Abstract
The aims of the current study were to examine the relationships between heart rate variability (HRV), salivary cortisol, sleep duration and training in young athletes. Eight athletes (16 ± 1 years) were monitored for 7 weeks during training and competition seasons. Subjects were training for endurance-based winter sports (cross-country skiing and biathlon). Training was divided into two zones (K1, easy training and K2, hard training). Heart rate and blood lactate during submaximal running tests (SRT), as well as cortisol, sleep duration and nocturnal HRV (RMSSD), were determined every other week. HRV and cortisol levels were correlated throughout the 7-week period (r = -0.552, P = 0.01), with the strongest correlation during week 7 (r = -0.879, P = 0.01). The relative changes in K1 and HRV showed a positive correlation from weeks 1-3 (r = 0.863, P = 0.006) and a negative correlation during weeks 3-5 (r = -0.760, P = 0.029). The relative change in sleep during weeks 1-3 were negatively correlated with cortisol (r = -0.762, P = 0.028) and K2 (r = -0.762, P = 0.028). In conclusion, HRV appears to reflect the recovery of young athletes during high loads of physical and/or physiological stress. Cortisol levels also reflected this recovery, but significant change required a longer period than HRV, suggesting that cortisol may be less sensitive to stress than HRV. Moreover, our results indicated that during the competition season, recovery for young endurance athletes increased in duration and additional sleep may be beneficial.
Key points.
Nocturnal HRV appears to correlate with salivary levels of cortisol in young endurance athletes.
Recovery during the competition season, despite a decrease in physical training, may require additional time.
Throughout the race season, young athletes may benefit from increased sleep.
Key words: Recovery, endurance training, physiological stress, individual adaptations, submaximal tests, autonomic nervous system
Introduction
During the past four decades, increasing emphasis has been placed on training young athletes, many of whom now train all year round (Brenner, 2016). At the same time, most investigations on responses to endurance training have involved adult subjects and considerably less is known about the trainability and development of younger individuals (Naughton et al., 2000; Murray, 2017). Moreover, in addition to the stress of daily training, the added stress of their studies affects the recovery of young athletes. Therefore, research on the training of young athletes, focused on reaching a high level of performance, is necessary for attaining maximal gains and allowing young athletes to succeed in elite sports (Brenner, 2016; Murray, 2017).
Physiological processes that occur during sleep are a fundamental aspect of an athlete’s recovery and subsequent ability to train and compete at maximal capacity (Samuels, 2008; Brand and Kirov, 2011). However, both the quality and quantity of sleep by young athletes has been declining (Samuels, 2008; Copenhaver and Diamond, 2017) for a number of reasons, including training schedules, education, social events and travel plans (Copenhaven and Diamond, 2017; Simpson et al., 2017) as well as chronic and acute stress. It is known that the human response to stress is largely regulated by the autonomic nervous system (ANS) and, therefore, can be observed easily and non-invasively by measuring beat-to-beat variation in resting heart rates, also known as heart rate variability (HRV) (Electrophysiology, 1996; McEwen, 2007). Numerous studies have investigated the effects of endurance training on HRV (Pichot et al., 2000; Hautala et al., 2001; Carter et al., 2003; Kiviniemi et al., 2007) and recent studies suggest that nocturnal recordings help further evaluate an individual’s accumulated training load (Pichot et al., 2000; Hynynen et al., 2010). HRV measurements during sleep provide a measurement that is independent of external factors and therefore, enhances their reliability (Pichot et al., 2000; Buchheit et al., 2004; Nummela et al., 2010). Furthermore, nocturnal HRV appears to have a dose-response relationship with increased exercise intensity causing a reduction in nocturnal HRV (Hynynen et al., 2010). Thus, sleep duration and nocturnal HRV measurements may be effective measures to monitor recovery in young athletes.
Cortisol is one of the most frequently investigated hormones as a measure of overtraining and stress. Extended periods of increased or decreased levels of cortisol have a negative impact on health and therefore, may hinder athletic performance (Duclos et al., 2007). The relationship between cortisol and exercise as well as the different methods used for measuring cortisol secretion have conflicting results (Neary et al., 2002; Duclos et al., 2007). However, several studies have shown a strong relationship between serum and salivary cortisol levels indicating that salivary cortisol is a reliable measurement method, a good biomarker for physiological stress and a non-invasive option for monitoring athletes (Neary et al., 2002; Gustafsson et al., 2008; Hellhammer et al., 2009). During a 37-week follow up, salivary cortisol increased with increased training but when training was reduced, no change was observed and no relationship was found between performances (Chatard et al., 2002). In addition, a repeated exercise prescription does not appear to elicit the same changes in resting levels of cortisol with previous research finding that cortisol decreased considerably between two different maximal tests (Hedelin et al., 2000). Maximal tests are a good measure of performance but require a highly intensive exertion, reducing their application to everyday training (Lamberts et al., 2011; Capostagno et al., 2016). As a result, submaximal tests are used more frequently to monitor athletes and predict performance (Lamberts et al., 2011). Submaximal treadmill tests have been compared to cycle tests in athletes training for triathlon, and findings showed that both testing modes could be used interchangeably (Basset and Boulay, 2003). Running is a common training mode for both cross-country skiers and biathletes, allowing SRT to be a valid test for monitor training in this study.
Although monitoring training load at the elite level is common practice, there is no well-defined boundary between effective and ineffective training adaptations (Gustafsson et al., 2008). Previous research has shown that the utilization of HRV measurements in sport is challenging due to inconsistent procedures making comparison of results problematic as well as time constraints reducing the overall athlete compliance, especially over a long season (Rave et al., 2018). Increases in technology have introduced monitoring options that are easily accessible and collected with minimal effort. However, these measures are often performed in home environments. Therefore, investigating “real-life” values is highly relevant and may provide future understanding that is highly applicable for athletes and coaches. Accordingly, this study was designed to characterize the relationships between HRV, salivary levels of cortisol, sleep duration, and blood lactate during submaximal running tests (SRT) in young athletes during their training and competition seasons. Our main hypothesis was that nocturnal HRV exhibits a negative relationship to salivary cortisol levels in the morning. We also hypothesized that a decrease in sleep duration alone or in combination with more intense and prolonged training reduces nocturnal HRV and elevates morning cortisol levels.
Methods
Participants
Eight well-trained young endurance athletes participated in this study. The participants were all athletes at a sports academy high school competing and training for cross-country skiing (6 subjects) or biathlon (2 subjects) year round and participating at the national level. Characteristics of the athletes are presented in Table 1. All subjects were fully informed of the study procedures and gave written consent to participate in the project. The ethics committee of the University of Jyväskylä, Finland, approved the study and the measurements were performed in accordance with the declaration of Helsinki.
Table 1.
Characteristics of the subjects (means ± SD).
| Women (n = 5) | Men (n = 3) | |
|---|---|---|
| Age (yrs) | 16 ± 1 | 16 ± 1 |
| Body mass (kg) | 58 ± 5 | 67 ± 5 |
| Body fat (%)a | 16.2 ± 8.8 | 14.5 ± 11.1 |
| Training hours (y)b | 500 ± 76 | 600 ± 71 |
a Assessed on the basis of bioimpedence measurements.
b recorded in electronic training diaries.
Study Design
This study was performed during a 7-week period (November-December) that involved both a training (T1) and early competition (T2) training phase. ANS state was assessed with nocturnal HRV analysis, collected using a ballistiocardiographic (BCG) sleep-tracking device (Emfit QS, Jyväskylä, Finland). Additional assessment tests occurred on four separate occasions: twice during the training season (weeks 1, 3; T1) and twice during the competition season (weeks 5, 7; T2). During each test week, subjects participated in SRT and saliva samples were collected for three consecutive days; one day before SRT, the test day and one day after SRT. Athletes recorded their own individual training plans during this time and training characteristics (easy training, hard training and training load) were evaluated via electronic training diaries. Body fat percentage was measured using the bioimpedance method (InBody 720, Inbody CO., Cerritos, California, USA) in the beginning of the training period.
HRV and Sleep Analysis
Previous research has demonstrated that determination of HRV on the basis of BCG is both accurate and reliable (Shin et al., 2011; Wang et al., 2015). The Emfit QS device (EMFIT QS, Emfit OY, Jyväskylä, Finland) consists of a contactless pressure sensor (542mm x 70mm x 1.4mm) that utilizes BCG to interpret repeated movements of the human body, such as heartbeat, by representing them graphically (Pinheiro et al., 2010). Evaluation of this device during one night of sleep under real-life conditions revealed good agreement with the measurements provided by a reference device that employs electrocardiography and has been validated in laboratory studies, with only very minor differences in the mean HR and HRV values obtained (Vesterinen et al., 2020). Thus, although the reliability of this device for monitoring these parameters during sleep has yet to be established, it would appear to provide a simple and effective tool for automatic daily analysis of HRV.
Therefore, the time spent sleeping, nature of the sleep, and associated HR and HRV were monitored with an Emfit QS device for 7 weeks here. To minimize the distance to the heart and thereby maximize signal quality, this device was placed under the mattress near the chest in a manner such that the subject was unaware of its presence. The device began to record automatically at a sampling rate of 100 Hz when it sensed body weight and continued throughout the night, stopping when the subject got out of bed in the morning.
This monitoring of nocturnal HRV and HR was collected in continuous 3-minute periods and data from periods in which the signal was poor and/or disrupted was excluded. The magnitude of the HRV is expressed relative to time, utilizing the root-mean-squared difference between successive RR intervals (RMSSD, ms). For this purpose, the average RMSSD for each 3-minute period was calculated and these averages used to visualize the nocturnal HRV values graphically (Figure 1). The endpoints of the best linear fit for each night were considered to be the average RMSSD values for evening and morning sleep and the latter taken to be the HRV RMSSD value (HRVNOC) during that night of sleep. Although previously the average 3-minute values for the entire night have been used to calculate this value (Vesterinen et al., 2020), the current investigation focused on an individual’s current state of recovery and readiness to train, so the morning value was considered to be more relevant. For monitoring sleep patterns outside the laboratory, wrist actigraphy is the approach most widely used and best validated (Van De Water et al., 2011), but, at the same time, devices incorporated into the bed are highly convenient. These eliminate the need for attachment of electrodes or sensors to the body, providing a valuable option for longer-term monitoring of sleep at home. Such devices identify the different classes of sleep, as well as periods of wakefulness, with good accuracy (Yi et al., 2019).
Figure 1.
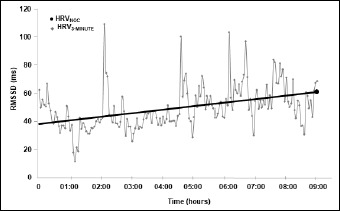
A representative example of the 3-minute sampling of the root mean square of successive differences between RR intervals (RMSSD) for heart rate variability (HRV) during sleep. The line shows the best fit used to calculate nocturnal HRV (HRVNOC).
The sleep duration and HRVNOC values analyzed here are presented automatically on the user interface of the Emfit QS device, providing easy daily access to both coaches and athletes. These values were also calculated as 3-day averages for comparison with cortisol values and the periods of SRT testing.
Cortisol analysis
Cortisol levels were analyzed from morning saliva samples. At the beginning of the testing period, all subjects were instructed on how to handle and collect the saliva samples. Collection occurred immediately after waking up before eating, drinking or brushing teeth. The passive drool-method was used for saliva collection and small cups were provided so that subjects could take 100 mL of water to wash out their mouth before collecting the sample. Subjects were asked to provide at least 3 mL of saliva for each sample as well as record the date, the time of day and how long it took to complete the procedure. Saliva samples were taken on 3 consecutive days, allowing for a sample the day before, the day of and one day after the SRT measurements occurred. Saliva samples were immediately placed into subjects’ freezers and collected every other week throughout the testing period.
Saliva samples were analyzed using the chemiluminescence method with the IMMULITE 2000 XPi Analyzer (Siemens Healthcare Diagnostics Products Ltd., Glyn Rhonwy, Llanberis, UK). The sensitivity of the saliva assay for cortisol was 5.5 nmol/l with inter-assay precision 8.2 % at 12.5 nmol/l. This method provides a noninvasive, easily repeatable and practical way to assess the cortisol response. Saliva cortisol values were analyzed in 3-day average values to coincide with the HRV values and SRT testing periods.
Training Analysis
Individual training plans were followed throughout the testing period. Subjects were asked to write down all training sessions daily including the intensity, duration and exercise mode in their electronic training diaries (elogger.net, Espoo, Finland). Subjects had participated in prior maximal graded exercise tests that provided individually determined heart rate zones to guide training intensity on a daily basis with their individual heart rate monitors. Endurance training intensities were based on a 5-zone training distribution with zone 1 and 2 representing basic training (estimated: ≤ 2 mM blood lactate) and zone 3-5 representing all high intensity training. Training was analyzed according to the electronic training diaries. Easy training (zone 1 and 2) was defined as all training below aerobic threshold (K1, minutes) and hard training (zone 3-5) was defined as all training above aerobic threshold (K2). Weekly distribution of specific training modes for K1 and K2 training during the 7-week testing period are presented in Figure 2. Training load was quantified using a modified version of Lucia’s simplified TRIMP system (Anta and Esteve-Lanao, 2011). Training load was calculated with the following equation:
| TL = 1 x K1 + 2.5 x K2 |
TL = training load, K1 = time training under aerobic threshold, K2 = time training at/above aerobic threshold. Training values were expressed in minutes. The training load, K1 and K2 were calculated to determine weekly values and three-day average values when STR and cortisol measurements occurred.
Figure 2.
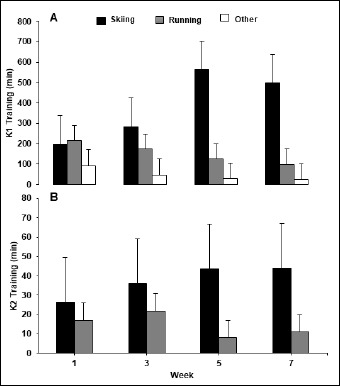
Graphs A and B represent the weekly distribution of exercise training modes for easy (K1) and hard (K2) training during all weeks of testing.
Submaximal Running Test
The SRT was 16-minutes in length and included 4 stages. Tests were performed on a Tunturi GO Run 50 Treadmill (Tunturi Fitness, Flevoland, Netherlands). The SRT was standardized for speed (women: 10 km/h, men: 11.7 km/h) with inclination increasing every 4 minutes, starting at 2%, then 4%, 7%, and 9%. One familiarization SRT was conducted so that all subjects were familiar with the test protocol. The SRT used in this study was designed for junior cross-country skiers and biathletes. Although we do not have validation of this protocol, it is a classic method used as a control test by athletes and coaches in Finland. Due to the homogenous group, the standardized protocol was appropriate and submaximal intensities were reached for all subjects. Heart rate (HR) was monitored with a HR-monitor (Polar V800, Polar Electro Oy, Kempele, Finland) and HR values were recorded when 15 s of each load remained. Subjects briefly stopped running and blood samples (20 μL) were taken from the fingertip every 4 minutes to determine blood lactate concentrations (Biosen C_line Lactate Analyzer. EKF Diagnostic, Magdeburg, Germany). Sample collection time (approx. 15 s) was included in the 4 minutes of upcoming stage.
Statistical Analysis
All statistical analyses were performed in the SPSS for Windows software (IBM SPSS Statistics 24 (SPSS, Inc., Chicago, IL, USA)). Since the number of subjects was small, Friedman’s non-parametric test for related samples was applied to analyze changes in training, HRV and salivary cortisol levels. Post-hoc analyses were performed with the Wilcox signed rank test. Spearman’s correlation coefficient was used to determine the relationship between 3-day average HRV and cortisol levels, as well as between HRV, sleep duration, cortisol levels and training characteristics. In addition, the relative changes in HRV, cortisol levels, sleep, SRT and training characteristics from week to week were also investigated using Spearman’s correlation coefficient. All values of HRV, sleep duration and cortisol level utilized were 3-day averages, whereas weekly averages were employed in the case of SRT and training characteristics. All values shown are means ± SD and statistical significance defined as p < 0.05.
Results
During all weeks of testing, HRV and salivary levels of cortisol were inversely related. Although the inter-individual differences were pronounced (Figure 3), the highest HRV and lowest level of cortisol were observed during week 5.
Figure 3.
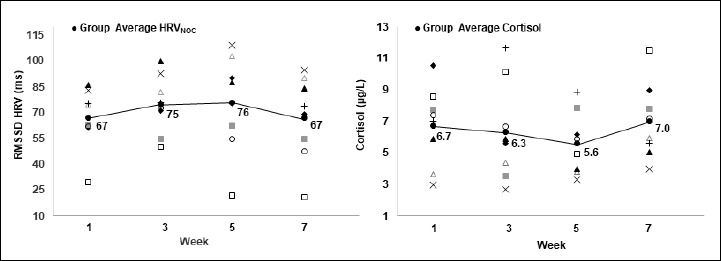
Average individual 3-day values for the root mean square of successive differences between RR intervals (RMSSD) and morning salivary levels of cortisol at different time-points during the 7-week study.
Relationships between HRV, Cortisol levels, sleep and training
Average values for HRV, levels of cortisol, sleep and weekly training values are shown in Table 2. Weekly correlations between these parameters are shown in Table 3. Figure 4 is a scatter plot of the relationship between HRV and cortisol during all weeks of testing combined. Figure 5 shows average daily values of HRV and cortisol throughout the testing period.
Table 2.
HRV, morning salivary levels of cortisol, nocturnal heart rate and sleep, amounts of hard and easy training, overall training load, and blood level of lactate and heart rate during submaximal running tests (SRT) at different time-points during the 7-week study period (means ± SD).
| T1, week 1 | T1, week 3 | T2, week 5 | T2, week 7 | |
|---|---|---|---|---|
| RMSSD (ms)a | 66.9 ± 17.8 | 75.0 ± 17.0* | 75.6 ± 28.7 | 67.0 ± 24.8* |
| Cortisol (μg/L)a | 6.7 ± 2.5 | 6.3 ± 3.1 | 5.6 ± 2.0 | 7.0 ± 2.4 |
| Hard training (min)b | 44 ± 30 | 64 ± 28 | 45 ± 17 | 53 ± 14* |
| Easy training (min)b | 508 ± 190 | 555 ± 151 | 743 ± 251 | 577 ± 136 |
| Training loadb | 616 ± 246 | 714 ± 209 | 856 ± 283 | 710 ± 132* |
| Blood lactate (mmol/L)c | 3.8 ± 1.0 | 3.6 ± 0.6 | 3.5 ± 0.6 | 3.7 ± 1.1 |
| Heart rate (bpm)c | 183 ± 6 | 184 ± 8 | 180 ± 9 | 182 ± 9 |
| Sleep (min)a | 482 ± 29** | 513 ± 36 | 513 ± 52 | 527 ± 44 |
| Nocturnal heart rate (bpm)a | 58 ± 5** | 55 ± 4 | 55 ± 3 | 54 ± 5 |
a Average 3-day values for the nocturnal root mean square of successive differences between RR intervals (RMSSD), nocturnal heart rate, night sleep and saliva levels of cortisol.
b Weekly values.
c Average during the final stage of the SRT.
* p < 0.05 compared to the previous week.
** p < 0.05 when compared to week 7.
Table 3.
Relationships (Spearman’s correlations) between HRVNOC and morning salivary level of cortisol, the amounts of hard and easy training, and overall training load during the different weeks of the study.
| Week | Cortisol (μg/L) | Hard training (min) | Easy training (min) | Training load |
|---|---|---|---|---|
| 1 | -0.833* | -0.287 | -0.071 | -0.095 |
| 2 | -0.238 | -0.036 | -0.048 | -0.132 |
| 3 | -0.524 | 0.024 | -0.238 | -0.143 |
| 4 | -0.833* | -0.571 | -0.264 | -0.143 |
* p < 0.05
Figure 4.
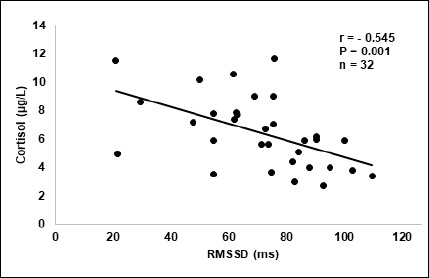
Graphs A and B represent the weekly distribution of exercise training modes for easy (K1) and hard (K2) training during all weeks of testing.
Figure 5.
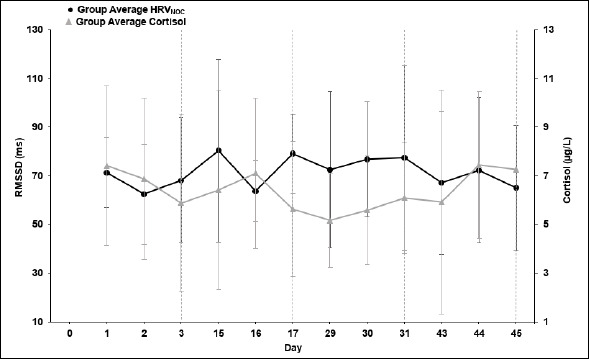
Daily values for the average root mean square of successive differences between RR intervals (RMSSD, ms) and morning salivary level of cortisol during the 7-week testing period.
As can be seen, the most pronounced correlation between HRV and morning cortisol levels was observed during week 1 and week 7 (r = -0.833, p < 0.05) (Table 3). The HRV values were significantly different between week 1 and week 3 (p < 0.05) and between week 5 and week 7 (p < 0.05). In addition, sleep and nocturnal sleep HR were both significantly different between week 1 and week 7 (p < 0.05) (Table 2). Differences between test weeks for cortisol and training values were not significant.
Throughout the training period (T1, weeks 1-3), a negative correlation was observed for relative changes in cortisol levels and sleep (r = -0.762, p < 0.05). The same relationship was demonstrated for K2 and sleep (week 1-3, r = -0.762, p < 0.05). During the shift from the training to the early competition season (week 3-5), cortisol displayed a positive relationship with K2 (r = 0.810, p < 0.05). When looking at HRV, a positive relationship was displayed for relative changes from week 5 to week 7 with sleep (r = 0.786, p < 05) and a negative relationship was observed for differences between weeks 1-7 (r = -0.714, p < 0.05) with sleep HR. In addition, the relative changes in K2 and HRV showed a positive correlation weeks 3-7 (r = 0.736, p < 0.05) and the same pattern was observed for relative changes in HRV and TL (week 3-7, r = 0.810, p < 0.05).
Associations between cortisol levels, training, and heart rate and levels of blood lactate during Submaximal Running Tests (SRT)
Cortisol’s response to changes in SRT varied throughout the testing period. Relative differences in week 3-5 demonstrated a negative relationship to both HR (r = -0.929, p < 0.01) and blood lactate (r = -0.857, p < 0.05). However, during the end of the testing period (week 5-7), the differences between cortisol levels displayed a positive relationship with the changes in the SRT HR values (r= 0.929, p < 0.01). In addition, a negative relationship between blood lactate and cortisol was observed during week 7 (r = -0.714, p < 0.05).
Monitoring of the distribution of training revealed that volume of K2 was the highest during week 3, while K1 and training load were both greatest during week 5, i.e., at the beginning of the competition season (Table 2). Distribution of training modes for K1 and K2 throughout the testing period can be viewed in Figure 2.
Discussion
The major findings of the current investigation were as follows: 1) HRVNOC and salivary cortisol correlated significantly during test weeks that displayed the lowest HRVNOC and highest salivary cortisol levels. 2) The decline in HRV from week 3 to week 7 was correlated with a reduced volume of intense training/training load, suggesting that this decline was not due to training stress, but rather the increase in fatigue/stress associated with the beginning of the competition period. 3) When the athletes were focused on training (weeks 1-3), the change in sleep duration was negatively correlated to both K2 and salivary cortisol levels, indicating that a reduction in the amount of sleep may be associated with elevated weekly strain. Finally, 4) although the amount of easy training and training load increased, a reduction in the volume of hard training is reflected in salivary cortisol levels with a positive relationship between changes in K2 and cortisol during weeks 3-5. Thus, young endurance athletes appear to handle large amounts of easy training when the volume of hard training is reduced.
During the 7-week study period, HRVNOC and cortisol displayed a negative relationship (Figure 4). When observing each test week separately, we found strong negative correlations between HRVNOC and cortisol during week 1 and 7 and moderate negative correlations for all remaining test weeks (Table 3). This indicates that cortisol and HRVNOC have a negative relationship for young endurance athletes.
The evaluation of three-day nocturnal RMSSD for the HRV analysis used in the current study is based on previous findings. Although daytime recordings are commonly used, it has been suggested that night readings enhance reliability since external factors are no longer affecting an individual during sleep (Pichot et al., 2000; Buchheit et al., 2004; Nummela et al., 2010). Earlier research has found that the time domain variable (RMSSD) has shown similar recovery times and changes to the commonly used high-frequency variables of HRV (Hautala et al., 2001; Carter et al., 2003), indicating RMSSD effectively evaluates change in autonomic regulation. Additionally, RMSSD measures have shown a high correlation to the high-frequency variability (Otzenberger et al., 1998; Esco et al., 2018) with various breathing frequencies having minimal effects on RMSSD values (Electrophysiology, 1996). Moreover, similar ballistocardiographic-based measures of nocturnal HRV have been compared to electrocardiographic measures and results showed that both HRV and HR data agreed with electrocardiography data that was also tested in real-life conditions (Vesterinen et al., 2020). These findings support the idea that HRVNOC may be a useful method to evaluate individual response for endurance training. Daily (Hautala et al., 2001; Carter et al., 2003; Kiviniemi et al., 2007; Hynynen et al., 2010; Herzig et al., 2017) and weekly HRV (Pichot et al., 2000; Nummela et al., 2010) values, which are both responsive to training, are commonly analyzed. However, since daily measurements can be influenced by pronounced diurnal variations, weekly averages may provide a better indication of adaptation to training and are recommended for use (Plews et al., 2013). At the same time, utilizing the combined values for nocturnal and morning HRV over several days instead of weekly values provides a better measure of rapid responses by the autonomic nervous system (Nuuttila et al., 2017). Additionally, although several studies found no sex differences in resting HRV values response to training of cross-country skiers (Hedelin et al., 2000, Schäfer et al., 2015), the averaging of several HRV values may help diminish the effect of individual confounders, such as gender, on HRV (Schäfer et al., 2015). Therefore, we analyzed the nocturnal morning RMSSD for three successive days, an approach that does not require special software and involves calculations that can be made easily, allowing its use not only in the laboratory, but in real life as well (Hynynen et al., 2010).
One important factor that may influence the daily changes in HRV values as well as the current state of recovery is sleep (Shinar et al., 2006). Sleep and overall levels of fatigue are highly interconnected, and sleep appears to have a critical role in the daily functioning during the adolescent years (Brand and Kirov, 2011). Sleep duration is a frequently and easily investigated measure for overall health and recommendations suggest that adolescents (13-18 years of age) should obtain 8-10 hours of sleep each night (Paruthi et al., 2016). In addition, athletes are advised to obtain additional sleep and ample research has reported the detrimental effects of sleep loss on human performance; demonstrating sleep is a valuable factor to observe in athletes (Fullagar et al., 2015; Simpson et al., 2017). Although monitored each night, sleep was not controlled during this study. Therefore, personal commitments (i.e. socializing, studying) and individual sleeping habits likely influenced the relationships between sleep and other investigated variables.
Previous research has discovered that even elite athletes are often unable to obtain the recommended amount of sleep (Roberts et al., 2019) with a recent review finding greater deficiencies in athletes’ sleep during competition periods (O’Donnell et al., 2018). Our findings differ from this tendency, with the greatest volume of sleep occurring during the competition period (Table 2). Additionally, the sleep duration during this study consistently remained within the recommended 8-10 hours. Although the quality of sleep was not monitored, previous research with young gymnasts found the overall quality of sleep was unaffected during a competition period (Sartor et al., 2017). Therefore, the positive correlation between changes in sleep and HRVNOC observed during the competition period (r = 0.786) indicates that individuals who obtained more sleep may have also experienced an enhanced recovery.
During this 7-week training period, training characteristics had a mixed effect on HRVNOC . Previous research has found conflicting responses with increases, decreases and no changes in HRV all occurring with an increased training load (Pichot et al., 2000; Hautala et al., 2001; Carter et al., 2003; Hynynen et al., 2010). It is evident that the exact mechanisms behind the effects of endurance training on HRV are not well-defined (Herzig et al., 2017). Moderate amounts of exercise have been shown to enhance vagal-related HRV indexes (Buchheit et al., 2004). Thus, the increase in HRVNOC from week 1 to week 3, when physical training increased, supports this previous finding. Moreover, HRVNOC was the highest, during week 5, when training load also reached its highest values, indicating there was a good tolerance to the present training stimulus. However, we found that the changes from week 3 to week 7 showed a positive correlation with decreases in volume of hard training, training load and HRV. Previous research found a decrease in nocturnal HRV values after both moderate and heavy endurance training sessions (Hynynen et al., 2010). Additionally, a progressive decrease in HRV values were found following a 3-week period of intensive training (Pichot et al., 2000). In the present study, the volume of hard training and training load were reduced, indicating that the decrease in HRVNOC was not associated to current training induced stress. Since the period of training analyzed in this study occurred during the initial phase of competition, all subjects also had the common goal of preparing for the early stage of their competition season. Therefore, as a group, weekly training followed similar and expected training programs with an intentional increase in ski-specific training throughout the study to reduce training stress as competitions approached. Similar to physical stress (physical work, fatigue, dietary stress), physiological stress (emotional, anxiety, cognitive), such as anticipatory stress, may have increased during the competition phase due to race-induced pressure and mental preparations that occur prior to competition. An independent measure of anxiety was not included in this study; as a result, although it appears, it is hard to identify if the alterations in HRVNOC and cortisol were induced by competition stress. Nevertheless, previous research has shown that acute stress, due to an anticipatory task, had an impact on HRV during sleep and was associated with a decreased parasympathetic modulation, and therefore, resulted in lower HRV values (Hall et al., 2004), further supporting our current finding.
Salivary cortisol levels are frequently used as a biomarker of psychological stress and have shown a moderate association to perceived stress (Hellhammer et al., 2009). Previous literature has investigated salivary cortisol level’s response to exercise and found cortisol only significantly increased after high-intensity exercise with no changes occurring after low and moderate exercise (VanBruggen et al., 2011). Additionally, when investigating cortisol levels in over-trained and control athletes no significant differences between groups were found (Hynynen et al., 2006). Furthermore, both baseline and response to training cortisol levels are influenced by genetics (Feitosa et al., 2002) so individual variation has an added effect on cortisol values. In the present study, changes during week 3 to week 5 showed that salivary cortisol appeared to respond to the reduced volume of hard training by demonstrating a positive correlation (r = 0.810). The fact that week 5 included the highest amount of physical training and lowest salivary cortisol levels suggests that young athletes appear to handle large volumes of easy training and high training loads as long as the amount of hard training is reduced (Table 3). This current finding supports previous research that found, an increase of low-intensity training, equivalent to about 100% increase in training load, showed no changes in cortisol, although decreased performance occurred (Jürimäe et al., 2004). Our study displayed the greatest increase in cortisol from week 5 to week 7, during the early competition period, suggesting changes in salivary cortisol may be more related to the early season race schedule rather than amount of hard training. During week 6, the competition season began with 7/8 subjects participating in their first race. Therefore, when interpreting cortisol results, the stress from racing is an important factor to consider. Previous research has found an increase in morning and afternoon salivary cortisol levels during a competition day, regardless of similar training volume and intensity, indicating competition may alter the physiology of stress-related hormones (Iellamo et al., 2003).
In addition, a decrease in sleep quality and duration has been associated with raised cortisol concentrations as well as an increase in activity of the sympathetic nervous system (Spiegel et al., 1999). In the present study, the training stimulus and sleep duration remained similar each week; therefore, our findings support the idea that competition stress may have increased morning cortisol levels. However, the cortisol values presented in our study were not collected on race day, so it is hard to know if a competition-induced stress was still present. The analysis of 3-day average salivary cortisol levels used in the present study shifts the focus to the total stress that was occurring each week rather than the stress response of an individual competition. When the subjects were focused on training (week 1 to week 3), changes in sleep duration revealed a negative relationship with both K2 and cortisol, proposing a decrease in sleep may be associated to an increased amount of weekly strain. This agrees with findings that found high intensity training negatively affected both subjective sleep parameters and recovery-related ratings (Kölling et al., 2016).
Furthermore, in endurance sports, the parasympathetic form of overtraining syndrome often dominates (Lehmann et al., 1993). Therefore, the increase in physical training during week 5, followed by the competition stress during week 6, may have resulted in a delayed fatiguing affect that was displayed during week 7. Pro-longed stress causes an increase in cortisol as well a decrease parasympathetic activity (McEwen, 2007) which may explain why an increase in cortisol was found as well as a decrease in HRV during week 7. Additionally, an anti-inflammatory process occurs due to training as well as muscle damage. Therefore, the elevation of cortisol may be associated to the greater training volume during week 5 or a result of a maximal race effort causing added stress and increased stimulation of glycogen re-synthesis (Kirwan et al., 1998).
In the present study, performance/recovery status was followed with SRT. As illustrated in Figure 2, running is a common exercise mode in both hard and easy training; therefore, a SRT was applicable for monitoring fatigue with this group of subjects. The easily repeatable design (based on speed and inclination) of this testing protocol provides a test that can be conducted in various training environments, such as at training camps or after long travels, to help athletes and coaches determine current levels of fatigue. Although the lack of individualized exercise intensities may reduce the reliability of the test, the repeated design provides valuable heart rate and lactate data at standardized exercise intensities during the 7-week period and significant changes in this data would indicate that levels of fatigue should be further investigated. Previous literature supports the application of submaximal tests for monitoring and predicting performance (Lamberts et al., 2004), but details the importance of implementing multiple variables so adequate insight of individual status is applied when interpreting results (Capostagno et al., 2016). As a result, we investigated the relationships between SRT heart rate, SRT blood lactate, morning salivary cortisol, HRVNOC, and physical training. During controlled submaximal intensities, HR has shown to remain constant with the lowest variation occurring at 90% HRmax values (Lamberts et al., 2004). In the current study, changes of SRT heart rate (around 90% VO2max) between week 5 and 7 displayed a strong relationship with changes in cortisol (r = 0.929). Cortisol demonstrated an additional relationship between week 3 and 7 with a negative correlation to changes in SRT heart rate (r = -0.929) and blood lactate (r = -0.857). Common assumptions about changes in HR at submaximal intensities suggest that an increase in aerobic fitness is linked to decreases in HR, while increases in HR are associated with a decline in fitness, dehydration or overtraining (Lamberts et al., 2004). Earlier research additionally suggests reduced submaximal HR is only a sign of effective endurance training when no decline in maximal performance is present (Hedelin et al., 2000). Therefore, without maximal HR values it is hard to evaluate the relationship between SRT HR and resting cortisol values, which also have mixed results. In addition, in order to detect significant changes in SRT, it is recommended that the HR values are approximately 7 bpm different at 90% HRmax workload (Lamberts et al., 2004) and therefore, fluctuations during the present study were too small to interpret any training induced changes.
Since variation in response to training stress is an apparent difference, it is logical to assume that monitoring variables, such as HRV, that also have an individualized response to training stress would be beneficial for optimizing performance. Research has investigated the response to endurance training and numerous factors have helped explain these differences such as, genotype, training background, gender, age, training load, etc (Carter et al., 2003; Buchheit et al., 2004; Nummela et al., 2010). In addition, large differences were observed despite prescribing the same amount of volume and modifying intensity training individually (Nummela et al., 2010). During this study, although physical training was not standardized, comparable training occurred due to group training and competition schedules. Present findings showed similar weekly trends for both HRV and cortisol but individual differences were high, agreeing with previous findings (Figure 3). Due to this high intra-individuality, previous research has investigated and implemented HRV-guided training into endurance training programs. HRV-guided training resulted in a lower frequency of high-intensity exercises and therefore, a decreased training load (Kiviniemi et al., 2007). When the timing and amount of high intensity exercise is adjusted, a slight change in the training periodization occurs. A large training focus for young endurance athletes is building their aerobic capacity and an improved endurance comes from accumulated years of effective training. As a result, further research should follow the long-term effects on HRV and endurance training before implementing a HRV-guided approach to training in young athletes.
Limitations
Limitations of this study may have occurred due to the small sample size and the grouping of both genders, as well as the decreased standardization due to the collections of nocturnal HRV, sleep duration and morning cortisol values occurring at home. There may be various factors such as poor or disrupted sleep that are not associated to training but still affect morning cortisol and HRV values. Additionally, the assessment of physical training and training load came from self-accessed training diaries; therefore, they were solely based on subjective estimations of the training-induced stress that was occurring during this period. Finally, our subjects’ level of psycho-physiological stress may have risen when the period of competition began, due, e.g., to the pressure to perform well. This potential change was not evaluated independently and may have influenced the HRV, sleep duration and saliva cortisol levels. However, autonomic stress reactions do not differ between the source of stress, and most likely, the greatest influence is the overall stress/recovery balance.
Practical applications
Young athletes are in a developmental period and therefore, may display a greater sensitivity to stress (McEwen, 2007). Application of HRV values to monitor training could identify better-individualized training profiles (Nummela et al., 2010). This would be valuable information for coaches and athletes and when no access to laboratory settings is required, it can be used daily as well as at training camps where training load increases. Thus, our findings suggest three-day average HRV values may provide an accurate representation of young athletes’ current recovery status of the autonomic nervous system and would be a beneficial value to follow during the high stress training and competition periods.
In addition, it appears sleep is another tool that could be utilized to further facilitate success in young athletes. Although we did not examine sleep quality or the circadian rhythm of sleep in detail, our findings on sleep duration provide insights of potential value to many athletes. Our findings provide support for previous suggestions that improved awareness of the negative consequences of sub-optimal sleep, in particular between races during the season of competition, can help athletes optimize their training (Simpson et al., 2017). During the period of competition, the weekly training load becomes slightly more constant in order to maximize preparation for important competitions. As a result, daily stress often increases and, therefore, young endurance athletes may benefit from additional sleep during this period.
Conclusion
In conclusion, the present study shows that nocturnal HRV appears to correlate negatively with salivary levels of cortisol in young endurance athletes. Coaches and athletes should be aware that as the training season ended, the decline in physical training correlated to the decrease in HRV, suggesting more time is needed to reach full recovery once the competition season has begun. This indication that full recovery from competition requires more time is supported further by the positive relationship between sleep duration and HRV during the competition season which implies additional sleep may also be beneficial for performance. In addition, changes in cortisol during the competition season suggest that an increased stress occurs but whether this stress is specific to competition is still unknown. Future research should be designed to determine which specific variables best reflect recovery and should be utilized to monitor this aspect of training. Additional focus should also be placed on determining what variables and changes are most closely associated with improved performance, so that further characterization of these patterns and variables can help young athletes improve their performance.
Acknowledgements
The authors would like to thank the athletes, coaches and research assistants who participated in this study for their collaboration, cooperation and enthusiasm throughout the entire process. The Amer Cultural Foundation, Finland, supported this work. The experiments comply with the current laws of the country in which they were performed. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author who was an organizer of the study.
Biographies
Christina MISHICA
Employment
PhD student for the Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland
Degree
MSc
Research interests
Exercise physiology, with a focus on the development of young endurance athletes, especially in cross-country skiing
E-mail: christina.m.mishica@jyu.fi
Heikki KYRÖLÄINEN
Employment
Professor for the Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland
Degree
PhD
Research interests
Exercise physiology and biomechanics
E-mail: heikki.kyrolainen@jyu.fi
Esa HYNYNEN
Employment
Specialist, Sports Physiology at KIHU – Research Institute for Olympic Sports, Jyväskylä, Finland
Degree
PhD
Research interests
Exercise physiology, with a focus on performance, heart rate variability and stress, especially in cross-country skiing and competitive walking.
E-mail: esa.hynynen@kihu.fi
Ari NUMMELA
Employment
Chief Specialist, Sports Physiology at KIHU – Research Institute for Olympic Sports, Jyväskylä, Finland
Degree
PhD
Research interests
Exercise physiology, with a focus on speed and endurance training, performance testing, training load/recovery and altitude training.
E-mail: ari.nummela@kihu.fi
Hans-Christer HOLMBERG
Employment
Professor for the Department of Health, Education and Technology, Luleä University of Technology, Luleå Sweden
Degree
PhD
Research interests
Exercise physiology and biomechanics, with a focus on utilizing an integrated approach to transfer knowledge between research and elite sport, especially in cross-county and alpine skiing.
E-mail: integrativephysiobiomech@gmail.com
Vesa LINNAMO
Employment
Professor for the Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland
Degree
PhD
Research interests
Biomechanics, with a focus on motor control and neuromuscular adaptation, especially in Nordic winter sports.
E-mail: vesa.linnamo@jyu.fi
References
- Anta R.C., Esteve-Lanao J. (2011) Training load quantification in triathlon. Journal of Human Sport and Exercise 6, 218-232. https://doi.org/10.4100/jhse.2011.62.03 10.4100/jhse.2011.62.03 [DOI] [Google Scholar]
- Basset F.A., Boulay M.R. (2003) Treadmill and cycle ergometer tests are interchangeable to monitor triathletes annual training. Journal of Sports Science & Medicine 2, 110. https://pubmed.ncbi.nlm.nih.gov/24627663/ [PMC free article] [PubMed] [Google Scholar]
- Brand S., Kirov R. (2011) Sleep and its importance in adolescence and in common adolescent somatic and psychiatric conditions. International Journal of General Medicine 4, 425-442. https://doi.org/10.2147/IJGM.S11557 10.2147/IJGM.S11557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner J.S. (2016) Sports specialization and intensive training in young athletes. Pediatrics 138, 154-157. https://doi.org/10.1542/peds.2016-2148 10.1542/peds.2016-2148 [DOI] [PubMed] [Google Scholar]
- Buchheit M., Simon C., Piquard F., Ehrhart J., Brandenberger G. (2004) Effects of increased training load on vagal-related indexes of heart rate variability: a novel sleep approach. American Journal of Physiology-Heart and Circulatory Physiology 287, 2813-2818. https://doi.org/10.1152/ajpheart.00490.2004 10.1152/ajpheart.00490.2004 [DOI] [PubMed] [Google Scholar]
- Capostagno B., Lambert M.I., Lamberts R.P. (2016) A systematic review of submaximal cycle tests to predict, monitor, and optimize cycling performance. International Journal of Sports Physiology and Performance 11, 707-714. https://doi.org/10.1123/ijspp.2016-0174 10.1123/ijspp.2016-0174 [DOI] [PubMed] [Google Scholar]
- Carter J.B., Banister E.W., Blaber A.P. (2003) The effect of age and gender on heart rate variability after endurance training. Medicine and Science in Sports and Exercise 35, 1333-1340. https://doi.org/10.1249/01.MSS.0000079046.01763.8F 10.1249/01.MSS.0000079046.01763.8F [DOI] [PubMed] [Google Scholar]
- Chatard J.C., Atlaoui D., Lac G., Duclos M., Hooper S., Mackinnon L. (2002) Cortisol, DHEA, performance and training in elite swimmers. International Journal of Sports Medicine 23, 510-515. https://doi.org/10.1055/s-2002-35073 10.1055/s-2002-35073 [DOI] [PubMed] [Google Scholar]
- Copenhaver E.A., Diamond A.B. (2017) The value of sleep on athletic performance, injury, and recovery in the young athlete. Pediatric Annals 46, 106-111. https://doi.org/10.3928/19382359-20170221-01 10.3928/19382359-20170221-01 [DOI] [PubMed] [Google Scholar]
- Duclos M., Guinot M., Le Bouc Y. (2007) Cortisol and GH: odd and controversial ideas. Applied Physiology, Nutrition, and Metabolism 32, 895-903. https://doi.org/10.1139/H07-064 10.1139/H07-064 [DOI] [PubMed] [Google Scholar]
- Electrophysiology Task Force of the European Society of Cardiology the North American Society of Pacing. (1996) Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93, 1043-1065. https://doi.org/10.1161/01.CIR.93.5.1043 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Esco M.R., Williford H.N., Flatt A.A., Freeborn T.J., Nakamura F.Y. (2018) Ultra-shortened time-domain HRV parameters at rest and following exercise in athletes: an alternative to frequency computation of sympathovagal balance. European Journal of Applied Physiology 118, 175-184. https://doi.org/10.1007/s00421-017-3759-x 10.1007/s00421-017-3759-x [DOI] [PubMed] [Google Scholar]
- Feitosa M.F., Rice T., Rosmond R., Borecki I.B., An P., Gagnon J., Leon A.S., Skinner J.S., Wilmore J.H., Bouchard C., Rao D.C. (2002) A genetic study of cortisol measured before and after endurance training: the HERITAGE Family Study. Metabolism-Clinical and Experimental 51, 360-365. https://doi.org/10.1053/meta.2002.30519 10.1053/meta.2002.30519 [DOI] [PubMed] [Google Scholar]
- Fullagar H.H., Skorski S., Duffield R., Hammes D., Coutts A.J., Meyer T. (2015) Sleep and athletic performance: the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Medicine 45, 161-186. https://doi.org/10.1007/s40279-014-0260-0 10.1007/s40279-014-0260-0 [DOI] [PubMed] [Google Scholar]
- Gustafsson H., Holmberg H.C., Hassmen P. (2008) An elite endurance athlete’s recovery from underperformance aided by a multidisciplinary sport science support team. European Journal of Sport Science 8, 267-276. https://doi.org/10.1080/17461390802195652 10.1080/17461390802195652 [DOI] [Google Scholar]
- Hall M., Vasko R., Buysse D., Ombao H., Chen Q., Cashmere J.D., Kupfer D., Thayer J.F. (2004) Acute stress affects heart rate variability during sleep. Psychosomatic Medicine 66, 56-62. https://doi.org/10.1097/01.PSY.0000106884.58744.09 10.1097/01.PSY.0000106884.58744.09 [DOI] [PubMed] [Google Scholar]
- Hautala A., Tulppo M.P., Mäkikallio T.H., Laukkanen R., Nissilä S., Huikuri H.V. (2001) Changes in cardiac autonomic regulation after prolonged maximal exercise. Clinical Physiology 21, 238-245. https://doi.org/10.1046/j.1365-2281.2001.00309.x 10.1046/j.1365-2281.2001.00309.x [DOI] [PubMed] [Google Scholar]
- Hedelin R., Kenttä G., Wiklund U., Bjerle P.E.R., Henriksson-Larsén K. (2000) Short-term overtraining: effects on performance, circulatory responses, and heart rate variability. Medicine and Science in Sports and Exercise 32, 1480-1484. https://doi.org/10.1097/00005768-200008000-00017 10.1097/00005768-200008000-00017 [DOI] [PubMed] [Google Scholar]
- Hedelin R., Wiklund U., Bjerle P., Henriksson-Larsén K. (2000) Pre-and post-season heart rate variability in adolescent cross-country skiers. Scandinavian journal of medicine & science in sports 10(5), 298-303. https://doi.org/10.1034/j.1600-0838.2000.010005298.x 10.1034/j.1600-0838.2000.010005298.x [DOI] [PubMed] [Google Scholar]
- Hellhammer D.H., Wüst S., Kudielka B.M. (2009) Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34, 163-171. https://doi.org/10.1016/j.psyneuen.2008.10.026 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Herzig D., Testorelli M., Olstad D.S., Erlacher D., Achermann P., Eser P., Wilhelm M. (2017) Heart-rate variability during deep sleep in world-class alpine skiers: a time-efficient alternative to morning supine measurements. International Journal of Sports Physiology and Performance 12, 648-654. https://doi.org/10.1123/ijspp.2016-0257 10.1123/ijspp.2016-0257 [DOI] [PubMed] [Google Scholar]
- Hynynen E., Uusitalo A., Konttinen N., Rusko H. (2006) Heart rate variability during night sleep and after awakening in overtrained athletes. Medicine and Science in Sports and Exercise 38, 313. https://doi.org/10.1249/01.mss.0000184631.27641.b5 10.1249/01.mss.0000184631.27641.b5 [DOI] [PubMed] [Google Scholar]
- Hynynen E., Vesterinen V., Rusko H., Nummela A. (2010) Effects of moderate and heavy endurance exercise on nocturnal HRV. International Journal of Sports Medicine 31, 428-432. https://doi.org/10.1055/s-0030-1249625 10.1055/s-0030-1249625 [DOI] [PubMed] [Google Scholar]
- Iellamo F., Pigozzi F., Parisi A., Di Salvo V. (2003) The stress of competition dissociates neural and cortisol homeostasis in elite athletes. Journal of Sports Medicine and Physical Fitness 43, 539. [PubMed] [Google Scholar]
- Jürimäe J., Mäestu J., Purge P., Jürimäe T. (2004) Changes in stress and recovery after heavy training in rowers. Journal of Science and Medicine in Sport 7, 335-339. https://doi.org/10.1016/S1440-2440(04)80028-8 10.1016/S1440-2440(04)80028-8 [DOI] [PubMed] [Google Scholar]
- Kirwan J.P., Costill D.L., Flynn M.G., Mitchell J.B., Fink W.J., Neufer P.D., Houmard J.A. (1988) Physiological responses to successive days of intense training in competitive swimmers. Medicine & Science in Sports & Exercise 20, 255-259. https://doi.org/10.1249/00005768-198806000-00007 10.1249/00005768-198806000-00007 [DOI] [PubMed] [Google Scholar]
- Kiviniemi A.M., Hautala A.J., Kinnunen H., Tulppo M.P. (2007) Endurance training guided individually by daily heart rate variability measurements. European Journal of Applied Physiology 101, 743-751. https://doi.org/10.1007/s00421-007-0552-2 10.1007/s00421-007-0552-2 [DOI] [PubMed] [Google Scholar]
- Kölling S., Wiewelhove T., Raeder C., Endler S., Ferrauti A., Meyer T., Kellmann M. (2016) Sleep monitoring of a six-day microcycle in strength and high-intensity training. European Journal of Sport Science 16, 507-515. https://doi.org/10.1080/17461391.2015.1041062 10.1080/17461391.2015.1041062 [DOI] [PubMed] [Google Scholar]
- Lamberts R.P., Lemmink K.A., Durandt J.J., Lambert M.I. (2004) Variation in heart rate during submaximal exercise: implications for monitoring training. The Journal of Strength & Conditioning Research 18, 641-645. https://doi.org/10.1519/00124278-200408000-00044 10.1519/00124278-200408000-00044 [DOI] [PubMed] [Google Scholar]
- Lamberts R.P., Swart J., Noakes T.D., Lambert M.I. (2011) A novel submaximal cycle test to monitor fatigue and predict cycling performance. British Journal of Sports Medicine 45, 797-804. https://doi.org/10.1136/bjsm.2009.061325 10.1136/bjsm.2009.061325 [DOI] [PubMed] [Google Scholar]
- Lehmann M., Foster C., Keul J. (1993) Overtraining in endurance athletes: a brief review. Medicine & Science in Sports & Exercise 25, 854-862. https://doi.org/10.1249/00005768-199307000-00015 10.1249/00005768-199307000-00015 [DOI] [PubMed] [Google Scholar]
- McEwen B.S. (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews 87, 873-904. https://doi.org/10.1152/physrev.00041.2006 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- Murray A. (2017) Managing the training load in adolescent athletes. International Journal of Sports Physiology and Performance 12, 42-49. https://doi.org/10.1123/ijspp.2016-0334 10.1123/ijspp.2016-0334 [DOI] [PubMed] [Google Scholar]
- Naughton G., Farpour-Lambert N.J., Carlson J., Bradney M., Van Praagh E., (2000) Physiological issues surrounding the performance of adolescent athletes. Sports Medicine, 30, 309-325. https://doi.org/10.2165/00007256-200030050-00001 10.2165/00007256-200030050-00001 [DOI] [PubMed] [Google Scholar]
- Neary J.P., Malbon L., McKenzie D.C. (2002) Relationship between serum, saliva and urinary cortisol and its implication during recovery from training. Journal of Science and Medicine in Sport 5, 108-114. https://doi.org/10.1016/S1440-2440(02)80031-7 10.1016/S1440-2440(02)80031-7 [DOI] [PubMed] [Google Scholar]
- Nummela A., Hynynen E., Kaikkonen P., Rusko H. (2010) Endurance performance and nocturnal HRV indices. International Journal of Sports Medicine 31, 154-159. https://doi.org/10.1055/s-0029-1243221 10.1055/s-0029-1243221 [DOI] [PubMed] [Google Scholar]
- Nuuttila O.P., Nikander A., Polomoshnov D., Laukkanen J.A., Häkkinen K. (2017) Effects of HRV-guided vs. predetermined block training on performance, HRV and serum hormones. International Journal of Sports Medicine 38(12), 909-920. https://doi.org/10.1055/s-0043-115122 10.1055/s-0043-115122 [DOI] [PubMed] [Google Scholar]
- O’Donnell S., Beaven C.M, Driller M.W. (2018) From pillow to podium: a review on understanding sleep for elite athletes. Nature and Science of Sleep 10, 243. https://doi.org/10.2147/NSS.S158598 10.2147/NSS.S158598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otzenberger H., Gronfier C., Simon C., Charloux A., Ehrhart J., Piquard F., Brandenberger G. (1998) Dynamic heart rate variability: a tool for exploring sympathovagal balance continuously during sleep in men. American Journal of Physiology-Heart and Circulatory Physiology 275, 946-950. https://doi.org/10.1152/ajpheart.1998.275.3.H946 10.1152/ajpheart.1998.275.3.H946 [DOI] [PubMed] [Google Scholar]
- Paruthi S., Brooks L.J., D’Ambrosio C., Hall W.A., Kotagal S., Lloyd R.M., Malow B.A., Maski K., Nichols C., Quan S.F., Rosen C.L. (2016) Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine 12, 785-786. https://doi.org/10.5664/jcsm.6288 10.5664/jcsm.6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot V., Roche F., Gaspoz J.M., Enjolras F., Antoniadis A., Minini P., Costes F., Busso T., Lacour J.R., Barthelemy J.C. (2000) Relation between heart rate variability and training load in middle-distance runners. Medicine and Science in Sports and Exercise 32, 1729-1736. https://doi.org/10.1097/00005768-200010000-00011 10.1097/00005768-200010000-00011 [DOI] [PubMed] [Google Scholar]
- Pinheiro E., Postolache O., Girão P. (2010) Theory and developments in an unobtrusive cardiovascular system representation: ballistocardiography. The Open Biomedical Engineering Journal 4, 201. https://doi.org/10.2174/1874120701004010201 10.2174/1874120701004010201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plews D.J., Laursen P.B., Kilding A.E., Buchheit M. (2013) Evaluating training adaptation with heart-rate measures: a methodological comparison. International Journal of Sports Physiology and Performance, 8, 688-691. https://doi.org/10.1123/ijspp.8.6.688 10.1123/ijspp.8.6.688 [DOI] [PubMed] [Google Scholar]
- Rave G., Fortrat J.O., Dawson B., Carre F., Dupont G., Saeidi A., Boullosa D., Zouhal H. (2018) Heart rate recovery and heart rate variability: use and relevance in European professional soccer. International Journal of Performance Analysis in Sport, 18, 168-183. https://doi.org/10.1080/24748668.2018.1460053 10.1080/24748668.2018.1460053 [DOI] [Google Scholar]
- Roberts S.S.H., Teo W.P., Warmington S.A. (2019) Effects of training and competition on the sleep of elite athletes: a systematic review and meta-analysis. British Journal of Sports Medicine 53, 513-522. https://doi.org/10.1136/bjsports-2018-099322 10.1136/bjsports-2018-099322 [DOI] [PubMed] [Google Scholar]
- Samuels C. (2008) Sleep, recovery, and performance: the new frontier in high-performance athletics. Neurologic Clinics 26, 169-180. https://doi.org/10.1016/j.ncl.2007.11.012 10.1016/j.ncl.2007.11.012 [DOI] [PubMed] [Google Scholar]
- Sartor F., Capuzzoni S., Rospo G., La Torre A., Vailati F., Vailati E. (2017) Influence of competition day on cognitive control and HRV in young male gymnasts. The Journal of Strength & Conditioning Research 31, 1982-1993. https://doi.org/10.1519/JSC.0000000000001652 10.1519/JSC.0000000000001652 [DOI] [PubMed] [Google Scholar]
- Schäfer D., Gjerdalen G.F., Solberg E.E., Khokhlova M., Badtieva V., Herzig D., Trachsel L.D., Noack P., Karavirta L., Eser P., Saner H., (2015) Sex differences in heart rate variability: a longitudinal study in international elite cross-country skiers. European Journal of Applied Physiology, 115, 2107-2114. https://doi.org/10.1007/s00421-015-3190-0 10.1007/s00421-015-3190-0 [DOI] [PubMed] [Google Scholar]
- Shin J.H., Hwang S.H., Chang M.H., Park K.S. (2011) Heart rate variability analysis using a ballistocardiogram during valsalva manoeuvre and post exercise. Physiological Measurement 32, 1239. https://doi.org/10.1088/0967-3334/32/8/015 10.1088/0967-3334/32/8/015 [DOI] [PubMed] [Google Scholar]
- Shinar Z., Akselrod S., Dagan Y., Baharav A. (2006) Autonomic changes during wake-sleep transition: a heart rate variability based approach. Autonomic Neuroscience 130, 17-27. https://doi.org/10.1016/j.autneu.2006.04.006 10.1016/j.autneu.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Simpson N.S., Gibbs E.L., Matheson G.O. (2017) Optimizing sleep to maximize performance: implications and recommendations for elite athletes. Scandinavian Journal of Medicine & Science in Sports 27, 266-274. https://doi.org/10.1111/sms.12703 10.1111/sms.12703 [DOI] [PubMed] [Google Scholar]
- Spiegel K., Leproult R., Van Cauter E. (1999) Impact of sleep debt on metabolic and endocrine function. The Lancet 354, 1435-1439. https://doi.org/10.1016/S0140-6736(99)01376-8 10.1016/S0140-6736(99)01376-8 [DOI] [PubMed] [Google Scholar]
- Van De Water A.T., Holmes A., Hurley D.A. (2011) Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography-a systematic review. Journal of Sleep Research 20, 183-200. https://doi.org/10.1111/j.1365-2869.2009.00814.x 10.1111/j.1365-2869.2009.00814.x [DOI] [PubMed] [Google Scholar]
- VanBruggen M.D., Hackney A.C., McMurray R.G., Ondrak K.S. (2011) The relationship between serum and salivary cortisol levels in response to different intensities of exercise. International Journal of Sports Physiology and Performance 6, 396-407. https://doi.org/10.1123/ijspp.6.3.396 10.1123/ijspp.6.3.396 [DOI] [PubMed] [Google Scholar]
- Vesterinen V., Rinkinen N., Nummela A. (2020) A contact-free, ballistocardiography-based monitoring system (Emfit QS) for measuring nocturnal heart rate and heart rate variability: validation study. JMIR Biomedical Engineering 5, e16620. https://doi.org/10.2196/16620 10.2196/16620 [DOI] [Google Scholar]
- Wang K., Zhu T., Zhang X., Yu C., Cao X., Tang J., Wan Z. (2015) Comparison of heart rate variability measurements between ballistocardiogram and electrocardiography (Chinese). Zhonghua Xin Xue Guan Bing Za Zhi 43, 448-451. [PubMed] [Google Scholar]
- Yi R., Enayati M., Keller J.M., Popescu M., Skubic M. (2019) Non-invasive in-home sleep stage classification using a ballistocardiography bed sensor. In: 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Chicago, USA, 1-4. IEEE. https://doi.org/10.1109/BHI.2019.8834535 10.1109/BHI.2019.8834535 [DOI] [Google Scholar]


