Peptidyl-arginine deiminases (PADs) catalyse the post-translational modification of arginine residues in numerous proteins. The human type III isoform (PAD3) is specifically expressed in skin tissue and is required for normal skin and hair formation. The structure of human PAD3 has been determined at 2.8 Å resolution, giving insight into the substrate specificity of the enzyme.
Keywords: peptidyl-arginine deiminase, protein citrullination, calcium binding, hair follicles, post-translational modifications
Abstract
The Ca2+-dependent enzyme peptidyl-arginine deiminase type III (PAD3) catalyses the deimination of arginine residues to form citrulline residues in proteins such as keratin, filaggrin and trichohyalin. This is an important post-translation modification that is required for normal hair and skin formation in follicles and keratocytes. The structure of apo human PAD3 was determined by X-ray crystallography to a resolution of 2.8 Å. The structure of PAD3 revealed a similar overall architecture to other PAD isoforms: the N-terminal and middle domains of PAD3 show sequence and structural variety, whereas the sequence and structure of the C-terminal catalytic domain is highly conserved. Structural analysis indicates that PAD3 is a dimer in solution, as is also the case for the PAD2 and PAD4 isoforms but not the PAD1 isoform.
1. Introduction
Deimination (or citrullination) is a post-translational modification catalysed by peptidyl-arginine deiminases (PADs), the consequences of which are not yet fully understood (Rogers et al., 1977 ▸; Kubilus et al., 1979 ▸, 1980 ▸; Kubilus & Baden, 1983 ▸; Rothnagel & Rogers, 1984 ▸; Mondal & Thompson, 2019 ▸). Five paralogous human genes encoding highly conserved PAD enzymes (numbered PAD1–4 and PAD6) have been identified (Dong et al., 2006 ▸; Chavanas et al., 2008 ▸). These genes are clustered in a single chromosomal locus at 1p35–36 in the human genome. Peptidyl-arginine deiminase activity has been detected in most organs, tissues and cells of all vertebrates. The PAD isoforms are controlled at the transcriptional, translational and activity levels, and they each have particular substrate specificities (Kizawa et al., 2008 ▸; Knuckley et al., 2010 ▸; Assohou-Luty et al., 2014 ▸).
PADs deiminate arginine residues in proteins, converting them into citrulline residues, in a Ca2+-dependent manner (Shirai et al., 2001 ▸; Das et al., 2004 ▸; Knuckley et al., 2007 ▸). Although the exact physiological functions of protein deimination have not been clarified, the modification of the positively charged guanidino group of arginine to the neutral ureido group of citrulline dramatically alters the charge of the targeted substrates, which decreases the electrostatic interactions (both salt bridge and cation–π) between arginine and other amino-acid residues, impairing the formation of functional tertiary structures (Vossenaar et al., 2003 ▸).
The human PAD isoforms are highly conserved, with between 45% and 58% pairwise amino-acid sequence identity between them. The structures of the PAD1, PAD2 and PAD4 proteins have been determined, and they share a common architecture (Arita et al., 2004 ▸; Slade et al., 2015 ▸; Saijo et al., 2016 ▸). Their structure is composed of three domains: an N-terminal cupredoxin-like domain (also referred to as an IgG1 domain), a central IgG-like domain and a C-terminal catalytic domain. The N-terminal and central domains have a lower shared sequence identity than the catalytic C-terminal domain.
PAD3 is specifically expressed in skin tissue and hair follicles (Steinert et al., 2003 ▸; Méchin et al., 2005 ▸; Kizawa et al., 2008 ▸) and several in vivo substrates of the enzyme have been identified, in particular structural proteins: intermediate filament (IF) proteins such as keratins and IF-associated proteins such as filaggrin (Senshu et al., 1996 ▸), trichohyalin (Tarcsa et al., 1996 ▸) and S100A3 (a cysteine-rich Ca2+-binding protein present in hair cuticles; Kizawa et al., 2008 ▸). Generally, they are abundant proteins and have a high percentage of arginine in their primary sequence: trichohyalin contains 23% arginine and filaggrin contains 11% arginine. In vitro, PAD3 specifically deiminates a single arginine residue (Arg51) in S100A3, but the origin of this target specificity is unclear. Defects in PAD3 activity have been linked to hair and skin diseases such as uncombable hair syndrome (Basmanav et al., 2016 ▸), psoriasis (Ishida-Yamamoto et al., 2000 ▸), bullous congenital ichthyosiform erythroderma (Chavanas et al., 2006 ▸) and central centrifugal cicatricial alopecia (Malki et al., 2019 ▸).
Understanding the detailed molecular role of PAD3 in skin and hair physiology has been hindered by the lack of structural information about this isoform and its specific recognition of protein substrates. Crystals of PAD3 that diffracted to 2.95 Å resolution have previously been reported by Unno et al. (2012 ▸). However, a structure of this protein has not previously been published or submitted to the Protein Data Bank. As an initial step to understand the mechanism of specific protein deimination in the hair follicle and the skin, we here describe the first crystal structure of PAD3.
2. Materials and methods
2.1. Macromolecule production
The synthetic gene coding for human PAD3 (UniProt Q9ULW8) was cloned into the pDEST17 vector (Gateway system, Invitrogen, USA). The plasmid was transformed into Escherichia coli BL21(DE3)pLysS cells for expression (Table 1 ▸). The cells were grown at 310 K in Luria–Bertani broth containing 50 mg ml−1 ampicillin and 35 mg ml−1 chloramphenicol until mid-log phase (OD600 = 0.5–0.7). Protein expression was induced by the addition of 0.25 mM isopropyl β-d-1-thiogalactopyranoside, and the cells were then grown at 291 K. The cells were harvested by centrifugation (3500g for 20 min) and resuspended in lysis buffer (50 mM HEPES pH 7.8, 500 mM NaCl, 10 mM imidazole) before being lysed using a cell disruptor. Cell debris was spun out of the lysate at 40 000g for 40 min at 277 K. His6-tagged PAD3 was purified from the soluble lysate fraction by Ni2+-affinity chromatography using a 5 ml HisTrap HP column (GE Healthcare). To remove any nonspecific binding, the column was washed with (i) ten column volumes (CV) of binding buffer (50 mM HEPES pH 7.8, 500 mM NaCl, 10 mM β-mercaptoethanol, 5% glycerol, 10 mM imidazole), (ii) 5 CV wash buffer 1 (50 mM HEPES pH 7.8, 1 M NaCl, 10 mM β-mercaptoethanol, 20 mM imidazole, 5% glycerol), (iii) 5 CV wash buffer 2 (50 mM HEPES pH 7.8, 1 M NaCl, 10 mM β-mercaptoethanol, 20 mM imidazole, 5% glycerol, 10 mM ATP, 20 mM MgCl2, 20 mM KCl) and (iv) 5 CV wash buffer 3 (50 mM HEPES pH 7.8, 1 M NaCl, 10 mM β-mercaptoethanol, 20 mM imidazole, 10 mM ATP, 20 mM MgCl2, 150 mM KCl).
Table 1. Macromolecule-production information.
| Source organism | Homo sapiens |
| Expression vector | pDEST17 |
| Expression host | E. coli BL21(DE3)pLysS |
| Complete amino-acid sequence of the construct produced | MSYYHHHHHHLESTSLYKKAGFMSLQRIVRVSLEHPTSAVCVAGVETLVDIYGSVPEGTEMFEVYGTPGVDIYISPNMERGRERADTRRWRFDATLEIIVVMNSPSNDLNDSHVQISYHSSHEPLPLAYAVLYLTCVDISLDCDLNCEGRQDRNFVDKRQWVWGPSGYGGILLVNCDRDDPSCDVQDNCDQHVHCLQDLEDMSVMVLRTQGPAALFDDHKLVLHTSSYDAKRAQVFHICGPEDVCEAYRHVLGQDKVSYEVPRLHGDEERFFVEGLSFPDAGFTGLISFHVTLLDDSNEDFSASPIFTDTVVFRVAPWIMTPSTLPPLEVYVCRVRNNTCFVDAVAELARKAGCKLTICPQAENRNDRWIQDEMELGYVQAPHKTLPVVFDSPRNGELQDFPYKRILGPDFGYVTREPRDRSVSGLDSFGNLEVSPPVVANGKEYPLGRILIGGNLPGSSGRRVTQVVRDFLHAQKVQPPVELFVDWLAVGHVDEFLSFVPAPDGKGFRMLLASPGACFKLFQEKQKCGHGRALLFQGVVDDEQVKTISINQVLSNKDLINYNKFVQSCIDWNREVLKRELGLAECDIIDIPQLFKTERKKATAFFPDLVNMLVLGKHLGIPKPFGPIINGCCCLEEKVRSLLEPLGLHCTFIDDFTPYHMLHGEVHCGTNVCRKPFSFKWWNMVP |
The protein was eluted with a 100 ml gradient from 20 mM to 1 M imidazole in 50 mM HEPES pH 7.8, 500 mM NaCl, 10 mM β-mercaptoethanol, 5% glycerol. Fractions containing His6-tagged PAD3 were pooled, dialyzed into ion-exchange chromatography (IEX) binding buffer (50 mM Tris pH 7.8, 50 mM NaCl, 2.5% glycerol, 2 mM DTT, 1 mM EDTA) and loaded onto a Source 30Q column (GE Healthcare) pre-equilibrated with IEX binding buffer for the anion-exchange chromatography purification step. The column was washed with 10 CV IEX binding buffer. The protein was then eluted in 100 ml IEX binding buffer with a gradient from 0 to 1 M NaCl. Fractions containing His6-tagged PAD3 were pooled and dialysed into size-exclusion chromatography buffer (20 mM Tris pH 7.8, 100 mM NaCl, 2 mM DTT, 1 mM EDTA). The protein was concentrated, spun at 20 000g for 10 min and filtered before loading onto an S200 16/600 size-exclusion column (GE Healthcare) for the final size-exclusion chromatography purification step. Fractions containing His6-tagged PAD3 were pooled and concentrated to 10 mg ml−1 for crystallization. Protein purity was verified using SDS–PAGE. Our expression and purification methods are similar to those previously reported by Unno et al. (2012 ▸), with differences in the choice of expression vector and the strain of E. coli used.
2.2. Crystallization
All crystallization trials were carried out using the sitting-drop vapour-diffusion method in 96-well Intelli-Plates (Hampton Research) containing 50 µl reservoir solution. For each condition, two crystallization drops, one with 0.25 µl protein solution plus 0.25 µl reservoir solution and the other with 0.3 µl protein solution plus 0.15 µl reservoir solution, were set up. Crystallization trays were maintained at 291 K. Precipitant screening was initially carried out using the MORPHEUS screen (Gorrec, 2009 ▸) as well as the JCSG-plus and PACT premier screens (Newman et al., 2005 ▸). Crystals of PAD3 were grown in condition E6 of the MORPHEUS screen (Gorrec, 2009 ▸), which comprises 0.12 M ethylene glycols, 0.1 M Buffer System 2 [1.0 M sodium HEPES; MOPS (acid); pH 7.5] and 50%(v/v) Precipitant Mix 2 [40%(v/v) ethylene glycol, 20%(w/v) PEG 8000] (Table 2 ▸). As a comparison, Unno and coworkers previously reported the crystallization of PAD3 at a concentration of 2–8 mg ml−1 in 0.1 M HEPES buffer pH 7.5, 0.2 M NaCl, 25%(v/v) PEG 400 (Unno et al., 2012 ▸).
Table 2. Crystallization.
| Method | Vapour diffusion, sitting drop |
| Plate type | Intelli-Plates (Hampton Research) |
| Temperature (K) | 291 |
| Protein concentration (mg ml−1) | 10 |
| Buffer composition of protein solution | 20 mM Tris pH 7.8, 100 mM NaCl, 2 mM DTT, 1 mM EDTA |
| Composition of reservoir solution | Na HEPES + MOPS (acid), ethylene glycols, PEG 8000 |
| Volume and ratio of drop | 0.5 µl (1:1 protein:reservoir), 0.45 µl (2:1 protein:reservoir) |
| Volume of reservoir (µl) | 50 |
2.3. Data collection and processing
Individual crystals were harvested directly from the MORPHEUS screen (Gorrec, 2009 ▸) and flash-cooled at 110 K. X-ray diffraction data were collected from native crystals on beamline MX2 at the Australian Synchrotron at a wavelength of 0.979 Å. The crystals diffracted to a maximum resolution of 2.8 Å. Data were collected with an oscillation angle of 0.1° and an exposure time of 0.1 s per frame, with a total rotation range of 360° (Table 3 ▸). X-ray diffraction data were integrated using the XDS suite (Kabsch, 2010 ▸) and data reduction was performed using AIMLESS (Evans & Murshudov, 2013 ▸).
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | Beamline MX2, Australian Synchrotron |
| Wavelength (Å) | 0.979 |
| Temperature (K) | 100 |
| Detector | Dectris EIGER X 16M |
| Space group | H32 |
| a, b, c (Å) | 115.02, 115.02, 328.49 |
| α, β, γ (°) | 90, 90, 120 |
| Resolution range (Å) | 49.24–2.80 (2.95–2.80) |
| Total No. of reflections | 390648 (53222) |
| No. of unique reflections | 21054 (3035) |
| Completeness (%) | 100 (100) |
| Multiplicity | 18.6 (17.5) |
| 〈I/σ(I)〉 | 8.0 (0.8)† |
| CC1/2 | 0.993 (0.334) |
| R meas | 0.575 (9.11) |
| R p.i.m. | 0.132 (2.16) |
| Overall B factor from Wilson plot (Å2) | 66.14 |
The resolution cutoff was chosen based on the point at which the CC1/2 value dropped to 0.3, resulting in 〈I/σ(I)〉 being <2.0 in the outer shell of data.
2.4. Structure solution and refinement
The structure was determined by molecular replacement using Phaser in the Phenix package (version 1.13_2998; Liebschner et al., 2019 ▸). Human PAD4 (PDB entry 2dew; Arita et al., 2006 ▸) was used as the search model. The crystals were indexed in space group H32, contained one monomer of protein in the asymmetric unit and seem to be related to the crystal form previously reported by Unno et al. (2012 ▸), which had similar cell lengths and was indexed in space group H3 with two protein monomers in the asymmetric unit. PAD3 is 664 amino acids in length, but the final model contained only 595 residues, as several surface loops could not be modelled due to weak electron density. The model was refined to a final R work and R free of 0.234 and 0.274, respectively, with good geometry (Table 4 ▸). The structure was built and refined with iterative cycles of model building in Coot (Emsley et al., 2010 ▸) and refinement using phenix.refine (version 1.19.2-4158; Afonine et al., 2012 ▸; Liebschner et al., 2019 ▸). The final rounds of maximum-likelihood refinement were carried out using TLS using TLS groups determined by the TLSMD server (version 1.4.0; Painter & Merritt, 2006a ▸,b ▸). Model validation was performed using MolProbity (Williams et al., 2018 ▸).
Table 4. Structure refinement.
Values in parentheses are for the outer shell.
| Resolution range (Å) | 49.24–2.80 (2.95–2.80) |
| Completeness (%) | 99.8 |
| No. of reflections, working set | 19978 (2769) |
| No. of reflections, test set | 1032 (155) |
| Final R cryst | 0.234 (0.354) |
| Final R free | 0.274 (0.336) |
| No. of non-H atoms | |
| Protein | 4253 |
| Ligand | 0 |
| Water | 0 |
| Total | 4253 |
| R.m.s. deviations | |
| Bonds (Å) | 0.004 |
| Angles (°) | 0.076 |
| Average B factors (Å2) | |
| Protein | 61.9 |
| Ramachandran plot | |
| Most favoured (%) | 93.4 |
| Allowed (%) | 100 |
3. Results and discussion
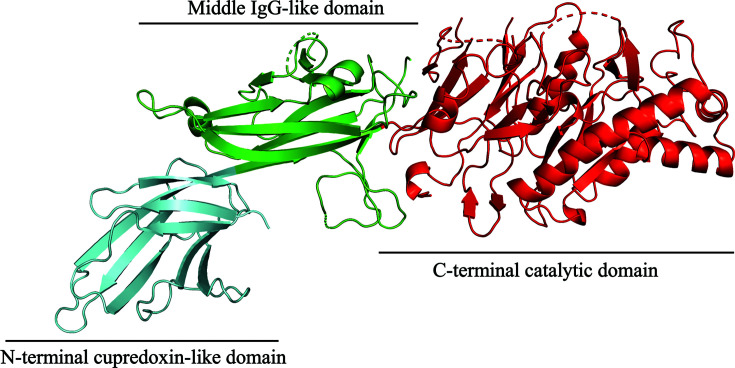
The PAD3 monomer adopts an overall elongated and curved shape, approximately 130 × 45 × 45 Å in size (Fig. 1 ▸), and like other PAD isoforms consists of three domains: an N-terminal domain (residues 1–113; shown in cyan), a central domain (residues 115–273; shown in green) and a C-terminal catalytic domain (residues 293–664; shown in red). The N-terminal domain has a cupredoxin-like fold: a β-sandwich composed of eight distorted parallel and antiparallel β-strands (β1–β8) connected by seven loops. The central domain has an immunoglobin-like (IgG) fold (Bruschi et al., 2017 ▸) and is composed of nine β-strands (β9–β17), arranged in two sheets, and two small α-helices. One of the helices connects β10 to β11 in a loop–helix–loop motif and the other links β11 to β12. The C-terminal domain is composed of five ββαβ modules that are arranged circularly in a pseudo-fivefold-symmetric α/β propeller. Together, the first β-strand of each individual module forms the active-site cleft that binds the substrate at the core of the domain in other isoforms.
Figure 1.
Cartoon representation of the PAD3 monomer structure. The N-terminal cupredoxin-like domain, the central IgG-like domain and the C-terminal catalytic domain are coloured cyan, green and red, respectively.
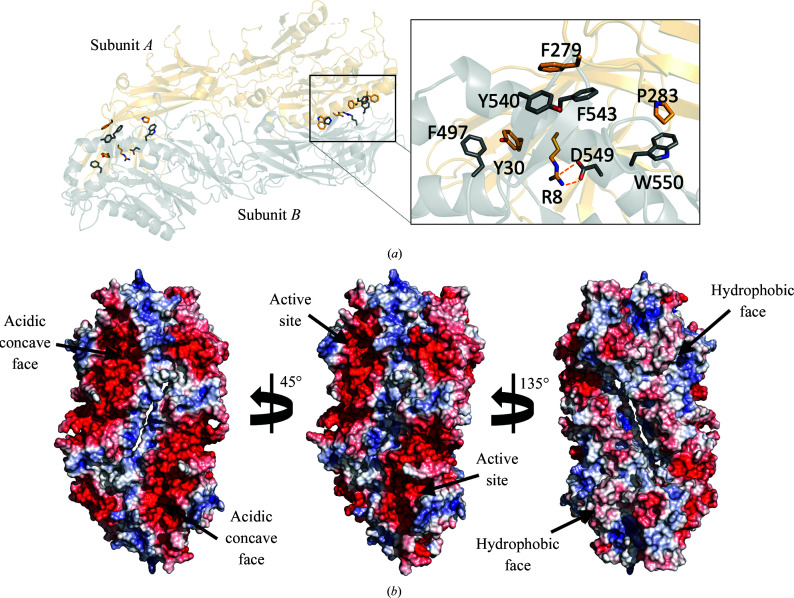
As in other PAD enzymes of known structure (Arita et al., 2004 ▸; Saijo et al., 2016 ▸; Slade et al., 2015 ▸), the crystal packing of the PAD3 molecules shows that two molecules from adjacent asymmetric units form a head-to-tail dimer with an extensive interaction interface of 2388 Å2 as calculated using PISA (Krissinel & Henrick, 2007 ▸; Fig. 2 ▸). This dimeric arrangement is similar to that seen in PAD2 and PAD4, and the extent of the interaction interface is similar to that in PAD2 (2551 Å2; PDB entries 4n20 and 4n2b) and PAD4 (2314 Å2; PDB entries 1wd8 and 1wd9). PAD1 is the exception in this protein family, crystallizing as a monomer (PDB entry 5hp5; Table 5 ▸). A previous study (Saijo et al., 2016 ▸) confirmed using SAXS experiments that PAD3 also forms a dimer in solution, like PAD2 and PAD4, whereas PAD1 is monomeric in solution. Saijo and coworkers also reported that the N-terminal loop of PAD1 appears to be the feature that prevents the formation of a native homodimer. Previous mutagenesis experiments have shown that PAD4 dimer-interface mutants can produce a monomeric form of this protein, but that it retains only 25–50% of the activity of the native dimeric form (Liu et al., 2011 ▸). The monomers in the PAD3 crystal are related by a crystallographic twofold axis. The calculated electrostatic surface potential of the PAD3 monomer shows that the protein has two distinct faces: one is acidic and solvent-exposed, whereas the other is mostly basic and hydrophobic in character, forming a mainly hydrophobic dimerization interface flanked by a conserved salt bridge between Arg8 of subunit A and Asp549 of subunit B (Fig. 2 ▸ a). The acidic face contains a concave groove that forms the active-site cleft, and the equivalent region is subject to a conformational change upon Ca2+ binding in PAD4 (Arita et al., 2004 ▸).
Figure 2.
The PAD3 dimer. (a) Cartoon representation of two PAD3 molecules arranged in a head-to-tail manner. Residues involved in the head-to-tail dimer interface are represented as sticks. Residues Arg8/Asp549 form a salt bridge and Phe279/Tyr540/Phe543, Phe497/Tyr30 and Pro283/Trp550 form hydrophobic clusters. (b) The whole dimer is shown with a solvent-accessible surface coloured by electrostatic potential, showing the two faces of the molecule. Electrostatic potentials were calculated using APBS (Baker et al., 2001 ▸; Jurrus et al., 2018 ▸; red =−90 k B T/e c, blue = +90 k B T/e c).
Table 5. Dimer interfaces of the PAD enzyme structures.
| PAD3 | PAD4, apo | PAD2, apo | PAD4, Ca2+ | PAD2, Ca2+ | PAD1, Ca2+ | |
|---|---|---|---|---|---|---|
| PDB code | 6ce1 | 1wd8 | 4n20 | 1wd9 | 4n2b | 5hp5 |
| No. of residues | ||||||
| Dimer interface | 78 (13.1%) | 77 (13.8%) | 81 (12.7%) | 72 (11.9%) | 78 (11.9%) | 25 (3.7%) |
| Surface | 570 (96%) | 507 (90.7%) | 587 (91.9%) | 541 (89.7%) | 599 (91.7%) | 613 (91.6%) |
| Total | 594 (100%) | 559 (100%) | 639 (100%) | 603 (100%) | 653 (100%) | 669 (100%) |
| Solvent-accessible area (Å2) | ||||||
| Dimer interface | 2388 (8.7%) | 2314 (8.5%) | 2552 (8.8%) | 2106 (7.8%) | 2480 (8.5%) | 1159 (3.9%) |
| Total | 27582 (100%) | 27227 (100%) | 28985 (100%) | 26948 (100%) | 29152 (100%) | 29656 (100%) |
| Complex-formation significance score | 1 | 1 | 0.189 | 1 | 0.135 | 0 |
PAD3 and PAD4 share 56% sequence identity, and the overall r.m.s.d. between their two structures when overlaid is 2.65 Å (Table 6 ▸). Domain-based sequence alignments between PAD3 and the other PAD isozymes show that the IgG-like domains have the lowest sequence identity (25–50%), whereas the C-terminal domains have the highest sequence identity (60–70%) (Table 6 ▸). In keeping with these statistics, the C-terminal domains of the PADs are structurally more conserved than the IgG-like domains, which show a greater structural variety.
Table 6. Structural superpositions of PAD3 with other PAD isozymes.
| Overall (3–664) | N-terminal cupredoxin-like domain (3–115) | Middle IgG-like domain (116–292) | C-terminal catalytic domain (293–664) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PDB code | Sequence identity (%) | R.m.s.d. (Å) | Sequence identity (%) | R.m.s.d. (Å) | Sequence identity (%) | R.m.s.d. (Å) | Sequence identity (%) | R.m.s.d. (Å) | |
| PAD1, Ca2+ | 5hp5 | 58 | 2.98 | 40 | 2.32 | 48 | 2.69 | 69 | 1.91 |
| PAD2 | 4n20 | 52 | 3.73 | 24 | 2.82 | 46 | 2.58 | 64 | 2.38 |
| PAD2, Ca2+ | 4n2b | 52 | 3.73 | 24 | 2.82 | 46 | 3.73 | 64 | 2.12 |
| PAD4 | 1wd8 | 56 | 2.65 | 35 | 2.72 | 49 | 2.92 | 69 | 2.00 |
| PAD4, Ca2+ | 1wd9 | 56 | 2.67 | 35 | 2.22 | 49 | 2.62 | 69 | 2.41 |
The active site of PAD3 is formed by a conserved catalytic tetrad composed of Asp350, His470, Asp472 and Cys646. The loop connecting strands β35 and β36 is poorly ordered, and is in a conformation that positions Cys646 at a distance of ∼10 Å from the inferred active-site centre. Thus, this structure shows a catalytically inactive state of the enzyme. There are known conformational differences in the C-terminal domains of Ca2+-free and Ca2+-bound forms of other PAD isoforms that are essential for enzyme function: in PAD2, the equivalent active-site cysteine residue (Cys645) is distant from the active site in the calcium-free form, but Ca2+ binding moves it by ∼5 Å, thus facilitating nucleophilic attack by the cysteine on the Cζ atom of the substrate (Arita et al., 2004 ▸), and an equivalent conformational change is presumably also important in PAD3.
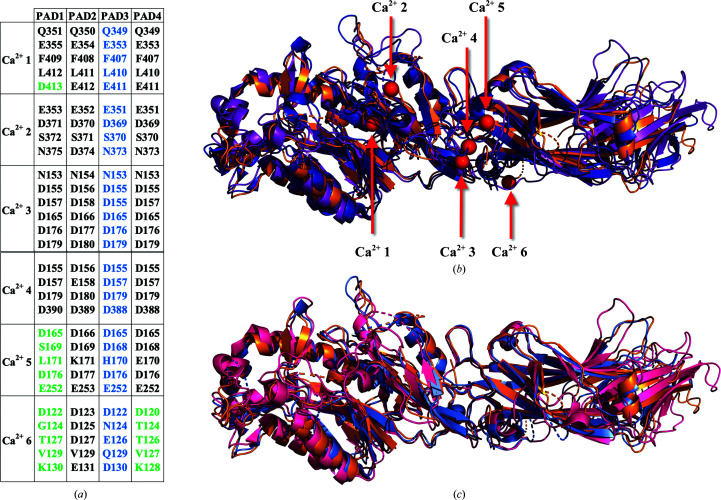
Previous PAD structures have shown up to six bound Ca2+ ions. In order to determine the putative Ca2+-binding sites of PAD3, we performed structural and sequence alignments of PAD1, PAD2, PAD3 and PAD4 (Fig. 3 ▸). Examination of the alignments indicates that the amino acids coordinating Ca2+ ions at sites 1, 2, 3 and 4 are conserved among all PADs, whereas site 5 is not present in PAD1 and site 6 is not present in either PAD1 or PAD4. The structure of PAD1 contains two Ca2+ ions, even without added Ca2+ in the crystallization conditions, but no such ions are evident in the structure of PAD3. Hence, in order to gain insight into the Ca2+-mediated regulatory processes that control PAD3 activity, we sought to determine the structure of PAD3 in its Ca2+-bound state. We attempted to do so by co-crystallization and also by soaking apo PAD3 crystals in crystallization solutions containing up to 50 mM CaCl2. However, neither approach produced good-quality crystals.
Figure 3.
The Ca2+-binding sites of PADs. (a) Ca2+-binding sites and their respective coordinating residues determined from X-ray crystallographic structures of PAD1 (PDB entry 5hp5), PAD2 (PDB entries 4n2b and 4n2c) and PAD4 (PDB entries 1wd8 and 1wd9). A structural alignment of PADs (PAD1, PAD2, PAD3 and PAD4) was conducted to determine the residues in PAD3 that are likely to be involved in Ca2+ coordination. The Ca2+-coordinating residues in PAD1, PAD2 and PAD4 that were visualized by X-ray crystallography (Arita et al., 2004 ▸; Slade et al., 2015 ▸; Saijo et al., 2016 ▸) are listed in black. The putative Ca2+-coordinating residues in PAD3 are listed in blue. The residues shown to not coordinate Ca2+ in PAD1 and PAD4 are listed in green. (b) Structure superposition of PAD3 (PDB entry 6ce1, light orange) with Ca2+-bound PAD2 (PDB entry 4n2b, purple) and Ca2+-bound PAD4 (PDB entry 1wd9, blue). All Ca2+ sites are shown in red for descriptive purposes. (c) Structure superposition of PAD3 (PDB entry 6ce1, light orange) with apo PAD2 (PDB entry 4n20, hot pink) and apo PAD4 (PDB entry 1wd8, light blue).
The crystal structure of apo PAD3 described here shows that like other PAD isoforms, PAD3 has a conserved architecture and forms a head-to-tail dimer similar to other PAD isoforms (Arita et al., 2004 ▸; Slade et al., 2015 ▸; Saijo et al., 2016 ▸). The Ca2+-free form of the enzyme corresponds to the low-activity state of the enzyme (Knuckley et al., 2010 ▸).
The biological function of PAD enzymes is only partially understood, with the molecular details of the specificity of PAD3 for its substrates (keratins, filaggrin and trichohyalin) remaining unclear, hampered by difficulties in obtaining soluble substrate proteins. Structures of PAD3 in complex with Ca2+ and substrate analogues are required to more precisely define the molecular determinants of substrate recognition in this protein.
Supplementary Material
PDB reference: peptidyl-arginine deiminase type III, 6ce1
Acknowledgments
We thank Jason Busby for his help in X-ray data processing and Ghader Bashiri, David Goldstone, Richard Kingston and Chris Squire for helpful discussions. This research was undertaken in part using the MX2 beamline at the Australian Synchrotron, which is part of ANSTO, and made use of the Australian Cancer Research Foundation (ACRF) detector. We thank all of the beamline staff for their technical support. We also thank Duane Harland and Jeff Plowman from AgResearch, Lincoln for their help, advice and the provision of scholarship funding for Othman Rechiche from the New Zealand Wool Consortium.
References
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Arita, K., Hashimoto, H., Shimizu, T., Nakashima, K., Yamada, M. & Sato, M. (2004). Nat. Struct. Mol. Biol. 11, 777–783. [DOI] [PubMed]
- Arita, K., Shimizu, T., Hashimoto, H., Hidaka, Y., Yamada, M. & Sato, M. (2006). Proc. Natl Acad. Sci. USA, 103, 5291–5296.6. [DOI] [PMC free article] [PubMed]
- Assohou-Luty, C., Raijmakers, R., Benckhuijsen, W. E., Stammen-Vogelzangs, J., de Ru, A., van Veelen, P. A., Franken, K. L., Drijfhout, J. W. & Pruijn, G. J. (2014). Biochim. Biophys. Acta, 1844, 829–836. [DOI] [PubMed]
- Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. (2001). Proc. Natl Acad. Sci. USA, 98, 10037–10041. [DOI] [PMC free article] [PubMed]
- Basmanav, F. B. Ü., Cau, L., Tafazzoli, A., Méchin, M.-C., Wolf, S., Romano, M. T., Valentin, F., Wiegmann, H., Huchenq, A., Kandil, R., Garcia Bartels, N., Kilic, A., George, S., Ralser, D. J., Bergner, S., Ferguson, D. J. P., Oprisoreanu, A. M., Wehner, M., Thiele, H., Altmüller, J., Nürnberg, P., Swan, D., Houniet, D., Büchner, A., Weibel, L., Wagner, N., Grimalt, R., Bygum, A., Serre, G., Blume-Peytavi, U., Sprecher, E., Schoch, S., Oji, V., Hamm, H., Farrant, P., Simon, M. & Betz, R. C. (2016). Am. J. Hum. Genet. 99, 1292–1304. [DOI] [PMC free article] [PubMed]
- Bruschi, M., Petretto, A., Bertelli, R., Galetti, M., Bonanni, A., Pratesi, F., Migliorini, P., Candiano, G., Vaglio, A. & Ghiggeri, G. M. (2017). Clin. Chim. Acta, 464, 12–16. [DOI] [PubMed]
- Chavanas, S., Adoue, V., Méchin, M.-C., Ying, S., Dong, S., Duplan, H., Charveron, M., Takahara, H., Serre, G. & Simon, M. (2008). PLoS One, 3, e3408. [DOI] [PMC free article] [PubMed]
- Chavanas, S., Méchin, M.-C., Nachat, R., Adoue, V., Coudane, F., Serre, G. & Simon, M. (2006). J. Dermatol. Sci. 44, 63–72. [DOI] [PubMed]
- Das, K., Butler, G. H., Kwiatkowski, V., Clark, A. D. Jr, Yadav, P. & Arnold, E. (2004). Structure, 12, 657–667. [DOI] [PubMed]
- Dong, S., Kanno, T., Yamaki, A., Kojima, T., Shiraiwa, M., Kawada, A., Méchin, M.-C., Chavanas, S., Serre, G., Simon, M. & Takahara, H. (2006). Biochem. J. 397, 449–459. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Evans, P. R. & Murshudov, G. N. (2013). Acta Cryst. D69, 1204–1214. [DOI] [PMC free article] [PubMed]
- Gorrec, F. (2009). J. Appl. Cryst. 42, 1035–1042. [DOI] [PMC free article] [PubMed]
- Ishida-Yamamoto, A., Takahashi, H., Iizuka, H., Senshu, T., Akiyama, K. & Nomura, K. (2000). J. Investig. Dermatol. 114, 701–705. [DOI] [PubMed]
- Jurrus, E., Engel, D., Star, K., Monson, K., Brandi, J., Felberg, L. E., Brookes, D. H., Wilson, L., Chen, J., Liles, K., Chun, M., Li, P., Gohara, D. W., Dolinsky, T., Konecny, R., Koes, D. R., Nielsen, J. E., Head-Gordon, T., Geng, W., Krasny, R., Wei, G.-W., Holst, M. J., McCammon, J. A. & Baker, N. A. (2018). Protein Sci. 27, 112–128. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kizawa, K., Takahara, H., Troxler, H., Kleinert, P., Mochida, U. & Heizmann, C. W. (2008). J. Biol. Chem. 283, 5004–5013. [DOI] [PubMed]
- Knuckley, B., Bhatia, M. & Thompson, P. R. (2007). Biochemistry, 46, 6578–6587. [DOI] [PMC free article] [PubMed]
- Knuckley, B., Causey, C. P., Jones, J. E., Bhatia, M., Dreyton, C. J., Osborne, T. C., Takahara, H. & Thompson, P. R. (2010). Biochemistry, 49, 4852–4863. [DOI] [PMC free article] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Kubilus, J. & Baden, H. P. (1983). Biochim. Biophys. Acta, 745, 285–291. [DOI] [PubMed]
- Kubilus, J., Waitkus, R. F. & Baden, H. P. (1980). Biochim. Biophys. Acta, 615, 246–251. [DOI] [PubMed]
- Kubilus, J., Waitkus, R. W. & Baden, H. P. (1979). Biochim. Biophys. Acta, 581, 114–121. [DOI] [PubMed]
- Liebschner, D., Afonine, P. V., Baker, M. L., Bunkóczi, G., Chen, V. B., Croll, T. I., Hintze, B., Hung, L.-W., Jain, S., McCoy, A. J., Moriarty, N. W., Oeffner, R. D., Poon, B. K., Prisant, M. G., Read, R. J., Richardson, J. S., Richardson, D. C., Sammito, M. D., Sobolev, O. V., Stockwell, D. H., Terwilliger, T. C., Urzhumtsev, A. G., Videau, L. L., Williams, C. J. & Adams, P. D. (2019). Acta Cryst. D75, 861–877.
- Liu, Y.-L., Chiang, Y.-H., Liu, G.-Y. & Hung, H.-C. (2011). PLoS One, 6, e21314. [DOI] [PMC free article] [PubMed]
- Malki, L., Sarig, O., Romano, M. T., Méchin, M. C., Peled, A., Pavlovsky, M., Warshauer, E., Samuelov, L., Uwakwe, L., Briskin, V., Mohamad, J., Gat, A., Isakov, O., Rabinowitz, T., Shomron, N., Adir, N., Simon, M., McMichael, A., Dlova, N. C., Betz, R. C. & Sprecher, E. (2019). N. Engl. J. Med. 380, 833–841. [DOI] [PubMed]
- Méchin, M.-C., Enji, M., Nachat, R., Chavanas, S., Charveron, M., Ishida-Yamamoto, A., Serre, G., Takahara, H. & Simon, M. (2005). Cell. Mol. Life Sci. 62, 1984–1995. [DOI] [PMC free article] [PubMed]
- Mondal, S. & Thompson, P. R. (2019). Acc. Chem. Res. 52, 818–832. [DOI] [PMC free article] [PubMed]
- Newman, J., Egan, D., Walter, T. S., Meged, R., Berry, I., Ben Jelloul, M., Sussman, J. L., Stuart, D. I. & Perrakis, A. (2005). Acta Cryst. D61, 1426–1431. [DOI] [PubMed]
- Painter, J. & Merritt, E. A. (2006a). Acta Cryst. D62, 439–450. [DOI] [PubMed]
- Painter, J. & Merritt, E. A. (2006b). J. Appl. Cryst. 39, 109–111.
- Rogers, G. E., Harding, H. W. & Llewellyn-Smith, I. J. (1977). Biochim. Biophys. Acta, 495, 159–175. [DOI] [PubMed]
- Rothnagel, J. A. & Rogers, G. E. (1984). Methods Enzymol. 107, 624–631. [DOI] [PubMed]
- Saijo, S., Nagai, A., Kinjo, S., Mashimo, R., Akimoto, M., Kizawa, K., Yabe-Wada, T., Shimizu, N., Takahara, H. & Unno, M. (2016). J. Mol. Biol. 428, 3058–3073. [DOI] [PubMed]
- Senshu, T., Kan, S., Ogawa, H., Manabe, M. & Asaga, H. (1996). Biochem. Biophys. Res. Commun. 225, 712–719. [DOI] [PubMed]
- Shirai, H., Blundell, T. L. & Mizuguchi, K. (2001). Trends Biochem. Sci. 26, 465–468. [DOI] [PubMed]
- Slade, D. J., Fang, P., Dreyton, C. J., Zhang, Y., Fuhrmann, J., Rempel, D., Bax, B. D., Coonrod, S. A., Lewis, H. D., Guo, M., Gross, M. L. & Thompson, P. R. (2015). ACS Chem. Biol. 10, 1043–1053. [DOI] [PMC free article] [PubMed]
- Steinert, P. M., Parry, D. A. & Marekov, L. N. (2003). J. Biol. Chem. 278, 41409–41419. [DOI] [PubMed]
- Tarcsa, E., Marekov, L. N., Mei, G., Melino, G., Lee, S. C. & Steinert, P. M. (1996). J. Biol. Chem. 271, 30709–30716. [DOI] [PubMed]
- Unno, M., Kizawa, K., Ishihara, M. & Takahara, H. (2012). Acta Cryst. F68, 668–670. [DOI] [PMC free article] [PubMed]
- Vossenaar, E. R., Zendman, A. J., van Venrooij, W. J. & Pruijn, G. J. (2003). Bioessays, 25, 1106–1118. [DOI] [PubMed]
- Williams, C. J., Headd, J. J., Moriarty, N. W., Prisant, M. G., Videau, L. L., Deis, L. N., Verma, V., Keedy, D. A., Hintze, B. J., Chen, V. B., Jain, S., Lewis, S. M., Arendall, W. B., Snoeyink, J., Adams, P. D., Lovell, S. C., Richardson, J. S. & Richardson, J. S. (2018). Protein Sci. 27, 293–315. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: peptidyl-arginine deiminase type III, 6ce1