ABSTRACT
Background
The human thrifty phenotype is characterized by a greater decrease in 24-h energy expenditure (24EE) during fasting due to relatively higher eucaloric 24EE in sedentary conditions, both of which are indicative of greater propensity to weight gain. Thriftiness is also associated with a smaller increase in 24EE (i.e., reduced adaptive thermogenesis) during overfeeding.
Objectives
We investigated whether short-term measures of adaptive thermogenesis during overfeeding with low/normal/high protein content characterize thriftiness.
Methods
In this secondary cross-sectional analysis of a single-arm crossover study, 24EE was measured using whole-room indirect calorimetry during energy balance, fasting, and different overfeeding conditions (low/3% protein, high/30% protein, and 3 normal/20% protein diets) with 200% of eucaloric requirements in 77 healthy individuals [63 men; BMI (in kg/m2): 26.4 ± 4.3; body fat by DXA: 27.7% ± 9.4%, mean ± SD] with normal glucose regulation. Relations between the 24EE during energy balance (adjusted for body composition) and 24EE during each overfeeding diet were analyzed using separate linear regression models. Participants were arbitrarily categorized as thrifty/spendthrift based on the median value (−177 kcal/d) of the difference in 24EE between fasting and energy balance conditions.
Results
Differences in 24EE during low/high-protein overfeeding diets (regression line slope = 0.76 and 0.68, respectively, both P < 0.05 compared with slope = 1) but not during the normal-protein overfeeding diets (all P > 0.05 compared with slope = 1) were dependent on baseline 24EE during energy balance. Specifically, individuals with higher eucaloric 24EE (thriftier phenotype) showed smaller increases in 24EE during protein-imbalanced overfeeding. Analyzed by group, thrifty individuals had smaller increases in 24EE by 42 and 237 kcal/d during low- and high-protein overfeeding, respectively, compared with spendthrift individuals who showed greater increases in 24EE by 100 and 302 kcal/d (P ≤ 0.03 compared with thrifty group).
Conclusions
During acute overfeeding conditions with low/high-protein content, thrifty participants have limited capacity to increase 24EE, indicating that impaired adaptive thermogenesis during protein-imbalanced diets further characterizes the thrifty phenotype and its susceptibility to weight gain. This trial was registered at clinicalTrials.gov as NCT00523627.
Keywords: energy expenditure, energy metabolism, fasting, overfeeding, dietary protein, metabolic rate
Introduction
The human thrifty genotype/phenotype concept was originally proposed to explain the increasing prevalence of type 2 diabetes by thrifty genes promoting hyperinsulinemia (1) and by acquired thriftiness through fetal malnutrition (2). In recent years, this concept has evolved to encompass the idea of energy conservation during famine and overnutrition that may have developed to promote survival when food was less abundant (3, 4). In studies on our unit, we have demonstrated that thriftiness can be quantified by measuring acute differences in 24-h energy expenditure (24EE) during 24-h fasting. In line with the concept of energy conservation, thrifty individuals show a relatively greater decrease in 24EE during acute fasting conditions (3, 4). In multiple, independent, longitudinal studies, our research group demonstrated that these thrifty individuals are more susceptible to gain weight both during sustained daily low-protein overfeeding in a highly controlled inpatient setting (5) and in free-living conditions (6), while being more resistant to diet-induced weight loss during sustained daily caloric restriction (7). Thriftiness, defined by a relatively lower sleeping metabolic rate during eucaloric conditions, was also associated with greater retention of fat mass after 6 mo in participants who underwent 8 wk of sustained overfeeding (8).
Thriftiness is also observed during conditions of positive energy balance such as short-term overfeeding (6, 9). In accordance with the theory of luxuskonsumption (10), later adapted to the concept of “adaptive thermogenesis” (11–13), participants with a blunted increase in 24EE during overfeeding (i.e., energy efficient and thus thrifty) might be more susceptible to gain weight in our modern food-abundant environment (14). Stock (15) and Dulloo and Jacquet (16) proposed that the individual differences in adaptive thermogenesis (and the related energy cost of weight gain) during sustained overfeeding are only modest during normal-protein overfeeding diets (dietary protein content: 15–20%), whereas they are “magnified” with protein-imbalanced diets such as low-protein (<5%) or high-protein (>20%) overfeeding.
We hypothesized that 24EE measured during overfeeding diets with low-protein (3%) or high-protein (30%) content would more clearly reveal the impairment in adaptive thermogenesis of thrifty participants compared with normal-protein (20%) diets. We tested our hypothesis in a cohort of 77 individuals with normal glucose regulation who underwent 24EE measurements during carefully controlled conditions of 24-h energy balance, fasting, and 5 different overfeeding conditions (200% eucaloric requirements) with different macronutrient compositions.
Methods
Participants
This unregistered secondary analysis, aimed to characterize the relations among acute metabolic responses to different overfeeding diets (primary outcomes), was performed using baseline data from an ongoing prospective cohort study (registered at clinicaltrials.gov as NCT00523627) designed to evaluate the longitudinal association between measures of energy metabolism during 24-h fasting and different overfeeding diets compared with long-term weight change (6, 17). The inclusion criteria for the current analysis were: 1) valid measurements of 24EE both during 24-h energy balance (with <20% absolute deviation from perfect energy balance) and 24-h fasting conditions and 2) valid measurement of 24EE during at least 1 overfeeding session. Valid data from n = 77 participants were included (Supplemental Figure 1). All individuals were 18 y or older and living nearby Phoenix. Participants were weight stable (<10% weight variation) for at least 6 mo prior to admission and were confirmed to be healthy by history, physical examination, and fasting blood tests. Upon admission, self-identified race was recorded using the following categories: American Indian/Alaska Native, Asian, Native Hawaiian or other Pacific Islander, Black or African American, White, more than 1 race, and unknown. Hispanic or non-Hispanic ethnicity was also recorded. The baseline data for our study were collected from 2008 to 2017. On admission to the clinical research unit, participants were placed on a standard, normal-protein, weight-maintaining diet (WMD; 50% carbohydrate, 30% fat, and 20% protein), using unit-specific equations based on weight and sex (18). Daily fasting body weight measured by calibrated scale was maintained within 1% of the admission weight by adjusting, if necessary, the energy intake of WMD by ±200 kcal (19). Body composition was assessed by DXA (DPX-1; Lunar Corp) on the second day after admission. Fat mass (FM) and fat-free mass (FFM) were calculated from weight and percentage body fat from DXA scan. All participants had normal glucose regulation based on an oral glucose tolerance test performed after 3 d on the WMD (20). All participants provided written informed consent prior to beginning the study. The institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases approved the study.
Measurement of energy metabolism
Twenty-four-hour energy expenditure was measured in a large, open-circuit indirect whole-room calorimeter, as previously described (7, 21). Briefly, volunteers entered the calorimeter at 08:00 after having fasted overnight and eating breakfast at 07:00. They remained therein for 23.25 h, during which 3 meals were provided at 11:00, 16:00, and 19:00 through an airlock. The 24-h respiratory quotient and 24EE were calculated from previously derived equations (22) by extrapolating to 24 h the average CO2 production and O2 consumption per minute during the 23.25 h in the calorimeter. While in the calorimeter, participants were asked to remain sedentary. Spontaneous physical activity (SPA) inside the calorimeter was measured by radar sensors and expressed as the percentage of time when activity was detected (23). The components of 24EE were calculated as previously described (24).
Dietary interventions
The experimental protocol for the acute dietary interventions given for 24 h during the baseline visit of the longitudinal study NCT00523627 is shown in Supplemental Figure 2 and has been previously described in detail (6). To closely achieve energy balance during the 24EE assessment in the whole-room calorimeter, 24EE was measured twice during eucaloric conditions and the same macronutrient composition of the WMD (20% protein). The first eucaloric energy expenditure (EE) assessment was obtained while participants resided for 24 h in the calorimeter with total energy intake calculated using a unit-specific formula to achieve 24-h energy balance in the confined environment of the calorimeter (21). Subsequently, after 2 d, participants had another eucaloric EE assessment inside the calorimeter when the total energy intake was equal to the 24EE value calculated during the first eucaloric EE assessment.
The 24EE measured in this second eucaloric session was considered the baseline measurement obtained in conditions close to perfect energy balance during balanced eucaloric feeding (50% carbohydrate, 30% fat, and 20% protein). The subsequent dietary interventions, 24-h fasting and the 5 overfeeding diets, given for 24 h while in the calorimeter were done in a random order and with a 3-d period on the WMD between each assessment to avoid carryover effects. Individuals were always studied in the same whole-room calorimeter. During 24-h fasting, only noncaloric noncaffeinated beverages (e.g., water) were permitted. The prescribed 24-h energy intake for the overfeeding sessions (i.e., 200% of energy requirements) was calculated as twice the 24EE value obtained during the second eucaloric (baseline) assessment. Three overfeeding diets contained 20% protein but varied in carbohydrate and fat contents (Supplemental Figure 3) as follows: 1) balanced overfeeding (BOF) with 50% carbohydrate and 30% fat as in the WMD; 2) high-fat, normal-protein overfeeding (FNP) with 60% fat and 20% carbohydrate; and 3) high-carbohydrate, normal-protein overfeeding (CNP) with 75% carbohydrate and 5% fat. In addition, 2 overfeeding diets varied in protein content as follows: 1) low-protein, high-fat overfeeding (LPF) with 3% protein, 46% fat, and 51% carbohydrate and 2) high-protein, high-fat overfeeding (HPF) with 30% protein, 44% fat, and 26% carbohydrate. After each session inside the calorimeter, the metabolic kitchen weighed food leftovers and calculated the actual energy intake consumed during the 24 h in the calorimeter (Table 1). If <95% of the total kcal given was consumed by the volunteer during the 24-h session, the EE data from that overfeeding session were excluded from the analysis. In addition, some EE data were not available due to technical or logistical issues. A detailed overview of reasons for missing EE data is shown in Supplemental Table 1. The HPF diet was added to the study protocol after the first 20 individuals completed their baseline admission; therefore, the sample size of this diet was smaller compared with the other overfeeding diets.
TABLE 1.
Measures of energy metabolism and intake during each overfeeding condition1
| Characteristic | Low-protein overfeeding (n = 66) | High-protein overfeeding (n = 53) | High-fat overfeeding (n = 65) | High-carbohydrate overfeeding (n = 66) | Balanced overfeeding (n = 67) |
|---|---|---|---|---|---|
| RQ, ratio | 0.91 ± 0.05 (0.8, 1.01) | 0.85 ± 0.03 (0.78, 0.95) | 0.83 ± 0.04 (0.75, 0.91) | 0.94 ± 0.04 (0.84, 1.03) | 0.89 ± 0.03 (0.82, 0.97) |
| 24EE, kcal/d | 2090 ± 333 (1432, 2895) | 2274 ± 332 (1527, 2959) | 2156 ± 324 (1532, 3114) | 2278 ± 346 (1573, 3214) | 2241 ± 368 (1481, 3251) |
| SLEEP, kcal/d | 1643 ± 283 (1071, 2636) | 1920 ± 296 (1181, 2747) | 1778 ± 287 (1208, 2530) | 1774 ± 292 (1174, 2441) | 1485 ± 250 (1007, 2301) |
| EE0, kcal/15 h | 1380 ± 211 (967, 1992) | 1465 ± 225 (928, 1958) | 1416 ± 211 (1021, 2110) | 1517 ± 231 (1058, 2238) | 1803 ± 312 (1173, 2739) |
| AFT, kcal/15 h | 348 ± 114 (42, 600) | 274 ± 133 (0, 680) | 299 ± 108 (90, 541) | 409 ± 118 (170, 750) | 363 ± 127 (147, 765) |
| SPA, % of time | 5.9 ± 3.7 (1.1, 20.3) | 6.6 ± 3.6 (0.9, 15.6) | 6.1 ± 3.3 (0.8, 16.7) | 6.8 ± 3.7 (0.9, 17) | 6.1 ± 3.2 (0.9, 14.5) |
| Actual intake,2 kcal/d | 4041 ± 630 (2854, 5625) | 3982 ± 569 (2854, 5464) | 3991 ± 579 (2914, 5377) | 3995 ± 578 (2858, 5620) | 4037 ± 620 (2854, 5615) |
| Fold increase in intake from energy balance3 | 1.99 ± 0.02 (1.89, 2.04) | 2.00 ± 0.01 (1.94, 2.01) | 1.99 ± 0.03 (1.9, 2.03) | 1.99 ± 0.03 (1.9, 2) | 1.96 ± 0.24 (0, 2.04) |
| Δ adjusted 24EE from energy balance during overfeeding,4 kcal/d | 72 ± 80 (–127, 270) | 272 ± 107 (41, 496) | 145 ± 104 (–183, 453) | 256 ± 93 (53, 498) | 230 ± 92 (–14, 529) |
Values are presented as mean ± SD (minimum, maximum).
Actual intake calculated as given minus leftover kcal measured by the metabolic kitchen.
Fold increase in intake from energy balance calculated as the actual 24-h intake during overfeeding divided by the actual 24-h intake during energy balance.
Residual 24EE values during 24-h fasting, energy balance, and overfeeding conditions were calculated separately by linear regression models, including age, sex, ethnicity, fat mass, fat-free mass, spontaneous physical activity, ambient temperature, and calorimeter room (room 1 or 2) as covariates. Adjusted 24EE values were then calculated as the residuals from the regression models plus the mean of unadjusted values of the whole cohort. The difference in adjusted 24EE during overfeeding was calculated as the adjusted 24EE during each overfeeding diet minus the adjusted 24EE during energy balance. 24EE, 24-h energy expenditure; AFT, awake-fed thermogenesis; EE0, EE in the inactive, awake state; RQ, respiratory quotient; SLEEP, sleeping metabolic rate; SPA, spontaneous physical activity.
Statistical analysis
Statistical analysis was performed using the SAS statistical software package (SAS Enterprise Guide Version 7.15; SAS Institute). Unless otherwise specified, data are expressed as mean ± SD or mean with 95% confidence interval (CI). The Pearson correlation coefficient was used to quantify associations between continuous variables with Gaussian distribution assessed by the Shapiro–Wilk test. Statistical significance was set for P < 0.05. The Bonferroni correction for multiple tests was applied to the statistical significance of main regression analyses.
Multivariable linear regression analyses were performed separately during 24-h energy balance, fasting, and overfeeding conditions to obtain residual values of 24EE in each dietary condition after accounting for known determinants, including demographic, environmental, and body composition measures (22, 25, 26). Specifically, we included age, sex, ethnicity, FM, FFM, SPA (only 24EE analyses), ambient temperature, and calorimeter room (room 1 or 2) as covariates in the regression models. Adjusted 24EE values were then calculated as the residuals from the abovementioned regression models via the SAS LSMEANS procedure plus the mean of unadjusted 24EE values calculated in the whole cohort. This method to calculate adjusted 24EE values has been previously established by Ravussin et al. (27) to normalize the absolute rate of EE by body composition and other known physiologic determinants. Unless otherwise indicated, throughout the text, all 24EE results refer to adjusted values. The differences in 24EE from energy balance during 24-h fasting and overfeeding conditions were calculated as the adjusted 24EE measure during fasting and overfeeding conditions minus the adjusted 24EE during energy balance, respectively.
Linear regression analyses were performed to evaluate the relations between adjusted 24EE during eucaloric conditions (independent variable) compared with adjusted 24EE during each overfeeding condition (dependent variable). Specifically, the slope of the linear regression equation was computed for each overfeeding diet and tested compared with a slope = 1 using the Student t test. Linear regression analyses were followed up by linear mixed-model analyses that provide unbiased results for slopes in the presence of missing at random values across different diets while taking into account repeated dietary measurements within the same individual. Two different mixed-model analyses were performed: 1) in the whole cohort (Supplemental Table 2) and 2) excluding HPF data (Supplemental Table 3) as this diet was added later to the study protocol, and missing values were due to study design.
For graphical purposes and ease of interpretation, the aforementioned regression analyses using continuous 24EE data were followed up with confirmatory analyses using metabolic groups identified by binary categorization of continuous 24EE distribution. Specifically, in these confirmatory analyses, thrifty/spendthrift participants were arbitrarily defined as those with a higher/lower-than-median decrease in adjusted 24EE during 24-h fasting from the energy balance condition, respectively, as done previously to conceptualize these metabolic phenotypes into 2 distinct groups with the same sample size (28–31). Similarly, additional analyses of the top 5 thriftiest and top 5 most spendthrift participants (e.g., those with the greatest and the smallest decrease in adjusted 24EE during 24-h fasting, respectively) were also carried out to visualize results from the extremes of data distribution. The Student paired t test was used to evaluate the individual difference in 24EE measures during each overfeeding diet from energy balance conditions, whereas the unpaired t test was used to compare 24EE measures between the aforementioned metabolic groups (thrifty/spendthrift).
Results
Characteristics of the study cohort are presented in Table 2. The mean ± SD deviation from exact energy balance (intake = 24EE) during the baseline 24EE assessment was 35 ± 86 kcal/d, equivalent to 1.9% ± 4.3% of measured 24EE. On average, thrifty participants—characterized by a greater decrease in 24EE during fasting—had a relatively higher 24EE than spendthrift participants during energy balance (+110 kcal/d; 95% CI: 59, 162; P < 0.0001) but similar 24EE during 24-h fasting conditions (P = 0.14) (Supplemental Figure 4A). This is also confirmed in a correlation analysis of quantitative/continuous data, where participants with higher adjusted 24EE during energy balance decreased 24EE more during 24-h fasting (i.e., thriftier participants) compared with participants with lower adjusted 24EE during energy balance (Supplemental Figure 5), as shown previously (29).
TABLE 2.
Clinical characteristics of the study cohort1
| Characteristic | Total (n = 77) | Spendthrift (n = 38) | Thrifty (n = 39) | P value |
|---|---|---|---|---|
| Male, n (%) | 63 (81.8) | 31 (81.6) | 32 (82.1) | 0.96 |
| Ethnicity, n (%) | 0.43 | |||
| African American | 18 (23.4) | 11 (29.0) | 7 (18.0) | |
| Caucasian | 26 (33.8) | 10 (26.3) | 16 (41.0) | |
| Native American | 23 (29.9) | 11 (29.0) | 12 (30.8) | |
| Hispanic | 10 (13.0) | 6 (15.8) | 4 (10.3) | |
| Age, y | 37.2 ± 10.2 (18, 55) | 36.7 ± 10.2 (18, 55) | 37.7 ± 10.3 (19, 53) | 0.66 |
| Body composition measures | ||||
| Height, cm | 173 ± 8 (157, 196) | 173 ± 8 (157, 196) | 172 ± 7 (157, 185) | 0.54 |
| Body weight, kg | 78.9 ± 14.1 (47.5, 127.1) | 79 ± 14 (56, 127.1) | 78.9 ± 14.3 (47.5, 107.8) | 0.97 |
| BMI, kg/m2 | 26.4 ± 4.3 (17.8, 44) | 26.3 ± 4.2 (20.6, 44) | 26.5 ± 4.5 (17.8, 39.1) | 0.77 |
| Body fat, % | 27.7 ± 9.4 (6.9, 53.8) | 27.7 ± 11.2 (7.3, 53.8) | 27.6 ± 7.5 (6.9, 52.8) | 0.96 |
| FM, kg | 22.4 ± 10.2 (4.9, 56.9) | 22.5 ± 11.4 (4.9, 54.1) | 22.2 ± 9.1 (5.9, 56.9) | 0.88 |
| FFM, kg | 56.6 ± 9.7 (33.9, 79.4) | 56.4 ± 9.7 (34.2, 77) | 56.7 ± 9.9 (33.9, 79.4) | 0.91 |
| Energy expenditure measures during energy balance | ||||
| RQ, ratio | 0.86 ± 0.04 (0.76, 1.02) | 0.87 ± 0.03 (0.8, 0.94) | 0.86 ± 0.04 (0.76, 1.02) | 0.47 |
| 24EE, kcal/d | 2015 ± 306 (1427, 2810) | 1960 ± 308 (1502, 2810) | 2070 ± 299 (1427, 2732) | 0.11 |
| Adjusted 24EE,2 kcal/d | 2015 ± 125 (1676, 2232) | 1960 ± 123 (1676, 2212) | 2070 ± 103 (1837, 2232) | <0.0001 |
| SLEEP, kcal/d | 1594 ± 246 (1131, 2303) | 1539 ± 220 (1193, 2227) | 1649 ± 261 (1131, 2303) | 0.049 |
| Adjusted SLEEP,2 kcal/d | 1594 ± 113 (1355, 1892) | 1548 ± 93 (1355, 1730) | 1640 ± 113 (1446, 1892) | 0.0002 |
| EE0, kcal/15 h | 1339 ± 203 (969, 1930) | 1310 ± 213 (1025, 1930) | 1366 ± 191 (969, 1733) | 0.22 |
| Adjusted EE0,2 kcal/15 h | 1339 ± 88 (1123, 1520) | 1309 ± 92 (1123, 1520) | 1367 ± 74 (1197, 1509) | 0.003 |
| AFT, kcal/15 h | 343 ± 103 (145, 624) | 349 ± 110 (171, 624) | 337 ± 97 (145, 557) | 0.62 |
| Adjusted AFT,2 kcal/15 h | 343 ± 83 (181, 553) | 343 ± 81 (208, 553) | 342 ± 86 (181, 503) | 0.96 |
| SPA, % of time | 5.9 ± 3.4 (0.7, 16.4) | 6.3 ± 3.9 (1.2, 16.4) | 5.6 ± 2.7 (0.7, 15.5) | 0.41 |
| 24-h energy intake, kcal/d | 2051 ± 304 (1512, 2921) | 2008 ± 313 (1529, 2921) | 2092 ± 293 (1512, 2709) | 0.23 |
| Energy balance, % | 1.9 ± 4.3 (−7.3, 19.7) | 2.6 ± 4.1 (−3.8, 19.7) | 1.2 ± 4.5 (−7.3, 16.4) | 0.17 |
| Energy expenditure measures during 24-h fasting | ||||
| RQ, ratio | 0.79 ± 0.03 (0.71, 0.9) | 0.79 ± 0.03 (0.73, 0.88) | 0.79 ± 0.03 (0.71, 0.9) | 0.88 |
| 24EE, kcal/d | 1851 ± 261 (1287, 2655) | 1858 ± 275 (1429, 2655) | 1845 ± 249 (1287, 2390) | 0.82 |
| Adjusted 24EE,2 kcal/d | 1851 ± 105 (1619, 2103) | 1869 ± 107 (1639, 2103) | 1834 ± 100 (1619, 2051) | 0.14 |
| Δ adjusted 24EE fromenergy balance tofasting,2 kcal/d | −164 ± 89 (−376, 104) | −91 ± 59 (−177, 104) | −236 ± 44 (−376, −178) | <0.0001 |
| SPA, % of time | 5.5 ± 3.2 (0.4, 18.3) | 5.4 ± 3.5 (1.1, 18.3) | 5.5 ± 3 (0.4, 12.7) | 0.88 |
Values are presented as mean ± SD (minimum, maximum) unless otherwise indicated. Classification of participants into the spendthrift or thrifty metabolic group was based on the median value of decrease in adjusted 24EE from energy balance during 24-h fasting (−177 kcal/d), such that thrifty participants were those with a greater-than-median decrease in 24EE. Differences between thrifty and spendthrift metabolic groups were assessed by Student unpaired t test for continuous variables and by χ2 test for categorical variables.
Adjusted 24-h energy expenditure was calculated by adding the average value calculated in the whole cohort to the residual values obtained from linear regression analysis with covariates age, sex, ethnicity, FM, FFM, SPA, ambient temperature, and calorimeter room. AFT, awake-fed thermogenesis; EE0, EE in the inactive, awake state; FM, fat mass; FFM, fat-free mass; RQ, respiratory quotient; SLEEP, sleeping metabolic rate; SPA, spontaneous physical activity; 24EE, 24-h energy expenditure.
Energy expenditure measures and the actual energy intake during each overfeeding condition are presented in Table 2. The actual 24-h energy intake in each overfeeding condition was almost exactly 2-fold higher than the energy intake during eucaloric conditions as per study design (Supplemental Figure 3D), such that all participants were approximately in the same state of energy surplus during all overfeeding sessions. The mean weight measured before each overfeeding session was within 1% of baseline weight. Spontaneous physical activity was similar across all overfeeding diets (P = 0.36). The changes in 24EE during 24-h overfeeding were highly variable across diets and individuals (range for LPF: –127 to +270 kcal/d; HPF: +41 to +496 kcal/d; FNP: –183 to +453 kcal/d; CNP: 53 to 498 kcal/d; BOF: –14 to +529 kcal/d), although the caloric content was similar among overfeeding conditions (Table 2).
Associations between differences in 24EE during fasting and overfeeding
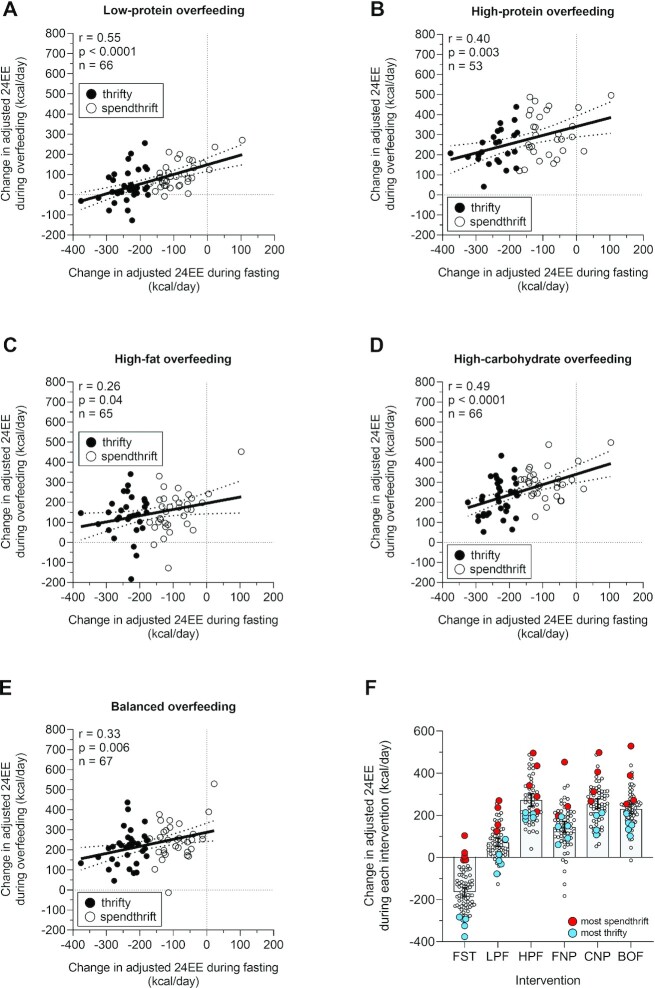
The difference in 24EE during 24-h fasting (which defines the thrifty compared with spendthrift phenotypes) correlated with the difference in 24EE during each overfeeding diet; that is, a greater decrease in 24EE during fasting (“thriftier phenotype”) was associated with smaller increase in 24EE during all overfeeding diets (all r ≥ 0.26, all P < 0.04, Figure 1A–E). Similar results were obtained when considering the differences in unadjusted 24EE expressed either as absolute (in kcal/d, data not shown) or as percentage of the 24EE during energy balance conditions (Supplemental Figure 6A–E).
FIGURE 1.
A greater decrease in 24EE during 24-h fasting (thriftier phenotype) correlates with less increases in 24EE during acute 24-h overfeeding with varying macronutrient content. Relations between the decrease in adjusted 24EE during 24-h fasting (x-axis, a greater decrease indicating greater thriftiness) and the increase in adjusted 24EE during (A) low-protein (n = 66), (B) high-protein (n = 53), (C) high-fat normal-protein (n = 65), (D) high-carbohydrate normal-protein (n = 66), and (E) balanced overfeeding (n = 67) (y-axis). The dotted lines in each plot denote the 95% confidence bands. Black circles denote thrifty individuals; white circles denote spendthrift individuals. (F) The difference in adjusted 24EE of the top 5 thriftiest (blue) and top 5 most spendthrift (red) participants during 24-h fasting and overfeeding conditions. Classification of the 5 thriftiest/5 most spendthrift participants was based on the decrease in adjusted 24EE from energy balance during 24-h fasting, such that the 5 thriftiest participants were those who had the greatest decrease in adjusted 24EE during 24-h fasting, whereas the 5 most spendthrift participants were those who had the smallest decrease (or increase) in adjusted 24EE during fasting. In panels A–E, the Pearson correlation coefficient was used to quantify associations between continuous variables. BOF, balanced overfeeding; CNP, high-carbohydrate overfeeding; FNP, high-fat overfeeding; HPF, high-protein overfeeding; LPF, low-protein overfeeding; 24EE, 24-h energy expenditure.
To further illustrate these results obtained considering the difference in 24EE during fasting as a continuous/quantitative variable, we arbitrarily analyzed data from the extremes of the continuous distribution, namely, the 5 thriftiest compared with the 5 most spendthrift participants based on the largest/smallest decrease in 24EE during 24-h fasting, respectively. Nearly all of the thriftiest participants, who decreased their 24EE by a mean of 313 kcal/d during 24-h fasting, were also those who had a lower-than-median increase in 24EE during each overfeeding diet (Figure 1F). Conversely, the most spendthrift participants, who on average even increased their adjusted 24EE by 21 kcal/d during 24-h fasting, overall had a greater-than-median increase in 24EE during each overfeeding diet (Figure 1F). Similar results were observed when the 5 thriftiest compared with the 5 most spendthrift participants were defined based on the percentage decrease in 24EE during 24-h fasting (Supplemental Figure 5F).
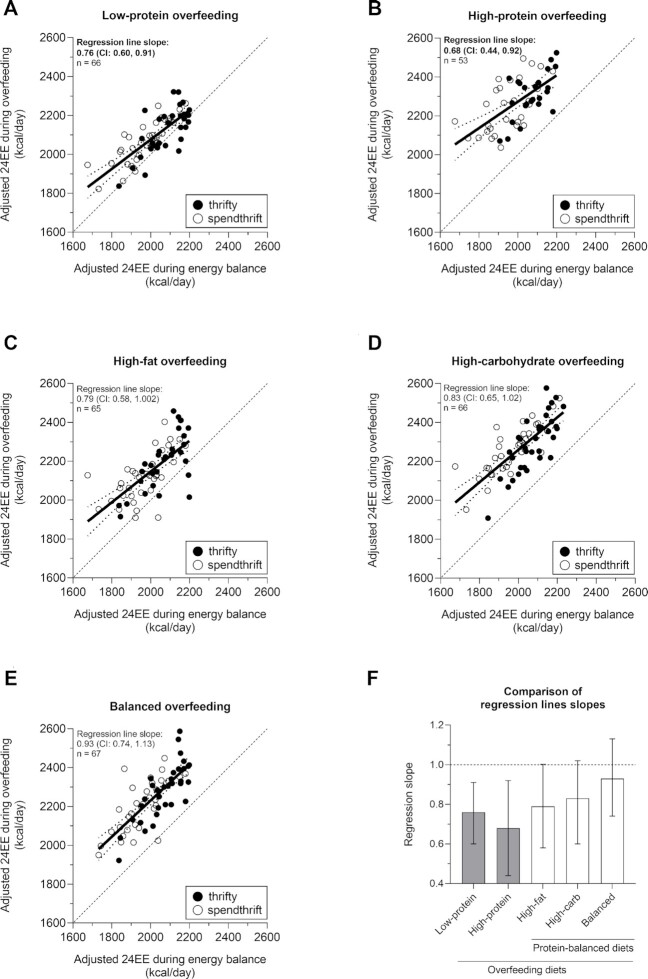
Relations between 24EE during energy balance and 24EE during acute overfeeding conditions
To determine whether the 24EE during each overfeeding condition was dependent on the baseline (i.e., energy balance) 24EE level, we plotted the 24EE during energy balance and 24EE during each overfeeding intervention (Figure 2A–E), and we performed linear regression analyses to evaluate whether the slope of the regression line was statistically different from the slope of the identity line (slope = 1). During LPF, the slope of the regression line was 0.76 (95% CI: 0.60, 0.91; Figure 2A), where the upper 95% limit of slope remains below the identity line (slope = 1), thus indicating a proportional effect. A similar effect was observed during HPF (slope = 0.68; 95% CI: 0.44, 0.92; Figure 2B) but not during the 3 normal-protein overfeeding diets (Figure 2C–E). The slope values during all the overfeeding diets are shown in Figure 2F. Results for LPF and HPF were still significant after Bonferroni correction (adjusted P = 0.01 and P = 0.04, respectively). Similar results were obtained using the linear mixed-model analysis (Supplemental Table 2). As randomness of missing values in the high-protein overfeeding diet could not be assumed due to study design, the mixed-model analysis was also performed excluding data from this diet, and similar results were obtained (Supplemental Table 3).
FIGURE 2.
Relatively higher 24EE during energy balance is associated with smaller increases in 24EE during acute, protein-imbalanced overfeeding but not during normal-protein overfeeding. Relations between adjusted 24EE during energy balance (x-axis) and during (A) low-protein (n = 66), (B) high-protein (n = 53), (C) high-fat normal-protein (n = 65), (D) high-carbohydrate normal-protein (n = 66), and (E) balanced overfeeding (n = 67) (y-axis). The dotted lines in each plot denote the 95% confidence bands. Black circles denote thrifty individuals; white circles denote spendthrift individuals. Linear regression analyses were performed to evaluate the associations between adjusted 24EE during eucaloric conditions compared with adjusted 24EE measures during overfeeding conditions. (F) Estimates of regression line slope values and their 95% CIs of panels A–E. The dotted horizontal line denotes a slope of 1 (i.e., the increase in 24EE during overfeeding is the same over the entire range of 24EE values during energy balance conditions). The protein-imbalanced overfeeding diets LPF and HPF had a slope significantly lower than 1 (both P < 0.05) by 1-sample Student t test (i.e., the increase in 24EE during overfeeding is smaller when 24EE during eucaloric conditions is higher). Results for LPF and HPF were still significant after adjustment for multiple comparisons using the Bonferroni method. BOF, balanced overfeeding; CNP, high-carbohydrate overfeeding; FNP, high-fat overfeeding; HPF, high-protein overfeeding; LPF, low-protein overfeeding; 24EE, 24-h energy expenditure.
Altogether, the results indicate that, only during the 2 protein-imbalanced overfeeding diets (low/high-protein content), individuals with relatively higher 24EE during energy balance (i.e., thriftier participants) showed smaller overfeeding-induced increases in 24EE compared with participants with relatively lower 24EE during energy balance (i.e., more spendthrift participants).
For illustrative purposes and to follow up the regression analyses using continuous 24EE data, we also performed a groupwise comparison of thrifty compared with spendthrift participants as arbitrarily defined by the median decrease (−177 kcal/d) in 24EE from energy balance during 24-h fasting (Table 1). During LPF (Figure 3A), on average, thrifty participants increased 24EE by 42 kcal/d (95% CI: 12, 71; P = 0.008) or 1.0% of their actual energy intake, whereas spendthrift participants increased by 100 kcal/d (95% CI: 77, 124; P < 0.0001) or 2.6% of their energy intake, which is more than double that of thrifty participants (100 minus 42 kcal/d = 58 kcal/d; 95% CI: 22, 96; P = 0.002). Because of their greater increase in 24EE during LPF, on average, spendthrift participants achieved the same level of 24EE of thrifty participants during LPF (P = 0.06) despite having lower 24EE during energy balance conditions (Figure 3A).
FIGURE 3.
Blunted increase in adjusted 24EE during overfeeding with diets with low/high-protein content in thrifty compared with spendthrift participants. Comparison of the difference in adjusted 24EE from energy balance during (A) low-protein and (B) high-protein overfeeding between thrifty (n = 39) and spendthrift (n = 38) participants. The height of black bars denotes the average 24EE during energy balance, whereas the height of gray bars denotes the average 24EE during overfeeding. The distance between the black and the gray bars denotes the average overfeeding-induced difference in 24EE. Error bars denote the 95% CI. Statistical significance between metabolic groups was determined by Student unpaired t tests. Three P values are shown in each panel: the upper one compares adjusted 24EE during overfeeding, the bottom one compares 24EE during energy balance, and the middle one compares the increase in 24EE from energy balance to overfeeding. Classification of participants as either thrifty or spendthrift is based on the median value of the adjusted decrease in 24EE from energy balance during 24-h fasting, such that thrifty participants were identified as those with a greater-than-median decrease in adjusted 24EE (<−177 kcal/d). 24EE, 24-h energy expenditure.
Similar results were observed during HPF (Figure 3B), when on average, thrifty participants increased their 24EE by only 237 kcal/d (95% CI: 199, 276; P < 0.0001) or 5.7% of their energy intake, whereas spendthrift participants increased by 302 kcal/d (95% CI: 259, 344; P < 0.0001) or 7.8% of their energy intake. Again, spendthrift participants increased their 24EE during HPF by an average of 302 minus 237 = 65 kcal/d more compared with that of thrifty participants (95% CI: 8, 122; P = 0.03), such that 24EE during HPF was similar between groups (P = 0.10) (Figure 3B).
Groupwise results for the other overfeeding diets are shown in Supplemental Figure 4B–D.
Discussion
In a cohort of 77 healthy participants with normal glucose regulation, there was a high interindividual variability in the 24EE responses to acute 24-h overfeeding despite strictly controlled conditions of energy surplus and restricted SPA in the confined environment of a whole-room calorimeter. After accounting for known 24EE determinants, we found that a relatively greater decrease in 24EE during acute fasting conditions was associated with higher 24EE during eucaloric conditions (both indicative of a thrifty phenotype), the latter being associated with smaller increases in 24EE only during overfeeding diets (200% energy requirements) with low- and high-protein content, indicating that thrifty individuals have a reduced capacity of energy dissipation in acute states of positive energy balance during protein-imbalanced overfeeding diets.
Metabolic thriftiness—acquired through fetal malnutrition?
The human “thrifty phenotype hypothesis” evolved from the original theory by Neel (1) ascribing today's high prevalence of type 2 diabetes to “thrifty genes” promoting hyperinsulinemia to a concept of “acquired thriftiness” through poor fetal and early postnatal nutrition leading to lower birth weight as proposed by Hales and Barker (2). One aspect of human thriftiness is that of energy conservation during famine and overnutrition to preserve body mass and promote survival (4). The existence of the energy-conserving thrifty phenotype has been demonstrated by measuring the 24EE response to 24-h fasting in the setting of a whole-room calorimeter where thrifty individuals show a greater decrease in 24EE during fasting as a result of their relatively higher 24EE during eucaloric conditions (9, 29). Being thrifty is indicative of greater susceptibility to weight gain during controlled, long-term daily overfeeding (5) and in free-living conditions (6), greater resistance to diet-induced weight loss (7), and greater weight regain after weight loss (32). The greater fasting-induced decrease in 24EE of thrifty individuals is partly due to a greater decrease in sleeping EE (29), a proxy for resting metabolic rate (RMR) (33). Interestingly, individuals with reduced RMR during short-term fasting also have a lower birth weight (34), who in turn have reduced expression of genes involved in energy metabolism during energy balance conditions (35) indicating that poor fetal nutrition and thriftiness defined by the 24EE response to fasting are linked and characterize the same underlying phenotype.
Protein-imbalanced overfeeding diets best uncover metabolic thriftiness
In previous studies, we reported that thrifty individuals have greater abdominal adiposity and lower core body temperature consistent with a more efficient metabolism (28). We also reported that thrifty individuals have less capacity for cold-induced thermogenesis (30) and less cold-induced brown adipose tissue activation (31).
Furthermore, thriftiness has been associated with less increase in 24EE during short-term overfeeding (6, 9). In the present study, these results were confirmed in a larger cohort and during 5 different, macronutrient-imbalanced overfeeding paradigms, indicating that the greater metabolic efficiency during 24-h fasting is also indicative of reduced metabolic waste during 24-h overfeeding independently of the macronutrient composition of the hypercaloric diet (Figure 1). However, it was unclear which overfeeding diet would best elucidate thriftiness.
Here we report that thrifty individuals can be more reliably identified by protein-imbalanced overfeeding diets (30 and 300 g of proteins for low- and high-protein content, respectively; Supplemental Figure 2) compared with normal-protein diets. Specifically, we found that individuals with relatively higher 24EE during energy balance conditions [indicative of a thriftier phenotype (29)] showed smaller increases in 24EE during low/high-protein overfeeding compared with participants with relatively lower 24EE during energy balance conditions [indicative of a more spendthrift phenotype (29)] (Figure 2). We did not find this effect with any protein-balanced diet.
In a groupwise comparison of thrifty compared with spendthrift participants as arbitrarily defined by the median decrease in 24EE from energy balance during 24-h fasting, we confirmed that on average, thrifty individuals increased 24EE during low- and high-protein overfeeding by 62% and 27% less than spendthrift individuals, respectively (Figure 3). We also confirmed the proportional effects obtained by linear regression analyses: despite having a greater 24EE during energy balance conditions, thrifty individuals arrived at the same 24EE level than spendthrift individuals during protein-imbalanced overfeeding (Figure 3).
Altogether, our results indicate that, when challenged with protein-imbalanced overfeeding diets, thrifty participants show a blunted adaptive thermogenesis, that is, an impaired ability to further increase 24EE to dissipate extra ingested calories (Figure 4). Our findings suggest that protein-imbalanced diets may be better tools to uncover thrifty phenotypes compared with normal-protein diets.
FIGURE 4.

Updated concept of the human metabolic phenotypes, including the metabolic response to 24-h, protein-imbalanced overfeeding. In this complement concept of the metabolic phenotypes, which includes the metabolic response to 24 h of low-protein (3%) and high-protein (30%) overfeeding with twice energy requirements, the blunted, overfeeding-induced increase in 24EE of thrifty participants is a result of higher EE requirements during energy balance. Conversely, spendthrift participants have relatively lower 24EE during energy balance, and they increase 24EE more both during low- and high-protein overfeeding. The increase in 24EE in spendthrift participants during low-protein overfeeding is more than double compared with thrifty individuals. During both low- and high-protein overfeeding, thrifty and spendthrift individuals end up at the same 24EE level. 24EE, 24-h energy expenditure.
Our data strengthen previous studies searching for optimal overfeeding diets to uncover thriftiness. For instance, in one of his seminal articles reanalyzing human overfeeding studies, Stock (15) reported that protein-imbalanced diets may differentiate feed-efficient from feed-inefficient participants by triggering energy-dissipating mechanisms that are generally less pronounced during normal-protein overfeeding. Dulloo and Jacquet (16) reported that low-protein overfeeding may best uncover the underlying thrifty compared with spendthrift phenotype. This was confirmed by a previous study from our group showing that impaired adaptive thermogenesis during 24-h low-protein overfeeding predicted 6-mo follow-up weight gain and thus revealed thriftiness (36). Interestingly, in that latter study, less increase in 24EE during low-protein overfeeding was also associated with less fibroblast growth factor 21 (FGF21) secretion, which on average increased up to 300% after that diet and independently predicted greater follow-up weight gain. FGF21 has been previously reported as a signal of protein restriction (37). Therefore, a blunted FGF21 secretion in response to low-protein overfeeding may explain why this diet is useful in the identification of thrifty individuals. On the other hand, high-protein overfeeding may identify thrifty individuals as they are more likely to exhaust their thermogenic potential due to the high energetic costs to process the excess protein (38–41) and as 24EE of thrifty individuals during energy balance conditions is already at a higher level compared with that of spendthrift individuals (29).
Interestingly, another study found that thrifty individuals (identified as those with low birth weight) exhibited a greater increase in nighttime EE during 5-d 150% high-fat overfeeding, whereas the overall overfeeding-induced increase in 24EE was not significantly different between thrifty and spendthrift individuals in that study (42). These previous results for 24EE support our present data, in which we also did not find a significant difference in the increase in 24EE during high-fat overfeeding between thrifty and spendthrift individuals defined by the decrease in 24EE during 24-h fasting. However, based on that latter study, measuring nighttime EE during a high-fat overfeeding diet might similarly be useful to identify thrifty individuals.
In the current study, thrifty individuals had on average higher 24EE (adjusted for body composition and other known EE determinants) during energy balance conditions compared with spendthrift individuals. This finding is in line with what we previously demonstrated, namely, the greater fasting-induced decrease in 24EE in thrifty individuals is due to their higher 24EE during energy balance conditions instead of lower 24EE during fasting conditions (29). We think that these results for fasting 24EE do not invalidate the definition of a “thrifty” metabolism. In fact, compared with spendthrift individuals, thrifty individuals show greater decreases in 24EE from energy balance during fasting conditions (mean decreases in 24EE: −236 kcal/d compared with −91 kcal/d, respectively; P < 0.001; Supplemental Figure 4), which is consistent with a “thriftier” energy metabolism characterized by a greater extent of metabolic slowing and a larger decrease in metabolic rate during acute fasting conditions. Furthermore, a relatively higher EE has been associated with greater energy intake and overeating (43–47), altered eating behavior (48), and increased weight gain susceptibility in selected populations (49, 50).
However, our energy balance assessment was obtained in conditions of low-energy turnover due to the restricted physical activity inside the confined environment of the metabolic chamber. As a low level of energy turnover was associated with poor control of eating (51, 52), we speculate that the relatively higher 24EE in thrifty individuals may be associated with higher energy intake in sedentary conditions (3) and thus higher predisposition to weight gain (32, 53).
Limitations
Our study has limitations. Not all of the total n = 77 participants completed every overfeeding diet (i.e., only n = 53 participants completed the high-protein overfeeding diet as it was later introduced in the protocol), which could have influenced the results. However, we confirmed our main results using linear mixed-model analysis (Supplemental Tables 2 and 3) to take into account missing at random values resulting from unplanned technical and logistical issues related to study procedures that were not participant-specific. All individuals in our cohort had a normal glucose regulation, and thus we cannot extrapolate our findings to individuals with impaired glucose tolerance. However, we included only participants with normal glucose tolerance to rule out any effects of insulin resistance on the 24EE response to overfeeding diets ( 54). Last, our EE measurements were obtained during sedentary conditions inside a respiratory chamber, and thus we cannot rule out that physical activity, physical fitness, or higher energy turnover in free-living conditions may have influenced our results (55).
Conclusion
By measuring 24EE during 24-h fasting, our laboratory has established a reliable method to identify thrifty compared with spendthrift phenotypes that characterize the individual susceptibility to future weight gain. The present study extends the characterization of the thrifty phenotype to protein-imbalanced overfeeding conditions (low- and high-protein overfeeding) and demonstrates that thrifty participants have reduced capacity to increase their metabolic rate during these dietary paradigms due to their higher metabolic rate during eucaloric conditions, which limits their adaptive thermogenesis during overfeeding. As we have established longitudinally that the 24EE response to low-protein overfeeding predicts weight change, this metabolic characteristic may in part explain why thrifty individuals are susceptible to the development of obesity, especially when consuming these types of diets. As previously indicated by Stock (15) and Dulloo and Jacquet (16), low- and high-protein overfeeding paradigms may thus be useful tools to identify the human metabolic phenotype indicative of propensity to weight gain as they can best differentiate thrifty from spendthrift individuals and might have clinical relevance in the assessment of individual susceptibility to obesity.
Supplementary Material
Acknowledgments
We thank the dietary, nursing, and technical staff of the National Institutes of Health Clinical Research Unit in Phoenix, AZ, for their assistance in conducting the inpatient studies. Most of all, we thank the volunteers for their participation in the study.
The authors’ contributions were as follows—TH: carried out the initial analyses and wrote the manuscript; TH, AB, TA, JK, and PP: interpreted the results; JK and PP: edited the initial draft of the manuscript; PP: designed the study; TH and PP: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. Author disclosures: The authors report no conflicts of interest.
Notes
Supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). PP was supported by the program “Rita Levi Montalcini for young researchers” from the Italian Minister of Education and Research (Ministero dell'Istruzione, dell'Università e della Ricerca). TH was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project 413490537.
Supplemental Figures 1–6 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BOF, balanced overfeeding; CNP, high-carbohydrate overfeeding; EE, energy expenditure; FFM, fat-free mass; FGF21, fibroblast growth factor 21; FM, fat mass; FNP, high-fat overfeeding; HPF, high-protein overfeeding; LPF, low-protein overfeeding; RMR, resting metabolic rate; SPA, spontaneous physical activity; WMD, weight-maintaining diet; 24EE, 24-h energy expenditure.
Contributor Information
Tim Hollstein, Obesity and Diabetes Clinical Research Section, Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ, USA; Division of Endocrinology, Diabetology and Clinical Nutrition, Department of Internal Medicine 1, University of Kiel, Kiel, Germany; Institute of Diabetes and Clinical Metabolic Research, Kiel, Germany.
Alessio Basolo, Obesity and Diabetes Clinical Research Section, Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ, USA.
Takafumi Ando, Obesity and Diabetes Clinical Research Section, Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ, USA; Human Behavior Research Team, Human-Centered Mobility Research Center, Information Technology and Human Factors, National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan.
Jonathan Krakoff, Obesity and Diabetes Clinical Research Section, Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ, USA.
Paolo Piaggi, Obesity and Diabetes Clinical Research Section, Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ, USA; Department of Information Engineering, University of Pisa, Pisa, Italy.
Data Availability
Data described in the article will be made available upon request pending application and approval by the Institutional Review Board of the NIDDK.
References
- 1.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”?. Am J Hum Genet. 1962;14(4):353. [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. [DOI] [PubMed] [Google Scholar]
- 3.Piaggi P. Metabolic determinants of weight gain in humans. Obesity. 2019;27(5):691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J Endocrinol Invest. 2018;41(1):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollstein T, Ando T, Basolo A, Krakoff J, Votruba SB, Piaggi P. Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: further evidence for spendthrift and thrifty metabolic phenotypes. Am J Clin Nutr. 2019;110(3):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlogl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Diabetes. 2015;64(11):3680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, Votruba SB. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes. 2015;64(8):2859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johannsen DL, Marlatt KL, Conley KE, Smith SR, Ravussin E. Metabolic adaptation is not observed after 8 weeks of overfeeding but energy expenditure variability is associated with weight recovery. Am J Clin Nutr. 2009;110(4):805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes. 2001;25(5):593–600. [DOI] [PubMed] [Google Scholar]
- 10.Neumann RO. Experimentelle Beiträge zur Lehre von dem täglichen Nahrungsbedarf des Menschen unter besonderer Berücksichtigung der notwendigen Eiweissmenge. Kiel: R. Oldenbourg; 1902; [Google Scholar]
- 11.Miller D, Mumford P. Luxuskonsumption. In: Energy balance in man. Apfelbaum Meditor. Paris: Masson; 1973:195–207. [Google Scholar]
- 12.Miller DS, Mumford P, Stock MJ. Gluttony. Am J Clin Nutr. 1967;20(11):1223–9. [DOI] [PubMed] [Google Scholar]
- 13.Stirling JL, Stock MJ. Metabolic origins of thermogenesis induced by diet. 1968;220(5169):801–2. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell N, Stock M. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci. 1983;64(1):19–23. [DOI] [PubMed] [Google Scholar]
- 15.Stock MJ. Gluttony and thermogenesis revisited. Int J Obes. 1999;23(11):1105–17. [DOI] [PubMed] [Google Scholar]
- 16.Dulloo AG, Jacquet J. Low-protein overfeeding: a tool to unmask susceptibility to obesity in humans. Int J Obes. 1999;23(11):1118–21. [DOI] [PubMed] [Google Scholar]
- 17.Begaye B, Vinales KL, Hollstein T, Ando T, Walter M, Bogardus C, Krakoff J, Piaggi P. Impaired metabolic flexibility to high-fat overfeeding predicts future weight gain in healthy adults. Diabetes. 2020;69(2):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86(3):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DS, Mumford P. Gluttony. 1. An experimental study of overeating low- or high-protein diets. Am J Clin Nutr. 1967;20(11):1212–22. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association . 2. Classification and diagnosis of diabetes. Diabetes Care. Jan 2015;38(Supplement 1):S8–S16.. DOI: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 21.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab. 2013;98(7):2791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schutz Y, Ravussin E, Diethelm R, Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. Int J Obes. 1982;6(1):23–8. [PubMed] [Google Scholar]
- 24.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62(12):4043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. The J Clin Endocrinol Metab. 2013;98(4):E703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinitz S, Hollstein T, Ando T, Walter M, Basolo A, Krakoff J, Votruba SB, Piaggi P. Early adaptive thermogenesis is a determinant of weight loss after six weeks of caloric restriction in overweight subjects. Metabolism. 2020;110(9):154303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–72. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt M, Schlogl M, Bonfiglio S, Votruba SB, Krakoff J, Thearle MS. Lower core body temperature and greater body fat are components of a human thrifty phenotype. Int J Obes. 2016;40(5):754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollstein T, Basolo A, Ando T, Votruba SB, Walter M, Krakoff J, Piaggi P. Recharacterizing the metabolic state of energy balance in thrifty and spendthrift phenotypes. J Clin Endocrinol Metab. 2020;105(5):1375–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollstein T, Heinitz S, Ando T, Rodzevik TL, Basolo A, Walter M, Chang DC, Krakoff J, PiaggiP. Metabolic responses to 24-hour fasting and mild cold exposure in overweight individuals are correlated and accompanied by changes in FGF21 concentration. Diabetes. 2020;69(7):1382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollstein T, Vinales K, Chen KY, Cypess AM, Basolo A, Schlögl M, Krakoff J, Piaggi P. Reduced brown adipose tissue activity during cold exposure is a metabolic feature of the human thrifty phenotype. Metabolism. 2021;117:154709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollstein T, Heinitz S, Basolo A, Krakoff J, Votruba SB, Piaggi P. Reduced metabolic efficiency in sedentary eucaloric conditions predicts greater weight regain in adults with obesity following sustained weight loss. Int J Obes. 2021;45(4):840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wouters-Adriaens MPE, Westerterp KR. Basal metabolic rate as a proxy for overnight energy expenditure: the effect of age. Br J Nutr. 2006;95(6):1166–70. [DOI] [PubMed] [Google Scholar]
- 34.Jørgensen SW, Brøns C, Bluck L, Hjort L, Færch K, Thankamony A, Gillberg L, Friedrichsen M, Dunger DB, Vaag AA. Metabolic response to 36 hours of fasting in young men born small vs appropriate for gestational age. Diabetologia. 2015;58(1):178–87. [DOI] [PubMed] [Google Scholar]
- 35.Gillberg L, Rönn T, Jørgensen SW, Perfilyev A, Hjort L, Nilsson E, Brøns C, Vaag A, Ling C. Fasting unmasks differential fat and muscle transcriptional regulation of metabolic gene sets in low versus normal birth weight men. EBioMedicine. 2019;47:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinales KL, Begaye B, Bogardus C, Walter M, Krakoff J, Piaggi P. FGF21 is a hormonal mediator of the human “thrifty” metabolic phenotype. Diabetes. 2019;68(2):318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MWet al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flatt J. The biochemistry of energy expenditure. Rec Adv Obes Res. 1978;2:211–28. [Google Scholar]
- 39.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, Most M, Brock C, Mancuso S, Redman LM. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev. 1996;36(4):391–7. [DOI] [PubMed] [Google Scholar]
- 41.Bier DM. The energy costs of protein metabolism: lean and mean on Uncle Sam's team. In: The role of protein and amino acids in sustaining and enhancing performance. Edington MA, editor. Washington (DC): National Academies Press (US); 1999:109–19. [PubMed] [Google Scholar]
- 42.Brøns C, Lilleøre SK, Astrup A, Vaag A. Disproportionately increased 24-h energy expenditure and fat oxidation in young men with low birth weight during a high-fat overfeeding challenge. Eur J Nutr. 2016;55(6):2045–52. [DOI] [PubMed] [Google Scholar]
- 43.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Näslund E, Blundell JE. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr. 2013;97(1):7–14. [DOI] [PubMed] [Google Scholar]
- 44.McNeil J, Lamothe G, Cameron JD, Riou M-È, Cadieux S, Lafrenière J, Goldfield G, Willbond S, Prud'homme D, Doucet É. Investigating predictors of eating: is resting metabolic rate really the strongest proxy of energy intake?. Am J Clin Nutr. 2017;106(5):1206–12. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins M, Finlayson G, Duarte C, Gibbons C, Johnstone AM, Whybrow S, Horgan GW, Blundell JE, Stubbs RJ. Biological and psychological mediators of the relationships between fat mass, fat-free mass and energy intake. Int J Obes. 2019;43(2):233. [DOI] [PubMed] [Google Scholar]
- 46.Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int J Obes. 2014;38(2):243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab. 2015;100(8):3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stinson EJ, Graham AL, Thearle MS, Gluck ME, Krakoff J, Piaggi P. Cognitive dietary restraint, disinhibition, and hunger are associated with 24-h energy expenditure. Int J Obes. 2019;43(7):1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr. 2006;83(5):1076–81. [DOI] [PubMed] [Google Scholar]
- 50.Treuth MS, Butte NF, Sorkin JD. Predictors of body fat gain in nonobese girls with a familial predisposition to obesity. Am J Clin Nutr. 2003;78(6):1212–8. [DOI] [PubMed] [Google Scholar]
- 51.Hägele FA, Büsing F, Nas A, Hasler M, Müller MJ, Blundell JE, Bosy-Westphal A. Appetite control is improved by acute increases in energy turnover at different levels of energy balance. J Clin Endocrinol Metab. 2019;104(10):4481–91. [DOI] [PubMed] [Google Scholar]
- 52.Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work. Am J Clin Nutr. 1956;4(2):169–75. [DOI] [PubMed] [Google Scholar]
- 53.Basolo A, Votruba SB, Heinitz S, Krakoff J, Piaggi P. Deviations in energy sensing predict long-term weight change in overweight Native Americans. Metabolism. 2018;82:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segal KR, Albu J, Chun A, Edano A, Legaspi B, Pi-Sunyer FX. Independent effects of obesity and insulin resistance on postprandial thermogenesis in men. J Clin Invest. 1992;89(3):824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ando T, Piaggi P, Bogardus C, Krakoff J. VO2max is associated with measures of energy expenditure in sedentary condition but does not predict weight change. Metabolism. 2019;90:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article will be made available upon request pending application and approval by the Institutional Review Board of the NIDDK.