ABSTRACT
Background
Flavonoids are a diverse group of plant constituents with demonstrated neuroprotective and anti-tumor effects. Flavonoid intake may decrease the risk of glioma, but the possibility of an association has not yet been investigated in humans.
Objectives
We evaluated the association between dietary flavonoid consumption and the risk of glioma.
Methods
We followed participants in the female Nurses’ Health Study (1984–2014; n = 81,688) and Nurses’ Health Study II (1991–2017; n = 95,228) and the male Health Professionals Follow-Up Study (1986–2014; n = 49,885). We used multivariable-adjusted Cox proportional hazards regression models to evaluate the associations between average long-term (up to 30 years) or recent (up to 12 years) dietary flavonoid intake (total flavonoids and each of 6 subclasses) and risks of incident glioma. Flavonoid intake was derived from validated quadrennial FFQs. Incident glioma was self-reported and confirmed by a medical record review or was determined by a medical record review after death.
Results
We documented 536 incident cases of glioma across 5,936,386 person-years of follow-up. Long-term total flavonoid, flavan-3-ol, and polymeric flavonoid (polymer) intakes were associated with decreased glioma risks in pooled analyses comparing the highest to lowest quintiles of consumption [HR, 0.79 (95% CI, 0.59–1.05; P-trend = 0.04) for total flavonoids; 0.76 (95% CI, 0.57–1.01; P-trend = 0.04) for flavan-3-ols; and 0.82 (95% CI, 0.61–1.09; P-trend = 0.05) for polymers]. Associations with recent intake were weaker. There were no associations with other flavonoid subclasses. After additional adjustment for tea consumption, there were no associations between flavan-3-ol or polymer consumption and glioma.
Conclusions
Increased dietary intakes of flavan-3-ol and polymeric flavonoids, especially those predominant in tea, were associated with decreased glioma risks in a prospective cohort of men and women.
Keywords: glioma, flavonoids, epidemiology, tea, flavan-3-ols
Introduction
Dietary risk factors for glioma are not well established. Recent studies showed that tea intake may be associated with a decreased glioma risk (1–3), although the mechanism is unknown. Hypotheses regarding the mechanisms linking tea intake to glioma risks include the anti-tumor effects of caffeine and bioactive constituents of tea, including flavonoids. Research into the association between dietary caffeine intake and the risk of glioma is mixed: our own research showed that tea, but not caffeinated coffee intake, was associated with a decreased glioma risk (2). These findings prompted us to investigate whether dietary flavonoids, which are abundant in tea and other plant foods, but not coffee, are associated with glioma risks.
Flavonoids are a diverse group of constituents found in plant foods and may be protective against glioma. To our knowledge, no studies have investigated dietary flavonoid intakes and glioma risks in humans. Studies on vegetable and fruit intakes and glioma risks have shown a decreased risk, although the results are mixed and, as with tea, the responsible compounds are unknown (4).
Experiments using human glioma cell lines in vitro show a promising anti-tumor role for flavonoids (5). Numerous flavonoid metabolites are known to cross the blood-brain barrier, although with varying efficacies (6); this phenomenon is supported by an abundance of research, including both prospective studies and randomized controlled trials establishing an association between dietary flavonoid intake and improved cognitive function (7–10). Specifically, flavan-3-ols and flavonols are 2 subclasses of flavonoids that have shown promising anti-glioma effects in vitro. Therefore, specific dietary flavonoids are a promising and unexplored nutrient potentially related to the glioma incidence.
In the present study, we evaluated both recent and long-term habitual flavonoid and flavonoid subclass intakes in association with glioma risks in 3 large prospective cohort studies: the Nurses’ Health Study (NHS), the Nurses’ Health Study II (NHSII), and the Health Professionals Follow-Up Study (HPFS). Because the association between flavonoid intake and the risk of glioma has not been studied previously, we included all flavonoid subclasses in this analysis.
Methods
Study population
Detailed information about the NHS, NHSII, and HPFS cohorts has been previously published (11, 12). In brief, the NHS enrolled 121,700 registered female nurses, aged 30–55, in 1976; the NHSII enrolled 116,429 registered female nurses, aged 25–42, in 1989; and the HPFS enrolled 51,529 male health professionals, aged 40–75, in 1986. Participants provided updated lifestyle habits and new medical diagnoses via a mailed, biennial questionnaire. Participants provided updated dietary information via a mailed, quadrennial FFQ. In the present study, we used the year of the first FFQ with an adequate assessment of flavonoid-rich foods as the baseline: 1984 for the NHS, 1991 for the NHSII, and 1986 for the HPFS. We excluded participants who died before the baseline, participants who were diagnosed with glioma before the baseline, and participants with missing or incomplete dietary data for the baseline FFQ (Supplementary Figure 1). After these exclusions, 226,801 (NHS, n = 81,688; NHSII, n = 95,228; HPFS, n = 49,885) participants were included in the analysis.
Assessment of flavonoid intake
Dietary intake data were assessed using a validated FFQ with 130 (NHS) or 131 (NHSII, HPFS) food and beverage items. Participants in the NHS cohort completed a 130-item FFQ in 1984 and similar FFQs in 1986 and every 4 years thereafter. Participants in the NHSII completed the FFQ in 1991 and every 4 years thereafter. Those in the HPFS completed the FFQ in 1986 and every 4 years thereafter. In each FFQ, participants reported how often they consumed a serving of various food items, with 9 possible responses ranging from “never or less than once per month” to “6 or more times per day.” The validity of the FFQ was evaluated previously in a subsample of participants from the NHS and HPFS; these studies indicate that the FFQ provides reasonably valid measurements of nutrient intake (13–16). The intake of an individual flavonoid compound was calculated as the sum of the consumption frequency of each food multiplied by the content of the specific flavonoid per portion size. Details on how the flavonoid content of a specific food was acquired has been described previously in detail (17). Flavonoid intake was derived from food and beverage sources only and was energy-adjusted using the residual method (18).
In the present study, we included total flavonoids and 6 major subclasses of flavonoids: flavan-3-ol monomers (catechins and epicatechins), polymeric flavonoids (polymers, including proanthocyanidins, theaflavins, and thearubigins), flavonols (quercetin, kaempferol, myricetin, isorhamnetin), anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin, petunidin, peonidin), flavanones (eriodictyol, hesperetin, naringenin), and flavones (apigenin, luteolin).
Participants with missing dietary data at baseline were excluded from the analysis. For missing dietary data on a follow-up questionnaire, data from the most recent prior FFQ were carried forward for up to 2 cycles (8 years) and were otherwise set to missing.
Assessment of covariates
All models were adjusted for the following covariates: age, total caloric intake (quintile), BMI (<25 kg/m2, 25–29.9 kg/m2, or ≥30 kg/m2), smoking status (never, past, or current), and hyperlipidemia (ever diagnosed compared with never diagnosed). Participants provided updated data on weight, smoking status, and hyperlipidemia with each biennial questionnaire; BMI was calculated from the height reported at baseline. Missing covariate values were modeled as a missing indicator for categorical variables. Median income was derived from the census tract–level median income for the 2000 census and was available for NHS and NHSII only. Median income was added to the NHS and NHSII multivariable models in a secondary analysis.
Two separate models included the above covariates with the addition of 1) average long-term tea consumption and 2) average long-term caffeine consumption. Tea consumption was derived from quadrennial FFQs, without reference to the type of tea or brewing method. Herbal or decaffeinated teas were distinguished from caffeinated teas starting with the 1998 questionnaire for the NHS, the 1995 questionnaire for the NHSII, and the 1998 questionnaire for the HPFS. For this analysis, we included all types of tea (caffeinated and herbal or decaffeinated). We could not distinguish between black and green tea. Total caffeine was calculated by summing the amount of caffeine in coffee, tea, soda, decaffeinated coffee, chocolate, and candies as reported on quadrennial FFQs. For use as a covariate in multivariable regression models, both tea and caffeine were included as continuous variables.
Race was self-reported by study participants. We analyzed the distribution of self-reported racial categories (white or other/unknown) among cases and controls, but did not include race as a covariate in the adjusted models due to the limited diversity of the study population (>90% white).
Assessment of glioma cases
The primary outcome variable for the present study was incident glioma and the secondary outcome variable was glioblastoma (GBM), an aggressive and common subtype of glioma. Primary brain malignancy cases were determined by self-report in biennial questionnaires and confirmed by a medical record review or were determined by a medical record review after death. Deaths were documented through the National Death Index, next of kin, and postal authorities. Deaths due to primary brain malignancy were confirmed with medical records. We included only cases with confirmed International Classification of Diseases, Ninth Edition, Clinical Modification diagnoses of 191.x (malignant neoplasm of the brain), which we further limited to glioma only. Data on tumor subtypes (any glioma compared with GBM) were extracted from medical records.
Ethics
The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Statistical analysis
We used multivariable-adjusted Cox proportional hazards regression models to evaluate the associations between recent or long-term dietary flavonoid intake (total flavonoids and each of 6 subclasses) and risks of incident glioma. Recent flavonoid intake was calculated as the average of reported intakes on the most recent 3 questionnaires, either from the 12 years preceding a diagnosis of glioma or preceding the end of follow-up (e.g., recent intake for 1994 was calculated as the average of reported intakes from 1986, 1990, and 1994). Long-term flavonoid intake was calculated as the average of reported intakes on all questionnaires preceding a diagnosis or the end of follow-up (up to 26 years). For the first 3 questionnaires (first 12 years of follow-up), both cumulative long-term and recent flavonoid intakes were calculated as the average of the first 1, 2, or 3 questionnaires. The duration of the preclinical period of glioma is unknown, so we included both recent and long-term intakes in the main analyses.
Follow-up began at the first questionnaire return date and ended with a glioma diagnosis, death, or the end of follow-up (30 June 2014 for the NHS, 30 June 30 2017 for the NHSII, and 31 December 2014 for the HPFS). The analyses were conducted separately for each cohort and combined by a meta-analysis using the fixed-effect model, with P heterogeneity calculated for each model. We used quintile medians in Cox proportional hazards regression models to estimate linear trend P values.
In addition to recent and long-term flavonoid intakes, we also performed lagged models in a secondary analysis that excluded flavonoid intake in the 8 years preceding a diagnosis or the end of follow-up, to mitigate the possible effects of behavior changes in the years preceding a glioma diagnosis. Another secondary analysis model was limited to GBM, an aggressive and common subtype of glioma.
Based on our finding that tea was the primary dietary contributor to the 2 flavonoid subclasses associated with a decreased risk of glioma, we repeated the main analysis evaluating the association between long-term flavan-3-ol or polymer intake, with mutual adjustment for long-term tea consumption. Tea consumption was highly correlated with flavan-3-ol and polymer consumption (Pearson correlation coefficient, R = 0.93 for flavan-3ol and R = 0.90 for polymer). Thus, this was an attempt to distinguish whether the consumption of tea itself or of specific flavonoids was driving the observed associations with a decreased glioma risk in the main analyses.
In another secondary analysis, we divided these 2 subclasses (flavan-3-ols and polymers) into flavonoids that are predominant in tea compared with non-tea sources, in an attempt to distinguish whether tea consumption or consumption of the flavonoid subclass itself was driving the association with glioma. Among polymers, we classified theaflavins and thearubigins (which are found almost exclusively in tea) as tea polymers and proanthocynidin polymers (which are found in both tea and non-tea sources, but are more substantial in non-tea sources) as non-tea polymers. There was significant overlap between tea and non-tea sources of flavan-3-ols; we classified epicatechin 3-gallate, epigallocatechin, and epigallocatechin 3-gallate (which are found in substantial amounts exclusively in tea) as tea flavan-3-ols and epicatechin, catechin, and gallocatechin (which are found in both tea and non-tea sources, but are more substantial in non-tea sources) as non-tea flavan-3-ols. Because the range of intakes of these tea and non-tea sources of flavonoid differed, we analyzed these subgroups using the linear trend analysis described above, per 100 mg/day increase in flavan-3-ol or 400 mg/day increase in polymer intake, based on the range of flavonoid intake from the first to fifth quintile.
Finally, in a third model we repeated the main analysis evaluating the association between long-term flavan-3-ol or polymer intake with mutual adjustment for long-term caffeine consumption, since caffeine is a constituent of tea that may be associated with a decreased glioma risk. Caffeine consumption was weakly correlated with flavan-3-ol and polymer consumption (Pearson correlation coefficient, R = 0.12 for flavan-3-ol and R = 0.09 for polymer).
All statistical analyses were performed using the SAS 9.3 statistical package (SAS Institute), and all P values were derived from 2-sided tests.
Results
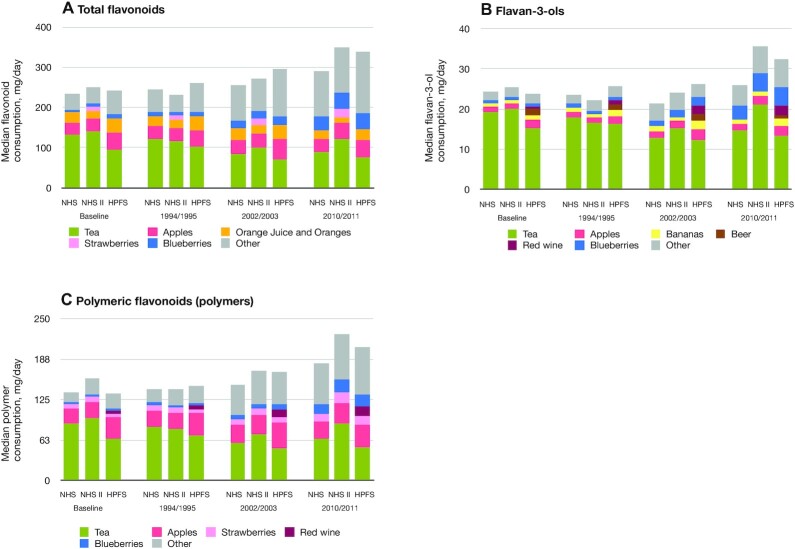
We documented 536 glioma cases (232 in the NHS, 93 in the NHSII, and 211 in the HPFS) and 361 GBM cases during 5,936,386 person-years of follow-up. Participants with incident glioma tended to be older and consume fewer total flavonoids, flavan-3-ols, and polymers at baseline (Table 1). Tea, apples, bananas, blueberries, beer, and red wine were top contributors to dietary flavan-3-ol consumption; tea, apples, strawberries, and red wine were top contributors to dietary polymer consumption (Figure 1).
TABLE 1.
Age-adjusted demographics of study participants by cohort at baseline: 1984 for NHS, 1991 for NHSII, and 1986 for HPFS1
| NHS | NHSII | HPFS | ||||
|---|---|---|---|---|---|---|
| Incident glioma cases | Overall cohort | Incident glioma cases | Overall cohort | Incident glioma cases | Overall cohort | |
| Participants, n | 232 | 81,688 | 93 | 95,228 | 211 | 49,885 |
| Age, y | 53.5 ± 6.7 | 51.0 ± 7.2 | 37.9 ± 4.7 | 36.6 ± 4.7 | 55.7 ± 8.9 | 57.3 ± 9.8 |
| BMI, kg/m2 | 25.2 ± 3.4 | 25.1 ± 4.8 | 24.8 ± 2.8 | 24.6 ± 5.3 | 25.8 ± 2.6 | 25.5 ± 3.4 |
| Race,2 % | ||||||
| White | 96 | 94 | 94 | 94 | 91 | 91 |
| Other/unknown | 4 | 6 | 6 | 6 | 9 | 9 |
| Diagnosis of hyperlipidemia, % | 5 | 4 | 7 | 10 | 10 | 13 |
| Smoking status, % | ||||||
| Never smoker | 45 | 44 | 63 | 65 | 46 | 44 |
| Former smoker | 34 | 32 | 27 | 22 | 41 | 42 |
| Current smoker | 21 | 24 | 8 | 12 | 7 | 10 |
| Unknown | 0 | 0 | 2 | 0 | 6 | 4 |
| Median family income,3 USD | 62,413 ± 14,910 | 64,628 ± 25,809 | 61,926 ± 14,114 | 61,319 ± 22,568 | — | — |
| Total flavonoids,4 mg/day | 306 ± 229 | 346 ± 332 | 368 ± 208 | 379 ± 374 | 313 ± 187 | 324 ± 280 |
| Flavan-3-ols, mg/day | 45.0 ± 50.8 | 55.2 ± 73.9 | 57.1 ± 45.7 | 60.8 ± 82.5 | 44.6 ± 41.0 | 45.5 ± 60.2 |
| Polymers, mg/day | 187 ± 174 | 223 ± 248 | 248 ± 154 | 257 ± 278 | 192 ± 140 | 199 ± 208 |
| Flavonols, mg/day | 12.8 ± 6.7 | 14.1 ± 10.3 | 19.7 ± 7.5 | 18.5 ± 13.3 | 14.0 ± 6.0 | 14.1 ± 9.0 |
| Anthocyanidins, mg/day | 11.2 ± 10.7 | 10.1 ± 12.5 | 12.9 ± 8.9 | 10.9 ± 14.2 | 11.8 ± 8.5 | 12.0 ± 14.9 |
| Flavanones, mg/day | 49.2 ± 33.3 | 43.1 ± 35.9 | 31.1 ± 17.6 | 33.0 ± 33.5 | 50.0 ± 24.1 | 52.1 ± 45.2 |
| Flavones, mg/day | 2.1 ± 1.1 | 1.9 ± 1.5 | 1.5 ± 0.6 | 1.5 ± 1.1 | 2.6 ± 1.3 | 2.5 ± 1.7 |
| Total calories, kcal | 1715 ± 362 | 1742 ± 531 | 1833 ± 330 | 1789 ± 548 | 1952 ± 414 | 1986 ± 620 |
| Caffeine intake, mg/day | 298 ± 172 | 315 ± 233 | 237 ± 142 | 244 ± 224 | 249 ± 175 | 239 ± 250 |
| Tea intake, cups/day5 | 0.5 ± 0.6 | 0.7 ± 1.1 | 0.6 ± 0.7 | 0.7 ± 1.1 | 0.4 ± 0.5 | 0.4 ± 0.8 |
| Coffee intake, cups/day | 1.7 ± 1.3 | 1.7 ± 1.7 | 1.3 ± 1.0 | 1.2 ± 1.5 | 1.4 ± 1.1 | 1.3 ± 1.6 |
All values, except age, are age-adjusted to the distribution of the cohort. Data are given as mean ± SD unless otherwise specified. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; USD, US dollar.
Race was self-reported by study participants.
Median income was derived from the census tract–level median income for the 2000 census, and was only available for the NHS and NHSII cohorts.
Values for total flavonoids and all subclasses were energy adjusted using the residual method.
One cup of tea or coffee is 8 fluid ounces.
FIGURE 1.
Top food contributors to total flavonoids or flavonoid subclasses, by year and cohort: (A) total flavonoids, (B) flavan-3-ols, and (C) polymeric flavonoids (polymers). HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Multivariable-adjusted associations between long-term and recent flavonoid intakes and incidences of glioma are shown in Table 2. Average long-term total flavonoid consumption was associated with a decreased risk of glioma in the pooled analysis (HR for highest compared to lowest quintile, 0.79; 95% CI, 0.59–1.05; P-trend = 0.04). Average long-term flavan-3-ol intake was associated with a decreased risk of glioma in the pooled analysis (HR, 0.76; 95% CI, 0.57–1.01; P-trend = 0.04). Average long-term flavan-3-ol intake was associated with a decreased risk of glioma in women (HR, 0.68; 95% CI, 0.47–0.97; P-trend = 0.07), but not in men (HR, 0.92; 95% CI, 0.58–1.45; P-trend = 0.31). Average long-term polymer intake was associated with a suggestive decreased risk of glioma in the pooled analysis (HR, 0.82; 95% CI, 0.61–1.09; P-trend = 0.05) and in women, but not men. There were no associations between other flavonoid subclasses and glioma risks (Table 2).
TABLE 2.
Multivariable-adjusted risk of glioma in women's (NHS, NHSII), men's (HPFS), and pooled (NHS, NHSII, HPFS) cohorts by total flavonoid and flavonoid subclass intake, using Cox proportional hazard modeling1
| Dietary flavonoid intake | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend2 | |
| Total flavonoids | ||||||
| Women (325 cases) | ||||||
| Recent3 intake | ||||||
| HR (95% CI) | Reference | 1.26 (0.89–1.79) | 1.09 (0.76–1.57) | 1.03 (0.71–1.48) | 1.02 (0.71–1.48) | 0.65 |
| Cases, n | 55 | 77 | 68 | 65 | 60 | — |
| Median (range), mg/day | 121.4 (0.5–160.4) | 194.3 (160.5–228.5) | 266.3 (228.6–313.2) | 376.2 (313.3–475.3) | 690.4 (475.4–4108) | — |
| Long-term4 intake | ||||||
| HR (95% CI) | Reference | 1.04 (0.73–1.48) | 1.12 (0.80–1.58) | 0.84 (0.58–1.21) | 0.84 (0.58–1.22) | 0.16 |
| Cases, n | 61 | 70 | 79 | 59 | 56 | — |
| Median (range), mg/day | 123.4 (0.6–162.8) | 196.3 (162.9–230.6) | 268.6 (230.7–315.3) | 377.7 (315.4–476.6) | 683.3 (476.7–4108) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.97 (0.63–1.49) | 1.02 (0.67–1.57) | 0.85 (0.54–1.32) | 0.80 (0.51–1.25) | 0.23 |
| Cases, n | 42 | 44 | 48 | 40 | 37 | — |
| Median (range), mg/day | 131.3 (4.1–172.8) | 208.8 (172.9–243.7) | 281.8 (243.8–325.1) | 381.7 (325.2–464.8) | 629.5 (464.9–4117) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.89 (0.58–1.36) | 1.10 (0.72–1.67) | 0.86 (0.56–1.34) | 0.70 (0.44–1.11) | 0.10 |
| Cases, n | 43 | 42 | 51 | 42 | 33 | — |
| Median (range), mg/day | 131.5 (4.1–172.0) | 206.7 (172.1–240.5) | 277.9 (240.6–318.7) | 372.7 (318.8–453.5) | 615.2 (453.6–3517) | — |
| Pooled5 (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 1.13 (0.86–1.49) | 1.06 (0.80–1.40) | 0.95 (0.72–1.26) | 0.93 (0.69–1.23) | 0.30 |
| Cases, n | 97 | 121 | 116 | 105 | 97 | — |
| Median (range), mg/day | 125.3 (1.9–165.3) | 200.0 (165.4–234.5) | 272.4 (234.6–317.9) | 378.3 (318.0–471.2) | 666.4 (471.3–4112) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.98 (0.74–1.28) | 1.11 (0.85–1.45) | 0.85 (0.64–1.12) | 0.79 (0.59–1.05) | 0.04 |
| Cases, n | 104 | 112 | 130 | 101 | 89 | — |
| Median (range), mg/day | 126.6 (2.0–166.4) | 200.4 (166.5–234.5) | 271.9 (234.6–316.6) | 375.7 (316.7–467.5) | 656.5 (467.6–3,875) | — |
| Flavan-3-ols | ||||||
| Women (325 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.92 (0.66–1.28) | 0.83 (0.59–1.16) | 0.82 (0.58–1.15) | 0.79 (0.55–1.12) | 0.32 |
| Cases, n | 71 | 70 | 64 | 63 | 57 | — |
| Median (range), mg/day | 9.5 (0–13.3) | 17.0 (13.4–21.5) | 27.7 (21.6–36.8) | 50.6 (36.9–73.5) | 124.4 (73.6–951.3) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.85 (0.61–1.18) | 0.82 (0.58–1.14) | 0.74 (0.53–1.05) | 0.68 (0.47–0.97) | 0.07 |
| Cases, n | 74 | 69 | 68 | 61 | 53 | — |
| Median (range), mg/day | 9.9 (0–14.0) | 18.2 (14.1–23.3) | 30.2 (23.4–40.0) | 54.5 (40.1–77.1) | 125.2 (77.2–946.6) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 1.08 (0.70–1.67) | 1.22 (0.80–1.87) | 0.95 (0.61–1.49) | 0.90 (0.57–1.41) | 0.33 |
| Cases, n | 40 | 44 | 51 | 39 | 37 | — |
| Median (range), mg/day | 10.7 (0.1–14.9) | 18.8 (15.0–23.1) | 28.3 (23.2–35.3) | 45.5 (35.4–62.7) | 99.3 (62.8–951.3) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.16 (0.76–1.79) | 1.28 (0.83–1.96) | 0.97 (0.61–1.53) | 0.92 (0.58–1.45) | 0.31 |
| Cases, n | 39 | 47 | 50 | 38 | 37 | — |
| Median (range), mg/day | 10.9 (0.1–15.2) | 19.1 (15.3–23.5) | 28.8 (23.6–36.0) | 46.5 (36.1–64.1) | 100.5 (64.2–819.7) | — |
| Pooled (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.97 (0.75–1.27) | 0.97 (0.74–1.26) | 0.86 (0.66–1.14) | 0.83 (0.63–1.09) | 0.18 |
| Cases, n | 111 | 114 | 115 | 102 | 94 | — |
| Median (range), mg/day | 10.0 (0–13.9) | 17.7 (14.0–22.1) | 28.0 (22.2–36.2) | 48.6 (36.3–69.2) | 114.5 (69.3–951.3) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.95 (0.73–1.24) | 0.97 (0.74–1.26) | 0.82 (0.62–1.07) | 0.76 (0.57–1.01) | 0.04 |
| Cases, n | 113 | 116 | 118 | 99 | 90 | — |
| Median (range), mg/day | 10.3 (0.1–14.5) | 18.5 (14.6–23.4) | 29.7 (23.5–38.4) | 51.3 (38.5–72.0) | 115.5 (72.1–896.6) | — |
| Polymers | ||||||
| Women (325 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.80 (0.56–1.14) | 1.05 (0.75–1.46) | 0.81 (0.57–1.15) | 0.85 (0.60–1.21) | 0.54 |
| Cases, n | 67 | 59 | 79 | 60 | 60 | — |
| Median (range), mg/day | 62.5 (0–86.9) | 109.6 (87.0–133.0) | 160.0 (133.1–192.7) | 238.3 (192.8–312.1) | 474.7 (312.2–3189) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.88 (0.63–1.24) | 1.01 (0.72–1.41) | 0.76 (0.53–1.09) | 0.74 (0.51–1.06) | 0.08 |
| Cases, n | 68 | 67 | 77 | 59 | 54 | — |
| Median (range), mg/day | 64.3 (0–88.8) | 111.3 (88.9–134.7) | 161.9 (134.8–195.3) | 241.7 (195.4–315.5) | 471.0 (315.6–3189) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 1.20 (0.78–1.82) | 0.92 (0.58–1.44) | 1.02 (0.65–1.58) | 0.86 (0.54–1.35) | 0.30 |
| Cases, n | 40 | 50 | 39 | 45 | 37 | — |
| Median (range), mg/day | 62.2 (0–88.0) | 111.1 (88.1–135.0) | 160.9 (135.1–192.0) | 231.7 (192.1–292.0) | 415.8 (292.1–3245) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.35 (0.88–2.07) | 1.09 (0.69–1.73) | 1.05 (0.66–1.66) | 0.96 (0.61–1.53) | 0.37 |
| Cases, n | 36 | 54 | 41 | 42 | 38 | — |
| Median (range), mg/day | 62.8 (0–87.6) | 110.1 (87.7–133.1) | 157.9 (133.2–187.3) | 226.3 (187.4–286.2) | 408.8 (286.3–2752) | — |
| Pooled (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.94 (0.72–1.24) | 1.00 (0.77–1.31) | 0.88 (0.67–1.16) | 0.85 (0.65–1.13) | 0.27 |
| Cases, n | 107 | 109 | 118 | 105 | 97 | — |
| Median (range), mg/day | 62.4 (0–87.4) | 110.2 (87.5–133.8) | 160.4 (133.9–192.5) | 235.7 (192.6–304.2) | 451.5 (304.3–3211) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.04 (0.80–1.36) | 1.04 (0.79–1.36) | 0.86 (0.65–1.14) | 0.82 (0.61–1.09) | 0.05 |
| Cases, n | 104 | 121 | 118 | 101 | 92 | — |
| Median (range), mg/day | 63.7 (0–88.3) | 110.8 (88.4–134.1) | 160.3 (134.2–192.1) | 235.6 (191.2–304.0) | 446.5 (304.1–3017) | — |
| Flavan-3-ols and proanthocyanidins | ||||||
| Women (325 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.80 (0.56–1.14) | 0.96 (0.68–1.34) | 0.84 (0.59–1.19) | 0.86 (0.60–1.23) | 0.62 |
| Cases, n | 67 | 58 | 74 | 63 | 63 | — |
| Median (range), mg/day | 62.3 (0.1–83.1) | 101.0 (83.2–118.3) | 136.5 (118.4–157.1) | 182.3 (157.2–217.3) | 277.0 (217.4–1448) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.95 (0.67–1.35) | 0.97 (0.69–1.37) | 0.80 (0.56–1.15) | 0.92 (0.64–1.30) | 0.55 |
| Cases, n | 64 | 66 | 71 | 59 | 65 | — |
| Median (range), mg/day | 63.6 (0.2–83.9) | 100.9 (84.0–116.9) | 134.0 (117.0–152.3) | 174.7 (152.4–206.2) | 260.3 (206.3–1358) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.95 (0.61–1.47) | 0.98 (0.63–1.51) | 0.98 (0.63–1.51) | 0.90 (0.58–1.42) | 0.70 |
| Cases, n | 41 | 41 | 43 | 45 | 41 | — |
| Median (range), mg/day | 67.6 (0.2–91.0) | 110.9 (91.1–130.1) | 150.1 (130.2–172.6) | 199.6 (172.7–236.5) | 299.2 (236.6–1464) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.00 (0.65–1.54) | 0.97 (0.62–1.50) | 1.09 (0.71–1.67) | 0.77 (0.48–1.22) | 0.29 |
| Cases, n | 41 | 44 | 42 | 49 | 35 | — |
| Median (range), mg/day | 67.9 (0.2–90.5) | 109 (90.6–127.1) | 145 (127.2–165.6) | 190 (165.7–222.5) | 278 (222.6–1315) | — |
| Pooled (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.86 (0.65–1.13) | 0.96 (0.74–1.26) | 0.89 (0.68–1.17) | 0.88 (0.66–1.16) | 0.53 |
| Cases, n | 108 | 99 | 117 | 108 | 104 | — |
| Median (range), mg/day | 64.4 (0.1–86.2) | 104.9 (86.3–123.0) | 141.8 (123.1–163.2) | 189.1 (163.3–224.8) | 285.7 (224.0–1454) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.97 (0.74–1.27) | 0.97 (0.74–1.27) | 0.91 (0.69–1.20) | 0.86 (0.65–1.14) | 0.26 |
| Cases, n | 105 | 110 | 113 | 108 | 100 | — |
| Median (range), mg/day | 65.3 (0.2–86.5) | 104.1 (86.6–120.9) | 138.3 (121.0–157.6) | 180.7 (157.7–212.6) | 267.3 (212.7–1341) | — |
| Flavonols | ||||||
| Women (325 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 1.31 (0.91–1.89) | 1.16 (0.79–1.69) | 1.23 (0.85–1.79) | 1.24 (0.85–1.81) | 0.49 |
| Cases, n | 49 | 72 | 65 | 71 | 68 | — |
| Median (range), mg/day | 7.6 (0.1–9.6) | 11.4 (9.7–13.1) | 15.1 (13.2–17.3) | 20.1 (17.4–24.2) | 31.0 (24.3–205.3) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.02 (0.71–1.48) | 1.11 (0.77–1.59) | 1.13 (0.79–1.61) | 1.01 (0.70–1.46) | 0.86 |
| Cases, n | 55 | 62 | 72 | 73 | 63 | — |
| Median (range), mg/day | 7.5 (0.1–9.5) | 11.2 (9.6–12.9) | 14.6 (13.0–16.6) | 19.1 (16.7–22.7) | 28.9 (22.8–156.9) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.88 (0.56–1.37) | 1.01 (0.65–1.56) | 1.13 (0.74–1.74) | 0.76 (0.47–1.22) | 0.41 |
| Cases, n | 40 | 38 | 44 | 54 | 35 | — |
| Median (range), mg/day | 8.1 (0.5–10.3) | 12.2 (10.4–14.2) | 16.1 (14.3–18.4) | 21.2 (18.5–25.0) | 31.6 (25.1–173.0) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.92 (0.58–1.44) | 1.22 (0.80–1.87) | 1.07 (0.69–1.65) | 0.72 (0.44–1.16) | 0.19 |
| Cases, n | 39 | 39 | 52 | 49 | 32 | — |
| Median (range), mg/day | 8.0 (0.5–10.2) | 12.0 (10.3–13.7) | 15.5 (13.8–17.5) | 20.0 (17.6–23.3) | 29.1 (23.4–134.5) | — |
| Pooled (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 1.12 (0.84–1.48) | 1.09 (0.82–1.45) | 1.19 (0.90–1.57) | 1.03 (0.76–1.38) | 0.96 |
| Cases, n | 89 | 110 | 109 | 125 | 103 | |
| Median (range), mg/day | 7.8 (0.2–9.9) | 11.7 (10.0–13.6) | 15.5 (13.7–17.7) | 20.5 (17.8–24.5) | 31.2 (24.6–192.6) | |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.98 (0.74–1.30) | 1.15 (0.88–1.52) | 1.10 (0.83–1.46) | 0.89 (0.66–1.19) | 0.51 |
| Cases, n | 94 | 101 | 124 | 122 | 95 | — |
| Median (range), mg/day | 7.7 (0.2–9.8) | 11.5 (9.9–13.2) | 15.0 (13.3–17.0) | 19.5 (17.1–23.0) | 29.0 (23.1–148.0) | — |
| Anthocyanidins | ||||||
| Women (325 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 1.07 (0.73–1.56) | 1.15 (0.80–1.66) | 1.09 (0.74–1.59) | 1.25 (0.86–1.82) | 0.21 |
| Cases, n | 52 | 64 | 68 | 66 | 75 | — |
| Median (range), mg/day | 2.7 (0.0–4.3) | 6.1 (4.4–8.2) | 10.7 (8.3–13.4) | 16.7 (13.5–21.6) | 31.7 (21.7–842.3) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.02 (0.70–1.50) | 1.00 (0.68–1.46) | 1.23 (0.85–1.77) | 1.11 (0.76–1.61) | 0.24 |
| Cases, n | 53 | 63 | 61 | 77 | 71 | — |
| Median (range), mg/day | 2.9 (0–4.4) | 6.0 (4.5–7.9) | 10.0 (8.0–12.4) | 15.2 (12.5–19.1) | 26.3 (19.2–600.9) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.94 (0.62–1.45) | 0.84 (0.54–1.31) | 0.84 (0.54–1.32) | 0.95 (0.60–1.49) | 0.89 |
| Cases, n | 42 | 45 | 40 | 39 | 45 | — |
| Median (range), mg/day | 2.8 (0–4.5) | 6.3 (4.6–8.5) | 11.1 (8.6–14.1) | 17.7 (14.2–22.9) | 34.0 (23.0–824.0) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.83 (0.54–1.27) | 0.70 (0.44–1.09) | 0.81 (0.52–1.25) | 0.85 (0.55–1.31) | 0.74 |
| Cases, n | 46 | 43 | 36 | 41 | 45 | — |
| Median (range), mg/day | 2.9 (0–4.5) | 6.2 (4.6–8.1) | 10.4 (8.2–13.0) | 16.1 (13.1–20.3) | 28.3 (20.4–580.1) | — |
| Pooled (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 1.01 (0.76–1.34) | 1.01 (0.76–1.34) | 0.98 (0.73–1.31) | 1.12 (0.84–1.49) | 0.35 |
| Cases, n | 94 | 109 | 108 | 105 | 120 | — |
| Median (range), mg/day | 2.7 (0–4.4) | 6.2 (4.5–8.3) | 10.8 (8.4–13.7) | 17.1 (13.8–22.1) | 32.6 (22.2–835.1) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.93 (0.70–1.24) | 0.86 (0.64–1.15) | 1.03 (0.78–1.37) | 0.99 (0.74–1.31) | 0.47 |
| Cases, n | 99 | 106 | 97 | 118 | 116 | — |
| Median (range), mg/day | 2.9 (0–4.4) | 6.1 (4.5–8.0) | 10.2 (8.1–12.6) | 15.6 (12.7–19.6) | 27.1 (19.7–592.7) | — |
| Flavanones | ||||||
| Women (325 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.85 (0.59–1.22) | 0.95 (0.67–1.34) | 0.88 (0.62–1.26) | 1.00 (0.71–1.41) | 0.83 |
| Cases, n | 63 | 56 | 66 | 65 | 75 | — |
| Median (range), mg/day | 7.4 (0–13.5) | 19.6 (13.6–26.0) | 32.8 (26.1–40.3) | 49.0 (40.4–59.9) | 77.9 (60.0–658.8) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.01 (0.70–1.45) | 0.99 (0.69–1.42) | 0.95 (0.66–1.35) | 1.04 (0.73–1.48) | 0.94 |
| Cases, n | 59 | 63 | 65 | 66 | 72 | — |
| Median (range), mg/day | 9.1 (0–15.6) | 21.7 (15.7–27.7) | 34.2 (27.8–41.1) | 49.1 (41.2–59.3) | 75.7 (59.4–659.0) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.96 (0.60–1.54) | 1.33 (0.86–2.05) | 1.13 (0.72–1.76) | 0.99 (0.63–1.56) | 0.98 |
| Cases, n | 36 | 36 | 51 | 46 | 42 | — |
| Median (range), mg/day | 10.0 (0–18.6) | 27.5 (18.7–36.5) | 45.6 (36.6–55.0) | 65.4 (55.1–78.3) | 100.0 (78.4–713.8) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.02 (0.64–1.61) | 1.08 (0.68–1.70) | 1.18 (0.76–1.83) | 1.07 (0.68–1.68) | 0.65 |
| Cases, n | 36 | 39 | 42 | 48 | 46 | — |
| Median (range), mg/day | 11.3 (0–20.4) | 29.3 (20.5–37.9) | 46.7 (38.0–55.6) | 65.5 (55.7–77.8) | 98.3 (77.9–713.8) | — |
| Pooled (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.89 (0.67–1.18) | 1.08 (0.82–1.42) | 0.97 (0.74–1.28) | 0.99 (0.75–1.31) | 0.87 |
| Cases, n | 99 | 92 | 117 | 111 | 117 | — |
| Median (range), mg/day | 8.4 (0–15.5) | 22.7 (15.6–30.1) | 37.8 (30.2–46.1) | 55.4 (46.2–67.2) | 86.6 (67.3–680.4) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.01 (0.76–1.34) | 1.02 (0.77–1.36) | 1.03 (0.78–1.36) | 1.05 (0.80–1.38) | 0.70 |
| Cases, n | 95 | 102 | 107 | 114 | 118 | — |
| Median (range), mg/day | 10.0 (0–17.5) | 24.7 (17.6–31.7) | 39.1 (31.8–46.8) | 55.6 (46.9–66.6) | 84.6 (66.7–680.6) | — |
| Flavones | ||||||
| Women (325 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.90 (0.62–1.29) | 0.97 (0.68–1.38) | 0.88 (0.61–1.26) | 1.00 (0.70–1.41) | 0.91 |
| Cases, n | 60 | 58 | 65 | 65 | 77 | — |
| Median (range), mg/day | 0.74 (0–1.0) | 1.3 (1.1–1.5) | 1.8 (1.6–2.1) | 2.4 (2.2–2.9) | 3.5 (3.0–84.9) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 1.03 (0.72–1.49) | 1.04 (0.73–1.49) | 1.05 (0.73–1.51) | 0.91 (0.63–1.31) | 0.53 |
| Cases, n | 56 | 63 | 68 | 74 | 64 | — |
| Median (range), mg/day | 0.77 (0–1.1) | 1.3 (1.2–1.5) | 1.8 (1.6–2.1) | 2.4 (2.2–2.7) | 3.3 (2.8–74.9) | — |
| Men (211 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.82 (0.51–1.32) | 1.27 (0.82–1.95) | 1.02 (0.65–1.61) | 1.23 (0.79–1.91) | 0.21 |
| Cases, n | 37 | 32 | 51 | 41 | 50 | — |
| Median (range), mg/day | 0.90 (0–1.4) | 1.7 (1.5–2.1) | 2.4 (2.2–2.8) | 3.2 (2.9–3.7) | 4.7 (3.8–112.6) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.65 (0.40–1.06) | 1.05 (0.68–1.62) | 1.11 (0.72–1.70) | 1.09 (0.70–1.67) | 0.20 |
| Cases, n | 40 | 28 | 46 | 49 | 48 | — |
| Median (range), mg/day | 0.98 (0–1.4) | 1.7 (1.5–2.0) | 2.4 (2.1–2.7) | 3.1 (2.8–3.6) | 4.4 (3.7–75.3) | — |
| Pooled (536 cases) | ||||||
| Recent intake | ||||||
| HR (95% CI) | Reference | 0.87 (0.65–1.16) | 1.08 (0.82–1.42) | 0.93 (0.70–1.23) | 1.08 (0.82–1.42) | 0.33 |
| Cases, n | 97 | 90 | 116 | 106 | 127 | — |
| Median (range), mg/day | 0.80 (0–1.2) | 1.5 (1.3–1.7) | 2.0 (1.8–2.4) | 2.7 (2.5–3.2) | 4.0 (3.3–95.8) | — |
| Long-term intake | ||||||
| HR (95% CI) | Reference | 0.88 (0.65–1.17) | 1.05 (0.79–1.38) | 1.08 (0.82–1.42) | 0.98 (0.74–1.29) | 0.61 |
| Cases, n | 96 | 91 | 114 | 123 | 112 | — |
| Median (range), mg/day | 0.85 (0–1.2) | 1.5 (1.3–1.7) | 2.0 (1.8–2.3) | 2.7 (2.4–3.1) | 3.7 (3.2–75.1) | — |
Cox proportional hazard models using energy-adjusted flavonoid intake and adjusted for age, total caloric intake (quintile), BMI (<25 kg/m2, 25–29.9 kg/m2, or ≥30 kg/m2), smoking status (never, past, or current), and hyperlipidemia (ever diagnosed compared with never diagnosed). HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; Q, quintile.
P trends were calculated using quintile median values in Cox proportional hazards regression models to estimate linear trends.
Recent flavonoid intake was calculated as the average of reported intake in the 12 years prior to diagnosis or the end of follow-up.
Long-term flavonoid intake was calculated as the average of reported intake in all years (up to 30) prior to diagnosis or the end of follow-up.
The pooled analysis consists of data from women (NHS, NHSII) and men (HPFS), combined by meta-analysis using fixed-effects models.
An additional adjustment for caffeine did not materially change the associations between flavan-3-ol or polymer intakes and the risk of glioma (Table 3). Neither cumulative long-term flavan-3-ol nor cumulative long-term polymer intake was significantly associated with the risk of glioma after an additional adjustment for tea consumption (Table 3), suggesting that the association was driven by components of tea. The addition of median income as a covariate in these models did not materially change the results. The association between flavan-3-ol intake and the risk of glioma was more pronounced in the NHS compared to the NHSII [HR for highest compared to lowest quintile, long-term intake: NHS HR, 0.57 (95% CI, 0.37–0.88; P-trend = 0.01); NHSII HR, 0.96 (95% CI, 0.51–1.80; P-trend = 0.80); P-heterogeneity was >0.05 in all pooled analyses].
TABLE 3.
Multivariable-adjusted risk of glioma in women's (NHS, NHSII), men's (HPFS), and pooled (NHS, NHSII, HPFS) cohorts by flavan-3-ol and polymeric flavonoid (polymer) intake, with additional adjustments for total caffeine and total tea intake1
| Hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend2 | |
| Flavan-3-ols | ||||||
| Women (325 cases) | ||||||
| Long-term intake,3 adjusted for tea | 1 (Reference) | 0.86 (0.61–1.19) | 0.86 (0.61–1.21) | 0.86 (0.58–1.27) | 1.02 (0.55–1.92) | 0.81 |
| Long-term intake, adjusted for caffeine | 1 (Reference) | 0.85 (0.61–1.18) | 0.82 (0.59–1.15) | 0.74 (0.53–1.05) | 0.69 (0.48–0.99) | 0.08 |
| Men (211 cases) | ||||||
| Long-term intake, adjusted for tea | 1 (Reference) | 1.17 (0.76–1.81) | 1.32 (0.86–2.02) | 1.07 (0.66–1.72) | 1.33 (0.67–2.64) | 0.55 |
| Long-term intake, adjusted for caffeine | 1 (Reference) | 1.15 (0.75–1.78) | 1.27 (0.83–1.95) | 0.96 (0.61–1.52) | 0.93 (0.59–1.47) | 0.35 |
| Pooled4 (536 cases) | ||||||
| Long-term intake, adjusted for tea | 1 (Reference) | 0.96 (0.74–1.25) | 1.01 (0.78–1.33) | 0.94 (0.69–1.27) | 1.16 (0.73–1.84) | 0.58 |
| Long-term intake, adjusted for caffeine | 1 (Reference) | 0.95 (0.73–1.24) | 0.97 (0.74–1.26) | 0.82 (0.62–1.07) | 0.77 (0.58–1.02) | 0.05 |
| Polymers | ||||||
| Women (325 cases) | ||||||
| Long-term intake, adjusted for tea | 1 (Reference) | 0.90 (0.64–1.27) | 1.06 (0.76–1.49) | 0.87 (0.59–1.28) | 1.09 (0.61–1.95) | 0.72 |
| Long-term intake, adjusted for caffeine | 1 (Reference) | 0.88 (0.62–1.24) | 1.00 (0.72–1.40) | 0.76 (0.53–1.08) | 0.74 (0.52–1.07) | 0.09 |
| Men (211 cases) | ||||||
| Long-term intake, adjusted for tea | 1 (Reference) | 1.37 (0.89–2.10) | 1.13 (0.71–1.79) | 1.15 (0.71–1.85) | 1.34 (0.72–2.49) | 0.59 |
| Long-term intake, adjusted for caffeine | 1 (Reference) | 1.32 (0.86–2.03) | 1.07 (0.67–1.69) | 1.02 (0.65–1.62) | 0.96 (0.60–1.53) | 0.38 |
| Pooled (536 cases) | ||||||
| Long-term intake, adjusted for tea | 1 (Reference) | 1.06 (0.81–1.38) | 1.09 (0.83–1.43) | 0.97 (0.72–1.31) | 1.20 (0.79–1.83) | 0.54 |
| Long-term intake, adjusted for caffeine | 1 (Reference) | 1.03 (0.79–1.35) | 1.02 (0.78–1.34) | 0.85 (0.64–1.12) | 0.82 (0.61–1.09) | 0.06 |
Cox proportional hazards models using energy-adjusted flavonoid intake and adjusted for age, total caloric intake (quintile), BMI (<25 kg/m2, 25–29.9 kg/m2, or ≥30 kg/m2), smoking status (never, past, or current), hyperlipidemia (ever diagnosed compared with never diagnosed), and either total caffeine or total tea intake. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; Q, quintile.
P trends were calculated using quintile median values in Cox proportional hazards regression models to estimate linear trends.
Long-term flavonoid intake was calculated as the average of reported intake in all years (up to 30) prior to diagnosis or the end of follow-up.
The pooled analysis includes data from women (NHS, NHSII) and men (HPFS), combined by a meta-analysis using fixed-effects models.
The results did not change materially with the lagged model (Supplementary Table 1) or with GBM as the primary outcome (Supplementary Table 2). There was an attenuation in the significance of associations of flavan-3-ols and polymers with the risk of glioma in both secondary analyses, which was expected given the smaller number of cases in these groups. In the lagged model, anthocyanidin intake was associated with an increased risk of glioma in the pooled analysis (HR, 1.34; 95% CI, 0.98–1.84; P-trend = 0.02).
Flavan-3-ols classified as tea-dominant were highly correlated with tea consumption (Pearson correlation coefficient, r = 0.90 for long-term tea flavan-3-ol and long-term tea intake); flavan-3-ols classified as non-tea were also highly correlated with tea consumption, although less so (r = 0.77). Polymers classified as tea-dominant were highly correlated with tea consumption (r = 0.78), while polymers classified as non–tea dominant were less strongly correlated with tea consumption (r = 0.12). Flavan-3-ol and polymer intakes were also highly correlated (r = 0.98).
Cumulative long-term tea flavan-3-ol intake was associated with a decreased risk of glioma in the pooled analysis (HR per 100 mg/day increase in intake = 0.74; 95% CI, 0.58–0.96), and there was a suggested association between long-term tea flavan-3-ol intake and decreased glioma risks in men and women separately (Supplementary Table 3). There was a suggested association between long-term non-tea flavan-3-ol consumption and a decreased glioma risk in the pooled cohort (HR, 0.46; 95% CI, 0.19–1.08). Cumulative long-term tea polymer intake was associated with a decreased risk of glioma in the pooled analysis (recent HR per 400 mg/day increase in intake = 0.78; 95% CI, 0.61–1.00) and in men (HR, 0.64; 95% CI, 0.41–1.00), with a suggested association in women (HR, 0.85; 95% CI, 0.64–1.14). Non-tea polymer intake was not associated with glioma risks in the men's, women's, or pooled cohorts (Supplementary Table 3).
Discussion
In a pooled analysis of 3 large cohorts of men and women, a cumulative long-term increased dietary flavan-3-ol and polymeric flavonoid intake was associated with a decreased risk of glioma. The strength of the associations for men and women varied, but the direction of associations was similar among cohorts. Tea was the largest dietary contributor to both flavan-3-ol and polymer subclasses, and tea intake was highly correlated with intakes of both subclasses. After adjustment for tea consumption, neither the flavan-3-ol nor the polymer intake was associated with the risk of glioma.
In the main analysis, cumulative long-term flavan-3-ol and polymer intakes were each associated with a decreased glioma risk in the pooled cohort. Because the duration of the preclinical period of glioma is poorly understood, we analyzed 3 different timelines in this study: cumulative long-term intake (average of all data preceding a diagnosis), recent intake (average of the most recent 12 years before a diagnosis), and a lagged model (long-term, excluding the most recent 8 years before a diagnosis). Our results suggest that the long-term habitual flavan-3-ol and polymer intake is most strongly associated with a decreased glioma risk; however, we did not have sufficient statistical power for a robust analysis of the specific timing of flavonoid consumption in relation to the risk of glioma.
We observed no association of other flavonoid subclasses with glioma risks in the long-term or recent flavonoid intake models. In the lagged model only, we observed an increased risk of glioma with increased anthocyanin intake; this finding may have been due to chance.
Although no prior studies have investigated the association between flavonoids and glioma risks, several prospective cohort studies, including our own earlier work, have found an inverse association between tea consumption and the risk of glioma (2, 3, 19–21). Given the significant contribution of tea to dietary flavan-3-ol and polymeric flavonoid intakes in our cohorts and the prior evidence for an inverse association between tea consumption and glioma, our findings add to the understanding of how tea consumption might be mechanistically linked to glioma through the actions of flavan-3-ols, theaflavins, and thearubigins.
We attempted to distinguish whether unique tea flavan-3-ols and polymers or all dietary flavan-3-ols and polymers were associated with glioma risks, and found that the association between flavan-3-ols and polymers and the risk of glioma was attenuated after mutual adjustment for tea intake. Additionally, we found that tea- but not non–tea predominant flavan-3-ols and polymers were associated with decreased glioma risks, although both sources of flavan-3-ols and polymers were associated with tea intake. The specific impact of flavan-3-ols and polymers, independent of tea intake, was difficult to disentangle, given substantial overlap in the flavan-3-ols found in both tea and non-tea dietary sources. Tea provides a much larger contribution of flavonoids in the diet than that derived from non-tea dietary sources, adding to the difficulty in comparing the 2 sources; thus, these results should be interpreted with caution and warrant follow-up. An additional adjustment for caffeine did not materially change the associations between flavan-3-ol or polymer intakes and glioma risks, suggesting that the observed associations are not explained by the biologic effects of caffeine.
Our observation of a decreased glioma risk with increased flavan-3-ol intake is supported by in vitro and mouse-model studies of the effects of individual flavonoids on glioma cell proliferation, which have shown that flavan-3-ols have antioxidative properties and the potential to disrupt metabolic pathways unique to glioma cells (22). Most studies on the effects of tea flavonoids in vitro or in mouse models have focused on epigallocatechin 3-gallate. For example, Zhang et al. (23) showed that glutamate dehydrogenase was elevated in human glioma cells, that glutamate dehydrogenase levels are predictive of survival in human glioma patients, and that glutamate dehydrogenase was inhibited in mice treated with epigallocatechin 3-gallate, leading to decreased tumor weights. Multiple other in vitro studies provide evidence to suggest that among the flavan-3-ols, epigallocatechin 3-gallate is the most likely to have significant anti-glioma properties, including increasing sensitivity to anti-cancer drug therapy (22, 24–26). Flavan-3-ol monomers have been shown to cross the blood-brain barrier, albeit inefficiently, further supporting the potential for a role of flavan-3-ols in glioma development in human (24).
Theaflavins are produced from flavonoid monomers (including epicatechin 3-gallate, epigallocatechin, and epigallocatechin 3-gallate) during the production of black and oolong tea, and have been shown to have comparable antioxidant effects as catechins (27). Given that theaflavins and tea monomers have similar properties, it makes sense that a similar association with the risk of glioma was observed between these 2 groups. In this study, intakes of all tea flavonoids were highly correlated, making it difficult to distinguish the independent effects of each subclass.
A limitation of this study is that we were unable to distinguish between green, black, or other types of tea, which have varying levels of flavonoids and would have allowed better distinction of the flavonoids that are driving the associations between subclasses and decreased glioma risks. We relied solely on reported intakes, but validation studies have shown a high correlation between FFQs and 24-hour dietary recalls. Another limitation of this study is its lack of generalizability to nonwhite populations, as this population was over 90% white. Strengths of our study include the prospective design, with over 500 cases in the pooled analyses and over 5 million person-years of follow-up. The longitudinal assessment of dietary habits allowed us to capture the effects of long-term flavonoid consumption, which appears to be more influential on the risk of glioma when compared to recent intake.
In conclusion, in this prospective study of men and women, habitual, long-term dietary intake of flavan-3-ol and polymeric flavonoids was associated with a decreased risk of glioma. These associations may be driven predominantly by tea flavan-3-ols (epigallocatechin, epicatechin 3-gallate, and epigallocatechin 3-gallate) and polymers (theaflavins and thearubigins); additional studies with more precise data on the types of tea consumed, or using metabolomic markers of flavonoid consumption, are warranted to further describe the associations between tea, flavonoids, and glioma risks.
Supplementary Material
Acknowledgments
We thank the participants and staff of the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AR, AZ, CA, CO, CT, DE, FL, GA, IA, ID, IL, IN, KY, LA, MA, MD, ME, MI, NC, ND, NE, NH, NJ, NY, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY.
The authors’ responsibilities were as follows – AMB, MJS, DJC: designed the research (project conception, development of overall research plan, and study oversight); AMB: analyzed data or performed the statistical analysis, wrote the paper (only authors who made a major contribution), and had primary responsibility for the final content; AC, EBR: provided subject matter expertise in contribution to study design; AC, EBR, MJS, DJC: reviewed and edited the paper; and all authors: read and approved the final manuscript. AC and EBR are on the scientific advisory committee for the United States Highbush Blueberry Council and receive research funding for a project unrelated to this manuscript. All other authors report no conflicts of interest.
Notes
This work was supported by the US National Institutes of Health grants (UM1 CA186107, P01 CA87969, U01 CA176726, and U01 CA167552).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors assume full responsibility for analyses and interpretation of these data.
Supplemental Figure 1 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: GBM, glioblastoma; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Contributor Information
Alaina M Bever, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Aedin Cassidy, Institute for Global Food Security, Queen's University Belfast, Belfast, United Kingdom.
Eric B Rimm, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Meir J Stampfer, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
David J Cote, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Data Availability
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Pranata R, Feraldho A, Lim MA, Henrina J, Vania R, Golden N, July J. Coffee and tea consumption and the risk of glioma: A systematic review and dose-response meta-analysis. Br J Nutr. 2021:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Cote DJ, Bever AM, Wilson KM, Smith TR, Smith-Warner SA, Stampfer MJ. A prospective study of tea and coffee intake and risk of glioma. Int J Cancer. 2020;146(9):2442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick CN, Smith SG, Giovannucci E, Michaud DS. Coffee, tea, caffeine intake and risk of adult glioma in 3 prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2010;19(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielecka J, Markiewicz-Żukowska R. The influence of nutritional and lifestyle factors on glioma incidence. Nutrients. 2020;12(6):1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braganhol E, Zamin LL, Canedo AD, Horn F, Tamajusuku ASK, Wink MR, Salbego C, Battastini AMO. Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer Drugs. 2006;17:663–71. [DOI] [PubMed] [Google Scholar]
- 6.Faria A, Pestana D, Teixeira D, Azevedo J, De Freitas V, Mateus N, Calhau C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell Mol Biol Lett. 2010;15(2):234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickman AM, Khan UA, Provenzano FA, Yeung L-K, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17(12):1798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastroiacovo D, Kwik-Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, Righetti R, Bocale R, Lechiara MC, Marini Cet al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study–A randomized controlled trial. Am J Clin Nutr. 2015;101(3):538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devore EE, Kang JH, Breteler MMB, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72(1):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland TM, Agarwal P, Wang Y, Leurgans SE, Bennett DA, Booth SL, Morris MC. Dietary flavonols and risk of Alzheimer dementia. Neurology. 2020;94:e1749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet North Am Ed. 1991;338(8765):464–8. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 13.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26., discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 16.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 17.Yue Y, Petimar J, Willett WC, Smith-Warner SA, Yuan C, Rosato S, Sampson L, Rosner B, Cassidy A, Rimm EBet al. Dietary flavonoids and flavonoid-rich foods: Validity and reproducibility of FFQ-derived intake estimates. Public Health Nutr. 2020;23(18):3295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett W, Stampfer MJ. Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. [DOI] [PubMed] [Google Scholar]
- 19.Creed JH, Smith-Warner SA, Gerke TA, Egan KM. A prospective study of coffee and tea consumption and the risk of glioma in the UK Biobank. Eur J Cancer. 2020;129:123–31. [DOI] [PubMed] [Google Scholar]
- 20.Burch JD, Craib KJ, Choi BC, Miller AB, Risch HA, Howe GR. An exploratory case-control study of brain tumors in adults. J Natl Cancer Inst. 1987;78:601–9. [PubMed] [Google Scholar]
- 21.Dubrow R, Darefsky AS, Freedman ND, Hollenbeck AR, Sinha R. Coffee, tea, soda, and caffeine intake in relation to risk of adult glioma in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2012;23(5):757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le CT, Leenders WPJ, Molenaar RJ, van Noorden CJF. Effects of the green tea polyphenol epigallocatechin-3-gallate on glioma: A critical evaluation of the literature. Nutr Cancer. 2018;70(3):317–33. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Wang G, Mao Q, Li S, Xiong W, Lin Y, Ge J. Glutamate dehydrogenase (GDH) regulates bioenergetics and redox homeostasis in human glioma. Oncotarget. 2016;5:1–12. [Google Scholar]
- 24.Vidak M, Rozman D, Komel R. Effects of flavonoids from food and dietary supplements on glial and glioblastoma multiforme cells. Molecules. 2015;20(10):19406–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shervington A, Pawar V, Menon S, Thakkar D, Patel R. The sensitization of glioma cells to cisplatin and tamoxifen by the use of catechin. Mol Biol Rep. 2009;36(5):1181–6. [DOI] [PubMed] [Google Scholar]
- 26.Chen TC, Wang W, Golden EB, Thomas S, Sivakumar W, Hofman FM, Louie SG, Schönthal AH. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011;302(2):100–8. [DOI] [PubMed] [Google Scholar]
- 27.Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131(9):2248–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.