portal vein in keeping with thrombosis (arrow).
Discussion
This case series describes the imaging and hematologic findings in six patients with vaccine-induced immune thrombotic thrombocytopenia following AstraZeneca vaccination. Similar to published data, we found that cerebral venous sinus thrombosis was the most common thrombosis site, followed by intra-abdominal thrombosis (5,6). Patients, as in our series, typically present with symptoms 5–28 days following vaccination with moderate to severe thrombocytopenia and thrombosis in unusual sites (4,6–8). Patients had a high d-dimer level, low platelet count, and atypical arterial or venous thrombosis; they developed symptoms 4 weeks or less following the first vaccine dose. Fibrinogen levels were mostly normal.
There are limited United Kingdom guidelines, which include those published by Royal Colleges and the British Society of Haematology (3,9,10). These will be revised and evolve with better clinical understanding. We identified asymptomatic intracardiac thrombus in one patient. An argument could be made for imaging additional asymptomatic regions in patients with VITT, especially for coexisting asymptomatic cerebral venous thrombosis, potentially altering oral anticoagulation choice. Reporting radiologists should remain alert to the possibility of additional thrombotic load, both in atypical sites and as incidental findings.
Current understanding is insufficient to know whether there is any genetic preexisting comorbidity or immune underlay predicting VITT.
Thrombotic thrombocytopenic purpura, another differential diagnosis, was not suspected because of patient history, absence of hemolysis, and no excess of red blood cell fragments on smear analysis. Vaccination stimulates the immune system and can promote nontolerance of self-antigens, resulting in immune thrombocytopenic purpura and hemolytic anemia.
A common denominator in all six patients was a high level of anti-PF4 antibodies, higher than typically seen in HIT (11). Proposed mechanisms of VITT include neoantigen formation between PF4 and vaccine proteins, leading to immunogenicity and high anti-PF4 titers. These antibodies, as in HIT, drive thrombosis by platelet, leukocyte, and endothelial activation. The VITT antibodies can mimic the effect of heparin by binding to a similar site on PF4, leading to thrombosis with platelet activation (12).
These patients were managed according to interim guidelines and discussion with the UK Expert Haematology Panel. All six patients received intravenous immunoglobulin, five of them were given steroids, and fondaparinux was the most common nonheparin anticoagulant. Therapeutic plasma exchange was used in three patients, either due to being refractory to initial management including intravenous immunoglobulin or extensive clot load. Those with cerebral venous sinus thrombosis or arterial ischemia were offered warfarin rather than novel oral anticoagulants. No patient had a fatal outcome. Primary care was advised against a second vaccine dose.
Additional multicenter studies are required to assess the incidence, pathophysiology, and location of thromboses to develop best practice guidelines.
Acknowledgments
Acknowledgements
Dr Naji Al-Khudairi, Dr Chris Ball, Dr Richard Beable, Dr Michelle Dinsey, Dr Janine Domjan, Dr Rachael Harrison, and Dr Jenny Latham contributed to the radiology reports for the images included in this case series.
A.G. and B.M. contributed equally to this work.
Disclosures of conflicts of interest: A.G. No relevant relationships. B.M. No relevant relationships. M.G. No relevant relationships. R.A. Grant from Celgene; payment or honoraria from Abbvie for podcast; payment or honoraria from Abbvie, Celgene, Gilead, Janssen, and Takeda for educational event attendance.
Abbreviations:
- HIT
- heparin-induced thrombocytopenia
- IQR
- interquartile range
- PF4
- platelet factor 4
- VITT
- vaccine-induced immune thrombotic thrombocytopenia
References
- 1. Voysey M , Clemens SAC , Madhi SA et al . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK . Lancet 2021. ; 397 ( 10269 ): 99 – 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramasamy MN , Minassian AM , Ewer KJ et al . Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial . Lancet 2021. ; 396 ( 10267 ): 1979 – 1993 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Expert Haematology Panel. Guidance from the Expert Haematology Panel (EHP) on Covid-19 Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT) version 2.0. https://b-s-h.org.uk/about-us/news/guidance-produced-by-the-expert-haematology-panel-ehp-focussed-on-vaccine-induced-thrombosis-and-thrombocytopenia-vitt/. Published 2021. Accessed June 2021.
- 4.Expert Haematology Panel. Guidance from the Expert Haematology Panel (EHP) on Covid-19 Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT) version 1.7. https://b-s-h.org.uk/media/19590/guidance-version-17-on-mngmt-of-vitt-20210420.pdf . Published 2021. Accessed June 2021.
- 5. Scully M , Singh D , Lown R et al . Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination . N Engl J Med . 2021. ; 384 ( 23 ): 2202 – 2211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cines DB,Bussel JB. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. 2021;384(23):2254–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz NH, Sørvoll IH , Michelsen AE et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021. 384(22): 2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A,Thiele T, Warkentin TE et al. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intercollegiate Multidisciplinary Guidance for Clinicians. Diagnosis and management of gastrointestinal manifestation of vaccine induced thrombosis & thrombocytopaenia. https://www.acpgbi.org.uk/content/uploads/2021/05/VITT-guidance-GI-manifestations-6-May-2021.pdf. Published 2021. Accessed June 2021.
- 10.College's Quality in Emergency Care (QEC) committee. Management of patients presenting to the Emergency Department/Acute Medicine with symptoms of Covid-19 Vaccine induced Thrombosis and Thrombocytopenia (VITT) 2021. https://www.rcem.ac.uk/RCEM/Quality-Policy/Clinical_Standards_Guidance/RCEM_Guidance_Folder/College_Guidelines. Published 2021. Accessed June 2021.
- 11.Greinacher A,Selleng K,Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114. [DOI] [PubMed] [Google Scholar]
- 12.Huynh A,Kelton JG, Arnold DM et al. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021. 10.1038/s41586-021-03744-4. Published online July 7, 2021. [DOI] [PubMed] [Google Scholar]