Abstract
The aim of this study was to optimizing the process variables, assessing the phytochemical constituents and antimicrobial activity of the extracted oil from Croton macrostachyus seed by Soxhlet extraction using n-hexane as a solvent. The maximum oil yield of 45.89 % extracted from Croton macrostachyus seed was obtained at a reaction temperature of 70 °C, particle size of 0.5 mm, reaction time of 5 h, and solute to solvent ratio of 0.06 g/ml. The physicochemical properties of the extracted Croton macrostachyus seed oil investigated were; specific gravity of 0.9, the density of 900 kg/m3 at 15 °C, refractive index of 1.461, acid value of 3.5 mg KOH/g, and free fatty acids of 1.75 mg KOH/g, match the relevant international standards of oil quality. The qualitative test analysis of the extracted Croton macrostachyus seed oil showed that the presence of different phytochemical constituents of an antimicrobial activity were flavonoids, alkaloids, tannins, saponins, terpenoids, and phenols. The functional groups and composition of the extracted oil from Croton macrostachyus seeds were determined using FT–IR and GC-MS respectively. The results exhibited that the oil obtained from Croton macrostachyus seeds can be used for modern medicine instead of traditional purposes.
Keywords: Croton macrostachyus seeds oil, Soxhlet extraction, Antimicrobial, Phytochemicals
Croton macrostachyus seeds oil, Soxhlet extraction, antimicrobial, Phytochemicals.
1. Introduction
For a thousand of years, Ethiopian peoples were used traditionally different parts of natural plants origin as a source of medicinal drugs. Recently many of these natural plants are used for modern drugs which their isolations methods were based on the uses of the agents in traditional medicine (Aylate et al., 2017; Gebrehiwot et al., 2018). The essential role of traditional medicines primarily in health care where about 80% of the world's inhabitants natural plant-based. Today in many parts of Ethiopian regions there is a manifestation of the expensive cost of modern antimicrobial drugs that have made ineffective management of infectious diseases that used for antibiotic resistance (Nigussie et al., 2021).
Antimicrobial agents play a great role in diminishing the caused by microorganisms like bacteria and fungus. However, reducing the infectious disease by microbial agents is a burden for the global community as resistant pathogens and extent of the effectiveness of antibiotics (Meresa et al., 2019; Saha et al., 2015). Therefore, a source of new antimicrobial agents with possibly novel mechanisms of different natural plant parts-based products can be used for this infectious disease (Abdulrasheed et al., 2016). Urgently, it needs alternative strategies to produce antimicrobial drug activity from different parts of plant, like Croton macrostachyus seed.
Croton macrostachyus (oromic local name Bakenissa) belongs to one of the largest family Euphorbiaceous called Croton under the subfamily Crotonoideae. Traditionally leaves and seeds of Croton macrostachyus were used for treating a range of ailments including intestinal, skin, and venereal diseases of humans and animals (Owade et al., 2019). A plant part of the seeds are used for diarrhea, malaria, internal worms, wound, gonorrhea, blackleg, clotting, blood clot, stomachache, tetanus, skin cancer, ringworm, febrile illness, headache, infection, liver problem, hookworm, leeches and poisoning in a rural community (Tadele, 2017). The seed of Croton macrostachyus oil is rich in saturated, monounsaturated, and polyunsaturated fatty acids and contained protein and oils up to 50% and 30–32% respectively (Bantie et al., 2014).
The objective of this study was to investigate the extraction of oil from Croton macrostachyus seeds using the solvent extraction method. The physicochemical properties, fatty acid compositions, and antimicrobials activities of the extracted oil were evaluated. The aim is to find the optimum operating conditions for the extraction of oil from Croton macrostachyus seed that maximize the oil yield via solvent extraction method.
2. Materials and methods
2.1. Materials and reagents
Croton macrostachyus oil was extracted from Croton macrostachyus seed acquired from Jimma university campus, Ethiopia in May 2020. The chemical n-hexane with a purity of 99 % was collected from Chem-Supply Kirkos Ltd. in Addis Ababa, Ethiopia. The chemical (n-hexane) of pure analytical grade was used.
2.2. Methods
Croton macrostachyus seeds were first cleaned of dirt, dust, sand, small stones and washed manually. Then cleaned Croton macrostachyus seeds were dried for two days by sunlight. The cleaned and dried 2 kg of Croton macrostachyus seeds were weighted and further dried in an oven at 105 °C for an hour to remove the rest of their moisture content until a constant weight was measured. Then seeds would have crushed using a crusher with a particle size of 0.5–1.5 mm for solvent extraction analysis. The prepared Croton macrostachyus seed was characterized using proximate analysis as ASTM standards to determine the parameters like moisture content, volatile matter, fixed carbon, and ash content.
2.2.1. Extraction of oil
Soxhlet apparatus was used to extracts the oil from Croton macrostachyus seed. A ground sample was placed in a thimble and loaded into the Soxhlet extraction unit. A solid to solvent ratio from 0.01 to 0.1 g/ml, extraction time from 3 to 5 h, temperature from 65 to 75 °C, and particle size from 0.5 to 1.5 mm were employed. Finally, the solvent and oil was separated using a rotary evaporator and the solvent was recovered using a condenser. The percentage oil yield was calculated using;
| (1) |
Where Wo is the weight of extracted oil (g), and Ws is the weight of the seed sample before extraction (g).
2.3. Determination of physicochemical properties of extracted Croton macrostachyus oil
The acid value, refractive index, free fatty, density, and specific gravity of extracted oil was analyzed according to standard methods described in (Kindermann et al., 2007).
Specific gravity; A clean and dry bottle of 25 ml capacity was weighed (W0) and the bottle was filled with the oil, stopper inserted and reweighed to give (W1). The oil was substituted with water after washing and drying the bottle and weighed to give (W2).
| (2) |
Determination of density; The density of oil was calculated from specific gravity (SG) of oil and density of water The density of oil was determined according the Eq. (3).
| (3) |
Acid value; A 2 g of oil was added to the mixture of 25 ml of toluene and 25 ml of ethanol in a 250 ml beaker. Then a few drops of phenolphthalein were added to the mixture. The mixture was titrated with 0.1 M KOH to the end point with consistent shaking for which a dark pink color was observed. The titer value in ml (n) was noted.
| (4) |
Where, n - the number of ml of 0.1 M potassium hydroxide required, and w - the weight of the oil (g).
Free Fatty Acid; 1.0 g of weighted oil transferred into a 250 ml conical flask. Three drops of phenolphthalein were added to the sample followed by 20 ml ethanol. The mixture was titrated with 0.1 M KOH solution until a pink color was developed. Free fatty acid was calculated as Eq. (5).
| (5) |
Refractive index; The temperature of the refractometer was adjusted at 20 °C and several drops of the sample were placed on the lower prism. Prisms were closed and tightened firmly with a screw head. It was allowed to stand until the sample comes to the temperature of the instrument. The instrument and light were adjusted to obtain the most distinct reading possible and the refractive index was determined.
2.4. Tested microorganisms
The types of bacteria and fungi reliable cultures used in this study were Bacillus cereus, Staphylococcus aureus, Escherichia coli, Aspergillus Niger, and Candida albicans obtained from “Microbial Type Culture collection”, Biotechnology Research Center, Jimma, Ethiopia.
Nutrient Muller Hinton agar medium was used to maintained bacterial cultures whereas Sabouraud Dextrose agar medium was used for fungal cultures. All bacterial and fungal strains were incubated for 24 h at 37 °C strains and incubated at 27 °C for 48 h respectively.
2.5. Antimicrobial activity
Antibacterial and antifungal activity of Croton macrostachyus seed oil extracted was determined by agar well diffusion method on Muller Hinton agar medium reported in (Tamil Selvi et al., 2011). The stock solution (500 mg/ml) was used to prepare the solutions of the extract and its fractions to give the concentrations of 250 mg/ml, 125 mg/ml, 62.5 mg/ml, and 31.25 mg/ml. All culture media and distilled water were sterilized at 121 °C for 15 min in an autoclave and to get a density of 0.5 Mc Farland and the inoculants were diluted with sterilized distilled water. All of the homogenized fluid agar media was mixed with 0.5 ml of each inoculum. About 25 ml of its solution was poured into sterile plastic petri dishes were allowed on the flat slab top to solidify the medium within 30 min. Four equidistant uniform wells per plate were prepared by standard cork borer of 5 mm in diameter was used to cut the surface of different plates on which 100 μl solution of each extract or its fraction was added to make the above different concentration. The antimicrobial activity was evaluated by measuring the zone of growth inhibition surrounding the discs. The experiment was repeated three times and the mean values were calculated. The dimethyl sulfoxide (DMSO) and Ketoconazole were used as a negative control against bacteria and positive for fungi respectively whereas amoxicillin and Ciprofloxacin were used as a positive and negative control for bacteria and fungi respectively.
2.5.1. Determination of minimum inhibitory concentration (MIC)
Minimum Inhibitory Concentration (MIC) of the oil was tested by the fold dilution method described in (Iraqui and Yadav, 2015). The extracted Croton macrostachyus seed oil was dissolved in dimethyl sulfoxide to the concentration of 1000 mg/ml to determine the zones of inhibition. For the minimum inhibitory concentration measurements, the stock solution (500 mg/ml) was serially diluted to give the concentrations of 250 mg/ml, 125 mg/ml, 62.5 mg/ml, and 31.25 mg/ml.
2.6. Phytochemical analysis
The presence of phytochemicals like alkaloids, flavonoids, saponins, tannins, terpenoids, and phenol in crude Croton macrostachyus seed oil was evaluated according to the method reported in (Ayoola et al., 2008).
Test for alkaloids: About 0.25 g of Croton macrostachyus seed oil was weighted and stirred with 5 ml of 1% HCl on a steam bath. Then 1 ml of the solution was taken and treated with some drops of Mayer's reagent and another 1 ml was taken to treat with Dragendorff's reagent. In both reagents the formation of turbidity or precipitation indicated the presence of alkaloids.
Test for saponins: 0.25 g of extracted the Croton macrostachyus seed oil and 5 ml of distilled water was dissolved in a test tube. Then the solution was shaken vigorously. The formation of froth indicated the presence of saponins.
Test for polyphenols (phenolic compounds): 0.25 g of extracted Croton macrostachyus seed oil was weighted and treated with few drops of 5% neutral ferric chloride solution. The formation of a greenish precipitate indicated the presence of phenol (Shetty et al., 2016).
Test for flavonoids: 0.25 g of n-hexane extracted Croton macrostachyus seed oil and 10 ml of ethyl acetate was mixed into a test tube and heated on a water bath for 3 min. The solution was cooled and filtered. Then about 4 ml of the filtrate was shaken with 1 ml of dilute ammonia solutions. The yellow color in the ammonia layer specified the presence of flavonoids.
Test for Terpenoids: Two ml of chloroform was added into a test tube having 0.25 g of hexane extracted Croton macrostachyus seed oil and about 3 ml concentrated sulfuric acid was dissolved into the mixture. A reddish-brown color was formed and shown the presence of terpenoids.
Test for tannins: 0.25 g of extracted Croton macrostachyus seed oil weighted were boiled in 10 ml of water in a test tube and filtered. Three drops of 0.1% ferric chloride were added to the filtrate. The brown greenish color was observed and indicated the presence of tannins.
2.7. Determination of fatty acid profile and functional group of Croton macrostachyus seed oil
Fatty acid composition of Croton macrostachyus seed oil extracted by using n-hexane was determined according to the AOCS 1998 official methods via Gas Chromatography mass spectrometer (Palo Alto, CA, USA, 7820A) equipped with flame ionization detector analysis. The oil composition was investigated after carboxyl acid alkyl radical esters mixed with a methanolic solution of potassium hydroxide using gas chromatography. The analysis was performed using nitrogen gas for heating the sample for the subsequent temperature profiles. First temperature of the sample was heated at 60 °C for one minute, then increased from 60 °C to 170 °C at a rate of 10 °C/min, 170–230 °C at a rate of three °C/min, and constantly heated for 15 min at 230 °C. Finally, the sample was injected at volumetric rate of 1 ml/min by a sampler injector at the temperatures of 225 °C and detected at 250 °C. Then fatty acids composition of Croton macrostachyus seed oil was identified based on peak retaining time.
Fourier transform infrared (FTIR) spectrometer (Bruker Tensor 270, Ettlingen, Germany) was used to determine various functional groups in the extracted oil and the spectra were recorded from 4000 to 400 cm−1. The analysis was done using KBr for extracted Croton macrostachyus seed oil.
2.8. Statistical analysis
The Design Expert software version 11.00 was used to perform the experiment according to randomized design with four operating parameters. Response Surface Methodology was used to investigate the influence of operating parameters on the extraction of oil and optimize the percentage of oil yield. A two-level-four-factor central composite design (CCD) was employed to study the effects of solute to solvent ratio, temperature, extraction time, and particle size on the extraction of oil from Croton macrostachyus seeds, and 29 randomized experiment runs were obtained (Jalili et al., 2018). A solute to solvent ratio from 0.01 to 0.1 g/ml, extraction time from 3 to 5 h, temperature from 65 to 75 °C, and particle size from 0.5 to 1.5 mm were investigated as independent variables.
3. Result and discussion
3.1. Proximate analysis of Croton macrostachyus seed
The proximate analysis of Croton macrostachyus seed was determined according to American standards Testing Methods (ASTM) and the results were shown in Table 1. The moisture content of seeds determines the ability of all seeds to be stored well. In addition to this, a seed was rich in volatiles but low in ash content and the value obtained for fixed carbon content is low due to the high contained of volatile mass dry based. The result shows that the sample is suitable for oil extraction without further drying of the seeds.
Table 1.
Proximate analysis of Croton macrostachyus seeds according to ASTM.
| Property | Croton macrostachyus Seed (%) | (ASTM) | Reference |
|---|---|---|---|
| Moisture content | 6.5 ± 0.05 | ASTM D 2016-74 | Apaydin-Varol and Pütün (2012) |
| Ash content | 3.24 ± 0.09 | ASTM D 1102-84 | |
| Volatile matter | 77.35 ± 0.1 | ASTM E 897-82 | |
| Fixed carbon contents | 12.91 ± 0.06 | From the difference |
3.2. Statistical analysis
Table 2 shows the models and their significant coefficients of the responses, where all the models are significant at a level of less than 0. 0001. The statistical analyses show that quadratic models fit very well into the data for the response and the responses were highly significant (p < 0.0001). The Model F value of 423.40 implies the model is significant for Croton macrostachyus seed oil yield. For Croton macrostachyus seed oil yield A, C, D, AC, AD, BD, CD, A2 B2, C2, and D2 were found to significantly affect the yield of extracted Croton macrostachyus seed oil, while B and AB were not significant. The analysis of variance for the lack of fit test did not show the inadequacy of the model concerning the Croton macrostachyus seed oil yield (P > 0.05), indicating that the model could adequately fit the experimental data.
Table 2.
Analysis of variance for response surface quadratic model of Croton macrostachyus seed oil yield.
| Sources | Sum of squares | Degree of freedom | Mean of squares | F-value | P-value | |
|---|---|---|---|---|---|---|
| Model | 361.30 | 14 | 25.81 | 423.40 | 0.0001 | significant |
| A-Temperature | 20.51 | 1 | 20.51 | 336.50 | 0.0001 | |
| B-Solute: solvent ratio | 0.2139 | 1 | 0.2139 | 3.51 | 0.0820 | |
| C-Extraction time | 71.46 | 1 | 71.46 | 1172.46 | 0.0001 | |
| D-Particle size | 150.50 | 1 | 150.50 | 2469.21 | 0.0001 | |
| AB | 0.1581 | 1 | 0.1581 | 2.59 | 0.1296 | |
| AC | 1.61 | 1 | 1.61 | 26.46 | 0.0001 | |
| AD | 5.81 | 1 | 5.81 | 95.29 | 0.0001 | |
| BC | 16.76 | 1 | 16.76 | 275.05 | 0.0001 | |
| BD | 0.9502 | 1 | 0.9502 | 15.59 | 0.0015 | |
| CD | 4.67 | 1 | 4.67 | 76.55 | 0.0001 | |
| A2 | 84.62 | 1 | 84.62 | 1388.27 | 0.0001 | |
| B2 | 3.13 | 1 | 3.13 | 51.39 | 0.0001 | |
| C2 | 12.27 | 1 | 12.27 | 201.39 | 0.0001 | |
| D2 | 14.84 | 1 | 14.84 | 243.42 | 0.0001 | |
| Residual | 0.8533 | 14 | 0.0610 | |||
| Lack of Fit | 0.7440 | 9 | 0.0827 | 3.78 | 0.0786 | Not significant |
3.3. Influence of process parameter on the extraction process
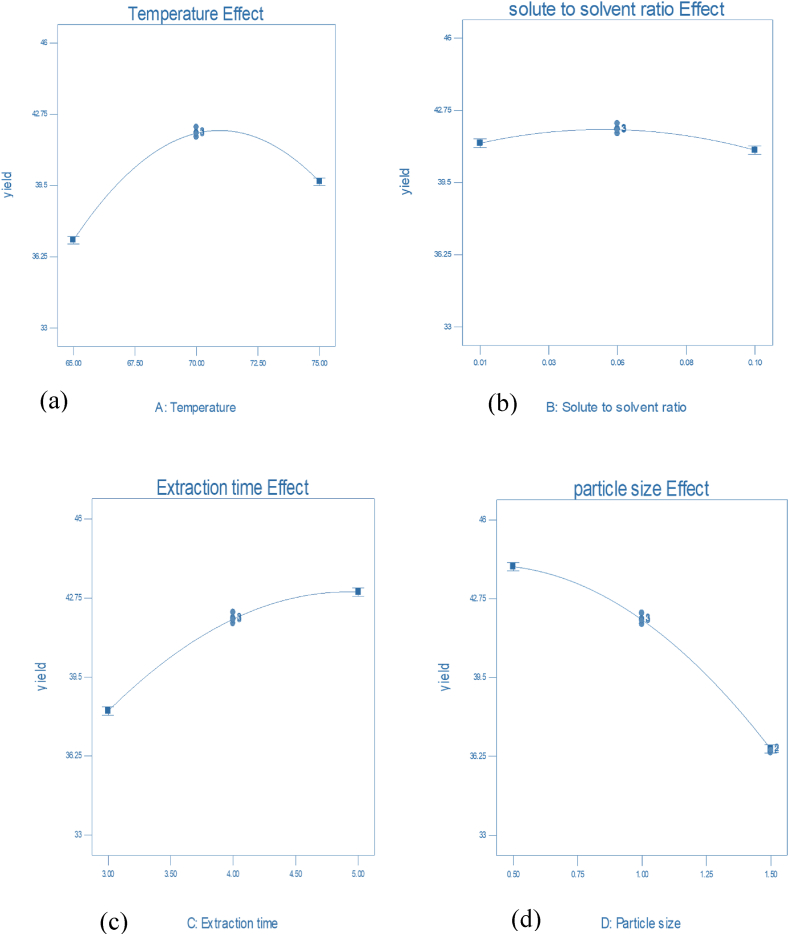
3.3.1. Effect of temperature on the extraction of oil
The fact is that oils are more soluble at elevated temperatures. As indicated the results in Figure 1(a) the obtained value analysis showed that an increase in temperature favors an increase in percentage of n-hexane extracted Croton macrostachyus oil yield. At the higher temperature, the viscosity of the solvent is reduced while the diffusivity as well as evaporation rate is increased. From the Soxhlet extraction using n-hexane, the temperature changed from 65 to 75 OC the percentage of extracted Croton macrostachyus seed oil yield changed from 33.12 to 45.89 %. This percentage indicates as the extraction temperature increases the percent oil yield also increases (Wu et al., 2018).
Figure 1.
Effects of (a) temperature (b) solute to solvent ratio (c) extraction time and (d) particle size on Croton macrostachyus seed oil yield.
3.3.2. Effect of solvent ratio on the extraction of oil
As indicated in Figure 1(b), the solute to solvent ratio from 0.01 to 0.05 g/ml had no significant effect on the extraction process.
3.3.3. Effect of time on the extraction of oil
The extraction time during the extraction process was significantly affected the yield of n-hexane extracted Croton macrostachyus seed oil. As the results shown in Figure 1(c) while the extraction time changed from 3 to 5 h the percentage of oil yield similarly changed from 33.12 to 45.89 %. Thus, the oil yield increased with an increase in extraction time and there was no considerable increase after 5 h. This indicates that the extraction time increases the percentage oil yield also increases (Haile et al., 2019).
3.3.4. Effect of particle size on the extraction of oil
Effect of particle size on extraction process of Croton macrostachyus seed oil yield using n-hexane as a solvent was highly significant as shown in Figure 1(d). As particle size gets smaller, the contacting area between the seed powder and solvent is increases, and the resistance diffusion of solute within the solid is low with the fact that the rate of material transfer is high. However, for very fine seed particle it is more difficult to get high Croton macrostachyus seed oil yield because of more liquid particle is dissolved in solid residues (Zerihun and Berhe, 2020).
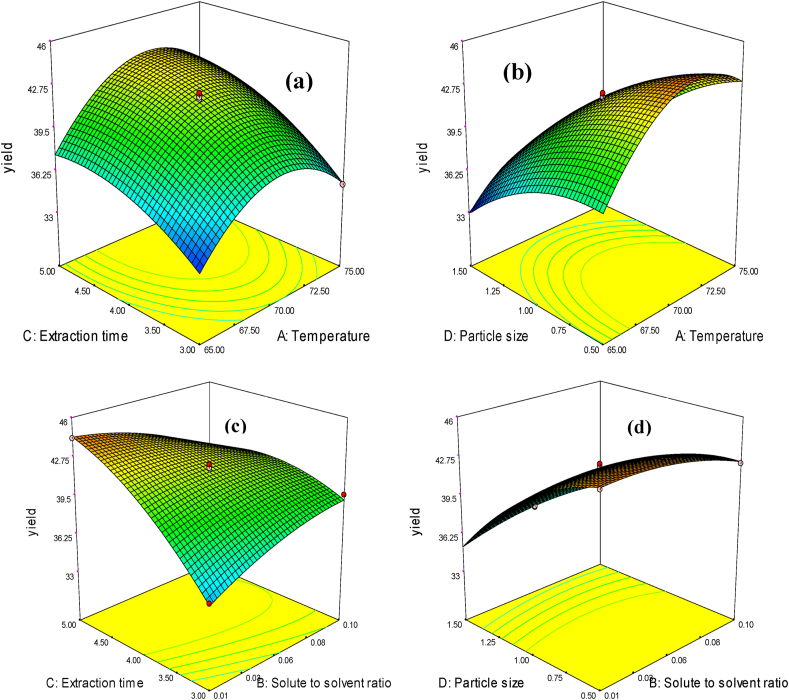
3.3.5. Interaction effects on the extracted Croton macrostachyus seed oil
The interaction effects of process parameters on Croton macrostachyus seed oil between temperature and solute to solvent ratio, extraction time and temperature, particle size and temperature, and particle size and extraction time for the quadratic models were significant effects on the extracted oil yield have been easily shown from Figure 2. As shown in Figure 2(a), it was revealed that the variation temperature was found to significantly affect the oil yield obtained from Croton macrostachyus as the extraction time was varied. The other interaction factors effect was shown in Figure 2(b) which is between particle size and temperature. So, it seems that the particle size should be controlled or reduced as much as possible to get the positive effect of temperature and extraction time. As revealed from this graph, at higher particle size a decrease in temperature 75 to 65 °C increased the extraction oil yield.
Figure 2.
Interaction effects of process variable between (a) extraction time and temperature (b) particle size and temperature (c) extraction time and solute to solvent ratio and (d) particle size and extraction time on Croton macrostachyus seed oil yield.
As indicated from Figure 2(c), the interaction effects between time and solute to solvent ratio significantly affect the oil yield obtained from Croton macrostachyus seed, due to variation of time and solute to solvent ratio. Thus, at high time and solute to solvent ratio, the solvent rapidly diffuses through the solute and shower easily this not leads to extraction since it is more vaporized and no more amount of extracted oil yield was obtained. As graphical representation shown in Figure 2(d), that the interaction between particle size and solute to solvent ratio is depicted by the shape of the curves. The reason is related to the mass transfer process. The concentration of solute will increase, the rate of extraction will progressively decrease, because the concentration gradient will be reduced, and the solution will generally become more viscous (Reshad et al., 2015).
3.3.6. Optimization of n-hexane extracted Croton macrostachyus seeds oil
Using optimization function in design expert software 11.00, the maximum and minimum oil yield was obtained. It was predicted that at the following operating condition; average temperature, particle size, extraction time, and average solute to solvent ratio, a maximum oil yield was obtained. A minimum oil yield of 33.12 % was obtained at a temperature of 65 °C particle size 1.5 mm, 4-hour extraction time, and 0.06 g/ml of Croton macrostachyus seed to solvent ratio. The optimization solutions for maximum yield are shown in Table 3 by using categorical factors.
Table 3.
Experimental results of Croton macrostachyus seed oil yield.
| Run | Temperature °C | Solute: solvent ratio (g/ml) | Extraction time hr. | Particle size (mm) | Oil yield % |
|---|---|---|---|---|---|
| 1 | 65.00 | 0.01 | 4.00 | 1.00 | 36.3 |
| 2 | 75.00 | 0.01 | 4.00 | 1.00 | 39.3 |
| 3 | 65.00 | 0.10 | 4.00 | 1.00 | 36.15 |
| 4 | 75.00 | 0.10 | 4.00 | 1.00 | 38.35 |
| 5 | 70.00 | 0.06 | 3.00 | 0.50 | 39.2 |
| 6 | 70.00 | 0.06 | 5.00 | 0.50 | 45.89 |
| 7 | 70.00 | 0.06 | 3.00 | 1.50 | 34.13 |
| 8 | 70.00 | 0.06 | 5.00 | 1.50 | 36.5 |
| 9 | 65.00 | 0.06 | 4.00 | 0.50 | 38.2 |
| 10 | 75.00 | 0.06 | 4.00 | 0.50 | 43.1 |
| 11 | 65.00 | 0.06 | 4.00 | 1.50 | 33.12 |
| 12 | 75.00 | 0.06 | 4.00 | 1.50 | 33.2 |
| 13 | 70.00 | 0.01 | 3.00 | 1.00 | 35.6 |
| 14 | 70.00 | 0.10 | 3.00 | 1.00 | 39.63 |
| 15 | 70.00 | 0.01 | 5.00 | 1.00 | 44.3 |
| 16 | 70.00 | 0.10 | 5.00 | 1.00 | 40.1 |
| 17 | 65.00 | 0.06 | 3.00 | 1.00 | 33.73 |
| 18 | 75.00 | 0.06 | 3.00 | 1.00 | 35.19 |
| 19 | 65.00 | 0.06 | 5.00 | 1.00 | 37.2 |
| 20 | 75.00 | 0.06 | 5.00 | 1.00 | 41.2 |
| 21 | 70.00 | 0.01 | 4.00 | 0.50 | 44.1 |
| 22 | 70.00 | 0.10 | 4.00 | 0.50 | 42.2 |
| 23 | 70.00 | 0.06 | 4.00 | 1.50 | 36.4 |
| 24 | 70.00 | 0.06 | 4.00 | 1.50 | 36.49 |
| 25 | 70.00 | 0.06 | 4.00 | 1.00 | 41.7 |
| 26 | 70.00 | 0.06 | 4.00 | 1.00 | 41.92 |
| 27 | 70.00 | 0.06 | 4.00 | 1.00 | 41.9 |
| 28 | 70.00 | 0.06 | 4.00 | 1.00 | 41.85 |
| 29 | 70.00 | 0.06 | 4.00 | 1.00 | 42.15 |
A mathematical model was developed to describe the relationship between operating variables and the response variable oil yield. The regression coefficients were calculated and the data were fitted to a second-order polynomial equation. The model was found the following:
The optimum operating conditions were found to be at 70 °C of extraction temperature (A), 0.01 mg/ml of solute to solvent ratio (B), 4 h of extraction time (C), and 0.5 mm of particle size (D). Under these optimized conditions the observed experimental value was 44.1 g oil/100 g and the validity of the model was confirmed.
3.4. Physiochemical properties of extracted Croton macrostachyus seed oil
The density, refractive index, specific gravity, acid value, free fatty acids of extracted Croton macrostachyus seed oil was analyzed and tabulated in Table 4. From this result, the specific gravity and density of Croton macrostachyus oil almost close to the specific gravity and density of water respectively, and the differences between oil are quite small. The high refractive index of oil also showed that the fatty acids in the oil will contain a high number of carbon atoms.
Table 4.
Physio-chemical properties of the obtained Croton macrostachyus seeds oil compares with the value of the standards.
| Property | Croton macrostachyus Seed oil | EN 14214 |
|---|---|---|
| Density at 15 °C, kg/m3 | 900 | 860–900 |
| refractive index | 1.461 | 1.44–1.49 |
| Specific gravity | 0.9 | 0.86–0.9 |
| Acid value, mg KOH/g | 3.5 | - |
| Free fatty acids, mg KOH/g | 1.75 | - |
The free fatty acid value is an indication for both possible hydrolytic degradation of the oil, lipase activity, and stimulation of oxidative deterioration of oils by enzymatic or chemical oxidation to form off-flavor components. The acid value is employed to ascertain the quality (condition) of oil.
3.5. Phytochemical analysis
In many parts of plants there are bioactive phytochemical components that responsible for the presence of phenol, flavonoids, alkaloid, terpene, saponins, and tannins. The presence of these phytochemical constituents in the extracted oil could be responsible for the antimicrobial activities of Croton macrostachyus seed oil (Gebrehiwot et al., 2018).
Based on the qualitative analysis of extracted Croton macrostachyus seed oil by using hexane as a solvent there was a change of tests for the presence of some phytochemical constituents of this oil. From these, the obtained oil was positive for the presence of alkaloids, tannins, flavonoids, saponins, polyphenols, and Terpenoids. The positive presence of the flavonoids indicates the contribution of their share for the observed antimicrobial activities especially by inhibiting free radical group of gram-positive bacteria which caused by its enzymatic activities by binding metal ions. The presence of phenolic compounds has been found to have a growth inhibition effect against different bacteria whereas presence of terpene in the oil has an effective inhibition growth in the antifungal activity. Thus, Croton macrostachyus seed oil has a properties of cytotoxicity and phototoxicity (Saha et al., 2015). The presence of tannin in the oil is used for antimicrobials by donating hydrogen atoms or electrons and acting as secondary antioxidants by retarding oxidating and bonding Fe2+ to restrict the reaction steps in the Fenton reaction. Both saponins and alkaloids also have an antimicrobial activity to inhibit the growth of both gram-positive and gram-negative test bacterial strains (Krzyczkowska and Kozłowska, 2017).
3.6. Antimicrobial activity and minimum inhibition concentration
The antimicrobial activity of hexane-extracted oil from Croton macrostachyus seed was tested against the bacterial and fungal strain. The results of the growth inhibition experiments are shown in Table 5 and expressed in terms of the diameter of the growth inhibition zone. The results were from three independent analyses and the mean of three values. The dimethyl sulfoxide (DMSO) and Ketoconazole were used as negative control against bacteria and positive for fungi respectively whereas amoxicillin and Ciprofloxacin were used as positive and negative control for bacteria and fungi respectively.
Table 5.
Shows the diameter of the zone of inhibition n-hexane extracted Croton macrostachyus seed oil against tested bacteria and fungus.
| Zone of inhibition (mm) | ||||||
|---|---|---|---|---|---|---|
| Sample tested | Concentration |
Bacterial strain |
fungal strain |
|||
| mg/ml | Bacillus cereus | Staphylococcus aureus | Escherichia coli | Aspergillus Niger | Candida albicans | |
|
Croton macrostachyus oil |
31.25 | 11.5 ± 1.2 | 7 ± 1.5 | 8.0 ± 1.1 | 10.5 ± 0.8 | 11.3 ± 1.2 |
| 62.5 | 12.2 ± 1.3 | 9 ± 1.4 | 12.2 ± 1.6 | 12.4 ± 0.95 | 12.2 ± 0.7 | |
| 125 | 13.3 ± 1.5 | 11.5 ± 01.2 | 13.7 ± 1.4 | 15.5 ± 1.1 | 14.9 ± 0.6 | |
| 250 | 18.2 ± 1.1 | 13.4 ± 1.1 | 15.1 ± 0.9 | 16.4 ± 1.2 | 16.2 ± 1.1 | |
| 500 |
20 ± 0.9 |
16.2 ± 1.2 |
17 ± 0.9 |
18.1 ± .9 |
17.1 ± 0.9 |
|
| Amoxicillin | 20 ± 0.35 |
19 ± 0.85 |
20.1 ± 0.8 |
NA |
NA |
|
| Ciprofloxacin | 19.8 ± .5 | 20.5 ± .3 | 19.2 ± .4 | NA | NA | |
| Dimethyl Sulfoxide (DMSO) | NA | NA | NA | 20.2 ± 0.3 | 20 ± 0.5 | |
| Ketoconazole | NA | NA | NA | 20.2 ± 0.3 | 20 ± 0.5 | |
NA not active.
The results obtained from the study showed that the Croton macrostachyus seed oil possesses antibacterial and antifungal activities against five pathogens tested consists of three bacteria and two fungi were in Table 5. When the extracted Croton macrostachyus seed oil was assayed against the tested pathogens, the mean zone of inhibition obtained was between 7 ± 1.5 mm and 20 ± 0.9 mm. As the concentration of Croton macrostachyus seed oil has increased the zone of inhibition was increased on both pathogenic. As shown from Table 5, the bacillus cereus was more sensitive than Staphylococcus aureus and Escherichia coli which shows more zone of inhibition was measured with respective concentration. On other hand, as the concentration of Croton macrostachyus seed oil increased, the obtained activities against the Aspergillus Niger and Candida albicans also increased. In general, the Croton macrostachyus seed oil showed high activity against Bacillus cereus (20 ± 0.9 mm), moderate activity against Escherichia coli (17 ± 0.9 mm), and low activity against Staphylococcus aureus (16.2 ± 1.2 mm) at the concentration of 500 mg/ml for all bacterial strain (Jiao et al., 2014). Regarding the extracted oil from Croton macrostachyus seed, it shows high activity against Bacillus cereus (20 ± 0.9 mm), which is higher than positive controls of Amoxicillin and Ciprofloxacin.
In addition to this Croton macrostachyus seed oil showed activity against the two fungal microorganisms. However, Croton macrostachyus seed oil showed the growth activity against Aspergillus Niger (18.1 ± .9 mm) was higher than the Candida albicans (17.1 ± 0.9 mm) at the concentration of 500 mg/ml. The extracted oil from Croton macrostachyus seed activity against the two fungal strain is lower than positive controls of dimethyl Sulfoxide and Ketoconazole. The antimicrobial activity of the Croton macrostachyus seed oil attributed due to various phytochemical constituents present in the oil extracts. It can be concluded that the presence of active components in Croton macrostachyus seed oil would help to develop the extracted oil as medicinal raw material.
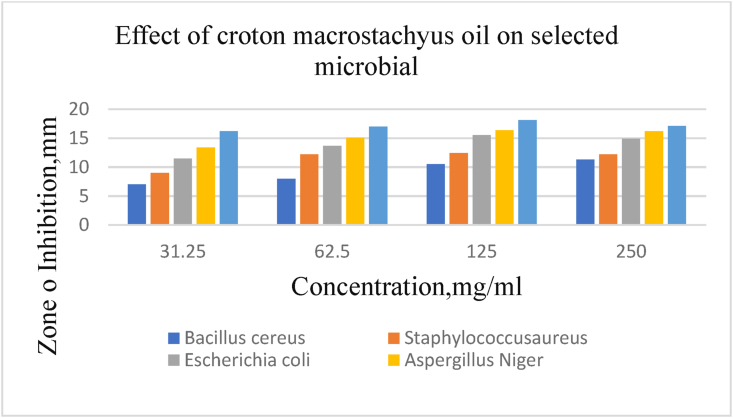
Minimum inhibition concentration (MIC) values between 31.25–125 mg/ml were obtained for the extracted oil in the tests with the bacterial agent while the range of 31.25–250 mg/ml was recorded against the fungal strains as shown from Figure 3. The results of MIC presented in Figure 3 exhibited that all microorganisms were very susceptible to the minimum inhibitory concentration of Croton macrostachyus seed oil (31.25 mg/ml). At the concentration of 31.25 mg/ml the MIC extracted oil from Croton macrostachyus seed showed that they had the induced the zone of inhibition between 7 ± 1.5 mm and 11.5 ± 1.2 mm against all bacterial and fungal strains. This indicates that at this concentration zone inhibition of all pathogen's activity was lower than the other concentrations. Comparing with literature results, strong activity is for MIC values between 250–500 (mg/ml), moderate activity MIC values between 62.5 –125% (mg/ml and weak activity above 31.25 (mg/ml) for both phytogenic bacteria (Okokon and Nwafor, 2010).
Figure 3.
Zone inhibition of n-hexane extracted Croton macrostachyus seed oil against tested bacteria and fungus.
3.7. Fatty acid profile
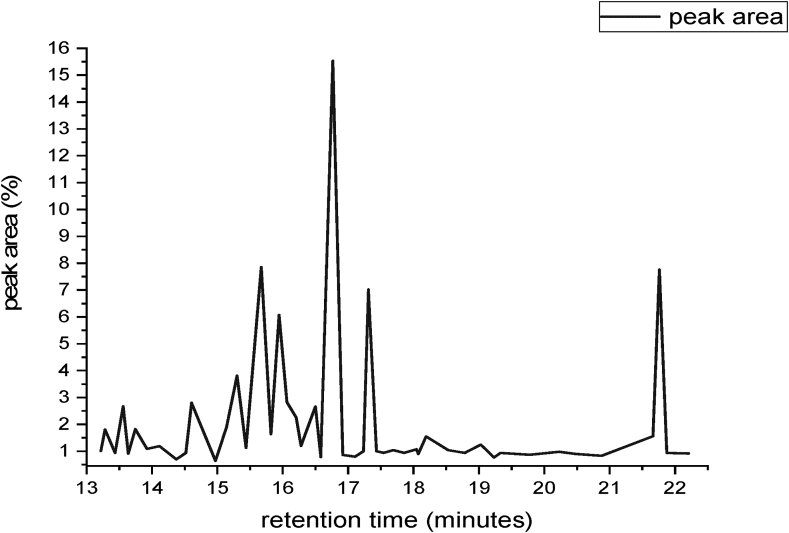
Fatty acid composition of extracted oil from Croton macrostachyus seed was detected by the GC-MS analysis method has shown in Figure 4. The main fatty acids present in extracted oils were, saturated and unsaturated compounds with straight aliphatic chains, identified through their retention times and peak area. The major components of the extracted oil from Croton macrostachyus were five saturated fatty acids, two monounsaturated fatty acids and two polyunsaturated fatty acids were identified and tabulated in Table 6. Among them, the most abundant fatty acid was monounsaturated oleic acid C18:1 (43.39 %), poly-unsaturated linoleic acid C18:2 (29.66 %), linolenic acid (4.78 %), saturated palmitic acid C16:0 (6.14 %), stearic acid C18:0 (4.93 %, heptadecanoic acid (1.5 %), and pentadecanoic acid (1.03 %). The other most important fatty acid was 4-Hexen-1-ol which acts as an attractant to many predatory insects. Polyunsaturated fatty acids (PUFAs) have positive impact on human health in the prevention of inflammatory, blood clothing, and autoimmune diseases like chronic inflammation, reactive arthritis, and ileitis's disease. Therefore Croton macrostachyus seed oils rich is responsible for stable to oxidative rancidity and stable deep-frying since the oil was rich in oleic acid and linoleic acid (Gebrehiwot et al., 2018). The total percentage of unsaturated fatty acid in the extracted oil from Croton macrostachyus seed was 77.83 % which was higher than Soybean oil (81.14 %) and Mustard oil (86.18 %), but lower than the total percentage of unsaturated fatty acid value of sunflower oil, Palm oil, and Coconut oil for values 62.4, 53.30 and 7.12 % respectively.
Figure 4.
Typical GC-MS chromatogram of Croton macrostachyus hexane extracted oil components.
Table 6.
A fatty acid profile of Croton macrostachyus seed oil.
| Fatty acid | Carbon structure | Saturation level | Total abundance % |
|---|---|---|---|
| Palmitic | 18:0 | Saturated | 6.14 |
| Heptadecanoic acid | 17:0 | Saturated | 1.5 |
| Pentadecanoic acid | 15:0 | saturated | 1.03 |
| Oleic | 18:1n-9 | Monounsaturated | 43.39 |
| Linoleic | 18:2n-6 | Polyunsaturated | 29.66 |
| Linolenic | 18:3n-3 | Polyunsaturated | 4.78 |
| Stearic | 16:0 | Monosaturated | 4.93 |
3.8. Analysis of Fourier transforms infrared (FT-IR)
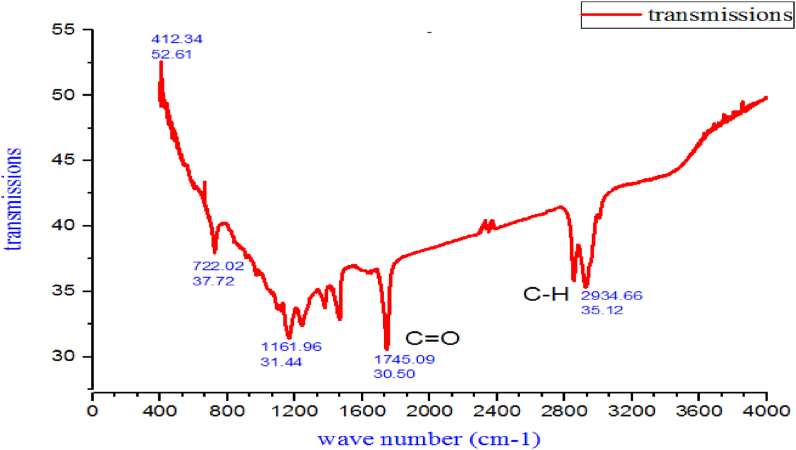
The FT-IR investigation was performed for qualitative analysis of the functional groups of the Croton macrostachyus seeds oil with the help of IR correlation charts. As indicated in Figure 5, the major functional groups oil extracted from Croton macrostachyus seeds oil was analyzed by FT- IR. The FTIR spectra was recorded at ambient temperature in the wavenumber and measurement of the samples was performed at a wavenumber range of 4000–400 cm−1.
Figure 5.
The FT-IR analysis of the extracted Croton macrostachyus seed oil.
The frequency of 2926 cm−1 and 2855 cm−1 indicates the asymmetrical stretching presence of the strong double bond group (Trans = C–H alkene) that represented by sp3 hybridized carbon molecules are found in the long carbon chain with a medium intensity of the functional group. The frequency of 1738 cm−1 shows bending of the vinyl C–H or cis = C–H bond of the olefinic group and it is associated with strong intensity. The frequency stretch of 1171–1197.17 cm−1 was assigned to the C–O ester carbonyl group with its characteristic strong intensity and stretching vibration. The frequency range of 1870 to 1540 cm−1 was attributed to the = C–H trans or cis – di substituted alkene group. Thus, it can be said that the functional groups present in Croton macrostachyus seeds oil are CH2 and CH3 of the saturated aliphatic compounds, the C=O of the dimerized carboxylic acid group, the C–O ester carbonyl group, and the trans or cis – di substituted alkene group (Wu et al., 2018).
4. Conclusion
Croton macrostachyus seeds are an affordable source of antibacterial and antifungal activity. The physicochemical properties of n-hexane extracted oil from Croton macrostachyus seed were analyzed and the optimum oil yield was obtained at the extraction temperature of 70 °C, particle size of 0.5 mm, extraction time of 5 h, and solute to solvent ratio 0.6 g/ml. The extraction temperature, particle size, and extraction time significantly affected the oil yield but solute to solvent ratio had no significant effect on the extraction process. The extracted oil from Croton macrostachyus seeds gave positive result against tested microorganisms with minimum inhibition concentration values between 31.25 and 500 mg/ml showed the highest zone of inhibition against microorganisms. The phytochemical analysis also revealed the presence of bioactive components like alkaloids, tannins, flavonoids, saponins, polyphenols, and terpenoids. The presence of these phytochemical constituents showed that the extracted Croton macrostachyus seed oil possesses antimicrobial activities against skin disease-causing definite pharmacological action on the human body. Thus it can be concluded that antimicrobial activity and its active components would be helpful in developing the Croton macrostachyus seed as medicinal raw material.
Declarations
Author contribution statement
Yigezu Mekonnen Bayisa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tafere Aga Bullo: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdulrasheed M., Ibrahim I., Mubarak M., Umar F. Comparison of antimicrobial activity of seed oil of garlic and Moringa oleifera against some food-borne microorganisms. Bayero J. Pure Appl. Sci. 2016;8(2):196. [Google Scholar]
- Apaydin-Varol E., Pütün A.E. Preparation and characterization of pyrolytic chars from different biomass samples. J. Anal. Appl. Pyrol. 2012;98:29–36. [Google Scholar]
- Aylate A., Agize M., Ekero D., Kiros A., Ayledo G., Gendiche K. In-vitro and in-vivo antibacterial activities of Croton macrostachyus methanol extract against E. Coli and S. Aureus. Adv. Anim. Vet. Sci. 2017;5(3):107–114. [Google Scholar]
- Ayoola G.A., Coker H.A.B., Adesegun S.A., Adepoju-bello A.A., Obaweya K., Ezennia E.C., Atangbayila T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop. J. Pharmaceut. Res. 2008;7:1019–1024. [Google Scholar]
- Bantie L., Assefa S., Teklehaimanot T., Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Compl. Alternative Med. 2014;14(1):1–10. doi: 10.1186/1472-6882-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrehiwot H., Zelelew D., Gebremariam A. Chemical analysis and medicinal activities of volatile components from the seeds of Croton macrostachyus plant. Int. J. Sci. Basic Appl. Res. 2018;37(2):316–330. [Google Scholar]
- Haile M., Duguma H.T., Chameno G., Kuyu C.G. Effects of location and extraction solvent on physico chemical properties of Moringa stenopetala seed oil. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraqui P., Yadav R.N.S. Evaluation of antibacterial and antifungal activities of leaf and seed extracts of Croton tiglium plant against skin disease causing microbes. Int. J. Res. Stud. Biosci. 2015;3(5):139–144. www.arcjournals.org [Google Scholar]
- Jalili F., Jafari S.M., Emam-Djomeh Z., Malekjani N., Farzaneh V. Optimization of ultrasound-assisted extraction of oil from Canola seeds with the use of response surface methodology. Food Anal. Methods. 2018;11(2):598–612. [Google Scholar]
- Jiao J., Li Z.G., Gai Q.Y., Li X.J., Wei F.Y., Fu Y.J., Ma W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chem. 2014;147:17–24. doi: 10.1016/j.foodchem.2013.09.079. [DOI] [PubMed] [Google Scholar]
- Kindermann M., Weis K., Lippert C. 2007. Tropentag 2007 University of Kassel-Witzenhausen and University of Göttingen; pp. 9–11. 2007. [Google Scholar]
- Krzyczkowska J., Kozłowska M. Effect of oils extracted from plant seeds on the growth and lipolytic activity of Yarrowia lipolytica Yeast. JAOCS - J. Am. Oil Chem. Soc. 2017;94(5):661–671. doi: 10.1007/s11746-017-2975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresa A., Ashebir Rekik, Worku G., Firehiwot Hirut, Abiy E., Samuel A.T. 2019. Ethno Medicinal Uses, Phytochemistry, and Anti-malarial Effect of Croton Ethno Medicinal Uses , Phytochemistry and Anti- Malarial Effect of Croton Macrostachyus ( Bisana ): A Review. Ethno Medicinal Uses , Phytochemistry and Anti-malarial Effect of Croton Ethno Medicinal Uses , Phytochemistry and Anti- Malarial Effect of Croton Macrostachyus ( Bisana ) [Google Scholar]
- Nigussie D., Davey G., Tufa T.B., Brewster M., Legesse B.A., Fekadu A., Makonnen E. Antibacterial and antifungal activities of Ethiopian medicinal plants: a systematic review. Front. Pharmacol. 2021;12:1–17. doi: 10.3389/fphar.2021.633921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okokon J.E., Nwafor P.A. Antimicrobial activity of root extract and crude fractions of Croton zambesicus. Pak. J. Pharm. Sci. 2010;23(1):114–118. [PubMed] [Google Scholar]
- Owade J.O., Gachuiri C.K., Abong G.O. Utilization of Croton seed as a possible animal Feed : a review. Online J. Anim. Feed Res. 2019;9(4):178–186. www.ojafr.ir [Google Scholar]
- Reshad A.S., Tiwari P., Goud V.V. Extraction of oil from rubber seeds for biodiesel application:Optimization of parameters. Fuel. 2015;150:636–644. [Google Scholar]
- Saha P., Talukdar A. Das, Ningthoujam S.S., Choudhury M.D., Nath D., Nahar L., Sarker S.D., Basar N. Chemical composition, antimicrobial and antioxidant properties of seed oil plants of North-East India: a review. Tang [Humanitas Medicine] 2015;5(3):17.1–17.22. [Google Scholar]
- Shetty S.B., Mahin-syed-ismail P., Varghese S., Thomas-george B., Kandathil P. Antimicrobial effects of Citrus sinensis peel extracts against dental caries bacteria : an in vitro study. 2016;8(1) doi: 10.4317/jced.52493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadele A. Ethiopian Public Health Institute; 2017. Ethiopian Herbal Medicine Research Article Profile. Part 1, 1–343. [Google Scholar]
- Tamil Selvi A., Dinesh M.G., Satyan R.S., Chandrasekaran B., Rose C. Leaf and Seed extracts of Bixa orellana L. Exert anti-microbial activity against bacterial pathogens. J. Appl. Pharmaceut. Sci. 2011;1(9):116–120. [Google Scholar]
- Wu H., Li C., Li Z., Liu R., Zhang A., Xiao Z., Ma L., Li J., Deng S. Simultaneous extraction of oil and tea saponin from Camellia oleifera Abel. Seeds under subcritical water conditions. Fuel Process. Technol. 2018;174:88–94. [Google Scholar]
- Zerihun M., Berhe H. Comparative assessment of some physicochemical properties of different sesame varieties and oil yield, Ethiopia. Bioprocess Eng. 2020;4(1):23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article